Abstract
Context: Quercetin, a flavonoid, has been tried in traditional medicine for treating many disorders and reported to have inhibitory action on PI3 kinase.
Objective: This study investigates the effect of quercetin on testosterone propionate induced polycystic ovary syndrome (PCOS) model, which shows both metabolic and endocrine features of PCOS.
Materials and methods: Female pre-pubertal Sprague–Dawley rats were randomly divided into four groups: normal control, PCOS control, quercetin, and metformin treated. PCOS was induced by testosterone propionate (10 mg/kg, s.c.) and treatments were carried out orally at the dose of 150 mg/kg from the 6th week. At the 6th and 10th week, blood was collected to investigate metabolic indices, and reproductive biochemical parameters including morphology of ovary, uterus, and estrous cyclicity were assessed. The ovaries were processed to determine CYP17A1 gene expression.
Results: The treatment with quercetin did not modify body weight gain but uterine (296.7 ± 5.11 versus 263.0 ± 8.60 mg) and ovary weights (49.5 ± 1.93 versus 37.8 ± 3.43 mg) were found to be decreased significantly (p <0.05) as compared with the PCOS control group. The PCOS control group showed hyperinsulinemia, hyperandrogenemia, and dyslipidemia. Treatment with quercetin showed statistically significant (p <0.01) improvement in insulin (12.46 ± 0.3 versus 10.0 ± 0.28 μU/ml), testosterone (0.65 ± 0.02 versus 0.29 ± 0.02 μU/ml), luteinising hormone (20.6 ± 0.28 versus 15.1 ± 0.36 U/ml), and lipid profile. Histological examination of ovary and uterus confirmed the disease occurrence and remission state in the diseased and treated groups, respectively. Quercetin also demonstrated PI3 kinase inhibition in a docking study and decreased CYP17A1 gene expression.
Discussion and conclusion: Thus, we can conclude that quercetin may have beneficial effect in PCOS by virtue of inhibition of PI3K which attributes to a decrease in the expression of CYP17A1 gene, having a key role in steroidogenesis.
Keywords: CYP17A1, cystic ovary, folliculogenesis, insulin resistance, quercetin, steroidogenesis
Introduction
Polycystic ovary syndrome (PCOS) is characterised by its three main consequences – hyperandrogenemia, hyperinsulinemia and/or abnormal ovulation. It is the most common endocrine disorder in women, with a prevalence of 6–10% based on the US National Institutes of Health (NIH) criteria and as high as 15% when the broader Rotterdam criteria are applied (Baillargeon et al. 2003). The complexity of PCOS can be well depicted by the fact that its etiology still remains ambiguous. Insulin resistance, glucose uptake, and glycogen synthesis declined along with exhibiting steroidogenic effect in ovaries during PCOS. Serine kinases (Li et al. 2002), m-TOR (Yaba and Demir 2012), AMP-activated protein kinase, sympathetic system (Lansdown and Rees 2012) and phosphatidylinositol 3-kinase (PI3K) (Munir et al. 2004) are the potential targets in the androgen synthesis and insulin pathway. PI3K inhibition in ovary leads to decreased androgen synthesis and its activation affects the positive actions of insulin like glucose uptake (Walker et al. 2000). Glucose uptake mediated by PI3K activation is not as much altered as the androgen levels by PI3K inhibition in PCOS. Therefore, PI3K inhibition can be a promising target in the treatment of PCOS.
Quercetin is the major flavonoid in the plant kingdom and is abundantly present in soybeans, vegetables, and fruits. Quercetin is a powerful bioactive constituent of the human diet, reported to have free radical scavenging, anti-inflammatory, anticancer, antihyperlipidemic, and antiplatelet effects (Juywiak et al. 2005). Quercetin is also found to produce inhibition of PI3K (Zhai et al. 2012). Thus, quercetin may produce beneficial effect in women with PCOS by acting on PI3K. In addition, insulin sensitisers used for treatment from a long time have recently demonstrated the risk of developing lactic acidosis in some patients and act only on insulin resistance pathway at periphery, not directly targeting ovarian cells. Hence, there is a need to identify new agents that would either replace insulin sensitisers or potentiate their beneficial effects by direct targeting steroidogenic pathway in ovarian theca cells. In the present study, we evaluated the effect of quercetin on testosterone propionate induced animal model of PCOS and its mechanism of action.
Materials and methods
Docking study
Docking experiments used to screen small molecules by orienting and scoring them in the binding site of a selected protein. Docking study was performed using SYBYL-X 1.3 and GOLD docking software. Quercetin was compared with other reported PI3K inhibitors such as wortmannin, PI103, GSK105915, and LY294002 based on fitness score, type of binding pattern, and energy values.
Animals and experimental protocol
Sprague–Dawley female (3-week-old) rats were obtained from the animal facility of Zydus Research Center, Ahmedabad, India. Animals were housed in a pathogen-free environment at the animal house of Institute of Pharmacy, Nirma University. All procedures described here were reviewed and approved by institutional animal ethical committee (IP/PCOL/MPH/13-1/005 dated on 5 August 2013).
Treatment protocol and blood collection time points
Sprague–Dawley female rats were randomly divided into four groups (n = 12): normal control, PCOS control, diseased animals treated with metformin (150 mg/kg, p.o.) and diseased animals treated with quercetin (150 mg/kg, p.o.). The PCOS was induced by subcutaneous injection of testosterone propionate (10 mg/kg, s.c.) dissolved in olive oil daily for 6 weeks (Hollman et al. 1995). The animals were examined for the development of PCOS at the end of 6 weeks by performing all the biochemical parameters from blood. Ovaries and uterus were isolated, weighed and histopathological studies were carried out to confirm PCOS development by observing polycystic ovaries and other histological changes in uterus. Treatment was continued in the remaining six animals for 6 weeks. At the end of the treatment, animals were subjected to morphological, biochemical and histopathological evaluations.
Evaluation parameters
Morphological changes
Body weight changes were measured for each group every week. Physical examination of hair growth was performed at the starting of the experiment and at the end of disease induction to check the presence of hirsutism in this model of PCOS.
Histological changes
Ovaries and uterus of sacrificed animals from each group were collected. These organs were stored in 10% formalin for fixation. After fixation, the organs were trimmed and embedded in paraffin block after dehydration. Sections were mounted on the glass slides by blocks and were stained using hematoxylin and eosin for histological examination. The histopathological slides were prepared and observed under microscope. The follicles, corpus luteum and luteomas were counted. Primordial follicle, primary follicle, preantral follicle, antral follicle and cystic follicle were observed.
Biochemical changes
At the end of the treatment, the animals were placed on an overnight fasting and the blood samples were collected and the serum was separated and stored at −20 °C until analysis was done. Serum samples were analysed for triglycerides and total cholesterol, high-density lipoprotein (HDL) levels using commercially available colorimetric diagnostic kits (Lab-Care Diagnostics Pvt. Ltd., Mumbai, India) using UV–visible spectrophotometer (Shimadzu UV-1601, Shimadzu Corp., Tokyo, Japan). Low-density lipoprotein (LDL) and very low-density lipoprotein (VLDL) were calculated from the values of total cholesterol, triglyceride, and HDL. Serum insulin, testosterone, and luteinising hormone (LH) were estimated by enzyme-linked immunosorbent assay technique using kits (NovaTec, Diagnostics Biochem Canada Inc., Dorchester, Canada and Erba Mannheim, Mannheim, Germany, respectively) with the help of Robonik readwell touch ELISA plate analyzer (Robonik India Private Limited, Mumbai, India).
Total RNA isolation and PCR
The primers were designed using nucleotide tool of NCBI database and were verified using Oligo Analyzer 3.1 software (Sigma, St. Louis, MO). The following designed primers were obtained from Eurofins Genomics Ltd (Bengaluru, India). The primers were used for CYP17A1 5′-ACTGAGGGTATCGTGGATGC-3′ (forward) and 5′-CCGTCAGGCTGGAGATAGAC-3′ (reverse). The primers were used for β-actin 5′-CAACTGGGACGATATGGAGAAG-3′ (forward) and 5′-CTCGAAGTCTAGGGCAACATAG-3′ (reverse). RNA was isolated from the ovaries using FastRNA® Pro Green kit from MP Biomedicals (Santa Ana, CA). The isolated RNAs were then quantified using UV-1800 Shimadzu spectrophotometer (Shimadzu Corp., Tokyo, Japan) at 250 nm and concentration was calculated. 10 pg–0.5 μg concentration of isolated RNA was subjected to cDNA synthesis using Revert Aid First Strand cDNA Synthesis Kit . After processing with PCR master mix (2×) (Thermo Scientific, Waltham, MA), cDNA strands were then subjected to Bio-Rad T-100TM Thermal cycler for DNA amplification with set protocol for cycles. The amplified PCR product was then run over gel electrophoresis along with GeneRulerTM 100 bp DNA Ladder (Fermentas Life Sciences, Waltham, MA) and gel was observed in an UV chamber.
Statistical analysis
The results were expressed as mean ± SEM. Statistical differences between groups were applied using Graph Pad Prism software version 6.0 (GraphPad Software, San Diego, CA). Results were analysed using one-way factorial analysis of variance ANOVA, and data were considered to be statistically significant at p value < 0.01 and <0.05.
Results
Docking scores
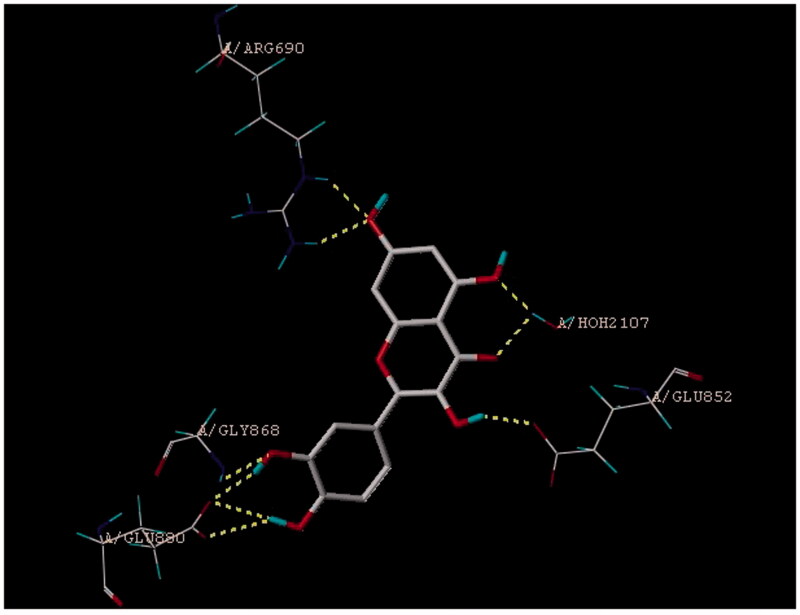
In the docking study, we have demonstrated that quercetin has perfect binding pose on PI3K substrate with four binding sites (Figure 1). Moreover, quercetin has shown highest binding affinity as compared with reported PI3K inhibitors such as wortmannin, PI103, GSK105915 and LY294002 based on the fitness score, type of binding pattern, and energy values (Table 1).
Figure 1.
Binding pose and binding sites of quercetin on PI3K.
Table 1.
Docking score of molecules from GOLD and SYBYL X-1.3.
| Molecule | GOLD score |
SYBYL X-1.3 score | |||
|---|---|---|---|---|---|
| Binding sites | Fitness | Docking Score | RMSD | ||
| Quercetin | ASP950, GLU880, LYS33, SER806 | 58.29 | −46.64 | 1.018 | 5.995 |
| Wortmannin | LYS833, VAL882, SER806 | 64.05 | −56.39 | 1.0317 | 4.269 |
| LY294002 | TYR867, ASP964, SER806 | 64.86 | −56.17 | 4.4323 | 4.681 |
| GSK105915 | ARG690, GLN846 | 47.98 | −54.68 | 2.347 | 4.832 |
| PI103 | TYR787, ASP964 | 56.71 | −53.26 | 2.886 | 5.709 |
Effect of treatment on physical parameters
In PCOS rats, body weight was found to be increased and by the treatment with metformin and quercetin the weight of animals was reduced, but it was not statistically significant. Therefore, the exact conclusion could not be drawn about body weight due to high variability. We have also observed slight increase in hair growth in PCOS animals and treatments have reduced it, but not significantly.
Effect of treatment on ovary and uterus
Testosterone administration was found to exhibit an increase in ovary and uterus weight as compared with the normal control group. Treatment with quercetin and metformin produced statistically significant decrease in ovary and uterus weight. Total follicles and corpus luteum count were found to be reduced in the disease control group and treatment with quercetin and metformin has produced significant increase in the number of normal follicles and corpus luteum (Table 2).
Table 2.
Effect of quercetin on uterus and ovaries weights, total follicles and corpus luteum count after treatment at 11th week.
| Group | NC | DC | DM | DQ |
|---|---|---|---|---|
| Ovary weight (mg) | 33.5 ± 2.40 | 49.5 ± 1.93# | 40.3 ± 1.58* | 37.8 ± 3.43* |
| Uterus weight (mg) | 266.3 ± 11.1 | 296.7 ± 5.11# | 263.8 ± 8.99* | 263.0 ± 8.60* |
| Total follicular count in ovarian section | 26.5 ± 1.5 | 12.8 ± 2.06# | 23.5 ± 3.1* | 22.0 ± 2.1* |
| Corpus luteum count in ovarian section | 12.5 ± 1.5 | 7.25 ± 1.32# | 8.9 ± 2.03* | 9.01 ± 1.9* |
Effect of treatment on histological parameters
In histopathological examination of ovary after induction of PCOS, testosterone propionate produced cystic formation, folliculogenesis, and decreased luteinisation as compared with normal animal (Figure 2A and B). Treatment with quercetin produced improvement in cyst formation, folliculogenesis, and luteinisation (Figure 2C), which was comparable with metformin (Figure 2D).
Figure 2.
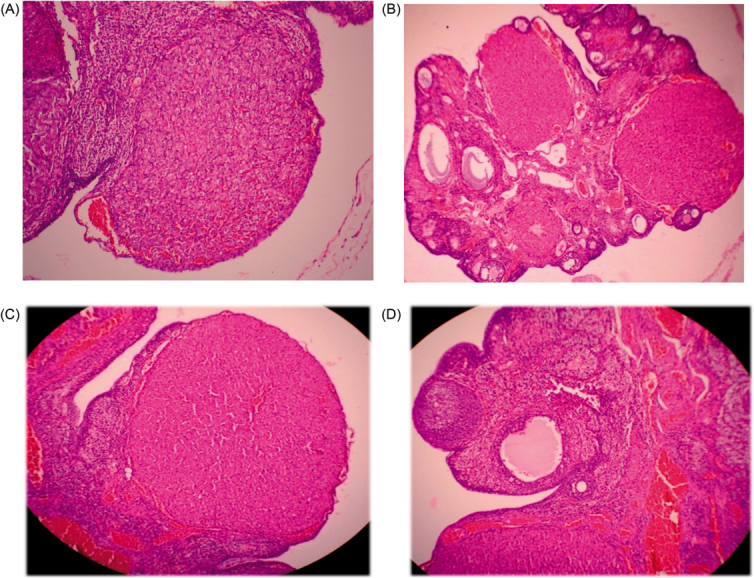
Histological changes in the ovary: (A) normal rat ovary; (B) cystic ovary after PCOS induction; (C) ovary treated with quercetin, nearly normal; (D) metformin-treated ovary close to normal.
The disease control group showed significant progression of disease in uterus by the development of adenomyosis as compared with normal myometrium in the normal control group (Figure 3A and B) and treatment with metformin and quercetin produced significant improvement in the histology of the uterus (Figure 3C and D).
Figure 3.
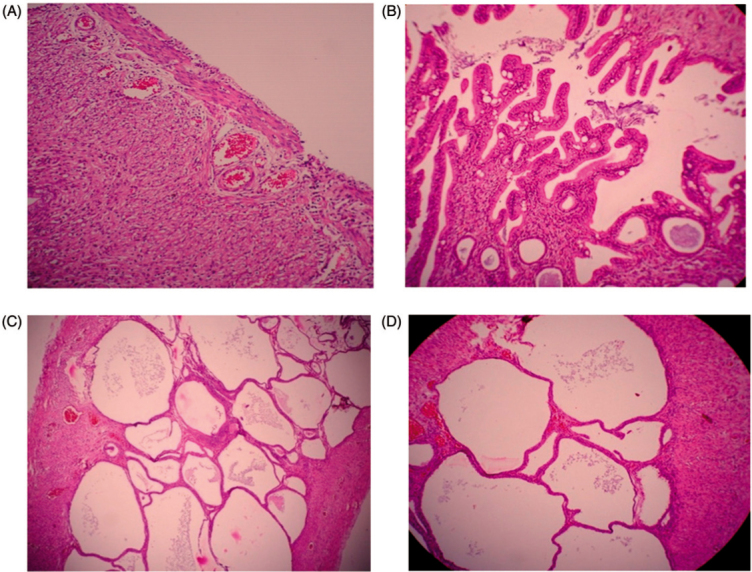
Histological changes in the uterus: (A) normal rat uterus; (B) diseased uterus after PCOS induction; (C) rat uterus treated with quercetin; (D) rat uterus treated with metformin.
Biochemical parameters
PCOS animals demonstrated dyslipidemia as early as 5 week of age, characterised by significantly increased plasma concentrations of cholesterol, triglyceride, LDL, and VLDL levels as compared with normal control animals. Treatment with quercetin and metformin produced significant reduction in elevated serum cholesterol, triglyceride, LDL, and VLDL levels as compared with the disease control group (p <0.05). While decreased HDL level was found to be improved as compared with the disease control group (Table 3).
Table 3.
Effect of quercetin on lipid parameters.
| Before treatment at 6th week (mean ± SEM) mg/dl |
|||||
|---|---|---|---|---|---|
| Groups | Cholesterol | Triglyceride | LDL | VLDL | HDL |
| NC | 96.0 ± 1.60 | 72.2 ± 10.66 | 34.9 ± 2.36 | 14.4 ± 0.87 | 46.7 ± 1.13 |
| DC | 228.8 ± 5.32# | 135.9 ± 3.12# | 176.4 ± 5.66# | 21.17 ± 1.07# | 25.2 ± 0.67# |
| DM | 225.6 ± 5.78# | 133.9 ± 3.69# | 173.7 ± 5.77# | 26.8 ± 1.12# | 25.1 ± 0.54# |
| DQ | 225.8 ± 5.77# | 124.6 ± 4.40# | 177.5 ± 5.43# | 24.9 ± 1.18# | 23.4 ± 0.54# |
| After treatment at 11th week (mean ± SEM) |
|||||
| NC | 97.7 ± 3.22 | 67.8 ± 14.7 | 36.1 ± 3.75 | 13.6 ± 1.20 | 48.05 ± 0.75 |
| DC | 208.9 ± 8.43# | 137.2 ± 19.19# | 177.0 ± 8.19# | 27.4 ± 1.57# | 26.8 ± 1.12# |
| DM | 147.6 ± 1.67* | 83.1 ± 14.6* | 89.4 ± 2.32* | 16.6 ± 1.20* | 41.6 ± 0.86* |
| DQ | 141.7 ± 3.10* | 74.6 ± 12.9* | 68.9 ± 4.07* | 14.9 ± 1.05* | 47.8 ± 0.98* |
*Significantly different from disease control (p < 0.01).
Significantly different from normal control (p < 0.01).
In the present study, insulin levels were found to be increased significantly (p <0.01) in the diseased group as compared with the normal control group. Treatment with quercetin and metformin produced significant decrease in insulin levels in both the groups (Table 4). In disease control rats, testosterone and luteinising hormone levels were increased significantly (p <0.01) as compared with normal control rats. After treatment with metformin and quercetin, both groups showed significantly (p <0.01) decreased testosterone and LH levels, respectively (Table 4).
Table 4.
Effect of quercetin serum insulin, testosterone, and luteinising hormone levels.
| Groups | Before treatment at 6th week (mean ± SEM) μU/ml |
||
|---|---|---|---|
| Insulin | Testosterone | Luteinising hormone | |
| NC | 8.54 ± 0.25 | 0.30 ± 0.02 | 13.41 ± 0.37 |
| DC | 12.04 ± 0.48# | 0.61 ± 0.02# | 20.38 ± 0.36# |
| DM | 13.86 ± 0.30# | 0.64 ± 0.02# | 21.64 ± 0.57# |
| DQ | 12.99 ± 0.31# | 0.65 ± 0.02# | 20.97 ± 0.57# |
| Groups | After treatment at the 11th week (mean ± SEM) |
||
| NC | 10.23 ± 0.34 | 0.26 ± 0.02 | 14.59 ± 0.36 |
| DC | 12.46 ± 0.30# | 0.65 ± 0.02# | 20.60 ± 0.28# |
| DM | 11.01 ± 0.31* | 0.34 ± 0.01* | 15.00 ± 0.36* |
| DQ | 10.00 ± 0.28* | 0.29 ± 0.02* | 15.10 ± 0.36* |
*Significantly different from disease control (p <0.01).
#Significantly different from normal control (p <0.01).
CYP17A1 gene expression in rat ovary
Results show that disease control animals showed a significant increase in CYP17A1 mRNA expression in the ovarian theca cells. Treatment with quercetin produced a significant decrease in CYP17A1 mRNA expression. While metformin did not alter CYP17A1 mRNA expression significantly (Figure 4).
Figure 4.
Gene expression of different groups using PCR: (A) CYP17A1 expression; (B) β-actin gene expression. In each image, first band was of the normal group, second was of the diseased group, third was of the metformin-treated group, and the fourth was the quercetin-treated group. The fifth band in each image is DNA ladder for the comparison of base pair size of PCR product expressed.
Discussion
The docking process involves a conformational search for ligand which compliments binding site, with the aim of identifying the best binding pose with the protein active site (Krishna et al. 2013). In the present study, we have presented the binding modes and binding site interactions of PI3 kinase with quercetin, and its score was compared with reported PI3 kinase inhibitors. Docking study of quercetin showed good binding score to PI3K. The core aglycone part of quercetin (pharmacophore) is reported to have binding affinity toward PI3K substrate and the glycone part enhances its binding further (Barber et al. 2008). The literature review of quercetin reveals that it inhibits PI3K (Walker et al. 2000). Thus, results of our docking study are in agreement with previous reports.
It has been reported that testosterone propionate-treated group weights of animals increase due to pathophysiology and hormonal imbalance in PCOS (Ota et al. 1983; Honnma et al. 2006). During the course of our study, we found weight gain in the testosterone propionate-treated group compared with the control group which may be due to deposition of fat in the abdominal region and insulin resistance. In our study, treatment with quercetin and metformin produced slight decrease in weight but could not produce significance reduction in weight gain. The slight reduction of weight may be because of the quercetin that has been reported to be useful in redistribution and uptake of fat and reported to be used in obesity (Aguirre et al. 2011). Increased hair growth is one of the symptoms of PCOS due to excess androgens. On an observational basis, we could confirm that quercetin was also useful in decreasing hirsutism. Moreover, in the present study, we observed increased uterus weight in the diseased group which may be due to the fat deposition in the abdominal region and inflammation caused by higher androgenic environment. On treatment with quercetin, the weight of uterus was found to be decreased. It may be due to amelioration by quercetin, which leads to decrease in inflammation by several of its mechanism. First and foremost, the mechanism could be caused by a decrease in the androgen level and by antioxidant property. PCOS rats exhibited formation of hollow cysts packed with follicular fluid in ovary similar to already studied ovarian histology by scientists. Also because of these cystic progressions, the ovary weight was observed to be higher in the diseased group during our study. On treatment with quercetin, we observed almost negligible cysts in the ovary and decrease in its weight. This may be due to improvement of hyperandrogenemia, and thus normalisation of hormonal imbalance. Improved hyperandrogenemia might be an outcome of decreased weight of ovary and cyst formation.
Histological examination in the present study showed ovaries with large, antral follicles, with a thickened theca interna cell layer; a diminished granulosa cell compartment were observed in disease control animals, which is consistent with previous findings (Kafali et al. 2004). This study also shows the absence of corpus luteum formation only when luteomas were seen, which do not favour the conception in the female rat, yielding to significant incapacity of ovulation. Also, the number of normal follicles of various stages decreases in disease condition. Foremost change in ovaries was formation of follicular cysts. Initially, degenerated follicles were formed and then it progressed towards cyst formation. The cystic follicles were devoid of nucleus or oocyte. Also, the absence of oocyte or corona radiate was observed in ovaries. Follicles which are degenerated or cystic were surrounded with increased hyperplasic luteinised cells. Second, increased theca cells with increased luteinisation surrounding it were found. Hyperthecosis with an increased number of widen stomas were observed. Decrease in granular cells, atrophy of interstitial cells, and hyperplasia in mesenchymal cells in ovarian cortex were also observed. The possible mechanism behind this is increased androgens and disrupted folliculogenesis due to disturbance in the estrous cycle (Wang et al. 2012; Linares et al. 2013). Treatment by quercetin increased normal follicles in ovaries as compared with disease control animal and also decreased cystic follicles and increased luteomas instead of corpus luteum, which may be due to decrease in androgen synthesis by quercetin.
Histopathological examinations of uterus from the disease control group showed that signs of increased inflammation were evident from adenomyosis (glands with their stoma invade myometrium from endometrium). Fibrous stoma and cluster of transitional cells were seen in the endometrium. Atrophy of endometrium and higher vacuolation were also observed. These histological changes were significantly improved by treatment with quercetin. We hypothesise that quercetin by reducing testosterone level inhibits the synthesis of estrogen and protects against atrophy of endometrium.
Hyperinsulinemia plays an important role in PCOS since it contributes to anovulation (Franks et al. 2000), impairs folliculogenesis, and affects follicular development (Musso et al. 2005). For these reasons, insulin-sensitising drug such as metformin is used in women with PCOS to improve insulin sensitivity (Vandermolen et al. 2001; Shah and Patel 2014). Although we did not directly assess insulin sensitivity, we measured markers of insulin sensitivity such as fasting serum insulin and fasting blood glucose. Our results showed that treatment with testosterone increased the serum insulin levels without affecting fasting glucose, while treatment with metformin and quercetin produced significant decrease in insulin levels as compared with PCOS controls. Our findings are in agreement with previous studies demonstrating that metformin increases peripheral insulin sensitivity in non-diabetic women with PCOS (Moghetti et al. 2000). Since quercetin also decreases insulin concentration and as in PCOS, insulin resistance at ovary plays a critical role than in pancreas or in skeleton muscles, it can be speculated that quercetin would regulate ovarian steroidogenesis by modulating insulin levels.
The data presented here show that rats from the testosterone-treated group exhibited significantly increased levels of both testosterone and luteinising hormone. The abnormal physiology like molecular derangement in the enzymes responsible for oestrogen synthesis may be responsible for hyperandrogenemia in diseased state. Moreover, lack of negative feedback at hypothalamus–pituitary axis leads to hypersecretion of LH. We can assume that in addition to the hyperandrogenised environment created by the daily injection of testosterone, the enhanced concentration of LH would result in unfavourable conditions for producing follicles. Evidence shows that women with PCOS show hypersecretion of both the hormones (Mendonca et al. 2004; Doi et al. 2005). These findings also support those of the current study. We demonstrate that the administration of quercetin produced a decrease in luteinising hormone and testosterone levels. As described earlier, LH stimulates androgen production in theca cells via activation of the PI3K/Akt pathway, which leads to excess androgen production via increase in 17α-hydroxylase activity (Fukuda et al. 2009). Correction in the hyperandrogenemia may perhaps be due to the inhibition of PI3K by quercetin. Inhibition of PI3K by Quercetin decreases the LH-induced Akt phosphorylation which results in the modulation of ovarian steroidogenesis.
Dyslipidemia is very common in PCOS and includes an artherogenic lipid profile characterised by elevated TG, cholesterol and LDL-C, and lowered HDL-C concentrations (Bahceci et al. 2007). In the present study, the PCOS rats exhibited fasting dyslipidemia. Hyperandrogenemia, hyperinsulinemia and hyperlipidemia are intimately interrelated, and each of these factors contributes to PCOS. Insulin resistance and hyperandrogenemia may also result in increased catecholamine-induced lipolysis and release of fatty acids from visceral adipose tissue into the circulation (Duez et al. 2008). Therefore, correction of hyperinsulinemia and hyperandrogenemia may improve dyslipidemia associated with PCOS. In the present study, treatment with quercetin produced decrease in lipid levels and also increased HDL levels. We therefore can assume that quercetin has a beneficial effect in reproductive and metabolic abnormalities.
Numerous reports have described that an activation of the intracellular signalling of PI3K/Akt plays important role in androgen production in theca cells (Carvalho et al. 2003; Fukuda et al. 2009). PI3K is involved in LH-induced Akt phosphorylation in theca cells which results in an increase in ovarian CYP17A1 gene expression. CYP17A1 gene is responsible for the increase in 17-α hydroxylase enzyme activity in patients with PCOS (Bogovich and Richards 1982; Wickenheisser et al. 2005). This enzyme plays key role in steroid synthesis by converting the progesterone to androgens, and thereby increases the level of androgens. In the present study, we observed increased expression of CYP17A1 gene in the PCOS control group. Treatment with quercetin produced a decrease in CYP17A1 gene expression. It is possible that PI3K inhibition by quercetin leads to a decrease in CYP17A1 gene expression in theca cells which may be responsible for a decrease in 17α-hydroxylase activity which likely plays a significant role in PCOS. However, metformin do not produce any significant reduction in CYP17A1 gene expression. Therefore, we could speculate that metformin would regulate ovarian steroidogenesis by modulating insulin levels.
Conclusion
In conclusion, our data suggest that quercetin shows protective effect in PCOS and its associated consequences like hyperandrogenemia, cystic ovaries, decreased, or abrupt follicles as evident from serum biochemical parameters, histological examination, morphological examination and gene expression study performed. The inhibition of PI3K by quercetin was evident in gene docking study. This study was evocative of the decreasing expression of CYP17A1 which is responsible for the activity of 17α-hydroxylase, a key enzyme for androgen synthesis. Moreover, the other beneficial side of quercetin could be regulation of ovarian steroidogenesis, both by direct action of quercetin on the ovarian theca cells and by modulating insulin levels. Thus, in brief, the efficacy of quercetin in treating PCOS is by inhibiting PI3K in ovarian theca cells which causes the decline in the androgen production by decreasing the expression of CYP17A1 gene.
Declaration of interest
The authors report that they have no conflicts of interest. The authors would like to acknowledge the funding provided by Institute of Pharmacy, Nirma University in the form of post-graduate contingency.
Correction Statement
This article was originally published with errors, which have now been corrected in the online version. Please see Correction (http://dx.doi.org/10.1080/13880209.2023.2233815).
References
- Aguirre L, Arias N, Macarulla MT, et al. 2011. Beneficial effects of quercetin on obesity and diabetes. Open Nutraceuticals J. 4:189–198. [Google Scholar]
- Bahceci M, Aydemir M, Tuzcu A.. 2007. Effects of oral fat and glucose tolerance test on serum lipid profile, apolipoprotein, and CRP concentration, and insulin resistance in patients with polycystic ovary syndrome. Fertil Steril. 87:1363–1368. [DOI] [PubMed] [Google Scholar]
- Baillargeon JP, Iuorno MJ, Nestler JE.. 2003. Insulin sensitizers for polycystic ovary syndrome. Clin Obstet Gynecol. 46:325–340. [DOI] [PubMed] [Google Scholar]
- Barber TM, Bennett AJ, Groves CJ, et al. 2008. Association of variants in the fat mass and obesity associated (FTO) gene with polycystic ovary syndrome. Diabetologia 51:1153–1158. [DOI] [PubMed] [Google Scholar]
- Bogovich K, Richards JS.. 1982. Androgen biosynthesis in developing ovarian follicles: evidence that luteinizing hormone regulates thecal 17 alpha-hydroxylase and C17-20-lyase activities. Endocrinology 111:1201–1208. [DOI] [PubMed] [Google Scholar]
- Carvalho CR, Carvalheira JB, Lima MH, et al. 2003. Novel signal transduction pathway for luteinizing hormone and its interaction with insulin: activation of Janus kinase/signal transducer and activator of transcription and phosphoinositol 3-kinase/Akt pathways. Endocrinology 144:638–647. [DOI] [PubMed] [Google Scholar]
- Doi SA, Al-Zaid M, Towers PA, et al. 2005. Irregular cycles and steroid hormones in polycystic ovary syndrome. Hum Reprod. 20:2402–2408. [DOI] [PubMed] [Google Scholar]
- Duez H, Lamarche B, Valéro R, et al. 2008. Both intestinal and hepatic lipoprotein production are stimulated by an acute elevation of plasma free fatty acids in humans. Circulation 117:2369–2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franks S, Mason H, Willis D.. 2000. Follicular dynamics in the polycystic ovary syndrome. Mol Cell Endocrinol. 25:49–52. [DOI] [PubMed] [Google Scholar]
- Fukuda S, Orisaka M, Tajima K, et al. 2009. Luteinizing hormone-induced Akt phosphorylation and androgen production are modulated by MAP kinase in bovine theca cells. J Ovarian Res. 2:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollman PC, De Vries JH, van Leeuwen SD, et al. 1995. Absorption of dietary quercetin glycosides and quercetin in healthy ileostomy volunteers. Am J Clin Nutr. 62:1276–1282. [DOI] [PubMed] [Google Scholar]
- Honnma H, Endo T, Henmi H, et al. 2006. Altered expression of Fas/Fas ligand/caspase 8 and membrane type 1-matrix metalloproteinase in atretic follicles within dehydroepiandrosterone-induced polycystic ovaries in rats. Apoptosis 11:1525–1533. [DOI] [PubMed] [Google Scholar]
- Juywiak S, Wójcicki J, Mokrzycki K, et al. 2005. Effect of quercetin on experimental hyperlipidemia and atherosclerosis in rabbits. Pharmacol Rep. 57:604–609. [PubMed] [Google Scholar]
- Kafali H, Iriadam M, Ozardali I, et al. 2004. Letrozole-induced polycystic ovaries in the rat: a new model for cystic ovarian disease. Arch Med Res. 35:103–108. [DOI] [PubMed] [Google Scholar]
- Krishna PS, Vani K, Prasad MR, et al. 2013. In silico molecular docking analysis of prodigiosin and cycloprodigiosin as COX-2 inhibitors. Springerplus 2:172–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansdown A, Rees DA.. 2012. The sympathetic nervous system in polycystic ovary syndrome: a novel therapeutic target ? Clin Endocrinol (Oxf). 77:791–801. [DOI] [PubMed] [Google Scholar]
- Li M, Youngren JF, Dunaif A, et al. 2002. Decreased insulin receptor (IR) autophosphorylation in fibroblasts from patients with PCOS: effects of serine kinase inhibitors and IR activators. J Clin Endocrinol Metab. 87:4088–4093. [DOI] [PubMed] [Google Scholar]
- Linares R, Hernandez D, Moran C, et al. 2013. Unilateral or bilateral vagotomy induces ovulation in both ovaries of rats with polycystic ovarian syndrome. Reprod Biol Endocrinol. 11:68–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendonca HC, Montenegro RM, Foss MC, et al. 2004. Positive correlation of serum leptin with estradiol levels in patients with polycystic ovary syndrome. Braz J Med Biol Res. 37:729–736. [DOI] [PubMed] [Google Scholar]
- Moghetti P, Castello R, Negri C, et al. 2000. Metformin effects on clinical features, endocrine and metabolic profiles, and insulin sensitivity in polycystic ovary syndrome: a randomized, double-blind, placebo-controlled 6-month trial, followed by open, long-term clinical evaluation. J Clin Endocrinol Metab. 85:139–146. [DOI] [PubMed] [Google Scholar]
- Munir I, Yen H, Geller DH, et al. 2004. Insulin augmentation of 17a-hydroxylase activity is mediated by phosphatidyl inositol 3-kinase but not extracellular signal-regulated kinase-1/2 in human ovarian theca cells. Endocrinology 145:175–183. [DOI] [PubMed] [Google Scholar]
- Musso C, Shawker T, Cochran E, et al. 2005. Clinical evidence that hyperinsulinaemia independent of gonadotropins stimulates ovarian growth. Clin Endocrinol (Oxf). 63:73–78. [DOI] [PubMed] [Google Scholar]
- Ota H, Fukushima M, Maki M.. 1983. Endocrinological and histological aspects of the process of polycystic ovary formation in the rat treated with testosterone propionate. Tohoku J Exp Med. 140:121–131. [DOI] [PubMed] [Google Scholar]
- Shah KN, Patel SS.. 2014. Phosphatidylinositide 3-kinase: a newer molecular target in metabolic and hormonal pathway of polycystic ovary syndrome. Exp Clin Endocrinol Diabetes 122:261–267. [DOI] [PubMed] [Google Scholar]
- Vandermolen DT, Ratts VS, Evans WS, et al. 2001. Metformin increases the ovulatory rate and pregnancy rate from clomiphene citrate in patients with polycystic ovary syndrome who are resistant to clomiphene citrate alone. Fertil Steril. 75:310–315. [DOI] [PubMed] [Google Scholar]
- Walker EH, Pacold ME, Perisic O, et al. 2000. Structural determinants of phosphoinositide 3-kinase inhibition by wortmannin, LY294002, quercetin, myricetin, and staurosporine. Mol Cell. 6:909–919. [DOI] [PubMed] [Google Scholar]
- Wang F, Yu B, Yang W, et al. 2012. Polycystic ovary syndrome resembling histopathological alterations in ovaries from prenatal androgenized female rats. J Ovarian Res. 5:15–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickenheisser JK, Nelson-Degrave VL, McAllister JM.. 2005. Dysregulation of cytochrome P450 17alpha-hydroxylase messenger ribonucleic acid stability in theca cells isolated from women with polycystic ovary syndrome. J Clin Endocrinol Metab. 90:1720–1727. [DOI] [PubMed] [Google Scholar]
- Yaba A, Demir N.. 2012. The mechanism of mTOR (mammalian target of rapamycin) in a mouse model of polycystic ovary syndrome (PCOS). J Ovarian Res. 5:38–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhai HL, Wu H, Xu H, et al. 2012. Trace glucose and lipid metabolism in high androgen and high-fat diet induced polycystic ovary syndrome rats. Reprod Biol Endocrinol. 10:5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]