Abstract
Introduction
Recently, a flow cytometric (FC) based test has been developed for detection of circulating fetal cells to replace the less accurate and reproducible Kleihauer-Betke test.
FC test is easier to perform, it can distinguish the origin of fetal cells, but it is expensive and available in highly specialized laboratories. We evaluated the introduction of high-performance liquid chromatography (HPLC) approach as initial screening to identify patients who need an additional FC test to better discriminate the nature of haemoglobin-F (HbF) positive cells.
Methods
Blood samples from 130 pregnant women suspected to have fetomaternal haemorrhage were analysed with HPLC and FC methods. The cut-off for HbF HPLC concentration was calculated. Statistical analyses for the evaluation of HPLC as a screening method were performed. The positivity cut-off of HbF to be used as decision-making value to continue the investigation was calculated.
Results
An excellent agreement (R2 > 0.90) was observed between the percentage of HbF obtained by HPLC and the percentage of fetal cells detected by FC. Results obtained from each assay were compared to define the HPLC threshold below which it is not necessary to continue the investigations, confirming the maternal nature of the HbF positive cells detected. Our study demonstrated that a cut-off of 1.0 % HbF obtained by HPLC was associated with the lowest rate of false negative results in our patient cohort.
Conclusions
This study provides a new FMH investigation approach that possibly leads to a reduction in times and costs of the analysis.
Keywords: Kleihauer-Betke test, Fetomaternal haemorrhage evaluation, High performance liquid chromatography, Flow cytometry, Haemoglobin-F
Highlights
-
•
HPLC screening test is cheap and could rapidly exclude fetomaternal bleeding.
-
•
Unlike the manual Kleihauer-Betke test, HPLC is a standardized automated approach.
-
•
Confirmation by flow cytometry could be required only for HPLC positive results.
1. Introduction
Fetomaternal haemorrhage (FMH) may occur at delivery or during pregnancy because of a sensitizing event causing fetal blood to enter maternal circulation, and leading to RhD immunisation if the mother is D-negative (RhD-) and the child is D-positive (RhD+). This can result in medical complication in case of a subsequent pregnancy [1,2]. The current practice of administering a standard dose of anti-D immunoglobulin to every RhD-pregnant woman at 28 weeks of gestation is associated with very low rate of Rh alloimmunization [3]. Since this dose neutralises up to 30 mL of RhD + fetal blood or 15 mL of fetal red blood cells, quantification of FMH is recommended to adapt the dose of anti-D in case of a massive FMH. It is therefore essential that an accurate identification and quantification of fetal red blood cells (RBCs) in peripheral maternal blood is provided to prevent maternal alloimmunization [3,4].
The Kleihauer-Betke test (KBT) for the assessment of fetal blood in maternal circulation has been normally employed in the diagnosis and prognosis of fetal anaemia and preterm labour, in case of intrauterine fetal demise and as a screening in the setting of trauma, which could lead to a break in the placental barrier and the consequent mixing of fetal haemoglobin (HbF) with maternal blood, as well as other events that can cause maternal immunisation [5]. Nevertheless, limitations in the sensitivity and precision associated with this widely used manual test have induced an increased diffusion of flow cytometric (FC) methods for fetal RBCs detection in maternal blood samples. Numerous studies confirmed that FC is more precise and easier to perform than the KBT, known to have poor reproducibility, multiple sources of variability and to be susceptible to false positive results related to the content of adult RBCs containing HbF, called F-cells, physiologically present at a range between 0.5 % and 0.7 % [[6], [7], [8], [9], [10], [11], [12], [13]].
The two parameters FC assay currently used is based on the identification of fetal cells using a commercial kit containing anti-HbF monoclonal antibodies and anti-carbonic anhydrase (CA) monoclonal antibodies [14]. Fetal RBCs and maternal F-cells differ in their content of HbF [[15], [16], [17]], but an appropriate discrimination of these cells based only upon the weaker fluorescence intensity of adult F-cells is difficult to achieve when targeting HbF alone. In contrast, CA expression reaches adult levels only 6 months after birth [18,19]: in this way it is possible to distinguish fetal RBCs from adult F-cells containing HbF. Therefore, combining the expression of HbF and CA, cells can be distinguished into fetal RBCs (HbF+, CA-), maternal F-cells (HbF+, CA+), and adult RBCs (HbF-, CA+) [14,20].
Although its advantages in terms of specificity and sensitivity, FC is expensive, time consuming, and it is not available in all care settings. To minimize these limitations, a first-step test to screen for fetal cells and to subsequently quantify them more accurately with FC in case of massive FMH.
High-performance liquid chromatography (HPLC), in particular cation-exchange HPLC, is accepted as the gold standard method for haemoglobin disorders diagnosis. HPLC uses cation exchange chromatography to retain the major haemoglobin species on the column. Separation is accomplished using an elution salt gradient and the retention times of the various haemoglobin species are visualized with a chromatogram. This analytical method allows separation of haemoglobin in its different physiological fractions, with accurate measurement of HbA2 and HbF, and sensitive detection of abnormal haemoglobin fractions for the diagnosis of haemoglobin variants. A non-pathological haemoglobin profile has approximately 97 % Hb A, 2.5 % Hb A2, and <1 % Hb F in HPLC [21,22].
We have evaluated the utility of HPLC as initial screening approach for FMH investigation comparing data obtained analysing samples in duplicate with FC and HPLC. The purposes of this investigation were: 1) to compare the ability of the two methods to detect a fetomaternal bleeding in peripheral blood samples; 2) to evaluate whether there was a correlation between HbF percentage determined by HPLC (HbF-HPLC) and fetal RBCs determined by FC (fetal RBCs-FC), and 3) to establish a HbF-HPLC cut-off as a screening value to detect samples that require a FC evaluation to identify a clinically significant FMH volume.
2. Methods
2.1. Patients and specimen selection
The study included the retrospective analysis of 130 pregnant women who underwent FMH evaluation at the Flow Cytometry Diagnostic Centre and Immunotherapy of the AOU Careggi of Florence, between May 2011 and January 2023.
All patients enrolled in the study were admitted for intrauterine fetal demise, fetal anaemia, caesarean delivery or trauma, and were evaluated in our laboratory to ascertain whether a fetomaternal bleeding had occurred. The analysed cohort did not include patients with sickle cell anaemia, heterozygosity for HbS or other Hb variant, or Hereditary Persistence of Fetal Haemoglobin (HPFH), all well-known conditions that can induce an increase in HbF.
Samples of whole venous blood were collected into EDTA anticoagulant and analysed in duplicate using both FC and HPLC.
2.2. Ethics
Procedures on donors’ specimens were approved by the Careggi University Hospital Ethical Committee and were in accordance with the declaration of Helsinki. Relevant demographic and diagnostic data were assembled through medical records review, including laboratory test results and clinical notes, according to internal policy of privacy observance.
2.3. Quantification of HbF by high-performance liquid chromatography
High-performance liquid chromatography test was performed on EDTA-anticoagulated whole blood samples. Samples were analysed on Biorad variant II HPLC.
The separation of the haemoglobin fractions takes place by means of a gradient between two mobile phases differing in salt concentration and pH. Physical characteristics, such as surface charge and the presence of hydrophilic and hydrophobic groups, determine the elution rate of each fraction.
Samples are automatically haemolysed and injected into the column during the gradient flow of mobile phases 1 and 2. The haemoglobin fractions migrate through the column at a rate determined by their physical properties and, as they exit the column, they pass through the spectrophotometric detector following the absorbance measurement at a wavelength of 413 nm ± 2 nm.
The instrument is calibrated for HbA2 and HbF measurement and is able to separate physiological haemoglobin fractions and many haemoglobin variants. The duration of the test is approximately 8 min for each sample.
All chromatograms were interpreted by determining HbA2 concentration for beta-thalassemia with also evaluating retention times, area percentages and windows of all the other structural variants. In each chromatogram, there were peaks for HbA, HbA2, and HbF. The lower limit of HbF detection is 0.4 %.
2.4. Quantification of fetal RBCs and maternal F cells by flow cytometry
The amount of fetal RBCs and adult F-cells in maternal whole blood samples was quantified by FC assay using a Fetal Cell Count™ Kit (IQ Products, Groningen, The Netherlands), including reagents for cell fixation, permeabilization, and antibodies for the fluorescence staining (anti-human HbF R-PE and anti-human CA FITC). The manufacturer's instructions were followed. To reduce the background signal, samples analysed more than 12 h but within two days from withdrawal were previously washed with PBS. Briefly, cells were fixed in the formaldehyde containing solution and then permeabilized before the fluorescent staining. RBCs suspension was subsequently incubated with anti-human HbF R-PE and anti-human CA FITC. After incubation, cells were washed to remove unfixed antibodies and resuspended in PBS for acquisition on a flow cytometer BD FACS Canto II (BD Biosciences, San Jose, CA, USA). A minimum of 100 000 events was recorded within the region gated at the erythrocytes and analysed for the expression of HbF and CA.
The analysis of recorded samples was performed using the BD FACSDiva software (BD Biosciences, San Jose, CA, USA). The FMH volume was estimated by taking into account the percentage of fetal RBCs (HbF+, CA-), maternal RBCs (HbF-, CA+) and maternal F-cells (HbF+, CA+). FMH volume was calculated using a simplified version of Mollison's formula [23] as reported by ANZSBT [24]:
| (1) |
ANZSBT proposes the formula above to evaluate FMH volume in whole blood samples. This formula is a simplified version of the formula described by Mollison. Abbreviations: FMH, fetomaternal haemorrhage; RBCs, red blood cells; mRBCs, maternal red blood cells; F-cells, maternal F-cells.
A sample was considered contaminated when a minimum volume of bleeding of 5 mL could be estimated [5].
2.5. Statistical analysis
Linear regression was performed in order to evaluate whether there was a correlation between HbF-HPLC and fetal RBCs-FC [25]. The Receiver Operator Characteristic (ROC) curve was then determined, aiming to evaluate a HPLC cut-off helpful to determine positive and negative samples [26].
3. Results
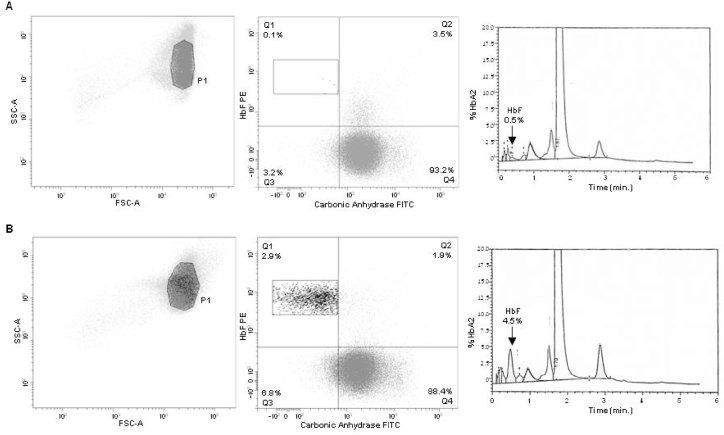
The haemoglobin fractions detected by HPLC were visualized with a chromatogram, where HbF is represented by a specific peak (Fig. 1-B).
Fig. 1.
Flow cytometric and high-performance liquid chromatography analysis of samples from two pregnant women. RBCs were analysed according to the procedures described in Materials and Method section. Gating Strategy for RBC identification, FC dot plots (HbF-PE vs. CA-FITC) divided into Q1, Q2, Q3 and Q4 gates containing fetal RBCs (HbF+, CA-), maternal F-cells (HbF+, CA+), unstained cells (HbF-, CA-) and adult RBCs (HbF-, CA+) and histograms obtained by HPLC analysis (black arrow corresponding to HbF peak) related to a negative (A) and a positive sample (B), respectively.
The FC analysis provided a HbF/CA plot where fetal RBCs, maternal RBCs and maternal F-cells populations were distinctly separated and located in the upper left, lower right, and upper right gates respectively (Fig. 1-A). This clear discrimination of fetal and maternal cells allowed the quantification of fetal RBCs percentage and the precise evaluation of fetomaternal bleeding, as described in the previous section.
Linear regression between FC and HPLC was performed in 130 samples. The distinction between positive and negative samples, 47 and 83 respectively, was made according to FMH volume estimated with the FC gold standard method, as described under the Methods section.
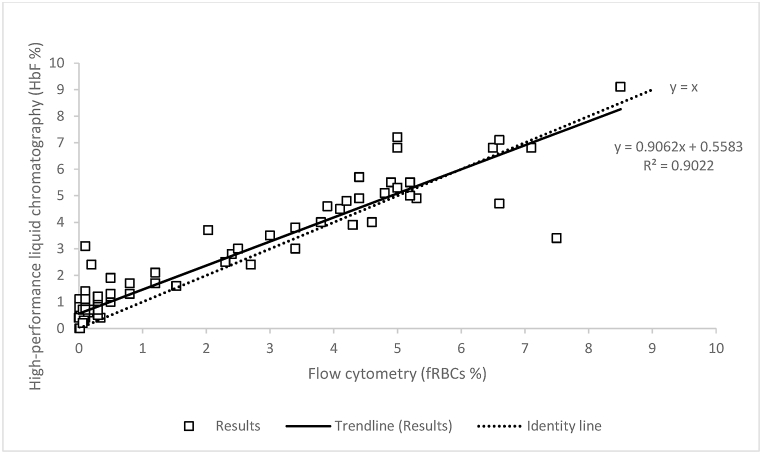
We evaluated whether fetal RBCs-FC was related to HbF-HPLC. The regression analysis showed a linear correlation between the two methods, with a positive slope and providing a R2 value > 0.90 (y = 0.9062x + 0.5583) (Fig. 2).
Fig. 2.
Regression analysis comparing high-performance liquid chromatography and flow cytometry performances in 130 samples. The correlation between the percentage of HbF (HbF%) detected by HPLC and the percentage of fetal RBCS (fRBCs%) detected by FC was evaluated. Plot represents, for each sample, values obtained with HPLC (Y axis) versus FC (X axis). The regression analysis showed a linear correlation, with a positive slope and R2 value > 0.90.
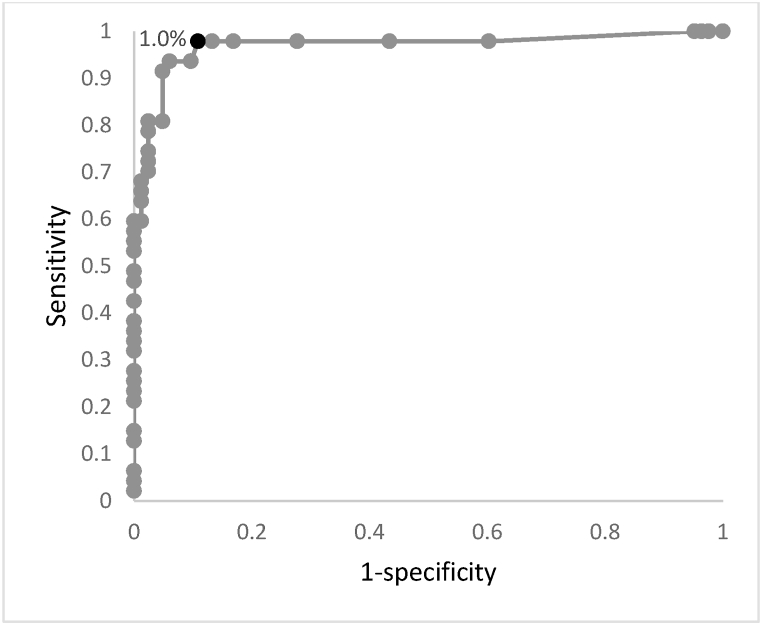
The aim of our study was, beyond providing a linear correlation, to propose a HPLC cut-off that could both maximise the ability to detect positive samples and minimize the proportion of false positive results. For this purpose, a ROC Curve was created with data referring to HbF-HPLC and assuming FC as the gold standard method to differentiate between positive and negative samples. As shown in Fig. 3, the chosen cut-off of HbF percentage is 1.0 %.
Fig. 3.
Threshold determination for the HPLC screening method. The black dot corresponds to 1.0 % cut-off value: the resulting sensitivity and specificity are respectively 0.98 and 0.89.
In order to achieve a higher negative predictive value for the HPLC screening test (which would bring to further diagnostic investigation), the chosen cut-off is 1.0 %. Such value allows a sensitivity 0.98, specificity 0.89, positive predictive value 0.84, negative predictive value 0.99 and accuracy 0.92.
We found only one discordant HbF-HPLC-/fetal RBCs-FC + result among the 130 analysed samples. In this case, HbF-HPLC was <1.0 % and FC-estimated bleeding volume was 8.2 mL. The FC-estimated bleeding volume was higher than the 5 mL FC cut-off [5], but it remained lower than the 15 mL threshold indicated by guidelines for additional anti-D administration [3,4], as further discussed.
4. Conclusions
Accurate detection and subsequent quantitation of FMH is fundamental in the management of pregnancy in the setting of diagnosis and prognosis of fetal anaemia, intrauterine fetal demise, traumas that could lead to the presence of fetal haemoglobin in maternal blood, and in case of suspected fetomaternal haemorrhage and maternal immunisation.
The well-established confirmatory test represented by FC [1], a method characterised by high sensitivity and specificity, is expensive and requires trained technicians and special equipment that is not available in all care settings. Therefore, the employment of a screening test would be a better option to avoid unnecessary FC testing. Although the manual KBT is still widely used for fetomaternal bleeding assessment, it is based on the visual microscopic counting of fetal RBCs on a blood film; consequently, it suffers from inter-observer and inter-laboratory variability. On the contrary, HPLC, although being less accurate than FC, is a fully automated approach, which is easier to standardise among different laboratories. Furthermore, HPLC is a widespread analytical method to measure Hba1c and for first level screening of thalassemia in diagnostic laboratories. For these reasons, we propose HPLC instead of KBT as a first step test in the contest of FMH evaluation.
In this study, we aimed to evaluate the correlation between FC and HPLC, in order to determine a cut-off percentage of HbF that could screen samples needing further investigation for FMH.
HPLC is currently used for separation and identification of the most common haemoglobin fractions. It can easily detect the percentage of HbF in the sample, but it is not able to determine whether it is fetal or maternal in origin. Therefore, positive HPLC results must be confirmed using FC for FMH assessment. FC exploits monoclonal antibodies against HbF and CA and leads to an accurate quantification of adult RBCs, fetal RBCs, and adult F-cells.
Comparing results obtained with FC and HPLC, an excellent agreement (R2 > 0.90) between HbF-HPLC and fetal RBCs-FC was observed. These results demonstrate the correlation between FC and HPLC for the detection of HbF in peripheral blood, allowing the application of the latter method as a screening of a potential FMH.
Furthermore, from the examination of data obtained through a ROC curve analysis, a HPLC threshold was proposed in order to advise FC only for samples above this threshold.
From our retrospective study we observed that a cut-off value of 1.0 % HbF was associated to a very low rate of false negative results: in our cohort, only a sample had a negative HbF-HPLC but a positive FC result with an estimated FMH volume of 8.2 mL, which was notably below the 15 mL threshold indicated by guidelines as the decision-making value for recommending an increased dose of the Rh immune globulin to prevent sensitisation [3,4]. Further studies involving a wider availability of clinical records will allow to better analysing the origin of false negative results.
Since the 1.0 % HbF threshold is associated with a negative predictive value (NPV) of 0.99, it can be applied in HPLC analysis to identify negative samples which do not need further testing, even if a certain percentage of false positive samples will require a FC detailed study: indeed, in samples analysed in duplicate, we noticed that HbF values obtained by HPLC approach were usually higher than fetal RBCs percentage obtained by FC. As described in literature, it is possible that physiological changes occurring during pregnancy may increase F-cells number [9,15]. In this setting, a higher baseline F-cells number causes an overestimation of HbF-HPLC, leading to a certain rate of false positive samples that need to be confirmed with FC. In spite of this, false positive results obtained with HPLC screening were a relatively small proportion (9/130) of our patient cohort and do not invalidate the final FMH volume estimation, because the following in-depth FC analysis can easily differentiate between maternal and fetal cells containing HbF.
In summary, HPLC employment as a screening approach appears to be useful in the laboratory routine as a first step to avoid unnecessary FC testing: considering the restrictive aspects in terms of resource allocation and cost-effectiveness, FC seems to be best suited as a confirmatory test for FMH.
Of note, many authors have investigated the correlation between analytical performances of different HPLC systems and capillary electrophoresis (CE) in separating and measuring Hb fractions. Even if a standardization of Hba2 measurement is not yet available [27], the performances of the two methods and different analytical platforms showed a good correlation [28], demonstrating that all HPLC platforms or CE platforms may be used to measure HbF in this type of experimental setting.
Likewise, new generation FC with greater sensibility which allow faster acquisition of data and more standardised analysis are already used in diagnostic routine; fetal RBCs-FC is a relatively simple FC test and validation in new instrument should be feasible.
In conclusion the application of HPLC as a screening test with an appropriate threshold could be a practical way to achieve a fully automated, fast performing and less expensive approach to exclude a fetomaternal bleeding and advise FC only for HPLC positive samples. A limitation in the present study is the impossibility to apply the described strategy to patients who suffer from sickle cell anaemia, HPFH and other conditions associated with an increase in HbF, since they were not included in the analysed cohort. Further studies involving a larger cohort of patients would be advantageous in clinical practice.
Data sharing statement
Data are available on request due to privacy/ethical restrictions.
CRediT authorship contribution statement
Benedetta Peruzzi: Writing – review & editing, Writing – original draft, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Serena Guerrieri: Writing – review & editing, Writing – original draft, Investigation, Formal analysis, Data curation. Tiziana Biagioli: Writing – review & editing, Writing – original draft, Resources, Investigation, Formal analysis, Data curation, Conceptualization. Luisa Lanzilao: Writing – review & editing, Formal analysis, Data curation. Sara Pratesi: Writing – review & editing, Investigation, Formal analysis. Sara Bencini: Resources, Investigation. Marinella Statello: Methodology. Alessia Carraresi: Methodology. Stefania Stefanelli: Methodology. Martina Tonelli: Methodology. Marco Brogi: Resources, Investigation. Manuela Capone: Resources, Investigation. Alessio Mazzoni: Writing – review & editing, Investigation. Anna Maria Grazia Gelli: Conceptualization. Alessandra Fanelli: Supervision. Roberto Caporale: Supervision, Resources, Investigation, Conceptualization. Francesco Annunziato: Supervision, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Data availability
The data that has been used is confidential.
References
- 1.Austin E., Bates S., De Silva M., Howarth D., Lubenko A., Rowley M., Scott M., Thomas E., White J., Williams M. Guidelines for the estimation of fetomaternal haemorrhage. Working Party of the British Committee for Standards in Haematology. Transfusion Task force. 2009;15:1–23. [Google Scholar]
- 2.Sebring E.S., Polesky H.F. Fetomaternal hemorrhage: incidence, risk factors, time of occurrence, and clinical effects. Transfusion. 1990;30(4):344–357. doi: 10.1046/j.1537-2995.1990.30490273444.x. [DOI] [PubMed] [Google Scholar]
- 3.Practice bulletin No. 181: prevention of Rh D alloimmunization. Obstet. Gynecol. 2017;130(2):e57–e70. doi: 10.1097/AOG.0000000000002232. [DOI] [PubMed] [Google Scholar]
- 4.Bennardello F., Coluzzi S., Curciarello G., Todros T., Villa S. Italian society of transfusion medicine and immunohaematology (SIMTI) and Italian society of gynaecology and obstetrics (SIGO) working group. Recommendations for the prevention and treatment of haemolytic disease of the foetus and newborn. Blood Transfus. 2015 Jan;13(1):109–134. doi: 10.2450/2014.0119-14. PMID: 25633877; PMCID: PMC4317097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krywko D.M., Yarrarapu S.N.S., Shunkwiler S.M. StatPearls. Treasure Island (FL) StatPearls Publishing; August 8, 2022. Kleihauer betke test. [Google Scholar]
- 6.Davis B.H., Olsen S., Bigelow N.C., Chen J.C. Detection of fetal red cells in fetomaternal hemorrhage using a fetal hemoglobin monoclonal antibody by flow cytometry. Transfusion. 1998;38(8):749–756. doi: 10.1046/j.1537-2995.1998.38898375514.x. [DOI] [PubMed] [Google Scholar]
- 7.Chambers E., Davies L., Evans S., Birchall J., Kumpel B. Comparison of haemoglobin F detection by the acid elution test, flow cytometry and high-performance liquid chromatography in maternal blood samples analysed for fetomaternal haemorrhage. Transfus. Med. 2012;22(3):199–204. doi: 10.1111/j.1365-3148.2012.01143.x. [DOI] [PubMed] [Google Scholar]
- 8.Duckett J.R., Constantine G. The Kleihauer technique: an accurate method of quantifying fetomaternal haemorrhage? Br. J. Obstet. Gynaecol. 1997;104(7):845–846. doi: 10.1111/j.1471-0528.1997.tb12032.x. [DOI] [PubMed] [Google Scholar]
- 9.Italia K.Y., Colah R., Mohanty D. Evaluation of F cells in sickle cell disorders by flow cytometry -- comparison with the Kleihauer-Betke's slide method. Int J Lab Hematol. 2007;29(6):409–414. doi: 10.1111/j.1365-2257.2006.00884.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Z., Ge J., Gong Z., Chen J., Wang C., Sun Y. Evaluation of machine learning-driven automated Kleihauer-Betke counting: a method comparison study. Int J Lab Hematol. 2021;43(3):372–377. doi: 10.1111/ijlh.13380. [DOI] [PubMed] [Google Scholar]
- 11.Kim Y.A., Makar R.S. Detection of fetomaternal hemorrhage. Am. J. Hematol. 2012;87(4):417–423. doi: 10.1002/ajh.22255. [DOI] [PubMed] [Google Scholar]
- 12.Kush M.L., Muench M.V., Harman C.R., Baschat A.A. Persistent fetal hemoglobin in maternal circulation complicating the diagnosis of fetomaternal hemorrhage. Obstet. Gynecol. 2005;105(4):872–874. doi: 10.1097/01.AOG.0000141646.58884.09. [DOI] [PubMed] [Google Scholar]
- 13.Navenot J.M., Merghoub T., Ducrocq R., Muller J.Y., Krishnamoorthy R., Blanchard D. New method for quantitative determination of fetal hemoglobin-containing red blood cells by flow cytometry: application to sickle-cell disease. Cytometry. 1998;32(3):186–190. [PubMed] [Google Scholar]
- 14.Porra V., Bernaud J., Gueret P., et al. Identification and quantification of fetal red blood cells in maternal blood by a dual-color flow cytometric method: evaluation of the Fetal Cell Count kit. Transfusion. 2007;47(7):1281–1289. doi: 10.1111/j.1537-2995.2007.01271.x. [DOI] [PubMed] [Google Scholar]
- 15.Rochette J., Craig J.E., Thein S.L. Fetal hemoglobin levels in adults. Blood Rev. 1994;8(4):213–224. doi: 10.1016/0268-960x(94)90109-0. [DOI] [PubMed] [Google Scholar]
- 16.Manca L., Masala B. Disorders of the synthesis of human fetal hemoglobin. IUBMB Life. 2008;60(2):94–111. doi: 10.1002/iub.4. [DOI] [PubMed] [Google Scholar]
- 17.Dziegiel M.H., Nielsen L.K., Berkowicz A. Detecting fetomaternal hemorrhage by flow cytometry. Curr. Opin. Hematol. 2006;13(6):490–495. doi: 10.1097/01.moh.0000245687.09215.c4. [DOI] [PubMed] [Google Scholar]
- 18.Aliakbar S., Brown P.R. Measurement of human erythrocyte CAI and CAII in adult, newborn, and fetal blood. Clin. Biochem. 1996;29(2):157–164. doi: 10.1016/0009-9120(95)02021-7. [DOI] [PubMed] [Google Scholar]
- 19.Brady H.J., Edwards M., Linch D.C., Knott L., Barlow J.H., Butterworth P.H. Expression of the human carbonic anhydrase I gene is activated late in fetal erythroid development and regulated by stage-specific trans-acting factors. Br. J. Haematol. 1990;76(1):135–142. doi: 10.1111/j.1365-2141.1990.tb07848.x. [DOI] [PubMed] [Google Scholar]
- 20.Othman J., Orellana D., Chen L.S., Russell M., Khoo T.L. The presence of F cells with a fetal phenotype in adults with hemoglobinopathies limits the utility of flow cytometry for quantitation of fetomaternal hemorrhage. Cytometry B Clin Cytom. 2018;94(4):695–698. doi: 10.1002/cyto.b.21598. [DOI] [PubMed] [Google Scholar]
- 21.Bisse E., Wieland H. High-performance liquid chromatographic separation of human haemoglobins. Simultaneous quantitation of foetal and glycated haemoglobins. J Chromatogr. 1988;434(1):95–110. doi: 10.1016/0378-4347(88)80065-3. [DOI] [PubMed] [Google Scholar]
- 22.Frömmel C. Newborn screening for sickle cell disease and other hemoglobinopathies: a short review on classical laboratory methods-isoelectric focusing, HPLC, and capillary electrophoresis. Int J Neonatal Screen. 2018;4(4):39. doi: 10.3390/ijns4040039. Published 2018 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mollison P.L. Quantitation of transplacental haemorrhage. Br. Med. J. 1972;3(5817):31–34. doi: 10.1136/bmj.3.5817.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guidelines for laboratory estimation of fetomaternal haemorrhage Australian and New Zealand Society of Blood Transfusion (ANZSBT) 2021 2nd edition September 2021. [Google Scholar]
- 25.Vidali M., Padoan A., Dittadi R., Brugnoni D., Carobene A., Mattioli S., Sciacovelli L., Ceriotti F. Protocollo operativo per la verifica della comparabilità dei risultati di laboratorio ottenuti su più procedure analitiche. Biochim. Clin. 2019;43:228–243. doi: 10.19186/BC_2019.047. [DOI] [Google Scholar]
- 26.D'Arrigo G., Provenzano F., Torino C., Zoccali C., Tripepi G. I test diagnostici e l'analisi della curva ROC [Diagnostic tests and ROC curves analysis] G. Ital. Nefrol. 2011;28(6):642–647. [PubMed] [Google Scholar]
- 27.Paleari R., Ceriotti F., Harteveld C.L., Strollo M., Bakker-Verweij G., Ter Huurne J., Bisoen S., Mosca A. Calibration by commutable control materials is able to reduce inter-method differences of current high-performance methods for HbA2. Clin. Chim. Acta. 2018 Feb;477:60–65. doi: 10.1016/j.cca.2017.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Degandt S., Coens R., Cauwelier B., Devos H., Langlois M., Emmerechts J. Evaluation of four hemoglobin separation analyzers for hemoglobinopathy diagnosis. J. Clin. Lab. Anal. 2018 Jan;32(1) doi: 10.1002/jcla.22224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that has been used is confidential.