Abstract
Germinating conidia of many phytopathogenic fungi must differentiate into an infection structure called the appressorium in order to penetrate into their hosts. This differentiation is known to require contact with a hard surface. However, the molecular basis for this requirement is not known. Induction of this differentiation in the avocado pathogen, Colletotrichum gloeosporioides, by chemical signals such as the host's surface wax or the fruit-ripening hormone, ethylene, requires contact of the conidia with a hard surface for about 2 h. To study molecular events triggered by hard-surface contact, we isolated several genes expressed during the early stage of hard-surface treatment by a differential-display method. The genes that encode Colletotrichum hard-surface induced proteins are designated chip genes. In this study, we report the characterization of CHIP2 and CHIP3 genes that would encode proteins with molecular masses of 65 and 64 kDa, respectively, that have no homology to any known proteins. The CHIP2 product would contain a putative nuclear localization signal, a leucine zipper motif, and a heptad repeat region which might dimerize into coiled-coil structure. The CHIP3 product would be a nine-transmembrane-domain-containing protein. RNA blots showed that CHIP2 and CHIP3 are induced by a 2-h hard-surface contact. However, disruption of these genes did not affect the appressorium-forming ability and did not cause a significant decrease in virulence on avocado or tomato fruits suggesting that C. gloeosporioides might have genes functionally redundant to CHIP2 and CHIP3 or that these genes induced by hard-surface contact control processes not directly involved in pathogenesis.
The germinating conidia of many plant-pathogenic fungi use physical or chemical signals from the plant surface to trigger differentiation of infection structures, appressoria, that are necessary for successful penetration of the host plant (11, 42). Anthracnose disease caused by Colletotrichum (Gloeosporium) of the Glomerella group is very common and destructive on numerous crop and ornamental plants worldwide. Conidia of Colletotrichum gloeosporioides germinate and form appressoria in response to chemical signals such as the host surface wax and the fruit-ripening hormone, ethylene (12, 13, 19, 36). The appressorium produces infection peg that penetrates the preformed defensive barriers, such as the cuticle and the underlying pectinaceous layer, probably using turgor-generated physical force (3, 8, 17) with assistance from the enzymes secreted in response to host signals (36, 47). Some of the genes involved in appressorium formation have been cloned from C. gloeosporioides, and the transcriptional regulation by plant signals has been detected (18, 19). Differential screening of a library produced by a subtractive hybridization approach yielded four genes expressed uniquely during appressorium formation induced by the host surface wax, and disruption of one of these genes drastically decreased its virulence on the host without manifesting any defects in appressorium formation (18, 19).
Hard-surface contact is known to be necessary to induce appressorium formation in many fungi (11). The molecular basis of this requirement is unknown. Response of C. gloeosporioides conidia to these host signals require a prior hard surface contact for about 2 h. Little is known about the genes expressed as a consequence of the early hard surface contact in any phytopathogen. Ca2+-calmodulin (CaM) signaling by hard-surface contact was suggested to be involved in the priming of C. gloeosporioides conidia that enables them to respond to the host signals to germinate and form appressoria. CAM gene expression has been shown to be induced by the hard-surface contact (22). CaM was also suggested to be involved in germination and appressorium formation of C. trifolii conidia since CaM antagonist inhibited this process (7, 9). In an effort to elucidate the molecular events triggered by the early hard-surface contact, we isolated several genes expressed during the early stage of hard-surface contact by a differential-display method. The genes that encode Colletotrichum hard-surface-induced proteins are designated chip genes and chip1 was identified as a gene encoding ubiquitin-conjugating enzyme (27). Here, we report the characterization of cDNAs and genes for CHIP2 and CHIP3 that would encode a putative DNA-binding protein that would probably be localized in the nucleus, and a putative nine-transmembrane-domain-containing protein, respectively. CHIP2 and CHIP3 are induced within a few hours of contact with the hard surface. The conidia of CHIP2- and CHIP3-disrupted mutants differentiated into appressoria on the hard surface when treated with the chemical signals as the wild type did and showed no measurable decrease in virulence on avocado or tomato fruits.
MATERIALS AND METHODS
Materials.
C. gloeosporioides, isolated from avocados, was provided by Dov Prusky (Volcani Centre, Bet Dagan, Israel); glycerol stock was kept at −80°C. Conidia produced on a potato dextrose agar (PDA) plate were obtained from 5- to 7-day-old cultures (19). Avocado fruits (Fuerte) were a generous gift from John A. Menge at the University of California, Riverside. Tomato fruits were purchased from a local grocery store.
Vectors, enzymes, and chemicals.
All plasmids were propagated in E. coli DH5α. All restriction and modifying enzymes, Taq polymerase, DH5α cells, and TRIzol reagent for RNA isolation were from Life Technologies (Gaithersburg, Md.). Expand High Fidelity Taq polymerase was from Boehringer Mannheim (Indianapolis, Ind.). Novozyme 234 was from InterSpex Products (Foster City, Calif.). Hygromycin was from Calbiochem (San Diego, Calif.). PCR primers were from Integrated DNA Technologies (Coralville, Iowa). The Rediprime random primed labeling kit was from Amersham (Arlington Heights, Ill.). Nytran membranes were from Schleicher & Schuell (Keene, N.H.). The Geneclean kit was from Bio 101 (La Jolla, Calif.).
RNA preparation and differential display of mRNA.
RNA preparation and differential display of mRNA were performed as described previously (27). A 532-bp segment amplified in the differential display with primer combinations of oligo(dT) primer HT11A and arbitrary 5′ decamer H-GP3 was subcloned into pCRII vector (Invitrogen, Carlsbad, Calif.) and designated CHIP2(532). A 209-bp segment amplified in the differential display with primer combinations of oligo(dT) primer HT11A and arbitrary 5′ decamer H-AP1 was subcloned into pCRII vector and designated CHIP3(209).
Isolation of CHIP2 and CHIP3 cDNA clones.
Full-length cDNA clones corresponding to CHIP2(532) and CHIP3(209) in the cDNA library constructed with RNA isolated from hard-surface treated conidia of C. gloeosporioides as described before (22) were identified using cDNA segments subcloned from the differential display as probes. The cDNA clones pCHIP2 and pCHIP3 thus obtained were completely sequenced and analyzed with a BLAST program from the National Center for Biotechnology Information (2). The protein motif and cell localization of CHIP2 and CHIP3 were predicted with MOTIF (Kyoto University), PSORTII algorithm (32), and the “hidden Markov model” (44).
RNA blot analysis.
RNA blot analysis was performed as described previously (22). Ethidium bromide staining showed that in all cases equal amounts of RNA were loaded.
Cloning of genomic DNA.
A genomic library of C. gloeosporioides constructed in λGEM11 (Promega) vector was screened by plaque hybridization with labeled CHIP2 or CHIP3 cDNA under high-stringency hybridization conditions at 65°C overnight in 6× SSPE (0.9 mM NaCl, 5 mM EDTA, 50 mM NaH2 PO4; pH 7.4), 5× Denhardt's solution (0.1% Ficoll, 0.1% polyvinylpyrrolidone, 0.1% bovine serum albumin), 0.1% sodium dodecyl sulfate (SDS), and 100 μg of sheared salmon sperm DNA per ml. A 3.5-kb SalI-digested fragment from a genomic clone of CHIP2 was ligated into pBluescript KS(−) (Stratagene) to yield pgCHIP2. A 2.9-kb SalI-digested fragment from a genomic clone of CHIP3 was ligated into pBluescript KS(−) to yield pgCHIP3.
Construction of gene replacement vector pCHIP2::hph.
The 5′ SalI site in pgCHIP2 was eliminated by removing the 200-bp ClaI fragment and by self-ligation. The 3′ SalI site was removed by digestion with SalI and filling in with Klenow and deoxynucleoside triphosphate, followed by self-ligation. To amplify a fragment of pgCHIP2 with a deletion of 830 bp from the coding region of CHIP2, an inverse PCR was done with a sense primer (5′-GGC CGG GTC GAC CAA GAC TCG CGT TCG AG-3′) and an antisense primer (5′-GGC CGG GTC GAC GTC ACG AGC CGC TTT CAC-3′) using Expand High Fidelity Taq DNA polymerase. The 5.3-kb PCR product was digested with SalI. The plasmid pCSN43, a pBluescript vector carrying the Escherichia coli hyg gene under the control of Aspergillus nidulans trpC promoter (41), was digested with SalI. The insert, purified with a Geneclean kit, was ligated to the inverse PCR product to generate a gene replacement vector, pCHIP2::hph.
Construction of gene replacement vector pCHIP3::hph.
The 2.9-kb genomic SalI fragment of CHIP3 was ligated to pBluescript KS(−) which had been digested with XhoI and SalI and then treated with alkaline phosphatase (BM), yielding a genomic clone, pgCHIP3. This genomic clone was digested with XhoI and then ligated to the SalI fragment carrying the E. coli hyg gene under the control of A. nidulans trpC promoter (41) to generate pCHIP3::hph.
C. gloeosporioides transformation.
Fungal transformation was performed using procedures based on those described previously (40, 43). Conidia (2 × 107) were inoculated into 500 ml of minimal medium containing a third of the concentration of the trace elements used in the experiments with Fusarium solani pisi (40), 1% glucose, and 0.6% yeast extract and then incubated at 30°C with shaking overnight in the dark. With full-strength trace elements, the germination and germ tube growth were affected, resulting in a very poor yield of protoplasts. The mycelia (5 g) were resuspended in 50 ml of 1 M sorbitol containing 300 mg of Novozyme 234, 60 mg of Driselase, 36 mg of bovine serum albumin, and 1.2 ml of β-glucuronidase, and the mixture was gently swirled at room temperature for 3.5 h. The protoplasts in the repeatedly washed preparation were counted using a hemacytometer and centrifuged as described above and then resuspended at 5 × 107 protoplasts/ml. Protoplasts (1 ml) were mixed with DNA (25 μg in 25 μl of STC), and after 15 min of incubation at room temperature, 12 ml of PTC (40% polyethylene glycol [PEG] 8000 in STC) was added. After an additional incubation for 20 min, 30 ml of TB3 (1% sucrose, 0.6% yeast extract, and 0.6% casein hydrolysate with 1 M sorbitol) was added, and the protoplasts were gently swirled for 3 h. The protoplast suspension was then centrifuged as before, and the pellet was resuspended in STC. The protoplasts were mixed with molten regeneration medium (minimal medium adjusted to 1.5% glucose, 1 M sorbitol, and 1% Bacto agar), and the mixture was poured onto hardened regeneration medium of the same composition but containing 1.5% Bacto agar and 300 μg of hygromycin B/ml; the total mixture (5 × 107 protoplasts) was divided into 20 plates. After 5 to 7 days, the hygromycin-resistant transformants were transferred to hardened complete medium (1% sucrose, 0.6% yeast extract, 0.6% casein hydrolysate) (CM) containing 200 μg of hygromycin B/ml.
Preparation of genomic DNA from transformants.
Transformants were cultured in 5 ml of CM containing hygromycin (50 μg/ml) for 3 to 4 days. After mycelia were harvested and lyophilized, DNA was extracted with 500 μl of isolation buffer (150 mM EDTA, 50 mM Tris [pH 8.0], 1% n-laurylsarcosine) by vortexing and centrifugation. DNA, extracted with a equal volume of phenol-chloroform, was precipitated with cold ethanol, resuspended in 200 μl of TE buffer (10 mM Tris, pH 8.0; 1 mM EDTA), and treated with RNase A for 1 h. DNA, precipitated by incubation with 140 μl of PEG (20% [wt/vol] PEG 8000, 2.5 M NaCl) on ice for 1 h, was collected by centrifugation and washed with 70% ethanol in water and dissolved in 100 μl of TE. Then, 5 μl was used for junction PCR.
Junction PCR for disruptants.
The primer pairs used for transformant screening for gene disruption were designed to test for junctions expected from homologous recombinations. The initial screening of transformants for disruption of CHIP genes was done by PCR in a programmable thermal controller (M.J. Research, Watertown, Mass.). Genomic DNA (40 ng) was mixed with 1× PCR buffer, 3 mM MgCl2, 0.2 mM concentrations of each deoxynucleoside triphosphate, 4% dimethyl sulfoxide, 0.1 μM concentrations of each primer, and Taq DNA polymerase in a total volume of 50 μl and then heated at 94°C for 15 min. The PCR amplification consisted of 30 cycles of denaturation at 94°C for 30 s, annealing at 56°C for 45 s, and polymerization at 72°C for 45 s, followed by a final extension step at 72°C for 10 min. The primers used for the identification of CHIP2 disruptants were from the 5′ end of CHIP2 gene (a sense primer, 5′-CTA TTT GGG CAA GCT CAA CTG-3′) and from the 3′ end of hph gene, (a sense primer, 5′-CTA GCT CCA GCC AAG CCC-3′). A 1,120-bp PCR product is expected from this pair of primers. For CHIP3 disruption, a sense primer from the CHIP3 gene, 5′-ATG ACT GGA TAC GAA GAC AG-3′, and a sense primer from the hph gene, 5′-CTA GCT CCA GCC AAG CCC-3′, were used to amplify an expected junction PCR product of 680 bp. All PCR products were electrophoresed on 1.2% agarose gels.
Genomic Southern blot analysis.
Genomic DNA (2 μg) of the wild type and of the disruptants of CHIP2 or CHIP3 prepared as described above was completely digested with SalI for CHIP2 or with EcoRV and SalI for CHIP3. The digests were fractionated on a 0.8% agarose gel, transferred to an Nytran nylon membrane, and hybridized at 65°C overnight to a 32P-labeled probe prepared with the Rediprime kit. After hybridization the membranes were washed for 20 min at ambient temperature in 2× SSPE–0.1% SDS. Additional washing was carried out with 0.1× SSPE–0.1% SDS at 65°C for 20 min. The membranes were exposed to X-ray film at −80°C.
Tests for germination and appressorium formation of wild-type and CHIP disruptants of C. gloeosporioides.
Germination and appressorium formation of C. gloeosporioides were tested on a cover glass surface as described previously (22, 36).
Tests for pathogenicity of the CHIP disruptants.
The pathogenicity test was similar to that described previously (18). The conidia of CHIP2 and CHIP3 disruptants and of the wild type were collected from PDA plates. After avocado and tomato fruits were surface sterilized as described previously (18), 7,500 conidia/cm2 were placed on each fruit in 200 μl of water. The fruits were incubated at room temperature for 6 to 10 days in a high-humidity chamber. When the fruits inoculated with the wild type showed lesions in the area where the spore suspension was placed, the fruits were longitudinally cut across the infection sites. The sections of avocado fruits were photographed with a Nikon camera (FM2) at shutter speed 1/1 without a filter under fluorescent light. Sections of tomato fruits were visually examined for lesion formation, thin sections were stained with lactophenol-cotton blue, and fungal penetration into the tissue was examined microscopically.
Nucleotide sequence accession numbers.
The GenBank nucleotide accession numbers for CHIP2 and CHIP3 cDNA were AF149296 and AF089807, respectively.
RESULTS
Differential display of RNA from conidia of C. gloeosporioides induced by hard-surface contact.
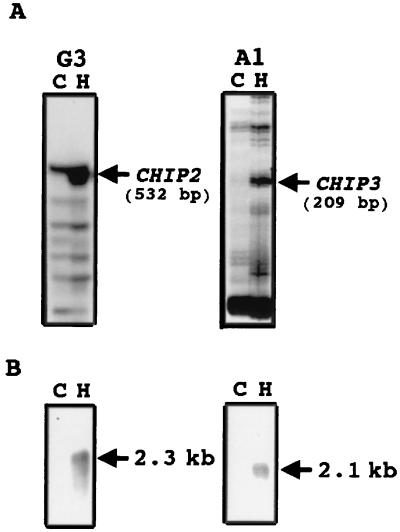
Total RNAs were reverse transcribed as described previously (27). When a PCR was performed using the reverse transcript as a template, a 532-bp PCR fragment was amplified in a reaction where oligo(dT), HT11A, and arbitrary 5′ decamer, H-GP3, were used as primers (Fig. 1A, left). Similarly, a 209-bp PCR fragment was amplified in a reaction where oligo(dT), HT11A, and arbitrary 5′ decamer H-AP1 were used as primers (Fig. 1A, right). When the 532-bp DNA PCR product was cloned and two independent clones were sequenced, they were found to have identical sequences. When this PCR product was used as a probe for an RNA blot analysis, a band at 2.3-kb strongly hybridized. This transcript, which was hardly detectable in the control, was strongly induced by 2 h of hard-surface treatment (Fig. 1B, left). The gene that encodes this transcript was designated CHIP2. Screening of a cDNA library prepared from hard-surface-treated conidia with the PCR clone of CHIP2 yielded pCHIP2; this 2,235-bp long clone contained a single open reading frame that would encode a protein of 567 amino acids with an estimated molecular mass of 65 kDa and a pI of 7.0.
FIG. 1.
(A) Area of a differential-display gel showing the amplified products obtained with primer combinations of oligo(dT) primer HT11A and arbitrary 5′ decamer H-GP3 (G3) on the left or H-AP1 (A1) on the right by using as templates cDNAs derived from conidia resting on a hard surface for 2 h (H) or an untreated control (C). (B) Northern blots showing induction of CHIP2 (left) and CHIP3 (right) by hard-surface contact in C. gloeosporioides conidia.
When the amino acid sequence of CHIP2 was compared with protein sequences in the GenBank database and the Saccharomyces genome database (Stanford University) using BLAST (2), it showed low homology (15 to 20%) with numerous proteins which have a coiled-coil structure, such as myosin heavy chains. Indeed, a coiled-coil structure in the domain ranging from Ala-212 to Asp-500 of CHIP2 (Fig. 2, arrows) was predicted by both PSORT II (32) and COILS, version 2.2 (28). The PSORT II algorithm (32) also predicted CHIP2 to have a nuclear targeting sequence, RK(X)11RPRR, in the N-terminal region (Fig. 2, underlined). This sequence is a bipartite-type nuclear targeting sequence which consists of two basic residues, a spacer region of ca. 10 amino acids, and a second basic cluster in which at least 3 of the next 5 amino acids are basic (10). A MOTIF algorithm (Kyoto University) predicted a leucine zipper motif, L-X(6)-L-X(6)-L-X(6)-L, in the heptad repeat region which might dimerize into a coiled-coil structure (Fig. 2, double underlined). These structural features suggest that CHIP2 might be a leucine-zipper-type transcription factor (34, 46). CHIP2 has a nuclear-export-signal-like sequence, L-(X)4-L-(X)2-L-(X)-L, in the N-terminal region (Fig. 2, dashed line) and has four E-rich boxes in the C-terminal region (Fig. 2, boxes).
FIG. 2.
(A) Deduced amino acid sequence of CHIP2. Putative nuclear localization signal (underlined), a nuclear-export-signal-like sequence (dashed underline), and a leucine zipper motif (double underline) are indicated. Amino acid residues (A212 to D500) representing a coiled coil structure are marked with arrows. Four E-rich regions are boxed.
When a 209-bp DNA band observed in the differential display (Fig. 1A, right) was PCR amplified and cloned and three independent clones were sequenced, they demonstrated identical sequences. To test whether this product was specifically amplified from a transcript induced by the hard-surface treatment, RNA blot analysis was performed using the amplified PCR product as a probe. RNA from conidia hard surface treated for 2 h showed a strongly hybridizing band at 2.1 kb, whereas this band was not detectable in the control (Fig. 1B, right), suggesting that the gene was strongly induced by hard-surface treatment. This gene was designated CHIP3. When the 209-bp PCR product of CHIP3 was used to screen a cDNA library prepared from hard-surface-treated conidia, a 1,971-bp clone was obtained. The nucleotide sequence of this clone showed that it would encode a protein of 559 amino acids with an estimated molecular mass of 64 kDa and a pI of 9.4.
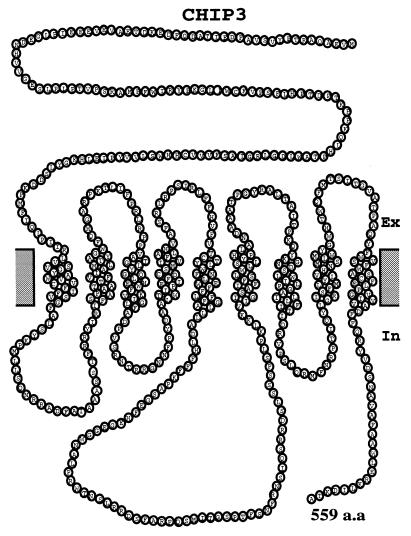
The deduced amino acid sequence did not show significant homology to any other known protein in the database (2). The hidden Markov model (44) predicted that CHIP3 has nine transmembrane domains (Fig. 3), suggesting that CHIP3 would be an integral membrane protein.
FIG. 3.
The nine transmembrane structure of CHIP3 as predicted by the hidden Markov model.
Induction of CHIP2 and CHIP3 transcript levels by hard-surface contact and ethylene treatment.
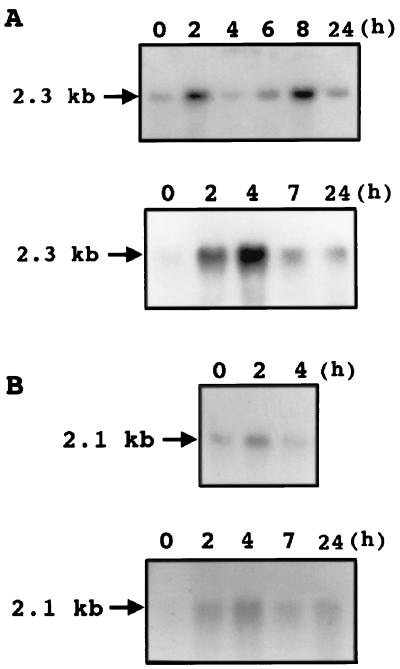
RNA blot analysis using total RNA obtained from conidia resting on a hard surface for different periods of time showed that CHIP2 was strongly induced in 2 h, with a subsequent decrease, followed by a further stronger induction at ca. 8 h and a subsequent decrease (Fig. 4A, top). Ethidium bromide staining of the rRNA bands showed that all lanes had equal amounts of RNA loaded and that the bimodal induction pattern for CHIP2 was very reproducible. Since ethylene is known to induce germination and appressorium formation of C. gloeosporioides conidia on a hard surface (12), the effect of ethylene on CHIP2 induction in conidia resting on a hard surface was tested. The induction of CHIP2 by ethylene on a hard surface was maximal at 4 h (Fig. 4A, bottom). A direct comparison of the RNA blots demonstrates that the CHIP2 transcript level was higher on the hard surface with ethylene than on the hard surface without ethylene (data not shown).
FIG. 4.
(A) RNA blots showing the time course of induction of CHIP2 by hard-surface contact (top) and by ethylene and hard-surface contact (bottom) in C. gloeosporioides conidia. (B) RNA blots showing the time course of induction of CHIP3 by hard-surface contact (top) and by ethylene and hard-surface contact (bottom) in C. gloeosporioides conidia. In both panels A and B, each lane had 20 μg of total RNA. 32P-labeled CHIP2 or CHIP3 cDNA was used as the probe. Experiments were repeated twice, and similar results were obtained. Ethylene was generated by adding 10 μM ethephon. Ethidium bromide staining showed equal loading of RNA.
The CHIP3 induction pattern was similar to that of CHIP2. The time course of transcript accumulation showed a strong induction that was maximal at 2 h, and then the transcript level decreased (Fig. 4B, top). Ethidium bromide staining of rRNA showed equal loading of RNA on all lanes. CHIP3 induction in conidia on the hard surface by ethylene was maximal at 4 h (Fig. 4B, bottom).
Generation of CHIP2-disrupted mutants.
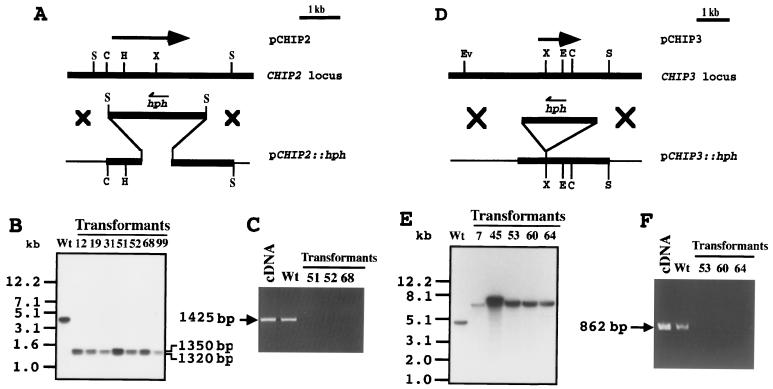
A single band was detected by Southern blot analysis of C. gloeosporioides genomic DNA using cDNA of CHIP2 as a probe, suggesting that CHIP2 might be a single-copy gene (Fig. 5B, lane Wt). To test whether this gene has a functional involvement in morphogenesis or in pathogenicity, a CHIP2 gene disruption was done. A gene disruption vector was constructed by replacing a 830-bp segment in the coding region of CHIP2 with a hygromycin resistance gene (Fig. 5A). This vector, pCHIP2::hph, was used to transform C. gloeosporioides and transformants were selected on hygromycin. DNA purified from each transformant was used for PCR with the primer from the 5′ end of the CHIP2 gene outside the region used for making the construct and a sense primer from the 3′ end of the hph gene to test for the presence of one of the junctions between the hph gene and the fungal genome expected from homologous recombination. Of 108 transformants examined, 7 showed the expected 1,120-bp junction PCR product (data not shown). Genomic Southern blot analysis showed that these transformants are gene-disrupted mutants (disruptants) by the observation that the 3.5-kb band produced by SalI digestion of the wild-type genomic DNA was replaced by the expected two bands at 1,320 and 1,350 bp in the transformants (Fig. 5B). To test for the expression of the CHIP2 gene, total RNA extracted from conidia of wild type and CHIP2 disruptants was subjected to reverse transcription-PCR (RT-PCR) with gene-specific primers from the coding region. The 1,425-nucleotide nt product expected from the native CHIP2 gene was not formed from the RNA from the gene-disrupted mutants, whereas the wild type yielded this product (Fig. 5C).
FIG. 5.
(A to C) Strategy used for CHIP2 gene disruption in C. gloeosporioides (A), genomic Southern blot analysis of the transformants (B), and RT-PCR analysis showing the absence of the disrupted-gene products (C). The physical map of the CHIP2 locus was estimated by restriction mapping, and cDNA of CHIP2 is indicated by an arrow above the genomic locus. The gene replacement vector pCHIP2::hph was constructed by replacing 830 bp of the CHIP2 coding region with hph gene as shown in Materials and Methods. Restriction sites: H, HindIII; X, XhoI; C, ClaI; S, SalI. Genomic DNAs (2 μg each) of the wild type and disruptants were completely digested with SalI and probed with the cDNA fragment. RT-PCR was performed with total RNA isolated from conidia treated on the hard surface for 2 h. (D to F) Strategy used for CHIP3 gene disruption in C. gloeosporioides (D), genomic Southern blot analysis (E), and RT-PCR analysis showing absence of the disrupted-gene product (F). The physical map of the CHIP3 locus was estimated by restriction mapping, and the cDNA of CHIP3 is indicated by an arrow above the genomic locus. The gene replacement vector pCHIP3::hph was constructed by inserting the hph gene into the coding region as described in Materials and Methods. Restriction sites: Ev, EcoRV; X, XhoI; E, EcoRI; C, ClaI; S, SalI. Genomic DNAs (2 μg each) of the wild type and disruptants were digested with EcoRV and SalI and probed with cDNA fragment. RT-PCR was performed with total RNA isolated from conidia treated on the hard surface for 2 h.
Generation of the CHIP3-disrupted mutants.
A single band was detected by the genomic Southern blot analysis, suggesting that CHIP3 exists as a single-copy gene in C. gloeosporioides genome (Fig. 5E, lane Wt). To explore possible biological functions of this gene product, a gene disruption was performed. To construct a gene replacement vector for CHIP3 disruption, the hygromycin resistance gene was inserted into the XhoI site of pgCHIP3, resulting in pCHIP3::hph (Fig. 5D). pCHIP3::hph was used to transform C. gloeosporioides, and transformants were selected on hygromycin. DNA purified from each transformant was used for junction PCR with a sense primer from the 5′ end of the CHIP3 gene outside of the region used for making the construct and a sense primer from the 3′ end of the hph gene. Of 36 transformants, 5 showed the expected junction PCR product (data not shown). Genomic Southern blot analysis confirmed that these transformants were real disruptants by the observation that the 5.0-kb band observed in the wild type was replaced by the expected larger 7.4-kb band in the mutant (Fig. 5E). Disruptant 45 appeared to have a double band, suggesting a possible ectopic integration in this mutant, and therefore it was excluded from further tests to avoid possible complications. To test for the expression of the CHIP3 gene, total RNA extracted from the conidia of the wild type and CHIP3 disruptants was subjected to RT-PCR with gene-specific primers from the coding region. The 862-bp PCR product expected from native CHIP3 gene was not formed from the RNA of the gene-disrupted mutants, whereas the wild type yielded this product (Fig. 5F).
Germination and appressorium formation of CHIP2 and CHIP3 disruptants.
Conidia of the disruptants of the two genes were similar to those of the wild-type C. gloeosporioides (data not shown). When the appressorium-forming ability of the conidia of CHIP2 and CHIP3 disruptants were tested on the hard surface with either ethylene or wax, the disruptants germinated and differentiated into appressoria exactly like the wild type did (Table 1). At a low conidial population density (<10 conidia/μl), the wild-type conidia germinated and formed appressoria on the glass surface without requiring host signals, and under such conditions CHIP2 and CHIP3 disruptants also germinated and formed appressoria (data not shown). Microscopic examination of the appressoria showed that the CHIP2 and CHIP3 disruptants had the same degree of melanization as the wild type. More than 95% of the conidia of CHIP2 and CHIP3 disruptants germinated in 0.5% yeast extract just as the wild-type conidia did. Mycelial growth of the disruptants on PDA was similar to that of the wild type.
TABLE 1.
Germination and appressorium formation of conidia of the wild type and CHIP disruptants
| Strain | % Germination or appressorium formationa ± SD with:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| H2O
|
Ethephon
|
Wax
|
Yeast extract
|
|||||
| Appressorium formation | Germination | Appressorium formation | Germination | Appressorium formation | Germination | Appressorium formation | Germination | |
| Wild type | 8.1 ± 2.8 | 11.2 ± 2.5 | 91.5 ± 1.5 | 95.0 ± 2.6 | 87.4 ± 5.0 | 92.1 ± 2.8 | 0 | 92.3 ± 1.8 |
| ΔCHIP2 | 5.3 ± 2.5 | 8.4 ± 5.4 | 92.3 ± 3.4 | 96.7 ± 4.5 | 90.3 ± 4.5 | 93.2 ± 6.4 | 0 | 94.7 ± 3.8 |
| ΔCHIP3 | 6.2 ± 1.2 | 10.4 ± 3.7 | 89.7 ± 7.1 | 93.5 ± 4.4 | 88.3 ± 2.5 | 95.2 ± 5.7 | 0 | 91.7 ± 2.7 |
Germination and appressorium formation were determined on a glass surface with the indicated additions to the medium as described in Materials and Methods.
Tests for pathogenicity of the CHIP2- and CHIP3 disruptants.
Although CHIP2 and CHIP3 gene disruption did not seem to affect the formation of melanized appressoria, their gene product(s) might be involved in host infection since previous reports indicate that gene disruptants that do not show obvious differences in appressorium formation can have decreased virulence (18, 50). To test for this possibility, the pathogenicity of the conidia of CHIP2 and CHIP3 disruptants was compared with that of wild-type conidia on avocado fruits. Once the wild type showed symptoms of infection, the fruits were cut longitudinally across the lesion. CHIP2 and CHIP3 disruptants and the wild-type C. gloeosporioides showed similar degrees of progression of infection into avocado fruits (data not shown). Both disruptants were tested for pathogenicity on tomato fruits, an alternate host for this pathogen. Both of them showed similar levels of virulence, as indicated by lesions and mycelial penetration into the tissue (data not shown).
DISCUSSION
As observed with many fungi (11), hard-surface contact has been shown to be essential for the conidia of C. gloeosporioides to germinate and form appressoria (13, 19). Hard-surface contact seems to prime the conidia of C. gloeosporioides by enabling them to respond to the chemical signals such as wax and ethylene that induce a set of genes necessary for the formation of appressoria (23). The only gene known to be induced during the early hard-surface contact is calmodulin gene (22). To elucidate the molecular events triggered by hard-surface contact, genes expressed in C. gloeosporioides conidia during hard-surface treatment were examined by an mRNA differential-display method; eight genes that were expressed preferentially up on hard-surface contact were identified, and they were designated chip genes (27). chip1, which encodes a ubiquitin-conjugating enzyme, was shown to be functionally equivalent to yeast ubc4 and ubc5 by complementation experiments. The chip1 product probably plays a role in the ubiquitin-proteasome system that plays a critical role in the selective protein degradation involved in the differentiation of the germ tube into appressorium. We tested two other genes, CHIP2 and CHIP3, that were identified by this differential-display approach.
CHIP2 gene product has features that suggest that it may be a transcription factor. For example, it has a nuclear localization signal, a nuclear-export-signal-like sequence, and very long heptad repeat region (A212 to D500) containing a leucine zipper domain. A bipartite-type nuclear localization signal motif found in the N-terminal region might function in nuclear targeting of CHIP2. The bipartite-type nuclear localization signals are found in many nuclear targeting proteins such as transcription factors (C-FOS, C-JUN, GCN4, etc.), and steroid hormone receptors (glucocorticoid, progesterone, etc.) (4, 30, 31, 33). Another interesting characteristic of CHIP2 is that the nuclear localization signal is followed by a nuclear-export-signal-like sequence that consists of a sequence enriched in hydrophobic amino acids, particularly leucine. In yeast cells, a nuclear-export-signal-containing protein is known to assemble into a trimeric complex with GTP-bound Ran and Crm1, which were originally described in fission yeasts for its chromosome region maintenance phenotype (1, 35). The nuclear-export-like signal of CHIP2 is similar to that of viruses and metazoa (5, 49). The parallel two-stranded α-helical coiled coil is the most frequently encountered subunit oligomerization motif in intracellular proteins (21, 28). This is found in various kinds of proteins, such as myosin, kinesin, tropomyosin, the leucine zipper domain of transcriptional activators, and the G protein β-subunit. As the coiled-coil motif of CHIP2 is fairly long (289 amino acids), it might dimerize or even assemble into an oligomerized structure. CHIP2 might be a transcription factor that is involved in some developmental processes in this fungus. Although transcriptional activation could not be demonstrated using the GAL4-CHIP2 hybrid protein, an N-terminal truncated (152 of 567 amino acids) CHIP2 expressed in E. coli was shown to bind DNA-cellulose resin, suggesting that CHIP2 has a DNA-binding activity (data not shown). It is possible that CHIP2 might be involved in cell endocytosis or cell division, as suggested for myosin families in S. cerevisiae (6, 24). Cells of the Myo1 mutant of Saccharomyces cerevisiae did not separate from one another, suggesting that this gene product is involved in cell separation (38, 48). MYO3 and MYO5 are involved in endocytosis, and single gene disruption of either gene has a less-obvious phenotypic alteration than does double disruption, suggesting that these genes can partially substitute for each other (14, 15).
Interestingly, RNA blot analysis showed that CHIP2 was induced at two discretely different stages, the first reaching a maximum level after 2 h of contact of the conidia with the hard surface, followed by a drastic decrease, and a second period of induction that peaked after 8 h. It is possible that this gene product is involved in the transcriptional activation of genes during the early hard-surface priming and other genes involved in later events involved in differentiation. Induction of CHIP2 by ethylene in conidia on the hard surface was much stronger than with hard-surface contact alone and reached a maximum at 4 h, after which the transcript level decreased drastically. Thus, in the presence of ethylene, the induction process is hastened in such way that the bimodal increase seen on the hard surface alone is not found in the presence of ethylene. The time course of induction of CHIP3 by hard-surface contact and ethylene showed that the CHIP3 transcript level is maximal at between 2 and 4 h of treatment. The presence of ethylene extended the period of high-level CHIP3 expression on the hard surface from 2 to 4 h. This time window of ethylene induction of CHIP2 and CHIP3 is exactly the period during which the conidia are known to become responsive to ethylene treatment (18). This observation suggests that CHIP2 and CHIP3 play an important role in some ethylene-induced processes.
Since CHIP2 and CHIP3 are found to be induced by hard-surface and ethylene treatment during the time period when such treatment is known to trigger appressorium formation, we suspected these genes could be involved in the differentiation process. To test this possibility, CHIP2 and CHIP3 disruptants were produced. Junction PCR and genomic Southern blotting–RT-PCR confirmed gene disruption. The conidia of CHIP2 and CHIP3 disruptants at a very low conidial population density (10/μl) without any host signal or at a higher population density (up to 100/μl) with ethylene or wax germinated and differentiated into appressoria on the hard surface. Light microscopic examination of the conidia and appressoria of CHIP2 and CHIP3 disruptants did not reveal any morphological differences from those of the wild type. The wild type and the gene disruptants developed pathogenic symptoms on the natural host in an identical manner, revealing no detectable effect on virulence. Although hard-surface contact and ethylene induced CHIP2 and CHIP3, their disruption did not show measurable effects on appressorium formation and pathogenicity. These results suggest either that CHIP2 and CHIP3 are not essential for pathogenesis or that functionally redundant genes can substitute in the disrupted mutants. Although genomic Southern blot analysis showed that there is only one copy each of CHIP2 and CHIP3 in the genome, it is possible that there are genes that have low homology to CHIP2 or CHIP3 but have the same function. Functional redundancy of important genes has been previously reported. It is also possible that CHIP2 and CHIP3 genes induced by the hard-surface treatment are involved in some other processes that are not essential for pathogenicity. Both possibilities have been suggested for other genes. For example, disruption of genes in pathogenic fungi encoding various degradative enzymes or toxins, singly or in combination, did not provide unambiguous evidence for their function in pathogenicity, although such enzymes are thought to be important for infection (16, 20, 25, 37). Madhani et al. (29) showed that several downstream effectors are under the regulation of the Kss1 mitogen-activated protein kinase (MAPK) signaling pathway which controls dimorphic development. Although these effectors were apparently regulated by Kss1 MAPK signaling pathway, gene disruption of most of these genes did not affect the dimorphic development.
CHIP3 encodes a protein which has multiple transmembrane domains. The hidden Markov model algorithm predicted that CHIP3 might have nine transmembrane domains. Hydrophobicity profiles by the method by Kyte and Doolittle (26) also predicts that CHIP3 has nine hydrophobic domains, suggesting that CHIP3 might be an integral membrane protein. One of the best-studied classes of multiple transmembrane proteins is the heterotrimeric G protein coupled seven-transmembrane-spanning receptors (7TM). In yeasts, a 7TM is involved in responding to mating pheromones, ultimately regulating the action of a transcription factor to stimulate pheromone-induced transcription. Another example of multiple transmembrane proteins is sts1+ gene product in fission yeast which might have eight or nine putative transmembrane domains (39). sts1+ gene disruptants exhibited pleiotropic defects, such as cold sensitivity for growth and supersensitivity to divalent cations. Recently, an ATP-driven efflux pump in Magnaporthe grisea, which has 12 transmembrane domains, was shown to be a pathogenicity factor (45). The biological function of CHIP3 that most probably generates an integral membrane protein remains to be elucidated.
ACKNOWLEDGMENTS
We are indebted to J. A. Menge and Elinor Pond for generously providing us with avocado and to Linda Rogers for helpful comments and technical assistance. We also thank Todd Walls for assistance in preparing the manuscript.
This work was supported in part by National Science Foundation grant no. IBN-9816868.
REFERENCES
- 1.Adachi Y, Yanagida M. Higher-order chromosome structure is affected by cold-sensitive mutations in a Schizosaccharomyces pombe gene crm1+ which encodes a 115-kD protein preferentially localized in the nucleus and its periphery. J Cell Biol. 1989;108:1195–1207. doi: 10.1083/jcb.108.4.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman J. Gapper BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bechinger C, Giebel K-F, Schnell M, Leiderer P, Deising H B, Bastmeyer M. Optical measurements of invasive forces exerted by appressoria of a plant pathogenic fungus. Science. 1999;285:1896–1899. doi: 10.1126/science.285.5435.1896. [DOI] [PubMed] [Google Scholar]
- 4.Bohmann D, Bos T J, Admon A, Nishimura T, Vogt P K, Tjian R. Human proto-oncogene c-jun encodes a DNA binding protein with structural and functional properties of transcription factor AP-1. Science. 1987;238:1386–1392. doi: 10.1126/science.2825349. [DOI] [PubMed] [Google Scholar]
- 5.Bogerd H P, Fridell R A, Benson R E, Hua J, Cullen B R. Protein sequence requirements for function of the human T-cell leukemia virus type 1 Rex nuclear export signal delineated by a novel in vivo randomization-selection assay. Mol Cell Biol. 1996;16:4207–4214. doi: 10.1128/mcb.16.8.4207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown S S. Myosins in yeast. Curr Opin Cell Biol. 1997;11:142–151. doi: 10.1016/s0955-0674(97)80150-0. [DOI] [PubMed] [Google Scholar]
- 7.Buhr T L, Dickman M B. Gene expression analysis during conidial germ tube and appressorium development in Colletotrichum trifolii. App Env Microbiol. 1997;63:2378–2383. doi: 10.1128/aem.63.6.2378-2383.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Jong J C, McCormack B J, Smirnoff N, Talbot N J. Glycerol generates turgor in rice blast. Nature. 1997;389:244–245. [Google Scholar]
- 9.Dickman M B, Buhr T L, Warwar V, Truesdell G M, Huang C X. Molecular signals during the early stages of alfalfa anthracnose. Can J Bot. 1995;73:S1169–S1177. [Google Scholar]
- 10.Dingwall C, Laskey R A. Nuclear targeting sequences-a consensus? Trends Biochem Sci. 1991;16:478–481. doi: 10.1016/0968-0004(91)90184-w. [DOI] [PubMed] [Google Scholar]
- 11.Emmett R W, Parbery D G. Appressoria. Annu Rev Phytopathol. 1975;13:147–167. [Google Scholar]
- 12.Flaishman M A, Kolattukudy P E. Timing of fungal invasion using host's ripening hormone as a signal. Proc Natl Acad Sci USA. 1994;91:6579–6583. doi: 10.1073/pnas.91.14.6579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flaishman M A, Hwang C-H, Kolattukudy P E. Involvement of protein phosphorylation in the induction of appressorium formation in Colletotrichum gloeosporioides by its host surface wax and ethylene. Physiol Mol Plant Pathol. 1995;47:103–117. [Google Scholar]
- 14.Geli M I, Riezman H. Role of type I myosins in receptor-mediated endocytosis in yeast. Science. 1996;272:533–535. doi: 10.1126/science.272.5261.533. [DOI] [PubMed] [Google Scholar]
- 15.Goodson H V, Spudich J A. Identification and molecular characterization of a yeast myosin I. Cell Motil Cytoskeleton. 1995;30:73–84. doi: 10.1002/cm.970300109. [DOI] [PubMed] [Google Scholar]
- 16.Guo W, González-Candelas L, Kolattukudy P E. Identification of a novel pelD gene expressed uniquely in planta by Fusarium solani f. sp. pisi (Nectria haematococca, mating type VI) and characterization of its protein product as an endo-pectate lyase. Arch Biochem Biophys. 1996;332:305–312. doi: 10.1006/abbi.1996.0346. [DOI] [PubMed] [Google Scholar]
- 17.Howard R J, Ferrari J A, Roach D H, Money N P. Penetration of hard substrates by a fungus employing enormous turgor pressures. Proc Natl Acad Sci USA. 1991;88:11281–11284. doi: 10.1073/pnas.88.24.11281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hwang C-S, Flaishman M A, Kolattukudy P E. Cloning of a gene expressed during appressorium formation by Colletotrichum gloeosporioides and a marked decrease in virulence by disruption of this gene. Plant Cell. 1995;7:183–193. doi: 10.1105/tpc.7.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hwang C-S, Kolattukudy P E. Isolation and characterization of genes expressed uniquely during appressorium formation by Colletotrichum gloeosporioides conidia induced by the host surface wax. Mol Gen Genet. 1995;247:282–294. doi: 10.1007/BF00293196. [DOI] [PubMed] [Google Scholar]
- 20.Jaton-Ogay K, Paris S, Huerre M, Quadroni M, Falchetto R, Tongli G, Latge J P, Monod M. Cloning and disruption of the gene encoding an extracellular metalloprotease of Aspergillus fumigatus. Mol Microbiol. 1991;14:917–928. doi: 10.1111/j.1365-2958.1994.tb01327.x. [DOI] [PubMed] [Google Scholar]
- 21.Kammerer R A, Schulthess T, Landwehr R, Lustig A, Engel J, Aebi U, Steinmetz M O. An autonomous folding unit mediates the assembly of two-stranded coiled coils. Proc Natl Acad Sci USA. 1998;95:13419–13424. doi: 10.1073/pnas.95.23.13419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kim Y-K, Li D, Kolattukudy P E. Induction of Ca2+-calmodulin signaling by hard-surface contact primes Colletotrichum gloeosporioides conidia to germinate and form appressoria. J Bacteriol. 1998;180:5144–5150. doi: 10.1128/jb.180.19.5144-5150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kolattukudy, P. E., Y.-K. Kim, D. Li, Z.-M. Liu, and L. Rogers. Early molecular communication between Colletotrichum gloeosporioides and its host. In M. Dickman, S. Freeman, and D. Prusky (ed.), Host specificity, pathology and host pathogen interaction of Colletotrichum, in press. The American Phytopathological Society, St. Paul, Minn.
- 24.Kölling R, Nguyen T, Chen E Y, Botstein D. A new yeast gene with a myosin-like heptad repeat structure. Mol Gen Genet. 1993;237:359–369. doi: 10.1007/BF00279439. [DOI] [PubMed] [Google Scholar]
- 25.Kwon-Chung K J. Gene disruption to evaluate the role of fungal candidate virulence genes. Curr Opin Microbiol. 1998;1:381–389. doi: 10.1016/s1369-5274(98)80053-2. [DOI] [PubMed] [Google Scholar]
- 26.Kyte J, Doolittle R F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982;157:105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z-M, Kolattukudy P E. Identification of a gene product induced by hard-surface contact of Colletotrichum gloeosporioides conidia as a uiquitin-conjugating enzyme by yeast complementation. J Bacteriol. 1998;180:3592–3597. doi: 10.1128/jb.180.14.3592-3597.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lupas A. Prediction and analysis of coiled-coil structures. Methods Enzymol. 1996;266:513–525. doi: 10.1016/s0076-6879(96)66032-7. [DOI] [PubMed] [Google Scholar]
- 29.Madhani H D, Galitski T, Lander E S, Fink G R. Effectors of a developmental mitogen-activated protein kinase cascade revealed by expression signatures of signaling mutants. Proc Natl Acad Sci USA. 1999;96:12530–12535. doi: 10.1073/pnas.96.22.12530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Maksymowych A B, Hsu T C, Litwack G. A nevel, highly conserved structural motif is present in all members of the steroid receptor superfamily. Receptor. 1992;2:225–240. [PubMed] [Google Scholar]
- 31.Nakabeppu Y, Nathans D. The basic region of Fos mediates specific DNA binding. EMBO J. 1989;8:3833–3841. doi: 10.1002/j.1460-2075.1989.tb08561.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nakai K, Horton P. PSORT: a program for detecting sorting signals in proteins and predicting their subcellular localization. Trends Biochem Sci. 1999;24:34–36. doi: 10.1016/s0968-0004(98)01336-x. [DOI] [PubMed] [Google Scholar]
- 33.O'Shea E K, Klemm J D, Kim P S, Alber T. X-ray structure of the GCN4 leucine zipper, a two-stranded, parallel coiled coil. Science. 1991;254:539–544. doi: 10.1126/science.1948029. [DOI] [PubMed] [Google Scholar]
- 34.O'Shea E K, Rutkowski R, Kim P S. Evidence that the leucine zipper is a coiled coil. Science. 1989;243:538–542. doi: 10.1126/science.2911757. [DOI] [PubMed] [Google Scholar]
- 35.Ohno M, Fornerod M, Mattaj I W. Nucleocytoplasmic transport: the last 200 nanometers. Cell. 1998;92:327–336. doi: 10.1016/s0092-8674(00)80926-5. [DOI] [PubMed] [Google Scholar]
- 36.Podila G K, Rogers L M, Kolattukudy P E. Chemical signals from avocado surface wax trigger germination and appressorium formation in Colletotrichum gloeosporioides. Plant Physiol. 1993;103:267–272. doi: 10.1104/pp.103.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ramesh M V, Kolattukudy P E. Disruption of the serine proteinase gene (sep) in Aspergillus flavus leads to a compensatory increase in the expression of a metalloproteinase gene (mep20) J Bacteriol. 1996;178:3899–3907. doi: 10.1128/jb.178.13.3899-3907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rodriguez J R, Paterson B M. Yeast myosin heavy chain mutant: maintenance of the cell type specific budding pattern and the normal deposition of chitin and cell wall components requires an intact myosin heavy chain gene. Cell Motil Cytoskeleton. 1990;17:301–308. doi: 10.1002/cm.970170405. [DOI] [PubMed] [Google Scholar]
- 39.Shimanuki M, Goebl M, Yanagida M, Toda T. Fission yeast sts1+ gene encodes a protein similar to the chicken lamin B receptor and is implicated in pleiotropic drug-sensitivity, divalent cation-sensitivity, and osmoregulation. Mol Biol Cell. 1992;3:263–273. doi: 10.1091/mbc.3.3.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Soliday C L, Dickman M B, Kolattukudy P E. Structure of the cutinase gene and detection of promoter activity in the 5′-flanking region by fungal transformation. J Bacteriol. 1989;171:1942–1951. doi: 10.1128/jb.171.4.1942-1951.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Staben C, Jensen B, Singer M, Pollock J, Schechtman M, Kinsey J, Selker E. Use of a bacterial hygromycin B resistance gene as a dominant selectable marker in Neurospora crassa transformation. Fungal Genet Newsl. 1989;36:79–81. [Google Scholar]
- 42.Staples R C, Hoch H C. Infection structures—form and function. Exp Mycol. 1987;11:163–169. [Google Scholar]
- 43.Sweigard J A, Chumley F G, Valent B. Disruption of a Magnaporthe grisea cutinase gene. Mol Gen Genet. 1992;232:183–190. [PubMed] [Google Scholar]
- 44.Tusnády G E, Simon I. Principles governing amino acid composition of integral membrane proteins: application to topology prediction. J Mol Biol. 1998;283:489–506. doi: 10.1006/jmbi.1998.2107. [DOI] [PubMed] [Google Scholar]
- 45.Urban M, Bhargava T, Hamer J E. An ATP-driven efflux pump is a novel pathogenicity factor in rice blast disease. EMBO J. 1999;18:512–521. doi: 10.1093/emboj/18.3.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vinson C R, Sigler P B, McKnight S L. Scissors-grip model for DNA recognition by a family of leucine zipper proteins. Science. 1989;246:911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- 47.Wattad C, Kobiler D, Dinoor A, Prusky D. Pectate lyase of Colletotrichum gloeosporioides attacking avocado fruits: cDNA cloning and involvement in pathogenicity. Physiol Mol Plant Pathol. 1997;50:197–212. [Google Scholar]
- 48.Watts F Z, Shiels G, Orr E. The yeast MYO1 gene encoding a myosin-like protein required for cell division. EMBO J. 1987;6:3499–3505. doi: 10.1002/j.1460-2075.1987.tb02675.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yan C, Lee L H, Davis L I. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 1998;17:7416–7429. doi: 10.1093/emboj/17.24.7416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J-R, Staiger C J, Hamer J E. Inactivation of the mitogen-activated protein kinase Mps1 from the rice blast fungus prevents penetration of host cells but allows activation of plant defense responses. Proc Natl Acad Sci USA. 1998;95:12713–12718. doi: 10.1073/pnas.95.21.12713. [DOI] [PMC free article] [PubMed] [Google Scholar]