Abstract
BACKGROUND
Atrial fibrillation (AF), the most common cardiac arrhythmia, is widely associated with inflammation, vascular dysfunction, and elevated levels of the vascular leak-inducing cytokine, vascular endothelial growth factor (VEGF). Mechanisms underlying AF are poorly understood and current treatments only manage this progressive disease, rather than arresting the underlying pathology. The authors previously identified edema-induced disruption of sodium channel (NaV1.5)-rich intercalated disk nanodomains as a novel mechanism for AF initiation secondary to acute inflammation. Therefore, we hypothesized that protecting the vascular barrier can prevent vascular leak-induced atrial arrhythmias.
OBJECTIVES
In this study the authors tested the hypothesis that protecting the vascular barrier can prevent vascular leak-induced atrial arrhythmias. They identified 2 molecular targets for vascular barrier protection, connexin43 (Cx43) hemichannels and pannexin-1 (Panx1) channels, which have been implicated in cytokine-induced vascular leak.
METHODS
The authors undertook in vivo electrocardiography, electron microscopy, and super-resolution light microscopy studies in mice acutely treated with a clinically relevant level of VEGF.
RESULTS
AF incidence was increased in untreated mice exposed to VEGF relative to vehicle control subjects. VEGF also increased the average number of AF episodes. VEGF shifted NaV1.5 signal to longer distances from Cx43 gap junctions, measured by a distance transformation-based spatial analysis of 3-dimensional confocal images of intercalated disks. Similar effects were observed with NaV1.5 localized near mechanical junctions composed of neural cadherin. Blocking connexin43 hemichannels (αCT11 peptide) or Panx1 channels (PxIL2P peptide) significantly reduced the duration of AF episodes compared with VEGF alone with no treatment. Concurrently, both peptide therapies preserved NaV1.5 distance from gap junctions to control levels and reduced mechanical junction-adjacent intermembrane distance in these hearts. Notably, similar antiarrhythmic efficacy was also achieved with clinically-relevant small-molecule inhibitors of Cx43 and Panx1.
CONCLUSIONS
These results highlight vascular barrier protection as an antiarrhythmic strategy following inflammation-induced vascular leak.
Keywords: antiarrhythmic therapy, arrhythmia, atrial fibrillation, inflammation, vasculature
Atrial fibrillation (AF) is the most common type of cardiac arrhythmia, affecting 3 to 6 million people in the United States.1 Left untreated, AF progresses from spontaneous episodes to permanent events, and predisposes patients to stroke and more severe cardiovascular disease, impairing quality of life. Currently available treatments primarily entail managing symptoms and often involve more invasive procedures with greater risks and limited efficacy. Interventions such as electrical cardioversion and catheter ablation are expensive and require major clinical infrastructure, and yet, AF recurrence of up to 80% has been reported following these therapies.2,3 And although antiarrhythmic drugs used to control rate and rhythm improve symptoms, many have potential deleterious and life-threatening side effects.4 Interestingly, combining such pharmacological treatments with procedures does not provide a substantial improvement in care nor does it mitigate the AF recurrence rate (~65%).3 The critical barrier to progress is that available AF therapies do not target the underlying disease process, and thus are not effective treatment options.
Notably, AF is associated with inflammation, vascular dysfunction, and cardiac structural remodeling.5-7 Early-stage AF patients have elevated serum levels of proinflammatory cytokines—vascular endothelial growth factor (VEGF), TNFα, and IL6 to name a few—which promote vascular leak and edema.8 We have previously identified a novel arrhythmia mechanism in which VEGF-induced vascular leak promotes atrial arrhythmias by disrupting sodium channel (NaV1.5)-rich intercalated disk (ID) nanodomains.9 Therefore, we hypothesized that protecting the vascular endothelial barrier can prevent vascular leak-induced atrial arrhythmias. Although inflammatory cytokines act through myriad pathways, confounding therapeutic targeting, downstream bottlenecks in the signaling process leading to breakdown of endothelial tight junctions could present attractive alternatives. Connexin 43 (Cx43) hemichannels (HCs) and pannexin1 (Panx1) channels play an important role in vascular endothelial barrier integrity, responding to signaling molecules and regulating vascular permeability.10-12 During periods of stress, or in regions of injury, these nonspecific channels remain open as part of the inflammatory response. Several studies have reported vasculoprotective effects with Cx43 HC and Panx1 channel inhibition in both cardiac13-16 and noncardiac tissues.17,18 Thus, we investigated the potential of these inhibitors as antiarrhythmic therapies in our vascular leak-induced atrial arrhythmia model (Video 1). Here, we provide structural and functional evidence demonstrating that inhibiting these channels exhibits cardioprotective effects.
METHODS
All animal procedures were approved by the Institutional Animal Care and Use Committee at The Ohio State University and performed in accordance with the Guide for the Care and Use of Laboratory Animals published by the U.S. National Institutes of Health (NIH Publication No. 85-23, revised 2011).
IN VIVO ELECTROCARDIOGRAM.
Continuous electrocardiogram (ECG) recordings (PL3504 PowerLab 4/35, ADInstruments) were obtained from mice anesthetized with isoflurane (1%-1.5%) as previously described.19 Briefly, after baseline recording (5 minutes), animals received either intraperitoneal VEGF (50 ng/kg—which corresponds to a serum level of 500 ng/L; Sigma) or vehicle (phosphate buffered saline [PBS]). For animals receiving Cx43 HC or Panx1 channel blockers, αCT11 (100 μmol/L, 9 mg/kg), PxIL2P (1.6 μmol/L, 0.32 mg/kg), mefloquine (25 μmol/L, 0.83 mg/kg), or spironolactone (20 μmol/L, 0.67 mg/kg) was administered following the 5-minute baseline recording, followed by VEGF at 10 minutes. After an additional 20 minutes, animals were injected intra-peritoneally with epinephrine (1.5 mg/kg; Sigma) and caffeine (120 mg/kg; Sigma) challenge, and ECG recording continued for 40 minutes. ECG recordings were analyzed using the LabChart 8 software (ADInstruments). At the conclusion of each ECG recording, the heart was excised, leading to euthanasia by exsanguination. The isolated hearts were prepared in one of the following 2 ways:
Cryopreservation: Hearts were embedded in optimal cutting temperature compound and frozen using liquid nitrogen for cryosectioning and fluorescent immunolabeling as in previous studies.20-23 These samples were used for light microscopy experiments as described in the following text.
Fixation for transmission electron microscopy (TEM): Isolated hearts were perfused fixed with 2% paraformaldehyde. Atria were then dissected and fixed overnight in 2.5% glutaraldehyde at 4 °C for resin embedding and ultramicrotomy as previously described.21,22
PRIMARY ANTIBODIES.
The following primary antibodies were used for Western immunoblotting and fluorescence microscopy studies:
Cx43 rabbit polyclonal (Sigma C6219)
Cx43 mouse monoclonal (Sigma MABT903)
Neural cadherin mouse monoclonal (BD Bio-sciences 610920)
Cardiac isoform of the voltage-gated sodium channel (NaV1.5) rabbit polyclonal (custom antibody)21
The antirabbit Cx43 antibody, which binds to the Cx43 C-terminus, was used for hearts from VEGF and vehicle control experiments. The antimouse Cx43 antibody binds to the N-terminus and was thus used in animals receiving peptide drugs (including the Cx43 C-terminal mimetic peptide αCT11) and small-molecule HC blockers to avoid conflating endogenous Cx43 with the therapeutic peptide.
FLUORESCENT IMMUNOLABELING.
Immunofluorescent labeling of cryosections (5-μm thickness) of fresh-frozen myocardium was performed, as previously described.19,21,22,24 Briefly, cryosections were fixed with paraformaldehyde (2%, 5 minutes at room temperature), permeabilized with Triton X-100 (0.2% in PBS for 15 minutes at room temperature), and treated with blocking agent (1% BSA, 0.1% triton in PBS for 2 hours at room temperature) before labeling with primary antibodies (overnight at 4 °C). Samples were then washed in PBS (3 × 5 minutes in PBS at room temperature) before labeling with secondary antibodies.
For confocal microscopy, samples were then labeled with goat antimouse, and goat antirabbit secondary antibodies conjugated to Alexa 488, Alexa 568, and Alexa 647 were used (1:8,000; Thermo Fisher Scientific). Samples were then washed in PBS (3 × 5 minutes in PBS at room temperature) and mounted in ProLong Gold (Invitrogen). For stochastic optical reconstruction microscopy (STORM), samples were labeled with Alexa 647 and Biotium CF 568 fluorophores. STORM samples were then washed in PBS (3 × 5 minutes in PBS at room temperature) and optically cleared using Scale U2 buffer (48 hours at 4 °C) before imaging.20,21,23
TRANSMISSION ELECTRON MICROSCOPY.
EM images of the ID, particularly gap junctions (GJs) and mechanical junctions (MJs), were obtained at 60,000× magnification on an FEI Tecnai G2 Spirit electron microscope. Intermembrane distance at various ID sites was quantified using ImageJ (U.S. National Institutes of Health), as previously described.21,22,25 Briefly, distance was measured manually between outer leaflets of apposed membranes at right angle to the membrane at sites located 50 nm away from the edges of GJs and MJs.
CONFOCAL IMAGING.
Confocal imaging was performed using an A1R-HD laser scanning confocal microscope equipped with 4 solid-state lasers (405, 488, 560, and 640 nm; 30 mW each), a 63×/1.4 numerical aperture oil immersion objective, 2 GaAsP detectors, and 2 high-sensitivity photomultiplier tube detectors (Nikon). Individual fluorophores were imaged sequentially with the excitation wavelength switching at the end of each frame. Images were collected as z-stacks with fluorophores images sequentially (line-wise) to achieve optimal spectral separation, followed by 3-dimensional (3D) deconvolution. Images were analyzed using our distance transformation-based spatial analysis approach, spatial pattern analysis using closest events (SPACE).26,27
SINGLE MOLECULE LOCALIZATION.
STORM imaging was performed using a Vutara 352 microscope (Bruker Nano Surfaces) equipped with biplane 3D detection, and fast sCMOS imaging achieving 20 nm lateral and 50 nm axial resolution, as previously described.20,21,:28,29 Individual fluorophore molecules were localized with a precision of 10 nm. The 2 color channels were precisely registered using localized positions of several TetraSpeck Fluorescent Microspheres (Thermo Fisher Scientific) scattered throughout the field of view, with the procedure being repeated at the start of each imaging session. Protein clustering and spatial organization were quantitatively assessed from single molecule localization data using STORM-RLA, a machine learning-based cluster analysis approach, as previously described.20
STATISTICAL ANALYSIS.
Treatments were applied in an unblinded fashion for all studies. The Kolmogorov-Smirnov test was applied to compare distributions of measurements, while the Wilcoxon rank sum test (for TEM data) and a weighted Student’s t-test (for SPACE outputs) were used for comparisons of central tendency. Fisher’s exact test was used to test differences in nominal data. For multiple comparisons, the Bonferroni correction was applied. A value of P < 0.05 was considered statistically significant. All values are reported as mean ± SE unless otherwise noted.
RESULTS
PROTECTING VASCULAR ENDOTHELIAL BARRIER REDUCES AF SUSCEPTIBILITY IN VEGF-TREATED HEARTS.
We have previously shown that proinflammatory cytokine-mediated vascular barrier breakdown acutely increased AF inducibility,9 a finding we recapitulate here (Figure 1). Notably, VEGF significantly increased the incidence and duration of atrial arrhythmias that degenerated into AF (Table 1) It should be noted here that the shorter mean and median durations observed for atrial arrhythmias, which degenerated into AF in VEGF-treated hearts vs vehicle control subjects, reflect the marked increase in the incidence of arrhythmias brought on by VEGF (5.5 per animal tested vs. 0.33 per animal tested in vehicle control subjects), which included numerous short episodes, some of which rapidly degenerated into AF. Notably, there was a wide difference in number of arrhythmias observed in the VEGF group (110 episodes/20 animals tested) compared with the control group (5 episodes/15 animals tested), which resulted from the marked increase in the number of short episodes in the VEGF group as well as a substantial increase in the longer episodes (15 episodes ≥30 s/20 animals in the VEGF group vs. 4 episodes ≥30s/15 animals in the control group). The resulting imbalance in statistical power limited our ability to statistically differentiate specific aspects of arrhythmia burden in these groups. Therefore, we compared the cumulative distributions of arrhythmia durations using the 2-sample Kolmogorov-Smirnov test, which revealed significantly increased arrhythmia burden following acute VEGF insult (P < 0.01 comparing control vs VEGF).
FIGURE 1. Antiarrhythmic Efficacy of Preserving Vascular Barrier Function.
(A) Representative in vivo electrocardiogram (ECG) traces. Periodic high-frequency fluctuations reflect the animal breathing. (B) Summary plot showing number of atrial fibrillation (AF) episodes as percent of mice positive for AF (control n = 15, vascular endothelial growth factor [VEGF] n = 20, peptides/small molecules n = 10 mice/group), *P < 0.05 vs VEGF by Fisher exact test. (C) Box and whisker plot of AF episode duration, *P < 0.05 vs VEGF by 2-sample Kolmogorov-Smirnov test, †P < 0.05 vs VEGF by Wilcoxon rank sum test. Dashed black ellipse highlights outliers in the VEGF group, which are longer AF episodes more numerous than all AF episodes observed in other groups. See Table 1 for details.
TABLE 1.
Characteristics of Atrial Arrhythmias That Degenerated Into AF
| Control | VEGF | αCT11 | PxIL2P | Mefloquine | Spironolactone | |
|---|---|---|---|---|---|---|
| Experiments | 15 | 20 | 10 | 10 | 10 | 10 |
| Animals that experienced AF | 3 | 14 | 4 | 6 | 1 | 1 |
| AF episodes | 5 | 110 | 6 | 16 | 10 | 8 |
| AF episodes ≥30 s | 4 | 15 | 2 | 5 | 1 | 0 |
| Average episodes/animal tested | 0.33 | 5.50 | 0.60 | 1.60 | 1.00 | 0.80 |
| Average episodes ≥30 s/animal tested | 0.27 | 0.75 | 0.2 | 0.5 | 0.1 | 0 |
| Average episodes/animal experiencing AF | 1.67 | 7.86 | 1.5 | 2.67 | 10.0 | 8.0 |
| Average episodes ≥30s/animal experiencing AF | 1.33 | 1.07 | 0.5 | 0.83 | 1 | 0 |
| Mean duration, s | 126.8 | 37.7 | 89.9 | 23.8 | 8.5 | 4.5 |
| SD, s | 109.8 | 104.8 | 152.2 | 36.0 | 10.5 | 3.6 |
| P value vs VEGF (2-sample KS test comparing mean durations; P values multiplied by Bonferroni’s correction factor for each group, α = 0.05) | 1.10 × 10−7 | – | 3.79 × 10−8 | 5.68 × 10−16 | 6.60 × 10−24 | 2.40 × 10−22 |
| Median duration, s | 93.9 | 4.4 | 17.2 | 3.8 | 2.4 | 4.0 |
| P value vs VEGF (Wilcoxon’s test comparing mean durations; P values multiplied by Bonferroni’s correction factor for each group, α = 0.05) | 1.74 | – | 0.415 | 6.62 × 10−7 | 1.96 × 10−15 | 1.95 × 10−13 |
| Max duration, s | 272.0 | 620.5 | 387.8 | 125.5 | 30.5 | 12.1 |
Values are n unless otherwise specified. Number, duration, and characteristics of atrial arrhythmias observed in our in vivo studies. A 2-sample Kolmogorov-Smirnov (KS) test was used to compare the distributions of arrhythmia durations between groups. Wilcoxon rank sum test was applied to compare median arrhythmia durations between groups.
AF = atrial fibrillation; VEGF = vascular endothelial growth factor.
Thus, we examined whether inhibiting vascular leak was sufficient to prevent atrial arrhythmias in mice injected with clinically-relevant pathological levels of VEGF, as reported in early-stage AF patients.5,6,30-32 Previous studies identify opening of Cx43 HCs and Panx1 channels in the vascular endothelium as key steps in inflammation-induced vascular leak9. Importantly, peptide inhibitors of Cx43 HCs (αCT11)14 and Panx1 channels (PxIL2P)33 have been shown to blunt pathological vascular leak in noncardiac tissues. Adult male mice were pretreated with peptide inhibitors of Cx43 HCs (αCT11; 100 μmol/L) and Panx1 (PxIL2P; 1.6 μmol/L) before the acute VEGF insult. Representative in vivo ECG traces in Figure 1A illustrate atrial arrhythmia elicited by catecholamine challenge in an untreated mouse exposed to VEGF and maintenance of normal sinus rhythm in mice treated with αCT11 or PxIL2P peptide before VEGF insult. The AF phenotype was quantified by assessing inducibility, defined as percent of mice tested under each condition that experienced AF (Figure 1B), and the duration AF episodes (Figure 1C). VEGF alone significantly exacerbated the AF phenotype relative to vehicle control subjects, inducing AF in 70% of mice tested (14 of 20 vs 3 of 10 in vehicle control subjects) (Figure 1B) and significantly increasing the number and duration of AF episodes (Figure 1C, Table 1). Although the decrease in AF incidence or burden (total arrhythmia duration per hour of observation) (Supplemental Figure 1) in peptide-treated mice compared with mice exposed to VEGF alone did not reach statistical significance, both peptide treatments markedly reduced the number (6 episodes in 10 αCT11-treated mice and 16 episodes in 10 PxIL2P-treated mice vs 110 episodes in 20 untreated mice exposed to VEGF) (Table 1) and maximum duration (387.8 seconds after αCT11 treatment, 125.5 seconds after PxIL2P treatment vs 620.5 seconds in untreated mice exposed to VEGF) (Table 1) of AF episodes compared with VEGF alone with no treatment. We also observed several brief runs of atrial flutter in all groups including untreated control subjects. Incidence and burden of atrial flutter showed no significant differences across treatment groups (Supplemental Figure 2). Taken together, these data suggest that vascular barrier protection by Cx43 HC and Panx1 channel inhibition can prevent AF in a setting of acute inflammatory insult.
PROTECTING VASCULAR ENDOTHELIAL BARRIER PRESERVES ID STRUCTURAL INTEGRITY FOLLOWING ACUTE VEGF INSULT.
To investigate if the antiarrhythmic effects of Cx43 HC and Panx1 channel inhibition occur by preventing vascular leak and the ensuing edema-induced ID nanodomain disruption, we performed TEM to assess ID structure in mice pretreated with either peptide inhibitor and subsequently subjected to acute VEGF insult. Representative TEM images show intermembrane spacing at GJ- and MJ-adjacent sites for all treatment groups (yellow highlighted areas in Figures 2A and B). Overall, acute VEGF insult increased median intermembrane spacing at perinexal sites as well as near MJs in highly heterogeneous fashion (Figures 2C and 2D). We note here that new measurements for the control and VEGF groups were collected from the same images used in our previous study9; therefore, representative images published in that study are repeated here in Figure 2A where they are compared against new data obtained from our treatment groups. Both peptide treatments preserved close membrane apposition at MJ-adjacent, but not perinexal, intermembrane sites after VEGF insult compared with no treatment (Figure 2C). In keeping with these central tendencies, distributions of intermembrane distances near MJs, but not at perinexi, were left-shifted in the peptide-treated hearts compared with hearts exposed to VEGF alone (Figure 2D). However, it should be noted that VEGF alone without treatment prompted the emergence of a mode around 100 nm in perinexal intermembrane spacing, which was not present in control subjects or in peptide-treated hearts.
FIGURE 2. Ultrastructural Effects of Vascular Endothelial Barrier Protection.
Representative transmission electron microscopy images of intercalated discs murine atria. (A) Untreated control and VEGF. Reproduced with permission from Mezache et al9 (2020). (B) αCT11 and PxIL2P. Cyan arrows point to gap junctions and black arrows point to mechanical junctions (MJs). Intermembrane spacing is highlighted in yellow in (A) and (B) to facilitate recognition of features being measured. (C) Summary plots of median intermembrane distance at gap junction adjacent perinexal sites (solid bars) and MJ-adjacent (striped bars) ID sites (>100 measurements/group/location from n = 3 hearts/group, * P < 0.05 vs VEGF at perinexi, †P < 0.05 vs VEGF at MJ-adjacent sites). (D) Cumulative distribution of intermembrane distance at perinexal and MJ-adjacent sites. Note the emergence of a second mode at ~100 nm in perinexal intermembrane distance in the VEGF group, a feature not present in αCT11/PxIL2P-treated hearts. Abbreviations as in Figure 1.
PROTECTING VASCULAR ENDOTHELIAL BARRIER PRESERVES NaV1.5 LOCALIZATION WITHIN THE ID FOLLOWING ACUTE VEGF INSULT.
We previously showed NaV1.5 migration away from GJs and MJs following acute VEGF treatment9; so, we used confocal imaging to examine perinexal and MJ-adjacent NaV1.5 distribution in mouse atria pretreated with either Cx43 HC or Panx1 channel peptide inhibitors. Representative 3D confocal images of atrial en face IDs illustrate NaV1.5 distribution relative to ID landmarks, GJs and MJs, across all treatment groups (Figures 3A and 3B). Overall, consistent with our previous results obtained using super-resolution microscopy,9 our distance transformation-based spatial analysis26,27 detected translocation of NaV1.5 away from GJs and MJs from diffraction-limited confocal images of VEGF-exposed hearts compared with vehicle control subjects (Figures 3C and 3D). Both peptide therapies preserved NaV1.5 distance from GJs, but not MJs, to control levels as compared with atria treated with VEGF alone (Figures 3C and 3D). Furthermore, by employing our image analysis approach, spatial pattern analysis using closest events (SPACE) (which evaluates relative localization by applying point process analysis to nearest neighbor distances between colabeled signals)27, we compared the observed distribution of nearest-neighbor distances between immunosignals for colabeled proteins (Figure 3C, solid lines) with the distribution predicted under complete spatial randomness (Figure 3C, dashed lines), and obtained measures of nonrandom attraction/repulsion between the colabeled proteins. VEGF decreased nonrandom attraction of NaV1.5 to GJs and MJs, while both peptide therapies preserved NaV1.5 distance from GJs at control levels (Figures 3C and 3D).
FIGURE 3. Confocal imaging of Intercalated Discs.
Representative 3-dimensional confocal images of en face intercalated discs from murine atria. (A) Untreated control subjects and VEGF-treated. (B) Connexin 43 (Cx43) hemichannels and pannexin 1 (Panx1) channel peptide inhibitors (αCT11 and PxIL2P, respectively). (C) Cumulative distribution of cardiac isoform of the voltage-gated sodium channel (NaV1.5) distance from Cx43 or neural cadherin (N-cad). Solid lines and dashed lines, respectively, represent weighted average curves for observed and predicted random nearest-neighbor distance distributions (measurements from each image were weighted by number of signal-positive voxels contained therein). Shaded areas around the curves represent SDs. (D) Summary plot of NaV1.5 attraction to gap and mechanical junctions from n = 3 hearts/group, *P < 0.05 vs VEGF by weighted Student’s t-test. Abbreviations as in Figure 1.
Thus, spatial analysis of 3D confocal images provides measures of NaV1.5 organization relative to specific ID landmarks, GJs and MJs. However, under physiological conditions, the distance between clusters of NaV1.5 channels and these ID landmarks often falls below Abbe’s diffraction limit. Thus, to directly assess NaV1.5 organization within these nanodomains and obtain orthogonal validation of the confocal image results, we turned to STORM single molecule localization microscopy and STORM-RLA machine learning-based cluster analysis. The subdiffraction resolution of STORM coupled with localization of molecules allows for more discrete measure of NaV1.5 protein density at our ID regions of interest, GJs and MJs. Representative 3D en face views of atrial IDs show retention of NaV1.5 cluster density within 50 nm of GJs and MJs in peptide pretreated hearts compared with untreated control subjects and VEGF-treated (Figures 4A and 4B). Overall, both peptide therapies maintained NaV1.5 distribution nearer to GJs compared with VEGF (Figure 4C, Supplemental Figures 3 to 5). However, there was no significant effect of either peptide on NaV1.5 distribution near MJs. These results are consistent with spatial analysis of the diffraction-limited 3D confocal images presented in the previous text.
FIGURE 4. Stochastic Optical Reconstruction Microscopy Imaging of Atrial IDs—NaV1.5.
Representative 3-dimensional stochastic optical reconstruction microscopy images of en face IDs immunolabeled for NaV1.5 along with Cx43 and N-cad from (A) control and VEGF-treated, (B) peptide pretreated murine atria. Stochastic optical reconstruction microscopy data are rendered as point clouds with each localized molecule represented as a 50-nm sphere. Although 20-nm resolution was achieved, the 50-nm size was chosen for rendering to guarantee visibility in print. (C) Cumulative distribution of NaV1.5 expression relative to gap junctions (Cx43) and mechanical junctions (N-cad) from n = 3 hearts/group, *†P < 0.05 vs VEGF at perinexi and mechanical junction-adjacent sites, respectively. Abbreviations as in Figures 1 and 3.
SMALL-MOLECULE ALTERNATIVES TO PEPTIDE INHIBITORS REDUCE AF SUSCEPTIBILITY FOLLOWING ACUTE VEGF INSULT.
Although peptide inhibitors offer greater selectivity, in vivo delivery of these drugs poses significant challenges, limiting clinical translatability. Therefore, we identified small-molecule alternatives to the Cx43 HC and Panx1 channel peptide inhibitors, mefloquine and spironolactone, respectively. Figure 5 presents the additional results obtained with these small-molecule drugs, comparing them with control, untreated, and peptide-treated cases (results for these latter groups are repeated from Figure 1 for easy comparison). Although there are many Cx43 HC and Panx1 channel blockers, mefloquine and spironolactone at the doses used here afford the highest potency with the lowest likelihood of adverse effects, especially gap junction inhibition.13,16,17,34-50 Mice were pretreated with either mefloquine or spironolactone for 10 minutes, followed by acute VEGF insult. Both small-molecule treatments significantly reduced AF incidence (Figure 5B, Table 1), episode duration (8.5 ± 10.5 seconds in mefloquine-treated mice, 4.5 ± 3.6 seconds in spironolactone-treated mice vs 37.7 ± 104.8 seconds in untreated mice exposed to VEGF) (Figure 5C, Table 1), burden (Supplemental Figure 1), and number of episodes (10 episodes in 10 mefloquine-treated mice, 8 episodes in 10 spironolactone-treated mice vs 110 episodes in 20 untreated mice exposed to VEGF) (Table 1). Together, these results suggest that protecting the vascular endothelial barrier reduces atrial arrhythmia susceptibility by preventing edema-induced ID remodeling.
FIGURE 5. Antiarrhythmic Efficacy of Small-Molecule Alternatives to Peptide Inhibitors.
(A) Representative in vivo ECG traces. (B) Summary plot showing number of AF episodes as percent of mice positive for AF, *P < 0.05 vs VEGF by Fisher exact test. (C) Box and whisker plot summarizing duration of AF episodes observed (control n = 15, VEGF n = 20, peptides/small molecules n = 10 mice/group), *P < 0.05 vs VEGF by 2 Sample Kolmogorov-Smirnov test, †P < 0.05 vs VEGF by Wilcoxon rank sum test. Dashed black ellipse highlights outliers in the VEGF group, which are longer AF episodes more numerous than all AF episodes observed in other groups. See Table 1 for details. Abbreviations as in Figure 1.
DISCUSSION
Patients presenting with new-onset AF experience inflammation, vascular dysfunction, and tissue edema and have acutely elevated levels of vascular leak-inducing cytokines, such as VEGF.5,6,11,30-32 We previously identified a novel arrhythmia mechanism by which inflammation-induced vascular leak disrupts NaV1.5-rich ID nanodomains, slowing conduction and promoting atrial arrhythmias.9 Indeed, AF is a common sequela of vascular barrier breakdown in many pathologies. We recognize that inhibiting proinflammatory cytokine receptors is an ostensibly evident therapy strategy; however, the inflammatory response acts through myriad interconnected pathways, confounding therapeutic targeting. Further, systemic inhibition of inflammatory receptors could interfere with vital physiological processes such as repair after injury or clearing infection. Therefore, we sought to target specific components within endothelial cells, which are critically involved in vascular barrier breakdown, regardless of which inflammatory cytokine may initiate the response. Inflammation-induced vascular barrier breakdown results from dynamic disassembly of tight junctions between vascular endothelial cells in response to inflammation.51 Key upstream steps involved in this process include the opening of Cx43 HCs52 and Panx1 channels10,33 located on the endothelial cell membrane. Under pathological conditions, these large nonspecific channels remain open as part of the inflammatory response, activating release of ATP and other small signaling molecules, triggering tight junction breakdown. Notably, endothelial Panx1 has been shown to promote synthesis and release of inflammatory cytokines contributing to positive feedback amplification of inflammatory signaling,12 further highlighting these channels as a potential therapeutic target. Here, we demonstrate that inhibiting these channels prevents atrial arrhythmias by preserving ID nanodomain structure and protein organization.
Several studies have identified Cx43 HCs and Panx1 channels as crucial contributors to the development of arrhythmogenic substrates.53-57 And, while the mechanism by which these channels increase the risk of cardiac arrhythmias has been largely obscure, inhibiting these channels has demonstrated vasculoprotective and cardioprotective effects. In retina, vascular barrier protection by Cx43 HC and Panx1 channel inhibition prevents retinal damage following ischemic injury and improves vision in patients with age-related macular degeneration and diabetic retinopathy.17,18 Inhibition of Cx43 HCs and Panx1 channels has also been shown to reduce inflammation and fibrosis and preserve myocardial function following ischemia-reperfusion injury/myocardial infarction.14,57-59 Here, we demonstrate that blocking vascular endothelial Cx43 HCs and Panx1 channels prevents vascular leak, thereby preserving ID nanodomains and reducing arrhythmia susceptibility.
Multiple peptide and small-molecule drugs can block Cx43 HCs and Panx1 channels. Cx43 HCs can be inhibited by Gap1916 and αCT11,14,42,60 and small-molecule drugs such as mefloquine47 and tonabersat.17 Although Gap19 has demonstrated protective effects following myocardial ischemia/reperfusion injury, αCT11 inhibits Cx43 HCs by promoting their accretion into GJ plaques, improving coupling between vascular endothelial cells.61 Furthermore, αCT11 is currently under clinical development for noncardiac indications (NCT05031806), demonstrating broad but useful indication of the potential for clinical translation in the context of our proposed application. Similarly, Panx1 channels can be inhibited by the PxIL2P peptide33 and small molecules such as spironolactone13 and probenecid.44 Whereas peptide drugs offer greater selectivity, small-molecule inhibitors enable easier in vivo delivery. Consistent with other studies, our in vivo ECG experiments showed a reduction in atrial arrhythmia burden in mice pretreated with either Cx43 HC or Panx1 channel peptide inhibitor following acute VEGF insult. Similar antiarrhythmic effects were observed with clinically relevant small-molecule alternatives, mefloquine and spironolactone, at doses selected to maximize inhibition while minimizing any off-target effects. Notably, others, who evaluated spironolactone in AF as an aldosterone antagonist, reported antiarrhythmic efficacy in early-stage15,62,63 but not persistent/permanent64 AF patients. This highlights the relevance of a targeted therapeutic approach based on a logical, mechanistic understanding of the underlying drivers of AF. Spironolactone was distinctly effective in reducing the risk of new-onset AF, mirroring levels of vascular leak-inducing cytokines, which are only elevated in early-stage AF patients,5,6,30-32 suggesting that it may be uniquely useful in treating early-stage AF. Thus, our results, along with those of the aforementioned clinical trials,15,62-64 suggest that spironolactone and mefloquine merit consideration for antiarrhythmic therapy specifically in early stages of AF in a setting of elevated inflammation.
Studies have shown Cx43 HC and Panx1 channel involvement in vascular endothelial permeability,10,52,65,66 promoting hyperpermeability by the release of key signaling molecules such as ATP in response to inflammatory conditions. As we previously identified in our novel AF mechanism,9 ID disruption occurs downstream of elevated vascular leak induced by the proinflammatory cytokine VEGF, similar to the ID nanodomain swelling observed in AF patients.7 Other studies have also demonstrated ID nanodomain swelling following acute interstitial edema.22,23,67,68 Our TEM studies revealed mitigation of ID nanodomain swelling, particularly MJ-adjacent regions, in hearts pretreated with either Cx43 HC or Panx1 channel inhibitor following acute VEGF insult. Taken together, these results suggest that preventing inflammation-induced vascular leak preserves ID nanodomain ultrastructure, thereby averting development of a structural proarrhythmic substrate.
Previous studies, including work by our group, have linked the importance of these ID nanodomains in cardiac impulse propagation with the enrichment of NaV1.5 sodium channels at GJ-adjacent perinexi and MJ-adjacent sites.20-23,69 Consistent with these reports, we previously demonstrated that VEGF-induced ID disruption and consequent NaV1.5 translocation away from these sites was sufficient to induce proarrhythmic conduction slowing.9 Therefore, we used confocal and super-resolution microscopy to investigate NaV1.5 organization at the ID in mice pretreated with Cx43 HC and Panx1 channel inhibitors. Notably, confocal microscopy coupled with spatial analysis26,27 was able to detect VEGF-induced NaV1.5 translocation previously identified using super-resolution imaging, specifically, stimulated emission depletion and STORM single molecule localization microscopy.9 Consistent with SPACE results from analysis of diffraction-limited confocal images, quantitative analysis of STORM-derived single-molecule localizations revealed that our therapeutic peptides preserved measures of NaV1.5 attraction to GJs, but not MJs, and NaV1.5 cluster density at perinexal sites similar to those of the control subjects. On a technical level, these results together suggest that spatial analysis of diffraction-limited images could provide clues regarding remodeling occurring at subdiffraction spatial scales. From a translational perspective, our peptide therapies preserved NaV1.5 cluster density near GJs, but did not fully prevent perinexal widening, although they did abolish the emergence of a subpopulation of extremely damaged perinexi with intermembrane distance in excess of 100 nm. Conversely, the peptide therapies maintained normal MJ-adjacent intermembrane distance but not NaV1.5 clustering at these sites. Nevertheless, the combined effect of each of the peptide therapies tested was sufficient to ameliorate VEGF-induced atrial arrhythmias in mice. Notably, pretreatment with the peptide and small-molecule inhibitors was chosen for 2 reasons: 1) AF is episodic in its earliest stages; and 2) VEGF, unlike other inflammatory cytokines, tends to be elevated and exert its effects over short time courses. Thus, we envision pretreatment as occurring between AF episodes. Additionally, we also envision such therapy benefiting patient subpopulations such as those at risk of postoperative AF.
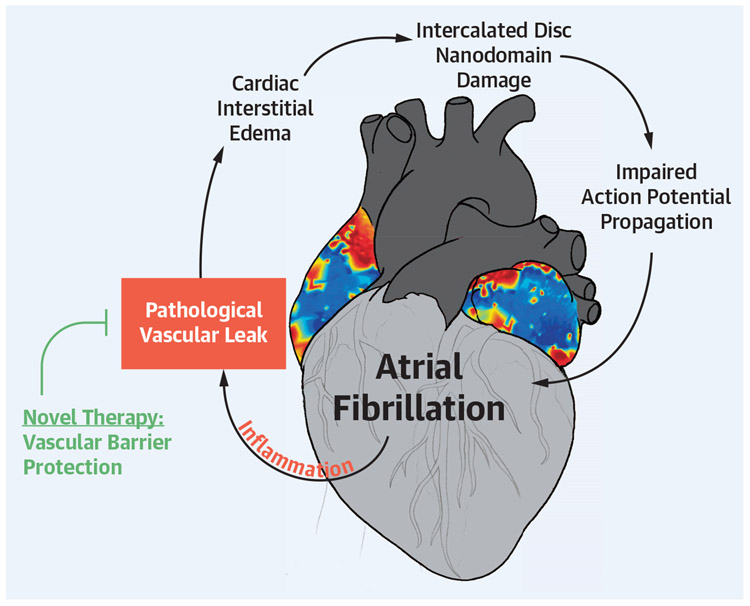
This points to an important mechanistic implication. Specifically, we demonstrate the antiarrhythmic efficacy of preserving ID nanodomains, which have been shown to play key roles in cardiac action potential propagation by supporting ephaptic coupling: Narrow extracellular cleft width and dense NaV1.5 clusters are fundamental requirements for this phenomenon,22,70,71 and previous work by us and others has demonstrated that disruption of these nanodomains results in arrhythmogenic conduction defects.9,21 Our electron and light microscopy data suggest that a mitigation of damage to ID nanodomains thought capable of supporting ephaptic coupling is sufficient to protect against arrhythmias. In other words, our therapies did not completely prevent VEGF-induced remodeling of ultrastructure and protein organization within these ID nanodomains; however, they limited heterogeneity to a degree compatible with normal atrial rhythm. Although this mechanism does not explain initiation of AF in all cases, our previous and present work demonstrate that acute inflammatory insults can induce disruption of ID nanodomains, sufficient to promote AF in otherwise healthy hearts. Our work also demonstrates that preventing such disruption through vascular barrier protection can prevent such atrial arrhythmias in the context of inflammation. It should also be noted that some aspects of the mechanism underlying conduction defects secondary to ID swelling and NaV1.5 reorganization is unclear. Our present work, therefore, underscores the need for further investigation to clearly delineate these mechanisms. Nevertheless, collectively, these data suggest protecting the vascular endothelial barrier by inhibiting Cx43 HCs and Panx1 channels prevents ID ultrastructural remodeling and protein reorganization, thereby maintaining functional cardiac ephapses, and preventing the dynamic formation of a structural substrate for arrhythmia (Central Illustration).
CENTRAL ILLUSTRATION. Proposed Strategy for Mechanistically Derived Antiarrhythmic Therapy.
Modified with permission from Mezache et al9 (2020). AF = atrial fibrillation; ID = intercalated disc; VEGF = vascular endothelial growth factor.
STUDY LIMITATIONS.
An important concern when targeting Cx43 in the endothelium is the potential impact on Cx43-mediated GJ coupling between cardiomyocytes. However, αCT11 inhibits Cx43 HCs by promoting their accretion into GJ plaques, avoiding any GJ uncoupling.72-75 Although small-molecule drugs are easier to deliver in vivo, their reduced specificity often yields off-target effects. Among small-molecule inhibitors of Cx43 HCs and Panx1 channels, however, mefloquine and spironolactone, respectively, present the greatest potency with minimal off-target effects.13,16,17,34,35,37,38,40-44,47-50 Particularly in the case of Cx43 HC inhibition, the selected dose of mefloquine has no impact on GJ coupling.36,39,42,45-47 Although mefloquine and spironolactone are not highly selective blockers of Cx43 HCs and Panx1 channels, the use of these therapies is not intended to treat all paroxysmal AF patients. Rather, we propose the use of these small-molecule inhibitors in the context of AF following acute inflammatory insult. Further, mefloquine and spironolactone are intended for use in patients experiencing AF related to an inflammatory-driven etiology for a limited period until the inflammatory burden is reduced, such as in postoperative patients. Although the dose regimes chosen in the present work are at the low end of the clinically used dose,39,42,45,47,76 further work is needed to determine the lowest effective dose for each drug and to investigate possible combination therapy with HC and Panx1 channel blockers. Interestingly, some studies have demonstrated inhibitory effects of mefloquine on Panx1 channels.77-79 Additionally, a retrospective analysis of patients using spironolactone may elucidate the relationship between dose and AF risk. Based on our ECG studies, the antiarrhythmic effects of mefloquine suggest that combined targeting of Cx43 HCs and Panx1 channels is synergistic, although further investigation is needed to fully understand drug interactions.
CONCLUSIONS
We demonstrate vascular endothelial barrier protection as a mechanistically derived antiarrhythmic strategy for the prevention of AF in its early stages. This approach, for which we demonstrate both efficacy and mode of action, provides a logical treatment option for arrhythmia prevention in patients at risk for new-onset arrhythmias, such as postoperative AF. This approach has considerable implications for standard of care for early-stage AF patients, particularly those with underlying pathologies associated with vascular dysfunction.
Supplementary Material
PERSPECTIVES.
COMPETENCY IN MEDICAL KNOWLEDGE:
Our novel results demonstrate a mechanistically-guided therapeutic approach for the prevention of inflammation-induced AF by protecting the vascular endothelial barrier and thereby preventing damage to intercalated disc nanodomains vital for cell-to-cell communication.
TRANSLATIONAL OUTLOOK:
We provide mechanistic evidence to demonstrate that novel therapeutic strategies, entailing inhibition of Cx43 HCs and Panx1 channels in the vascular endothelium using clinically relevant drugs, can effectively prevent AF in a setting of acute inflammation. This work holds important implications for the prevention of postoperative AF as well as new-onset/early-stage AF associated with inflammatory etiologies.
ACKNOWLEDGMENTS
The authors wish to thank Prof Robert Gourdie for extremely valuable discussions on the biology of Cx43 HCs and the use of mimetic peptides to inhibit them. Immense thanks are also owed to Ms Chloe ‘Cleo’ Samohano for designing and producing the animated video abstract, in particular for her exquisite attention to detail to ensure accurate representation of cardiac structure at various spatial scales.
FUNDING SUPPORT AND AUTHOR DISCLOSURES
This work was supported by grants from the National Institutes of Health (R01 HL148736 and R01 HL165751 awarded to Dr Veeraraghavan), and the American Heart Association (20TPA35460040 awarded to Dr Veeraraghavan and a predoctoral fellowship awarded to Dr Mezache). The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
ABBREVIATIONS AND ACRONYMS
- AF
atrial fibrillation
- Cx43
connexin 43
- GJ
gap junction
- ID
intercalated disc
- MJ
mechanical junction
- NaV1.5
cardiac isoform of the voltage-gated sodium channel
- Panx1
pannexin 1
- SPACE
spatial pattern analysis using closest events, a spatial image analysis approach
- STORM
stochastic optical reconstruction microscopy
- VEGF
vascular endothelial growth factor
Footnotes
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, including patient consent where appropriate. For more information, visit the Author Center.
APPENDIX For an expanded results section and supplemental figures and a video, please see the online version of this paper.
REFERENCES
- 1.Kornej J, Borschel CS, Benjamin EJ, Schnabel RB. Epidemiology of atrial fibrillation in the 21st century: novel methods and new insights. Circ Res. 2020;127:4–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biffi M, Boriani G, Bartolotti M, et al. Atrial fibrillation recurrence after internal cardioversion: prognostic importance of electrophysiological parameters. Heart. 2002;87:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fetsch T, Bauer P, Engberding R, et al. for the Prevention of Atrial Fibrillation after Cardioversion Investigators. Prevention of atrial fibrillation after cardioversion: results of the PAFAC trial. Eur Heart J. 2004;25:1385–1394. [DOI] [PubMed] [Google Scholar]
- 4.Mankad P, Kalahasty G. Antiarrhythmic drugs: risks and benefits. Med Clin North Am. 2019;103:821–834. [DOI] [PubMed] [Google Scholar]
- 5.Chung NA, Belgore F, Li-Saw-Hee FL, et al. Is the hypercoagulable state in atrial fibrillation mediated by vascular endothelial growth factor? Stroke. 2002;33:2187–2191. [DOI] [PubMed] [Google Scholar]
- 6.Li J, Solus J, Chen Q, et al. Role of inflammation and oxidative stress in atrial fibrillation. Heart Rhythm. 2010;7:438–444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raisch TB, Yanoff MS, Larsen TR, et al. Intercalated disk extracellular nanodomain expansion in patients with atrial fibrillation. Front Physiol. 2018;9:398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hadi HA, Alsheikh-Ali AA, Mahmeed WA, Suwaidi JM. Inflammatory cytokines and atrial fibrillation:current and prospective views. J Inflamm Res. 2010;3:75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mezache L, Struckman HL, Greer-Short A, et al. Vascular endothelial growth factor promotes atrial arrhythmias by inducing acute intercalated disk remodeling. Sci Rep. 2020;10:20463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sharma AK, Charles EJ, Zhao Y, et al. Pannexin-1 channels on endothelial cells mediate vascular inflammation during lung ischemia-reperfusion injury. Am J Physiol Lung Cell Mol Physiol. 2018;315:L301–L312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weis SM. Vascular permeability in cardiovascular disease and cancer. Curr Opin Hematol. 2008;15:243–249. [DOI] [PubMed] [Google Scholar]
- 12.Yang Y, Delalio LJ, Best AK, et al. Endothelial pannexin 1 channels control inflammation by regulating intracellular calcium. J Immunol. 2020;204:2995–3007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Good ME, Chiu YH, Poon IKH, et al. Pannexin 1 channels as an unexpected new target of the antihypertensive drug spironolactone. Circ Res. 2018;122:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jiang J, Hoagland D, Palatinus JA, et al. Interaction of alpha carboxyl terminus 1 peptide with the connexin 43 carboxyl terminus preserves left ventricular function after ischemia-reperfusion injury. J Am Heart Assoc. 2019;8:e012385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lou YM, Zheng ZL, Xie LY, et al. Effects of spironolactone on hypoxia-inducible factor-1alpha in the patients receiving coronary artery bypass grafting. J Cardiovasc Pharmacol. 2021;78:e101–e104. [DOI] [PubMed] [Google Scholar]
- 16.Wang N, De Vuyst E, Ponsaerts R, et al. Selective inhibition of Cx43 hemichannels by Gap19 and its impact on myocardial ischemia/reperfusion injury. Basic Res Cardiol. 2013;108:309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mat Nor MN, Rupenthal ID, Green CR, Acosta ML. Connexin hemichannel block using orally delivered tonabersat improves outcomes in animal models of retinal disease. Neurotherapeutics. 2020;17:371–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dvoriantchikova G, Ivanov D, Barakat D, et al. Genetic ablation of Pannexin1 protects retinal neurons from ischemic injury. PLoS One. 2012;7:e31991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koleske M, Bonilla I, Thomas J, et al. Tetrodotoxin-sensitive Navs contribute to early and delayed afterdepolarizations in long QT arrhythmia models. J Gen Physiol. 2018;150(7):991–1002. 10.1085/jgp.201711909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Veeraraghavan R, Gourdie RG. Stochastic optical reconstruction microscopy-based relative localization analysis (STORM-RLA) for quantitative nanoscale assessment of spatial protein organization. Mol Biol Cell. 2016;27:3583–3590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Veeraraghavan R, Hoeker GS, Alvarez-Laviada A, et al. The adhesion function of the sodium channel beta subunit (beta1) contributes to cardiac action potential propagation. Elife. 2018;7:e37610. 10.7554/eLife.37610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Veeraraghavan R, Lin J, Hoeker GS, Keener JP, Gourdie RG, Poelzing S. Sodium channels in the Cx43 gap junction perinexus may constitute a cardiac ephapse: an experimental and modeling study. Pflugers Arch. 2015;467:2093–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Veeraraghavan R, Lin J, Keener JP, Gourdie R, Poelzing S. Potassium channels in the Cx43 gap junction perinexus modulate ephaptic coupling: an experimental and modeling study. Pflugers Arch. 2016;468:1651–1661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Radwański PB, Ho H-T, Veeraraghavan R, et al. Neuronal Na+ channels are integral components of pro-arrhythmic Na+/Ca2+ signaling nanodomain that promotes cardiac arrhythmias during β-adrenergic stimulation. J Am Coll Cardiol Basic Trans Science. 2016;1:251–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Struckman HL, Moise N, King DR, et al. Unraveling impacts of chamber-specific differences in intercalated disc ultrastructure and molecular organization on cardiac conduction. J Am Coll Cardiol EP. Published online July 12, 2023. 10.1016/j.jacep.2023.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bogdanov V, Soltisz AM, Moise N, et al. Distributed synthesis of sarcolemmal and sarcoplasmic reticulum membrane proteins in cardiac myocytes. Basic Res Cardiol. 2021;116:63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Soltisz AM, Craigmile PF, Veeraraghavan R. Spatial pattern analysis using closest events (space)-a nearest neighbor point pattern analysis framework for assessing spatial relationships from image data. bioRxiv. 2023:2023.05.17.541131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bonilla IM, Belevych AE, Baine S, et al. Enhancement of cardiac store operated calcium entry (SOCE) within novel intercalated disk microdomains in arrhythmic disease. Sci Rep. 2019;9:10179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Struckman HL, Baine S, Thomas J, et al. Super-resolution imaging using a novel high-fidelity antibody reveals close association of the neuronal sodium channel NaV1.6 with ryanodine receptors in cardiac muscle. Microsc Microanal. 2020:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ogi H, Nakano Y, Niida S, et al. Is structural remodeling of fibrillated atria the consequence of tissue hypoxia? Circ J. 2010;74:1815–1821. [DOI] [PubMed] [Google Scholar]
- 31.Scridon A, Morel E, Nonin-Babary E, Girerd N, Fernandez C, Chevalier P. Increased intracardiac vascular endothelial growth factor levels in patients with paroxysmal, but not persistent atrial fibrillation. Europace. 2012;14:948–953. [DOI] [PubMed] [Google Scholar]
- 32.Seko Y, Nishimura H, Takahashi N, Ashida T, Nagai R. Serum levels of vascular endothelial growth factor and transforming growth factor-beta1 in patients with atrial fibrillation undergoing defibrillation therapy. Jpn Heart J. 2000;41:27–32. [DOI] [PubMed] [Google Scholar]
- 33.Billaud M, Chiu YH, Lohman AW, et al. A molecular signature in the pannexin1 intracellular loop confers channel activation by the alpha1 adrenoreceptor in smooth muscle cells. Sci Signal. 2015;8:ra17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. [DOI] [PubMed] [Google Scholar]
- 35.Contreras JE, Saez JC, Bukauskas FF, Bennett MV. Gating and regulation of connexin 43 (Cx43) hemichannels. Proc Natl Acad Sci U S A. 2003;100:11388–11393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cruikshank SJ, Hopperstad M, Younger M, Connors BW, Spray DC, Srinivas M. Potent block of Cx36 and Cx50 gap junction channels by mefloquine. Proc Natl Acad Sci U S A. 2004;101:12364–12369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Desplantez T, Verma V, Leybaert L, Evans WH, Weingart R. Gap26, a connexin mimetic peptide, inhibits currents carried by connexin43 hemichannels and gap junction channels. Pharmacol Res. 2012;65:546–552. [DOI] [PubMed] [Google Scholar]
- 38.Evans WH, Leybaert L. Mimetic peptides as blockers of connexin channel-facilitated intercellular communication. Cell Commun Adhes. 2007;14:265–273. [DOI] [PubMed] [Google Scholar]
- 39.Jones R, Kunsman G, Levine B, Smith M, Stahl C. Mefloquine distribution in postmortem cases. Forensic Sci Int. 1994;68:29–32. [DOI] [PubMed] [Google Scholar]
- 40.Kim Y, Griffin JM, Nor MNM, et al. Tonabersat prevents inflammatory damage in the central nervous system by blocking connexin43 hemichannels. Neurotherapeutics. 2017;14:1148–1165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mugisho OO, Rupenthal ID, Paquet-Durand F, Acosta ML, Green CR. Targeting connexin hemichannels to control the inflammasome: the correlation between connexin43 and NLRP3 expression in chronic eye disease. Expert Opin Ther Targets. 2019;23:855–863. [DOI] [PubMed] [Google Scholar]
- 42.Rhett JM, Jourdan J, Gourdie RG. Connexin 43 connexon to gap junction transition is regulated by zonula occludens-1. Mol Biol Cell. 2011;22:1516–1528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Salameh A, Dhein S. Pharmacology of gap junctions. New pharmacological targets for treatment of arrhythmia, seizure and cancer? Biochim Biophys Acta. 2005;1719:36–58. [DOI] [PubMed] [Google Scholar]
- 44.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol. 2008;295:C761–C767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Skeberdis VA, Rimkute L, Skeberdyte A, Paulauskas N, Bukauskas FF. pH-dependent modulation of connexin-based gap junctional uncouplers. J Physiol. 2011;589:3495–3506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Srinivas M, Hopperstad MG, Spray DC. Quinine blocks specific gap junction channel subtypes. Proc Natl Acad Sci U S A. 2001;98:10942–10947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tong D, Li TY, Naus KE, Bai D, Kidder GM. In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci. 2007;120:4016–4024. [DOI] [PubMed] [Google Scholar]
- 48.Wang J, Ma M, Locovei S, Keane RW, Dahl G. Modulation of membrane channel currents by gap junction protein mimetic peptides: size matters. Am J Physiol Cell Physiol. 2007;293:C1112–C1119. [DOI] [PubMed] [Google Scholar]
- 49.Wang N, De Bock M, Antoons G, et al. Connexin mimetic peptides inhibit Cx43 hemichannel opening triggered by voltage and intracellular Ca2+ elevation. Basic Res Cardiol. 2012;107:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ye ZC, Wyeth MS, Baltan-Tekkok S, Ransom BR. Functional hemichannels in astrocytes: a novel mechanism of glutamate release. J Neurosci. 2003;23:3588–3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wolpe AG, Ruddiman CA, Hall PJ, Isakson BE. Polarized proteins in endothelium and their contribution to function. J Vasc Res. 2021;58:65–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hautefort A, Pfenniger A, Kwak BR. Endothelial connexins in vascular function. Vasc Biol. 2019;1:H117–H124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Andelova K, Egan Benova T, Szeiffova Bacova B, et al. Cardiac connexin-43 hemichannels and pannexin1 channels: provocative antiarrhythmic targets. Int J Mol Sci. 2020;22(1):260. 10.3390/ijms22010260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Meens MJ, Kwak BR, Duffy HS. Role of connexins and pannexins in cardiovascular physiology. Cell Mol Life Sci. 2015;72:2779–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rodriguez-Sinovas A, Sanchez JA, Fernandez-Sanz C, Ruiz-Meana M, Garcia-Dorado D. Connexin and pannexin as modulators of myocardial injury. Biochim Biophys Acta. 2012;1818:1962–1970. [DOI] [PubMed] [Google Scholar]
- 56.Rusiecka OM, Montgomery J, Morel S, et al. Canonical and non-canonical roles of connexin43 in cardioprotection. Biomolecules. 2020;10(9):1225. 10.3390/biom10091225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saez PJ, Shoji KF, Aguirre A, Saez JC. Regulation of hemichannels and gap junction channels by cytokines in antigen-presenting cells. Mediators Inflamm. 2014;2014:742734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dolmatova E, Spagnol G, Boassa D, et al. Cardiomyocyte ATP release through pannexin 1 aids in early fibroblast activation. Am J Physiol Heart Circ Physiol. 2012;303:H1208–H1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Good ME, Young AP, Wolpe AG, et al. Endothelial pannexin 1 regulates cardiac response to myocardial infarction. Circ Res. 2021;128:1211–1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.King DR, Sedovy MW, Leng X, et al. Mechanisms of connexin regulating peptides. Int J Mol Sci. 2021;22(19):10186. 10.3390/ijms221910186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Obert E, Strauss R, Brandon C, et al. Targeting the tight junction protein, zonula occludens-1, with the connexin43 mimetic peptide, alphaCT1, reduces VEGF-dependent RPE pathophysiology. J Mol Med (Berl). 2017;95:535–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chung YW, Yang YH, Wu CK, et al. Spironolactone is associated with reduced risk of new-onset atrial fibrillation in patients receiving renal replacement therapy. Int J Cardiol. 2016;202:962–966. [DOI] [PubMed] [Google Scholar]
- 63.Dabrowski R, Borowiec A, Smolis-Bak E, et al. Effect of combined spironolactone-beta-blocker +/− enalapril treatment on occurrence of symptomatic atrial fibrillation episodes in patients with a history of paroxysmal atrial fibrillation (SPIR-AF study). Am J Cardiol. 2010;106:1609–1614. [DOI] [PubMed] [Google Scholar]
- 64.Shantsila E, Shahid F, Sun Y, et al. Spironolactone in atrial fibrillation with preserved cardiac fraction: the IMPRESS-AF trial. J Am Heart Assoc. 2020;9:e016239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maier-Begandt D, Comstra HS, Molina SA, et al. A venous-specific purinergic signaling cascade initiated by Pannexin 1 regulates TNFalpha-induced increases in endothelial permeability. Sci Signal. 2021;14(672):eaba2940. 10.1126/scisignal.aba2940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.D’Hondt C, Iyyathurai J, Himpens B, Leybaert L, Bultynck G. Cx43-hemichannel function and regulation in physiology and pathophysiology:insights from the bovine corneal endothelial cell system and beyond. Front Physiol. 2014;5:348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Veeraraghavan R, Salama ME, Poelzing S. Interstitial volume modulates the conduction velocity-gap junction relationship. Am J Physiol Heart Circ Physiol. 2012;302:H278–H286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.George SA, Calhoun PJ, Gourdie RG, Smyth JW, Poelzing S. TNFalpha modulates cardiac conduction by altering electrical coupling between myocytes. Front Physiol. 2017;8:334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Leo-Macias A, Agullo-Pascual E, Sanchez-Alonso JL, et al. Nanoscale visualization of functional adhesion/excitability nodes at the intercalated disc. Nat Commun. 2016;7:10342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Nowak MB, Greer-Short A, Wan X, et al. Intercellular sodium regulates repolarization in cardiac tissue with sodium channel gain of function. Biophys J. 2020;118:2829–2843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nowak MB, Veeraraghavan R, Poelzing S, Weinberg SH. Cellular size, gap junctions, and sodium channel properties govern developmental changes in cardiac conduction. Front Physiol. 2021;12:731025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Barker RJ, Price RL, Gourdie RG. Increased association of ZO-1 with connexin43 during remodeling of cardiac gap junctions. Circ Res. 2002;90:317–324. [DOI] [PubMed] [Google Scholar]
- 73.Hunter AW, Barker RJ, Zhu C, Gourdie RG. Zonula occludens-1 alters connexin43 gap junction size and organization by influencing channel accretion. Mol Biol Cell. 2005;16:5686–5698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.O’Quinn MP, Palatinus JA, Harris BS, Hewett KW, Gourdie RG. A peptide mimetic of the connexin43 carboxyl terminus reduces gap junction remodeling and induced arrhythmia following ventricular injury. Circ Res. 2011;108:704–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Palatinus JA, Rhett JM, Gourdie RG. The connexin43 carboxyl terminus and cardiac gap junction organization. Biochim Biophys Acta. 2012;1818:1831–1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayo Clinic. Spironolactone (oral route): proper use. Accessed October 26, 2023. https://www.mayoclinic.org/drugs-supplements/spironolactone-oral-route/proper-use/drg-20071534
- 77.Dahl G, Qiu F, Wang J. The bizarre pharmacology of the ATP release channel pannexin1. Neuropharmacology. 2013;75:583–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Iglesias R, Spray DC, Scemes E. Mefloquine blockade of Pannexin1 currents: resolution of a conflict. Cell Commun Adhes. 2009;16:131–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Rusiecka OM, Tournier M, Molica F, Kwak BR. Pannexin1 channels-a potential therapeutic target in inflammation. Front Cell Dev Biol. 2022;10:1020826. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.