Abstract
As climate change causes the environment to shift away from the local optimum that populations have adapted to, fitness declines are predicted to occur. Recently, methods known as genomic offsets (GOs) have become a popular tool to predict population responses to climate change from landscape genomic data. Populations with a high GO have been interpreted to have a high “genomic vulnerability” to climate change. GOs are often implicitly interpreted as a fitness offset, or a change in fitness of an individual or population in a new environment compared to a reference. However, there are several different types of fitness offset that can be calculated, and the appropriate choice depends on the management goals. This study uses hypothetical and empirical data to explore situations in which different types of fitness offsets may or may not be correlated with each other or with a GO. The examples reveal that even when GOs predict fitness offsets in a common garden experiment, this does not necessarily validate their ability to predict fitness offsets to environmental change. Conceptual examples are also used to show how a large GO can arise under a positive fitness offset, and thus cannot be interpreted as a population vulnerability. These issues can be resolved with robust validation experiments that can evaluate which fitness offsets are correlated with GOs.
Keywords: validation, evaluation, machine learning, genomic offset, climate change, novel climates
Background
Many species are locally adapted to environmental gradients. Such local adaptation complicates our ability to predict species vulnerability to climate change, because the vulnerability of populations within a species will depend on the details of local adaptation. Within the last 8 years, a set of methods known as “genomic offsets” (GOs) have been developed to make such predictions. A GO is typically defined as the instantaneous degree of maladaptation of a genome when moved from the environment to which it is adapted into a new environment (Fitzpatrick & Keller, 2015; Hoffmann et al., 2021; Láruson et al., 2022; Rellstab et al., 2021). GOs are also defined as the amount of genetic change that would be required for the population to adapt to a new environment (Rellstab et al., 2021). The calculation of GOs is often based on some kind of combined signal across putatively adaptive loci identified as outliers in genotype-environment associations, and there are several new methods available for performing the calculations (Gain et al., 2023; Gain & François, 2021; Capblancq & Forester, 2021; Fitzpatrick & Keller, 2015; Rellstab et al., 2016; Rochat et al., 2021).
Populations within a species that are estimated to have the largest GOs to future climate conditions have been interpreted as having a high “genomic vulnerability” to climate change (Bay et al., 2018; Ruegg et al., 2018). Many of the recent papers that have used GOs to make predictions about the maladaptation of populations to climate change across a species range have been published in high-profile venues (Bay et al., 2018; Brauer et al., 2023; Gain et al., 2023; Chen et al., 2022; Fitzpatrick et al., 2021; Ingvarsson & Bernhardsson, 2020; Rhoné et al., 2020; Ruegg et al., 2018; Sang et al., 2022). In addition, a few in situ or in silico evaluation experiments have observed a significant relationship between GO predictions and the fitness of different genotypes moved into a common garden, and are lending credibility to the GO framework (Gain et al., 2023; Fitzpatrick et al., 2021; Láruson et al., 2022; Lind et al., 2024; Rhoné et al., 2020).
The study of “maladaptation,” however, has many conceptual issues that arise from defining the comparison: maladapted “relative to what?” (Brady et al., 2019; Crespi, 2000; Orr, 2009). By using the term “maladaptation” when discussing GOs and their interpretation, these studies often explicitly or implicitly interpret GOs as a fitness disparity or fitness offset. A fitness offset is the change in fitness of a genotype or population in a new situation compared to a reference situation. There are several different types of fitness offset that depend on how one defines the reference, and that the appropriate choice depends on whether one seeks to predict population vulnerability to climate change or to predict the most-fit genotype in a particular common garden environment.
The goals of this Comment and Opinion are to mathematically define different types of fitness offsets and to clarify their applications, to explore how different patterns of local adaptation lead to strong or weak correlations among different types of fitness offsets, and to elucidate the situations in which the interpretation of GOs and other kinds of genomic forecasts (built on patterns of allele frequency across a landscape) may be related or unrelated to different types of fitness offsets. By delineating these different situations, the field of genomic forecasting can be directed toward more accurate inference. The first section explores conceptual issues in order to determine what kind of prediction is actually needed for different applications. The next section explores what a GO might measure and whether that measurement is consistent with interpretation. These conceptual and interpretation issues highlight shortcomings of the GO forecasting framework that have yet to be adequately addressed by the field, and the final section shows how the field can more rigorously evaluate forecasts through carefully designed experiments.
Conceptual issues in local adaptation, maladaptation, and forecasts
A major unaddressed issue with GOs, which combine signals across multiple loci in the genome, is the question of what biological quantity the GO estimates. Here, I synthesize conceptual issues in local adaptation (with regards to “home-away” vs. “local-foreign” criteria) (Kawecki & Ebert, 2004) and conceptual issues in maladaptation (with regards to absolute and relative fitness) (Brady et al., 2019; Crespi, 2000; Orr, 2009) to show that what kind of fitness offset a GO might be estimating is not at all straightforward. Here, the term “genotype” is defined as a locally adapted population associated with a specific habitat, and “fitness” is defined as the absolute lifetime reproductive success. While lifetime reproductive success is not feasible to estimate for many species, here I assume that it is known without error to illustrate how the conceptual issues arise.
The “home vs. away” criterion for local adaptation emphasizes the comparison of a single genotype in different habitats/environments and is met when a genotype has higher fitness in its own (home) habitat than in other habitats (away). In contrast, the “local vs. foreign” criterion for local adaptation emphasizes the comparison of genotypes within a given habitat and is met when the genotype has higher fitness in its own (local) habitat than other (foreign) genotypes in the same habitat. Both “local vs. foreign” and “home vs. away” patterns contribute to an overall pattern of genotypes having higher fitness in sympatry than in allopatry, and jointly contribute to the degree of local adaptation (Blanquart et al., 2013). Some of the conceptual issues with GOs stem from the fact that home-away and local-foreign comparisons can lead to different fitness offsets, as shown below. Therefore, the appropriate criterion depends on the application.
What kind of prediction is needed?
The first question that one should ask when constructing a forecast such as a GO is: what do we want to predict? For practical conservation applications, we may need a model that can predict whether a population will be maladapted to future climate, or we may need a model that can predict the most successful genotypes for a restoration site. In an ideal world, we would be able to predict the absolute fitnesses of multiple genotypes in multiple environments (Figure 1A), and this data would be used to construct predictions for both types of questions.
Figure 1.
Different ways to measure a fitness offset. (A) Absolute fitnesses of each genotype in each common garden. (B–D) Home-away fitness offset and its derivatives (home-away relative fitness and home-away relative fitness offset) compare the fitness of a genotype in a new environment to its fitness in its home environment. Colors represent a genotype, dark color shades represent the home environment and lighter shades represent the away environment for that genotype. (E–G) Local-foreign fitness offset and its derivatives (local-foreign relative fitness and local-foreign relative fitness offset) compare the fitness of a foreign genotype to the fitness of a local genotype within a common garden site. Colors represent a common garden, dark color shades represent the local genotype and lighter shades represent the foreign genotypes.
For predicting whether a population will be maladapted to future climate, we aim to forecast the change in absolute fitness of a genotype in a future environment (“away”) relative to its current environment (“home”). If a genotype has a fitness change when the environment changes from A (home) to A’ (away), then this change would be measured as △ = (fitness in A’) − (fitness in A). If climate change results in a population being more fit, then △ will be positive. If climate change results in a population being less fit, then △ will be negative. This type of forecast is a home-away fitness offset for each genotype (Figure 1B). Thus, home-away fitness offsets are what investigators should aim to predict when they are trying to inform population vulnerability to climate change.
In contrast, for predicting successful genotypes for a site restoration, we aim to forecast the difference in fitness between “foreign” genotypes and an optimal “local” genotype. If a local genotype (G) has higher fitness than a foreign genotype (G’) at site A, then the local-foreign fitness offset is measured as △ = (fitness of G’) − (fitness of G) (Figure 1E). Negative values indicate that the foreign genotype has lower fitness than the local genotype. Across the metapopulation, absolute fitness, home-away fitness offsets, and local-foreign fitness offsets do not necessarily equate (compare Figure 1A, B, and E), which highlights the importance of choosing the right kind of fitness offset for the application.
The absolute fitnesses shown in Figure 1A can be used to calculate relative fitnesses. Relative fitness is measured as the absolute fitness of some focal entity divided by the absolute fitness of a comparative or reference entity (Brady et al., 2019). Thus, the question with relative fitness is: “relative to what?” (Brady et al., 2019). A home-away relative fitness is defined as the fitness of the focal genotype in a new environment relative to its fitness in the home environment (Figure 1C) and is relevant for predicting the relative change in fitness due to environmental change. A local-foreign relative fitness is defined as the fitness of foreign genotypes relative to the local genotype in a reference environment (Figure 1F) and is relevant for predicting the fitnesses of different genotypes relative to each other for a restoration. The concepts of fitness offsets and relative fitness can be combined to calculate home-away relative fitness offset (Figure 1D) and a local-foreign relative fitness offset (Figure 1G), in which the fitness offset is standardized by the reference. By the definitions in Figure 1, a matrix of “relative fitnesses” will always be perfectly correlated with their respective “relative fitness offsets,” so subsequent text will focus on the latter to stay consistent with the idea of offsets.
Open questions regarding the interpretation of GOs
What might GOs predict?
The second question that one should ask when constructing a forecast is: can the data on which the forecasting model is being built capture what we want to predict? For restoration or projections of climate vulnerability, one may want a forecast of absolute fitnesses or absolute fitness offsets, which could be used to infer population growth rates (Brady et al., 2019). Models built on allele frequencies (such as GO models), however, will be more closely tied to the relative fitness among genotypes (Brady et al., 2019). When gene flow, mutation, and drift are weak relative to selection, population genomic theory predicts that allele frequency patterns at a geographic site evolve via a model of the relative fitness of genotypes within a site (a type of “local-foreign” comparison) (Brady et al., 2019; Orr, 2009). Local-foreign patterns of fitness within the site will determine the allele frequency at a location because the spread of an allele depends on the absolute fitness of that allele in relation to the absolute fitness of alternate alleles at the same location, given that gene flow and drift are weak relative to selection (Brady et al., 2019; Orr, 2009). Therefore, we might expect genomic forecasts built on allele frequencies to measure something more consistent with a local-foreign relative fitness (Figure 1F) or a local-foreign relative fitness offset (Figure 1G). Importantly, local-foreign relative fitness or local-foreign fitness offsets do not necessarily equate with home-away relative fitnesses or home-away fitness offsets (compare Figure 1G with Figure 1C and Figure 1F with Figure 1D, also see below).
In addition, across a wider parameter space, there may not be a strong relationship between relative fitnesses offsets and fitness offsets, because the slope of the relationship between the two depends on the absolute fitness of the reference genotype. This is shown in Figure 2, where a large number of absolute fitnesses were randomly drawn from a log-normal distribution (e.g., high variance in reproductive success). These were the reference genotypes for a relative fitness calculation. Each reference genotype was then moved to another environment in which its new absolute fitness was randomly chosen to be between 1% and 99% of the fitness in its home environment (i.e., the “fitness in new context” in Figure 2; equivalently the new context could be another genotype compared to the reference). When individuals have high variance in reproductive success (some individuals have very high absolute fitness and others have very low absolute fitness) there is a positive but weak relationship between relative fitnesses and fitness offsets (relationship between all points in Figure 2). This result occurs because the slope of the relationship between absolute fitness offset and relative fitness depends on absolute fitness of the reference (Figure 2, note slope for points with shared colors).
Figure 2.
The relationship between absolute fitness offset (x-axis) and relative fitness offset (y-axis) depends on absolute fitness of the genotype in the reference situation (shade/color). If all genotypes have the exact same absolute fitness in their reference (e.g., home/local) situation, then fitness offsets and relative fitness offsets will be perfectly correlated (e.g., note linear correlations for a single point shade/color). Real populations have some variation in reproductive success, in which some individuals have very high fitness and some have very low fitness (a mix of light yellow and dark purple points). This in turn weakens the relationship between fitness offset and relative fitness offset.
Are GOs interpreted in a way that is consistent with what they might (or might not) predict?
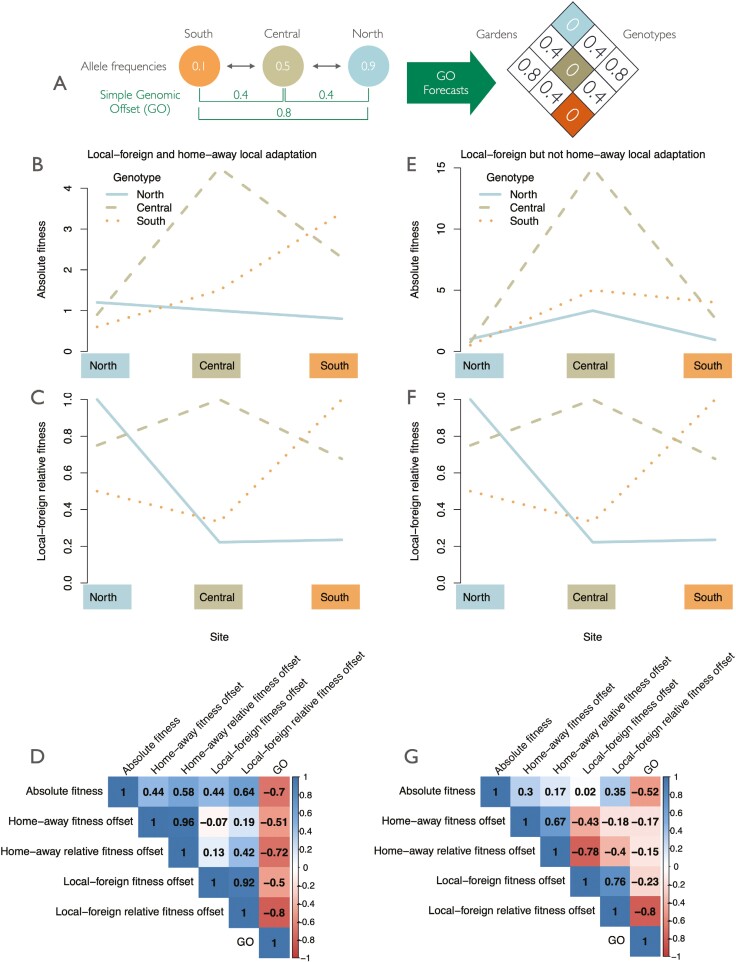
The third question that one should ask when constructing a model is: is what the model forecasts consistent with the way the forecasts are being interpreted? For forecasts built on GOs specifically, whether the forecasts are being interpreted correctly is a major question that the field should discuss. Using a simple GO calculation as the allele frequency difference between sites at a single locus along an environmental gradient (Rellstab et al., 2016, Figure 3A), we can hypothetically explore how different kinds of fitness offsets correlate with this simple GO and with each other in a full reciprocal transplant experiment for two hypothetical metapopulations. Correlations are calculated among the reciprocal transplant matrices (absolute fitness, fitness offset, and relative fitness offset matrices based on calculations in Figure 1) and the GO in Figure 3A.
Figure 3.
Relationships among fitness offsets. The correlations among different types of fitness offsets when the metapopulation evolves both local-foreign and home-away local adaptation (left), or evolves local-foreign but not home-away local adaptation (right). (A) A simple genomic offset (GO) calculated as the allele frequency difference at a single locus among pairs of populations. (B) In the first hypothetical metapopulation, patterns of absolute fitness meet the home-away and local-foreign local adaptation. (C) Local-foreign relative fitness patterns derived from panel B. (D) Pearson correlations among the reciprocal transplant matrices for the various types of fitness offsets derived from panel B (equations shown in Figure 1) and the GO. (E) In the second hypothetical metapopulation, patterns of absolute fitness meet the local-foreign criteria for local adaptation because all genotypes have highest fitness when compared to other genotypes within their site, but not home-away local adaptation, because North and South genotypes have highest fitness in the highly productive central site. (F) Patterns of local-foreign relative fitness are equivalent to those shown in the first example. (G) Correlations among the reciprocal transplant matrices for the various types of fitness offsets derived from panel E and the GO.
In the first hypothetical metapopulation, local adaptation follows both the home-away and local-foreign criteria for local adaptation (genotypes both have higher fitness in their home environment than when they are moved elsewhere, and local genotypes have higher fitness than foreign genotypes) (absolute fitnesses in Figure 3B; local-foreign relative fitnesses shown in Figure 3C). The metapopulation in Figure 3B meets the established mathematical definition of local adaptation in a metapopulation, with mean fitness in sympatry 1.85 units higher than in allopatry (measured as △SA of Blanquart et al., 2013). Although, as expected, the GO is negatively correlated with both absolute fitness and fitness offsets across the reciprocal transplant (higher GOs translate to higher fitness declines), it is correlated to a higher degree with relative fitness offsets than with absolute fitness offsets (Figure 3D).
In the second hypothetical metapopulation, local adaptation follows the local-foreign criteria but not the home-away criteria due to high variation in productivity among sites: the central site has the highest productivity and the northern site has the lowest productivity (Figure 3E). Note that the patterns of relative fitness within sites (local-foreign relative fitness) are equivalent in both metapopulations (compare Figure 3C with Figure 3F), which could lead to similar patterns of allele frequency in the metapopulation given all else equal. The metapopulation in Figure 3E meets the established mathematical definition of local adaptation in a metapopulation, with mean fitness in sympatry 4.46 units higher than in allopatry (measured as △SA of Blanquart et al., 2013). In this “high productivity variation” scenario, the correlations between the GO and home-away fitness offset are relatively weak, because local adaptation does not follow the home-away criteria (Figure 3G). In addition, the correlation between the GO and local-foreign fitness offset is also relatively weak, due to the higher variation in fitness disrupting the relationship between the absolute and relative fitness offsets (as shown in Figure 2). However, the GO correlation with local-foreign relative fitness offset is the same in both metapopulations, since the patterns of local-foreign relative fitness were preserved in this hypothetical example (as shown in Figure 3C and F).
We can also use Figure 3E to illustrate an important problem with the interpretation of GOs as the degree of maladaptation to future climate change. Let’s assume that the future climate of the northern population will be like that of the central population, so we can use the fitness of the northern population in the central site to predict the effect of climate change on the northern site. As the climate changes, the fitness of the northern site will increase and there will be no maladaptation, but nevertheless, the pairwise GO is positive (Figure 3A) which has been interpreted in many studies as a maladaptation.
Empirical patterns
Both hypothetical data and real data have benefits and downsides. The benefit of hypothetical data is that it allows us to conduct thought experiments and delineate the situations in which an analysis may or may not produce the correct outcome. The downside of hypothetical data is, well, it’s hypothetical, and hypothetical patterns like those shown in Figure 3E may not be common in nature. The benefit of empirical data is that it is obtained by direct observation. The downside of empirical data is that it is extremely difficult to estimate lifetime reproductive success in the field, and any reported measures can be subject to criticism that they do not adequately estimate lifetime reproductive success.
To calculate correlations among different kinds of fitness offsets, at minimum a 3 × 3 fully factorial reciprocal transplant design is needed. To find empirical datasets, I evaluated studies cited in a recent review on transplant studies in climate change (Nooten & Hughes, 2017). Of the dozens of studies they reviewed, 11 of them were designed in a way that informed genetic vs. environmental drivers. Of those 11, two studies had a design large enough to calculate correlation among different kinds of fitness offsets (Bennington et al., 2012; Etterson & Shaw, 2001), and a third study was found through a literature search (Johnson et al., 2022). Fitness proxies (percent cover, fecundity as seed production, or proportion survival) were extracted from figures in each paper using Web Plot Digitizer (Rohatgi, 2022) and fitness offsets were calculated according to the equations in Figure 1 (see Supplementary Material, R Markdown for details).
Figure 4 shows the results for Etterson & Shaw (2001), who conducted a 3 × 3 reciprocal transplant experiment on the North American prairie legume Chamaecrista fasciculata and estimated total lifetime fecundity as seed production (Figure 4A). The dataset exhibits local adaptation as genotypes produce on average 611.33 more seeds in sympatry than allopatry (measured as △SA of Blanquart et al., 2013). In this dataset, the local-foreign fitness offsets have a weak correlation with home-away fitness offsets (r = 0.15, Figure 4B). This relationship arises because the dataset shows patterns of local-foreign relative fitness (each genotype has the highest fitness compared to foreign genotypes at its local site, Figure 4C), but the home-away relative fitness patterns are different due to the higher fecundity of the Oklahoma genotype in the Kansas common garden compared to its home site in Oklahoma (Figure 4D), where all genotypes had lower fecundity (Figure 4A). Thus, this dataset shares some similarities with the “high productivity scenario” shown in Figure 3E.
Figure 4.
Relationships among fitness offsets from a 3 × 3 reciprocal transplant in the North American prairie legume Chamaecrista fasciculata (Etterson & Shaw, 2001). (A) Total lifetime fecundity of each genotype in each site was measured as the number of seeds produced. (B) Correlation matrix among different kinds of fitness offsets. (C) Relative fitness of each genotype relative to the local genotype within each garden (equation in Figure 1F). (D) Relative fitness of a single genotype at each site relative to its fitness at its home site (equation Figure 1C). Abbreviations: gen: genotype; CG: common garden, MN: Minnesota; KS: Kansas; OK: Oklahoma.
The other two datasets show cases in which the metapopulation more consistently meets both home-away and local-foreign criteria for local adaptation, and both datasets show strong correlations among different kinds of fitness offsets (see Supplementary Material, R Markdown for details).
Summary of conceptual and interpretation issues
These hypothetical and empirical examples highlight important considerations. First, not all conservation applications will want to calculate the same kind of fitness offset. Second, not all types of fitness offset are necessarily correlated with each other or with relative fitness. Third, GOs will not predict the same thing in every study system, because the type of fitness offset they are most correlated with depends on the patterns of local adaptation in the metapopulation (the examples presented here are only a subset of possible scenarios, and readers can explore different scenarios using functions in Supplementary Material, R Markdown). Fourth, current GO methods only return positive values, even for populations whose fitness will increase when moved to a new environment, and should not be interpreted as a degree of maladaptation or vulnerability. Fifth, because common garden experiments that evaluate GOs base the performance of a GO on its association with the fitness of different genotypes within a site (Gain et al., 2023; Fitzpatrick et al., 2021; Lind et al., 2024; Rhoné et al., 2020), these experiments measure the ability of a GO to predict a local-foreign relative fitness offset within a common garden. Sixth, GOs are likely to be predictive of local-foreign relative fitness offsets based on first principles because they are calculated from allele frequencies, and this can explain why common garden evaluations find good performance (Gain et al., 2023; Fitzpatrick et al., 2021; Lind et al., 2024; Rhoné et al., 2020). However, correlating a GO with fitness proxies of genotypes within a common garden does not necessarily validate the use of GOs for estimating genomic vulnerability to climate change, because the former evaluates a local-foreign relative fitness offset while the latter seeks to predict a home-away fitness offset.
Experimental design considerations
The conceptual issues surrounding local-foreign offsets vs. home-away offsets have important implications for how we design experiments for validating GOs for management and conservation, because local-foreign patterns are more relevant for restoration and the home-away patterns are more relevant for predicting climate change. Most validation experiments have been based on multiple genotypes in a single common garden for feasibility (Gain et al., 2023; Fitzpatrick et al., 2021; Lind et al., 2024; Rhoné et al., 2020), and so only evaluate the ability of GOs to predict the fitness of genotypes relative to each other within a site (local-foreign patterns). To evaluate the ability of GOs to predict the fitness of a genotype when the environment changes (home-away patterns), then multiple common gardens are needed. In addition, to more thoroughly investigate the relationship among different offset measures empirically, multiple common gardens with more than two genotypes in more than two different environments are needed.
Experimental designs with multiple common gardens and multiple genotypes per common garden will be useful to evaluate the ability of GOs to predict both home-away and local-foreign offsets, especially if the environments in some of the common gardens are relevant to future conditions. A conceptual example is shown for a metapopulation with eight demes along an environmental gradient, with a simple model of shifting future climate (Figure 5A). By considering the current and future environment for each genotype, one can identify common gardens that will capture current (“C”) and future (“F”) conditions for at least a few genotypes (“C” and “F” in Figure 5B). Then, one could raise many genotypes in a handful of common gardens (yellow cells in Figure 5B), since it is usually easier to raise more genotypes in a single treatment than to create many different treatments. With this design, one can evaluate the ability of GOs to predict the fitnesses of genotypes relative to each other within the common garden (a “local-foreign” comparison, Figure 5B, right side), as well as the change in fitness for genotypes when they are moved to a new environment (a “home-away” comparison, Figure 5B, bottom). The design also allows calculation of fitness offsets on an absolute and relative scale.
Figure 5.
Experimental design considerations. (A) A simple stepping-stone model of a metapopulation along an environmental gradient experiences linear climate change. (B) Experimental treatments are chosen to best represent current and future conditions at a number of sites. This example assumes it is easier to grow many genotypes in a few common gardens than vice versa. In each environmental treatment, comparison of the fitnesses of genotypes within a common garden (yellow/shaded boxes in the same row) gives insight to local-foreign fitness offsets (right arrows). For each genotype raised in their home environment (bold C), comparison of the fitnesses in other environmental treatments (yellow/shaded boxes in the same column) gives insight to home-away fitness offsets (down arrows).
This is a simple scenario, and for more realistic scenarios the environmental change will be multivariate with novel climates likely to emerge (Lotterhos et al., 2021; Williams et al., 2007). Investigators can leverage quantitative frameworks for studying climate novelty to calculate nearest-neighbor distances between current and future climates in multivariate space to choose experimental levels (Mahony et al., 2017).
Synopsis: how should GOs be interpreted?
The interpretation of GOs has limitations that the field has yet to adequately recognize and address. Current GO estimates are built on allele frequency differences across environments, and for this reason, GOs may be more applicable to predicting the relative success of genotypes in a restoration project (i.e., predictive of local-foreign fitness offsets) than to predicting the effects of climate change on populations (i.e., predictive of home-away fitness offsets), but this remains to be tested experimentally. This manuscript has explored some conditions under which GO predictions can be misleading, such as when there are large differences in productivity among sites, and adds to other literature that shows large differences in genetic drift among sites can mislead GOs (Láruson et al., 2022). How common these conditions are in nature is an open question. The assumptions of genomic offset methods are vaguely described in the literature as populations being locally adapted or residing within their adaptive optima. The development of rigorous tests of assumptions that a dataset should meet before proceeding with a GO analysis is an important direction for future research.
When one closely examines the mathematics underlying GOs, many of them measure some kind of genome-wide allele frequency difference between two environments, or they measure some kind of multivariate environmental distance between one environment (in space and time) and another environment (in space and time), with individual environmental variables weighted by how much evolved genetic patterns turnover or covary with that variable. Whether these types of measurements can accurately identify areas of a species range where climate change will have the greatest impacts requires more investigation.
Although there are some open questions regarding the interpretation of GOs, the term “genomic vulnerability” is problematic for several reasons and should be avoided. The term is not consistent with established definitions of vulnerability and adds confusion to the literature. The International Union for Conservation of Nature (IUCN) Species Survival Commission guidelines for assessing species’ vulnerability to climate change consider three primary facets of vulnerability and their uncertainty: (a) exposure to rapid changes in their physical environment, (b) sensitivity to that exposure, and (c) the ability of the species to adjust or moderate damage to that exposure (Foden, 2016; Foden et al., 2019). Thus, establishing vulnerability consists of different aspects beyond what genomic offsets may measure.
Carefully planned experiments with multiple genotypes in multiple environments will give important insights into these conceptual and interpretation issues. Both relative fitness and absolute fitness can tell different stories about the degree of (mal)adaptation, as relative fitness is more closely tied to changes in allele frequency and absolute fitness is more closely tied to population growth and persistence (Brady et al., 2019). Studies that report and interpret results in light of these different perspectives will advance our understanding of GO methods and lead to more robust conservation applications.
Supplementary Material
Acknowledgments
The author would like to thank Marlene Jahnke and Zea Segnitz for their comments on the manuscript, and to Brandon Lind and Stephen Keller for helpful discussions on the subject. The Editor, an anonymous reviewer, and Matthew Fitzpatrick provided valuable feedback through the peer review process that helped to refine the manuscript, and their time is greatly appreciated.
Data and code availability
The Supplementary Material, R Markdown reproduces the calculations and visualizations in Figures 2 and 3 and allows users to explore additional scenarios.
Author contributions
This manuscript was conceptualized and written by K.E.L.
Funding
K.E.L. was supported by a CAREER award from the National Science Foundation (2043905).
Conflict of interest: The author declares no conflict of interest.
References
- Bay, R. A., Harrigan, R. J., Underwood, V. L., Gibbs, H. L., Smith, T. B., & Ruegg, K. (2018). Genomic signals of selection predict climate-driven population declines in a migratory bird. Science, 359(6371), 83–86. 10.1126/science.aan4380 [DOI] [PubMed] [Google Scholar]
- Bennington, C. C., Fetcher, N., Vavrek, M. C., Shaver, G. R., Cummings, K. J., & McGraw, J. B. (2012). Home site advantage in two long-lived arctic plant species: Results from two 30-year reciprocal transplant studies. The Journal of Ecology, 100(4), 841–851. 10.1111/j.1365-2745.2012.01984.x [DOI] [Google Scholar]
- Blanquart, F., Kaltz, O., Nuismer, S. L., & Gandon, S. (2013). A practical guide to measuring local adaptation. Ecology Letters, 16(9), 1195–1205. 10.1111/ele.12150 [DOI] [PubMed] [Google Scholar]
- Brady, S. P., Bolnick, D. I., Barrett, R. D. H., Chapman, L., Crispo, E., Derry, A. M., Eckert, C. G., Fraser, D. J., Fussmann, G. F., Gonzalez, A., Guichard, F., Lamy, T., Lane, J., McAdam, A. G., Newman, A. E. M., Paccard, A., Robertson, B., Rolshausen, G., Schulte, P. M., … Hendry, A. (2019). Understanding maladaptation by uniting ecological and evolutionary perspectives. The American Naturalist, 194(4), 495–515. 10.1086/705020 [DOI] [PubMed] [Google Scholar]
- Brauer, C. J., Sandoval-Castillo, J., Gates, K., Hammer, M. P., Unmack, P. J., Bernatchez, L., & Beheregaray, L. B. (2023). Natural hybridization reduces vulnerability to climate change. Nature Climate Change, 13(3), 282–289. [Google Scholar]
- Capblancq, T., & Forester, B. R. (2021). Redundancy analysis: A Swiss Army Knife for landscape genomics. Methods in Ecology and Evolution, 12(12), 2298–2309. 10.1111/2041-210x.13722 [DOI] [Google Scholar]
- Chen, Y., Jiang, Z., Fan, P., Ericson, P. G. P., Song, G., Luo, X., Lei, F., & Qu, Y. (2022). The combination of genomic offset and niche modelling provides insights into climate change-driven vulnerability. Nature Communications, 13(1), 4821. 10.1038/s41467-022-32546-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crespi, B. J. (2000). The evolution of maladaptation. Heredity, 84(Pt 6), 623–629. 10.1046/j.1365-2540.2000.00746.x [DOI] [PubMed] [Google Scholar]
- Etterson, J. R., & Shaw, R. G. (2001). Constraint to adaptive evolution in response to global warming. Science, 294(5540), 151–154. 10.1126/science.1063656 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, M. C., Chhatre, V. E., Soolanayakanahally, R. Y., & Keller, S. R. (2021). Experimental support for genomic prediction of climate maladaptation using the machine learning approach Gradient Forests. Molecular Ecology Resources, 21(8), 2749–2765. 10.1111/1755-0998.13374 [DOI] [PubMed] [Google Scholar]
- Fitzpatrick, M. C., & Keller, S. R. (2015). Ecological genomics meets community-level modelling of biodiversity: Mapping the genomic landscape of current and future environmental adaptation. Ecology Letters, 18(1), 1–16. 10.1111/ele.12376 [DOI] [PubMed] [Google Scholar]
- Foden, W. B. (2016). IUCN SSC guidelines for assessing species’ vulnerability to climate change. IUCN. [Google Scholar]
- Foden, W. B., Young, B. E., Akçakaya, H. R., Garcia, R. A., Hoffmann, A. A., Stein, B. A., Thomas, C. D., Wheatley, C. J., Bickford, D., Carr, J. A., Hole, D. G., Martin, T. G., Pacifici, M., Pearce-Higgins, J. W., Platts, P. J., Visconti, P., Watson, J. E. M., & Huntley, B. (2019). Climate change vulnerability assessment of species. Wiley Interdisciplinary Reviews: Climate Change, 10(1), e551. [Google Scholar]
- Gain, C., & François, O. (2021). LEA 3: Factor models in population genetics and ecological genomics with R. Molecular Ecology Resources, 21(8), 2738–2748. 10.1111/1755-0998.13366 [DOI] [PubMed] [Google Scholar]
- Gain, C. E., Rhon E, B. E. E., Cubry, P., Salazar, I., Forbes, F., Vigouroux, Y., Jay, F., & Fran Cois, O. (2023). A quantitative theory for genomic offset statistics. Molecular Biology and Evolution, 40(6): msad140, 1–10. 10.1093/molbev/msad140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann, A. A., Weeks, A. R., & Sgrò, C. M. (2021). Opportunities and challenges in assessing climate change vulnerability through genomics. Cell, 184(6), 1420–1425. 10.1016/j.cell.2021.02.006 [DOI] [PubMed] [Google Scholar]
- Ingvarsson, P. K., & Bernhardsson, C. (2020). Genome-wide signatures of environmental adaptation in European aspen () under current and future climate conditions. Evolutionary Applications, 13(1), 132–142. 10.1111/eva.12792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson, L. C., Galliart, M. B., Alsdurf, J. D., Maricle, B. R., Baer, S. G., Bello, N. M., Gibson, D. J., & Smith, A. B. (2022). Reciprocal transplant gardens as gold standard to detect local adaptation in grassland species: New opportunities moving into the 21st century. The Journal of Ecology, 110(5), 1054–1071. 10.1111/1365-2745.13695 [DOI] [Google Scholar]
- Kawecki, T. J., & Ebert, D. (2004). Conceptual issues in local adaptation. Ecology Letters, 7(12), 1225–1241. 10.1111/j.1461-0248.2004.00684.x [DOI] [Google Scholar]
- Láruson, J., Fitzpatrick, M. C., Keller, S. R., Haller, B. C., & Lotterhos, K. E. (2022). Seeing the forest for the trees: Assessing genetic offset predictions from gradient forest. Evolutionary Applications, 15(3), 403–416. 10.1111/eva.13354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lind, B. M., Candido-Ribeiro, R., Singh, P., Lu, M., Vidakovic, D. O., Booker, T. R., Whitlock, M. C., Yeaman, S., Isabel, N., & Aitken, S. N. (2024). How useful is genomic data for predicting maladaptation to future climate? Global Change Biology. 10.1101/2023.02.10.528022 [DOI] [PubMed]
- Lotterhos, K. E., Láruson, J., & Jiang, L. -Q. (2021). Novel and disappearing climates in the global surface ocean from 1800 to 2100. Scientific Reports, 11(1), 15535. 10.1038/s41598-021-94872-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahony, C. R., Cannon, A. J., Wang, T., & Aitken, S. N. (2017). A closer look at novel climates: New methods and insights at continental to landscape scales. Global Change Biology, 23(9), 3934–3955. 10.1111/gcb.13645 [DOI] [PubMed] [Google Scholar]
- Nooten, S. S., & Hughes, L. (2017). The power of the transplant: Direct assessment of climate change impacts. Climatic Change, 144(2), 237–255. 10.1007/s10584-017-2037-6 [DOI] [Google Scholar]
- Orr, H. A. (2009). Fitness and its role in evolutionary genetics. Nature Reviews. Genetics, 10(8), 531–539. 10.1038/nrg2603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellstab, C., Dauphin, B., & Exposito-Alonso, M. (2021). Prospects and limitations of genomic offset in conservation management. Evolutionary Applications, 14(5), 1202–1212. 10.1111/eva.13205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rellstab, C., Zoller, S., Walthert, L., Lesur, I., Pluess, A. R., Graf, R., Bodénès, C., Sperisen, C., Kremer, A., & Gugerli, F. (2016). Signatures of local adaptation in candidate genes of oaks (Quercus spp.) with respect to present and futsure climatic conditions. Molecular Ecology, 25(23), 5907–5924. 10.1111/mec.13889 [DOI] [PubMed] [Google Scholar]
- Rhoné, B., Defrance, D., Berthouly-Salazar, C., Mariac, C., Cubry, P., Couderc, M., Dequincey, A., Assoumanne, A., Kane, N. A., Sultan, B., Barnaud, A., & Vigouroux, Y. (2020). Pearl millet genomic vulnerability to climate change in West Africa highlights the need for regional collaboration. Nature Communications, 11(1), 5274. 10.1038/s41467-020-19066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rochat, E., Selmoni, O., & Joost, S. (2021). Spatial Areas of Genotype Probability (SPAG): Predicting the spatial distribution of adaptive genetic variants under future climatic conditions. Diversity and Distributions, 27, 1076–1090. 10.1111/ddi.13256 [DOI] [Google Scholar]
- Rohatgi, A. (2022). Webplotdigitizer: Version 4.6. https://automeris.io/WebPlotDigitizer
- Ruegg, K., Bay, R. A., Anderson, E. C., Saracco, J. F., Harrigan, R. J., Whitfield, M., Paxton, E. H., & Smith, T. B. (2018). Ecological genomics predicts climate vulnerability in an endangered southwestern songbird. Ecology Letters, 21(7), 1085–1096. 10.1111/ele.12977 [DOI] [PubMed] [Google Scholar]
- Sang, Y., Long, Z., Dan, X., Feng, J., Shi, T., Jia, C., Zhang, X., Lai, Q., Yang, G., Zhang, H., Xu, X., Liu, H., Jiang, Y., Ingvarsson, P. K., Liu, J., Mao, K., & Wang, J. (2022). Genomic insights into local adaptation and future climate-induced vulnerability of a keystone forest tree in East Asia. Nature Communications, 13(1), 6541. 10.1038/s41467-022-34206-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J. W., Jackson, S. T., & Kutzbach, J. E. (2007). Projected distributions of novel and disappearing climates by 2100 AD. Proceedings of the National Academy of Sciences of the United States of America, 104(14), 5738–5742. 10.1073/pnas.0606292104 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Supplementary Material, R Markdown reproduces the calculations and visualizations in Figures 2 and 3 and allows users to explore additional scenarios.