Abstract
Background
Streptococcus pneumoniae is the most common bacterial cause of community acquired pneumonia and the acute respiratory distress syndrome (ARDS). Some clinical trials have demonstrated a beneficial effect of corticosteroid therapy in community acquired pneumonia, COVID-19, and ARDS, but the mechanisms of this benefit remain unclear. The primary objective of this study was to investigate the effects of corticosteroids on the pulmonary biology of pneumococcal pneumonia in a mouse model. A secondary objective was to identify shared transcriptomic features of pneumococcal pneumonia and steroid treatment in the mouse model and clinical samples.
Methods
We carried out comprehensive physiologic, biochemical, and histological analyses in mice to identify the mechanisms of lung injury in Streptococcus pneumoniae with and without adjunctive steroid therapy. We also studied lower respiratory tract gene expression from a cohort of 15 mechanically ventilated patients (10 with Streptococcus pneumoniae and 5 controls) to compare with the transcriptional studies in the mice.
Results
In mice with pneumonia, dexamethasone in combination with ceftriaxone reduced (1) pulmonary edema formation, (2) alveolar protein permeability, (3) proinflammatory cytokine release, (4) histopathologic lung injury score, and (5) hypoxemia but did not increase bacterial burden. Transcriptomic analyses identified effects of steroid therapy in mice that were also observed in the clinical samples.
Conclusions
In combination with appropriate antibiotic therapy in mice, treatment of pneumococcal pneumonia with steroid therapy reduced hypoxemia, pulmonary edema, lung permeability, and histologic criteria of lung injury, and also altered inflammatory responses at the protein and gene expression level. The transcriptional studies in patients suggest that the mouse model replicates some of the features of pneumonia in patients with Streptococcus pneumoniae and steroid treatment. Overall, these studies provide evidence for the mechanisms that may explain the beneficial effects of glucocorticoid therapy in patients with community acquired pneumonia from Streptococcus Pneumoniae.
Supplementary Information
The online version contains supplementary material available at 10.1186/s13054-024-04956-6.
Keywords: Pneumonia, Acute respiratory distress syndrome, Glucocorticoids, Streptococcal infections
Introduction
Bacterial pneumonia is a frequent cause of severe respiratory failure and acute respiratory distress syndrome (ARDS) [1]. The most common cause of bacterial community acquired pneumonia (CAP) is Streptococcus pneumoniae [2, 3], which directly damages the lung epithelium and induces the release of a substantial number of cytokines and chemokines from epithelial cells and macrophages, resulting in protein exudation and edema formation in the lung [1, 4]. Despite effective antibiotic therapy, the dysregulated pathogen-host interaction may still cause life-threatening acute respiratory failure [5].
Corticosteroids are sometimes prescribed as an adjunctive therapy for severe pulmonary infections and ARDS. The clinical benefit of adjunctive steroid therapy for pneumococcal infections has been previously demonstrated in meningitis [6]. Recent clinical trials have demonstrated therapeutic benefits of dexamethasone, a long-acting glucocorticoid, on mortality in severe COVID-19 infection [7], non-COVID ARDS [8], and in severe CAP [9–11]. However, the mechanisms by which steroids improve outcomes in patients with severe pneumonia or ARDS are not well established, and few studies have comprehensively characterized the effects of steroids in the respiratory tract of critically ill patients [12, 13] or clinically relevant animal models using bacterial pathogens treated with antibiotic therapy [14, 15].
Corticosteroids are typically used to potently suppress systemic immune responses, but they have additional effects that may be relevant to lung injury, including effects on wound healing, modifying lung fluid balance and the extent of pulmonary edema, and metabolism [16, 17]. There is emerging evidence that corticosteroids have distinct effects on systemic vs. pulmonary inflammation [13, 18]. Given that corticosteroids have pleiotropic and lung-specific effects, elucidating the effects of corticosteroids on the injured lung in bacterial pneumonia is important for establishing a biologic rationale for further clinical application of steroids in severe lung infections. Understanding the specific mechanisms of corticosteroid benefit should help identify the patients with pneumonia or ARDS most likely to benefit from steroids.
To investigate the effects of corticosteroids in bacterial pneumonia, the primary objective was to carry out comprehensive biochemical, physiological, and histologic studies in mice to understand the effects of steroids in pneumococcal pneumonia. A secondary objective was to study lower respiratory tract gene expression in an observational cohort of mechanically ventilated patients with and without S. pneumoniae pneumonia to compare with transcriptional studies in the mice.
Methods
See the Additional file 2 for more detailed methods.
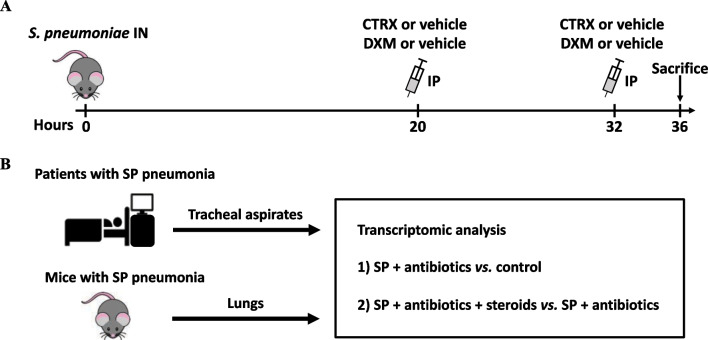
Experimental Model in Mice, Bacterial Infection, and Treatment: The protocol was approved by the University of California, San Francisco Institutional Animal Care and Use Committee (No.AN189182). C57BL/6 mice were randomly divided into four groups: (1) Healthy control; (2) S. pneumoniae; (3) S. pneumoniae + ceftriaxone; (4) S. pneumoniae + ceftriaxone + dexamethasone. Mice in groups 2–4 were anesthetized and inoculated intranasally with 108 CFU of live S. pneumoniae serotype 19F. Mice received 10 mg/kg of dexamethasone or vehicle control in combination with 150 mg/kg of ceftriaxone or vehicle control intraperitoneally 20 and 32 h after inoculation. In some experiments, body temperature, body weight, and oxygen saturation by pulse oximetry (SpO2) were measured 12, 20, and 32 h after inoculation. All mice were sacrificed 36 h after infection or earlier if pre-specified criteria indicating unacceptably severe illness were met. Figure 1A depicts experimental procedures.
Fig. 1.
Study design. A Mice were inoculated intranasally (IN) with 108 colony-forming units of Streptococcus pneumoniae (SP) and received 10 mg/kg of dexamethasone or vehicle control in conjunction with 150 mg/kg of ceftriaxone or vehicle control intraperitoneally (IP) 20 and 32 h after bacterial inoculation. Mice were sacrificed 36 h after infection. B Tracheal aspirate samples were collected from an observational cohort of mechanically ventilated adults within 72 h of admission to the ICU. Patients who had SP in a respiratory tract culture or met criteria for SP infection using an established metagenomic sequencing classifier model. Lungs were collected from mouse model of SP pneumonia. Abbreviations: IN intranasal injection; IP intraperitoneal injection; CTRX ceftriaxone; DXM dexamethasone; SP streptococcus pneumoniae
Lung Injury Endpoints: Mice underwent overdose of ketamine and xylazine, bilateral thoracotomy, and exsanguination by right ventricular puncture. In some mice, lungs were removed and homogenized to measure excess extravascular lung water (ELW) [19, 20]. In other mice, the lungs were lavaged, and bronchoalveolar lavage (BAL) protein was measured with the BCA Protein Assay (Thermo Fisher). BAL cell count was measured with a Coulter counter. Postmortem bacterial titers of BAL were measured by serial dilution and plaque counting on sheep blood agar plates. For histology, the lungs were fixed by 4% paraformaldehyde and stained with hematoxylin–eosin. Lung injury was assessed by an observer (XF) who was blinded to the experimental groups, based on the scoring system partly modified from the report of the American Thoracic Society [21]. BAL inflammatory cytokines and chemokines were measured using a FLEXMAP 3D™ (Luminex). Receptor for advanced glycation end products (RAGE) in BAL was measured using the Quantikine ELISA kit (R&D Systems). Alveolar fluid clearance was measured according to previously published methods [22, 23].
Observational patient cohort: We studied tracheal aspirate gene expression from samples collected from an observational cohort of mechanically ventilated adults within 72 h of admission to the ICU at UCSF Medical Center. The study was approved by the UCSF Institutional Review Board (17–24,056). We included all subjects who had S. pneumoniae in a respiratory tract culture or met criteria for S. pneumoniae infection using a metagenomic sequencing-based model as previously described [24]. We also included controls subjects who were mechanically ventilated for neurologic injury, were not immunocompromised, received no immunosuppressive medications, and had no evidence of respiratory disease on chest radiograph (Fig. 1B).
RNA Sequencing: In mice, the whole lung was placed in RNA Shield, frozen, homogenized, and then samples extracted using Quick-RNA mini prep plus kit (Zymo research). In humans, tracheal aspirate samples were collected on the day of enrollment and stored at -80C in RNAse-free conditions [25]. RNA was extracted using the Allprpep kit (Qiagen and then ribodepleted using FastSelect (Qiagen) before undergoing library preparation using the NEBNext Ultra II RNASeq Kit (New England Biolabs) and paired-end Illumina sequencing on an Novaseq 6000. Human and mouse samples were sequenced separately.
Bioinformatics analysis: Transcriptomic analysis was performed using established bioinformatics pipelines. Briefly, FASTQ files were aligned using STAR and count matrices were generated using tximport. Differential expression between groups was determined using limma-voom, and fgsea was used to identify pathways enriched in each experimental condition. CEMiTool was used to perform an unsupervised network analysis. A Benjamini–Hochberg adjusted p-value < 0.1 was considered significant for all analyses. Full details are provided in Additional file 3, Additional file 4, Additional file 5 and Additional file 6.
Statistical analysis: All data were tested for normality with Shapiro–Wilk tests. Comparisons of each group were made with one-way ANOVA followed by Tukey’s multiple comparisons tests for normally distributed parameters, or with Kruskal–Wallis test followed by Dunn’s multiple comparisons for skewed parameters. Two-way analysis of variance for repeated measures followed by Tukey’s multiple comparison test was used to evaluate the effect of time and group. All tests were two-tailed, and differences were considered to be statistically significant when p < 0.05.
Results
Dexamethasone prevents the progression of hypoxemia and pulmonary edema in mice with pneumococcal pneumonia
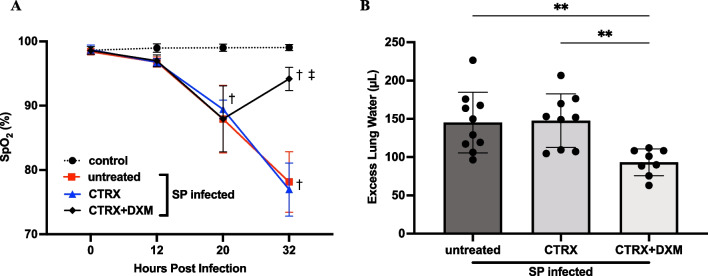
Oxygen Saturation. Mice inoculated intranasally with 108 CFU of S. pneumoniae developed hypoxemia at 20 h after infection (n = 5/group). The average oxygen saturation (SpO2) declined to approximately 87–91% (Fig. 2A). At 20 h, mice received either no treatment, ceftriaxone, or ceftriaxone plus dexamethasone. By 32 h, dexamethasone-treated mice had significantly higher oxygen saturation than those who did not receive dexamethasone. Thus, dexamethasone treatment attenuated progression of arterial hypoxemia 12 h after treatment, whereas ceftriaxone treatment did not prevent the progression of hypoxemia. Importantly, the arterial hypoxemia in the mice treated with antibiotics alone reached a mean value of 76% at 32 h whereas dexamethasone treatment increased oxygen saturation to a mean level above 90%.
Fig. 2.
Dexamethasone prevents the progression of hypoxemia and pulmonary edema in mice with pneumococcal pneumonia. A Arterial oxygen saturation (SpO2) was measured 12, 20, and 32 h after bacterial inoculation. SpO2 in the infected mice declined 20 h after infection. Dexamethasone-treated mice had higher SpO2 32 h after infection than mice without dexamethasone. n = 5/group. B Excess lung water, a measure of edema in the interstitial and alveolar spaces, were reduced with combination treatment of dexamethasone and ceftriaxone when compared to untreated mice and ceftriaxone alone. n = 7–10/group. All data are shown as means ± SD. Statistical differences between groups were calculated with (A) two-way repeated ANOVA followed by Tukey’s multiple comparison test, or B one-way ANOVA followed by Tukey’s multiple comparison test. †P < 0.05 compared with control. ‡P < 0.05 compared with groups without dexamethasone. **P < 0.01. Abbreviations: CTRX ceftriaxone; DXM dexamethasone; SpO2; arterial oxygen saturation; SP streptococcus pneumoniae
Pulmonary Edema. Mice inoculated with S. pneumoniae had approximately 150μL of excess lung water (ELW), demonstrating that 108 of S. pneumoniae induced substantial pulmonary edema within 36 h after infection (n = 7–10/group). The results for hypoxemia and ELW by treatment group were similar, underscoring the relevance of ELW as a marker of physiologically important pulmonary edema (Fig. 2B). Treatment with ceftriaxone alone did not reduce ELW. However, the combination of ceftriaxone and dexamethasone significantly reduced ELW compared to the untreated mice and to ceftriaxone alone (Fig. 2B).
Temperature and Body Weight. Infected mice became hypothermic (approximately 30℃) at 12 h after infection and had lower body temperature than normal healthy control mice throughout the experiment. Mice in all infected groups had significant body weight loss compared with normal healthy controls. (n = 11–12/group) (Additional file 1: Fig. S1).
Dexamethasone reduces both alveolar protein permeability and accumulation of leukocytes in the airspaces
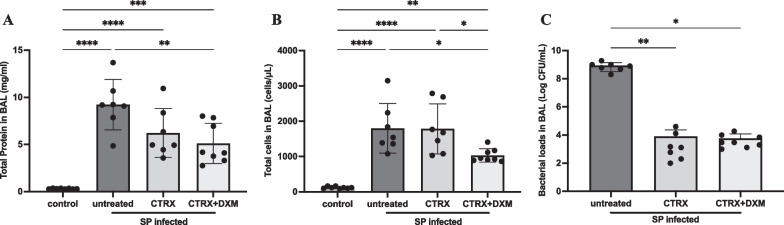
Mice with S. pneumoniae had higher protein concentrations in BAL compared with controls, indicating alveolar-capillary barrier disruption (n = 7–8/group) (Fig. 3A). The combination of ceftriaxone and dexamethasone significantly decreased BAL protein concentration by one way ANOVA; ceftriaxone alone also decreased BAL protein concentration, though not significantly compared to untreated mice. Total cell counts in BAL showed a substantial increase of white blood cells in the distal airspaces with S. pneumoniae (n = 7–8/group) (Fig. 3B). Similarly to the pulmonary edema results, treatment with ceftriaxone alone did not reduce total leukocyte accumulation. However, the combination of ceftriaxone and dexamethasone significantly reduced the accumulation of white blood cells compared to untreated mice and to ceftriaxone alone. Most of the leukocytes were neutrophils (Additional file 1: Fig. S2).
Fig. 3.
Dexamethasone reduces both alveolar protein permeability and cell infiltration. A Total protein concentration in bronchoalveolar lavage (BAL) fluid were elevated in the infected mice, indicating alveolar-capillary barrier disruption. The combination of dexamethasone and ceftriaxone reduced the BAL protein concentration, whereas ceftriaxone alone did not. n = 7–8/group. B Total cell number in BAL fluid was elevated in infected mice. The combination of dexamethasone and ceftriaxone reduced the total cell number compared with untreated and ceftriaxone alone. n = 7–8/group. C Ceftriaxone substantially reduced the bacterial loads 36 h after infection. The addition of dexamethasone to appropriate antibiotic treatment with ceftriaxone did not increase bacterial loads. n = 7–8/group. All data are shown as means ± SD. Statistical differences between groups were calculated with one-way ANOVA followed by Tukey’s multiple comparison test or Kruskal–Wallis followed by Dunn’s multiple comparison. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. Abbreviations: CFU colony forming unit; BAL bronchoalveolar lavage; SP streptococcus pneumoniae; CTRX ceftriaxone; DXM dexamethasone
Bacterial load in the bronchoalveolar lavage (BAL). Since dexamethasone may impair host defense, we measured the bacterial load in BAL (n = 7–8/group) (Fig. 3C). Ceftriaxone substantially reduced the bacterial loads 36 h after inoculation, as expected. The addition of dexamethasone to ceftriaxone treatment did not increase the bacterial burden in the BAL.
Dexamethasone improves lung histopathology in mice with pneumococcal pneumonia
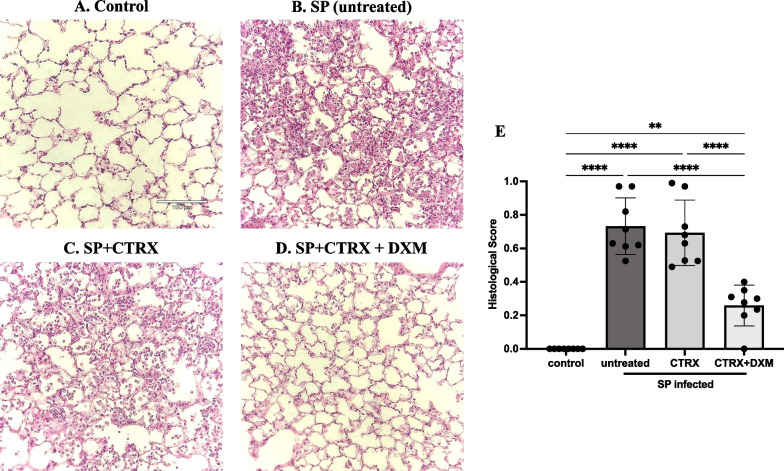
As quantified by an investigator blinded to the experimental condition, when compared to healthy mice (Fig. 4A), the lungs of mice inoculated with S. pneumoniae had severe inflammation including the infiltration of neutrophils into the airspaces and alveolar septal thickening and alveolar edema fluid at 36 h after infection (Fig. 4B). Treatment with ceftriaxone alone had no effect on histological markers of lung injury despite its capacity to eliminate bacteria (Fig. 4C). In contrast, the combination of ceftriaxone and dexamethasone significantly and markedly reduced histological markers of lung injury and inflammation and significantly improved the histopathological score compared to untreated controls and to ceftriaxone alone (Fig. 4D, E), findings that are consistent with the results of pulmonary edema, total protein concentration and white blood cells in the BAL (n = 4/group).
Fig. 4.
Dexamethasone improves histopathology in mice with pneumococcal pneumonia. A–D Representative images of lung histology; E Histological scores for each group. Compared with normal healthy control (A), the lungs of mice inoculated with Streptococcus pneumoniae (SP) had severe inflammation including the infiltration of neutrophils into the airspace, alveolar septal thickening, and alveolar edema fluid at 36 h after infection (B). Treatment with ceftriaxone had little effect on the inflammation despite of its capacity to eliminate the bacteria (C). The combination of dexamethasone and ceftriaxone further reduced the inflammation (D) and significantly improved the histopathological score comparable to healthy control (E). n = 4/group. Data are shown as means ± SD. Statistical differences between groups were calculated with with one-way ANOVA followed by Tukey’s multiple comparison test. **P < 0.01, ****P < 0.0001. Abbreviations: SP streptococcus pneumoniae; CTRX ceftriaxone; DXM dexamethasone.
Alveolar Epithelium—Functional and Biochemical Studies. To test the effect of dexamethasone on the vectorial fluid clearance capacity of alveolar epithelium [26, 27], we measured alveolar fluid clearance 36 h after infection (n = 9–14/group). Alveolar fluid clearance was substantially impaired with pneumococcal pneumonia, but there was no significant difference between the untreated and other treatment groups (Additional file 1: Fig. S3A), indicating that the favorable effects of dexamethasone combined with antibiotics on pulmonary edema, hypoxemia and histology were explained by a reduction in the formation of pulmonary edema not by an increase in the rate of alveolar fluid clearance.
In addition, we measured BAL levels of RAGE, a type I epithelial cell injury marker [28, 29] (n = 7–8/group). There was a significant increase in RAGE in BAL of pneumococcal infected mice (Additional file 1: Fig. S3B), indicating significant alveolar epithelial cell injury. However, the level of RAGE in BAL was not reduced by ceftriaxone or dexamethasone, a result that supports no significant effect of either treatment on the vectorial fluid transport capacity of the alveolar epithelium.
Dexamethasone attenuates the inflammatory responses in the air spaces of mice with pneumococcal pneumonia
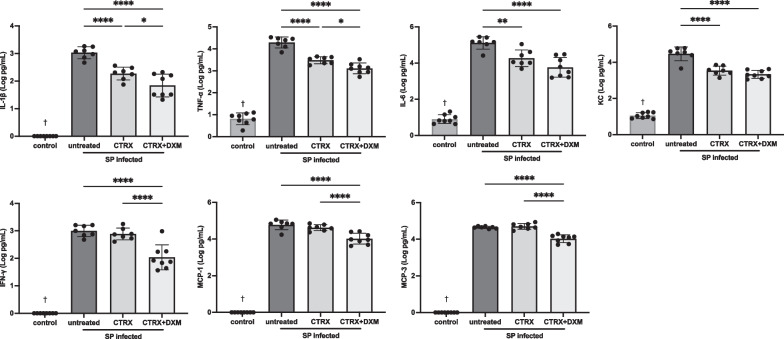
We measured cytokines and chemokines in BAL fluid using a multiplex assay to quantify the inflammatory responses with dexamethasone treatment for pneumococcal pneumonia (n = 7–8/group). Selected cytokines and chemokines with the high relevance to lung injury are displayed in Fig. 5. IL-1β, TNF-α, IL-6, and IFN-γ and chemokines including KC, MCP-1, and MCP-3 were significantly higher in infected mice compared with normal healthy control mice (Fig. 5). Ceftriaxone reduced IL-1β, TNF-α, IL-6, and KC, but did not impact the levels of IFN-γ, MCP-1, and MCP-3. Importantly, the combination of ceftriaxone and dexamethasone significantly attenuated all of these inflammatory cytokine and chemokine accumulation in the air spaces compared with untreated controls and significantly reduced IL-1β, TNF-α, IFN-γ, MCP-1, and MCP-3 as compared to ceftriaxone alone. Additional biomarkers were measured, several of which showed a decrease in BAL concentration with dexamethasone and antibiotic treatment (Additional file 1: Fig. S4). These results indicate that dexamethasone in combination with appropriate antibiotics reduces the release of pro-inflammatory cytokines and chemokines in pneumococcal pneumonia.
Fig. 5.
Dexamethasone attenuates the inflammatory response in mice with pneumococcal pneumonia. Inflammatory biomarkers including IL-1β, tumor necrosis factor-α (TNF-α), IL-6, keratinocyte chemoattractant (KC), interferon-γ (IFN-γ), monocyte chemoattractant protein-1 (MCP-1) and MCP-3 were significantly higher in infected mice compared with normal healthy control. Ceftriaxone reduced IL-1β, TNF-α, IL-6, and KC, but did not impact the levels of IFN-γ, MCP-1, and MCP-3. The combination of ceftriaxone and dexamethasone significantly attenuated all of these inflammatory cytokine and chemokine accumulation in the air spaces compared with untreated controls and significantly reduced IL-1β, TNF-α, IFN-γ, MCP-1, and MCP-3 as compared to ceftriaxone alone. n = 7–8/group. Data are shown as means ± SD. Statistical differences between groups were calculated with one-way ANOVA followed by Tukey’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001. †P < 0.05 compared with infected groups. Abbreviations: SP streptococcus pneumoniae; CTRX ceftriaxone; DXM dexamethasone; TNF-α tumor necrosis factor-α; KC keratinocyte chemoattractant; IFN-γ interferon-γ; MCP monocyte chemoattractant protein
Human subjects
Tracheal aspirate transcriptomes were available from 159 mechanically ventilated subjects, of whom 15 met inclusion criteria. Ten of the patients had clinically adjudicated S. pneumoniae pneumonia, in 5 patients the pathogen was detected by culture and in 5 patients by metagenomic sequencing [24, 30] (Additional file 2: Table S1). All patients were treated with antibiotics active against S. pneumoniae prior to sample collection, and 5 patients (3 culture-positive) also received corticosteroids prior to sample collection. Samples were also available from 5 s uninfected control subjects. The patients treated with steroids were numerically sicker by several clinical criteria (Additional file 2: Table S1).
RNA sequencing identifies reproducible effects of both pneumococcal pneumonia and steroid treatment in human patients and mice
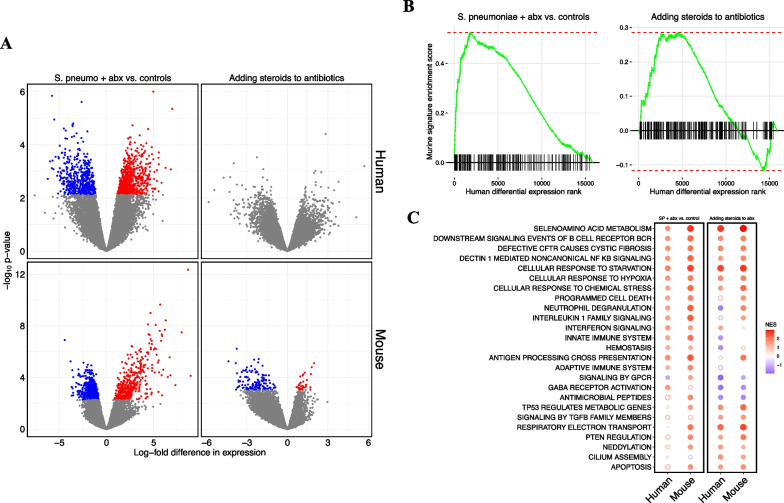
S. pneumoniae infection was associated with markedly altered gene expression in the lower respiratory tracts of both humans and mice (Fig. 6A). There were 1,184 genes differentially expressed between patients with S. pneumoniae who were not treated with steroids versus uninfected controls, and 951 genes differentially expressed between infected mice treated with ceftriaxone and uninfected controls. No individual gene was differentially expressed between pneumonia patients who received steroids versus those who did not, while 162 genes were differentially expressed in mice treated with dexamethasone and ceftriaxone compared to mice treated with ceftriaxone alone (Fig. 6A). Gene set enrichment analysis (GSEA) identified notable overlaps between gene expression from clinical samples and gene expression in the mouse model (Fig. 6B), suggesting that the experimental model replicated some of the biological features of clinical disease and steroid treatment. The top 100 genes upregulated in patients with S. pneumoniae pneumonia compared to controls were significantly enriched in the S. pneumoniae mouse model compared to control mice (p = 1.2 × 10–5). The top 100 genes upregulated in patients with pneumococcal pneumonia who received steroids compared to patients with pneumococcal pneumonia who did not receive steroids were significantly enriched in mice treated with dexamethasone and ceftriaxone compared to mice treated with ceftriaxone alone (p = 1.9 × 10–12).
Fig. 6.
RNA sequencing identifies reproducible effects of pneumococcal pneumonia and steroid treatment in humans and mice. A Volcano plots of differential gene expression. Each point represents an individual gene. The log2-fold difference in gene expression estimated by limma for each comparison is on the x-axis, and the p-value for each comparison is on the y axis. A positive difference in gene expression indicates the gene is more highly expressed in humans or mice with S. pneumoniae treated with antibiotics in the first column, and more highly expressed in humans or mice who received steroids in the second column. Grey points were not statistically significant (FDR < 0.1) after adjusting for multiple hypothesis testing. B Enrichment plots for mouse gene signatures in human differential expression. Genes were ranked along the x-axis by the estimated log-fold difference in gene expression between groups in human tracheal aspirate transcriptomes. A vertical line along the x-axis identifies the genes that are present in the mouse gene signature. The green line represents the running GSEA enrichment score at that position on the ranked gene list. C Dot plot of GSEA net enrichment scores (NES) for the differential expression data in panel B. Red indicates genes in selected Reactome pathways that are relatively more highly expressed in humans or mice with S. pneumoniae treated with antibiotics in first and second columns, and more highly expressed in humans or mice who received steroids in the third and fourth columns. A solid circle indicates GSEA FDR < 0.1. Abbreviations: SP streptococcus pneumoniae; NES net enrichment score
GSEA identified enrichment of several pathways shared between humans and mice (Fig. 6C). For example, in both mice and humans, S. pneumoniae infection, as expected, increased expression of cellular stress responses and upregulated adaptive and innate immune signaling pathways, including interferon and interleukin 1 family signaling, neutrophil degranulation, and B cell receptor signaling compared to uninfected controls. The addition of steroids to antibiotics increased the expression of pathways associated with injury resolution, including TGF-beta signaling, anti-inflammatory signaling by PTEN, and cilium assembly pathways in humans and in mice. Interestingly, several pathways that were more highly expressed in S. pneumoniae infection compared to controls, including stress and hypoxia responses, non-canonical NF-kB signaling, and B cell receptor signaling were increased further by the addition of steroids. Weighted gene co-expression network analysis (WGCNA) results are described in the Additional file 1.
Discussion
This study addresses several important gaps in our knowledge regarding the effects of corticosteroid treatment for bacterial pneumonia in the lungs. Few studies have directly evaluated the mechanistic effects of corticosteroids in an experimental model of pneumococcal pneumonia, a particularly important issue in view of the recent clinical trials reporting improved outcomes in patients with severe community acquired pneumonia when treated with corticosteroids [11]. Although patients recognized to have bacterial pneumonia are uniformly treated with antibiotics, animal models using bacterial pathogens do not routinely include the use of antibiotics, which limits their clinical relevance. This study identifies several mechanisms by which dexamethasone decreases lung injury in mice with pneumococcal pneumonia treated the antibiotics and supports the biological relevance of this model to humans.
Dexamethasone improved oxygenation, decreased pulmonary edema, and decreased histological evidence of lung injury when added to antibiotics. These results are consistent with recent clinical studies, in which early administration of dexamethasone reduced the duration of mechanical ventilation in ARDS patients and reduced the progression to mechanical ventilation in non-intubated patients with severe pneumonia [11, 31, 32]. Assessment of BAL fluid identified that the combination of antibiotics and dexamethasone reduced protein permeability and leukocyte accumulation into the distal air spaces. In contrast to the favorable effect on protein permeability, dexamethasone did not enhance alveolar fluid clearance, indicating that the favorable effects of dexamethasone combined with antibiotics were explained by a reduction in the formation of pulmonary edema, not by an increase in the rate of alveolar fluid clearance.
Because of their immunosuppressive effects, steroids can impair host bacterial clearance [17]. In one mouse study using a very early administration of glucocorticoids (one hour after infection) [14], high dose dexamethasone increased bacterial burden in the lung. In contrast, dexamethasone did not impair host bacterial clearance in the current study, possibly because dexamethasone was given 20 h after infection and at the same time antibiotic therapy was initiated. We selected this schedule in part to enhance the clinical relevance of the model, as patients with pneumonia usually come to medical attention when their infection has progressed enough to cause symptoms. These findings are consistent with the results of some recent clinical trials of steroids in COVID-19 ARDS [33, 34], non-COVID ARDS [32], and meta-analyses of steroid use in community-acquired pneumonia [35], in which steroid administration provided clinical benefit in patients with pneumonia of differing etiologies but was not associated with significantly higher rates of secondary infection.
Since dexamethasone has pleiotropic effects on the immune system [17], we investigated the impact of dexamethasone on the release of inflammatory cytokines and chemokines. The combination of dexamethasone and ceftriaxone attenuated the release of inflammatory cytokines in BAL fluid, including IL-1β, IL-6, TNF-α, and IFN-γ, and chemokines including KC, MCP-1, and MCP-3. These results are consistent with a prior study of corticosteroid treatment in ARDS patients [36]. Also, the combination of dexamethasone and ceftriaxone significantly reduced the release of IL-1β, TNF-α, IFN-γ, MCP-1, and MCP-3 compared with ceftriaxone alone. An experimental study using a mouse model of pneumococcal pneumonia reported that neutralization of IFN-γ accelerates recovery from lung injury [37]. Growing evidence suggest that MCP-1 is involved in lung inflammatory disorders [38]. The beneficial effect of dexamethasone on lung injury might be attributed in part through suppressing the release of these proinflammatory cytokines and chemokines. Transcriptomic analyses also identified additional pathways, including increased non-canonical NF-kB signaling and shifts in metabolic pathways, that may be important targets for future research.
Our mouse model reproduces several important clinical and histopathological features of severe bacterial pneumonia. Arterial hypoxemia was severe (76% on room air, Fig. 2A), pulmonary edema was markedly increased (Fig. 2B), and the histology showed extensive interstitial and alveolar edema and inflammation even in the mice treated with ceftriaxone (Fig. 4). Like many informative models of critical illness [39], our model does not reproduce all features of severe pneumococcal disease, including circulatory shock and mechanical ventilation. While these are important areas for future study, patients with shock were notably excluded from a recent trial that found a mortality benefit for steroids in severe CAP, and the majority of patients in this trial were not intubated at the time of enrollment [11], suggesting our model is clinically relevant.
There are some limitations of the mouse studies. We focused on the early phase of lung injury from pneumococcal pneumonia but did not address survival. This design made it possible to understand the earliest effects of steroid therapy on critical physiologic end points (oxygenation, lung fluid and protein balance), inflammatory responses, lung pathology, and bacterial burden. A recent clinical trial [11] reported a mortality benefit for corticosteroids in severe CAP, so we did not think that survival studies were as critical compared to in depth mechanistic analyses. Future studies will need to test the effects of steroids in other bacterial and viral pathogens in addition to the results from this study with S. pneumoniae.
Prior to this study, limited data were available on the biological overlap of experimental pneumonia in mice compared to patients with pneumococcal pneumonia. Transcriptomic analyses identified several pathways that were dysregulated by S. pneumoniae infection in both humans and mice, including signatures of inflammasome activation, including IL1 signaling and non-canonical NF-kB activation, and neutrophil degranulation in infected mice and humans. Several pathways were also modified by the addition of dexamethasone to ceftriaxone. While steroids decreased the expression of some pathways upregulated in S. pneumoniae-infected mice compared to controls, they also increased the expression of other pathways, suggesting the effects of steroids are more complex than simply reversing expression of dysregulated pathways. Notably, tracheal aspirate RNA sequencing from mechanically ventilated patients with S. pneumoniae infection identified similar gene expression changes to those observed in the mouse model, suggesting this experimental system replicates several biological features of clinical disease. In addition, some pathways that were modified by dexamethasone treatment in mice also were also modified in humans treated with steroids compared to those who were not. While the transcriptomic analyses identify potentially important pathways for future study, they have some limitations. First, the sample size from our clinical cohort is modest, which likely limited our power to identify individual genes that were significantly differentially expressed with steroids in humans. Second, steroid treatment was non-randomly assigned and likely more commonly administered in sicker patients. Third, our clinical respiratory tract samples obtained from tracheal aspirates, while our mouse experimental samples were from whole lung homogenate, which likely decreased our ability to detect differentially expressed genes overlapping between humans and mice. Despite these limitations, we were able to detect several changes at the pathway level that were shared between our experimental mouse model and the clinical samples.
The role of corticosteroids in the treatment of severe pneumonia and ARDS remains unclear. Our results identify several potential mechanisms for the therapeutic benefit of corticosteroids observed in the DEXA-ARDS, RECOVERY, and CAPE COD trials [11, 32, 34]. Notably, older trials found contradictory evidence for the role of steroids in these conditions [40]. One important challenge in comparing trials of corticosteroids for ARDS is the considerable heterogeneity in inclusion/exclusion criteria and timing of treatment. The recent, positive trials all notably initiated treatment early in the clinical course. In this study, mice received steroids (or placebo) 20 h after inoculation, which is likely a model of early treatment. Further study is required to understand the effects of delayed steroid treatment and the effects of steroids in other ARDS models.
Conclusions
In summary, adjunctive therapy with both antibiotics and dexamethasone reduces the severity of hypoxemia, pulmonary edema, and histologic evidence of lung injury in experimental pneumococcal pneumonia compared to antibiotics alone, in part through attenuating the release of inflammatory cytokines and chemokines. Importantly, steroid therapy in combination with antibiotics does not increase bacterial burden in the early phase of pneumococcal pneumonia in mice. Although the clinical studies were carried out in a modest number of patients, transcriptomic profiling further supports the clinical relevance of this mouse model, which replicates several features of pneumococcal pneumonia and steroid therapy in humans. In conclusion, steroid therapy has a sound mechanistic basis as an adjunctive treatment for severe community acquired bacterial pneumonia from S. Pneumoniae with the use of appropriate antibiotics.
Supplementary Information
Additional file 1. Supplemental Figures.
Additional file 4. Differential expression results.
Acknowledgements
We thank the patients who agreed to be part of these studies.
Abbreviations
- ARDS
Acute respiratory distress syndrome
- CAP
Community acquired pneumonia
- ELW
Excess extravascular lung water
- BAL
Bronchoalveolar lavage
- RAGE
Receptor for advanced glycation
- GSEA
Gene set enrichment analysis
- WGCNA
Weighted gene co-expression network analysis
Author contributions
HT and KDW designed the study, conducted the study, collected the data, analyzed the data, wrote the manuscript, and revised the manuscript. AS reviewed clinical data, performed bioinformatics analyses, wrote the manuscript, and revised the manuscript. SM contributed to study design, conducted experiments, analyzed the data, and revised the manuscript. RG generated transcriptomic data. XF and MM conducted the study, collected the data, and revised the manuscript. JEG, CRL, CSC, and MAM supervised the study, interpreted all data, and revised the manuscript.
Funding
NHLBI HL 123004 (MAM), HL140026 (MAM and CSC), R24AA019661 (CSC), 5T32GM008440-24 (KDW), and K23HL163491 (AS).
Availability of data and materials
Sequencing data for murine experiments is available at GSE246398. Because some tracheal aspirates samples were collected under a waiver of informed consent, the UCSF IRB prohibits the public sharing of genomic data from the human subjects included in this study. Processed gene count data for humans and mice are available in Additional file 3. Researchers interested in studying the genomic data from human subjects should contact the corresponding author (Hiroki.Taenaka@ucsf.edu).
Code availability
All code used for bioinformatics analyses is available at https://github.com/AartikSarma/PneumoniaSteroids
Declarations
Ethics approval and informed consent
The study protocol was approved by the University of California San Francisco (UCSF) Institutional Review Board (17-24056). If a patient was able to consent or had a surrogate available, a study physician or research coordinator obtained written informed consent at the time of enrollment. For subjects who could not consent at enrollment, the UCSF IRB granted an initial waiver of informed consent for biospecimen and clinical data collection, as previously described [25]. The UCSF IRB also approved a full waiver of consent for patients who died prior to regaining an ability provide informed consent, but restricted data sharing for samples collected from these patients. Because some of the subjects included in the present study were enrolled under a waiver of consent, our IRB protocol prohibits the public release of raw sequencing data, which may contain personally identifiable genetic information. The animal experiments were approved by the UCSF Institutional Animal Care and Use Committee (AN189182), were performed in compliance with the National Institute of Health Guide for Care and Use of Laboratory Animals and were reported in accordance with the Animal Research: Report of In Vivo Experiments (ARRIVE) guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hiroki Taenaka, Katherine D. Wick and Aartik Sarma have contributed equally to this work.
References
- 1.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, Herridge M, Randolph AG, Calfee CS. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Troeger C, Blacker B, Khalil IA, Rao PC, Cao J, Zimsen SRM, Albertson SB, Deshpande A, Farag T, Abebe Z, et al. Estimates of the global, regional, and national morbidity, mortality, and aetiologies of lower respiratory infections in 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Infect Dis. 2018;18(11):1191–1210. doi: 10.1016/S1473-3099(18)30310-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ortqvist A, Hedlund J, Kalin M. Streptococcus pneumoniae: epidemiology, risk factors, and clinical features. Semin Respir Crit Care Med. 2005;26(6):563–574. doi: 10.1055/s-2005-925523. [DOI] [PubMed] [Google Scholar]
- 4.Weiser JN, Ferreira DM, Paton JC. Streptococcus pneumoniae: transmission, colonization and invasion. Nat Rev Microbiol. 2018;16(6):355–367. doi: 10.1038/s41579-018-0001-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.File TM, Ramirez JA. Community-acquired pneumonia. N Engl J Med. 2023;389(7):632–641. doi: 10.1056/NEJMcp2303286. [DOI] [PubMed] [Google Scholar]
- 6.Brouwer MC, McIntyre P, de Gans J, Prasad K, van de Beek D. Corticosteroids for acute bacterial meningitis. Cochrane Database Syst Rev 2010(9).
- 7.Recovery Collaborative Group, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med 2021;384(8):693–704. [DOI] [PMC free article] [PubMed]
- 8.Villar J, Ferrando C, Martinez D, Ambros A, Munoz T, Soler JA, Aguilar G, Alba F, Gonzalez-Higueras E, Conesa LA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 9.Stern A, Skalsky K, Avni T, Carrara E, Leibovici L, Paul M. Corticosteroids for pneumonia. Cochrane Database Syst Rev. 2017;12(12):CD007720. doi: 10.1002/14651858.CD007720.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Briel M, Spoorenberg SMC, Snijders D, Torres A, Fernandez-Serrano S, Meduri GU, Gabarrus A, Blum CA, Confalonieri M, Kasenda B, et al. Corticosteroids in patients hospitalized with community-acquired pneumonia: systematic review and individual patient data metaanalysis. Clin Infect Dis. 2018;66(3):346–354. doi: 10.1093/cid/cix801. [DOI] [PubMed] [Google Scholar]
- 11.Dequin P-F, Meziani F, Quenot J-P, Kamel T, Ricard J-D, Badie J, Reignier J, Heming N, Plantefève G, Souweine B, et al. Hydrocortisone in severe community-acquired pneumonia. N Engl J Med. 2023;388(21):1931–1941. doi: 10.1056/NEJMoa2215145. [DOI] [PubMed] [Google Scholar]
- 12.Brabander Jd, Boers LS, Kullberg RFJ, Zhang S, Nossent EJ, Heunks LMA, Vlaar APJ, Bonta PI, Schultz MJ, Poll Tvd et al. Persistent alveolar inflammatory response in critically ill patients with COVID-19 is associated with mortality. Thorax 2023;78(9): 912–921. [DOI] [PubMed]
- 13.Neyton LPA, Patel RK, Sarma A, Willmore A, Haller SC, Kangelaris KN, Eckalbar WL, Erle DJ, Krummel MF, Hendrickson CM et al: Distinct pulmonary and systemic effects of dexamethasone in severe COVID-19. Res Sq 2023. [DOI] [PMC free article] [PubMed]
- 14.Wen SH, Wu HJ, Lin L, Chong L, Zhu LL, Zhang WX, Zhang HL, Li CC. Adjunctive dexamethasone therapy improves lung injury by inhibiting inflammation and reducing RIP3 expression during Staphylococcus aureus pneumonia in mice. Int Immunopharmacol. 2014;23(2):709–718. doi: 10.1016/j.intimp.2014.10.027. [DOI] [PubMed] [Google Scholar]
- 15.Sibila O, Luna CM, Agustí C, Baquero S, Gando S, Patrón JR, Morato JG, Absi R, Bassi N, Torres A. Effects of glucocorticoids in ventilated piglets with severe pneumonia. Eur Respir J. 2008;32(4):1037–1046. doi: 10.1183/09031936.00009208. [DOI] [PubMed] [Google Scholar]
- 16.Dagenais A, Denis C, Vives MF, Girouard S, Massé C, Nguyen T, Yamagata T, Grygorczyk C, Kothary R, Berthiaume Y. Modulation of alpha-ENaC and alpha1-Na+-K+-ATPase by cAMP and dexamethasone in alveolar epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2001;281(1):L217–230. doi: 10.1152/ajplung.2001.281.1.L217. [DOI] [PubMed] [Google Scholar]
- 17.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bartko J, Stiebellehner L, Derhaschnig U, Schoergenhofer C, Schwameis M, Prosch H, Jilma B. Dissociation between systemic and pulmonary anti-inflammatory effects of dexamethasone in humans. Br J Clin Pharmacol. 2016;81(5):865–877. doi: 10.1111/bcp.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Su X, Lee JW, Matthay ZA, Mednick G, Uchida T, Fang X, Gupta N, Matthay MA. Activation of the alpha7 nAChR reduces acid-induced acute lung injury in mice and rats. Am J Respir Cell Mol Biol. 2007;37(2):186–192. doi: 10.1165/rcmb.2006-0240OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gotts JE, Bernard O, Chun L, Croze RH, Ross JT, Nesseler N, Wu X, Abbott J, Fang X, Calfee CS, et al. Clinically relevant model of pneumococcal pneumonia, ARDS, and nonpulmonary organ dysfunction in mice. Am J Physiol Lung Cell Mol Physiol. 2019;317(5):L717–L736. doi: 10.1152/ajplung.00132.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. An official American thoracic society workshop report: features and measurements of experimental acute lung injury in animals. Am J Respir Cell Mol Biol. 2011;44(5):725–738. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukuda N, Folkesson HG, Matthay MA. Relationship of interstitial fluid volume to alveolar fluid clearance in mice: ventilated vs. in situ studies. J Appl Physiol. 2000;89(2):672–679. doi: 10.1152/jappl.2000.89.2.672. [DOI] [PubMed] [Google Scholar]
- 23.Flodby P, Kim YH, Beard LL, Gao D, Ji Y, Kage H, Liebler JM, Minoo P, Kim KJ, Borok Z, et al. Knockout mice reveal a major role for alveolar epithelial type I cells in alveolar fluid clearance. Am J Respir Cell Mol Biol. 2016;55(3):395–406. doi: 10.1165/rcmb.2016-0005OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Langelier C, Kalantar KL, Moazed F, Wilson MR, Crawford ED, Deiss T, Belzer A, Bolourchi S, Caldera S, Fung M, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U S A. 2018;115(52):E12353–e12362. doi: 10.1073/pnas.1809700115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sarma A, Christenson SA, Byrne A, Mick E, Pisco AO, DeVoe C, Deiss T, Ghale R, Zha BS, Tsitsiklis A, et al. Tracheal aspirate RNA sequencing identifies distinct immunological features of COVID-19 ARDS. Nat Commun. 2021;12(1):5152. doi: 10.1038/s41467-021-25040-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huppert LA, Matthay MA. Alveolar Fluid Clearance in Pathologically Relevant Conditions: In Vitro and In Vivo Models of Acute Respiratory Distress Syndrome. Front Immunol 2017; 8:371. [DOI] [PMC free article] [PubMed]
- 27.Taenaka H, Matthay MA. Mechanisms of impaired alveolar fluid clearance. Anat Rec (Hoboken) 2023. [DOI] [PMC free article] [PubMed]
- 28.Uchida T, Shirasawa M, Ware LB, Kojima K, Hata Y, Makita K, Mednick G, Matthay ZA, Matthay MA. Receptor for advanced glycation end-products is a marker of type I cell injury in acute lung injury. Am J Respir Crit Care Med. 2006;173(9):1008–1015. doi: 10.1164/rccm.200509-1477OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fraser RM, Charlotte S, Mark JG, Mark RT, Alastair GP. The pulmonary endothelium in acute respiratory distress syndrome: insights and therapeutic opportunities. Thorax. 2016;71(5):462. doi: 10.1136/thoraxjnl-2015-207461. [DOI] [PubMed] [Google Scholar]
- 30.Kalantar KL, Neyton L, Abdelghany M, Mick E, Jauregui A, Caldera S, Serpa PH, Ghale R, Albright J, Sarma A, et al. Integrated host-microbe plasma metagenomics for sepsis diagnosis in a prospective cohort of critically ill adults. Nat Microbiol. 2022;7(11):1805–1816. doi: 10.1038/s41564-022-01237-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Vidal C, Calbo E, Pascual V, Ferrer C, Quintana S, Garau J. Effects of systemic steroids in patients with severe community-acquired pneumonia. Eur Respir J. 2007;30(5):951–956. doi: 10.1183/09031936.00027607. [DOI] [PubMed] [Google Scholar]
- 32.Villar J, Ferrando C, Martínez D, Ambrós A, Muñoz T, Soler JA, Aguilar G, Alba F, González-Higueras E, Conesa LA, et al. Dexamethasone treatment for the acute respiratory distress syndrome: a multicentre, randomised controlled trial. Lancet Respir Med. 2020;8(3):267–276. doi: 10.1016/S2213-2600(19)30417-5. [DOI] [PubMed] [Google Scholar]
- 33.Matthay MA, Wick KD. Corticosteroids, COVID-19 pneumonia, and acute respiratory distress syndrome. J Clin Investig. 2020;130(12):6218–6221. doi: 10.1172/JCI143331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, et al. Dexamethasone in hospitalized patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Saleem N, Kulkarni A, Snow TAC, Ambler G, Singer M, Arulkumaran N. Effect of corticosteroids on mortality and clinical cure in community-acquired pneumonia: a systematic review, meta-analysis, and meta-regression of randomized control trials. Chest. 2023;163(3):484–497. doi: 10.1016/j.chest.2022.08.2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Meduri GU, Headley S, Tolley E, Shelby M, Stentz F, Postlethwaite A. Plasma and BAL cytokine response to corticosteroid rescue treatment in late ARDS. Chest. 1995;108(5):1315–1325. doi: 10.1378/chest.108.5.1315. [DOI] [PubMed] [Google Scholar]
- 37.Mock JR, Tune MK, Dial CF, Torres-Castillo J, Hagan RS, Doerschuk CM. Effects of IFN-γ on immune cell kinetics during the resolution of acute lung injury. Physiol Rep. 2020;8(3):e14368. doi: 10.14814/phy2.14368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rose CE, Jr, Sung SS, Fu SM. Significant involvement of CCL2 (MCP-1) in inflammatory disorders of the lung. Microcirculation. 2003;10(3–4):273–288. doi: 10.1038/sj.mn.7800193. [DOI] [PubMed] [Google Scholar]
- 39.Matthay MA, Schmidt EP, Bastarache JA, Calfee CS, Frevert CW, Martin TR. The translational value of rodent models of sepsis. Am J Respir Crit Care Med. 2024;209(5):488–490. doi: 10.1164/rccm.202308-1489VP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hough CL. Steroids for acute respiratory distress syndrome? Clin Chest Med. 2014;35(4):781–795. doi: 10.1016/j.ccm.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Supplemental Figures.
Additional file 4. Differential expression results.
Data Availability Statement
Sequencing data for murine experiments is available at GSE246398. Because some tracheal aspirates samples were collected under a waiver of informed consent, the UCSF IRB prohibits the public sharing of genomic data from the human subjects included in this study. Processed gene count data for humans and mice are available in Additional file 3. Researchers interested in studying the genomic data from human subjects should contact the corresponding author (Hiroki.Taenaka@ucsf.edu).
All code used for bioinformatics analyses is available at https://github.com/AartikSarma/PneumoniaSteroids