Abstract
Background
Shigellosis is one of the significant causes of diarrhea in Bangladesh. It is a global health problem; approximately 1.3 million people die yearly from Shigellosis. The current treatment method, using different antibiotics against Shigellosis is ineffective. Moreover, it becomes a worrying situation due to the emergence of antibiotic-resistant pathogenic microbes responsible for these diarrheal diseases.
Methodology
Previous immunoinformatics study predicted a potential peptide from the Ferric enterobactin protein (FepA) of Shigella spp. In this study, we have chemically synthesized the FepA peptide. As a highly immunogenic, FepA peptide conjugated with KLH has been tested in mice model with complete and incomplete adjuvants as a vaccine candidate.
Results
Immunological analysis showed that all vaccinated mice were immunologically boosted, which was statistically significant (P-value 0.0325) compared to control mice. Immunological analysis for bacterial neutralization test result was also statistically significant (P-value 0.0468), where each ELISA plate was coated with 1 × 107S. flexneri cells. The Challenge test with 1 × 1012S. flexneri cells to each vaccinated and controlled mice showed that 37.5 % of control (non-vaccinated) mice died within seven days after the challenge was given while 100 % of vaccinated mice remained strong and stout. The analyses of the post-challenge weight loss of the mice were also significant (P-value 0.0367) as the weight loss percentage in control mice was much higher than in the vaccinated mice. The pathological and phenotypic appearances of vaccinated mice were also clearly differentiable compared with control mice. Thus all these immunological analysis and pathological appearances directly supported our FepA peptide as a potential immune booster.
Conclusion
This study provides evidence that the FepA peptide is a highly immunogenic vaccine candidate against S. flexneri. Therefore, these findings inspire future trials for the evaluation of the suitability of this vaccine candidate against Shigellosis.
Keywords: Shigellosis, Diarrhea, Ferric enterobactin protein (FepA), Immunogenic, Vaccine, Bangladesh, Etc
Introduction
Shigella is a Gram-negative, facultatively anaerobic, non-motile, nonsporulating, and rod-shaped true Enterobacteria family that can invade hosts via spoiled food or contaminated water. They can survive in the stomach with extreme acidic conditions and create diarrheal diseases like Shigellosis [1], [2], [3].
Four species of Shigella, i.e. S. dysenteriae, S. flexneri, S. boydii, and S. sonnei, are responsible for Shigellosis that created a global human health problem [4], [5], [6]. All these four species are subdivided into serotypes based on O-specific polysaccharides of the lipopolysaccharides (LPS). They may be associated with life-threatening complications like bloody mucoid stools, abdominal pain, abdominal cramps, loose stools washing away essential minerals, and dehydration [4], [7], [8]. They may multiply within colonic epithelial cells, form colonies in the intestinal epithelium, and cause cell death. In chronic conditions, they may kill adjacent epithelial cells causing mucosal ulcers, internal bleeding, and inflammation that may lead to patients’ death. All over the world, about 1.3 million people die every year due to shigellosis [4], [9].
In developing countries like ours (Bangladesh), diarrheal diseases are one of the significant causes of mortality among not only children but also adult people. The mortality and disability of diarrheal disease are ranked fourth as the subject of death and second as a productive life lost every year [10], [11]. The annual burden of Shigella or Shigellosis worldwide was predicted to be 164.7 million cases and 163.2 million cases in developing countries [12]. Million people die (approximately 1.3) worldwide every year; among them, more than 95,000 children < 5 years old die due to shigellosis in Bangladesh [9], [13]. Shigellosis is not only a significant health problem in poor and developing counties but also for developed countries. Different studies have shown that S. sonnei has been mainly responsible for dysentery in developed countries worldwide. Phylogenetic analysis shows that modern-day S. sonnei populations arose from a common ancestor about 500 years ago and diversified into several lineages with identical characteristics [14]. The predominance of this diversification occurred in Europe and other developed countries. On the other hand, S. flexneri is now emerging as a problem in the developing world, affecting public health and economic development [14], [15].
Under consideration of different parameters like affecting rate, mortality, and clinical and economic losses, it can be said that Shigellosis is one of the significant health problems in the world. They become more challenging day to day as Shigella become resistant to commonly used antibiotics like ampicillin, tetracycline, streptomycin, nalidixic acid, and sulfamethoxazole-trimethoprim [16], [17]. Currently, third-generation antibiotic ciprofloxacin and fluoroquinolone have been used to treat shigellosis and dysentery, especially in the case of children. But when S. flexneri exhibits resistance against this third-generation antibiotic, the medical health care system will be broken, and they will no longer help treat diarrheal diseases. We should take alternative measures to cope with the more powerful S. flexneri. Vaccine development is a possible way that can elicit long-term immunological responses to fight against Shigella. To develop a safe and effective vaccine, it is essential to understand the pathogen’s structure, types, and pathogenesis. It is also required to analyze their pathogenesis in animal models replicating human symptoms [18], [19]. Ferric enterobactin protein (FepA) is a membrane transport protein found in E. coli O157 and Shigella spp. The outer membrane protein FepA in Shigella is highly immunogenic, and FepA protein may be tested on mice models to identify its immunogenicity against S. flexneri [20]. Highly immunogenic recombinant FepA peptide can be designed to evaluate immunity against E. coli and S. flexneri.
Our previous immunoinformatics study has developed a modified FepA peptide form a 746 amino acid containing protein, which can be used as a vaccine candidate. The modified FepA peptide, as a peptide vaccine, was tested on adult mice as they are readily available, inexpensive, easy to handle, and have a short life cycle. Although, as nonhuman primates, rats, guinea pigs, rabbits, and macaques, monkeys can be used as animal models that replicate humans [21], [22].
Many approaches and attempts were taken to develop Shigella vaccines. Although some of them elicit immune responses, they have some potential risks. Unfortunately, there is no experimental vaccine available so far. As far as our knowledge, no report suggests using FepA peptide as a vaccine candidate for trial on animal models to identify its immunogenicity against S. flexneri. [23].
This study demonstrated the potential application of in silico designed FepA peptide vaccine against S. flexneri and targeted FepA protein as one of the best vaccine candidates covering all the S. flexneri throughout the world.
Materials and methods
Mice Collection
Four to six weeks old male Swiss albino mice were collected from the animal house of the International Centre for Diarrheal Disease Research, Bangladesh. They were nourished in a controlled environment with appropriate feeding. After one week, these mice were grouped into three groups (Experimental-A: The experimental group A mice were injected with our synthesized FepA peptide with complete and incomplete adjuvant; Control-B: The control group B mice were injected with PBS + complete/incomplete adjuvant; and Neutral Control-C: The neutral control group C mice were injected nothing). The neutral control group was used to observe if the weight loss of the mice occurred for other environmental factors. Each group consisted of eight mice. As this study included animals, the guidelines of this study were approved by the ethical review committee of the Mawlana Bhashani Science and Technology University (Approval no: MBSTU/ ERC/ ECC/157/04/2022).
Synthesis of FepA peptide
We have synthesized FepA peptide by Fmoc-solid phase peptide synthesis (Fmoc-SPPS) methodology [24], [25]. We incorporated a cysteine residue in the sequence that has reactive free thiol group suitable for conjugation with maleimide activated mcKLH.
Conjugation of the peptide with KLH
At first, 2 mg of sulfhydryl-containing FepA peptide had been dissolved in 400 µl conjugation buffer. Then, 400 µl water had been added to 2 mg of maleimide activated mcKLH to make a 10 mg/ml solution. The peptide and activated mcKLH had been mixed immediately and allowed to react for 2 h at room temperature. mcKLH forms a suspension that typically appears translucent to whitish blue. Conjugate was purified by gel filtration to remove EDTA. Because, an anti-coagulant, EDTA, should not be injected into laboratory animals [26], [27]. Zeba Spin Desalting Columns (Thermo scientific; Product No. 89889) have been used for gel filtration. The sample has been applied on top of the resin and centrifuged at 1000 × g for 2 min. A precipitate was formed during conjugation that has been centrifuged. Only the supernatant was collected that had been purified. Finally, the clarified supernatant was combined with the precipitate [28].
Final hapten preparation with adjuvant
After purification, the total amounts of conjugate (800 µl where 400 µl hapten and 400 µl mcKLH) were divided into four centrifuged tubes that can be injected in 3 booster dose. We injected 50 µl of conjugated peptide in each mouse (consisting 50 µg peptide). The conjugated peptide was mixed with equal amount of complete and incomplete adjuvants (0.425g/ml). The complete adjuvant (Imject™ Freund's Complete Adjuvant) was injected in first dose and incomplete adjuvant (Imject™ Freund's Incomplete Adjuvant) formulation was injected in the booster doses.
PBS preparation with adjuvant (Placebo)
450 µl PBS dissolved with equal amount of complete or incomplete adjuvants.
Immunization of mice and serum collection
The final immunogen with an adjuvant is ready for injection in experimental (Group-A) mice. Before injecting, the pre-immune blood had been collected from each group of mice as a blank for the ELISA experiment. Then for each mouse, 50 µg of immunogen containing an equal amount of complete adjuvant was injected into the intraperitoneal space. After seven days, the first immunized blood had been collected from the mice’s facial veins. Serum was collected from clotted blood sample and stored in a separate microcentrifuge tube at − 80 °C. Likewise, 2nd, 3rd, and 4th dose of vaccine mixed vigorously with incomplete adjuvant (booster dose) were injected 14th, 28th, and 42nd day after 1st dose. The serum samples were collected on the 21st, 35th and 49th day. At the same time, Group-B mice were injected with PBS containing complete and incomplete adjuvant. The Group-C mice were kept as a neutral control, injected nothing only caring them with appropriate feeding and controlled environment like Group-A and B mice.
Collection of bacterial strain
S. flexneri was collected from the microbiology laboratory of the Department of Biotechnology and Genetic Engineering, MBSTU, and confirmed by Biochemical test and further confirmed by PCR amplifying the wzy gene.
Biochemical test
To find out S. flexneri different kinds of well-isolated NLF single colonies from MacConkey's agar were subjected to varying types of biochemical tests such as Kligler Iron Agar (KIA), Motility-Indole-Urease (MIU), and Citrate were performed [29].
Polymerase chain reaction (PCR)
Template DNA was prepared according to the heat and shock method, where 1 ml of each enriched broth was centrifuged at 13000 rpm for 7 min and washed with 1 ml of normal saline/ PBS [30]. Then the pellet was mixed with 200 µl of distilled water, boiled at 100 °C for 15 min, and cooled down immediately on ice for 10 min to disrupt the cells. Bacterial debris was removed by centrifugation at 13000 rpm for 10 min, and the supernatant was collected, which was stored at −20 °C. The PCR primer used in this study to amplify the ‘wzy/rfc’ gene for detecting S flexneri is shown in table S1 [31].
Assessment of IgG antibody in the mice sera by immunological analysis
An enzyme-linked immunosorbent assay was performed to determine the FepA-specific antibody response in experimental and control mice serum samples. The ELISA plate wells were incubated overnight with 100 µl coating buffer at 4 °C, where 100 ng of FepA peptide was present. The plate wells were washed thrice with PBS for 5 min and blocked with 5 % skimmed milk in PBS for 1–2 h at room temperature. After washing thrice with PBS, the antigen (FepA peptide) coated each well was incubated with 100 µl of appropriate diluted (100 times) of respective test sera for 1 h at 30 °C. After proper washing, each well was incubated with optimally diluted 100 µl of secondary antibody for 1 h at 30 °C. After washing, 100 µl of TMB with peroxide solution was added and reacted for 30 min. The reaction was stopped with 100 µl of 2 M H2SO4. The absorbance of each well has been measured at 450 nm. The result has been assessed for statistical significance using Graph pad prism version 8. The paired t-test was performed for the OD of vaccinated mice in comparison to control mice.
Antibody titer determination
To evaluate antibody titer, the final vaccinated serum samples were diluted to 100 to 1600 times. The FepA peptide (100 ng/well) was used to coat the ELISA plate. And the titer is defined as the maximum dilution of final serum samples where the absorbance value was 0.2, after subtracting the average value of the blank wells [32].
Immunized serum bactericidal assay (SBA)
At first baby rabbit complement (BRC) was prepared by incubating the serum of a one-month-old baby Rabbit in the water bath at 56 °C for 30 min. Serum bactericidal assay was performed by adding 217.5 µl of diluted target bacteria (∼105 CFU/ml), 30 µl (12.5 % final concentration) of diluted heat-inactivated baby rabbit complement, and 2.5 µl of experimental immunized serum (100 times diluted) in a culture tube. We have used a control for this experiment that consist of the well contained only bacteria and baby rabbit complement (BRC); no test serum was added to these culture wells. Then all these reaction mixtures were incubated for 2 h at 37 °C. Then 10 µl of reaction mixture from each well was spotted on LB agar media. Then the agar plates were incubated overnight at 29 °C. Next day, 100 µg/ml triphenyl tetrazolium chloride (TTC) containing agar overlaid on the culture plates and incubated additional 2 h at 37 °C. Due to the activity of TCC, the bacterial colony turns red color. Then the bacterial colonies were counted manually [33].
Confirmation of Shigella specific antibody in the mice sera
Each well of ELISA plate was coated with 1 × 107 bacteria and incubate overnight at 4 °C following the strategy used in a previous study [34]. The bacterial count was performed by spread plate method and used a spectrophotometer to measure the OD of the liquid culture broth. Then a standard Enzyme-Linked Immunosorbent Assay was performed to confirm Shigella-specific antibody in the mice sera.
S. flexneri challenge test
The mice were challenged by Shigella isolate at the intraperitoneal route after three weeks of vaccination. So, the approximate age of these mice was 12–14 weeks during the challenge study. For the challenge, mice (vaccinated and non-vaccinated) were injected with 1 × 1012 S. flexneri bacterial cells in their peritoneal space. After the challenge, all the grouped mice were monitored carefully for about ten days [35].
Results
Selection of the peptide and formulation
We have selected the peptide candidate from the whole FepA protein of Shigella from our previous immunoinformatics study [36]. Where T-cell epitopes of FepA protein were predicted by the NetCTL and IEDB server using a previously utilized protocol [37]. A 15aa epitope of FepA has 99.0 % population coverage based on MHC class I and MHC class II and is significantly non-toxic, non-allergic, and immunogenic [36]. This epitope was also shown to have B-cell inducing ability shown bioinformatically. These in-silico studies have boosted our encouragement for a wet-lab experiment with the proposed FepA peptide as a vaccine candidate against S. flexneri.
Assessment of IgG titer in the vaccinated mice
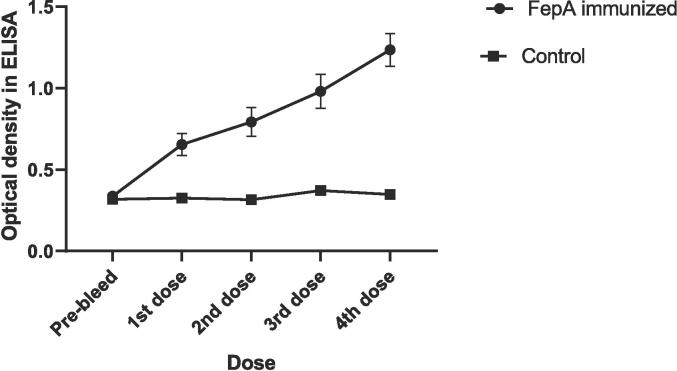
Immunological analysis was performed to identify the produced antibody against the FepA peptide. Immunological analysis shows that the antibody response against FepA peptide gradually increased after hapten's 1st, 2nd, 3rd, and 4th dose with complete/incomplete adjuvant in experimental mice. At the same time, the antibody response in control mice (PBS) was not gradually increased, although they were injected with PBS containing adjuvant without hapten of interest. There is a significant relationship (P-value 0.0325) between the immunizations of immunized mice against control mice. That means the FepA peptide had a significant role in boosting antibody response against S. flexneri. The average absorbance in experimental serum was 0.336 ± 0.07 (pre-bleed), 0.655 ± 0.19 (1st dose), 0.793 ± 0.246 (2nd dose), 0.980 ± 0.293 (3rd dose), and 1.235 ± 0.286 (4th dose) and in control serum the mean absorbance was 0.318 ± 0.066 (pre-bleed), 0.326 ± 0.056 (1st dose), 0.316 ± 0.048 (2nd dose), 0.371 ± 0.054 (3rd dose), 0.348 ± 0.066(4th dose) (Fig. 1). The final vaccinated 800 times diluted serum samples show the antibody titers (absorbance 0.2) after performing a titration experiment of these sera.
Fig. 1.
A graph showing the absorbance in different immunization for experimental and control mice. Based on optical density (OD) in ELISA, (i) FepA immunized mice showed increasing immune response with respect to the increased number of booster doses; (ii) Control mice injected with PBS showed a steady state immune response. The immune response in pre-vaccinated mice and control mice was almost similar. Upon vaccination, FepA immunized mice showed a significant (P-value 0.0325) immune response concerning the control mice.
Confirmation of the S. flexneri specific antibody in mice sera
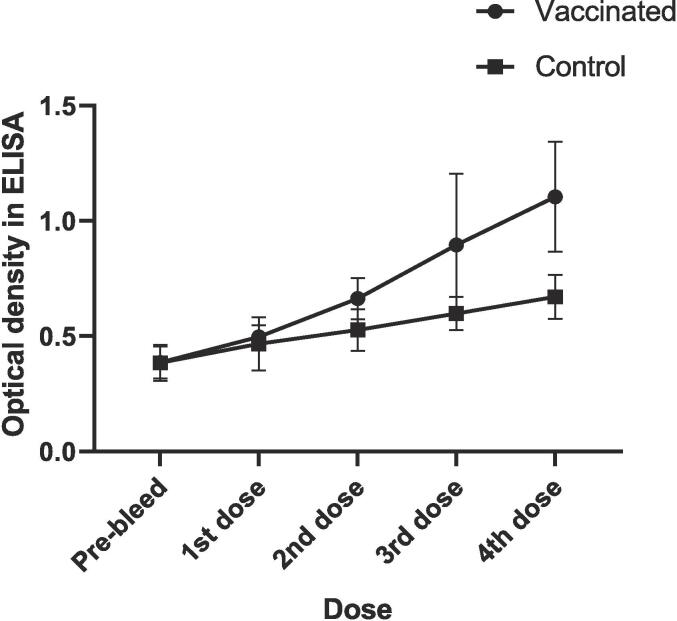
Immunological analysis was also performed to determine the exact antibody against whole bacterial cells (S. flexneri). The immunological analysis showed that the antibody response was gradually increased against whole bacterial cells. The serum collected after the 4th boosting of hapten with incomplete adjuvant in experimental mice was given the highest OD against bacterial cells. In addition, the antibody response in control mice (PBS) was also gradually increased, although their immunization rate was significantly lower than in experimental mice (paired t-test between experimental and control mice; P-value 0.0468). The average OD in experimental serum was 0.386 ± 0.07 (pre-bleed), 0.497 ± 0.0496 (1st dose), 0.663 ± 0.089 (2nd dose), 0.896 ± 0.307 (3rd dose), and 1.105 ± 0.238 (4th dose) in comparison to the control serum the mean OD was 0.384 ± 0.077 (pre-bleed), 0.467 ± 0.115 (1st dose), 0.527 ± 0.089 (2nd dose), 0.598 ± 0.0719 (3rd dose), 0.670 ± 0.094 (4th dose) (Fig. 2).
Fig. 2.
A graph showing the recognition of bacterial isolate by the immunized serum. Each ELISA plate well was sensitized with 1 × 107S. flexneri cells. Based on optical density (OD) in ELISA, the antibody response was gradually increased against whole bacterial cells in both vaccinated and control mice. Here the antibody response in the serum of vaccinated mice was significantly (P-value 0.0468) higher than the control mice.
Serum bactericidal assay to measure the inhibition by FepA peptide-specific antibody
The control reaction mixtures containing only BRC showed 150–180 CFU per spot (10 µl). While, the FepA peptide immunized serum containing reaction mixtures showed bacterial (S. flexneri) counts of 65–68 CFU/per spot (10 µl). Therefore, the immunized serum containing BRC inhibits more bacterial growth than the control reaction mixture containing only BRC. Thus we can conclude that the 100 times diluted FepA immunized serum reduce the growth of S. flexneri on average 59.69 % than the control reaction mixture.
Identification of S. flexneri by biochemical test
In Kliger’s Iron Agar S. flexneri showed an alkaline slant and acidic butt condition. In Motility Indole Urease test S. flexneri showed non-motility pattern. They neither utilized Simmon’s citrate nor produced H2S gas and did not utilize urea. The results of the biochemical analysis are shown in (Table 1). Based on the biochemical test S. flexneri was confirmed.
Table 1.
Biochemical characteristics of Shigella isolate used in this study.
| KIA |
MIU |
Citrate |
|||||
|---|---|---|---|---|---|---|---|
| Slant | Butt | Gas | Motility | Indole | Urease | Slant | Butt |
| K | A | 0 | 0 | 0 | 0 | 0 | 0 |
*KIA: Kliger Iron Agar; MIU: Motility-Indole-Urease; K: Alkaline; A: Acidic.
Molecular detection of S. flexneri
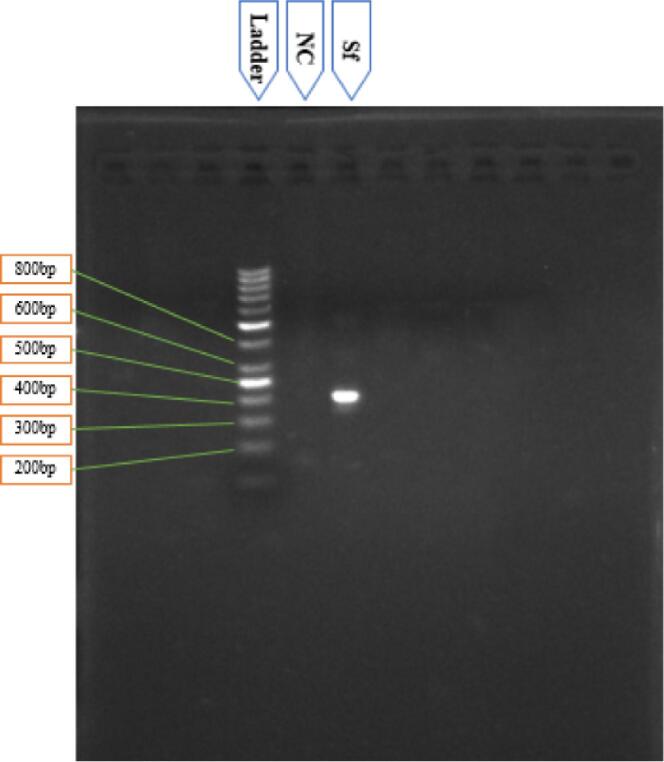
S. flexneri was further confirmed through PCR analysis using a specific primer for S. flexneri. A 538 bp band was generated by amplifying the “wzy” gene that confirmed the collected bacterial strain as S. flexneri (Fig. 3).
Fig. 3.
Amplification of “wzy” gene (538 bp) for detecting S. flexneri isolates. L: 1kbp DNA ladder. The same sample was undergone with S. sonnei (Ss) and S. dysenteriae (Sd) specific PCR reaction, but the result was negative. That indirectly confirmed that the collected isolated was not contaminated with other Shigella strains (Ss & Sd).
Physical abnormalities of the mice after post-vaccination challenge test
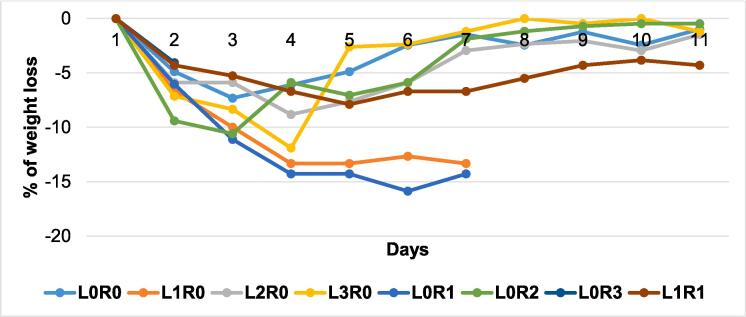
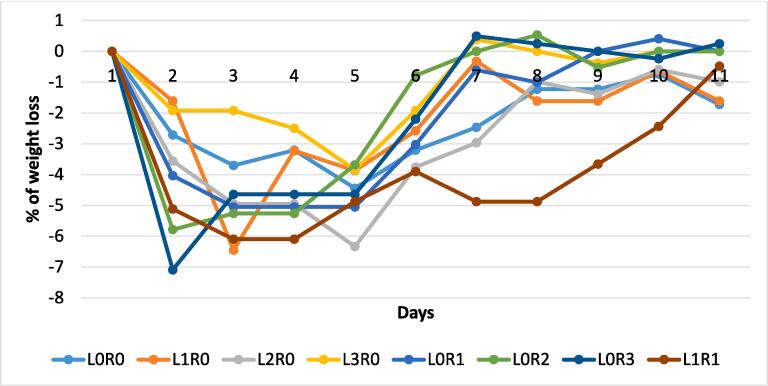
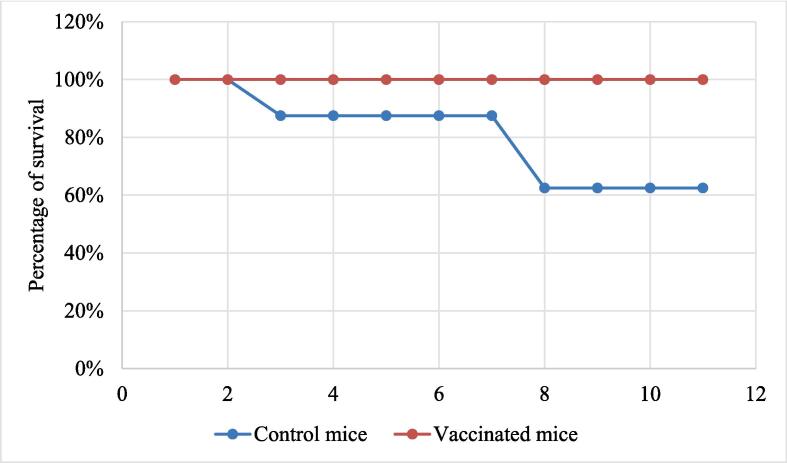
After vaccination, both immunized and control mice were injected with 1 × 1012 S. flexneri isolates. In response to this challenge, the control mice were seriously ill & weak and infection occur with diarrhea having loose stool (Table 2, Fig. 4). On the other hand, the immunized mice were much more regular in physical behavior, although they were observed a little weak after the following days of the given challenge (Table 3, Fig. 5). Sickness and food reluctance resulted in decreased bodyweight of both groups of mice (control and experimental) as shown in Fig. 4, and Fig. 5. The neutral control mice were not given challenge with S. flexneri strains, and there was no weight loss observed as observed in the experimental mice. Though all the vaccinated mice were alive after the challenge with a lethal dose of S. flexneri, 37.5 % of the control mice died within seven days after the challenge (Fig. 6).
Table 2.
Anthropometric and behavioral changes occurred in the control unvaccinated mice after challenge with S. flexneri strain.
| Days | Weight Gm ± SD |
Body temp. | Heart rate | Sleeping hours | Daily activities |
|---|---|---|---|---|---|
| Day0 | 36.85 ± 5.442 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day1 | 34.6 ± 4.980 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Seriously ill & weak |
| Day2 | 34.44 ± 5.342 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Seriously ill & weak (1 dead) |
| Day3 | 34.07 ± 5.947 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Seriously ill & weak |
| Day4 | 35.76 ± 8.120 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Seriously ill & weak |
| Day5 | 34.96 ± 6.591 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Weak |
| Day6 | 35.51 ± 6.824 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Weak |
| Day7 | 39.34 ± 3.616 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Moderate (2 dead) |
| Day8 | 39.56 ± 3.614 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Moderate |
| Day9 | 39.5 ± 3.777 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day10 | 39.58 ± 3.509 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
*The dead mice have been excluded from calculation
Fig. 4.
Graphical representation of weight loss among S. flexneri injected control mice. L means marked on the left ear; R means marked on the right ear, and 0–3, is the number of markings; (eight mice used in each group). (i) each line of the graph represent the percent of weight loss of non-vaccinated control mice after challenge given; (ii) incomplete lines represent the death of mice due to challenge by Shigella.
Table 3.
Anthropometric and behavioral changes occurred in the vaccinated mice after challenge with S. flexneri strain.
| Days | Weight Gm ± SD |
Body temp. | Heart rate | Sleeping hours | Daily activities |
|---|---|---|---|---|---|
| Day0 | 42.93 ± 7.202 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day1 | 41.23 ± 7.115 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Weak |
| Day2 | 40.94 ± 7.252 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Weak |
| Day3 | 41.05 ± 6.954 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Moderate |
| Day4 | 40.93 ± 6.705 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day5 | 41.76 ± 6.932 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day6 | 42.36 ± 7.162 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day7 | 42.46 ± 7.289 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day8 | 42.48 ± 7.296 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day9 | 42.71 ± 7.295 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
| Day10 | 43.83 ± 7.639 | 36.9 ± 0.5 | 400 – 600/m | 12–14 | Normal |
Fig. 5.
Graphical representation of weight loss among S. flexneri injected vaccinated mice. L means marked on the left ear; R means marked on the right ear; 0–3 is the number of markings; (eight mice used in each group). (i) each line of graph represents the percent of weight loss of vaccinated mice after challenge given.
Fig. 6.
Survival rate of the control and vaccinated mice after challenging with lethal dose of S. flexneri. (i) After challenge, 100% vaccinate mice were survived (red line) (ii) among non-vaccinated control mice, 37.5% of them died within seven days after the challenge (blue line). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
However, immunized and control mice gradually lost weight in response to S. flexneri infection (Table S2, and S3). But the weight loss percentage was higher in control mice than in vaccinated mice. Statistical analysis shows a positive relationship (P-value 0.0367) between control and immunized mice weight loss percentage (Figure S1).
Confirmation of infection by S. flexneri
We collected stool from mice after challenge with S. flexneri. The stool samples were subjected to bacteriological and molecular study (PCR) and found positive (Figure S2). So, the infection happened all the control mouse due to the challenge of S. flexneri.
Discussion
As Shigellosis is a fast-spread and fatal disease, there is an immediate need to develop a safe and effective vaccine against S. flexneri. It is alarming that more than 160 million cases are reported worldwide per year, affected mainly children below five years old, adults, military personnel, tourists, and pilgrims who are also susceptible to Shigella infection [38]. Despite several recent attempts, no licensed vaccine is available that prevents Shigellosis. Shigellosis mainly infects lower and middle-class people, resulting in many deaths and a high disease burden in poor and middle-income countries like ours [39]. Vaccination offers the greatest hope of improving this miserable condition as an effective and substantial strategy against Shigellosis. There is an urgent need to develop a broad-spectrum vaccine that will be effective in all serotypes of Shigella spp. To develop a vaccine, we must identify any candidate that will be highly immunogenic and elicit a long-term immunological response. From this point of view, we selected the Ferric enterobactin protein (FepA), a membrane transport protein found in Escherichia coli O157 and Shigella spp., [40] as a vaccine candidate using the Bioinformatics tool. The FepA protein (outer membrane protein) in Shigella is highly immunogenic [41], [43], with some modification and incomplete/complete adjuvant FepA hapten as a vaccine candidate tested on mice model to determine its immune responses against S. flexneri. Our previous study on multi-epitope-based synthetic vaccines also showed to produce neutralizing antibodies on Vero-2 cell culture against SARS-CoV-2 [42].
For experimental mice, FepA hapten with complete/incomplete adjuvant was given four sequential doses, and for control mice, PBS with adjuvant was sequentially injected. The immunological analysis showed that the immune response in experimental mice was much higher than in control mice, making the experiment statistically significant (P-value 0.0325). The highest OD in experimental mice serum was 0.445 (pre-bleed), 0.936 (1st dose), 0.966 (2nd dose), 1.526 (3rd dose), and 1.669 (4th dose) and in control serum the highest OD was 0.423 (pre-bleed), 0.412 (1st dose), 0.404 (2nd dose), 0.46 (3rd dose), 0.458 (4th dose) respectively (Fig. 1). This experiment shows that FepA hapten elicits B-cell immune response. Then we perform another immunological analysis using whole bacterial (S. flexneri) cells to sensitize each well of ELISA plate. The immunological profile showed that the antibody response against bacterial cells gradually increases with the increase of dosing rate. The serum collected after 4th boosting was given the highest OD against bacterial cells. But due to the naïve immune response, the antibody response in control mice (PBS) was also gradually increased. However, their immune response rate was significantly (P-value 0.0468) lower than the experimental mice. The highest OD in experimental serum was 0.4742 (pre-bleed), 0.548 (1st dose), 0.751 (2nd dose), 1.2295 (3rd dose), and 1.3756 (4th dose) and in control serum the mean OD was 0.5002 (pre-bleed), 0.5911 (1st dose), 0.6033 (2nd dose), 0.7004 (3rd dose), 0.7908 (4th dose), respectively (Fig. 2). So, it has been clearly shown that the antibody generated against the FepA peptide can recognize the bacterial cell. This is evidence that the small synthetic peptide can elicit a specific immune response in mice. The immune response is not as vigorous as a big protein; however, a specific immune response might be more important than the non-specific vigorous immune response to combat the pathogenic effects of bacteria. Serum bactericidal assay also showed that FepA peptide-specific antibody containing serum inhibit more than 59.69 % of S. flexneri bacterial growth.
From these experiments, it was clear that the FepA peptide has the potential to stimulate an immune response against S. flexneri. So post-vaccination challenge test was performed to determine the physiological effects of experimental and control mice. For this purpose, the immunized and control mice were injected with PCR confirmed 1 × 1012 S. flexneri cells. Not surprisingly, the control mice were seriously ill and weak and affected with diarrhea, heavy loose stool, reduced food and water intake and thereby loss the body weight (Fig. 4). Moreover, 37.5 % of mice died within seven days after the challenge was given (Fig. 6). On the other hand, after giving the challenge the vaccinated mice only lost their body weight, however, their survival rate was 100%, respectively (Fig. 5, Fig. 6). The stool samples of infected mice were microbiologically analyzed and found S. flexneri isolated that we used for challenge responsible for their diarrhea (Figure S2).
At the same time, the immunized mice were moderate in physical behavior; although they were observed to be a little bit weak the following days after the challenge was given, they returned to normal condition very quickly. However, immunized and control mice gradually lost weight in response to the S. flexneri challenge, but the immunized mice regained their weight quickly and effectively. The frequency of weight loss percentage in control mice was much higher than in the immunized mice, which is statistically significant (P-value 0.0367) (Figure S1).
Therefore, this study strongly supports the FepA peptide as a highly immunogenic candidate for S. flexneri and may be considered a potential vaccine that can also be included in a multi-epitope-based vaccine. So FepA peptide can be used as a potential vaccine against Shigellosis and may also elicit protection against all Shigella spp.
Conclusion
FepA peptide vaccinated mice were well tolerated against the given challenge (1012 S. flexneri), confirming its immune-modularity activity and were statistically significant. In conclusion, we predict that recombinant FepA peptide may serve as a potent vaccine molecule as it has revealed its immunogenic and protective efficacy against S. flexneri. As it is highly conserved in other strains, it should also be effective against S. dysenteriae, S. boydii, and S. sonnei. To develop the FepA peptide as an effective vaccine, further studies, such as different immunization procedures with human-compatible adjuvants, cross-immunity, and additional clinical trials, should be required to make the FepA peptide applicable in humans and large animals. So we roseate that many scientists will come forward and further research will be performed to make our FepA peptide a potential vaccine candidate against Shigellosis.
Ethics approval
The study has been approved by Ethical review committee of Mawlana Bhashani Science and Technology University. The ethical approval number: MBSTU/ ERC/ ECC/157/04/2022.
Data availability statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
CRediT authorship contribution statement
Md. Rayhan Ali: Writing – original draft, Visualization, Methodology, Investigation, Formal analysis. Shahin Mahmud: Methodology, Investigation, Data curation. Md. Omar Faruque: Methodology, Investigation, Formal analysis, Data curation. Md. Imam Hossain: Supervision, Project administration. Mohammed Akhter Hossain: Methodology, Funding acquisition. K.M. Kaderi Kibria: Writing – review & editing, Supervision, Resources, Project administration, Investigation, Funding acquisition, Formal analysis, Conceptualization.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [K. M. Kaderi Kibria reports financial support was provided by Government of the People’s Republic of Bangladesh Ministry of Education. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.].
Acknowledgement
We thank Dr. Kaisar Ali Talukdar for providing the Shigella strain in our previous project. This study was funded by ministry of education, Bangladesh (through BANBEIS, LS2017618).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2024.100493.
Contributor Information
Mohammed Akhter Hossain, Email: akhter.hossain@florey.edu.au.
K.M. Kaderi Kibria, Email: km_kibria@yahoo.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
The data are available along with the manuscript.
References
- 1.Chapela M.J., Garrido-Maestu A., Cabado A.G. Detection of foodborne pathogens by qPCR: A practical approach for food industry applications. Cogent Food Agric. 2015 doi: 10.1080/23311932.2015.1013771. [DOI] [Google Scholar]
- 2.Jeffery S. Van Der Putten WH. Soil Borne Human Diseases. 2011 [Google Scholar]
- 3.Clontz L. Microbial Limit and Bioburden Tests. 2008 doi: 10.1201/noe1420053487. [DOI] [Google Scholar]
- 4.Niyogi S.K. Shigellosis. J Microbiol. 2005;43:133–143. [PubMed] [Google Scholar]
- 5.Noriega F.R., Liao F.M., Formal S.B., Fasano A., Levine M.M. Prevalence of Shigella enterotoxin 1 among shigella clinical isolates of diverse serotypes. J Infect Dis. 1995 doi: 10.1093/infdis/172.5.1408. [DOI] [PubMed] [Google Scholar]
- 6.Parsot C. Shigella spp. and enteroinvasive Escherichia coli pathogenicity factors. FEMS Microbiol Lett. 2005 doi: 10.1016/j.femsle.2005.08.046. [DOI] [PubMed] [Google Scholar]
- 7.Taylor D.N., Trofa A.C., Sadoff J., Chu C., Bryla D., Shiloach J., et al. Synthesis, characterization, and clinical evaluation of conjugate vaccines composed of the O-specific polysaccharides of Shigella dysenteriae type 1, Shigella flexneri type 2a, and Shigella sonnei (Plesiomonas shigelloides) bound to bacterial toxoids. Infect Immun. 1993 doi: 10.1128/iai.61.9.3678-3687.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alam N.H., Ashraf H. Treatment of infectious diarrhea in children. Pediatr Drugs. 2003 doi: 10.2165/00128072-200305030-00002. [DOI] [PubMed] [Google Scholar]
- 9.Baker S., The H.C. Recent insights into Shigella: A major contributor to the global diarrhoeal disease burden. Curr Opin Infect Dis. 2018 doi: 10.1097/QCO.0000000000000475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chowdhury F., Khan I.A., Patel S., Siddiq A.U., Saha N.C., Khan A.I., et al. Diarrheal illness and healthcare seeking behavior among a population at high risk for diarrhea in Dhaka. Bangladesh PLoS One. 2015 doi: 10.1371/journal.pone.0130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pires S.M., Fischer-Walker C.L., Lanata C.F., Devleesschauwer B., Hall A.J., Kirk M.D., et al. Aetiology-specific estimates of the global and regional incidence and mortality of diarrhoeal diseases commonly transmitted through food. PLoS One. 2015 doi: 10.1371/journal.pone.0142927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang X.Y., Tao F., Xiao D., Lee H., Deen J., Gong J., et al. Trend and disease burden of bacillary dysentery in China (1991–2000) Bull World Health Organ. 2006 doi: 10.2471/BLT.05.023853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Talukder K.A., Khajanchi B.K., Islam M.A., Dutta D.K., Islam Z., Khan S.I., et al. The emerging strains of Shigella dysenteriae type 2 in Bangladesh are clonal. Epidemiol Infect. 2006 doi: 10.1017/S0950268806006029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Holt K.E., Baker S., Weill F.X., Holmes E.C., Kitchen A., Yu J., et al. Shigella sonnei genome sequencing and phylogenetic analysis indicate recent global dissemination from Europe. Nat Genet. 2012 doi: 10.1038/ng.2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Anandan S., Sethuvel D.P.M., Gajendiren R., Verghese V.P., Walia K., Veeraraghavan B., et al. and 2015: Trends in South India. GERMS. 2014;2017 doi: 10.18683/germs.2017.1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Trung V.N., Le P.V., Le C.H., Weintraub A. Antibiotic resistance in diarrheagenic Escherichia coli and Shigella strains isolated from children in Hanoi. Vietnam Antimicrob Agents Chemother. 2005 doi: 10.1128/AAC.49.2.816-819.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lima A.A.M., Lima N.L., Pinho M.C.N., Barros E.A., Teixeira M.J., Martins M.C.V., et al. to 1993. Antimicrob Agents Chemother. 1988;1995 doi: 10.1128/aac.39.1.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Walker R.I., Wierzba T.F., Mani S., Bourgeois A.L. Vaccines against Shigella and enterotoxigenic Escherichia coli: A summary of the VASE Conference. Vaccine. 2016;2017 doi: 10.1016/j.vaccine.2017.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Riddle M.S., Kaminski R.W., Di Paolo C., Porter C.K., Gutierrez R.L., Clarkson K.A., et al. Safety and immunogenicity of a candidate bioconjugate vaccine against Shigella flexneri 2a administered to healthy adults: A single-blind, randomized phase i study. Clin Vaccine Immunol. 2016 doi: 10.1128/CVI.00224-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clarke T.E., Tari L.W., Vogel H.J. Structural biology of bacterial iron uptake systems. Curr Top Med Chem. 2001;1:7–30. doi: 10.2174/1568026013395623. [DOI] [PubMed] [Google Scholar]
- 21.Brault A.C., Domi A., McDonald E.M., Talmi-Frank D., McCurley N., Basu R., et al. A Zika Vaccine Targeting NS1 Protein Protects Immunocompetent Adult Mice in a Lethal Challenge Model. Sci Rep. 2017 doi: 10.1038/s41598-017-15039-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hensley L.E., Young H.A., Jahrling P.B., Geisbert T.W. Proinflammatory response during Ebola virus infection of primate models: Possible involvement of the tumor necrosis factor receptor superfamily. Immunol Lett. 2002 doi: 10.1016/S0165-2478(01)00327-3. [DOI] [PubMed] [Google Scholar]
- 23.Ashkenazi S., Cohen D. An update on vaccines against Shigella. Ther Adv Vaccines. 2013;1:113–123. doi: 10.1177/2051013613500428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hossain M.A., Lin F., Zhang S., Ferraro T., Bathgate R.A., Tregear G.W., et al. Regioselective disulfide solid phase synthesis, chemical characterization and in vitro receptor binding activity of equine relaxin. Int J Pept Res Ther. 2006 doi: 10.1007/s10989-006-9020-9. [DOI] [Google Scholar]
- 25.Liu M., White B.F., Praveen P., Li W., Lin F., Wu H., et al. Engineering of a Biologically Active Insulin Dimer. J Med Chem. 2021 doi: 10.1021/acs.jmedchem.1c01594. [DOI] [PubMed] [Google Scholar]
- 26.Si L., Meng K., Tian Z., Sun J., Li H., Zhang Z., et al. Triterpenoids manipulate a broad range of virus-host fusion via wrapping the HR2 domain prevalent in viral envelopes. Sci Adv. 2018 doi: 10.1126/sciadv.aau8408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nardi M.A., Liu L.X., Karpatkin S. GPIIIa-(49–66) is a major pathophysiologically relevant antigenic determinant for anti-platelet GPIIIA of HIV-1-related immunologic thrombocytopenia. Proc Natl Acad Sci U S A. 1997 doi: 10.1073/pnas.94.14.7589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiener J., Kokotek D., Rosowski S., Lickert H., Meier M. Preparation of single- and double-oligonucleotide antibody conjugates and their application for protein analytics. Sci Rep. 2020 doi: 10.1038/s41598-020-58238-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iyekhoetin O.M., Nnanna I.I., Blessing O.I., Idowu B.O.O. Benin City; Eur J Sci Res: 2011. Antibiograms and mutagenicity evaluation of hospital wastewaters from University of Benin Teaching Hospital (UBTH) [Google Scholar]
- 30.George S., Xu Y., Rodger G., Morgan M., Sanderson N.D., Hoosdally S.J., et al. DNA thermo-protection facilitates whole-genome sequencing of mycobacteria direct from clinical samples. J Clin Microbiol. 2020 doi: 10.1128/JCM.00670-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ojha S.C., Yean Yean C., Ismail A., Banga Singh K.K. A pentaplex PCR assay for the detection and differentiation of Shigella species. Biomed Res Int. 2013 doi: 10.1155/2013/412370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo L., Overholser J., Darby H., Ede N.J., Kaumaya P.T.P. A newly discovered PD-L1 B-cell epitope peptide vaccine (PDL1-Vaxx) exhibits potent immune responses and effective anti-tumor immunity in multiple syngeneic mice models and (synergizes) in combination with a dual HER-2 B-cell vaccine (B-Vaxx) Oncoimmunology. 2022 doi: 10.1080/2162402X.2022.2127691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nahm M.H., Yu J., Weerts H.P., Wenzel H., Tamilselvi C.S., Chandrasekaran L., et al. Development, Interlaboratory Evaluations, and Application of a Simple. High-Throughput Shigella Serum Bactericidal Assay MSphere. 2018 doi: 10.1128/msphere.00146-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Floderus E., Pál T., Karlsson K., Lindberg A.A. Identification of Shigella and enteroinvasive Escherichia coli strains by a virulence-specific, monoclonal antibody-based enzyme immunoassay. Eur J Clin Microbiol Infect Dis. 1995 doi: 10.1007/BF02111868. [DOI] [PubMed] [Google Scholar]
- 35.Saleh A.A., Saad M.A., Ryan I., Amin M., Shindy M.I., Hassan W.A., et al. Safety and immunogenicity evaluation of inactivated whole-virus-SARS-COV-2 as emerging vaccine development in Egypt. Antib Ther. 2021 doi: 10.1093/abt/tbab012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ullah H., Mahmud S., Hossain M.J., Bin I.MS., Kibria K.M.K. Immunoinformatic identification of the epitope-based vaccine candidates from Maltoporin, FepA and OmpW of Shigella Spp, with molecular docking confirmation. Infect Genet Evol. 2021 doi: 10.1016/j.meegid.2021.105129. [DOI] [PubMed] [Google Scholar]
- 37.Bin I.MS., Miah M., Hossain M.E., Kibria K.M.K. A conserved multi-epitope-based vaccine designed by targeting hemagglutinin protein of highly pathogenic avian H5 influenza viruses. 3. Biotech. 2020 doi: 10.1007/s13205-020-02544-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chitradevi S.T.S., Kaur G., Sivaramakrishna U., Singh D., Bansal A. Development of recombinant vaccine candidate molecule against Shigella infection. Vaccine. 2016 doi: 10.1016/j.vaccine.2016.08.034. [DOI] [PubMed] [Google Scholar]
- 39.DeRoeck D., Clemens J.D., Nyamete A., Mahoney R.T. Policymakers’ views regarding the introduction of new-generation vaccines against typhoid fever, shigellosis and cholera in Asia. Vaccine. 2005 doi: 10.1016/j.vaccine.2004.11.044. [DOI] [PubMed] [Google Scholar]
- 40.Mey A.R., Wyckoff E.E., Oglesby A.G., Rab E., Taylor R.K., Payne S.M. Identification of the Vibrio cholerae enterobactin receptors VctA and IrgA: IrgA is not required for virulence. Infect Immun. 2002 doi: 10.1128/IAI.70.7.3419-3426.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Alwis R., Liang L., Taghavian O., Werner E., The H.C., Thu T.N.H., et al. The identification of novel immunogenic antigens as potential Shigella vaccine components. Genome Med. 2021 doi: 10.1186/s13073-020-00824-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kibria K.M.K., Faruque M.O., Islam M.S.b., Ullah H., Mahmud S., Miah M., et al. A conserved subunit vaccine designed against SARS-CoV-2 variants showed evidence in neutralizing the virus. Appl Microbiol Biotechnol. 2022 doi: 10.1007/s00253-022-11988-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berlanda Scorza F., Colucci A.M., Maggiore L., Sanzone S., Rossi O., Ferlenghi I., et al. High yield production process for Shigella outer membrane particles. PLoS One. 2012 doi: 10.1371/journal.pone.0035616. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data are available along with the manuscript.