Abstract
Biological oxidation of cyclic alcohols normally results in formation of the corresponding dicarboxylic acids, which are further metabolized and enter the central carbon metabolism in the cell. We isolated an Acinetobacter sp. from an industrial wastewater bioreactor that utilized cyclohexanol as a sole carbon source. A cosmid library was constructed from Acinetobacter sp. strain SE19, and oxidation of cyclohexanol to adipic acid was demonstrated in recombinant Escherichia coli carrying a SE19 DNA segment. A region that was essential for cyclohexanol oxidation was localized to a 14-kb fragment on the cosmid DNA. Several putative open reading frames (ORFs) that were expected to encode enzymes catalyzing the conversion of cyclohexanol to adipic acid were identified. Whereas one ORF showed high homology to cyclohexanone monooxygenase from Acinetobacter sp. strain NCIB 9871, most of the ORFs showed only moderate homology to proteins in GenBank. In order to assign functions of the various ORFs, in vitro transposon mutagenesis was performed using the cosmid DNA as a target. A set of transposon mutants with a single insertion in each of the ORFs was screened for cyclohexanol oxidation in E. coli. Several of the transposon mutants accumulated a variety of cyclohexanol oxidation intermediates. The in vitro transposon mutagenesis technique was shown to be a powerful tool for rapidly assigning gene functions to all ORFs in the pathway.
Microbes have the ability to use a variety of xenobiotic compounds and convert them to cellular metabolites. The enzymes that catalyze some of the degradative reactions in xenobiotic metabolism can potentially be used for biotransformations. For example, nitrile hydratase, which is involved in the degradation of acrylonitrile, is used for the industrial production of acrylamide (33). Similarly, some of the oxygenases that are involved in the degradation of xylene or styrene have been used for the synthesis of a wide range of chemicals (23). The genes involved in these degradative pathways often are physically linked and may be chromosomal or episomal in nature.
A number of microorganisms are capable of oxidizing cyclic alcohols to dicarboxylic acids. The biochemical metabolism of cyclohexane and cyclohexanol to adipic acid in organisms including Acinetobacter, Pseudomonas, and Xanthobacter sp. has been studied (9, 28, 29). The biochemical pathway for the conversion of cyclohexanol to adipic acid involves cyclohexanol → cyclohexanone → ɛ-caprolactone → 6-hydroxyhexanoic acid → 6-oxohexanoic acid → adipic acid (9). Some of the enzyme activities in this pathway have been demonstrated, including cyclohexanol dehydrogenase, NADPH-linked cyclohexanone monooxygenase, ɛ-caprolactone hydrolase, and NAD (NADP)-linked 6-hydroxyhexanoic acid dehydrogenase (28). The broad spectrum of activity of cyclohexanone monooxygenase has been extensively studied (4) and its gene sequence from Acinetobacter sp. strain NCIB 9871 has been reported (7). However, very little is known about other genes and the genetic organization of the cyclohexanol oxidative pathway. In this paper, we report the genetic analysis of a gene cluster involved in the degradation of cyclohexanol from Acinetobacter sp. strain SE19.
We have isolated a 14-kb DNA fragment from Acinetobacter sp. strain SE19 that is involved in cyclohexanol oxidation. A recombinant Escherichia coli containing the 14-kb gene cluster was able to convert cyclohexanol to adipic acid. The 14-kb gene cluster contains genes encoding two alcohol dehydrogenases, an aldehyde dehydrogenase, a monooxygenase, a hydrolase, and a transcriptional regulator. In vitro transposon mutagenesis was used to construct a series of random insertions in the 14-kb gene cluster. Biochemical characterization of the transposon mutants allowed us to identify the in vivo functions of these genes in E. coli.
MATERIALS AND METHODS
Strain isolation and 16S rDNA typing.
The bacterial strain that grows on cyclohexanol used in this study was isolated from an enrichment of activated sludge obtained from an industrial wastewater bioreactor. The enrichment culture was established by inoculating 1 ml of activated sludge into 20 ml of S12 medium (50 mM potassium phosphate buffer [pH 7.0], 10 mM ammonium sulfate, 2 mM MgCl2, 0.7 mM CaCl2, 50 μM MnCl2, 1 μM FeCl3, 1 μM ZnCl3, 1.72 μM CuSO4, 2.53 μM CoCl2, 2.42 μM Na2MoO2, 0.0001% FeSO4) in a 125-ml screw-cap Erlenmeyer flask. It was supplemented with 100-ppm cyclopentanol added directly to the culture medium and was incubated at 35°C with reciprocal shaking. This culture was maintained by adding 100-ppm cyclopentanol every 2 to 3 days. The culture was diluted every 2 to 10 days by replacing 10 ml of the culture with the same volume of S12 medium. After 15 days of incubation, serial dilutions of the enrichment culture were spread onto Luria-Bertani (LB) (27) agar plates. Single colonies were screened for the ability to grow on S12 liquid with cyclohexanol as the sole carbon and energy source. One of the isolates, SE19, was selected for further characterization.
The 16S rRNA genes of SE19 isolates were amplified by PCR (16) and analyzed as follows. Several colonies of SE19 from LB plates were suspended in 200 μl of lysis buffer (1% Triton X-100, 20 mM Tris [pH 8.5], 2 mM EDTA). The mixture was heated to 95°C for 10 min and then centrifuged to remove cellular debris. The 16S rRNA gene sequences in the supernatant were amplified by PCR with forward HK12 primer GAGTTTGATCCTGGCTCAG (complementary to positions 11 to 28 on the E. coli 16S rDNA) and reverse HK13 primer TACCTTGTTACGACTT (complementary to positions 1512 to 1496 on the E. coli 16S rDNA). The amplified 16S rDNA was purified using a QIAquick PCR purification kit according to the manufacturer's instructions (Qiagen, Valencia, Calif.) and sequenced on an automated ABI sequencer. The 16S rRNA gene sequence of each isolate was used as the query sequence for a BLASTN search (2).
Construction of Acinetobacter sp. strain SE19 cosmid library.
Acinetobacter sp. strain SE19 was grown in 25 ml of LB medium for 6 h at 37°C with aeration. Cells were pelleted by centrifugation and frozen at −80°C. Chromosomal DNA was prepared with special care taken to avoid shearing of DNA. The cell pellet was gently resuspended in 5 ml of 50 mM Tris–10 mM EDTA (pH 8), and lysozyme was added to a final concentration of 2 mg/ml. The suspension was incubated at 37°C for 1 h. Sodium dodecyl sulfate (SDS) was then added to a final concentration of 1%, and proteinase K was added at 100 μg/ml. The suspension was incubated at 55°C for 2 h. The clear lysate was extracted with an equal volume of phenol-chloroform-isoamyl alcohol (25:24:1). After the lysate was centrifuged at 17,000 × g for 20 min, the aqueous phase was carefully removed and transferred to a new tube. Two volumes of ethanol were added, and the DNA was gently spooled with a sealed glass Pasteur pipette. The DNA was dipped into a tube containing 70% ethanol. After being air dried, the DNA was resuspended in 400 μl of Tris-EDTA (10 mM Tris–1 mM EDTA, pH 8) with RNaseA (100 μg/ml) and stored at 4°C. The concentration and purity of DNA were determined spectrophotometrically by calculating the ratio of the optical density at 260 nm to that at 280 nm. A diluted aliquot of DNA was run on a 0.5% agarose gel to determine the intact nature of DNA.
A cosmid library of SE19 was constructed using SuperCos 1 cosmid vector kit (Stratagene, La Jolla, Calif.). The packaged Acinetobacter genomic DNA library contained a titer of 5.6 × 104 CFU per μg of DNA as determined by transfecting E. coli XL1BlueMR. Cosmid DNA was isolated from six randomly chosen E. coli transformants and found to contain large inserts of DNA (30 to 40 kb).
Screening of cosmids for the cyclohexanone monooxygenase gene.
The cosmid library of Acinetobacter sp. strain SE19 was screened by PCR based on the homology of the cyclohexanone monooxygenase gene. Two primers, monoL (GAGTCTGAGCATATGTCACAAAAAATGGATTTTG) and monoR (GAGTCTGAGGGATCCTTAGGCATTGGCAGGTTGCTTGAT), were designed based on the published sequence of the cyclohexanone monooxygenase gene of Acinetobacter sp. strain NCIB 9871 (7). PCR was performed in a Perkin-Elmer (Foster City, Calif.) GeneAmp9600. The cells were lysed by incubating them at 94°C for 5 min and then cycled 25 times at 92°C for 1 min, 50°C for 45 s, and 72°C for 1 min. Positive clones were characterized further as outlined below.
Sequencing the cosmid clones.
Cosmid clones were sequenced by a shotgun approach. Cosmid DNA was sheared in a Nebulizer (Inhalation Plastics Inc., Chicago, Ill.) at 20 lb/in2 for 45 s, and the 1- to 3-kb DNA fraction was excised from a 0.8% agarose gel. Purified DNA was treated with T4 DNA polymerase and T4 polynucleotide kinase by following the manufacturer's (GIBCO/BRL, Gaithersburg, Md.) instructions. The inserts were ligated to pUC18 vector using Ready-To-Go pUC18SmaI/BAP+ ligase (GIBCO/BRL). The ligated DNA was transformed into E. coli DH5α cells and plated on LB plates with ampicillin and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). The majority of transformants were white, and those containing inserts were sequenced with the standard M13 −20 forward primer and the M13 −48 reverse primer of pUC18. The segments were assembled using the Sequencher 3.0 program (Gene Codes Corporation, Ann Arbor, Mich.).
Southern hybridization.
Southern hybridization was used to map the ends of the cosmids. Cosmid DNA was digested with EcoRI, which has a recognition site in the insert in the cyclohexanone monooxygenase gene. The digest was separated on a 0.9% agarose gel and transferred to a PolyScreen polyvinylidene difluoride membrane (NEN Research Products, Boston, Mass.) by alkaline downward capillary blotting. The full-length cyclohexanone monooxygenase gene probe was prepared with a PCR DIG labeling kit (Boehringer Mannheim, Indianapolis, Ind.) using digoxigenin-labeled dexoynucleoside triphosphate. Hybridization was carried out overnight at 37°C in Easy Hyb solution (Boehringer Mannheim). The blot was washed once with 2× SSC (1× SSC is 0.15 M NaCl plus 0.15 M sodium citrate)–0.1% SDS and once with 0.1× SSC–0.1% SDS and developed with a DIG luminescence detection kit (Boehringer Mannheim).
In vitro transposon mutagenesis.
In vitro transposon mutagenesis was performed with 8F6 cosmid DNA using the Primer Island transposition kit (Perkin-Elmer) by following the manufacturer's instructions. The mutagenized DNA was electroporated into E. coli DH10B and plated onto LB plates with ampicillin (100 μg/ml) and trimethoprim (100 μg/ml). The insertion sites of the AT2 transposon in the isolated mutants were identified by direct sequencing of the cosmid DNA prepared from the mutants using PI(+) primer to the end of the AT2 transposon. The sequences from the transposon mutants were aligned with the 17,417-bp contig or the SuperCos1 vector sequence by the FASTA program (1) to identify the transposon insertion sites. Sequence data obtained from mapping the insertions were also used to confirm initial results of shotgun library sequencing and sequence assembly.
Identification of adipic acid production.
E. coli cells carrying cosmids (E. coli cosmid clones) containing the gene cluster were grown in M9 minimal medium (27) supplemented with 0.4% glucose as the carbon source. Cells were grown at 30°C with shaking for 2 h and 330-ppm cyclohexanol was added. Cells were further incubated at 30°C, and aliquots were taken at 2, 4, and 20 h after addition of cyclohexanol. A control culture consisting of the host strains transformed only with the SuperCos1 vector was grown under the same conditions. Samples were frozen at −80°C and thawed at 37°C. Cells were pelleted and supernatants were passed through 0.22-μm-pore-size Acrodisc liquid chromatography (LC) polyvinylidene difluoride syringe filters (Gelman Sciences, Ann Arbor, Mich.). The filtered supernatants were analyzed by high pressure liquid chromatography (HPLC) and LC/mass spectrometry (MS).
The HPLC system used was a Hewlett Packard 1100 series with photodiode array detector. An HPLC organic acid analysis column (Aminex HPX-87H ion exclusion column; 300 by 7.8 mm) was purchased from Bio-Rad Laboratories (Hercules, Calif.). The column temperature was controlled at 40°C. The mobile phase was 0.004 M sulfuric acid at a flow rate of 0.6 ml/min. Samples (100 μl) were injected, and 210 nm was used for detection. Standard samples were prepared with known amounts of adipic acid (Sigma, St. Louis, Mo.) in the medium ranging from 25 to 1,000 ppm. The retention time of adipic acid produced in metabolite samples was identical to that of the standard. Diluted metabolite samples were also spiked with 100-ppm adipic acid standard. The peak of the adipic acid standard was superimposed on the adipic acid peak in the samples.
Electrospray LC/MS analysis was used to confirm the presence of adipic acid in the samples. For these analyses, a reverse-phase HPLC method with a Prodigy C18 column (Phenomenex; 2.0 by 250 mm, 5-μm particles) and an acetonitrile–0.5% (vol/vol) aqueous acetic acid mobile phase was used. The HPLC/MS instrument couples a Hewlett-Packard 1100 liquid chromatograph to a Finnigan TSQ-700 mass spectrometer equipped with electrospray interface. The flow rate was 0.25 ml/min, with a postcolumn 50:1 splitter yielding an ultimate flow to the mass spectrometer of 5 μl/min. Electrospray mass spectra were obtained in the negative-ion detection mode. The negative-ion electrospray mass spectrum of adipic acid has a base ion appearing at 145 Da, which corresponds to [MW-H]− (where the molecular weight [MW] = 146 for adipic acid). Confirmation of adipic acid in samples required agreement with the peak retention time and mass spectrum for the adipic acid standard.
Identification of intermediates of cyclohexanol oxidation.
Cosmid DNA prepared from mutant strains was transformed into XL1BlueMR cells by electroporation. Adipic acid production by the mutants was assayed by HPLC as described above. The samples were also concentrated for gas chromatography (GC)/MS analysis for detection of all metabolites of cyclohexanol. Samples were acidified to pH 2 upon addition of HCl. The acidified samples were extracted twice with methylene chloride. The combined methylene chloride extracts were dried with anhydrous magnesium sulfate, and filtered extracts were evaporated to dryness at room temperature under a gentle stream of nitrogen. The samples were analyzed using two methods. The first method analyzes underivatized samples using a “polar” FFAP capillary GC column (50 by 0.32 mm; 0.52-μm film thickness; Hewlett-Packard). The extraction residues were dissolved in 1 ml of methylene chloride and analyzed by high-sensitivity splitless injection GC/MS using the FFAP column for the separation. The FFAP column has been found to provide good chromatographic efficiencies for the separation of monocarboxylic acids and omega-substituted carboxylic acids (e.g., hydroxy acids). The second method analyzes derivatized samples using a nonpolar column. The extraction residues were derivatized with 0.5 ml of BSTFA [bis(trimethylsilyl)trifluoroacetamide] silylation reagent (SUPELCO, Bellefonte, Pa.) and analyzed by split injection GC/MS using a 30-m MDN-5S capillary column (SUPELCO; 0.5-μm film thickness) for the separation. Derivatization with BSTFA reagent has been found to be a valid method for detection of polar compounds such as trimethylsilyl esters and/or ethers.
Nucleotide sequence accession number.
The nucleotide sequence reported in the paper has been deposited in GenBank under accession no. AF282240.
RESULTS
Isolation and growth characteristics of Acinetobacter sp. strain SE19.
An enrichment culture of bacteria that degrade cyclopentanol was established using sludge from an industrial aerobic wastewater bioreactor. Three strains were isolated from the cyclopentanol enrichment by nonselective plating on LB plates. Two isolates were able to grow on both cyclopentanol and cyclohexanol. Analysis of the 1.5-kb 16S rRNA gene sequences from the two strains indicated that they belonged to the genera Acinetobacter and Rhodococcus. We selected Acinetobacter sp. strain SE19 for further characterization. Strain SE19 was phylogenetically related to Acinetobacter haemolyticus (99% identity) and Acinetobacter junii (99% identity) based on 16S rRNA sequence analysis.
Although originally isolated from a cyclopentanol enrichment culture, strain SE19 grew better on cyclohexanol than on cyclopentanol (Table 1). The strain was unable to grow on the cyclic alkanes tested ranging from six to eight member rings. The ability of SE19 to grow on cyclic alcohols or ketones was dependent on the ring size, with six-member rings as the preferred substrates. Genes in the cyclohexanol oxidation pathway in SE19 are inducible by cyclohexanone as indicated by oxygen uptake assays (5, 32) (data not shown).
TABLE 1.
Growth substrates of Acinetobacter sp. strain SE19
| Carbon sourcea | Growthb |
|---|---|
| Cyclopentanol | + |
| Cyclopentanone | + |
| 2-Methylcyclopentanone | − |
| Valerolactone | + |
| Cyclohexane | − |
| Methylcyclohexane | − |
| Cyclohexene | − |
| Cyclohexanol | ++ |
| Cyclohexanone | ++ |
| Cyclohex-2-ene-1-one | + |
| 2-Methylcyclohexanone | + |
| Caprolactone | ++ |
| Cycloheptane | − |
| Cycloheptanol | − |
| Cycloheptanone | − |
| Cyclooctane | − |
| Cyclooctanol | − |
| Cyclooctanone | − |
| No carbon source | − |
Growth was examined on S12 plates with the indicated compound as the sole carbon and energy source. The compound was supplied as a vapor by placing 5 μl of the volatile compound (Sigma) on the interior of the petri dish lid. The petri dish was sealed with Parafilm and incubated with the compound on the lid.
++, strong growth; +, weak growth; −, no growth.
Identification and characterization of cyclohexanol oxidation genes.
The cosmid library of Acinetobacter sp. strain SE19 was screened based on the homology of the cyclohexanone monooxygenase gene. Five positive clones (5B12, 5F5, 8F6, 14B3, and 14D7) were identified among the 960 clones screened. These cosmids contained inserts of 35 to 40 kb that were homologous to the cyclohexanone monooxygenase gene from Acinetobacter sp. strain NCIB 9871 (7). Shotgun libraries of 5B12 and 8F6 were constructed, and sequences of 200 to 300 clones from each library were assembled into a contig of 17,417 bp containing the cyclohexanone monooxygenase gene. The sequence assembly was confirmed by restriction mapping and direct sequencing of regions from the cosmid DNA template.
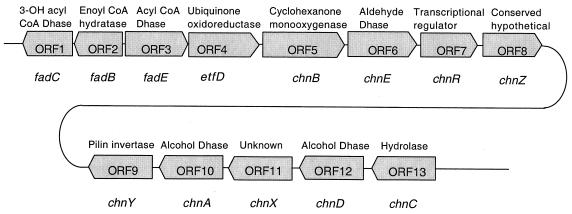
The DNA sequences were translated in all reading frames, and the products were compared for similarity to all publicly available protein sequences contained in the nonredundant database using the BLASTX algorithm (2). Thirteen open reading frames (ORFs) (ORFs 1 to 13; Fig. 1) were identified (12) on the 17-kb gene cluster. The homology search results for each ORF are shown in Table 2. Based on the BLAST results, it was determined that the five enzymes required for oxidation of cyclohexanol to adipic acid were most likely encoded by ORF5, ORF6, ORF10, ORF 12, and ORF13 (Table 2). ORF5 (chnB, encoding a monooxygenase) had the greatest homology with the ORF encoding cyclohexanone monooxygenase from Acinetobacter sp. strain NCIB 9871 (7). ORF6 (chnE, encoding an aldehyde dehydrogenase) had the greatest homology with the ORF encoding toluenesulfonate aldehyde dehydrogenase from Comamonas testosteroni (15). ORF10 (chnA, encoding an alcohol dehydrogenase) had the greatest homology with the ORF encoding 2,5-dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase from Sphingomonas paucimobilis (22). ORF12 (chnD, encoding an NAD-dependent alcohol dehydrogenase) had the greatest homology with the ORF encoding the NAD-dependent alcohol dehydrogenase from Sulfolobus solfataricus (3). ORF13 (chnC, encoding a hydrolase) had the greatest homology with the ORF encoding acetyl hydrolase from Streptomyces hygroscopicus (25).
FIG. 1.
Gene organization of the 17-kb cluster required for conversion of cyclohexanol to adipic acid in Acinetobacter sp. strain SE19. Thirteen ORFs (ORF1 to ORF13) were identified on the cluster. The exact locations of the ORFs in reference to the 17,417-bp-contig are listed in Table 2. ORF8 and ORF9 are contiguous, and the curved line between them is solely for the purpose of the diagram. The block arrows indicate direction of transcription of the genes, whose designations are listed below the ORFs. The protein homologous to the product of each ORF identified from the BLAST search is shown above the ORF. Dhase, dehydrogenase; CoA, coenzyme A.
TABLE 2.
Homology of the ORFs with proteins in the nonredundant protein databasesd
| ORF | Gene name | Locatione (bp) | Homologous protein (source species) | % Identitya | % Similarityb | E valuec |
|---|---|---|---|---|---|---|
| 1 | fadC | 726–184 | 3-Hydroxybutyryl-CoAf dehydrogenase (Bacillus subtilis) | 36 | 51 | 4e − 39 |
| 2 | fadB | 1506–730 | Enoyl-CoA dehydratase (Pseudomonas putida) | 48 | 64 | 1e − 60 |
| 3 | fadE | 1777–2931 | Acyl-CoA dehydrogenase (Bacillus subtilis) | 42 | 59 | 2e − 77 |
| 4 | etfD | 3132–4850 | Ubiquinone oxidoreductase (Acinetobacter calcoaceticus) | 91 | 95 | 0.0 |
| 5 | chnB | 5318–6949 | Cyclohexanone monooxygenase (Acinetobacter sp.) | 99 | 99 | 0.0 |
| 6 | chnE | 6954–8450 | Toluenesulfonate aldehyde dehydrogenase (Comamonas testosteroni) | 38 | 57 | 1e − 105 |
| 7 | chnR | 8512–9453 | AraC-like transcriptional regulator (Bacillus subtilis) | 34 | 54 | 7e − 10 |
| 8 | chnZ | 9775–10434 | Conserved hypothetical protein 1 (Actinobacillus pleuropneumoniae) | 75 | 85 | 2e − 99 |
| 9 | chnY | 11575–10601 | Pilin gene inverting protein (Moraxella bovis) | 29 | 48 | 2e − 37 |
| 10 | chnA | 12690–11935 | 2,5-Dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase (Sphingomonas paucimobilis) | 41 | 58 | 2e − 47 |
| 11 | chnX | 14015–13152 | LimA (Dictyostelium discoideum) | 0.26 | ||
| 12 | chnD | 15208–14150 | NAD-dependent alcohol dehydrogenase (Sulfolobus solfataricus) | 32 | 52 | 1e − 60 |
| 13 | chnC | 16201–15299 | Acetyl-hydrolase (Streptomyces hygroscopicus) | 36 | 51 | 2e − 32 |
% Identity, percentage of amino acids that are identical between the two proteins.
% Similarity, percentage of amino acids that are identical or conserved between the two proteins.
Expect value, which estimates the statistical significance of the match by specifying the number of matches, with a given score, that are expected in a search of a database of this size absolutely by chance.
Homology search was performed by the BLASTX algorithm provided by the National Center for Biotechnology Information prior to the deposit of AB006902 (12).
Location in reference to the 17,417-bp contig. The first ATG or GTG was chosen as the start codon of the putative proteins.
CoA, coenzyme A.
The monooxygenase ChnB presumably catalyzes the conversion of cyclohexanone to caprolactone. The aldehyde dehydrogenase ChnE presumably catalyzes the conversion of 6-oxohexanoic acid to adipic acid. The two alcohol dehydrogenases ChnA and ChnD belonged to two different families of dehydrogenases. ChnA had 251 amino acid residues and belonged to short-chain zinc-independent alcohol dehydrogenases (17, 26). ChnD had 353 amino acid residues and belonged to zinc-dependent long-chain alcohol dehydrogenases (26). ChnD also had high homology (41% identity and 54% similarity) to alcohol dehydrogenase HCADH from Arthrobacter oxydans (8), which is not in the current BLAST database, whereas ChnA had no significant homology to HCADH. HCADH was reported (8) to catalyze the conversion of 6-hydroxyhexanoic acid to 6-oxohexanoic acid in Arthrobacter oxydans. Therefore, we postulate that ChnD is a homolog of HCADH and catalyzes the conversion of 6-hydroxyhexanoic acid to 6-oxohexanoic acid in Acinetobacter sp. strain SE19. ChnA is proposed to catalyze the conversion of cyclohexanol to cyclohexanone. The hydrolase ChnC presumably catalyzes the conversion of caprolactone to 6-hydroxyhexanoic acid.
Conversion of cyclohexanol to adipic acid by E. coli cosmid clones.
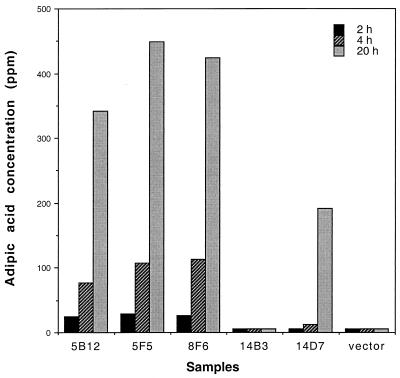
Five E. coli cosmid clones containing the gene cluster were assayed for adipic acid production. Cells were grown in M9-glucose medium with cyclohexanol as described in Materials and Methods. Four out of five cosmid clones (5B12, 5F5, 8F6, and 14D7) produced adipic acid. Adipic acid was detected after 2 h of incubation and had increased more than 10-fold by 20 h (Fig. 2). The adipic acid concentration in the cultures with positive clones ranged from 200 to 400 ppm based on HPLC/UV (210 nm) analysis. The identity of adipic acid was further confirmed by electrospray HPLC/MS analysis. No adipic acid was detected in the 14B3 strain and in the SuperCos1 vector control strain. Southern analysis revealed that rearrangement of Acinetobacter chromosomal DNA adjacent to the monooxygenase gene in cosmid 14B3 had occurred (data not shown). Adipic acid was also produced when cyclohexanone or ɛ-caprolactone was used as the substrate for the E. coli strain containing cosmid 8F6 (data not shown).
FIG. 2.
Adipic acid production from recombinant E. coli strains. The x axis represents samples prepared from E. coli XL1BlueMR containing different cosmids. Samples were taken after 2, 4, or 24 h of incubation following addition of cyclohexanol. The y axis represents the amount of adipic acid produced from each sample, as determined by HPLC.
Localization of the region essential for adipic acid production from the cosmids.
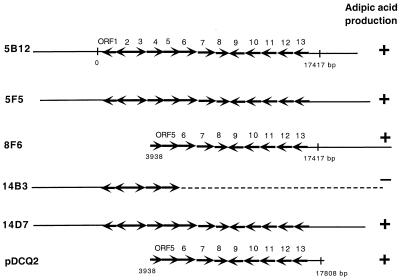
Southern hybridization results (data not shown) indicated that the cosmid clone 5B12 had about 20 kb upstream of the monooxygenase gene and that cosmid clone 8F6 had about 30 kb downstream of the monooxygenase gene. That the flanking region of the monooxygenase gene was essential for adipic acid production was suggested by the fact that cosmid clone 14B3 failed to produce adipic acid due to DNA rearrangement adjacent to the monooxygenase gene.
Sequencing the left junction of cosmid 8F6 identified that the insert starts at bp 3938, which is in the middle of the ORF4 spanning from bp 3132 to 4850 (Fig. 3). The region spanning ORF1, ORF 2, ORF3, and the N-terminal-coding portion of ORF4 is not present on 8F6. The ability of cosmid 8F6 to convert cyclohexanol to adipic acid indicated that ORF1, ORF2, ORF3, and ORF4 were not essential for adipic acid production. Subclone pDCQ2 was constructed from cosmid 8F6 to localize the essential region of the 35-kb insert on 8F6. A restriction map of 8F6 indicated a BglII site less than 1 kb away from the 3′ end of the sequenced 17-kb contig. The 8F6 cosmid DNA was digested with NotI and BglII, and the 14-kb fragment containing ORF5 to ORF13 was ligated with pBluescript SK(+) vector treated with NotI and BamHI. The resulting clone, designated pDCQ2 (Fig. 3), was transformed into an XL1BlueMR host. The HPLC results showed that the E. coli host containing pDCQ2 converted cyclohexanol to adipic acid, whereas the pBluescript vector control did not. These results demonstrated that the 14-kb fragment on pDCQ2 was sufficient for conversion of cyclohexanol to adipic acid. Sequencing pDCQ2 using the outward primer to the right end of the original 17-kb contig and the primer to the vector extends our DNA sequence to the BglII site (bp 17808), which was only 391 bp away from the original end of the 17,417-bp contig. No additional ORF was identified at the end of the contig. Therefore, ORFs 5 to 13 or a subset of ORFs 5 to 13 on the insert of pDCQ2 (from bp 3938 to 17808) are sufficient for conversion of cyclohexanol to adipic acid.
FIG. 3.
Localization of the region essential for adipic acid production. Solid horizontal lines, spanning of the Acinetobacter chromosomal DNA on each of the cosmid clones or the subclone; dashed line, region of the rearranged chromosomal DNA; region with arrows, 13 ORFs identified on the 17,417-bp cluster. The numbers above the arrows correspond to ORFs in Table 2. The vertical lines represent the beginning (0) and the end (17417 bp) of the reported cluster. ORF4 was truncated on 8F6 and pDCQ2 and started at bp 3938. The 3′ end of the insert on pDCQ2 was at bp 17808, which was 391 bp downstream of the reported 17,417-bp cluster. +, production of adipic acid; −, no production of adipic acid.
Functional analysis of the genes contained in the localized region essential for adipic acid production.
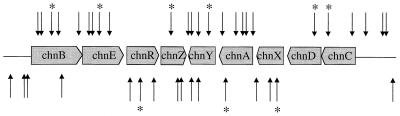
In vitro transposon mutagenesis was used to confirm putative functions of the genes involved in conversion of cyclohexanol to adipic acid. A collection of mutants was generated by mutagenizing cosmid 8F6 DNA, and insertion sites in 120 mutants were mapped by sequencing the transposon junctions in the mutants. Forty-one insertions that mapped within the 14-kb fragment are shown in Fig. 4. Other insertions occurred either on the SuperCos1 vector or on another part of the insert on 8F6. The number of insertions obtained within the 14-kb fragment was proportional to the length of the fragment. One insertion mutant of each ORF was selected for further phenotypic characterization. To determine which of the genes are required for conversion of cyclohexanol to adipic acid, we first assayed the mutants for adipic acid production using HPLC/UV (210 nm) analysis. Results are summarized in Table 3. Mutants with insertions in the conserved hypothetical protein ChnZ, pilin invertase ChnY, or an unknown protein, ChnX, still produced adipic acid at levels comparable to that at which it was produced by mutant 8F6ty25, which had an insertion on the SuperCos1 vector. These data suggest that ChnZ, ChnY, and ChnX were not required for adipic acid production. Mutants with insertions in cyclohexanone monooxygenase ChnB, alcohol dehydrogenase ChnA, NAD-dependent alcohol dehydrogenase ChnD, hydrolase ChnC, or transcriptional activator ChnR no longer produced adipic acid, suggesting that the corresponding genes were needed for adipic acid production from cyclohexanol. The 8F6ty9 mutant, with an insertion in the aldehyde dehydrogenase ChnE, produced about 30% as much adipic acid as the 8F6ty25 control. The low level of adipic acid produced suggested that ChnE was involved in adipic acid production; however, loss of its activity in the 8F6ty9 mutant may be compensated by an endogenous dehydrogenase(s) in E. coli.
FIG. 4.
Mapping of the transposon insertions on cosmid 8F6 DNA. Boxes, genes organized on the 14-kb cluster that encode enzymes for adipic acid production; space between the boxes, intergenic region; line, upstream and downstream regions of the genes on the 14-kb cluster in 8F6; horizontal arrows, direction of transcription of the genes; vertical arrows, approximate locations of the transposon insertion sites obtained within the 14-kb region mapped by sequencing 120 mutants. Other insertions occurred either on the SuperCos1 vector or on the insert outside of the 14-kb region. The arrows above the genes represent the transposon insertions where the left end of the transposon was adjacent to the 5′ ends of the genes; the arrows below the genes represent transposon insertions in the opposite orientation. ∗, mutant representatives chosen for further characterization, as listed in Table 3.
TABLE 3.
Characterization of the representatives of transposon mutants
| Cosmid | Insertion sitea (bp) | Mutated gene | Adipic acid productionb | Major metabolitec | Function of mutated gened |
|---|---|---|---|---|---|
| 8F6 parent | None | None | + | Adipic acid | N/A |
| 8F6ty1 | 8925 | chnR | − | Cyclohexanol | Transcriptional activator |
| 8F6ty2 | 11990 | chnA | − | Cyclohexanol | Cyclohexanol dehydrogenase |
| 8F6ty9 | 7370 | chnE | +/− | 6-hydroxy hexanoic acid | 6-Oxohexanoic acid dehydrogenase |
| 8F6ty18 | 6070 | chnB | − | Cyclohexanone | Cyclohexanone monooxygenase |
| 8F6ty24 | 15510 | chnC | − | Caprolactone | Caprolactone hydrolase |
| 8F6ty25 | Vector | Vector | + | Adipic acid | Not essential |
| 8F6ty26 | 10030 | chnZ | + | Adipic acid | Not essential |
| 8F6ty29 | 11390 | chnY | + | Adipic acid | Not essential |
| 8F6ty38 | 13950 | chnX | + | Adipic acid | Not essential |
| 8F6ty95 | 15180 | chnD | − | 6-hydroxy hexanoic acid | 6-Hydroxyhexanoic acid dehydrogenase |
| Vector | None | None | − | Cyclohexanol | N/A |
Location in reference to the 17,417-bp contig.
Conversion of cyclohexanol to adipic acid was assayed by HPLC. +, production; −, no production; ±, decreased production.
Metabolites of cyclohexanol were detected by GC/MS.
N/A, does not contain mutated gene; not essential, not required for conversion of cyclohexanol to adipic acid.
To further identify the biochemical roles of ChnB, ChnE, ChnA, ChnD, ChnC, and ChnR, supernatants from cultures of these mutants were analyzed for cyclohexanol metabolites by GC/MS analysis (Table 3).
(i) ChnB.
ChnB has high homology to a known cyclohexanone monooxygenase from Acinetobacter sp. strain NCIB 9871 (7, 30). Accumulation of cyclohexanone as the major intermediate in the ChnB insertion mutant also confirmed that the enzymatic reaction catalyzed by the cyclohexanone monooxygenase was blocked in the ChnB mutant. Therefore, chnB encodes the cyclohexanone monooxygenase that is responsible for the conversion of cyclohexanone to caprolactone.
(ii) ChnE.
ChnE has high homology to other aldehyde dehydrogenases in GenBank. chnE is likely to encode 6-oxohexanoic acid dehydrogenase, which catalyzes the conversion of 6-hydroxyhexanoic acid to 6-oxohexanoic acid. 6-Hydroxyhexanoic acid was detected as the major intermediate accumulated in the ChnE mutant. Adipic acid was also produced at 30% of the wild-type level. The inability to detect free 6-oxohexanoic acid in the ChnE mutant could be due to several possible reasons including both biological and chemical factors (more details in Discussion).
(iii) ChnA.
ChnA has high homology to a class of short-chain zinc-independent alcohol dehydrogenases (17, 26). GC/MS analysis (Table 3) showed that cyclohexanol was detected as the major intermediate accumulated in the ChnA mutant. This suggested that the enzymatic reaction catalyzed by cyclohexanol dehydrogenase was blocked in the ChnA mutant. Therefore, chnA encodes the cyclohexanol dehydrogenase that is responsible for the conversion of cyclohexanol to cyclohexanone.
(iv) ChnD.
ChnD has high homology to a class of zinc-dependent long-chain alcohol dehydrogenases (26) such as HCADH of Arthrobacter. HCADH has been shown to be a 6-hydroxyhexanoic acid (6-hydroxycaproic acid) dehydrogenase in Arthrobacter (8). GC/MS analysis (Table 3) showed that 6-hydroxyhexanoic acid accumulated in the ChnD mutant. This suggested that the enzymatic reaction catalyzed by 6-hydroxyhexanoic acid dehydrogenase was blocked in the ChnD mutant.
(v) ChnC.
ChnC has high homology to known hydrolases. Accumulation of caprolactone in the ChnC mutant suggested that hydrolysis of caprolactone was blocked in the mutant. Therefore, chnC encodes the caprolactone hydrolase that is responsible for the conversion of caprolactone to 6-hydroxyhexanoic acid.
(vi) ChnR.
ChnR has homology to AraC-like transcriptional regulators (11, 18). Cyclohexanol accumulated in the ChnR mutant, although it had a wild-type copy of the chnA gene encoding the catalytic enzyme cyclohexanol dehydrogenase. This suggested that the chnA gene could not be turned on in the ChnR null mutant, and the cyclohexanol substrate was not used. Therefore, chnR is likely to encode a transcriptional activator that is involved in the induction of the cyclohexanol oxidation pathway and is needed for the expression of chnA and possibly another gene(s) in the pathway.
DISCUSSION
In this paper, we report the first gene cluster encoding all the required enzymes for conversion of cyclohexanol to adipic acid. Since many genes of metabolic pathways are often physically linked, a cosmid library approach was used to clone the genes involved in cyclohexanol oxidation from Acinetobacter sp. strain SE19. Nine full-length genes residing on the 14-kb insert conferred on E. coli the ability to convert cyclohexanol to adipic acid. These nine genes were arranged in two sets. One set contained the first four genes transcribed in the same direction, and the other set contained the remaining five genes transcribed in the opposite direction. The intergenic regions of the genes ranged from a few base pairs to a few hundred base pairs. These two divergent sets of genes were separated by an ORF with homology to that encoding a pilin invertase (10), which is involved in inverting the orientation of adjacent DNA during antigenic variation of pilins in Moraxella bovis. It is conceivable that the ancestral forms of chn genes were organized in the same orientation and that the putative invertase might have been responsible for the change of orientation of one set of genes during evolution. The organization of the cyclohexanol oxidative genes on the 14-kb sequence in Acinetobacter sp. strain SE19 differs from the gene organization in Arthrobacter oxydans (8). In Arthrobacter, only three genes in the cyclohexanol oxidation pathway have been cloned. The 6-hydroxyhexanoic acid dehydrogenase (HCADH) gene was divergently transcribed from the caprolactone hydrolase (CLH) gene. The cyclohexanone monooxygenase gene was downstream of the CLH gene.
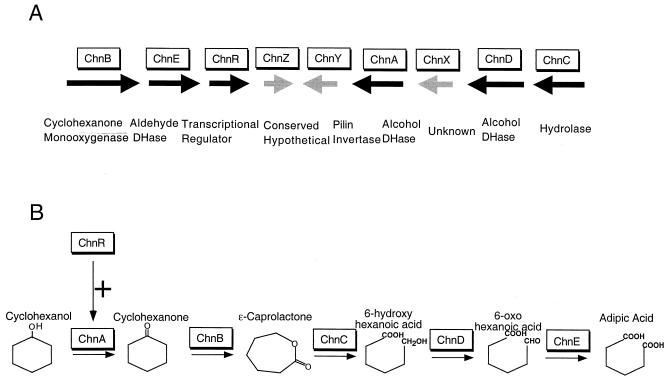
The function of the genes on the 14-kb fragment was determined by in vitro transposon mutagenesis. This high-throughput genetic technique can be easily applied to rapidly assigning functions to genes of any metabolic pathway. The functions of all nine genes involved in the conversion of cyclohexanol to adipic acid were determined simultaneously in one experiment that generated a library of mutants with a single insertion in each gene. The representative insertion mutants were characterized by GC/MS analysis of the accumulated intermediates in the mutants. Three of the genes (chnZ, chnY, and chnX) were not involved in the conversion of cyclohexanol to adipic acid. Six of the genes (chnB, chnE, chnA, chnD, chnC, and chnR) are required for adipic acid formation. Among the six essential genes, chnR encodes a transcriptional activator required for the induction of the cyclohexanol oxidation pathway. The other five genes encode enzymes for the five steps leading from cyclohexanol to adipic acid (Fig. 5). Two of them, ChnA and ChnD, were putatively identified as alcohol dehydrogenases. The in vivo substrate specificity of ChnA and ChnD was unambiguously determined by the differently accumulated intermediates in the ChnA and ChnD mutants. ChnA is the cyclohexanol dehydrogenase, since cyclohexanol accumulated in the ChnA mutant. ChnD is the 6-hydroxyhexanoic acid dehydrogenase, since 6-hydroxyhexanoic acid accumulated in the ChnD mutant.
FIG. 5.
Functional assignment of genes in the cyclohexanol oxidation pathway. (A) Identification of the essential genes for conversion of cyclohexanol to adipic acid. The genetic organization of the nine genes on the 14-kb insert of pDCQ2 is illustrated. Black arrows, six genes shown to be essential for conversion of cyclohexanol to adipic acid; gray arrows, three genes that are not essential for the conversion. Direction of arrows indicates direction of transcription of the genes. Enzymes encoded by the genes are in boxes above the corresponding genes. (B) Assignment of the biochemical function of the six genes essential for conversion of cyclohexanol to adipic acid. Five of the essential genes encode enzymes, and one encodes a regulatory protein. The five steps of the reactions are represented by horizontal arrows. The enzymes (boxes) are assigned to each required reaction based on GC/MS results (Table 3). The vertical arrow with the plus sign represents positive regulation of the first step of the reaction by the transcriptional regulator ChnR.
The genetic approach used in the present study for assigning gene function in vivo allowed us to gain insights into function, specificity, and regulation of genes, bypassing the need for expression, purification, and assay development for each individual protein. Overproduction of recombinant proteins followed by biochemical characterization is often labor-intensive. Some proteins are not expressed in soluble forms in E. coli. In addition, in vitro enzymatic assay conditions, such as the substrate concentration and substrate competition, may not mimic the conditions in vivo. The in vitro transposition and high-throughput phenotype analysis combine gene identification and functional confirmation in a single step, speeding up the gene discovery process.
In most insertion mutants, the pathway intermediate that accumulated was the compound prior to the step blocked by the transposon insertion. One exception was the ChnE mutant, in which no free 6-oxohexanoic acid was detected. Biologically produced aldehydes are unlikely to accumulate inside the cell due to their toxicity and chemical reactivity. The aldehyde dehydrogenases usually prevent toxicity of reactive aldehydes that formed as intermediates (14). In the ChnE mutant, the aldehyde dehydrogenase was disrupted and oxidation of 6-oxohexanoic acid to adipic acid would have been blocked; however, 6-oxohexanoic acid did not accumulate in the ChnE mutant. There are two possible detoxification mechanisms the cells could use in this case. First, a host aldehyde dehydrogenase could partially compensate the ChnE mutation, as suggested by the adipic acid level of about 30% detected in the ChnE mutant. A similar observation was reported by Cho et al. (8). Expression of the Arthrobacter 6-hydroxyhexanoic acid dehydrogenase (ChnD homolog) in E. coli was sufficient to convert 6-hydroxyhexanoic acid to adipic acid, suggesting that an endogenous E. coli aldehyde dehydrogenase enzyme was able to carry out the second step of converting 6-oxohexanoic acid to adipic acid. Second, the reaction catalyzed by the alcohol dehydrogenase might be reversible. The ready reversibility of the reaction catalyzed by benzyl alcohol dehydrogenase in Acinetobacter calcoaceticus has been reported (19). Without the oxidation of 6-oxohexanoic acid to adipic acid by the aldehyde dehydrogenase in the ChnE mutant, the equilibrium for the conversion of 6-hydroxyhexanoic acid to 6-oxohexanoic acid could be shifted to favor the reverse reaction. 6-Hydroxyhexanoic acid was thus detected as the major intermediate that accumulated in the ChnE mutant. In addition to enzymatic reactions to minimize its accumulation, the aldehyde 6-oxohexanoic acid is highly reactive and chemically unstable. Researchers have used hydrazine (aromatic amines) to trap the benzaldehyde for the enzymatic assay of benzyl alcohol dehydrogenase (19). Since aldehydes are known to react with amines, the small amount of 6-oxohexanoic acid that accumulated in the ChnE mutant might react with cellular amines such as amino acids and proteins. These mechanisms are most likely the reasons that free 6-oxohexanoic acid was not detected in the ChnE mutant, and this finding was consistent with the observation of a small amount of adipic acid in the ChnE mutant.
Cyclohexanol was added to the culture medium as the substrate for the cloned pathway. There are three scenarios to explain why a mutant strain could not convert cyclohexanol. The first is a mutation in a transport protein blocking substrate uptake. The second is a mutation in a positive regulator blocking pathway induction. The third is a mutation in the enzyme blocking the first step of the catalytic reactions. Cyclohexanol was detected as the major intermediate in two of our mutants, the ChnR and ChnA mutants. The ChnR mutant appears to fit the second scenario since ChnR has homology to the AraC/XylS family of transcriptional regulators. The ChnA mutant appears to fit the third scenario since ChnA has homology to alcohol dehydrogenases. A gene encoding a putative transport protein was not identified on the 17-kb gene cluster. It is likely that a specialized transport protein is not required for the uptake of cyclohexanol in Acinetobacter as cyclohexanol could diffuse into E. coli to produce adipic acid.
At the late stage of our manuscript preparation, identification of two new genes involved in cyclohexanol oxidation from Acinetobacter sp. strain NCIB 9871 was published (13). The reported 6.3-kb sequence contains the previously determined cyclohexanone monooxygenase gene (7) and two new downstream genes (chnE and chnR) encoding a 6-oxohexanoate dehydrogenase and a transcriptional activator. The genetic organization of the three genes chnBER is identical to the organization of the corresponding genes in our 17-kb cluster. The reported two new genes were 99 and 100% identical to two of the genes in our 17-kb gene cluster. Iwaki and colleagues expressed ChnE and ChnR individually in E. coli. The 6-oxohexanoate dehydrogenase activity of ChnE was confirmed with the crude protein extract (13). The activity of the transcriptional activator of ChnR was suggested by induction of a lacZ transcriptional fusion to the monooxygenase gene chnB and was shown to be required for the expression of the cyclohexanone monooxygenase gene (13). Our experimental evidence agrees with the role of ChnR as a transcriptional activator. Furthermore, our results show that the cyclohexanol dehydrogenase (ChnA) gene is also regulated by ChnR. Since ChnR regulates both chnA and chnB, the upstream regions of these two genes were compared. A stretch of eleven nucleotides, TTGTTTGGATC, was found to be identical within the upstream intergenic region of chnA and chnB. It is approximately 200 bp upstream of chnA and 390 bp upstream of chnB. It is not present upstream of chnC, encoding the caprolactone hydrolase. Whether the stretch of conserved nucleotides is involved in the binding of the activator ChnR remains to be tested. A comparison of the reported sequence (accession no. AB006902) with our 17,417-bp sequence indicated an over 99% nucleotide identity over the entire region (bp 1 to 6330 of their deposited sequence matched with bp 4781 to 11108 of our sequence). The minor differences could be due to sequencing error or strain variation between NCIB 9871 and SE19.
Adipic acid was accumulated at a much higher level in the heterologous E. coli host than in the native Acinetobacter sp. host. One possible reason could be the inability of E. coli to further metabolize adipic acid. E. coli cells containing the 17-kb gene cluster were able to convert cyclohexanol to adipic acid; however they were unable to grow on cyclohexanol or adipic acid as the sole carbon and energy source. Our data indicated that cyclohexanol was transported into E. coli, suggesting that lack of growth was likely due to the absence of enzymes able to further metabolize adipic acid. Adipic acid was proposed to be metabolized by a β-oxidation mechanism (6). Cells might employ a different set of enzymes or regulatory machinery for β-oxidation of fatty acids and dicarboxylic acids. The products of ORFs (ORF1, ORF2, and ORF3) identified on the 17-kb gene cluster showed homology to proteins involved in fatty acid degradation. It is feasible that they might be involved in further metabolism of adipic acid in Acinetobacter sp. strain SE19; however, they are not sufficient for adipic acid degradation.
Cycloalkanes are found naturally as components of petroleum (24) and produced industrially by hydrogenation of benzene (31). Whether the pathway we identified is dedicated to function in cycloalkane degradation is not known. The cyclohexanone monooxygenase ChnB also has significant homology to steroid monooxygenase from Rhodococcus (20). Some of the enzymes in the cycloalkane degradation pathway might also play a role in steroid metabolism. Enzymes involved in steroid and aromatic hydrocarbon catabolism have been shown to be coregulated (21). It is postulated that steroids might represent the original substrates of some of the enzymes, which evolved to act on chemicals with similar structures.
ACKNOWLEDGMENTS
We are grateful to Sylvia Stack and Ray Jackson at the DuPont sequencing facility for their excellent assistance in sequencing the 16S rRNA gene and the cosmid clones. We thank Miguel Rivera for screening the cosmid library and Carol McCutchen for editing and assembling the sequences. We appreciate John Buckholz's help with Electrospray HPLC/MS analysis. We thank Michael Bramucci, Steve Fahnestock, Tony Gatenby, and Patricia Brzostowicz for critical reading of the manuscript. We also thank Pierre Rouviere and Ethel Jackson for stimulating discussions during the course of this work.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Altschul S F, Madden T L, Schaffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ammendola S, Raia C A, Caruso C, Camardella L, D'Auria S, DeRosa M, Rossi M. Thermostable NAD(+)-dependent alcohol dehydrogenase from Sulfolobus solfataricus: gene and protein sequence determination and relationship to other alcohol dehydrogenases. Biochemistry. 1992;31:12514–12523. doi: 10.1021/bi00164a031. [DOI] [PubMed] [Google Scholar]
- 4.Branchaud B P, Walsh C T. Functional group diversity in enzymatic oxygenation reaction catalyzed by bacterial flavin-containing cyclohexanone oxygenase. J Am Chem Soc. 1985;107:2153–2161. [Google Scholar]
- 5.Brzostowicz P C, Gibson K L, Thomas S M, Blasko M S, Rouviere P E. Simultaneous identification of two cyclohexanone oxidation genes from an environmental Brevibacterium isolate using mRNA differential display. J Bacteriol. 2000;182:4241–4248. doi: 10.1128/jb.182.15.4241-4248.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chapman P J, Duggleby R G. Dicarboxylic acid catabolism by bacteria. Biochem J. 1967;103:7. doi: 10.1042/bj1030007c. c–9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen Y C J, Peoples O P, Walsh C T. Acinetobacter cyclohexanone monooxygenase: gene cloning and sequence determination. J Bacteriol. 1988;170:781–789. doi: 10.1128/jb.170.2.781-789.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho T, Takahashi Y, Yamamoto S. Manufacture of adipic acid by biotechnology. Bio Industry. 1991;8:671–678. [Google Scholar]
- 9.Donoghue N A, Trudgill P W. The metabolism of cyclohexanol by Acinetobacter NCIB 9871. Eur J Biochem. 1975;60:1–7. doi: 10.1111/j.1432-1033.1975.tb20968.x. [DOI] [PubMed] [Google Scholar]
- 10.Fulks K A, Marrs C F, Stevens S P, Green M R. Sequence analysis of the inversion region containing the pilin genes of Moraxella bovis. J Bacteriol. 1990;172:310–316. doi: 10.1128/jb.172.1.310-316.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gallegos M-T, Michan C, Ramos J L. The XylS/AraC family of regulators. Nucleic Acids Res. 1993;21:807–810. doi: 10.1093/nar/21.4.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 13.Iwaki H, Hasegawa Y, Teraoka M, Tokuyama T, Bergeron H, Lau P C K. Identification of a transcriptional activator (ChnR) and a 6-oxohexanoate dehydrogenase (ChnE) in the cyclohexanol catabolic pathway in Acinetobacter sp. strain NCIMB9871 and localization of the genes that encode them. Appl Environ Microbiol. 1999;65:5158–5162. doi: 10.1128/aem.65.11.5158-5162.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Janssen D B, van der Ploeg J, Pries F. Genetic adaptation of bacteria to halogenated aliphatic compounds. Environ Health Perspect. 1995;103:29–32. doi: 10.1289/ehp.95103s429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Junker F, Cook A M. Characterization of the p-toluenesulfonate operon tsaMBCD and tsaR in Comamonas testosteroni T-2. J Bacteriol. 1997;179:919–927. doi: 10.1128/jb.179.3.919-927.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kane M D, Poulsen L K, Stahl D A. Monitoring the enrichment and isolation of sulfate-reducing bacteria by using oligonucleotide hybridization probes designed from environmentally derived 16S rRNA sequences. Appl Environ Microbiol. 1993;59:682–686. doi: 10.1128/aem.59.3.682-686.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krozowski Z. The short-chain alcohol dehydrogenase superfamily: variations on a common theme. J Steroid Biochem Mol Biol. 1994;51:125–130. doi: 10.1016/0960-0760(94)90084-1. [DOI] [PubMed] [Google Scholar]
- 18.Kunst F, et al. The complete genome sequence of the gram-positive bacterium Bacillus subtilis. Nature. 1997;390:249–256. doi: 10.1038/36786. [DOI] [PubMed] [Google Scholar]
- 19.MacKintosh R W, Fewson C A. Benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase II from Acinetobacter calcoaceticus. Purification and preliminary characterization. Biochem J. 1988;250:743–751. doi: 10.1042/bj2500743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miyamoto M, Matsumoto J, Iwaya T, Itagaki E. Bacterial steroid monooxygenase catalyzing the Baeyer-Villiger oxidation of C21-ketosteroids from Rhodococcus rhodochrous: the isolation and characterization. Biochim Biophys Acta. 1995;1251:115–124. doi: 10.1016/0167-4838(95)00090-h. [DOI] [PubMed] [Google Scholar]
- 21.Mobus E, Jahn M, Schmid R, Jahn D, Maser E. Testosterone-regulated expression of enzymes involved in steroid and aromatic hydrocarbon catabolism in Comamonas testosteroni. J Bacteriol. 1997;179:5951–5955. doi: 10.1128/jb.179.18.5951-5955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nagata Y, Ohtomo R, Miyauchi K, Fukuda M, Yano K, Takagi M. Cloning and sequencing of a 2,5-dichloro-2,5-cyclohexadiene-1,4-diol dehydrogenase gene involved in the degradation of γ-hexachlorocyclohexane in Pseudomonas paucimobilis. J Bacteriol. 1994;176:3117–3125. doi: 10.1128/jb.176.11.3117-3125.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ogawa J, Shimizu S. Microbial enzymes: new industrial applications from traditional screening methods. Trends Biotechnol. 1999;17:13–21. doi: 10.1016/s0167-7799(98)01227-x. [DOI] [PubMed] [Google Scholar]
- 24.Perry J J. Microbial metabolism of cyclic alkanes, p. p63. In: Atlas R M, editor. Petroleum microbiology. New York, N.Y: McMillian Publishing Co.; 1984. [Google Scholar]
- 25.Raibaud A, Zalacain M, Holt T G, Tizard R, Thompson C J. Nucleotide sequence analysis reveals linked N-acetyl hydrolase, thioesterase, transport, and regulatory genes encoded by the bialaphos biosynthetic gene cluster of Streptomyces hygroscopicus. J Bacteriol. 1991;173:4454–4463. doi: 10.1128/jb.173.14.4454-4463.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reid M F, Fewson C A. Molecular characterization of microbial alcohol dehydrogenases. Crit Rev Microbiol. 1994;20:13–56. doi: 10.3109/10408419409113545. [DOI] [PubMed] [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 28.Tanaka H, Obata H, Tokuyama T, Ueno T, Yoshizako F, Nishmura A. Metabolism of cyclohexanol by Pseudomonas species. Hakkokogaku Kaishi. 1977;55:62–67. [Google Scholar]
- 29.Trower M K, Buckland M, Higgins R, Griffin M. Isolation and characterization of cyclohexane-metabolizing Xanthobacter sp. Appl Environ Microbiol. 1985;49:1282–1289. doi: 10.1128/aem.49.5.1282-1289.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trudgill P W. Cyclohexanone 1,2-monooxygenase from Acinetobacter NCIB 9871. Methods Enzymol. 1990;188:70–77. doi: 10.1016/0076-6879(90)88014-2. [DOI] [PubMed] [Google Scholar]
- 31.Weissermel K, Arpe H-J. Industrial organic chemistry. 3rd ed. New York, N.Y: VCH Publishers; 1997. [Google Scholar]
- 32.Whittaker J W, Orville A M, Lipscomb J D. Protocatechuate 3,4 dioxygenase from Brevibacterium fuscum. Methods Enzymol. 1990;188:83. doi: 10.1016/0076-6879(90)88016-4. [DOI] [PubMed] [Google Scholar]
- 33.Yamada H, Kobayashi M. Nitrile hydratase and its application to industrial production of acrylamide. Biosci Biotechnol Biochem. 1996;60:1391–1400. doi: 10.1271/bbb.60.1391. [DOI] [PubMed] [Google Scholar]