Abstract
Background
Movement smoothness is a potential kinematic biomarker of upper extremity (UE) movement quality and recovery after stroke; however, the measurement properties of available smoothness metrics have been poorly assessed in this group. We aimed to measure the reliability, responsiveness and construct validity of several smoothness metrics.
Methods
This ancillary study of the REM-AVC trial included 31 participants with hemiparesis in the subacute phase of stroke (median time since stroke: 38 days). Assessments performed at inclusion (Day 0, D0) and at the end of a rehabilitation program (Day 30, D30) included the UE Fugl Meyer Assessment (UE-FMA), the Action Research Arm Test (ARAT), and 3D motion analysis of the UE during three reach-to-point movements at a self-selected speed to a target located in front at shoulder height and at 90% of arm length. Four smoothness metrics were computed: a frequency domain smoothness metric, spectral arc length metric (SPARC); and three temporal domain smoothness metrics (TDSM): log dimensionless jerk (LDLJ); number of submovements (nSUB); and normalized average rectified jerk (NARJ).
Results
At D30, large clinical and kinematic improvements were observed. Only SPARC and LDLJ had an excellent reliability (intra-class correlation > 0.9) and a low measurement error (coefficient of variation < 10%). SPARC was responsive to changes in movement straightness (rSpearman=0.64) and to a lesser extent to changes in movement duration (rSpearman=0.51) while TDSM were very responsive to changes in movement duration (rSpearman>0.8) and not to changes in movement straightness (non-significant correlations). Most construct validity hypotheses tested were verified except for TDSM with low correlations with clinical metrics at D0 (rSpearman<0.5), ensuing low predictive validity with clinical metrics at D30 (non-significant correlations).
Conclusions
Responsiveness and construct validity of TDSM were hindered by movement duration and/or noise-sensitivity. Based on the present results and concordant literature, we recommend using SPARC rather than TDSM in reaching movements of uncontrolled duration in individuals with spastic paresis after stroke.
Trial Registration
NCT01383512, https://clinicaltrials.gov/, June 27, 2011.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01382-1.
Keywords: Measurement properties, Reaching, Kinematics, Smoothness, Stroke
Highlights
Reliability, responsiveness and construct validity of SPARC were satisfactory.
Responsiveness and construct validity of LDLJ, NARJ and nSUB were highly related to movement duration.
LDLJ had an excellent reliability and a low measurement error, but not NARJ and nSUB.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12984-024-01382-1.
Introduction
Spastic paresis of the upper extremity (UE) was reported in 48% of survivors at 1 week after stroke in a community-based population (n = 421), with full UE function achieved at discharge by 79% of those with mild paresis but only 18% of those with severe paresis [1]. Three main symptoms are well described in spastic paresis syndrome [2]: structural alterations relating to immobility (spastic myopathy, leading to muscle contractures) [3, 4], impaired motor control (stretch-sensitive paresis) of the agonist muscles [5, 6], and overactivity of antagonist muscles [7, 8], (including spasticity [8–10], spastic dystonia [11] and spastic cocontractions [12–15]).
Spastic paresis directly alters the movement trajectories and velocity with spatial (poor movement control, less efficient trajectories) and temporal (longer movement duration) discontinuities, resulting in a lack of smoothness [16–18]. Changes in the smoothness of the hand trajectory after stroke have been studied during reaching, grasping, and pointing movements [19], and the evaluation of smoothness has been suggested as a valid indicator of the quality of spontaneous motor recovery [20–23] and rehabilitation-induced recovery [18, 24–26].
The assessment of measurement properties of smoothness metrics is needed for the evaluation of changes in the poststroke spastic paretic UE. To date, many metrics have been used to explore movement recovery after stroke [27]. Research involving robotic rehabilitation systems in the last fifteen years has particularly contributed to the development of kinematic metrics, including smoothness, as potential biomarkers for movement recovery [24, 25, 27–29]. However, the use of smoothness metrics in clinical research remains limited, as those metrics require particular instrumentation and expertise that might be an obstacle for multicentric studies, are often insufficiently defined mathematically (some are even robot-specific metrics) and validated, and are often non-reproducible, non-dimensionless (i.e. highly relying on movement time), poorly robust against measurement noise, or are not related to the intermittency of movement [19, 27, 30].
New smoothness metrics that attempt to avoid those limitations have been developed and used to assess point-to-reach and point-to-grasp movement in healthy subjects and individuals after stroke [23, 31–33], namely the log dimensionless jerk (LDLJ), a smoothness metric conceived in the temporal domain and the spectral arc length metric (SPARC). The SPARC was conceived in the frequency domain by Balasubramanian and colleagues, notably to overcome the bias of movement duration and noise-sensitivity in previously developed smoothness metrics, who tested its content validity and described it as a robust to noise, sensitive, reliable, and practical metric after tests on mathematical models [30, 34].
In an earlier study, we compared the properties of four smoothness metrics currently used in the literature (SPARC, and three temporal domain smoothness metrics (TDSM): LDLJ, number of zero-crossings in the acceleration profile also called number of submovements (nSUB) and normalized average rectified jerk (NARJ)) during UE reaching movements in 32 middle-aged healthy participants [33]. In this setting, the SPARC had the lowest measurement error, and seemed independent of movement duration whereas the TDSM were highly time-dependent. A better understanding of the measurement properties of these metrics is still needed for patients with poststroke UE impairment. An international consensus was reached on the taxonomy, terminology and definitions of measurement properties within the COSMIN initiative (COnsensus-based Standards for the selection of health Measurement INstruments) setting a framework for the present study [35].
This study aimed to assess the measurement properties (reliability, responsiveness and construct validity) of the SPARC and three TDSM (NARJ, LDLJ and nSUB) for point-to-reach movements in people with moderate to severe impairment in the subacute phase of stroke, before and after a rehabilitation program.
Based on our previous work in healthy subjects [33] and literature, we hypothesized that the three TDSM would be more associated with movement duration while the SPARC would be more associated with movement straightness in the present context.
Methods
This was an ancillary study of the REM-AVC (Ré-Éducation Mécanisée après Accident Vasculaire Cérébral – Mechanized rehabilitation after cerebrovascular accident) multicenter single-blinded prospective randomized controlled trial, which compared the effects of 20 days (4 weeks, 5 days a week) of self-rehabilitation using a mechanized device with control self-exercises on UE impairment in people in the subacute phase of stroke. More details can be found in the original publication of the study [36]. It was conducted in accordance with the Declaration of Helsinki, Good Clinical Practice guidelines and local regulatory requirements (registration number, ID-RCB: NCT01383512, https://clinicaltrials.gov/, registered June 27, 2011), and was approved by the Brest University Hospital Institutional Review Board (n°653). All participants gave written consent to the use of their data.
Sample
Of the 218 individuals included in the REM-AVC trial, 37 participants in three centers underwent motion capture of their paretic UE. Six participants were excluded: two did not complete both motion capture assessments and four had uninterpretable data (many artefacts). Among the 31 included, the median (Q1 – Q3) age was 64 (54–72) years and 22 (71%) were males. Twenty-three (74%) participants had experienced an ischemic stroke, 8 a hemorrhagic stroke in the middle cerebral artery territory and the spastic paresis syndrome affected the dominant side in 14 (45%) participants. The median (Q1 – Q3) initial NIHSS score was 11 (7.5–15.5) points and the median (Q1 – Q3) time since stroke was 38 (25–62) days.
Clinical assessments
The clinical metrics were the upper extremity Fugl-Meyer assessment (UE-FMA, ranging from 0 to 66), the Action Research Arm Test (ARAT, ranging from 0 to 57), a composite Modified Ashworth Scale (cMAS – the sum of the scores of the elbow flexors and extensors, wrist and finger flexors) and the shoulder passive range of motion (PROM). Each outcome was assessed twice: at inclusion (Day 0, D0) and at the end of the rehabilitation protocol (Day 30, D30). All assessments were performed by a blinded investigator. The proximal subscore of UE-FMA (maximum score: 42) was secondarily calculated by excluding from the original score the hand and wrist (on 24 points) assessments as they are less involved in the smoothness of reaching movements.
Experimental set-up
Participants underwent two 3D motion analysis sessions at D0 and D30, during which they performed a reaching task (i.e. reach-to-point) with the impaired UE. Twenty-five reflective markers (14 mm) were placed on UE and trunk anatomical landmarks, by the same investigator at each session, following the International Society of Biomechanics recommendations [37] as illustrated in Fig. 1. Marker trajectories were recorded using a six, eight or nine camera motion capture system (Vicon, MX13 and FX20 camera models, Oxford, UK) at 120 Hz.
Fig. 1.
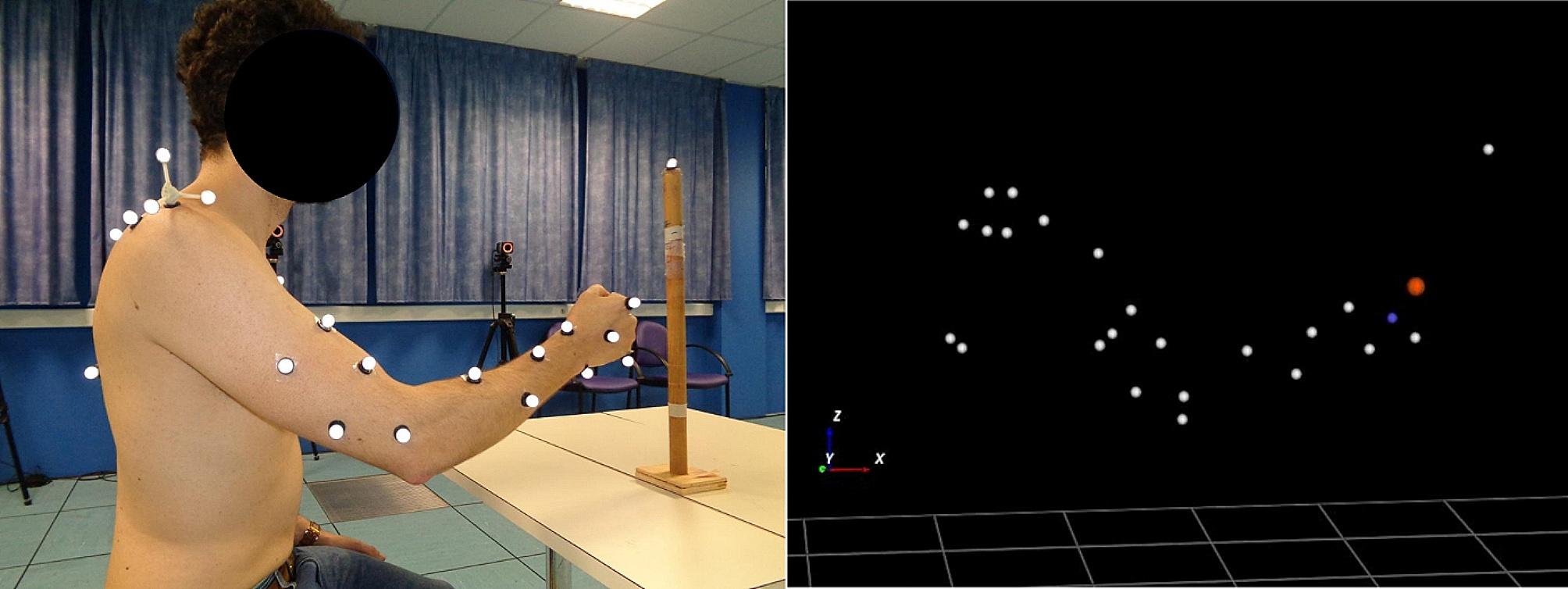
Marker placement (left) and 3D reconstruction (right) during motion analysis
Blue: mid-hand marker; red: head of the second metacarpal marker
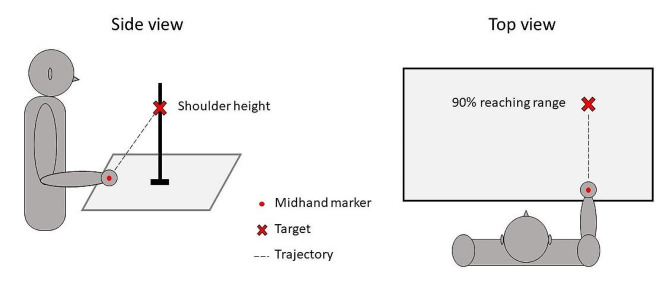
Participants were seated with their closed fist resting on a table and unconstrained trunk. The shoulder was at 0 degrees of flexion and abduction, and the elbow was flexed at 90 degrees in a neutral pronation-supination position. Participants were asked to reach with their closed fist, at comfortable speed, as close as possible to a single target indicated by a mark on a vertical stick and located in front of them, at 90% of the length of their upper limb and at the clavicle level. The set-up is represented in Fig. 2. The movement was repeated four times, the first attempt being considered as a training movement and thus not recorded. Thus for all participants, a total of 93 movements were recorded and analyzed at each session.
Fig. 2.
Representation of the motion analysis set-up at the starting position
Data analysis
The analyses presented here are focused on the mid-hand marker (placed over the middle of the third metacarpal bone, on the back of the hand). Each recorded trajectory was visually inspected twice by the same investigator to manually define the beginning and end of movements. The beginning of the movement was defined as the first ascending point of the trajectory in an upward direction. The end of the movement was the furthest point of the trajectory in the anteroposterior direction. If large artefacts were observed, the mid-hand marker was replaced by the marker placed over the head of the second metacarpal. Marker position data were computed using WorkStation 5.2.9 (Oxford Metrics, Oxford, UK).
A second order, zero-lag, low-pass Butterworth filter with a 6 Hz cutoff frequency was applied to the trajectories using Python [38] before analyses, except for the SPARC as recommended by its authors [30] because it has an in-built filter. The 6 Hz cut-off frequency was based on previous work studying the effect of filtering on TDSM [31]. The mean value of the three movements was used in the analyses for each outcome. Python was used for all calculations. First, second, and third derivatives of 3D trajectory of the mid-hand marker data were calculated to retrieve the velocity, acceleration, and jerk profiles. Peak velocity and peak acceleration were recorded.
Smoothness was quantified using the SPARC (with Vthreshold = 0.05 and ωcmax = 20 Hz as recommended by Balasubramanian et al. [30]) and three temporal domain smoothness metrics (TDSM): NARJ, LDLJ, and nSUB. SPARC and LDLJ are negative metrics (an increase in magnitude towards 0 indicates increased smoothness) whereas NARJ and nSUB are positive metrics (an increase in magnitude indicates a reduction in smoothness). A mathematical description of the metrics is available in a prior publication [33].
The index of curvature (IoC), a measure of movement straightness defined as the ratio of the arc length of the trajectory to the length of the straight line linking the first and the last movement points [39] was calculated. It is reported to approximate movement efficiency in the case of pathological movement [40]. The Python code provided by Balasubramanian et al. [30] for computing SPARC and LDLJ was edited to include the calculation of all the kinematic metrics.
COSMIN measurement properties
Reliability and measurement error
Reliability is the degree to which the instrument is free from measurement error. Measurement error is the error of a patient’s score that is not attributed to true changes in the construct. Participants could have a short break between tries. The investigator in charge of 3D motion analysis recording and treatment did not know the value of the kinematic or clinical metrics between tries or between sessions as all calculations were made after the end of the study.
Responsiveness
Responsiveness is the extent to which an instrument truly measures change, by comparing changes in an instrument of interest with changes in a gold standard. No gold standard is established in smoothness metrics; thus, we chose to measure changes in smoothness measures between D0 and D30 as compared with changes in related constructs. Movement smoothness is defined as a quality related to non-intermittency, and lack of movement smoothness can be explained by arrests or deviations in its trajectory, independently of its amplitude and duration [30]. Direct consequences of a lack of smoothness can be an increase in the spatial and temporal components of the movement. We hypothesized that changes in a metric measuring movement smoothness would be correlated with changes in IoC, and, to a lesser extent, with changes in movement duration because IoC, as a measure of trajectory (position as a function of time); explores both the spatial and temporal components of movement. As discussed by COSMIN experts and Angst [41], effect size is not a proper measure of responsiveness but it is still a complementary measure if previous hypotheses are made, in the present case that smoothness metrics should display an effect size closer to IoC than to movement duration.
Construct validity
Hypotheses-testing validity is the degree to which the scores of an instrument at one moment in time are consistent with hypotheses. We hypothesized that:
Smoothness metrics would be positively correlated with each other, with kinematic (IoC, movement duration) and clinical metrics (UE-FMA, UE-FMAp, ARAT) at D0 and at D30.
Smoothness metrics at D0 would be positively correlated with kinematic (IoC, movement duration) and clinical metrics (UE-FMA, UE-FMAp, ARAT) at D30 (predictive validity).
Smoothness metrics absolute values would be lower for LDLJ and SPARC, and higher for NARJ and nSUB, than those published in healthy subjects [33].
Criterion validity is the degree to which the scores of an instrument are an adequate reflection of a “gold standard”. No gold standard is available for smoothness metrics in the present context; thus, criterion validity was not assessed.
Statistics
As participants in both REM-AVC groups received the same amount of treatment and as no demographic, clinical or kinematic variables differed, participants were pooled into a single sample for the purpose of this study. Descriptive statistics were performed to calculate the median values and interquartile intervals.
The normality of data was assessed by visual inspection of the data distribution and a Shapiro‒Wilk test. As most data had a non-normal distribution due to ceiling effects and sample size, only nonparametric tests were used. Comparisons between D0 and D30 clinical and kinematic variables were performed using Wilcoxon tests, and effect sizes were calculated ([0.1–0.3[: small; [0.3–0.5[: medium; ≥0.5: large). A figure displaying mean and standard deviation of trajectory and velocity profiles was made for visual analysis.
Reliability and measurement error
Reliability of smoothness metrics was assessed by intra-class correlation (ICC) estimates and their 95% confident intervals (2 ways mixed effects, average measures (k = 3), absolute agreement). ICC values less than 0.5, between 0.5 and 0.75, between 0.75 and 0.9, and greater than 0.90 were interpreted as indicative of poor, moderate, good, and excellent reliability, respectively [42]. Measurement error was assessed by the median of intra-individual coefficients of variation (CoV, which is the ratio of the standard deviation to the mean of the three recorded tries) at D0 and at D30.
Responsiveness
Changes between D0 and D30 (Δ) were calculated as follow: Δ = D30 value – D0 value. Responsiveness was assessed with Spearman correlations between changes in smoothness metrics and changes in IoC and movement duration.
Construct validity
Hypotheses were tested using Spearman correlations between smoothness metrics and clinical metrics (UE-FMA, UE-FMAp and ARAT) and kinematic metrics (movement duration and IoC) at D0 and at D30; and between smoothness metrics at D0 and clinical and kinematic metrics at D30.
Spearman’s r was interpreted as weak if < 0.4, moderate if [0.4–0.6[, strong if [0.6–0.8[ and very strong if ≥ 0.8. All statistical analyses were performed using SPSS v20 (IBM, Armonk, NY).
Results
The only missing demographic, clinical or kinematic data was shoulder range of motion at D0 for one participant. Database for main metrics is available in Appendix A.
Clinical changes
Changes in clinical metrics are presented in Table 1. The UE-FMA total and proximal subscore and ARAT scores were improved at D30 (effect size: large). cMAS and shoulder PROM did not change between D0 and D30.
Table 1.
Baseline and final clinical metrics and comparison
| Clinical measures | Day 0 | Day 30 | Difference | Effect size | ||
|---|---|---|---|---|---|---|
| Median | Q1 – Q3 | Median | Q1 – Q3 | p-value | ||
| UE-FMA | 27 | 19–33 | 45 | 33–52 | < 0.0001 | 0.87 |
| UE-FMA: proximal subscore | 24 | 17–27 | 37 | 26–42 | < 0.0001 | 0.86 |
| ARAT | 10 | 3–19 | 31 | 19–45 | < 0.0001 | 0.85 |
| cMAS | 3 | 1–4 | 3 | 1–4 | 0.4 | - |
| Shoulder PROM | ||||||
| Anterior flexion | 150 | 110–170 | 150 | 120–170 | 0.3 | - |
| Abduction | 103 | 90–153 | 105 | 90–160 | 0.8 | - |
| External rotation | 40 | 15–55 | 40 | 20–60 | 0.2 | - |
Q: quartile, UE-FMA: upper extremity Fugl Meyer assessment, cMAS: composite modified Ashworth scale, ARAT: Action Research Arm Test, PROM: passive range of motion
Kinematic changes
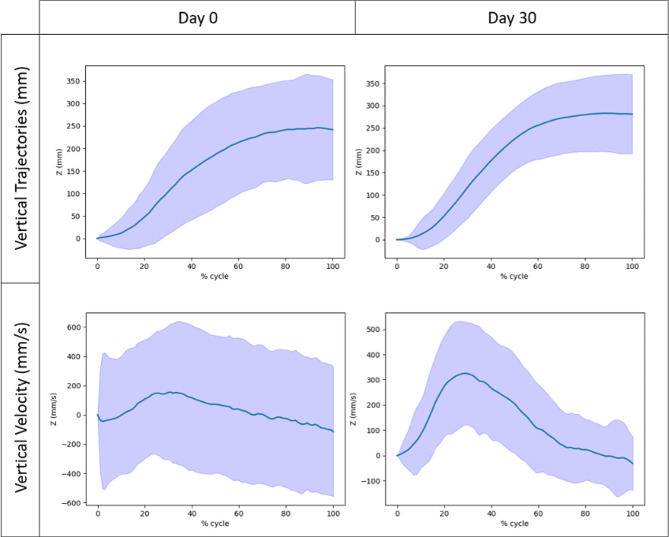
Changes in kinematic metrics are presented in Table 2. Trajectories and velocity profiles visually improved between D0 and D30 as illustrated in Fig. 3. Movements at D30 were significantly shorter in duration and trajectory (less distance covered to reach the target), straighter, faster and smoother according to all four smoothness metrics (medium (mean velocity) to large effect sizes). Peak velocity and peak acceleration did not change significantly.
Table 2.
Baseline and final kinematic metrics and comparison
| Kinematic metrics | D0 | D30 | Difference | Effect size | ||
|---|---|---|---|---|---|---|
| Median | Q1 – Q3 | Median | Q1 – Q3 | p-value | ||
| Duration (s) | 2.6 | 2.1–3.9 | 2 | 1.7–2.6 | 0.0003 | 0.64 |
| CoVintra | 16 | 11–25 | 13 | 7–24 | 0.9 | - |
| Trajectory length (mm) | 552 | 461–709 | 437 | 406–623 | 0.001 | 0.58 |
| CoVintra | 7 | 4–13 | 4 | 3–9 | 0.9 | - |
| IoC (%) | 35.2 | 22.9–79.2 | 13.8 | 10.7–23.1 | < 0.0001 | 0.80 |
| CoVintra | 25 | 19–48 | 35 | 17–45 | 0.8 | - |
| Mean velocity (mm/s) | 213 | 127–273 | 246 | 185–319 | 0.02 | 0.43 |
| CoVintra | 13 | 10–21 | 13 | 7–20 | 0.4 | - |
| Peak velocity (mm/s) | 600 | 391–721 | 682 | 480–745 | 0.4 | - |
| CoVintra | 12 | 8–20 | 11 | 7–15 | 0.06 | - |
| Peak acceleration (mm/s²) | 2768 | 1860–3650 | 3169 | 1899–4523 | 0.4 | - |
| CoVintra | 23 | 11–36 | 21 | 13–37 | 0.5 | - |
| SPARC | -1.82 | -2.14 – -1.70 | -1.61 | -1.78 – -1.51 | < 0.0001 | 0.76 |
| CoVintra | 8.9 | 6–14 | 4.1 | 2–10 | 0.03 | 0.39 |
| LDLJ | -10.7 | -12.7 – -9.83 | -9.36 | -11,0 – -8.05 | 0.0003 | 0.65 |
| CoVintra | 9.1 | 6–13 | 7.8 | 4–13 | 0.2 | - |
| nSUB | 15 | 12–28 | 11 | 7–17 | 0.0009 | 0.59 |
| CoVintra | 33 | 22–46 | 32 | 20–43 | 0.9 | - |
| NARJ 10− 5 (mm/s³) | 3.8 | 1.88–9.71 | 1.52 | 0.79–3.31 | 0.0003 | 0.65 |
| CoVintra | 48 | 28–75 | 38 | 19–57 | 0.2 | - |
Q: quartile, CoVintra: intra-individual coefficient of variation, SPARC: spectral arc length metric, LDLJ: log dimensionless jerk, nSUB: number of submovements, NARJ: normalized average rectified jerk
Fig. 3.
Trajectories and velocity profiles of day 0 (D0) and day 30 (D30) reaching movements. Blue line: mean value, lavender: standard deviation area
Reliability and measurement error
SPARC and LDLJ had close ICC estimates (0.912 [0.861;0.946] and 0.911 [0.861;0.945] respectively) indicating excellent reliability, followed by nSUB (0.891 [0.830;0.933]) indicating good reliability and NARJ (0.613 [0.391;0.763]) indicating moderate reliability. Among the smoothness metrics, SPARC had the smallest CoV both at D0 and at D30 and was the only metric for which CoV was significantly improved at D30 (medium effect size). CoV at D0 and at D30 were also less than 10% for LDLJ but was greater than 30% for NARJ and nSUB.
Responsiveness
Correlations between the changes in kinematic metrics between D0 and D30 are presented in Table 3. ΔSPARC was moderately correlated with the changes in movement duration and strongly correlated with ΔIoC. ΔTDSM were very strongly correlated with movement duration, but not with ΔIoC. ΔSPARC and ΔTDSM were moderately to strongly correlated. ΔTDSM were strongly to very strongly correlated with each other. Effect size of change between D0 and D30 for the SPARC (0.76) was closer to the one of IoC (0.80), while it was closer to the one of movement duration (0.64) for those of LDLJ (0.65), NARJ (0.65) and nSUB (0.59).
Table 3.
Spearman (r) correlations between changes in metrics from day 0 (D0) to day 30 (D30)
| ΔDuration | ΔIoC | ΔnSUB | ΔNARJ | ΔLDLJ | |
|---|---|---|---|---|---|
| ΔSPARC | -0.51** | -0.64** | -0.54** | -0.59** | 0.67** |
| ΔLDLJ | -0.81** | -0.26 | -0.84** | -0.73** | |
| ΔNARJ | 0.90** | 0.35 | 0.89** | ||
| ΔnSUB | 0.96** | 0.33 |
**: p < 0.01, Δ: D30-D0 value, IoC: index of curvature, SPARC: spectral arc length metric, LDLJ: log dimensionless jerk, nSUB: number of submovements, NARJ: normalized average rectified jerk
Construct validity
At D0.
SPARC was moderately correlated with UE-FMA and its proximal subscore and strongly correlated with ARAT. TDSM were weakly or insignificantly correlated with UE-FMA and moderately correlated with ARAT. SPARC was very strongly correlated with IoC and moderately correlated with movement duration; TDSM were moderately correlated with IoC and very strongly correlated with movement duration. Overall results at D0 are presented in Table 4.
Table 4.
Spearman correlations (r) between metrics at day 0
| UE-FMA | UE-FMAp | ARAT | Duration | IoC | |
|---|---|---|---|---|---|
| SPARC | 0.48** | 0.56** | 0.68** | -0.59** | -0.87** |
| LDLJ | 0.39* | 0.28 | 0.46** | -0.89** | -0.57** |
| NARJ | -0.36* | -0.23 | -0.46** | 0.87** | 0.56** |
| nSUB | -0.30 | -0.16 | -0.39* | 0.93** | 0.41* |
*: p < 0,05, **: p < 0.01, UE-FMA: upper limb Fugl Meyer score, UE-FMAp: proximal subscore of UE-FMA, ARAT: Action Research Arm Test, IoC: index of curvature, SPARC: spectral arc length metric, LDLJ: log dimensionless jerk, nSUB: number of submovements, NARJ: normalized average rectified jerk
-
2)
At D30.
All smoothness metrics were strongly correlated with UE-FMA and UE-FMAp scores and moderately correlated with ARAT scores. Movement duration was very strongly correlated with all TDSM and only moderately correlated with SPARC. IoC was moderately to strongly correlated with all smoothness metrics. Overall results at D30 are presented in Table 5.
Table 5.
Spearman correlations (r) between metrics at day 30
| UE-FMA | UE-FMAp | ARAT | Duration | IoC | |
|---|---|---|---|---|---|
| SPARC | 0.63** | 0.66** | 0.46** | -0.58** | -0.58** |
| LDLJ | 0.66** | 0.66** | 0.47** | -0.82** | -0.62** |
| NARJ | -0.74** | -0.73** | -0.55** | 0.89** | 0.68** |
| nSUB | -0.61** | -0.60** | -0.40* | 0.81** | 0.60** |
**: p < 0.01, UE-FMA: upper limb Fugl Meyer score, UE-FMAp: proximal subscore of UE-FMA, ARAT: Action Research Arm Test, IoC: index of curvature, SPARC: spectral arc length metric, LDLJ: log dimensionless jerk, nSUB: number of submovements, NARJ: normalized average rectified jerk
-
3)
Predictive validity.
SPARC at D0 was moderately correlated with UE-FMA, UE-FMAp and ARAT at D30, whereas TDSM at D0 were not correlated with clinical metrics at D30. SPARC at D0 was strongly correlated with IoC at D30, whereas TDSM at D0 were either not correlated (nSUB) or moderately correlated (LDLJ, NARJ) with IoC at D30. Weak (NARJ and nSUB) to moderate (SPARC and LDLJ) correlations were observed between smoothness metrics at D0 and movement duration at D30. Correlations between D0 smoothness metrics and D30 clinical and kinematic metrics are presented in Table 6.
Table 6.
Spearman correlations (r) between day 0 smoothness metrics and day 30 clinical and kinematic metrics
| D30/ D0 | UE-FMA | UE-FMAp | ARAT | Duration | IoC |
|---|---|---|---|---|---|
| SPARC | 0.57** | 0.54** | 0.58** | -0.55** | -0.69** |
| LDLJ | 0.21 | 0.17 | 0.31 | -0.42* | -0.40* |
| NARJ | -0.22 | -0.18 | -0.33 | 0.39* | 0.41* |
| nSUB | -0.18 | -0.10 | -0.26 | 0.39* | 0.26 |
D0: day 0; D30: day 30; *: p < 0,05, **: p < 0.01, UE-FMA: upper limb Fugl Meyer score, UE-FMAp: proximal subscore of UE-FMA, ARAT: Action Research Arm Test, IoC: index of curvature, SPARC: spectral arc length metric, LDLJ: log dimensionless jerk, nSUB: number of submovements, NARJ: normalized average rectified jerk
Supplementary results for correlations between smoothness metrics are presented in Appendix B.
Discussion
This study assessed four smoothness metrics in people with moderate to severe motor impairment before and after one month of intensive physical rehabilitation in the subacute phase of stroke. Large clinical and kinematic improvements occurred between D0 and D30. Reliability, responsiveness and construct validity of the smoothness metrics were assessed. Only SPARC and LDLJ had an excellent reliability. Measurement error was lowest for SPARC, followed by LDLJ. Changes in SPARC were correlated with changes in movement duration and more strongly with changes in IoC, while TDSM (NARJ, nSUB and LDLJ) responsiveness was skewed towards movement duration. Most construct validity hypotheses were verified except for TDSM with lower correlations than expected with clinical metrics at D0, ensuing low predictive correlations with clinical metrics at D30.
In the present study, most clinical and kinematic metrics improved from D0 to D30. cMAS and shoulder PROM did not change, but these measures are characterized by uncertain validity and sensitivity to change [43–46]. Movement duration, trajectory length, straightness and smoothness were abnormal at D0, as is generally observed after stroke [16, 47], and all improved significantly by D30 with large effect size. Kinematic outcomes can provide an accurate indication of UE motor recovery after stroke [48]. Participants with high-to-normal UE-FMA still showed deficits in movement kinematic outcomes in a study [39]. UE-FMA and SPARC improved in a longitudinal study of people with mild stroke [23], and is also suggested by our results in people with moderate-to-severe stroke. A recent meta-analysis found that smoothness (measured with nSUB) was the most responsive after stroke among few kinematic outcomes (movement duration, peak velocity, shoulder active range of motion (AROM), control strategy, IoC, elbow AROM and trunk AROM) and that it was as responsive to change as the UE-FMA, indicating that clinical and kinematic measures are complementary and provide a comprehensive and accurate follow-up of motor recovery [18].
Measurement properties
SPARC and LDLJ displayed an excellent reliability and a low measurement error, while nSUB and NARJ displayed only good and moderate reliabilities respectively and a high measurement error. This result was expected as SPARC and LDLJ were developed to be more reliable than previous metrics [30, 34].
SPARC changes from D0 to D30 were more strongly correlated with changes in movement straightness as assessed by IoC than with changes in movement duration, which can be interpreted as a satisfying responsiveness from a kinematic point of view. This result is complementary to clinical longitudinal validity of SPARC with UE-FMA observed by Saes et al. in 40 individuals followed from week 1 to week 26 after a mild stroke [23]. In contrast, the changes in TDSM were very strongly correlated with the changes in movement duration, but not with changes in movement straightness. The magnitude of change was closer to IoC for the SPARC and closer to movement duration for TDSM, which supports the aforementioned results. This difference in responsiveness suggests a bias of movement duration in the construct measured by TDSM, as was previously found in healthy subjects [34]. The explanation may come from the known noise sensitivity of TDSM [19, 30, 34] as additional noise is mechanically recorded with longer movement duration.
At D0, SPARC more strongly correlated with UE-FMA and ARAT scores than did TDSM. However, at D30, the correlations for SPARC and TDSM with UE-FMA and ARAT scores were of similar strength. Moreover, at D0, SPARC very strongly correlated with movement straightness and moderately with movement duration while TDSM very strongly correlated with movement duration and moderately with movement straightness. Again, differences in correlations were less important at D30. Finally, SPARC values at D0 were more strongly correlated with kinematic and clinical measures at D30 than the TDSM, suggesting stronger predictive validity of SPARC. These differences may be again explained by the known noise-sensitivity ensuing movement duration dependence of TDSM [19, 30, 34], which could have been a more pronounced issue at D0 as the movements were slower and thus may have generated a higher number of signal artefacts.
We found notably stronger correlations between each TDSM than between TDSM and SPARC both at D0 and at D30 (results in Appendix B), suggesting that the TDSM are measuring a very similar construct and that the SPARC is measuring a close but different construct. This is in line with data previously reported for theoretical models and healthy individuals [30, 31, 33]. Smoothness metrics values in the present study differed notably from those in healthy subjects [33] which verified our hypothesis. In particular, the SPARC values during reaching movements of healthy individuals (approximately -1.44 ± 0.02) reported in studies by Engdahl et al. [31], Saes et al. [23] and Bayle et al. [33] differ notably from the values found in the present study (D0: -1.82 and D30: -1.61). In addition, the values for the participants with mild stroke in the study by Saes et al. (week 1: -1.72, week 5: -1.53) [23] differed from both healthy individuals and the participants with a more severe stroke included in the present study. These findings support a discriminant aspect of the construct validity of SPARC.
Finally, the usefulness of adding movement smoothness to the stroke standard assessment is yet to be fully determined even if the addition of kinematic movement quantification has been strongly encouraged by an international consensus [49]. Overall, the measurement properties of smoothness metrics assessed in this clinical study complete the mathematical and simulated results of Mohamed Refai et al. [19] and reinforce the recommendation of the SPARC for the assessment of reaching tasks after stroke.
Study limitations
Only univariable analyses were conducted owing to the non-normal distribution of the data and the small number of participants. Thus, the correlations, despite their consistency, may be biased by confounding factors. The results may not be generalizable to the people with milder impairments or other types of abnormal movement. The ideal filtering for an optimal noise-to-signal ratio has not been determined for the different smoothness metrics even if we chose the best performing cut-off frequency from a previously published work including tests on filtering and smoothness metrics [31]. Another choice of filter may have improved TDSM performance, especially at D0. High intra-individual variability was observed at D0 for nSUB and NARJ which had higher CoVs than LDLJ and SPARC; thus, assessing more UE movements could have led to steadier results. Recording five trials for participants with more severe impairment could be a pragmatic compromise between data robustness and participant fatigue in future studies, as recently suggested [50].
Conclusion
The results of this study increase the knowledge of smoothness metrics reliability, responsiveness and construct validity for the assessment of upper limb reach-to-point movements in the subacute phase of stroke. We recommend using SPARC rather than LDLJ to assess the smoothness of reaching movements of uncontrolled duration based on our findings and concordant literature. NARJ and nSUB provide less valid and reliable results in this context. The gathering of validity evidence is an ongoing process, therefore future studies using SPARC or other smoothness metrics in different setups or populations should report their findings concerning measurement properties.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
We are very grateful to all participants of this study. We also wish to thank all our colleagues, research therapists and other healthcare professionals at the different study sites for their dedication.
Abbreviations
- ARAT
Action Research Arm Test
- cMAS
Composite modified Ashworth scale
- CoV
Coefficient of variation
- D0
Day 0 (inclusion in the rehabilitation program)
- D30
Day 30 (end of the rehabilitation program)
- Δ
D30 value minus D0 value
- IoC
Index of curvature
- LDLJ
Log dimensionless jerk
- NARJ
Normalized average rectified jerk
- nSUB
Number of submovements
- PROM
Passive range of motion
- SPARC
Spectral arc length metric
- TDSM
Temporal domain smoothness metrics
- UE-FMA
Upper limb Fugl Meyer Assessment
- UE-FMAp
Proximal subscore of UE-FMA
Author contributions
BM, ML, ORN, RG, and SB participated in data acquisition; GC and ML analyzed the data; GC, JM, JMG, ML, NB, and ORN interpreted results; GC, JR, LM and NB were major contributors in writing the manuscript. All authors read and approved the final manuscript.
Funding
Funding was provided by the French ministry of health: EMREM_AVC CHU BREST 20 220.
Data availability
The dataset supporting the conclusions of this article is included within the article (and its additional files), more details are available on reasonable request.
Declarations
Ethics approval and consent to participate
This work was conducted in accordance with the Declaration of Helsinki and was approved by the Brest University Hospital Institutional Review Board (n°653). Informed consent was obtained from all participants.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nakayama H, Jørgensen HS, Raaschou HO, Olsen TS. Recovery of upper extremity function in stroke patients: the Copenhagen Stroke Study. Arch Phys Med Rehabil. 1994;75(4):394–8. doi: 10.1016/0003-9993(94)90161-9. [DOI] [PubMed] [Google Scholar]
- 2.Baude M, Nielsen JB, Gracies JM. The neurophysiology of deforming spastic paresis: a revised taxonomy. Ann Phys Rehabil Med. 2019;62(6):426–30. doi: 10.1016/j.rehab.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 3.Pradines M, Ghedira M, Portero R, Masson I, Marciniak C, Hicklin D, et al. Ultrasound Structural changes in Triceps Surae after a 1-Year daily self-stretch program: a prospective Randomized Controlled Trial in Chronic Hemiparesis. Neurorehabil Neural Repair. 2019;33(4):245–59. doi: 10.1177/1545968319829455. [DOI] [PubMed] [Google Scholar]
- 4.Jalal N, Gracies JM, Zidi M. Mechanical and microstructural changes of skeletal muscle following immobilization and/or stroke. Biomech Model Mechanobiol. 2020;19(1):61–80. doi: 10.1007/s10237-019-01196-4. [DOI] [PubMed] [Google Scholar]
- 5.Gracies JM. Pathophysiology of spastic paresis. I: paresis and soft tissue changes. Muscle Nerve. 2005;31(5):535–51. doi: 10.1002/mus.20284. [DOI] [PubMed] [Google Scholar]
- 6.Vinti M, Bayle N, Hutin E, Burke D, Gracies JM. Stretch-sensitive paresis and effort perception in hemiparesis. J Neural Transm Vienna Austria 1996. 2015;122(8):1089–97. doi: 10.1007/s00702-015-1379-3. [DOI] [PubMed] [Google Scholar]
- 7.Colebatch JG, Gandevia SC. The distribution of muscular weakness in upper motor neuron lesions affecting the arm. Brain J Neurol. 1989;112(Pt 3):749–63. doi: 10.1093/brain/112.3.749. [DOI] [PubMed] [Google Scholar]
- 8.Gracies JM. Pathophysiology of spastic paresis. II: emergence of muscle overactivity. Muscle Nerve. 2005;31(5):552–71. doi: 10.1002/mus.20285. [DOI] [PubMed] [Google Scholar]
- 9.Lance JW. The control of muscle tone, reflexes, and movement: Robert Wartenberg Lecture. Neurology. 1980;30(12):1303–13. doi: 10.1212/WNL.30.12.1303. [DOI] [PubMed] [Google Scholar]
- 10.Mottram CJ, Suresh NL, Heckman CJ, Gorassini MA, Rymer WZ. Origins of abnormal excitability in biceps brachii motoneurons of spastic-paretic stroke survivors. J Neurophysiol. 2009;102(4):2026–38. doi: 10.1152/jn.00151.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lorentzen J, Pradines M, Gracies JM, Bo Nielsen J. On Denny-Brown’s ‘spastic dystonia’ - what is it and what causes it? Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2018;129(1):89–94. doi: 10.1016/j.clinph.2017.10.023. [DOI] [PubMed] [Google Scholar]
- 12.Vinti M, Costantino F, Bayle N, Simpson DM, Weisz DJ, Gracies JM. Spastic cocontraction in hemiparesis: effects of botulinum toxin. Muscle Nerve. 2012;46(6):926–31. doi: 10.1002/mus.23427. [DOI] [PubMed] [Google Scholar]
- 13.Vinti M, Couillandre A, Hausselle J, Bayle N, Primerano A, Merlo A, et al. Influence of effort intensity and gastrocnemius stretch on co-contraction and torque production in the healthy and paretic ankle. Clin Neurophysiol off J Int Fed Clin Neurophysiol. 2013;124(3):528–35. doi: 10.1016/j.clinph.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 14.Vinti M, Bayle N, Merlo A, Authier G, Pesenti S, Jouve JL, et al. Muscle shortening and spastic cocontraction in gastrocnemius Medialis and Peroneus Longus in very young Hemiparetic Children. BioMed Res Int. 2018;2018:2328601. doi: 10.1155/2018/2328601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chalard A, Amarantini D, Tisseyre J, Marque P, Tallet J, Gasq D. Spastic co-contraction, rather that spasticity, is associated with impaired active function in adults with acquired brain injury: a pilot study. J Rehabil Med. 2019;51(4):307–11. doi: 10.2340/16501977-2528. [DOI] [PubMed] [Google Scholar]
- 16.Collins KC, Kennedy NC, Clark A, Pomeroy VM. Kinematic Components of the Reach-to-Target Movement after Stroke for Focused Rehabilitation Interventions: systematic review and Meta-analysis. Front Neurol. 2018;9:472. doi: 10.3389/fneur.2018.00472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirstea MC, Levin MF. Compensatory strategies for reaching in stroke. Brain J Neurol. 2000;123(Pt 5):940–53. doi: 10.1093/brain/123.5.940. [DOI] [PubMed] [Google Scholar]
- 18.Villepinte C, Verma A, Dimeglio C, De Boissezon X, Gasq D. Responsiveness of kinematic and clinical measures of upper-limb motor function after stroke: a systematic review and meta-analysis. Ann Phys Rehabil Med. 2021;64(2):101366. doi: 10.1016/j.rehab.2020.02.005. [DOI] [PubMed] [Google Scholar]
- 19.Mohamed Refai MI, Saes M, Scheltinga BL, van Kordelaar J, Bussmann JBJ, Veltink PH, et al. Smoothness metrics for reaching performance after stroke. Part 1: which one to choose? J Neuroeng Rehabil. 2021;18(1):154. doi: 10.1186/s12984-021-00949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rohrer B, Fasoli S, Krebs HI, Hughes R, Volpe B, Frontera WR, et al. Movement smoothness changes during stroke recovery. J Neurosci off J Soc Neurosci. 2002;22(18):8297–304. doi: 10.1523/JNEUROSCI.22-18-08297.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hogan N, Sternad D. Sensitivity of smoothness measures to Movement Duration, Amplitude and arrests. J Mot Behav. 2009;41(6):529–34. doi: 10.3200/35-09-004-RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Liebermann DG, Levin MF, McIntyre J, Weiss PL, Berman S. Arm path fragmentation and spatiotemporal features of hand reaching in healthy subjects and stroke patients. In: 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology [Internet]. Buenos Aires: IEEE; 2010 [cited 2023 Mar 21]. pp. 5242–5. http://ieeexplore.ieee.org/document/5626297/. [DOI] [PubMed]
- 23.Saes M, Mohamed Refai MI, van Kordelaar J, Scheltinga BL, van Beijnum BJF, Bussmann JBJ, et al. Smoothness metric during reach-to-grasp after stroke: part 2. Longitudinal association with motor impairment. J Neuroeng Rehabil. 2021;18(1):144. doi: 10.1186/s12984-021-00937-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bosecker C, Dipietro L, Volpe B, Krebs HI. Kinematic robot-based evaluation scales and clinical counterparts to measure upper limb motor performance in patients with chronic stroke. Neurorehabil Neural Repair. 2010;24(1):62–9. doi: 10.1177/1545968309343214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yue Z, Zhang X, Wang J. Hand Rehabilitation Robotics on Poststroke Motor Recovery. Behav Neurol. 2017;2017:3908135. doi: 10.1155/2017/3908135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Germanotta M, Cortellini L, Insalaco S, Aprile I. Effects of Upper Limb Robot-Assisted Rehabilitation Compared with conventional therapy in patients with stroke: preliminary results on a Daily Task Assessed using motion analysis. Sensors. 2023;23(6):3089. doi: 10.3390/s23063089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schwarz A, Kanzler CM, Lambercy O, Luft AR, Veerbeek JM. Systematic review on kinematic assessments of Upper Limb movements after Stroke. Stroke. 2019;50(3):718–27. doi: 10.1161/STROKEAHA.118.023531. [DOI] [PubMed] [Google Scholar]
- 28.Slavens BA, Harris GF. The biomechanics of upper extremity kinematic and kinetic modeling: applications to rehabilitation engineering. Crit Rev Biomed Eng. 2008;36(2–3):93–125. doi: 10.1615/CritRevBiomedEng.v36.i2-3.20. [DOI] [PubMed] [Google Scholar]
- 29.Tran VD, Dario P, Mazzoleni S. Kinematic measures for upper limb robot-assisted therapy following stroke and correlations with clinical outcome measures: a review. Med Eng Phys. 2018;53:13–31. doi: 10.1016/j.medengphy.2017.12.005. [DOI] [PubMed] [Google Scholar]
- 30.Balasubramanian S, Melendez-Calderon A, Roby-Brami A, Burdet E. On the analysis of movement smoothness. J Neuroeng Rehabil. 2015;12:112. doi: 10.1186/s12984-015-0090-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Engdahl SM, Gates DH. Reliability of upper limb movement quality metrics during everyday tasks. Gait Posture. 2019;71:253–60. doi: 10.1016/j.gaitpost.2019.04.023. [DOI] [PubMed] [Google Scholar]
- 32.Gulde P, Hermsdörfer J. Smoothness Metrics in Complex Movement tasks. Front Neurol. 2018;9:615. doi: 10.3389/fneur.2018.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayle N, Lempereur M, Hutin E, Motavasseli D, Remy-Neris O, Gracies JM, et al. Comparison of various smoothness Metrics for Upper Limb movements in Middle-aged healthy subjects. Sensors. 2023;23(3):1158. doi: 10.3390/s23031158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Balasubramanian S, Melendez-Calderon A, Burdet E. A robust and sensitive metric for quantifying movement smoothness. IEEE Trans Biomed Eng. 2012;59(8):2126–36. doi: 10.1109/TBME.2011.2179545. [DOI] [PubMed] [Google Scholar]
- 35.Mokkink LB, Terwee CB, Patrick DL, Alonso J, Stratford PW, Knol DL, et al. The COSMIN study reached international consensus on taxonomy, terminology, and definitions of measurement properties for health-related patient-reported outcomes. J Clin Epidemiol. 2010;63(7):737–45. doi: 10.1016/j.jclinepi.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Rémy-Néris O, Le Jeannic A, Dion A, Médée B, Nowak E, Poiroux É, et al. Additional, mechanized Upper Limb Self-Rehabilitation in patients with Subacute Stroke: the REM-AVC randomized trial. Stroke. 2021;52(6):1938–47. doi: 10.1161/STROKEAHA.120.032545. [DOI] [PubMed] [Google Scholar]
- 37.Wu G, van der Helm FCT, Veeger HEJD, Makhsous M, Van Roy P, Anglin C, et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion–part II: shoulder, elbow, wrist and hand. J Biomech. 2005;38(5):981–92. doi: 10.1016/j.jbiomech.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 38.van Rossum G. Python tutorial, Technical Report CS-R9526, Centrum voor Wiskunde en Informatica (CWI), Amsterdam, May 1995.
- 39.Thrane G, Sunnerhagen KS, Persson HC, Opheim A, Alt Murphy M. Kinematic upper extremity performance in people with near or fully recovered sensorimotor function after stroke. Physiother Theory Pract. 2019;35(9):822–32. doi: 10.1080/09593985.2018.1458929. [DOI] [PubMed] [Google Scholar]
- 40.de los Reyes-Guzmán A, Dimbwadyo-Terrer I, Trincado-Alonso F, Monasterio-Huelin F, Torricelli D, Gil-Agudo A. Quantitative assessment based on kinematic measures of functional impairments during upper extremity movements: a review. Clin Biomech Bristol Avon. 2014;29(7):719–27. doi: 10.1016/j.clinbiomech.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 41.Angst F. The new COSMIN guidelines confront traditional concepts of responsiveness. BMC Med Res Methodol. 2011;11:152. doi: 10.1186/1471-2288-11-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koo TK, Li MY. A Guideline of selecting and reporting Intraclass correlation coefficients for Reliability Research. J Chiropr Med. 2016;15(2):155–63. doi: 10.1016/j.jcm.2016.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meseguer-Henarejos AB, Sánchez-Meca J, López-Pina JA, Carles-Hernández R. Inter- and intra-rater reliability of the Modified Ashworth Scale: a systematic review and meta-analysis. Eur J Phys Rehabil Med. 2018;54(4):576–90. doi: 10.23736/S1973-9087.17.04796-7. [DOI] [PubMed] [Google Scholar]
- 44.Alibiglou L, Rymer WZ, Harvey RL, Mirbagheri MM. The relation between Ashworth scores and neuromechanical measurements of spasticity following stroke. J Neuroeng Rehabil. 2008;5:18. doi: 10.1186/1743-0003-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pandyan AD, Price CIM, Barnes MP, Johnson GR. A biomechanical investigation into the validity of the modified Ashworth Scale as a measure of elbow spasticity. Clin Rehabil. 2003;17(3):290–3. doi: 10.1191/0269215503cr610oa. [DOI] [PubMed] [Google Scholar]
- 46.de Jong LD, Nieuwboer A, Aufdemkampe G. The hemiplegic arm: interrater reliability and concurrent validity of passive range of motion measurements. Disabil Rehabil. 2007;29(18):1442–8. doi: 10.1080/09638280601056145. [DOI] [PubMed] [Google Scholar]
- 47.van Dokkum L, Hauret I, Mottet D, Froger J, Métrot J, Laffont I. The contribution of kinematics in the assessment of upper limb motor recovery early after stroke. Neurorehabil Neural Repair. 2014;28(1):4–12. doi: 10.1177/1545968313498514. [DOI] [PubMed] [Google Scholar]
- 48.Thrane G, Sunnerhagen KS, Murphy MA. Upper limb kinematics during the first year after stroke: the stroke arm longitudinal study at the University of Gothenburg (SALGOT) J Neuroeng Rehabil. 2020;17(1):76. doi: 10.1186/s12984-020-00705-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kwakkel G, van Wegen EEH, Burridge JH, Winstein CJ, van Dokkum LEH, Alt Murphy M, Levin MF, Krakauer JW, ADVISORY group Standardized measurement of quality of Upper Limb Movement after Stroke: Consensus-based core recommendations from the second stroke recovery and Rehabilitation Roundtable. Neurorehabil Neural Repair. 2019;33(11):951–8. doi: 10.1177/1545968319886477. [DOI] [PubMed] [Google Scholar]
- 50.Frykberg GE, Grip H, Alt Murphy M. How many trials are needed in kinematic analysis of reach-to-grasp?-A study of the drinking task in persons with stroke and non-disabled controls. J Neuroeng Rehabil. 2021;18(1):101. doi: 10.1186/s12984-021-00895-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The dataset supporting the conclusions of this article is included within the article (and its additional files), more details are available on reasonable request.