Abstract
Background:
Comorbidities and poor sleep quality are prevalent among individuals with multiple sclerosis (MS). Our understanding of the effects of comorbidities on sleep quality in MS remains limited.
Objectives:
The objectives were to investigate whether the number and presence of specific comorbidities have associations with sleep quality and to assess the relative contribution of comorbidity groups to sleep quality.
Methods:
We collected data on sleep quality (using Pittsburgh Sleep Quality Index (PSQI)) and presence of comorbidities in people with MS (n = 1597). Associations between comorbidities and sleep quality were examined using linear regression and dominance analysis.
Results:
Having more comorbidities was associated with poorer sleep quality (p for trend < 0.001). All 13 groups of comorbidities explained 12.9% of the variance in PSQI from which half of the variance was contributed by mental health disorders. In total, 16 of the 28 comorbidities were associated with significantly worse sleep quality, with the strongest associations seen for ‘other autoimmune diseases’ (β = 1.98), depression (β = 1.76), anxiety (β = 1.72) and rheumatoid arthritis (β = 1.62).
Conclusions:
Many individual comorbidities are associated with poorer sleep quality, with mental health disorders making the largest relative contribution. Optimal management of comorbidities that make the greatest contributions could have the largest benefit for improving sleep in MS.
Keywords: Comorbidities, PSQI, dominance analysis, multiple sclerosis, sleep
Introduction
Multiple sclerosis (MS) is a multifaceted illness profoundly affecting individuals, caregivers and healthcare system.1–3 Given its complex nature, effective management demands a multidisciplinary approach. 4 People living with multiple sclerosis (PwMS) frequently contend with a wide array of comorbid medical conditions, including, but not limited to, depression and cardiovascular diseases (>90%). 5 These comorbidities have been linked to adverse health outcomes. 6
In our previous study, as well as in other studies, it has been consistently shown that PwMS experience a higher prevalence of poor sleep quality (~67%)7,8 when compared to the general population (33%–45%). 9 In our earlier study, poor sleep quality was higher among those with younger age, overweight or obese, a recent MS relapse, higher disability levels and distinct symptom clusters (e.g. feelings of anxiety and depression). 8 However, we did not investigate whether comorbidities were associated with sleep quality. A few studies have investigated the association between individual comorbidities and sleep quality in PwMS. Each study focused on one or two comorbidities, such as migraine 10 or depression,11,12 with both medical conditions reducing sleep quality. While useful, this does not allow an assessment of the total burden of all comorbidities on sleep quality nor an assessment of the relative contribution of individual comorbidities. A Canadian study on adults with MS aged 55 years and over, revealed that those with a higher number of physical comorbidities experienced poor sleep quality compared to those with no comorbidity, 13 but this study solely considered physical comorbidities. Also, their assessment of sleep quality relied on a single question, rather than utilizing a more comprehensive assessment of self-assessed sleep quality such as the Pittsburgh Sleep Quality Index (PSQI). 14 Investigating poor sleep quality is important because of its association with poorer health-related quality of life (HRQoL) in people with MS. 8 Understanding the total impact of comorbidities and their individual contributions allows clinicians to tailor their consultations with MS patients with sleep problems and optimize the management of sleep problems.
We examined the associations between the total number of and/or types of comorbidities and sleep quality by assessing multiple comorbidities among a large sample of Australians with MS. In addition, we sought to quantify the relative contributions of groups of comorbidities to sleep quality and identify individual comorbidities with the most robust association with sleep quality.
Methods
Population and data source
This cross-sectional analysis used data from the Australian Multiple Sclerosis Longitudinal Study (AMSLS). Approximately 96% of AMSLS participants were diagnosed with MS by a neurologist, based on McDonald’s criteria. 15 Ethical permissions for the AMSLS were obtained from the Tasmanian Health and Medical Human Research Ethics Committee, ensuring adherence to ethical standards. Prior to enrolment in the AMSLS, each participant provided informed consent, expressing their willingness to take part.
Sleep data were sourced from the 2020 MS Nurses and Sleep Survey (February to April 2020). A total of 2499 active participants were invited, and 1722 (68.9%) responded, with 1717 of them completing PSQI. Notably, 94.4% of participants completed the survey before the first official Australian lockdown for the COVID-19 pandemic, which took place on 23 April 2020.
Comorbidity data were sourced from Lifestyle and Environment Surveys (October to December: 2016, 2018 and 2020). For the 2016 Lifestyle and Environment Survey, we invited 3112 people, with 1519 completing the survey (48.8%) and 1487 (97.9%) responding to at least one comorbidity question. In the 2018 Lifestyle and Environment Survey, we invited 2678 individuals, with 1708 completing the survey (63.8%). Similarly, in the 2020 Lifestyle and Environment Survey, we invited 2576 people, and 1491 completed the survey (57.9%). Using the comorbidity data obtained, we created a 2020 comorbidity data set and 2018 data set. For the final analysis, we included 1597 participants who had provided both comorbidity and sleep data, excluding 120 participants with PSQI data but no comorbidity data.
Measurements
PSQI
The PSQI is a 19-item patient-reported outcome measure that evaluates sleep quality over the past 4 weeks. 14 It is derived from seven component scores which include subjective sleep quality, sleep latency, sleep duration, habitual sleep efficiency, sleep disturbance, sleep medication and daytime dysfunction with a PSQI global score ranging from 0 to 21. A PSQI global score greater than 5 indicates poor sleep quality. 14 The instrument exhibits excellent sensitivity (89.6%) and specificity (86.5%) as evaluated over an 18-month period in a cohort of healthy individuals and a cohort of people with depression and sleep disorders. 14 Moreover, PSQI has been validated for use in PwMS. 16 In our study, Cronbach’s alpha coefficient for the PSQI items was 0.7, which is considered acceptable. 17 Although the minimum clinically important difference for PSQI in PwMS has not been established, estimates range from 1.3 to 4.4 for patients who underwent a rotator cuff tear repair. 18
Comorbidities
We collected data on 30 individual comorbidities, selected based on the most prevalent comorbidities observed in prior studies among PwMS. 19 Participants were asked to respond ‘Yes’ or ‘No’ to each comorbidity question, indicating whether they currently have or have had that specific medical condition. If participants answered ‘Yes’, they were coded as having that comorbidity. Next, we asked participants if the comorbidity was confirmed by a doctor, with response options of ‘Yes’, ‘No’ or ‘Don’t know’. If a participant answered ‘Yes’, the comorbidity was considered doctor-diagnosed. Those who responded ‘No’ or ‘Don’t know’ were categorized as not having a doctor-diagnosed comorbidity. Unless specified as ‘doctor-diagnosed’, we employed the term ‘comorbidity’ to denote the medical conditions reported by participants (self-reported). Those who completed the 2016 survey were only asked to report new comorbidities in 2018 and 2020. Similarly, those who completed the 2018 survey for the first time were only asked to report new comorbidities in 2020. We assumed that once comorbidity was reported, it was present for the remainder of the follow-up due to the chronicity or recurrent nature of nearly all of these conditions. The 2016 and 2018 data were combined to create a 2018 comorbidity data set, which included 2178 participants. Subsequently, this 2018 comorbidity data set was merged with the 2020 data to establish a 2020 comorbidity data set, comprising 2394 participants.
Taking power into account, conditions with fewer than 10 observations were excluded from the analysis specifically Schizophrenia (n = 6) and Parkinson’s disease (n = 0). Consequently, we utilized 28 individual comorbidities to calculate the total number of comorbidities and form groups of comorbidities. In the final analysis, each participant’s total number of comorbidities and total number of doctor-diagnosed comorbidities ranged from 0 (indicating no comorbidity) to 16 (maximum number of comorbidities reported by a participant in this study). To categorize individual comorbidities, we utilized the International Classification of Diseases (ICD-10) as previously applied in a study using AMSLS data. 5 Based on this classification system, we created 13 groups of comorbidities for further analysis. We combined several rare and medically related autoimmune diseases under the category ‘other autoimmune diseases’. This category was also utilized when investigating groups of comorbidities.
Other measures
Body mass index (BMI, kg/m2) was calculated using weight and height. In addition to BMI, the following demographic and clinical variables were included in the analysis: age, the highest educational attainment, sex, MS type, MS duration since diagnosis, MS duration since symptom onset and current use of disease-modifying therapy. All measures were self-reported.
Statistical analyses
Data management and statistical analysis were carried out using Stata v.17 (College Station, TX, USA). A significance level of p < 0.05 was considered statistically significant. Descriptive statistics were used to present a comprehensive overview of the distribution of participants’ characteristics. Chi-square and independent t-tests were applied to determine differences between those who completed the survey and those who did not complete it.
Linear regression models were fitted to explore associations between the total number of comorbidities or individual comorbidities and PSQI. The dose–response relationships for the total number of comorbidities were assessed using test-for-trend analysis. Beta coefficients were obtained from linear regressions, whereas predicted values were obtained using post-estimation modelling using a linear combination of coefficients (lincom). The analyses were adjusted for age, sex and educational attainment, ensuring that the potential confounding factors were considered during the investigation of associations between comorbidities and sleep quality.
We performed a general dominance analysis to estimate the relative contributions (%) of each comorbidity group to PSQI. 20 Considering the presence of correlations between groups of comorbidities, dominance analysis was employed as it is specifically designed to handle situations where predictors are correlated. 21 This method uses the coefficient of determination (R2) to determine and compare the relative importance of each comorbidity group to sleep quality.
Sensitivity analysis
For the primary analysis, we utilized comorbidity data from 2020. However, recognizing that some comorbidities might have occurred in 2020 after the sleep data was collected (6–8 months earlier), we conducted a secondary analysis by repeating the same analysis using the 2018 comorbidity data instead of the 2020 comorbidity data.
Results
Descriptive characteristics of the participants
Table 1 presents the clinical and demographic characteristics of the study cohort. The included participants did not show statistically significant differences in terms of sex distribution, and education level compared to those who were not included. However, the included participants were slightly older (+2.2 years, i.e. 57.9 vs 55.7) and had a longer duration of MS (+1.4 years, i.e. 17.9 vs 16.5) compared to those who were not included.
Table 1.
Descriptive characteristics of PwMS (N = 1597).
| Variables | Total | Sleep Quality | |
|---|---|---|---|
| Good (PSQI ⩽ 5) (n = 509, 31.9%) | Poor (PSQI > 5) (n = 1088, 68.1%) | ||
| Age (years) | 57.9 (11.3) | 58.4 (11.6) | 57.7 (11.2) |
| Sex, n (%) | |||
| Male | 327 (20.5) | 126 (24.7) | 201 (18.5) |
| Female | 1270 (79.5) | 383 (75.3) | 887 (81.5) |
| MS duration since diagnosis (years) | 17.9 (9.5) | 18.2 (9.2) | 17.7 (9.7) |
| MS duration since symptom onset (years) | 23.6 (10.9) | 23.2 (10.8) | 23.8 (11.0) |
| MS onset type, n (%) | |||
| Relapsing-onset MS | 1032 (84.3) | 334 (84.8) | 698 (84.1) |
| Progressive-onset MS | 192 (15.7) | 60 (15.2) | 132 (15.9) |
| Disease-modifying therapy, n (%) | |||
| No | 570 (35.8) | 191 (37.7) | 379 (35.0) |
| Yes | 1020 (64.2) | 316 (62.3) | 704 (65.0) |
| Education, n (%) (N = 1586) | |||
| Primary and secondary school | 405 (25.5) | 113 (22.3) | 292 (27.0) |
| Occupational certificate/diploma | 506 (31.9) | 147 (29.1) | 359 (33.2) |
| Univ. bachelor’s degree | 335 (21.1) | 130 (25.7) | 205 (19.0) |
| Univ. postgraduate degree | 266 (16.4) | 89 (17.6) | 177 (16.4) |
| Others | 74 (4.7) | 27 (5.3) | 47 (4.5) |
| Body mass index (kg/m2), n (%) | |||
| Healthy (BMI = 18.5–24.9) | 580 (38.8) | 204 (43.9) | 376 (36.6) |
| Overweight (BMI = 25–29.9) | 504 (33.8) | 166 (35.7) | 338 (32.9) |
| Obese (BMI ⩾ 30) | 409 (27.4) | 95 (20.4) | 314 (30.5) |
| Total number of any comorbidities a , n (%) | |||
| 0 | 103 (6.4) | 55 (10.8) | 48 (4.4) |
| 1 | 189 (11.8) | 92 (18.1) | 97 (8.9) |
| 2-3 | 555 (34.8) | 194 (38.1) | 361 (33.2) |
| ⩾4 | 750 (47.0) | 168 (33.0) | 582 (53.5) |
| Total number of doctor-diagnosed comorbidities, n (%) | |||
| 0 | 169 (10.6) | 84 (16.5) | 85 (7.8) |
| 1 | 253 (15.8) | 106 (20.8) | 147 (13.5) |
| 2-3 | 561 (35.1) | 183 (36.0) | 378 (34.7) |
| ⩾4 | 614 (38.5) | 136 (26.7) | 478 (44.0) |
| Sleep quality | |||
| Pittsburgh sleep quality index | 8.0 (4.2) | 3.6 (1.4) | 10.1 (3.30) |
| Use of sleep medication in the past month | |||
| No | 1004 (63.1) | 470 (92.3) | 534 (49.3) |
| Less than once a week | 144 (9.0) | 27 (5.3) | 117 (10.8) |
| Once or twice a week | 130 (8.2) | 4 (0.8) | 126 (11.6) |
| Three or more times a week | 314 (19.7) | 8 (1.6) | 306 (28.3) |
MS: multiple sclerosis; BMI: body mass index, variables are continuous (expressed in mean and standard deviation) unless stated.
Refers to self-reported comorbidities.
Association between the number of comorbidities and sleep quality
After adjusting for age, sex and education level, a higher total number of comorbidities were associated with a greater PSQI, explaining 11.4% of the variation in PSQI (Table 2). Compared to those without any comorbidities, those with two to three comorbidities and ⩾4 comorbidities had a 2.05- and 3.86-units greater PSQI score, respectively. Consistent results were observed when considering only doctor-diagnosed comorbidities.
Table 2.
Association between the number of comorbidities and PSQI score among PwMS (N = 1597).
| Number of comorbidities | PSQI score (0-21) | |
|---|---|---|
| Predicted PSQI mean (95% CI) | Adjusted coefficients (β, 95% CI) a | |
| Any comorbidities b | ||
| 0 | 5.61 (4.84,6.38) | 0.00 (Reference) |
| 1 | 6.36 (5.80,6.93) | 0.86 (-0.09,1.82) |
| 2-3 | 7.45 (7.12,7.78) | 2.05 (1.22,2.89) |
| ⩾4 | 9.23 (8.94,9.51) | 3.86 (3.03,4.68) |
| Test for trend | p < 0.001 | |
| Doctor-diagnosed comorbidities c | ||
| 0 | 5.99 (5.38,6.59) | 0.00 (Reference) |
| 1 | 6.88 (6.39,7.38) | 1.07 (0.29,1.84) |
| 2-3 | 7.73 (7.40,8.06) | 2.01 (1.32,2.69) |
| ⩾4 | 9.39 (9.04,9.68) | 3.72 (3.03,4.41) |
| Test for trend | p < 0.001 | |
PSQI score: Pittsburgh Sleep Quality Index to measure sleep quality.
Bold font portrays statistical significance at a 95% confidence interval. The coefficients were obtained from the linear regression analysis and the predicted PSQI mean was obtained from the margins command (adjusted result). The coefficient of determination (R2) was 11.4% for the adjusted model.
The values were adjusted for age, sex and educational attainment.
Total comorbidities refer to any self-reported comorbidity.
Doctor-diagnosed comorbidities refer to any comorbidity the patient reported as diagnosed by a doctor.
Relative contribution of groups of comorbidities to sleep quality
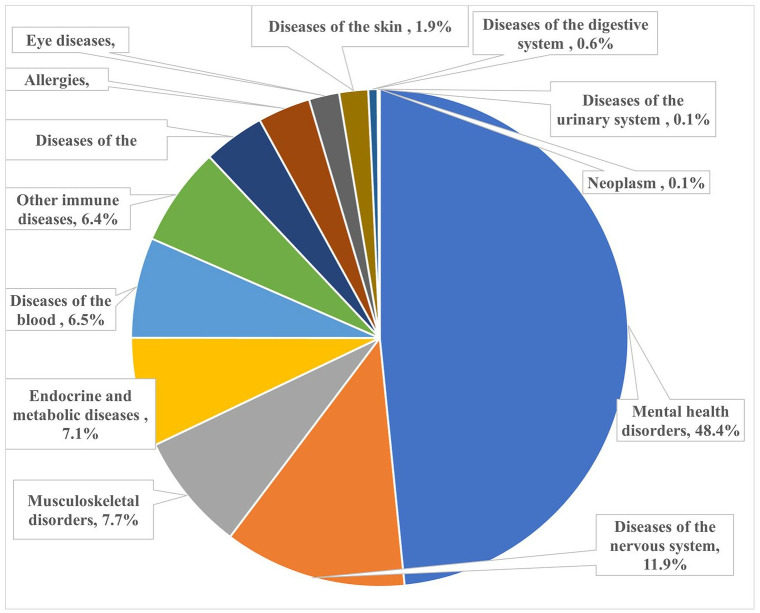
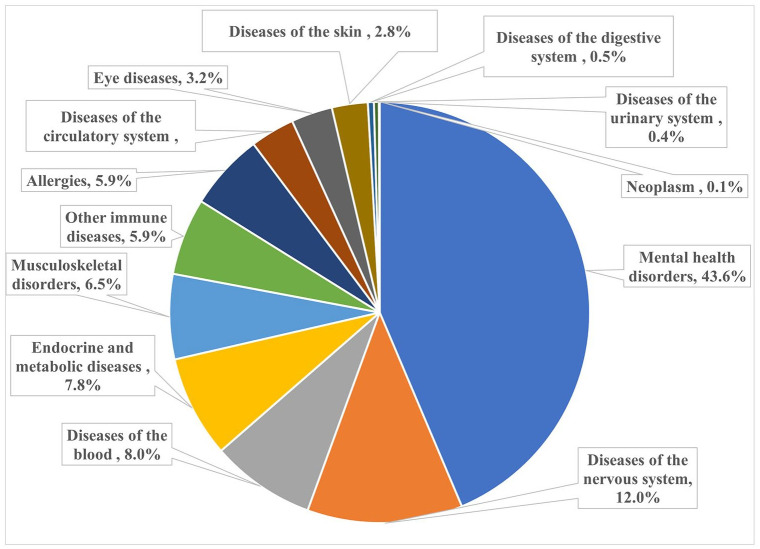
The 13 groups of comorbidities, collectively, explained 12.9% of the variance in PSQI scores, with mental health disorders, accounting for 48.4% (equivalent to 6.3% of the variance). Diseases of the nervous system and musculoskeletal disorders accounted for 11.9% and 7.7% of the variance, respectively (Table 3 and Figure 1). Similar results were obtained for doctor-diagnosed comorbidities (Figure 2).
Table 3.
Association between self-reported individual comorbidities and PSQI score among PwMS (N = 1597).
| Comorbidities | Comorbidities, n (%) a | Doctor-diagnosed, n (%) b | Predicted PSQI mean (95% CI) | Adjusted* | |
|---|---|---|---|---|---|
| (β, 95% CI) | R2 (%) | ||||
| Total variance (for 13 comorbidity groups) | 12.9 | ||||
| Predicted PSQI of those without comorbidities (reference) | 5.40 (4.63, 6.17) | ||||
| Mental health disorders | 6.3 | ||||
| Depression | 697 (43.6) | 585 (36.6) | 7.27 (6.39,8.16) | 1.76 (1.35,2.17) | |
| Anxiety | 662 (41.5) | 407 (25.5) | 7.23 (6.34,8.12) | 1.72 (1.31,2.13) | |
| Bipolar disorders | 18 (1.1) | 18 (1.1) | 6.07 (4.02,8.13) | 0.60 (-1.30,2.49) | |
| Diseases of the nervous system | 1.5 | ||||
| Migraine | 458 (28.7) | 308 (19.3) | 6.58 (5.66,7.50) | 1.09 (0.64,1.55) | |
| Epilepsy | 29 (1.8) | 27 (1.7) | 6.76 (5.04,8.49) | 1.29 (-0.24,2.81) | |
| Musculoskeletal disorders | 1.0 | ||||
| Osteoarthritis | 422 (26.4) | 397 (24.9) | 6.83 (5.92,7.75) | 1.42 (0.94,1.89) | |
| Osteoporosis | 250 (15.6) | 244 (15.3) | 5.49 (4.52,6.47) | 0.02 (-0.57,0.60) | |
| Rheumatoid arthritis | 51 (3.2) | 49 (3.1) | 7.09 (5.71,8.48) | 1.62 (0.48,2.76) | |
| Systemic lupus erythematosus | 18 (1.1) | 17 (1.0) | 7.01 (4.95, 9.06) | 1.53 (-0.36, 3.43) | |
| Endocrine and metabolic diseases | 1.0 | ||||
| High cholesterol | 423 (26.5) | 418 (26.2) | 6.30 (5.38,7.22) | 0.84 (0.37,1.30) | |
| Hypothyroidism | 136 (8.5) | 132 (8.3) | 6.09 (5.02,7.16) | 0.63 (-0.10,1.35) | |
| Type 2 diabetes mellitus | 92 (5.8) | 91 (5.7) | 6.97 (5.80,8.1) | 1.51 (0.64,2.38) | |
| Hyperthyroidism | 52 (3.3) | 51 (3.2) | 6.05 (4.67,7.43) | 0.57 (-0.56,1.70) | |
| Type 1 diabetes mellitus | 17 (1.1) | 17 (1.1) | 6.50 (4.39,8.61) | 1.02 (-0.93,2.97) | |
| Diseases of blood (anaemia) | 238 (14.9) | 232 (14.5) | 6.77 (5.79,7.75) | 1.29 (0.72,1.86) | 0.8 |
| Other autoimmune diseases† | 77 (4.8) | 74 (4.6) | 7.45 (6.23,8.67) | 1.98 (1.05,2.91) | 0.8 |
| Diseases of circulatory system | 0.5 | ||||
| Hypertension | 528 (33.1) | 516 (32.3) | 5.96 (5.05,6.86) | 0.50 (0.05,0.96) | |
| Heart disease | 146 (9.1) | 141 (8.8) | 6.73 (5.67,7.79) | 1.27 (0.56,1.98) | |
| Stroke | 46 (2.9) | 43 (2.7) | 5.99 (4.54,7.44) | 0.52 (-0.70,1.73) | |
| Peripheral vascular disease | 44 (2.8) | 42 (2.6) | 5.88 (4.42,7.35) | 0.41 (-0.83,1.65) | |
| Myocardial infarction | 35 (2.2) | 34 (2.1) | 6.95 (5.36,8.53) | 1.48 (0.10,2.85) | |
| Allergies | 661 (41.4) | 524 (32.8) | 6.15 (5.25,7.05) | 0.68 (0.26,1.10) | 0.4 |
| Eye diseases | 308 (19.3) | 294 (18.4) | 6.26 (5.31,7.21) | 0.80 (0.27,1.33) | 0.3 |
| Diseases of the skin (psoriasis) | 156 (9.8) | 129 (8.1) | 6.31 (5.27,7.35) | 0.83 (0.16,1.51) | 0.2 |
| Diseases of digestive system | 0.1 | ||||
| Inflammatory bowel disease | 57 (3.6) | 52 (3.3) | 6.76 (5.42,8.09) | 1.28 (0.20,2.36) | |
| Coeliac diseases | 29 (1.8) | 22 (1.4) | 5.21 (3.51,6.90) | -0.27 (-1.77,1.23) | |
| Diseases of the urinary system | 0.01 | ||||
| Chronic renal disease | 29 (1.8) | 25 (1.6) | 6.18 (4.49,7.88) | 0.71 (-0.79,2.22) | |
| Neoplasms (cancer) | 286 (17.9) | 282 (17.7) | 5.58 (4.62,6.53) | 0.10 (-0.43,0.64) | 0.01 |
Bold fonts indicate a statistically significant association between individual comorbidities and PSQI score at a 95% confidence interval. The R2 is the total variance (unadjusted) explained by 13 comorbidity groups, whereas individual variances (contributed by each comorbidity) were obtained from the general dominance analysis, that is, dominance stat. PSQI: Pittsburgh Sleep Quality Index. The predicted means (of PSQI) and coefficients were obtained from the linear combination of estimates in the model for any comorbidities.
Refers to the number and % of people with each comorbidity.
Refers to the number of comorbidities reported as doctor-diagnosed.
Alopecia, eczema, ankylosing spondylitis, antiphospholipid antibody syndrome, autoimmune thrombocytopenia purpura, granuloma annulare, Graves’ disease, Hashimoto’s thyroiditis, neuromyelitis optica, pemphigus, pernicious anaemia, primary sclerosing cholangitis, sarcoidosis, scleroderma, Sjogren’s disorder and vitiligo.
Indicates the regression was adjusted for age, sex and education.
Figure 1.
Relative contributions (%) of groups of comorbidities on PSQI score in MS.
Figure 2.
Relative contributions (%) of groups of doctor-diagnosed comorbidities on PSQI score in MS.
Association between individual comorbidities and PSQI score
After controlling for age, sex and education level, 16 out of 28 comorbidities were significantly associated with a greater mean PSQI score (Table 3). The effect sizes, compared to those without comorbidities, were most prominent for other autoimmune diseases (+1.98), depression (+1.76), anxiety (+1.72), rheumatoid arthritis (+1.62), type 2 diabetes mellitus (+1.51), myocardial infarction (+1.48), osteoarthritis (+1.42), anaemia (+1.29), inflammatory bowel disease (+1.28) and heart disease (+1.27). Significant associations were also observed for migraines, eye diseases, high cholesterol, allergies, psoriasis and hypertension. Similar results were obtained for doctor-diagnosed comorbidities (Supplementary Table 1).
Sensitivity analysis
Upon conducting the analysis using the 2018 comorbidity data instead of the 2020 comorbidity data, no significant differences in the results were observed in terms of both prevalence and effect sizes (Supplementary Tables 2 and 3 and Supplementary Figures 1 and 2).
Discussion
In this large MS cohort, most individual comorbidities were associated with poorer sleep quality. Collectively, the 13 comorbidity groups explained 12.9% of the variation in PSQI score, with mental health disorders explaining nearly half of that. Specifically, depression and anxiety exhibited the greatest impact on sleep quality, suggesting that optimal management of individual comorbidities could somewhat improve sleep quality in MS. Having more comorbidities was strongly associated with poorer sleep quality, with a clear dose–response relationship, emphasizing the need for comprehensive management of comorbidities to improve sleep outcomes.
Of the 28 comorbidities included in the analysis, 16 were significantly associated with worse sleep quality. Individuals with depression and anxiety exhibited higher PSQI scores (1.76 and 1.72 points higher, respectively) compared to those without comorbidities. In support of this, mental health disorders emerged as the most prominent comorbidity group, accounting for nearly half of the overall effect, underscoring the substantial influence of mental health disorders on sleep quality. Although the specific impact of comorbidity groups on sleep quality in MS has not been examined in previous studies, evidence supports that psychological burdens, including depression, exerting a deleterious effect on sleep in MS.12,11 In addition, evidence from Australia 5 and Turkey 22 indicated that depression impacts HRQoL in MS, suggesting the significant influence of mental health disorders on various aspects of well-being, with the Australian study using an HRQoL instrument that encompasses sleep. The association between mental health disorders (e.g. depression) and poor sleep is also biologically plausible in that individuals with depression exhibit slower transmission of serotonin, a neurotransmitter that plays a role in sleep. However, there is evidence that sleep may also influence depression. A longitudinal study conducted on children and adolescents without MS has demonstrated the bidirectional association between sleep and depression. 23 However, we found no studies exploring the bidirectional relationship between sleep and depression in MS. Indeed, clinically, sleep disturbance is a criterion that can be counted towards the diagnosis of both depression and anxiety in the Diagnostic and Statistical Manual of Mental Disorders (DSM-5), 24 which may partially explain their large effect size. As depression and anxiety can exist as independent comorbidities or secondary to MS, our findings suggest that identifying those with depression or anxiety and ensuring optimal management may contribute to improving sleep health.
On average, participants without comorbidities reported a mean PSQI score of 5.4. However, the presence of any comorbidity was associated with a higher PSQI score compared to those without comorbidities. For example, compared to those without any comorbidities, those with ⩾4 comorbidities had a 3.86-unit greater PSQI score. We found the number of comorbidities had a dose–response relationship with worse sleep quality. This is consistent with a previous study of physical comorbidities in PwMS living in Canada. 13 This holds significant value for healthcare workers in identifying and prioritizing the comorbidities that exert substantial influence on sleep quality to enhance the overall quality of sleep and potentially improve the well-being of people with MS.
While less prevalent (4.8%), other autoimmune diseases were significantly associated with poorer sleep quality, exhibiting a higher effect size (+1.98) compared to any other comorbidities. Numerous studies have established a link between autoimmune diseases and disturbed sleep in populations without MS.25,26 The contributions of other comorbidity groups, while relatively small compared to mental health disorders, still significantly affect sleep quality. Diseases of the nervous system, including migraine and epilepsy, explain 11.9% of the total variance of all comorbidity groups, and previous studies reported that higher migraine frequency was associated with worse sleep quality. 10 Addressing and effectively treating migraines may improve sleep outcomes, given their shared pathways with sleep circuits. 27 Despite smaller effect sizes compared to depression and anxiety, other individual comorbidities were also significantly associated with poor sleep quality. Anaemia was significantly associated with poorer sleep (effect size: +1.29). Previous research has established anaemia as a risk factor for adverse consequences in MS, primarily fatigue, which further impairs sleep quality. 11 Although these conditions have a low prevalence, myocardial infarction, heart disease, type 2 diabetes mellitus and inflammatory bowel disease were also associated with higher PSQI scores (above 1 PSQI unit), which were supported by previous studies.28,29 Similarly, allergies, hypertension, high cholesterol, eye disease and psoriasis were significantly associated with a higher PSQI score (effect sizes below 1 PSQI unit). While the individual impact of the comorbidities may be modest, the cumulative impact of comorbidities is likely to be substantial. Thus, it is essential for clinicians to adopt a holistic approach to managing comorbidities in individuals with MS, prioritizing those with the greatest contributions. In addition, a focus on health promotion may prevent or delay the onset of (additional) comorbidities. By implementing optimal management and health promotion strategies, individuals with MS can potentially achieve improved outcomes across various aspects of their health, including sleep.
All comorbidity groups explained 12.9% of the variance. It is important to recognize other factors that may play a role including MS symptoms (including fatigue), medications, lifestyle factors (e.g. caffeine consumption), environmental influences (like noise and light), stress and poor sleep hygiene (e.g. using electronic devices before bedtime).8,30,31 At the individual level, understanding and addressing these other factors will be important when assisting people with MS with poor sleep or when developing sleep interventions. Future research could include the conduct of trials that focus on optimizing the management of comorbidities and evaluating their impact on MS outcomes, including sleep quality. Previous studies focused on a limited set of comorbidities; however, we explored a wide range of comorbid conditions, allowing us to offer novel insights into the intricate interplay between comorbidities and sleep health in the context of MS. This information can guide the design of interventions to enhance sleep quality and help clinicians identify the root causes of their patients’ sleep experiences. As poor sleep has been associated with diminished HRQoL, 8 addressing these factors holds the potential to significantly improve overall quality of life for PwMS.
The inclusion of a large national sample is a strength of this study. Differences between active AMSLS participants who did and did not complete the surveys showed that those completing the surveys were slightly older and had a longer disease duration. While the AMSLS has been shown to be representative of the Australian MS population in 2010, 32 the overall sample is now older on average, with the recruitment of new, younger participants more challenging in this digital era. Having a slightly older population may have increased the number of comorbidities that were experienced. However, associations are generally robust to some selection bias, 33 and we adjusted our associations between comorbidities and sleep for age. In this study, female participation is higher (79.5%), which aligns with findings from previous studies. 5 In addition, we verified that the distribution of sex is similar between participants who completed the surveys and those who did not. Another limitation of this study was assuming that once comorbidity was reported, it was considered to be present for the remainder of the follow-up due to the chronic or recurrent nature of many of these conditions. While this assumption is reasonable for most included comorbidities, it may not hold true for some, such as anxiety and depression, which can change over time. As a result, we might have overestimated the prevalence of comorbidities that are not fixed over time, potentially leading to an underestimation of their effects on sleep. While we asked individuals about the presence of specific comorbidities, we did not include data on treatment, duration of comorbidities and complications associated with the comorbidities, which may affect the results. In future investigations, the inclusion of additional variables such as sleep hygiene, environmental conditions and caffeine consumption would be important to understand their roles in the association between comorbidities and poor sleep. Given we used comorbidity data collected approximately 6–8 months after the sleep data, some comorbidities may have developed after the sleep data was collected. Nevertheless, we observed similar findings when conducting a sensitivity analysis using the comorbidity data from 2018 (collected before the sleep data).
Conclusion
Many individual comorbidities assessed were associated with poor sleep quality, with depression and anxiety having the greatest impact on sleep, responsible for nearly half of the 12.9% variation in PSQI. Having more comorbidities was associated with worse sleep quality. Addressing comorbidities through optimal treatment and management holds the potential to substantially improve sleep quality for PwMS.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-docx-3-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-tif-4-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-tif-5-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Acknowledgments
The authors extend their heartfelt gratitude to the study participants for their invaluable cooperation, the Tasmania Graduate Research Scholarship and MS Australia for financial support. The authors are also thankful to the members of the MS Flagship for their valuable input, offering insightful comments and suggestions that significantly enriched our work. Furthermore, the authors like to express their special thanks to Dr Kirsty Hawkes (AMSLS Liaison Officer & Data Manager), Ms Carol Hurst (Research Officer) and Ms Hilary Waugh (Research Officer) for their exceptional assistance in managing and handling the data.
Footnotes
Author Contributions: Conceptualization and project design: B.D. and I.v.d.M.
Data management: B.D., I.v.d.M., B.T. and L.L.
Statistical analysis and interpretation of results: All authors.
Drafting of the manuscript: B.D., L.L. and I.v.d.M.
Critical revision of the manuscript for important intellectual content: All authors.
Secure funding: I.v.d.M.
Supervision: L.L.L., C.H., B.T. and I.v.d.M.
All authors approved the final work for submission and agree to be accountable for the aspects related to research integrity.
Data Availability Statement: The data used for the analysis in this study can be made available by the corresponding author upon reasonable request.
The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This study was supported by the MS Australia.
ORCID iDs: Baye Dagnew  https://orcid.org/0000-0002-2397-5930
https://orcid.org/0000-0002-2397-5930
Laura L Laslett  https://orcid.org/0000-0002-4336-0095
https://orcid.org/0000-0002-4336-0095
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Baye Dagnew, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia; College of Medicine and Health Sciences, University of Gondar, Gondar, Ethiopia.
Laura L Laslett, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Cynthia A Honan, School of Psychological Sciences, University of Tasmania, Launceston, TAS, Australia.
Leigh Blizzard, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Tania Winzenberg, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Bruce V Taylor, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
Ingrid van der Mei, Menzies Institute for Medical Research, University of Tasmania, Hobart, TAS, Australia.
References
- 1. Kobelt G, Pugliatti M. Cost of multiple sclerosis in Europe. Eur J Neurol 2005; 12: 63–67. [DOI] [PubMed] [Google Scholar]
- 2. Buhse M. Assessment of caregiver burden in families of persons with multiple sclerosis. J Neurosci Nurs 2008; 40(1): 25–31. [DOI] [PubMed] [Google Scholar]
- 3. Naci H, Fleurence R, Birt J, et al. Economic burden of multiple sclerosis: A systematic review of the literature. Pharmacoeconomics 2010; 28(5): 363–379. [DOI] [PubMed] [Google Scholar]
- 4. Perrin Ross A. Management of multiple sclerosis. Am J Manag Care 2013; 19(Suppl. 16): s301–s306. [PubMed] [Google Scholar]
- 5. Lo LMP, Taylor BV, Winzenberg T, et al. Estimating the relative contribution of comorbidities in predicting health-related quality of life of people with multiple sclerosis. J Neurol 2021; 268(2): 569–581. [DOI] [PubMed] [Google Scholar]
- 6. Al-Sakran L, Marrie RA, Blackburn D, et al. Impact of comorbidity on hospitalizations in individuals newly diagnosed with multiple sclerosis: A longitudinal population-based study. Mult Scler Relat Disord 2020; 40: 101955. [DOI] [PubMed] [Google Scholar]
- 7. Strober LB. Fatigue in multiple sclerosis: A look at the role of poor sleep. Front Neurol 2015; 6: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Laslett LL, Honan C, Turner JA, et al. Poor sleep and multiple sclerosis: Associations with symptoms of multiple sclerosis and quality of life. J Neurol Neurosurg Psychiatry 2022; 93: 1162–1165. [DOI] [PubMed] [Google Scholar]
- 9. Adams RJ, Appleton SL, Taylor AW, et al. Sleep health of Australian adults in 2016: Results of the 2016 sleep health foundation national survey. Sleep Health 2017; 3(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 10. Lin YK, Lin GY, Lee JT, et al. Associations between sleep quality and migraine frequency: A cross-sectional case-control study. Medicine 2016; 95(17): e3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Bøe Lunde HM, Aae TF, Indrevåg W, et al. Poor sleep in patients with multiple sclerosis. PLoS ONE 2012; 7(11): e49996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cederberg KLJ, Jeng B, Sasaki JE, et al. Demographic, clinical, and symptomatic correlates of subjective sleep quality in adults with multiple sclerosis. Mult Scler Relat Disord 2021; 55: 103204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Garland SN, Scurrey SRM, Ploughman M, et al. Factors associated with poor sleep in older adults with multiple sclerosis. Int J Behav Med 2017; 24(6): 937–945. [DOI] [PubMed] [Google Scholar]
- 14. Buysse DJ, Reynolds CF, Monk TH, et al. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res 1989; 28(2): 193–213. [DOI] [PubMed] [Google Scholar]
- 15. McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: Guidelines from the international panel on the diagnosis of multiple sclerosis. Ann Neurol 2001; 50(1): 121–127. [DOI] [PubMed] [Google Scholar]
- 16. Jerković A, Mikac U, Matijaca M, et al. Psychometric properties of the Pittsburgh Sleep Quality Index (PSQI) in patients with multiple sclerosis: Factor structure, reliability, correlates, and discrimination. J Clin Med 2022; 11(7): 2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Frey BB. The SAGE encyclopedia of educational research, measurement, and evaluation. Thousand Oaks, CA: Sage, 2018. [Google Scholar]
- 18. Longo UG, Berton A, De Salvatore S, et al. Minimal clinically important difference and patient acceptable symptom state for the Pittsburgh sleep quality index in patients who underwent rotator cuff tear repair. Int J Environ Res Public Health 2021; 18(16): 8666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Marrie RA, Cohen J, Stuve O, et al. A systematic review of the incidence and prevalence of comorbidity in multiple sclerosis: Overview. Mult Scler 2015; 21(3): 263–281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Budescu DV. Dominance analysis: A new approach to the problem of relative importance of predictors in multiple regression. Psychol Bull 1993; 114(3): 542–551. [Google Scholar]
- 21. Johnson JW. A heuristic method for estimating the relative weight of predictor variables in multiple regression. Multivariate Behav Res 2000; 35(1): 1–19. [DOI] [PubMed] [Google Scholar]
- 22. Gedik Z, Idiman E. Health-related quality of life in multiple sclerosis: Links to mental health, self-esteem, and self-compassion. Dusunen Adam: J Psychiatry Neurol Sci 2020; 33(1): 59–70. [Google Scholar]
- 23. Marino C, Andrade B, Montplaisir J, et al. Testing bidirectional, longitudinal associations between disturbed sleep and depressive symptoms in children and adolescents using cross-lagged models. JAMA Netw Open 2022; 5(8): e2227119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tolentino JC, Schmidt SL. DSM-5 criteria and depression severity: Implications for clinical practice. Front Psychiatry 2018; 9: 450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Blattner MS, de Bruin GS, Bucelli RC, et al. Sleep disturbances are common in patients with autoimmune encephalitis. J Neurol 2019; 266(4): 1007–1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Green ME, Bernet V, Cheung J. Thyroid dysfunction and sleep disorders. Front Endocrinol 2021; 12: 725829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holland PR, Barloese M, Fahrenkrug J. PACAP in hypothalamic regulation of sleep and circadian rhythm: Importance for headache. J Headache Pain 2018; 19(1): 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Almamari RSS, Muliira JK, Lazarus ER. Self-reported sleep quality and depression in post myocardial infarction patients attending cardiology outpatient clinics in Oman. Int J Nurs Sci 2019; 6(4): 371–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Khandelwal D, Dutta D, Chittawar S, et al. Sleep disorders in type 2 diabetes. Indian J Endocrinol Metab 2017; 21(5): 758–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Novak AM, Lev-Ari S. Resilience, stress, well-being, and sleep quality in multiple sclerosis. J Clin Med 2023; 12(2): 716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lanza G, Ferri R, Bella R, et al. The impact of drugs for multiple sclerosis on sleep. Mult Scler 2017; 23(1): 5–13. [DOI] [PubMed] [Google Scholar]
- 32. Taylor BV, Palmer A, Simpson S, Jr, et al. Assessing possible selection bias in a national voluntary MS longitudinal study in Australia. Mult Scler 2013; 19(12): 1627–1631. [DOI] [PubMed] [Google Scholar]
- 33. Ponsonby AL, Dwyer T, Couper D. Is this finding relevant? Generalisation and epidemiology. Aust N Z J Public Health 1996; 20(1): 54–56. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-docx-2-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-docx-3-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-tif-4-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal
Supplemental material, sj-tif-5-msj-10.1177_13524585241248278 for The association of comorbidities with sleep quality among Australians with multiple sclerosis: Insights from the Australian Multiple Sclerosis Longitudinal Study by Baye Dagnew, Laura L Laslett, Cynthia A Honan, Leigh Blizzard, Tania Winzenberg, Bruce V Taylor and Ingrid van der Mei in Multiple Sclerosis Journal