Abstract
Background:
The coronavirus disease 2019 (COVID-19) pandemic offered an epidemiological opportunity to evaluate if isolation and masking affected John Cunningham (JC) virus transmission.
Objective:
This study aimed to assess the proportion of natalizumab-treated patients who converted to a positive anti-JCV antibody serostatus before and during the pandemic.
Methods:
Data from TYSABRI Outreach: Unified Commitment to Health (TOUCH) for 22,375 US patients treated with natalizumab with anti-JCV antibody records were assessed in epochs annually from 2017 to 2022.
Results:
Pre-pandemic anti-JCV antibody serostatus change was observed for 7.4%–7.7%. During the first and second years of the pandemic, 7.3% and 7.2% of patients’ serostatus changed, respectively.
Conclusion:
The proportion of patients with anti-JCV antibody serostatus change did not significantly differ during the first 2 years of the pandemic compared with prior years. In contrast to seasonal influenza, masking and social distancing had no discernable effect on JCV serostatus change.
Keywords: John Cunningham virus, multiple sclerosis, natalizumab, COVID-19, progressive multifocal leukoencephalopathy, seroconversion, physical distancing, infections, rare disease
Introduction
The mode of transmission of John Cunningham virus (JCV) is not well understood, and the COVID-19 pandemic provided an unprecedented national epidemiological opportunity to evaluate the dynamics of JCV infection. It is thought that the majority of JCV infections occur early in childhood and remain quiescent in the kidneys, bone marrow, and lymphoid tissue.1,2 Latent JCV infection increases the risk of progressive multifocal leukoencephalopathy (PML) specifically in people with compromised immune systems. Natalizumab (Tysabri, Biogen), a disease-modifying therapy approved for the treatment of relapsing–remitting multiple sclerosis in 2006, is associated with a risk of PML. 3 The monitoring of PML risk during natalizumab treatment has been extensively studied, and JCV antibodies (Ab) can be used to stratify this risk. 4 Natalizumab-treated patients are routinely monitored to assess JCV Ab, typically at 6-month intervals.
Masking and social distancing practices during the COVID-19 pandemic were implemented in the United States in March 2020 and profoundly attenuated the spread of seasonal influenza in 2020–2021. 5 As the mode of JCV transmission is not well defined,1,2,6 we evaluated whether these social distancing practices recommended during the COVID-19 pandemic affected JCV transmission as assessed by JC Ab serostatus.
Methods
JCV Ab index data were extracted from the TYSABRI Outreach: Unified Commitment to Health (TOUCH) prescribing program for 22,375 patients with multiple sclerosis (MS) treated with natalizumab in the United States and with available anti-JCV Ab information between 1 April 2017 and 31 March 2022. Patients were assessed in annual epochs from 1 April to 31 March, in years 2017 through 2022, and were included in a given epoch if they had received > 1 dose of natalizumab in that year, had ⩾ 1 anti-JCV antibody record 3 months prior to April 1 of the specified year, and had ⩾ 1 anti-JCV antibody record after April 1 of that year. Serostatus change was defined as a change in anti-JCV antibody status from seronegative to seropositive using the STRATIFY JCV® DxSelect. 7 The percentage of patients with a serostatus change was defined as the number of patients with a serostatus change divided by the total number of patients included in the epoch (including both baseline negative and positive JCV serostatus).
To evaluate serostatus change differences across regions of the United States (Northeast, South, Central, and West), locations were determined from infusion site postal codes.
A sensitivity analysis was conducted to evaluate the number of patients with a serostatus change to positive within the natalizumab-treated patients with a negative JCV serostatus at baseline.
Results
The natalizumab-treated patients meeting inclusion criteria for each yearly epoch from 1 April 2017 to 31 March 2022 declined from 11,253 to 9774 (Table 1). The baseline characteristics were similar across all epochs except for baseline JCV serostatus; the percentage of patients with baseline negative JCV serostatus increased from 72.7% to 80.0% over the 5-year observation period.
Table 1.
Baseline demographics for the overall population by epoch (April 1–March 31). Annual JCV serostatus change (negative to positive) rates in the United States from 2017 to 2022 by gender and by age group.
| 2017–2018 (N = 11,253) |
2018–2019 (N = 10,449) |
2019–2020 (N = 10,169) |
2020–2021 (N = 9914) |
2021–2022 (N = 9774) |
|
|---|---|---|---|---|---|
| Baseline demographics | |||||
| Age (years), M (SD) | 40.8 (11.37) | 40.3 (11.26) | 40.1 (11.24) | 39.9 (11.18) | 39.7 (11.14) |
| Age group (years), n (%) | |||||
| <30 | 1949 (17.3) | 1904 (18.2) | 1920 (18.9) | 1896 (19.1) | 1877 (19.2) |
| 30–39 | 3319 (29.5) | 3178 (30.4) | 3092 (30.4) | 3115 (31.4) | 3143 (32.2) |
| 40–49 | 3340 (29.7) | 3068 (29.4) | 2986 (29.4) | 2893 (29.2) | 2821 (28.9) |
| 50–59 | 2057 (18.3) | 1806 (17.3) | 1710 (16.8) | 1558 (15.7) | 1499 (15.3) |
| >60 | 588 (5.2) | 493 (4.7) | 461 (4.5) | 452 (4.6) | 434 (4.4) |
| Gender | |||||
| Female, n (%) | 8225 (73.6) | 7837 (75.3) | 7668 (75.7) | 7531 (76.3) | 7502 (77.0) |
| Region, n (%) | |||||
| Northeast | 2809 (25.7) | 2609 (25.7) | 2503 (25.4) | 2409 (25.1) | 2396 (25.3) |
| Central | 2331 (21.3) | 2176 (21.5) | 2087 (21.2) | 2071 (21.5) | 1934 (20.4) |
| South | 2982 (27.2) | 2755 (27.2) | 2725 (27.6) | 2726 (28.4) | 2648 (28.0) |
| West | 2831 (25.9) | 2600 (25.6) | 2550 (25.9) | 2408 (25.1) | 2484 (26.3) |
| Negative baseline JCV serostatus, n (%) | 8178 (72.7) | 8056 (77.1) | 8033 (79.0) | 7869 (79.4) | 7817 (80.0) |
| Annual JCV serostatus change (negative to positive) rates a in the United States from 2017 to 2022 by gender | |||||
| 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | 2021–2022 | |
| Male, % (n/N) | 8.8% (258/2947) |
8.3% (214/2567) |
6.9% (170/2458) |
7.4% (173/2341) |
8.9% (199/2237) |
| Female, % (n/N) | 7.4% (605/8225) |
7.1% (557/7837) |
7.5% (577/7668) |
7.3% (550/7531) |
6.7% (505/7502) |
| Annual JCV serostatus change (negative to positive) rates a in the United States from 2017 to 2022 by age group (years) | |||||
| 2017–2018 | 2018–2019 | 2019–2020 | 2020–2021 | 2021–2022 | |
| <30, % (n/N) | 6.8% (133/1949) |
6.6% (25/1904) |
7.4% (142/1920) |
7.2% (136/1896) |
7.7% (144/1877) |
| 30–39, % (n/N) | 7.8% (260/3319) |
7.3% (233/3178) |
6.4% (198/3092) |
6.8% (208/3115) |
7.4% (232/3143) |
| 40–49, % (n/N) | 7.6% (255/3340) |
7.8% (240/3068) |
7.4% (220/2986) |
7.2% (207/2893) |
6.9% (195/2821) |
| 50–59, % (n/N) | 8.4% (173/2057) |
7.5% (137/1806) |
8.5% (146/1710) |
8.4% (131/1558) |
6.3% (94/1499) |
| >60, % (n/N) | 8.5% (50/588) |
8.5% (42/493) |
10.0% (46/461) |
9.3% (42/452) |
9.5% (41/434) |
Patients with missing information on age, gender, or region were excluded when calculating percentages.
Defined as the number of patients with a serostatus change divided by the total number of patients included in the epoch.
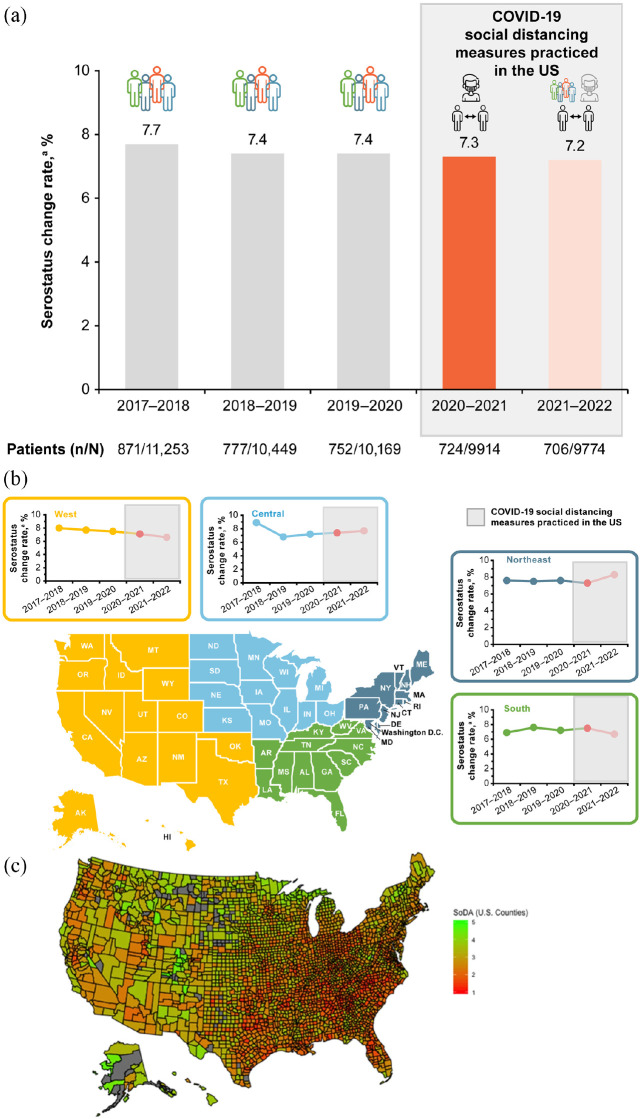
In the pre-pandemic years, from 1 April 2017 to 31 March 2018, anti-JCV antibody serostatus change was observed for 7.7% of patients, with a serostatus change for 7.4% and 7.4% of patients in the following 2 years (2018–2019 and 2019–2020, respectively; Figure 1(a)). During the first and second years of the COVID-19 pandemic (2020–2021 and 2021–2022), serostatus change was observed for 7.3% and 7.2% of patients, respectively (Figure 1(a)). When looking at JCV serostatus change per US region (Figure 1(b)), by gender, or by age group (Table 1), there were no discernable trends referable to the pandemic years. Seroconversion rates, in general, were higher in the oldest group of patients, both before and during the pandemic, as shown in Table 1
Figure 1.
(a) Annual JCV serostatus change (negative to positive) rates in the United States from 2017 to 2022 for the overall population. (b) Annual JCV serostatus change (negative to positive) rates in the United States from 2017 to 2022 by US region. COVID-19 social distancing measures implemented in 2020–2021 (shown in bright red) with restrictions lifting in 2021–2022 (shown in faint red). (c) The regional COVID-19 social distancing adherence (SoDA) on a county-level is shown to depict the extent to which these measures were adopted across the United States in Spring 2020. 8
aDefined as the number of patients with a serostatus change divided by the total number of patients included in the epoch.
bThe social distancing adherence map shown in Figure 1(c) was published in “Prediction of COVID-19 Social Distancing Adherence (SoDA) on the United States county-level” by M. Ingram, A. Zahabian, and C. Hur, Humanit Soc Sci Commun 8, 87 (2021) and is licensed under CC BY 4.0. This map represents an analysis by Ingram et al. of data from the Unacast Social Distancing Dataset from 16 March 2020 (first day of the national stray at home order) to 24 April 2020 (first day a state relaxed the social distancing guidelines). Higher SoDA scores (green) indicate greater adherence to social distancing guidelines. Counties that are gray did not have sufficient amount of cellphone data to be included.
The results of the sensitivity analysis, in which the serostatus change was calculated using only the JCV negative at baseline population, showed similar results as the entire population analysis and showed no noteworthy trend. During the pre-pandemic years, 1 April 2017 to 31 March 2018, anti-JCV antibody serostatus change was observed for 10.7% of patients, with serostatus change of 9.6% and 9.4% of patients in the following 2 years (2018–2019 and 2019–2020). During the first and second years of the COVID-19 pandemic (2020–2021 and 2021–2022), serostatus changed for 9.2% and 9.0% of patients, respectively.
Conclusion
As the mode and patterns of JC virus transmission are incompletely understood, the pandemic offered a unique opportunity to lend more clarity around the patterns of JC seroconversion. The proportion of natalizumab-treated patients with an anti-JCV serostatus change did not meaningfully differ during the first 2 years of the COVID-19 pandemic (2020–2022) in comparison with the three pre-pandemic years. These results suggest that, in contrast to the decreased incidence of seasonal influenza attributed to masking and social distancing during the pandemic, 5 these practices had no discernable effect on JCV serostatus changes. Considering that masking and social distancing measures were adopted to varying degrees across US regions, 8 it is notable that there were no geographic patterns to JCV serostatus change during the pandemic era.
The serostatus change rates observed in the sensitivity analyses are higher than the 3%–8% annual change rate that is reported in the prescribing information for natalizumab, 3 which may be due to different definitions of serostatus change. Whereas the prescribing information defines serostatus change as changing from anti-JCV antibody negative at baseline to antibody positive at a later timepoint and remaining positive at all subsequent timepoints, in these analyses, serostatus change is inclusively defined as switching from anti-JCV antibody negative at baseline to positive at any later timepoint and includes patients who may have tested intermittently negative during the epoch. Other potential explanations for differences in seroconversion rates between populations could include level of baseline antibody index; this information, however, is not included in the TOUCH database which captures seropositivity but not index values. 9
This study includes the largest population to examine the JCV transmission pattern before and during the COVID-19 pandemic. Limited conclusions can be drawn from these data, however, given the study was retrospective in nature, observational, and there was no adjustment for repeated measurements for yearly serostatus change calculations that could lead to within-individual association bias. Furthermore, while age, sex, and geographic region did not affect serostatus change during the period of social distancing at a population level, social distancing and masking practices on an individual patient level are unknown, and the dataset did not allow for stratification by race or other sociodemographic factors. Another limitation of the study is that the number of patients who discontinued natalizumab or switched to alternative therapies during the pandemic is unknown; however, a large sample size continued natalizumab during the study period and thus had longitudinal serostatus information available.
In the United States, the risk of JCV seroconversion was not attenuated during a period of social isolation and masking, which suggests that neither direct contact with a JCV carrier nor air transmission are likely the mode of JCV transmission. Given the latency of JCV in the kidneys, another hypothesized route of JCV transmission is through urinary shedding and urine-oral transmission. 6 Therefore, other such modes of transmission are possible, which may contribute to our findings. The increase in handwashing and sanitation practices during the COVID era also did not yield a reduction in JC seroconversion. While further studies are needed to understand JCV transmission, our results suggest that no changes in lifestyle of people with MS are warranted to endeavor to prevent JC seroconversion.
Acknowledgments
Medical writing and editorial support for the development of this manuscript, under the direction of the authors, was provided by Holly Engelman, PhD, and Celia Nelson of Ashfield MedComms, an Inizio Company, and funded by Biogen. Cara Farrell from Excel Scientific Solutions copyedited and styled the manuscript per journal requirements. The authors provided final approval of all content.
Footnotes
Data Availability Statement: Data sharing is not applicable to this article as no data sets were generated or analyzed during the current study.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: S.C.K. reports consulting or advisory work with Biogen, EMD Serono, Genentech, Genzyme/Sanofi, Novartis, Octave, and TG Therapeutics; nonpromotional speaking with Biogen, EMD Serono, and Genentech; grant and research support from Biogen, Bristol Myers Squibb (BMS), Novartis, and Sanofi. S.S., F.H., J.S., T.J.K., K.W., and R.L.A. are the employees of and may hold stock and/or stock options in Biogen.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was investigator-initiated and supported by Biogen.
Contributor Information
Stephen C Krieger, Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, 5 East 98th Street, Box 1138, New York, NY 10029, USA; Corinne Goldsmith Dickinson Center for Multiple Sclerosis, Icahn School of Medicine at Mount Sinai, New York, NY, USA.
Susie Sinks, Biogen, Cambridge, MA, USA.
Furong Huang, Biogen, Cambridge, MA, USA.
Julie Steverson, Biogen, Cambridge, MA, USA.
Tamar J Kalina, Biogen, Cambridge, MA, USA.
Kurt White, Biogen, Cambridge, MA, USA.
Robin L Avila, Biogen, Cambridge, MA, USA.
References
- 1. Atkinson AL, Atwood WJ. Fifty years of JC polyomavirus: A brief overview and remaining questions. Viruses 2020; 12(9): 969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pietropaolo V, Prezioso C, Bagnato F, et al. John Cunningham virus: An overview on biology and disease of the etiological agent of the progressive multifocal leukoencephalopathy. New Microbiol 2018; 41(3): 179–186. [PubMed] [Google Scholar]
- 3. Biogen. TYSABRI (natalizumab, Prescribing information). Cambridge, MA: Biogen, 2021. [Google Scholar]
- 4. Plavina T, Subramanyam M, Bloomgren G, et al. Anti-JC virus antibody levels in serum or plasma further define risk of natalizumab-associated progressive multifocal leukoencephalopathy. Ann Neurol 2014; 76(6): 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Centers for Disease Control Prevention. 2020–2021 flu season summary, https://www.cdc.gov/flu/season/faq-flu-season-2020-2021.htm (accessed 8 February 2023)
- 6. Berger JR, Miller CS, Mootoor Y, et al. JC virus detection in bodily fluids: Clues to transmission. Clin Infect Dis 2006; 43(1): e9–12. [DOI] [PubMed] [Google Scholar]
- 7. Lee P, Plavina T, Castro A, et al. A second-generation ELISA (STRATIFY JCV™ DxSelect™) for detection of JC virus antibodies in human serum and plasma to support progressive multifocal leukoencephalopathy risk stratification. J Clin Virol 2013; 57(2): 141–146. [DOI] [PubMed] [Google Scholar]
- 8. Ingram M, Zahabian A, Hur C. Prediction of COVID-19 social distancing adherence (SoDA) on the United States county-level. Humanit Soc Sci Commun 2021; 8: 87. [Google Scholar]
- 9. Hegen H, Auer M, Bsteh G, et al. Stability and predictive value of anti-JCV antibody index in multiple sclerosis: A 6-year longitudinal study. PLoS ONE 2017; 12(3): e0174005. [DOI] [PMC free article] [PubMed] [Google Scholar]