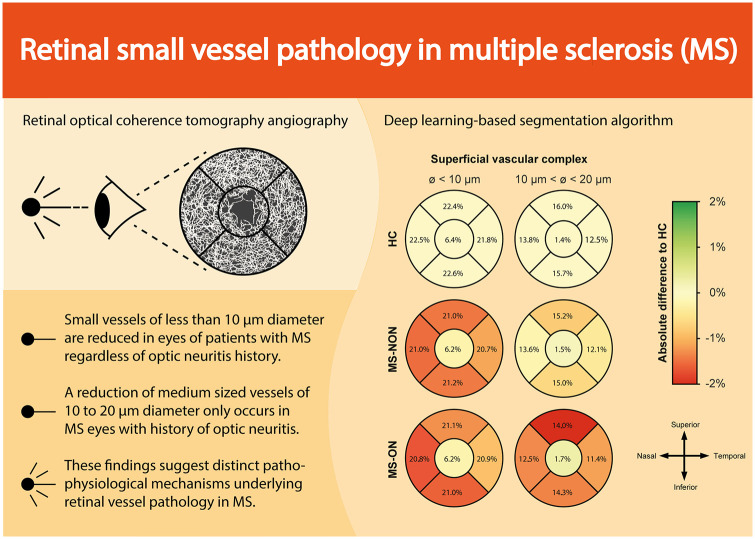
Abstract
Background:
Alterations of the superficial retinal vasculature are commonly observed in multiple sclerosis (MS) and can be visualized through optical coherence tomography angiography (OCTA).
Objectives:
This study aimed to examine changes in the retinal vasculature during MS and to integrate findings into current concepts of the underlying pathology.
Methods:
In this cross-sectional study, including 259 relapsing–remitting MS patients and 78 healthy controls, we analyzed OCTAs using deep-learning-based segmentation algorithm tools.
Results:
We identified a loss of small-sized vessels (diameter < 10 µm) in the superficial vascular complex in all MS eyes, irrespective of their optic neuritis (ON) history. This alteration was associated with MS disease burden and appears independent of retinal ganglion cell loss. In contrast, an observed reduction of medium-sized vessels (diameter 10–20 µm) was specific to eyes with a history of ON and was closely linked to ganglion cell atrophy.
Conclusion:
These findings suggest distinct atrophy patterns in retinal vessels in patients with MS. Further studies are necessary to investigate retinal vessel alterations and their underlying pathology in MS.
Keywords: Multiple sclerosis, pathophysiology, retinal microvasculature, optical coherence tomography angiography, deep learning, neuroophthalmology
Graphical Abstract.
Introduction
Alterations of the optic nerve and the retina are commonly observed in multiple sclerosis (MS), a chronic autoimmune disorder characterized by inflammation, demyelination and axonal loss in the central nervous system (CNS). 1 Optical coherence tomography (OCT) is a non-invasive imaging technique that allows high-resolution visualization of the retina. In patients with MS, inner retinal layer thinning is frequently found and has been linked to neurodegenerative processes in the CNS and might be indicative of a worse disease prognosis.2–7
Beyond retinal layer alterations, growing evidence suggests changes in the retinal vasculature in patients with MS. OCT angiography (OCTA), an extension of OCT imaging, allows for detailed visualization of the retinal vasculature. Here, successive scans are acquired at a single location of the retina. After removing stationary tissue signals, the residual signal represents moving erythrocytes in veins and arteries down to the capillary level. 8 OCTA has been increasingly employed for diagnosing and managing ophthalmologic conditions, such as age-related macular degeneration, diabetic retinopathy and glaucoma. In neurodegenerative diseases, such as Alzheimer’s and Parkinson’s disease, reduced vessel densities seem to correlate with disease severity. A reduction of blood flow in the optic nerve has been demonstrated for both arteritic (AION) and non-arteritic (NAION) optic neuropathies and correlates with the loss of peripheral visual field and visual acuity. However, it is not possible to differentiate between the two entities. 9 In MS patients with acute optic neuritis (ON), rarefication of superficial retinal vessels occurs in parallel to ON-related ganglion cell and inner plexiform layer (GCIP) atrophy within the initial 3 months.10–13 Recent data suggest that retinal vessel loss is also evident in eyes without an ON history in MS and might be associated with disease activity.13,14 It is controversially discussed whether superficial retinal vessel rarefication results from a primary retinal vessel pathology or results secondary to an altered retinal metabolism following ganglion cell loss. 10
In this study, we investigated changes in the retinal vasculature in MS patients with and without a history of ON by analyzing OCTA images with a deep learning (DL)-based segmentation algorithm. Furthermore, we correlated alterations of retinal vessels to changes in the retinal architecture and visual function, advancing current hypotheses of the underlying pathology.
Methods
Study design
In this cross-sectional cohort study, we included patients with relapsing–remitting MS (RRMS) according to the 2017 McDonald consensus criteria 15 and healthy controls (HCs) aged 18–75 years. RRMS patients were recruited as part of an ongoing observational study (TUM-MS study) at the Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health of the Technical University of Munich from 2018 to 2023. HCs were age-matched from an already existing cohort of volunteers in our department and were screened for relevant comorbidities. All participants underwent neurological examination including the expanded disability status scale (EDSS), visual testing, 10 retinal OCT and OCTA examination. Intraocular pressure was not measured. We obtained an extensive medical history of each participant, which included any diseases affecting the visual system. Participants’ eyes were stratified according to eyes with an ON history (MS-ON) and without an ON history (MS-NON). Eyes with subclinical ON were treated as eyes with clinical ON. Participants with any history of eye disease or affection other than ON, refractory errors exceeding more than 6 diopters, and insufficient OCT or OCTA quality were excluded.
Standard protocol approvals, registration and patient consent
This study adhered to the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines received approval from the ethics commission of the Technical University of Munich, School of Medicine (166/16S, 2023-526-S-KH) and followed the principles outlined in the Declaration of Helsinki. Written informed consent was obtained from all participants and patients.
OCT and OCTA image analysis
Conventional OCT images of the macula (Early Treatment Diabetic Retinopathy Study (ETDRS) grid extracted from a 30° × 25° macular scan) were acquired using a spectral-domain OCT (Heidelberg Engineering’s Spectralis OCT2) as previously described. 16 All images were checked for sufficient quality according to the OSCAR-IB criteria. 17 The vendor-provided software (Eye Explorer, v2.5.4) was used to automatically obtain the retinal layer segmentations, which were manually corrected if needed. Subclinical ON was considered in individuals with an inter-eye difference in peripapillary retinal nerve fibre layer (pRNFL) and the combined GCIP measures exceeding 5 and 4 µm, respectively. 3
We recorded OCTA data using the same spectral-domain OCT device with its angiography module (Heidelberg Engineering Spectralis OCT2) as described elsewhere. 10 En-face images and decorrelation signals were recorded within a 2.9 × 2.9 mm region focusing on the fovea centralis. To minimize motion artefacts, active eye tracking was ensured by a software built-in algorithm. All OCTA images were quality-controlled according to the OSCAR-MP criteria. 18
We assessed vessel densities of the superficial (SVC) and deep (DVC) vascular complex around the fovea in 2.5-mm eccentricity (area 4.9 mm2) using a previously introduced DL-based segmentation of the retinal vasculature. 19 Here, a neural network is trained using transfer learning on a large dataset of synthetically generated OCTA images before being employed for segmentation of real OCTA images. Previous studies have demonstrated the ability of this approach to extract high-quality segmentation maps of the retinal vasculature from OCTA images.19,20 In particular, the smallest blood vessels are identified with a higher accuracy and consistency compared to commonly used segmentation methods relying on vesselness filters or thresholding. 21 Based on the obtained vessel segmentations, we extract corresponding vascular grafts using the Voreen software.22,23 This allows us to characterize individual vessel segments with regard to their length and diameter. In this work, we differentiated densities of vessels with a diameter of < 10, 10–20 and > 20 µm. For further analysis. we divided the described macular into five sectors (centre, superior, temporal, inferior and nasal; see Figure 1) comparable to an ETDRS grid. The foveal avascular zone was calculated based on the DVC.
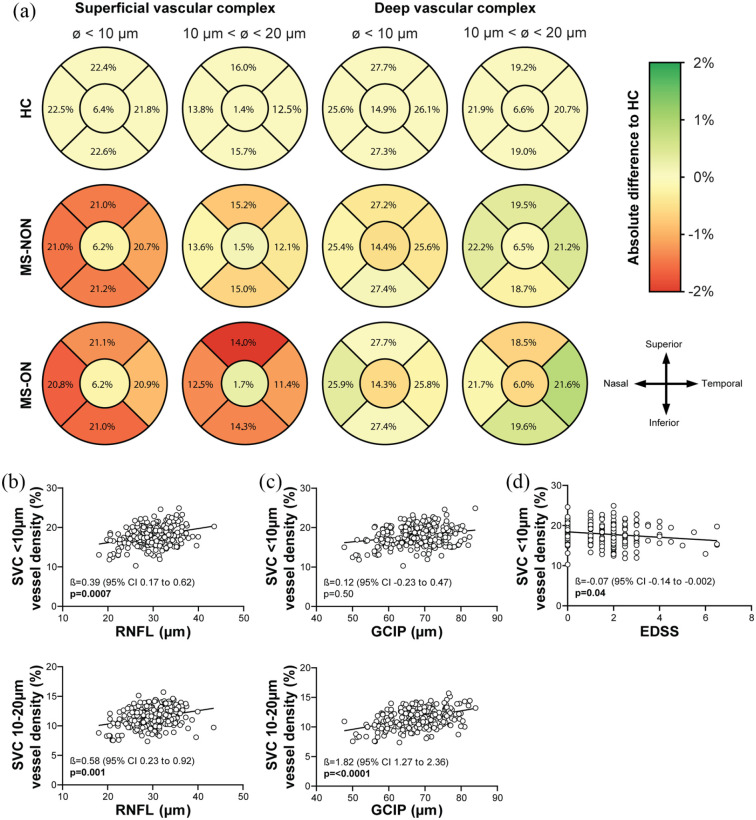
Figure 1.
Alterations of the retinal vasculature and its association with retinal layer atrophy and disease burden. (a) Following the Early Treatment Diabetic Retinopathy Study (ETDRS) grid, we divided the macula into five sectors: centre, nasal, superior, inferior and temporal. Alterations in vessel densities in both the left and right eyes are superimposed on the morphology of the left eye. An increase and decrease in vessel density are shown in green and red, respectively. In comparison to HC (upper panel), a reduction of the smallest vessels of the SVC (diameter < 10 µm) can be observed in all outer sectors in both MS-ON (middle panel) and MS-NON (lower panel) cohorts. A loss of medium-sized vessels (diameter 10–20 µm) is only observed in all outer sectors of the MS-ON cohort. No significant changes were seen in the DVC. (b) Atrophy of the RNFL is associated with small- and medium-sized vessels (diameter < 20 µm) in patients with MS irrespective of history of ON. (c) In contrast, GCIP loss is predominantly associated with reduced medium-sized vessels (diameter 10–20 µm). (d) A loss of small vessels is associated with more severe disability. (b–d) All p-values depicted were corrected for age and gender. The p-values for regression analysis of SVC < 10 µm vessel density and RNFL or GCIP (B + C upper panels) were corrected for SVC 10–20 µm vessel densities and vice versa (B + C lower panels).
P-values < 0.05 are marked as bold.
DVC: deep vascular complex; GCIP: ganglion cell combined inner plexiform layer; HC: healthy control; MS: multiple sclerosis; NON: no history of optic neuritis; ON: history of optic neuritis; RNFL: retinal nerve fibre layer; SVC: superficial vascular complex.
Statistical analysis
We performed statistical analyses using GraphPad Prism (v9.5.0). We applied the paired-eye statistical approach to account for inter-eye correlations. If values of both eyes were available for assignment to the same test group, the mean was used as one data point. If not, the remaining eye was used. 24 For demographics, we used the Mann–Whitney test for non-parametric data and the Fisher’s exact test for gender analysis. To quantify differences between the three cohorts, we performed an ordinary one-way analysis of variance (ANOVA) with Tukey’s multiple comparisons if data were distributed normally or a non-parametric Kruskal–Wallis test with Dunn’s multiple comparisons if not. Values are provided as median (25%–75% interquartile range (IQR)). We used multiple linear regression models, corrected for age and sex, to analyze associations between OCT and OCTA values on visual acuity, disease duration and burden (EDSS). Respective estimates (β-value) are given as regression parameters. We accepted a p-value of less than 0.05 as significant.
Results
Demographics and OCT characteristics
In this cross-sectional study, we included 337 participants (674 eyes) comprising 259 RRMS patients and 78 HCs. In total, 107 eyes (24 HCs, 72 MS-NON and 11 MS-ON) were excluded due to poor OCTA quality, resulting in 567 eyes of 337 participants included in the study. Participants were balanced concerning age and gender (median age RRMS 39 (26–54) years, RRMSNON 39 (30–51) and RRMSON 39 (31–46) years), HC 39 (31–49) years, p = 0.58; RRMS 72% female (RRMSNON 72% female, RRMSON 78% female), HC 76% female, p = 0.56). However, 30% of MS patients had at least one episode of ON (78 patients out of 259 patients). In 14 patients, this ON episode occurred within 3 months prior to OCTA analysis. Patients with RRMS revealed short disease durations (median 14 (2–60) months) and only mild clinical disability (median EDSS 1.5 (0–2.0)). MS-ON eyes showed a significantly lower visual acuity as compared to MS-NON and HC eyes (Table 1). As expected, both MS-ON and MS-NON eyes revealed atrophy of the RNFL, and the GCIP as compared to healthy eyes whereas RNFL and GCIP thinning was pronounced in MS-ON eyes. Inner nuclear layer thickening occurred in MS-ON eyes (Table 1).
Table 1.
Demographics, OCT and OCTA characteristics.
| HC n = 132 eyes |
MS-NON n = 352 eyes |
MS-ON n = 83 eyes |
ANOVA p-value | |
|---|---|---|---|---|
| Visual acuity a (LogMAR) | ||||
| HCVA | 0.00 (−0.05 to 0.00) | 0.0 (−0.11 to 0.01) | 0.01 (0.00 to 0.22) | <0.0001 b |
| LCVA | 0.60 (0.48 to 0.70) | 0.60 (0.48 to 0.70) | 0.70 (0.49 to 1.05) | 0.0002 c |
| OCT d (µm) | ||||
| RNFL | 33.3 (32.8 to 35.9) | 30.8 (28.0 to 33.3) | 27.9 (23.5 to 30.4) | <0.0001 e |
| GCIP | 71.3 (69.0 to 74.8) | 68.8 (64.0 to 72.5) | 62.3 (56.8 to 67.7) | <0.0001 f |
| INL | 34.5 (32.8 to 35.9) | 34.3 (33.1 to 36.2) | 35.4 (33.7 to 37.5) | 0.003 g |
| OCTA | ||||
| SVC (% vessel density) | 32.8 (30.8 to 34.3) | 31.1 (28.9 to 33.1) | 30.2 (27.2 to 32.5) | <0.0001 h |
| DVC (% vessel density) | 41.9 (40.4 to 43.4) | 42.0 (40.5 to 43.4) | 41.6 (40.1 to 43.5) | 0.59 |
| FAZ (mm2) | 0.10 (0.08 to 0.13) | 0.11 (0.08 to 0.13) | 0.11 (0.09 to 0.14) | 0.17 |
| OCTA stratified by vessel diameter (% vessel density) | ||||
| SVC < 10 µm | 19.4 (17.8 to 20.7) | 18.1 (16.3 to 19.9) | 17.9 (16.0 to 19.6) | <0.0001 i |
| SVC 10–20 µm | 12.1 (11.3 to 12.7) | 11.7 (10.4 to 12.6) | 10.8 (9.4 to 12.4) | 0.0001 j |
| SVC > 20 µm | 0.60 (0.34 to 0.99) | 0.56 (0.34 to 0.89) | 0.38 (0.21 to 0.74) | 0.01 k |
ANOVA: Analysis of variance; CI: confidence interval; DVC: deep vascular complex; FAZ: foveal avascular zone; GCIPL: ganglion cell combined inner plexiform layer; HC: healthy control; HCVA: high-contrast visual acuity; INL: inner nuclear layer; KWT: Kruskal–Wallis test; LCVA: low-contrast visual acuity; MS: multiple sclerosis; NON: no history of optic neuritis; OCT optical coherence tomography; OCTA optical coherence tomography angiography; ON: history of optic neuritis; RNFL: retinal nerve fibre layer; SVC: superficial vascular complex. p-values < 0.05 are marked bold.
nHC 92 eyes, nMS-NON 294 eyes and nMS-ON 71 eyes.
KWT; multiple comparisons: pHC vs MS-ON 0.02, pMS-NON vs ON < 0.0001.
KWT; multiple comparisons: pHC vs MS-ON 0.01, pMS-NON vs ON < 0.0001.
nHC 115 eyes, nMS-NON 349 eyes and nMS-ON 83 eyes.
One-way ANOVA; multiple comparisons: pHC vs MS-NON < 0.0001 (mean difference 4.4 CI 0.65–8.1), pHC vs MS-ON < 0.0001 (11.2 CI 6.7–15.8), pMS-NON vs ON < 0.0001 (6.9 CI 3.3–10.4).
One-way ANOVA; multiple comparisons: pHC vs MS-NON 0.002 (3.1 CI 1.0–5.2), pHC vs MS-ON < 0.0001 (9.0 CI 6.5–11.6), pMS-NON vs ON < 0.0001 (5.9 CI 3.9–7.9).
One-way ANOVA; multiple comparisons: pHC vs MS-ON 0.01 (−0.03 CI −0.06 to −0.006), pMS-NON vs ON 0.003 (−0.03 CI −0.05 to −0.009).
KWT; multiple comparisons: pHC vs MS-NON 0.0003, pHC vs MS-ON < 0.0001.
One-way ANOVA; multiple comparisons: pHC vs MS-NON < 0.0001 (1.3 CI 0.6–2.1), pHC vs MS-ON 0.0002 (1.6 CI 0.7–2.5).
One-way ANOVA; multiple comparisons: pHC vs MS-NON 0.08 (0.4 CI −0.04 to 0.9), pHC vs MS-ON < 0.0001 (1.1 CI 0.5–1.7), pMS-NON vs ON 0.006 (0.6 CI 0.1–1.1).
KWT; multiple comparisons: pHC vs MS-ON 0.03, pMS-NON vs ON 0.01.
Changes of the retinal vasculature in OCTA images
In the first step, we evaluated changes in retinal vessel densities across all vessel diameters. Here, we recognized a rarefication of SVC vessel densities in MS-NON and MS-ON as compared to HC eyes where no differences were detected between MS-NON and MS-ON eyes. Vessel measures of the DVC and FAZ were comparable across all three groups (Table 1). In the second step, we further analyzed alterations of the SVC by stratifying the retinal vasculature into different vessel diameters. We detected a comparable loss of small vessels (diameter < 10 µm) in MS-NON and MS-ON eyes as compared to healthy eyes which was evident in the majority of perifoveal quadrants (Figure 1(a) and Supplemental Figure). Interestingly, a rarefication of intermediate (diameter 10–20 µm) and large vessel (diameter > 20 µm, Table 1) occurred exclusively in MS-ON, but not in MS-NON eyes as compared to HC.
Association of superficial retinal vessel loss, the retinal architecture and disability
In the third step, we aimed to analyze whether changes in the SVC are associated with certain alterations of the retinal architecture. Here, thinning of the RNFL was associated with both loss of vessels with < 10 and 10–20 µm diameter (Figure 1(b)) regardless of an ON history (data not shown). Ganglion cell atrophy correlated with rarefication of medium-sized vessels (diameter 10–20 µm) but not with small vessel structures (diameter < 10 µm) as depicted in Figure 1(c).
In the last step, we searched for associations of retinal vessel thinning and disease burden in patients with RRMS. Here, thinning of small-sized (diameter < 10 µm, Figure 1(d)) but not medium-sized vessels (diameter 10–20 µm: β −0.08, p = 0.16) was associated with increased disability (EDSS). Both small- (diameter < 10 µm: β −4.94, p = 0.0006) and medium-sized vessels (diameter 10–20 µm: β −8.23, p = 0.0002) were associated with longer disease durations.
Discussion
The presented study uses advanced DL-based analysis of OCTA images to gain an in-depth view of pathological alterations of the retinal vasculature during MS. On one hand, we recognized a reduction of small-sized vessels (diameter < 10 µm) as a consistent finding in all MS eyes irrespective of an ON history. This change correlated with the MS disease burden and seemed to occur independently of retinal ganglion cell loss. On the other hand, rarefication of medium-sized vessels (diameter 10–20 µm) was predominantly detected in eyes with an ON history and was closely associated with ganglion cell loss. These findings suggest different pathophysiological mechanisms of retinal vessel pathology during MS.
Several groups have previously used OCTA to investigate retinal vessel changes in patients with MS as reviewed elsewhere.14,25 The rarefication of the SVC appears to be a consistent finding. To date, the underlying mechanism responsible for the rarefication of superficial vessels remains elusive. In this study, we used a novel DL-based segmentation algorithm that allows higher accuracy in identification and stratification of the smallest vessels. We found that medium-sized vessels (diameter 10–20 µm) were reduced mainly in eyes with a history of ON and accompanying ganglion cell atrophy. Moreover, vessel density measures of those vessels correlated with GCIP thickness. We hypothesize that rarefication of these vessels might thus be pathophysiologically linked to the decline of ganglion cells. These findings are in line with the literature and recent work from our group involving patients with acute ON. 10 In that study, we observed a concurrent decline of ganglion cells and a reduction in the SVC during the initial 3 months after an acute ON episode. Vessels of the SVC supply the RNFL and GCIP with blood and oxygen. 26 Consequently, the loss of ganglion cells and the reduced oxygen demand may potentially result in decreased perfusion of medium-sized superficial retinal vessels. As such, we hypothesize that the loss of medium-sized vessels may be a secondary phenomenon of an altered retinal metabolism due to ganglion cell atrophy.
As another finding, we detected a decreased perfusion of small-sized retinal vessels (< 10 µm) across all patients with MS that was linked to MS disease duration and disability but occurred largely independently of ganglion cell layer changes. Autopsy studies have revealed histopathological changes in the retina of most MS patients, including hypertrophy and loss of resident glial cells, activated microglia and disruption of tight junctions. 27 These alterations may collectively contribute to a compromised blood–retina barrier. We speculate that these changes might lead to impaired microcirculation and the loss of small-sized vessels. The latter phenomenon has been observed in other autoimmune diseases such as neuromyelitis optica spectrum disorders in which rarefication of perifoveal superficial vessel structures correlated with the damage of retinal astrocytes. 28 Our prior research has linked an ON-independent reduction of superficial vessels to inflammatory processes in the CNS. 13 Therefore, we suspect inflammation-related mechanisms within the retina that instigate fundamental changes in the retinal microvasculature. Numerous studies in the past have shown that MS leads to a change in the phenotype of microglia and astrocytes within the brain, spinal cord and retina. In advanced stages of the disease, these changes can also be found far away from demyelinated areas in the brain and might reflect a systemic, CNS intrinsic process. 29 It is assumed that chronically activated and altered glial cells drive local inflammatory processes, representing the pathophysiological correlate for progressive and relapse-independent disability progression.1,30 In this study, we speculate that altered retinal glial cells and an impaired blood–retina barrier might be the pathological correlate of retinal small vessel loss. 31 Further histopathological studies are necessary to investigate the involvement of resident glial cells in microvascular changes of the retina.
Our study has several limitations. OCTA is a relatively new technique and is highly sensitive to artefacts that can impact vessel density measurements. However, all examinations were carried out by experienced technicians, and we implemented a stringent approach by adhering to newly recommended criteria for OCTA quality control. 18 In addition, due to its methodology, OCTA can only provide information about perfusion and cannot distinguish between a genuine loss of vessels and merely vessel constriction. Finally, despite being extensively benchmarked in previous studies, our method to segment the retinal vasculature and characterize individual vessel segments performs worse in image regions with very high vessel density and overlapping segments.19,20 However, by excluding images with severe flow projection artefacts and analyzing the individual vascular complexes separately, we were able to mitigate the impact of this shortcoming. Furthermore, participants underwent no ophthalmological check-up, including intraocular pressure measurement, to confirm the lack of any subclinical eye disease or affection other than ON, as stated by the participant.
In conclusion, a distinct atrophy pattern of superficial small- and medium-sized retinal vessels can be observed during MS. Additional research is required to incorporate retinal vessel abnormalities into the pathophysiological framework of MS.
Supplemental Material
Supplemental material, sj-tif-1-msj-10.1177_13524585241247775 for Retinal small vessel pathology is associated with disease burden in multiple sclerosis by Rebecca Wicklein, Linus Kreitner, Anna Wild, Lilian Aly, Daniel Rueckert, Bernhard Hemmer, Thomas Korn, Martin J Menten and Benjamin Knier in Multiple Sclerosis Journal
Acknowledgments
The authors thank Mira Radic, Elisabeth Wolf, Nicolas Banze, Christina Noll and Eva Feodora Romahn for expert assistance during OCTA acquisition and analysis.
Footnotes
Author Contributions: B.K. contributed to conception and design of the study. R.W., L.K., A.W., L.A., D.R., B.H., T.K. M.J.M and B.K. involved in acquisition and analysis of data. R.W., M.J.M. and B.K drafted a significant portion of the article or figures. All authors read and approved the final article.
Data Availability: The data are not accessible to the public due to privacy and ethical considerations. However, on a reasonable request from a qualified investigator, the data can be shared in an anonymized format.
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: R.W. received a poster grant by Novartis. L.A. received a research grant by Novartis and travel grants by Novartis, Sanofi and Horizon therapeutics. B.H. has served on scientific advisory boards for Novartis; he has served as DMSC member for AllergyCare, Sandoz, Polpharma, Biocon and TG therapeutics; he or his institution have received speaker honoraria from Desitin; his institution received research grants from Regeneron and Roche for MS research. He holds part of two patents; one for the detection of antibodies against KIR4.1 in a subpopulation of patients with MS and one for genetic determinants of neutralizing antibodies to interferon. All conflicts are not relevant to the topic of the study. B.K. received travel support and speaking honoraria from Novartis Deutschland GmbH, Teva Deutschland, Merck, and Heidelberg Engineering and served at the advisory board of Merck. He received a research grant from Novartis. L.K., A.W., D.R., T.K. and M.J.M. have nothing to disclose.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: R.W. received an intramural research grant from the Technical University of Munich, School of Medicine and was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC–2145–SyNergy ID 390857198). L.K. was supported by ERC Advanced Grant Deep4MI (884622). L.A. received funding by Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (SyNergy). D.R. and M.J.M. were supported by the Munich Centre for Machine Learning. B.H. was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) under Germany’s Excellence Strategy within the framework of the Munich Cluster for Systems Neurology (EXC 2145 SyNergy – ID 390857198, Macroscale Hub) and the European Union’s Horizon 2020 Research and Innovation Programme (grant MultipleMS, EU RIA 733161). T.K. was supported by the Deutsche Forschungsgemeinschaft (SFB1054 (ID 210592381), TRR128 (ID 213904703), TRR274 (ID 408885537), TRR355 (ID 490846870), GRK2668 (ID 435874434) and EXC 2145 (SyNergy, ID 390857198)) and by the Hertie Network of Clinical Neuroscience. B.K. is funded by the Deutsche Forschungsgemeinschaft DFG (528297171), the Else Kröner-Fresenius-Stiftung (Else Kröner-Fresenius Exzellenzstipendium), the Gemeinnützige Hertie Foundation (medMS programme) and received a research award from Novartis (Oppenheim award 2020).
Supplemental Material: Supplemental material for this article is available online.
ORCID iD: Benjamin Knier  https://orcid.org/0000-0003-4187-9472
https://orcid.org/0000-0003-4187-9472
Contributor Information
Rebecca Wicklein, Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany.
Linus Kreitner, Institute for AI and Informatics in Medicine, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany.
Anna Wild, Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany.
Lilian Aly, Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany.
Daniel Rueckert, Institute for AI and Informatics in Medicine, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany; BioMedIA, Imperial College London, London, UK.
Bernhard Hemmer, Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany; Munich Cluster of Systems Neurology (SyNergy), Munich, Germany.
Thomas Korn, Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany; Munich Cluster of Systems Neurology (SyNergy), Munich, Germany; Institute for Experimental Neuroimmunology, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany.
Martin J Menten, Institute for AI and Informatics in Medicine, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany; BioMedIA, Imperial College London, London, UK.
Benjamin Knier, Department of Neurology, Klinikum rechts der Isar, TUM School of Medicine and Health, Technical University of Munich, Munich, Germany; Department of Neurology and Geriatric Neurology, Diakonie Klinikum Schwäbisch Hall, Schwäbisch Hall, Germany.
References
- 1. Hemmer B, Kerschensteiner M, Korn T. Role of the innate and adaptive immune responses in the course of multiple sclerosis. Lancet Neurol 2015; 14(4): 406–419. [DOI] [PubMed] [Google Scholar]
- 2. Lambe J, Fitzgerald KC, Murphy OC, et al. Association of spectral-domain OCT with long-term disability worsening in multiple sclerosis. Neurology 2021; 96: e2058–e2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Nolan-Kenney RC, Liu M, Akhand O, et al. Optimal intereye difference thresholds by optical coherence tomography in multiple sclerosis: An international study. Ann Neurol 2019; 85(5): 618–629. [DOI] [PubMed] [Google Scholar]
- 4. Petzold A, Fraser CL, Abegg M, et al. Diagnosis and classification of optic neuritis. Lancet Neurol 2022; 21: 1120–1134. [DOI] [PubMed] [Google Scholar]
- 5. Romahn EF, Wiltgen T, Bussas M, et al. Association of retinal vessel pathology and brain atrophy in relapsing-remitting multiple sclerosis. Front Immunol 2023; 14: 1284986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wauschkuhn J, Solorza Buenrostro G, Aly L, et al. Retinal ganglion cell loss is associated with future disability worsening in early relapsing-remitting multiple sclerosis. Eur J Neurol 2023; 30(4): 982–990. [DOI] [PubMed] [Google Scholar]
- 7. Zimmermann HG, Knier B, Oberwahrenbrock T, et al. Association of retinal ganglion cell layer thickness with future disease activity in patients with clinically isolated syndrome. JAMA Neurol 2018; 75: 1071–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Javed A, Khanna A, Palmer E, et al. Optical coherence tomography angiography: A review of the current literature. J Int Med Res 2023; 51(7): 3000605231187933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Suh A, Ong J, Kamran SA, et al. Retina oculomics in neurodegenerative disease. Ann Biomed Eng 2023; 51(12): 2708–2721. [DOI] [PubMed] [Google Scholar]
- 10. Aly L, Noll C, Wicklein R, et al. Dynamics of retinal vessel loss after acute optic neuritis in patients with relapsing multiple sclerosis. Neurol Neuroimmunol Neuroinflamm 2022; 9(3): e1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Feucht N, Maier M, Lepennetier G, et al. Optical coherence tomography angiography indicates associations of the retinal vascular network and disease activity in multiple sclerosis. Mult Scler 2019; 25(2): 224–234. [DOI] [PubMed] [Google Scholar]
- 12. Murphy OC, Kwakyi O, Iftikhar M, et al. Alterations in the retinal vasculature occur in multiple sclerosis and exhibit novel correlations with disability and visual function measures. Mult Scler 2020; 26(7): 815–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Noll C, Hiltensperger M, Aly L, et al. Association of the retinal vasculature, intrathecal immunity, and disability in multiple sclerosis. Front Immunol 2022; 13: 997043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mohammadi S, Gouravani M, Salehi MA, et al. Optical coherence tomography angiography measurements in multiple sclerosis: A systematic review and meta-analysis. J Neuroinflammation 2023; 20: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol 2018; 17: 162–173. [DOI] [PubMed] [Google Scholar]
- 16. Knier B, Leppenetier G, Wetzlmair C, et al. Association of retinal architecture, intrathecal immunity, and clinical course in multiple sclerosis. JAMA Neurol 2017; 74: 847–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tewarie P, Balk L, Costello F, et al. The OSCAR-IB consensus criteria for retinal OCT quality assessment. PLoS ONE 2012; 7(4): e34823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wicklein R, Yam C, Noll C, et al. The OSCAR-MP consensus criteria for quality assessment of retinal optical coherence tomography angiography. Neurol Neuroimmunol Neuroinflamm 2023; 10(6): e200169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kreitner L, Ezhov I, Rueckert D, et al. Automated analysis of diabetic retinopathy using vessel segmentation maps as inductive bias, 2022, https://arxiv.org/pdf/2210.16053.pdf
- 20. Kreitner L, Paetzold JC, Rauch N, et al. Detailed retinal vessel segmentation without human annotations using simulated optical coherence tomography angiographs. IEEE Trans Med Imaging 2024; Jan 15:PP. [DOI] [PubMed] [Google Scholar]
- 21. Giarratano Y, Bianchi E, Gray C, et al. Automated segmentation of optical coherence tomography angiography images: Benchmark data and clinically relevant metrics. Transl Vis Sci Technol 2020; 9(13): 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Meyer-Spradow J, Ropinski T, Mensmann J, et al. Voreen: A rapid-prototyping environment for ray-casting-based volume visualizations. IEEE Comput Graph Appl 2009; 29(6): 6–13. [DOI] [PubMed] [Google Scholar]
- 23. Paetzold JC, McGinnis J, Shit S, et al. Whole brain vessel graphs: A dataset and benchmark for graph learning and neuroscience. In: Proceedings of the neural information processing systems track on datasets and benchmarks (ed Vanschoren J, Yeung S.), virtual, December 2021. [Google Scholar]
- 24. Cruz- Herranz A, Balk LJ, Oberwahrenbrock T, et al. The APOSTEL recommendations for reporting quantitative optical coherence tomography studies. Neurology 2016; 86: 2303–2309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Pellegrini M, Vagge A, Ferro Desideri LF, et al. Optical coherence tomography angiography in neurodegenerative disorders. J Clin Med 2020; 9: 1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Campbell JP, Zhang M, Hwang TS, et al. Detailed vascular anatomy of the human retina by projection-resolved optical coherence tomography angiography. Sci Rep 2017; 7: 42201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Green AJ, McQuaid S, Hauser SL, et al. Ocular pathology in multiple sclerosis: Retinal atrophy and inflammation irrespective of disease duration. Brain 2010; 133(Pt 6): 1591–1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Aly L, Strauß EM, Feucht N, et al. Optical coherence tomography angiography indicates subclinical retinal disease in neuromyelitis optica spectrum disorders. Mult Scler 2022; 28(4): 522–531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kutzelnigg A, Lucchinetti CF, Stadelmann C, et al. Cortical demyelination and diffuse white matter injury in multiple sclerosis. Brain 2005; 128(Pt 11): 2705–2712. [DOI] [PubMed] [Google Scholar]
- 30. Healy LM, Stratton JA, Kuhlmann T, et al. The role of glial cells in multiple sclerosis disease progression. Nat Rev Neurol 2022; 18(4): 237–248. [DOI] [PubMed] [Google Scholar]
- 31. Maisam Afzali A, Stüve L, Pfaller M, et al. Aquaporin-4 prevents exaggerated astrocytosis and structural damage in retinal inflammation. J Mol Med 2022; 100(6): 933–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-tif-1-msj-10.1177_13524585241247775 for Retinal small vessel pathology is associated with disease burden in multiple sclerosis by Rebecca Wicklein, Linus Kreitner, Anna Wild, Lilian Aly, Daniel Rueckert, Bernhard Hemmer, Thomas Korn, Martin J Menten and Benjamin Knier in Multiple Sclerosis Journal