Abstract
Background and aims:
Cerebral microbleeds are magnetic imaging resonance (MRI) markers of hemorrhage-prone cerebral small vessel disease that predict future risk of ischemic stroke and intracranial hemorrhage (ICrH). There exist concerns about the net benefit of antithrombotic therapy in patients with microbleeds. We aimed to investigate the effects of an oral factor-XIa inhibitor (asundexian), that is hypothesized to inhibit thrombosis without compromising hemostasis, on the development of new microbleeds over time and interactions between microbleeds and asundexian treatment on clinical outcomes. We additionally assessed associations between baseline microbleeds and the risks of clinical and neuroimaging outcomes in patients with non-cardioembolic ischemic stroke.
Methods:
This is a secondary analysis of the PACIFIC-STROKE, international, multi-center Phase 2b double-blind, randomized clinical trial. PACIFIC-STROKE enrolled patients aged ⩾ 45 years with mild-to-moderate non-cardioembolic ischemic stroke who presented within 48 h of symptom onset for whom antiplatelet therapy was intended. Microbleeds were centrally adjudicated, and participants with an interpretable T2*-weighted sequence at their baseline MRI were included in this analysis. Patients were randomized to asundexian (10/20/50 mg daily) versus placebo plus standard antiplatelet treatment. Regression models were used to estimate the effects of (1) all pooled asundexian doses and (2) asundexian 50 mg daily on new microbleed formation on 26-week MRIs. Cox proportional hazards or regression models were additionally used to estimate interactions between treatment assignment and microbleeds for ischemic stroke/transient ischemic attack (TIA) (primary outcome), and ICrH, all-cause mortality, hemorrhagic transformation (HT), and new microbleeds (secondary outcomes).
Results:
Of 1746 participants (mean age, 67.0 ± 10.0; 34% female) with baseline MRIs, 604 (35%) had microbleeds. During a median follow-up of 10.6 months, 7.0% (n = 122) had ischemic stroke/TIA, 0.5% (n = 8) ICrH, and 2.1% (n = 37) died. New microbleeds developed in 10.3% (n = 155) of participants with adequate follow-up MRIs and HT in 31.4% (n = 345). In the total sample of patients with adequate baseline and 26-week follow-up MRIs (n = 1507), new microbleeds occurred in 10.2% of patients assigned to any asundexian dose and 10.5% of patients assigned to placebo (OR, 0.96; 95% CI, 0.66–1.41). There were no interactions between microbleeds and treatment assignment for any of the outcomes (p for interaction > 0.05). The rates of new microbleeds, HT, and ICrH were numerically less in patients with microbleeds assigned to asundexian relative to placebo. The presence of microbleeds was associated with a higher risk of HT (aOR, 1.6; 95% CI, 1.2–2.1) and new microbleeds (aOR, 4.4; 95% CI, 3.0–6.3).
Conclusion:
Factor XIa inhibition with asundexian appears safe in patients with non-cardioembolic ischemic stroke and hemorrhage-prone cerebral small vessel disease marked by microbleeds on MRI. These preliminary findings will be confirmed in the ongoing OCEANIC-STROKE randomized trial.
Trial Registration:
ClinicalTrials.gov Identifier: NCT04304508.
Keywords: Brain microbleeds, clinical trial, ischemic stroke, prevention, stroke, antithrombotic
Introduction
Cerebral microbleeds are radiological markers of hemorrhage-prone cerebral small vessel disease detected on blood-sensitive magnetic resonance imaging (MRI) sequences. Microbleeds are present in about one-third of patients with ischemic stroke and indicate prior microhemorrhage.1,2 Microbleeds have been associated with a 1.2-fold increased risk of recurrent ischemic stroke and a 2.5-fold increased risk of intracerebral hemorrhage (ICH). 3 These associations have raised concerns about the net benefit of antithrombotic treatment in ischemic stroke patients with microbleeds and led some to advocate avoidance of anticoagulation in these patients. However, subgroup analyses of randomized trials conducted to date have not demonstrated deleterious treatment interactions between microbleeds and antithrombotic treatment for clinical outcomes.4–7
Factor XI/XIa inhibitors are new anticoagulants that are hypothesized to inhibit pathologic thrombus formation without compromising hemostasis. In the PACIFIC-STROKE randomized trial, Factor XIa inhibition with asundexian was suggested to reduce ischemic stroke/transient ischemic attack (TIA) in patients with acute non-cardioembolic ischemic stroke without significantly increasing bleeding when compared with placebo on top of background standard antiplatelet treatment. 8 We performed secondary analyses of the PACIFIC-STROKE trial with the primary aims of estimating the effect of asundexian relative to placebo on the development of new microbleeds during study participation and to investigate potential interactions between treatment assignment and microbleeds for the outcomes of ischemic stroke/TIA, intracranial hemorrhage (ICrH), mortality, hemorrhagic transformation (HT) of the qualifying infarct, and new microbleeds on follow-up MRI. In addition, we aimed to characterize the determinants of microbleeds in PACIFIC-STROKE participants and the association between microbleeds and the risks of the aforementioned outcomes. We hypothesized that asundexian treatment would not increase the risk of new microbleeds on follow-up MRI and that there would be no significant interactions between baseline microbleeds and treatment assignment for the outcomes of interest.
Methods
Study design
The design and main results of the PACIFIC-STROKE trial have been reported elsewhere. 8 The protocol was approved by the relevant health authorities and the institutional review boards at each participating site, and written informed consent was obtained from participants or their legally authorized representative. The trial is registered at clinicaltrials.gov (NCT04304508). Further details are found in the supplemental material.
Study participants
Individuals aged ⩾ 45 years with mild-to-moderate (National Institutes of Health Stroke Scale (NIHSS) =0–15) non-cardioembolic ischemic stroke who presented within 48 h of symptom onset and were intended for antiplatelet therapy were eligible. The main exclusion criteria were prior ischemic stroke within 30 days of index event, history of atrial fibrillation, active bleeding or history of major bleeding, uncontrolled hypertension, estimated glomerular filtration rate of <30 mL/min/1.73 m2, significant liver disease, or contraindication to MRI. To be included in the present secondary analyses, participants were additionally required to have an interpretable T2*-sequence allowing for microbleed detection available on their baseline MRI. This study followed the Consolidated Standards of Reporting Trials (CONSORT) reporting guideline. 9
Intervention
Eligible participants were randomly assigned in a 1:1:1:1 ratio to receive one of three asundexian dosages (10, 20, and 50 mg once daily) or placebo, in addition to usual background antiplatelet therapy. Participants were followed for 6–12 months.
Data collection
Demographic information, vascular risk factors, and neuroimaging findings were prospectively recorded at the time of study enrollment. 8 Race/ethnicity was self-reported. Both patients and investigators were masked to the treatment assignment during the study period.
All participants were required to undergo at least two MRIs that met study requirements. In addition to the baseline MRI (obtained following the index stroke either prior to randomization or within 72 h after randomization), participants underwent a final study MRI at 26 weeks or as soon as possible after early termination of assigned study treatment. The initial 829 participants additionally underwent MRI at 13 weeks according to a pre-planned interim safety review. Requisite MRI acquisition parameters are described in the supplementary methods.
MRI data were transmitted to the central MRI Core Laboratory at the University of Calgary for independent interpretation by two radiologists blinded to participant data and treatment assignment, with differences resolved by consensus. Incident infarcts, white matter hyperintensities, and microbleeds were defined according to Standards for Reporting Vascular Changes on Neuroimaging (STRIVE). 10 HT was reported on baseline MRIs obtained after the first dose of study drug.
Microbleeds were categorized as present or absent, and if present, strictly lobar (with or without cerebellar microbleeds), strictly deep (deep/brainstem, cerebellar microbleeds, or both), or mixed (concurrent lobar and deep/brainstem microbleeds). In addition, their severity was categorized as absent (0 microbleeds), mild (1–2 microbleeds), moderate (3–10), or severe (> 10 microbleeds).4,6 New microbleeds were identified by direct comparison of the baseline MRI with the follow-up study MRI. The inter-rater reliability for microbleed presence on an independent test set was 0.94 (95% confidence interval (CI) 0.81–0.99), indicating excellent agreement.
Outcomes
New microbleeds were defined as any newly detected microbleeds on follow-up MRI. The primary efficacy outcome for treatment interactions was the composite of symptomatic ischemic stroke and TIA. Secondary outcomes included ICrH, all-cause mortality, HT of the qualifying infarct, and new microbleeds. TIA was defined as acute focal neurologic deficit related to brain or retinal ischemia, which lasted < 24 h without any neuroimaging manifestation of infarcts. 8 ICrH was specified as any bleeding inside the skull except for HT of the qualifying infarct.8,11
Statistical analysis
We compared patient characteristics using t-test or Wilcoxon rank-sum test for continuous variables and Fisher’s exact or chi-square test for categorical variables as appropriate. Baseline characteristics associated with microbleed presence (p < 0.05) in univariable analyses were entered into a stepwise multivariable logistic regression model to identify variables that are independently associated with baseline microbleeds. An additional multivariable logistic regression model included imaging acquisition parameters, consisting of the type of T2*-weighted available for microbleed detection, magnet strength, slice thickness, and gap width was performed.
Multivariable Cox proportional hazards or logistic regression models, adjusting a priori for age, Asian ethnicity, hypertension, qualifying stroke subtype, and treatment assignment were used to estimate the contribution of microbleeds to risk of outcomes. In a sensitivity analysis, we repeated these analyses with additional adjustment for baseline characteristics that were determined to be independently associated with microbleeds.
Regression models were used to estimate the effects of (1) all pooled asundexian doses and (2) asundexian 50 mg daily on new microbleeds on follow-up MRIs at 26 weeks. Cox proportional hazards or logistic regression models were used to assess interactions between treatment assignment and microbleeds for the outcomes of interest. All the analyses followed the intention-to-treat paradigm.
The p-values were two-sided. Statistical significance was accepted at the 0.05 level. Statistical analyses were performed using SAS software (release 9.4).
Results
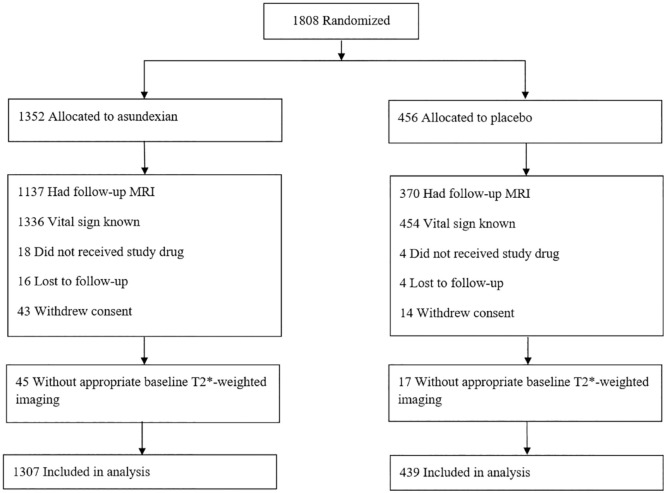
Overall, 1746 of 1808 (97%) PACIFIC-STROKE participants who were enrolled between June 15, 2020 and July 22, 2021 had an interpretable T2*-weighted sequence as part of their baseline MRI and were eligible for these analyses (Figure 1). The mean (SD) age was 67.0 (10.0) years and 34% were female. Clinical characteristics between included and excluded participants were similar. However, included participants were less likely to be White, enrolled from North America, and have an ultimate diagnosis of cardioembolic stroke subtype (Supplemental Table S1).
Figure 1.
CONSORT flow diagram.
Prevalent microbleeds were detected in 604 participants on baseline MRI (35%). Microbleed severity was mild in 387 (64%), moderate in 156 (26%), and severe in 61 (10%) of the participants with microbleeds. The location of microbleeds was strictly deep in 122 (20%), strictly lobar in 321 (53%), and mixed in 161 (27%).
Patients with microbleeds were older, more likely to be Asian, have a history of hypertension, heart failure, previous stroke/TIA, small vessel occlusion stroke subtype as their qualifying event, chronic macrohemorrhages, and more severe white matter disease on their MRI. In addition, they had higher presenting blood pressures and lower glomerular filtration rates (Supplemental Table S2). The MRI parameter associated with microbleed detection was reduced slice thickness, albeit a trend additionally existed for greater detection of microbleeds with susceptibility-weighted imaging/susceptibility-weighted angiography relative to gradient-recalled echo. Asian race (adjusted odds ratio (aOR), 1.65; 95% CI, 1.23–2.22), heart failure (aOR, 1.65; 95% CI, 1.05–2.59), previous stroke/TIA (aOR, 1.35; 95% CI, 1.01–1.80), and more severe white matter hyperintensities on MRI (aOR per one-unit increase on the Fazekas scale, 1.55; 95% CI, 1.43–1.68) were independently associated with microbleeds in multivariable regression model (Table 1). After additional adjustment for imaging acquisition parameters, these associations were largely unchanged (Supplemental Table S3).
Table 1.
Multivariable model of patient characteristics and imaging findings independently associated with cerebral microbleeds.
| No. of patients | ⩾1 microbleed(s), N (%) | Unadjusted odds ratio (95% CI) | p | Adjusted odds ratio (95% CI) a | p | |
|---|---|---|---|---|---|---|
| Age | 1746 | 604 | 1.02 (1.01–1.03) | <0.001 | 0.99 (0.98–1.00) | 0.120 |
| Race | ||||||
| White | 1445 | 467 | Reference | NA | Reference | NA |
| Black | 18 | 2 | 0.32 (0.08–1.25) | 0.101 | 0.40 (0.10–1.65) | 0.206 |
| Asian | 266 | 130 | 2.00 (1.54–2.61) | <0.001 | 1.65 (1.23–2.22) | <0.001 |
| Other | 3 | 0 | 0.30 (0.01–9.17) | 0.490 | 0.42 (0.01–13.65) | 0.625 |
| Current tobacco use | 471 | 145 | 0.79 (0.63–0.99) | 0.042 | 0.78 (0.60–1.01) | 0.060 |
| Heart failure | 100 | 46 | 1.66 (1.11–2.49) | 0.014 | 1.65 (1.05–2.59) | 0.029 |
| Previous stroke/transient ischemic attack | 274 | 121 | 1.62 (1.25–2.10) | <0.001 | 1.35 (1.01–1.80) | 0.041 |
| Qualifying stroke subtype | ||||||
| Large artery atherosclerosis | 310 | 105 | Reference | NA | Reference | NA |
| Small vessel occlusion | 786 | 315 | 1.31 (0.99–1.72) | 0.057 | 1.36 (1.00–1.85) | 0.052 |
| Other | 43 | 17 | 1.28 (0.66–2.46) | 0.465 | 1.34 (0.65–2.77) | 0.428 |
| Cryptogenic | 599 | 163 | 0.73 (0.54–0.98) | 0.037 | 0.93 (0.66–1.30) | 0.669 |
| Cardioembolism | 7 | 3 | 1.46 (0.32–6.66) | 0.622 | 2.34 (0.51–10.78) | 0.275 |
| Total Fazekas score | 1742 | 604 | 1.58 (1.47–1.70) | <0.001 | 1.55 (1.43–1.68) | <0.001 |
| Chronic macrohemorrhage | 28 | 19 | 4.09 (1.84–9.09) | <0.001 | 2.31 (0.92–5.78) | 0.073 |
CI: confidence interval; NA: not applicable.
Firth’s logistic regression is used due to the quasi-complete separation feature in race data.
Outcomes and effect of treatment assignment
During a median follow-up of 10.6 months (interquartile range (IQR) =8.1–12.4), 7% of the participants had recurrent ischemic stroke/TIA, 0.5% ICrH, and 2% died. In patients with adequate follow-up blood-sensitive MRIs sequences (n = 1507 (86.3%) median (IQR) baseline-to-follow-up MRI time = 25.9 (25.6–26.1) weeks), the rate of new microbleeds on follow-up MRI was 10.3% (Supplemental Figure S1). Among patients with baseline MRI after initiation of study drug within 72 h of randomization (n = 1100), any HT occurred in 31.4%.
Asundexian treatment was not associated with an increase in new microbleeds on follow-up MRI at 26 weeks. New microbleeds occurred in 10.2% (116/1137) of patients assigned to any asundexian dose strength (OR, 0.96; 95% CI, 0.66–1.41), 8.4% (31/368) of patients assigned to asundexian 50 mg daily (OR, 0.78; 95% CI, 0.48–1.28), and 10.5% (39/370) of patients assigned to placebo (reference).
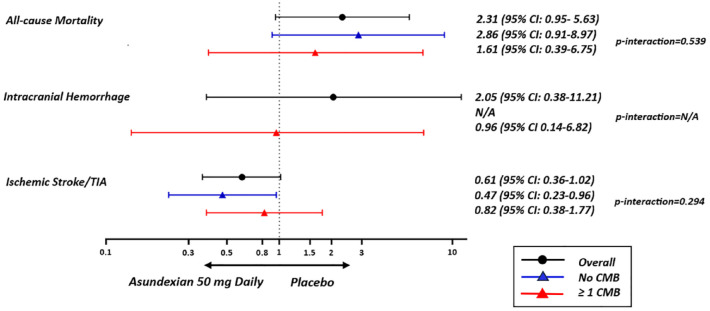
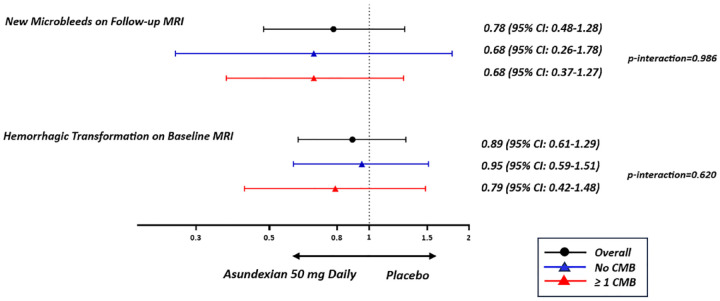
The effect of asundexian treatment—analyzed as either (1) any asundexian dose strength or (2) 50 mg daily versus placebo—was not modified by microbleed presence, severity, or distribution for any of the clinical or MRI outcomes (Figures 2 and 3, and Supplemental Tables S4–S13). The rates of ICrH ((1) 1% with all pooled asundexian doses, (2) 2% with asundexian 50 mg daily vs 2% with placebo), HT of the qualifying infarct ((1) 37%, (2) 32% vs 38%), and new microbleeds ((1) 19%, (2) 19% vs 25%) were numerically less in patients with microbleeds assigned to asundexian treatment compared to those assigned to placebo.
Figure 2.
Response to treatment according to microbleed status for clinical outcomes. CMB: cerebral microbleed.
Figure 3.
Response to treatment according to microbleed status for neuroimaging outcomes. CMB: cerebral microbleed.
Associations between baseline microbleeds and outcomes
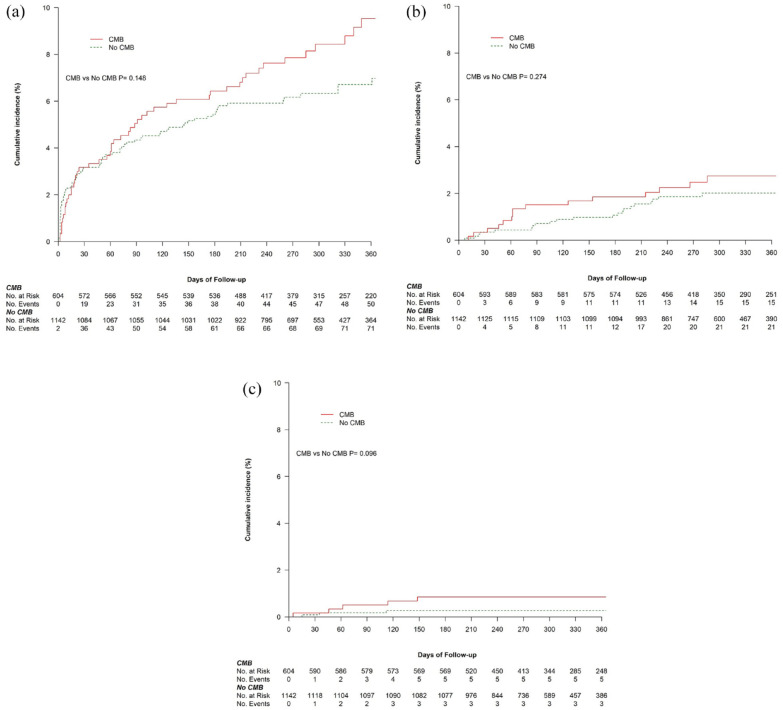
The risk of ischemic stroke/TIA, ICrH, and all-cause mortality did not differ between patients with and without microbleeds (Table 2, and Figure 4) or between subgroups of microbleed location (Supplemental Figures S2–S4). However, patients with three or more microbleeds had an 8.5-fold excess risk of ICrH during follow-up (1.8% vs 0.3%; adjusted hazard ratio (aHR), 8.49; 95% CI, 1.70–42.40) (Table 2, and Supplemental Figure S4b). The absolute risk of ischemic stroke/TIA (10.1%) remained substantially higher than ICrH (1.8%) in patients with three or more microbleeds, and this held true for all microbleed severity and location subgroups. In the sensitivity analysis with additional adjustment for baseline characteristics that were determined to be associated with microbleeds in this cohort, the results remained unchanged except for the emergence of a statistically significant higher risk of ischemic stroke/TIA in patients with strictly deep/mixed microbleed versus no microbleeds (aHR, 1.64; 95% CI, 1.02–2.64) (Supplemental Table S14).
Table 2.
Risk of ischemic stroke/TIA, ICrH, and all-cause mortality stratified by baseline microbleed status.
| No. of patients | Ischemic stroke/TIA |
Intracranial hemorrhage |
All-cause mortality |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Events, N (%) | HR, (95% CI) | aHR a , (95% CI) | Events, N (%) | HR, (95% CI) | aHR a , (95% CI) | Events, N (%) | HR, (95% CI) | aHR a , (95% CI) | ||
| CMB presence | ||||||||||
| None | 1142 | 72 (6.3) | Reference | Reference | 3 (0.3) | Reference | Reference | 21 (1.8) | Reference | Reference |
| CMB | 604 | 50 (8.3) | 1.30 (0.91–1.87) | 1.17 (0.81–1.69) | 5 (0.8) | 3.16 (0.75–13.21) | 3.89 (0.86–17.52) | 16 (2.6) | 1.43 (0.75–2.75) | 1.43 (0.74–2.78) |
| CMB burden | ||||||||||
| No CMB | 1142 | 72 (6.3) | Reference | Reference | 3 (0.3) | Reference | Reference | 21 (1.8) | Reference | Reference |
| 1–2 CMBs | 387 | 28 (7.2) | 1.14 (0.74–1.77) | 1.09 (0.70–1.69) | 1 (0.3) | 0.99 (0.10–9.50) | 1.27 (0.13–12.72) | 7 (1.8) | 0.98 (0.42–2.30) | 1.01 (0.42–2.38) |
| 3 or more CMBs | 217 | 22 (10.1) | 1.59 (0.99–2.56) | 1.30 (0.79–2.15) | 4 (1.8) | 6.99 (1.57–31.25) | 8.49 (1.70–42.40) | 9 (4.1) | 2.25 (1.03–4.92) | 2.17 (0.97–4.86) |
| CMB topography | ||||||||||
| No CMB | 1142 | 72 (6.3) | Reference | Reference | 3 (0.3) | Reference | Reference | 21 (1.8) | Reference | Reference |
| Strictly deep/mixed CMBs | 283 | 28 (9.9) | 1.56 (1.01–2.42) | 1.38 (0.88–2.17) | 3 (1.1) | 3.99 (0.81–19.76) | 5.36 (0.92–31.24) | 8 (2.8) | 1.54 (0.68–3.47) | 1.54 (0.66–3.57) |
| Strictly lobar CMBs | 321 | 22 (6.9) | 1.08 (0.67–1.74) | 0.98 (0.60–1.59) | 2 (0.6) | 2.40 (0.40–14.39) | 2.88 (0.45–18.25) | 8 (2.5) | 1.35 (0.60–3.04) | 1.34 (0.59–3.04) |
TIA: transient ischemic attack; HR: hazard ratio; CI: confidence interval; aHR: adjusted hazard ratio; CMB: cerebral microbleed.
Adjusted for age, Asian ethnicity, hypertension, qualifying stroke subtype, and treatment assignment.
Figure 4.
Kaplan–Meier curves for clinical outcomes stratified by cerebral microbleed status. (a) Ischemic stroke/TIA. (b) All-cause mortality. (c) ICrH. CMB: cerebral microbleed.
HT was more frequent in patients with microbleeds 37.2% (n = 149) versus those without 28% (n = 196; aOR, 1.57; 95% CI, 1.19–2.05) (Table 3). The strength of the association between microbleeds and HT was similar irrespective of microbleed severity. However, a more robust association with HT was observed among patients with strictly lobar microbleeds (aOR, 1.67; 95% CI, 1.20–2.33) relative to patients with strictly deep/mixed patterns (aOR, 1.44; 95% CI, 1.01–2.06). Patients with at least one microbleed at baseline were at a fourfold increased risk of developing new microbleeds on their follow-up MRI (20% vs 5%; aOR, 4.37; 95% CI, 3.02–6.32) (Table 3). In total, 39% of patients with three or more microbleeds on baseline MRI developed new microbleeds on follow-up MRI (aOR, 11.06; 95% CI, 7.21–16.97 relative to patients without microbleeds). The rate of new microbleeds was 26% in patients with strictly deep/mixed microbleeds (aOR, 6.21; 95% CI, 4.08–9.48) and 15% in patients with strictly lobar microbleeds (aOR, 3.03; 95% CI, 1.93–4.75). These results were essentially unchanged in sensitivity analyses (Supplemental Table S15).
Table 3.
Risk of HT and new cerebral microbleeds on MRI stratified by baseline microbleed status.
| Characteristic | Hemorrhagic transformation |
New microbleeds |
||||||
|---|---|---|---|---|---|---|---|---|
| No. of patients | Events, N (%) | OR, (95% CI) | aORa,b, (95% CI) | No. of patients | Events, N (%) | OR, (95% CI) | aORa,c, (95% CI) | |
| CMB presence | ||||||||
| None | 699 | 196 (28.0) | Reference | Reference | 982 | 48 (4.9) | Reference | Reference |
| CMB | 401 | 149 (37.2) | 1.52 (1.17–1 .97) | 1.57 (1.19–2.05) | 525 | 107 (20.4) | 4.98 (3.48–7.14) | 4.37 (3.02–6.32) |
| CMB burden | ||||||||
| No CMB | 699 | 196 (28.0) | Reference | Reference | 982 | 48 (4.9) | Reference | Reference |
| 1–2 CMBs | 245 | 91 (37.1) | 1.52 (1.12–2.06) | 1.55 (1.13–2.13) | 334 | 33 (9.9) | 2.13 (1.34–3.39) | 1.87 (1.16–3.00) |
| 3 or more CMBs | 156 | 58 (37.2) | 1.52 (1.06–2.19) | 1.58 (1.09–2.31) | 191 | 74 (38.7) | 12.31 (8.16–18.56) | 11.06 (7.21–16.97) |
| CMB topography | ||||||||
| No CMB | 699 | 196 (28.0) | Reference | Reference | 982 | 48 (4.9) | Reference | Reference |
| Strictly deep/mixed CMBs | 189 | 68 (36.0) | 1.44 (1.03–2.03) | 1.44 (1.01–2.06) | 246 | 65 (26.4) | 6.99 (4.66–10.48) | 6.21 (4.08–9.48) |
| Strictly lobar CMBs | 212 | 81 (38.2) | 1.59 (1.15–2.19) | 1.67 (1.2–2.33) | 279 | 42 (15.1) | 3.45 (2.23–5.34) | 3.03 (1.93–4.75) |
HT: hemorrhagic transformation; MRI: magnetic resonance imaging; OR: odds ratio; CI: confidence interval; aOR: adjusted odds ratio; CMB: cerebral microbleed.
Adjusted for age, Asian ethnicity, hypertension, qualifying stroke subtype, and treatment assignment.
All HTs (hemorrhagic infarction 1 and 2 and parenchymal hematoma 1 and 2) on baseline MRIs which were done after the initial dose of the study drug (up to 72 h after randomization).
New microbleeds were rated for patients who had an interpretable T2*.
Discussion
In this well-characterized cohort of patients with non-cardioembolic ischemic stroke presenting within 48 h of symptom onset, microbleeds were prevalent and associated with Asian race, history of heart failure and stroke/TIA, and higher severity of white matter disease. Treatment with asundexian did not increase new microbleed formation on follow-up MRI and no interactions were observed between microbleeds and asundexian treatment (vs placebo) for the clinical and radiological outcomes of interest. Although our analyses may have lacked power to definitively exclude an interaction, reassuring the rates of ICrH, HT, and new microbleeds was numerically less with asundexian treatment versus placebo in patients with microbleeds. A higher numerical rate of recurrent ischemic stroke/TIA, all-cause mortality, and ICrH was observed in patients with microbleeds, but these were not statistically significant. However, patients with microbleeds had a 1.5-fold greater risk of developing any HT and a fourfold greater risk of new microbleeds on follow-up MRI. In addition, patients with deep/mixed microbleeds were at a 1.6-fold increased risk of recurrent ischemic stroke/TIA in our broadened adjusted model.
Factor XI/XIa inhibitors are hypothesized to prevent ischemic vascular events without increasing the risk of bleeding. Consistent with this hypothesis, we observed no increase in ICrH, HT, or new microbleeds with asundexian when used on top of background antiplatelet treatment in patients with hemorrhage-prone cerebral small vessel disease marked by microbleeds on MRI. Consistent with prior microbleed subgroup analyses of randomized trials of antithrombotic treatment, we did not observe any treatment interaction between microbleeds and asundexian treatment for the clinical and MRI outcomes of interest.4,6,7
Our observed 35% prevalence of microbleeds is consistent with prior studies of patients with ischemic stroke/TIA.3,12 Contrary to prior studies, we did not find a significant association between microbleeds and recurrent ischemic stroke/TIA and all-cause mortality, although these clinical outcomes occurred more frequently in patients with microbleeds, and, in the case of ischemic stroke/TIA, with increasing microbleed number.3,4,6 Of note, our sensitivity analysis demonstrated that the presence of strictly deep/mixed microbleeds was associated with 1.6 times higher risk of ischemic stroke/TIA. Our analysis may have thus lacked sufficient follow-up time and the power to detect more differences relative to larger observational cohorts with longer follow-up durations. 3 Patients with three or more microbleeds—indicating a moderate–severe burden of disease—participating in PACIFIC-STROKE were at an 8.5-fold increased risk of ICrH during study follow-up. Yet, in keeping with prior pooled analyses of ischemic stroke/TIA cohorts, patients with microbleeds had substantially higher absolute risks of ischemic stroke/TIA than ICrH, irrespective of microbleed severity or location.1–3 These consistent findings challenge existing concerns surrounding the use of antithrombotic treatments in patients with ischemic stroke/TIA and microbleeds on MRI.
Patients with acute ischemic stroke and microbleeds on MRI have previously been reported to have higher risk of HT both with and without revascularization treatments, particularly in patients with higher microbleed severity.13–15 Similarly, we observed a 1.5-fold excess risk of any HT on MRI in patients with microbleeds. These observations suggest that the same underlying microangiopathic processes that increase vascular fragility and permeability leading to microbleed formation, also lead to an increased risk of HT of infarcted tissue. Our results suggest that patients with lobar microbleeds, indicative of underlying cerebral amyloid angiopathy, may be particularly at heightened risk of HT. Moreover, in line with previous observations, patients with baseline microbleeds were at greater risk of developing new microbleeds,16,17 and the association increased with greater microbleed severity. The rate of new microbleeds on MRI overall was 10% and was as high as 39% in patients with three or more baseline microbleeds suggesting active underlying microangiopathic processes in the acute/subacute phase of ischemic stroke.18,19
Our study has several advantages. It is first to investigate the effect of Factor XI/XIa inhibition (vs placebo) on cerebral microbleed formation and clinical outcomes in patients with hemorrhage-prone cerebral small vessel disease marked by microbleeds. We report results from a large international prospective sample of well-characterized non-cardioembolic ischemic stroke with a standardized MRI protocol. MRI rating was performed centrally by experienced radiologists blinded to patient data and treatment assignment, with excellent inter-rater reliability for microbleeds. Asundexian treatment assignment was randomized and concealed from both patients and health care providers.
Our study also has limitations. Our analyses may have been underpowered, particularly for the clinical outcomes of ICrH and mortality due to the small number of events and relatively short median follow-up time. Moreover, subgroup analyses of randomized trials can be prone to bias from inadequate randomization or multiple testing, leading to spurious findings. The trial eligibility criteria, potential for selection bias during recruitment, and the unavailability of adequate T2*-weighted sequences during follow-up MRI in all PACIFIC-STROKE participants could limit the generalizability of our findings. In this regard, prospective observational cohorts with standardized longitudinal assessments would be better suited to characterize the natural history of disease. Reassuringly, however, the prevalence of microbleeds in our cohort and their associations with baseline demographics and outcomes were quite consistent with the existing literature. About 3% of the PACIFIC-STROKE trial participants were excluded from this post hoc analysis due to unavailability of adequate baseline T2*-weighted sequences, which might have biased our results. However, other than the overrepresentation of Asian ethnicity among the included cohort, the included patients had similar baseline characteristics as those excluded from these analyses. In view of the higher rate of microbleeds and risk of ICrH in Asian patients with ischemic stroke, our sample may have been more susceptible to hemorrhagic events.
Conclusion
Asundexian treatment appears to be safe when used on top of antiplatelet treatment in patients with non-cardioembolic ischemic stroke and hemorrhage-prone cerebral small vessel disease marked by microbleeds on MRI. These results will be confirmed in the ongoing OCEANIC-STROKE randomized trial.
Supplemental Material
Supplemental material, sj-docx-1-wso-10.1177_17474930231216339 for Cerebral microbleeds and asundexian in non-cardioembolic ischemic stroke: Secondary analyses of the PACIFIC-STROKE randomized trial by Pargol Balali, Robert G Hart, Eric E Smith, Feryal Saad, Pablo Colorado, Robin Lemmens, Gian Marco De Marchis, Valeria Caso, Lizhen Xu, Laura Heenan, Stuart J Connolly, Hardi Mundl and Ashkan Shoamanesh in International Journal of Stroke
Acknowledgments
R.L. is a senior clinical investigator of FWO Flanders. A.S. is supported by the Martha and Owen Boris Chair in Stroke Research and Care and the Heart and Stroke Foundation of Canada.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: All co-authors or their institutions received financial support from Bayer for participation in the PACIFIC-STROKE trial except H.M. and P.C., who are employees of Bayer.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This trial was sponsored, designed, and carried out by Bayer; collaborators at the Population Health Research Institute at McMaster University (Hamilton, Canada) collected the data and independently analyzed and reported the results to the Trial Steering Committee.
Data availability: The de-identified, individual participant-level data can be made available to investigators for secondary analyses after review of a submitted proposal by the PACIFIC-Stroke steering committee. Requests should be addressed to the corresponding author (ashkan.shoamanesh@phri.ca) or the sponsor representative (hardi.mundl@bayer.com). Requesters will be required to complete a study questionnaire. All requests will be assessed by the Steering Committee to review and comment on any potential publication of data from the trial.
ORCID iDs: Eric E Smith  https://orcid.org/0000-0003-3956-1668
https://orcid.org/0000-0003-3956-1668
Valeria Caso  https://orcid.org/0000-0002-2020-8891
https://orcid.org/0000-0002-2020-8891
Hardi Mundl  https://orcid.org/0000-0001-6218-3058
https://orcid.org/0000-0001-6218-3058
Ashkan Shoamanesh  https://orcid.org/0000-0002-2802-1626
https://orcid.org/0000-0002-2802-1626
Supplemental material: Supplemental material for this article is available online.
References
- 1. Puy L, Pasi M, Rodrigues M, et al. Cerebral microbleeds: from depiction to interpretation. J Neurol Neurosurg Psychiatry 2021; 92: 598–607. [DOI] [PubMed] [Google Scholar]
- 2. Shoamanesh A, Kwok CS, Benavente O. Cerebral microbleeds: histopathological correlation of neuroimaging. Cerebrovasc Dis 2011; 32: 528–534. [DOI] [PubMed] [Google Scholar]
- 3. Wilson D, Ambler G, Lee K-J, et al. Cerebral microbleeds and stroke risk after ischaemic stroke or transient ischaemic attack: a pooled analysis of individual patient data from cohort studies. Lancet Neurol 2019; 18: 653–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Shoamanesh A, Hart RG, Connolly SJ, et al. Microbleeds and the effect of anticoagulation in patients with embolic stroke of undetermined source: an exploratory analysis of the NAVIGATE ESUS randomized clinical trial. JAMA Neurol 2021; 78: 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Park HK, Lee JS, Kim BJ, et al. Cilostazol versus aspirin in ischemic stroke with cerebral microbleeds versus prior intracerebral hemorrhage. Int J Stroke 2021; 16: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 6. Shoamanesh A, Pearce LA, Bazan C, et al. Microbleeds in the SPS3 Trial: stroke, mortality and treatment interactions. Ann Neurol 2017; 82: 196–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Al-Shahi Salman R, Dennis M, Sandercock P, et al. Effects of antiplatelet therapy after stroke due to intracerebral haemorrhage (RESTART): a randomised, open-label trial. Lancet 2019; 393: 2613–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Shoamanesh A, Mundl H, Smith EE, et al. Factor XIa inhibition with asundexian after acute non-cardioembolic ischaemic stroke (PACIFIC-Stroke): an international, randomised, double-blind, placebo-controlled, phase 2b trial. Lancet. Epub ahead of print 2 September 2022. DOI: 10.1016/S0140-6736(22)01588-4. [DOI] [PubMed] [Google Scholar]
- 9. Piaggio G, Elbourne DR, Pocock SJ, Evans SJ, Altman DG; CONSORT Group. Reporting of noninferiority and equivalence randomized trials: an extension of the CONSORT statement. JAMA 2006; 295: 1152–1160. [DOI] [PubMed] [Google Scholar]
- 10. Wardlaw JM, Smith EE, Biessels GJ, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet Neurol 2013; 12: 822–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Caceres JA, Goldstein JN. Intracranial hemorrhage. Emerg Med Clin North Am 2012; 30: 771–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cordonnier C, Al-Shahi Salman R, Wardlaw J. Spontaneous brain microbleeds: systematic review, subgroup analyses and standards for study design and reporting. Brain 2007; 130: 1988–2003. [DOI] [PubMed] [Google Scholar]
- 13. Shoamanesh A, Kwok CS, Lim PA, Benavente OR. Postthrombolysis intracranial hemorrhage risk of cerebral microbleeds in acute stroke patients: a systematic review and meta-analysis. Int J Stroke 2013; 8: 348–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tsivgoulis G, Zand R, Katsanos AH, et al. Risk of symptomatic intracerebral hemorrhage after intravenous thrombolysis in patients with Acute Ischemic Stroke and high cerebral microbleed burden: a meta-analysis. JAMA Neurol 2016; 73: 675–683. [DOI] [PubMed] [Google Scholar]
- 15. Liu C, Dong Z, Xu L, et al. MR image features predicting hemorrhagic transformation in acute cerebral infarction: a multimodal study. Neuroradiology 2015; 57: 1145–1152. [DOI] [PubMed] [Google Scholar]
- 16. Gregoire SM, Brown MM, Kallis C, Jäger HR, Yousry TA, Werring DJ. MRI detection of new microbleeds in patients with ischemic stroke: five-year cohort follow-up study. Stroke 2010; 41: 184–186. [DOI] [PubMed] [Google Scholar]
- 17. Orken DN, Uysal E, Timer E, Kuloglu-Pazarcı N, Mumcu S, Forta H. New cerebral microbleeds in ischemic stroke patients on warfarin treatment: two-year follow-up. Clin Neurol Neurosurg 2013; 115: 1682–1685. [DOI] [PubMed] [Google Scholar]
- 18. Shoamanesh A, Yan S, Charidimou A. New cerebral microbleeds and mechanism of post–thrombolysis remote intracerebral hemorrhage: “red meets white” revisited. Front Neurol 2015; 6: 203, https://www.frontiersin.org/articles/10.3389/fneur.2015.00203 (accessed 9 May 2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sharma M, Smith EE, Pearce LA, et al. Rivaroxaban versus aspirin for prevention of covert brain infarcts in patients with embolic stroke of undetermined source: NAVIGATE ESUS MRI substudy. Int J Stroke 2022; 17: 799–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-wso-10.1177_17474930231216339 for Cerebral microbleeds and asundexian in non-cardioembolic ischemic stroke: Secondary analyses of the PACIFIC-STROKE randomized trial by Pargol Balali, Robert G Hart, Eric E Smith, Feryal Saad, Pablo Colorado, Robin Lemmens, Gian Marco De Marchis, Valeria Caso, Lizhen Xu, Laura Heenan, Stuart J Connolly, Hardi Mundl and Ashkan Shoamanesh in International Journal of Stroke