Abstract
Direct oral anticoagulants (DOACs) have become the preferred option for treatment of venous thromboembolism due to their favorable profile compared with other agents such as vitamin K antagonists or low-molecular-weight heparin. However, findings from randomized controlled trials suggest efficacy and/or safety concerns with DOAC use in some clinical contexts. This illustrated review will summarize indications where DOACs have proven efficacy and safety, situations where they fall short, and situations where uncertainty remains compared with other treatments for venous thromboembolism.
Keywords: anticoagulants, direct oral anticoagulants, low-molecular-weight heparin, venous thromboembolism, Vitamin K Antagonists
Essentials
-
•
Randomized trials suggest that direct oral anticoagulants (DOACs) may not be as safe or effective for mangement of venous thromboembolism (VTE) in certain situations.
-
•
We review scenarios where DOACs are safe and effective, where they have reduced safety or efficacy, and when their safety and/or efficacy are uncertain.
-
•
DOACs are not advised for VTE in antiphospholipid syndrome or luminal cancers.
-
•
The safety and efficacy of DOACs for VTE remain uncertain in some conditions.
Capsule 1
Capsule 2
Capsule 3
Capsule 4
Capsule 5
Capsule 6
Capsule 7
Capsule 8
Acknowledgments
This illustrated review follows strict ethical guidelines, ensuring that all data, images, and figures used are properly attributed and cited to maintain accuracy and integrity. All authors contributed to the scientific content and review editing, with CDK leading the conceptualization and design of illustrations, and BB providing supervision, direction, and final approval. Capsules were created with BioRender.com.
Funding
The authors received no funding for this study.
Relationship disclosure
Outside the submitted work, Dr Bikdeli is supported by a Career Development Award from the American Heart Association and VIVA Physicians (#938814). B.B. was supported by the Scott Schoen and Nancy Adams IGNITE Award and is supported by the Mary Ann Tynan Research Scientist award from the Mary Horrigan Connors Center for Women’s Health and Gender Biology at Brigham and Women’s Hospital and the Heart and Vascular Center Junior Faculty Award from Brigham and Women’s Hospital. Dr Bikdeli reports that he was a consulting expert, on behalf of the plaintiff, for litigation related to 2 specific brand models of Inferior Vena Cava filters. Dr Bikdeli has neither been involved in the litigation in 2022-2024 nor has he received any compensation in 2022-2024. Dr Bikdeli reports that he is a member of the Medical Advisory Board for the North American Thrombosis Forum and serves in the Data Safety and Monitory Board of the NAIL-IT trial funded by the National Heart, Lung, and Blood Institute, and Translational Sciences. Dr Bikdeli is a collaborating consultant with the International Consulting Associates and the US Food and Drug Administration in a study to generate knowledge about utilization, predictors, retrieval, and safety of IVC filters. Dr Bikdeli receives compensation as an associate editor for the New England Journal of Medicine Journal Watch Cardiology, as an associate editor for Thrombosis Research, and as an executive associate editor for Journal of the American College of Cardiology and is a section editor for Thrombosis and Haemostasis (no compensation). Dr Piazza has received research support from Bristol-Myers Squibb/Pfizer Alliance, Bayer, Janssen, Alexion, Amgen, Esperion, Boston Scientific Corporation, and the National Heart, Lung, and Blood Institute (1R01HL164717-01) and consulting/advisory fees from BMS, Boston Scientific Corporation, Janssen, PERC, NAMSA, Regeneron, Penumbra, and Amgen.
Footnotes
Handling Editor: Dr Michael Makris
References
- 1.January C.T., Wann L.S., Calkins H., Chen L.Y., Cigarroa J.E., Cleveland J.C., et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice guidelines and the Heart Rhythm Society in collaboration with the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665. [DOI] [PubMed] [Google Scholar]
- 2.De Caterina R., Husted S., Wallentin L., Andreotti F., Arnesen H., Bachmann F., et al. New oral anticoagulants in atrial fibrillation and acute coronary syndromes: ESC Working Group on Thrombosis—Task Force on Anticoagulants in Heart Disease position paper. J Am Coll Cardiol. 2012;59:1413–1425. doi: 10.1016/j.jacc.2012.02.008. [DOI] [PubMed] [Google Scholar]
- 3.Ortel T.L., Neumann I., Ageno W., Beyth R., Clark N.P., Cuker A., et al. American Society of Hematology 2020 guidelines for management of venous thromboembolism: treatment of deep vein thrombosis and pulmonary embolism. Blood Adv. 2020;4:4693–4738. doi: 10.1182/bloodadvances.2020001830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stevens S.M., Woller S.C., Baumann Kreuziger L., Bounameaux H., Doerschug K., Geersing G.J., et al. Executive summary: antithrombotic therapy for VTE disease: second update of the CHEST guideline and expert panel report. Chest. 2021;160:2247–2259. doi: 10.1016/j.chest.2021.07.056. [DOI] [PubMed] [Google Scholar]
- 5.Agnelli G., Buller H.R., Cohen A., Curto M., Gallus A.S., Johnson M., et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. doi: 10.1056/NEJMoa1302507. [DOI] [PubMed] [Google Scholar]
- 6.EINSTEIN Investigators. Bauersachs R., Berkowitz S.D., Brenner B., Buller H.R., Decousus H., Gallus A.S., et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–2510. doi: 10.1056/NEJMoa1007903. [DOI] [PubMed] [Google Scholar]
- 7.EINSTEIN–PE Investigators. Büller H.R., Prins M.H., Lensin A.W., Decousus H., Jacobson B.F., Minar E., et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 8.Hokusai-VTE Investigators. Büller H.R., Décousus H., Grosso M.A., Mercuri M., Middeldorp S., Prins M.H., et al. Edoxaban versus warfarin for the treatment of symptomatic venous thromboembolism. N Engl J Med. 2013;369:1406–1415. doi: 10.1056/NEJMoa1306638. [DOI] [PubMed] [Google Scholar]
- 9.Schulman S., Kearon C., Kakkar A.K., Mismetti P., Schellong S., Eriksson H., et al. Dabigatran versus warfarin in the treatment of acute venous thromboembolism. N Engl J Med. 2009;361:2342–2352. doi: 10.1056/NEJMoa0906598. [DOI] [PubMed] [Google Scholar]
- 10.Agnelli G., Buller H.R., Cohen A., Curto M., Gallus A.S., Johnson M., et al. Apixaban for extended treatment of venous thromboembolism. N Engl J Med. 2013;368:699–708. doi: 10.1056/NEJMoa1207541. [DOI] [PubMed] [Google Scholar]
- 11.Weitz J.I., Lensing A.W., Prins M.H., Bauersachs R., Beyer-Westendorf J., Bounameaux H., et al. Rivaroxaban or aspirin for extended treatment of venous thromboembolism. N Engl J Med. 2017;376:1211–1222. doi: 10.1056/NEJMoa1700518. [DOI] [PubMed] [Google Scholar]
- 12.Schulman S., Kearon C., Kakkar A.K., Schellong S., Eriksson H., Baanstra D., et al. Extended use of dabigatran, warfarin, or placebo in venous thromboembolism. N Engl J Med. 2013;368:709–718. doi: 10.1056/NEJMoa1113697. [DOI] [PubMed] [Google Scholar]
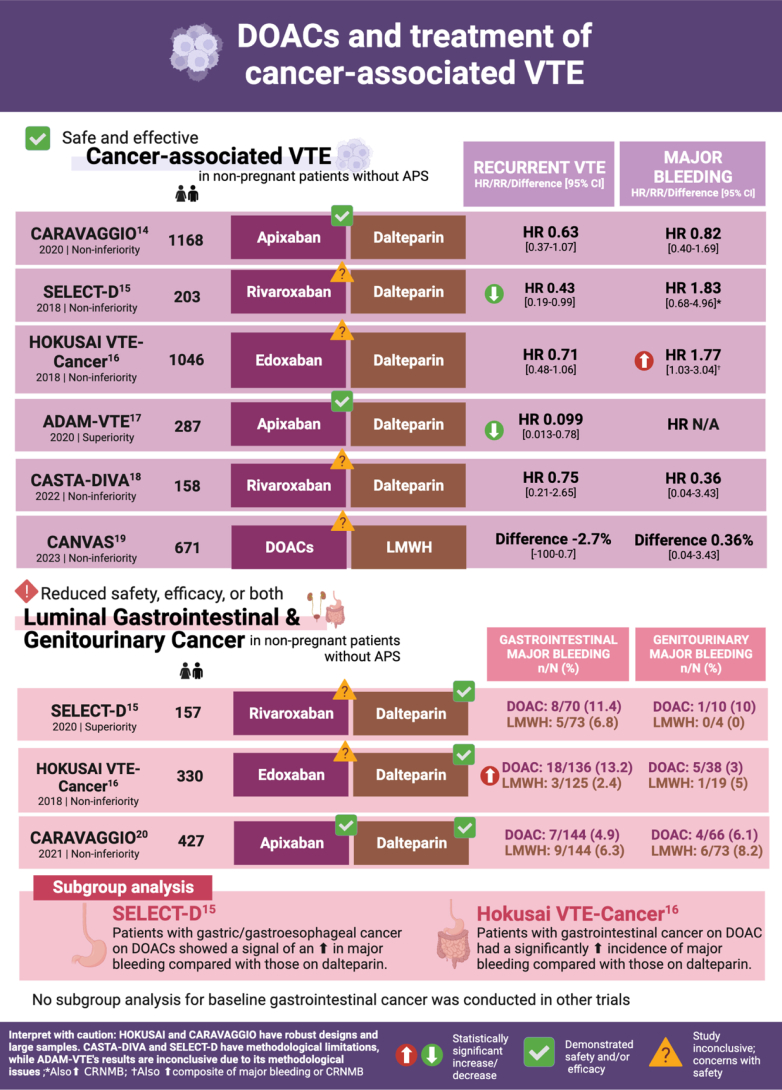
- 13.Agnelli G., Becattini C., Meyer G., Muñoz A., Huisman M.V., Connors J.M., et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599–1607. doi: 10.1056/NEJMoa1915103. [DOI] [PubMed] [Google Scholar]
- 14.Young A.M., Marshall A., Thirlwall J., Chapman O., Lokare A., Hill C., et al. Comparison of an oral factor Xa inhibitor with low molecular weight heparin in patients with cancer with venous thromboembolism: results of a randomized trial (SELECT-D) J Clin Oncol. 2018;36:2017–2023. doi: 10.1200/JCO.2018.78.8034. [DOI] [PubMed] [Google Scholar]
- 15.Raskob G.E., van Es N., Verhamme P., Carrier M., Di Nisio M., Garcia D., et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624. doi: 10.1056/NEJMoa1711948. [DOI] [PubMed] [Google Scholar]
- 16.McBane R.D., 2nd, Wysokinski W.E., Le-Rademacher J.G., Zemla T., Ashrani A., Tafur A., et al. Apixaban and dalteparin in active malignancy-associated venous thromboembolism: the ADAM VTE trial. J Thromb Haemost. 2020;18:411–421. doi: 10.1111/jth.14662. [DOI] [PubMed] [Google Scholar]
- 17.Planquette B., Bertoletti L., Charles-Nelson A., Laporte S., Grange C., Mahé I., et al. Rivaroxaban vs dalteparin in cancer-associated thromboembolism: a randomized trial. Chest. 2022;161:781–790. doi: 10.1016/j.chest.2021.09.037. [DOI] [PubMed] [Google Scholar]
- 18.Malec K., Broniatowska E., Undas A. Direct oral anticoagulants in patients with antiphospholipid syndrome: a cohort study. Lupus. 2020;29:37–44. doi: 10.1177/0961203319889156. [DOI] [PubMed] [Google Scholar]
- 19.Schrag D., Uno H., Rosovsky R., Rutherford C., Sanfilippo K., Villano J.L., et al. Direct oral anticoagulants vs low-molecular-weight heparin and recurrent VTE in patients with cancer: a randomized clinical trial. JAMA. 2023;329:1924–1933. doi: 10.1001/jama.2023.7843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ageno W., Vedovati M.C., Cohen A., Huisman M., Bauersachs R., Gussoni G., et al. Bleeding with apixaban and dalteparin in patients with cancer-associated venous thromboembolism: results from the Caravaggio study. Thromb Haemost. 2021;121:616–624. doi: 10.1055/s-0040-1720975. [DOI] [PubMed] [Google Scholar]
- 21.Legault K., Blostein M., Carrier M., Khan S., Schulman S., Shivakumar S., et al. A single-arm feasibility cohort study of rivaroxaban in antiphospholipid syndrome. Pilot Feasibility Stud. 2020;6:52. doi: 10.1186/s40814-020-00594-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen H., Hunt B.J., Efthymiou M., Arachchillage D.R., Mackie I.J., Clawson S., et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol. 2016;3:e426–e436. doi: 10.1016/S2352-3026(16)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pengo V., Denas G., Zoppellaro G., Jose S.P., Hoxha A., Ruffatti A., et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood. 2018;132:1365–1371. doi: 10.1182/blood-2018-04-848333. [DOI] [PubMed] [Google Scholar]
- 24.Ordi-Ros J., Sáez-Comet L., Pérez-Conesa M., Vidal X., Riera-Mestre A., Castro-Salomó A., et al. Rivaroxaban versus vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med. 2019;171:685–694. doi: 10.7326/M19-0291. [DOI] [PubMed] [Google Scholar]
- 25.Woller S.C., Stevens S.M., Kaplan D., Wang T.F., Branch D.W., Groat D., et al. Apixaban compared with warfarin to prevent thrombosis in thrombotic antiphospholipid syndrome: a randomized trial. Blood Adv. 2022;6:1661–1670. doi: 10.1182/bloodadvances.2021005808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Khairani C.D., Bejjani A., Piazza G., Jimenez D., Monreal M., Chatterjee S., et al. Direct oral anticoagulants vs vitamin K antagonists in patients with antiphospholipid syndromes: meta-analysis of randomized trials. J Am Coll Cardiol. 2023;81:16–30. doi: 10.1016/j.jacc.2022.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
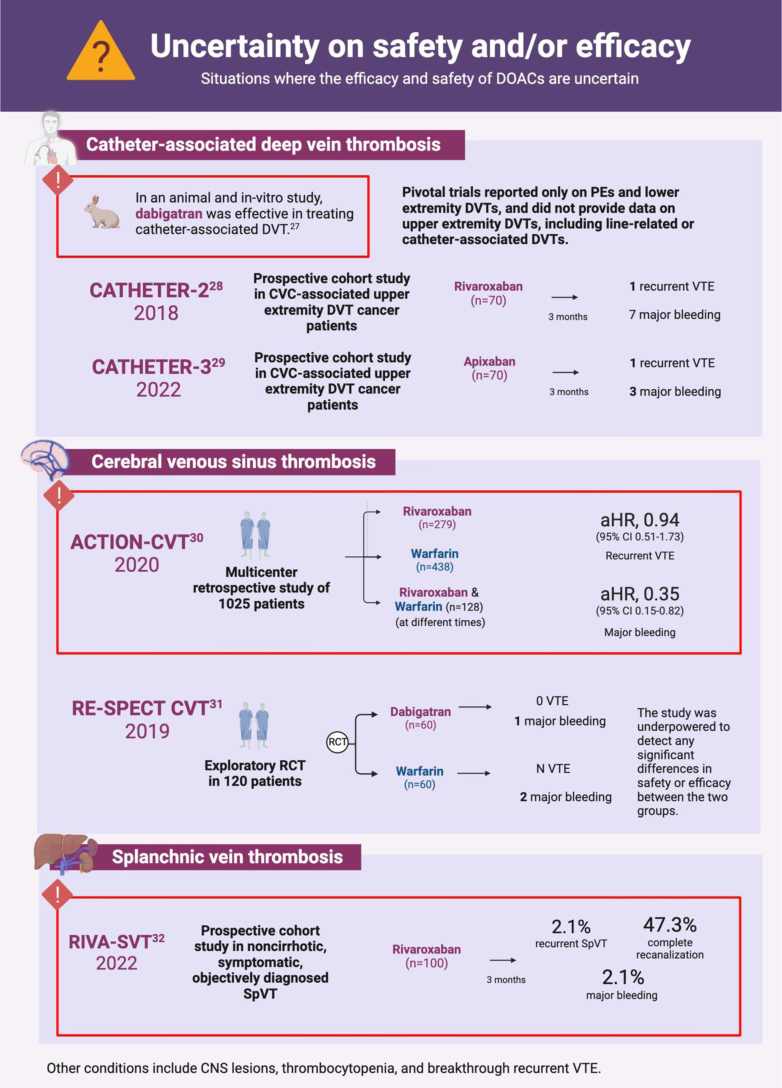
- 27.Yau J.W., Liao P., Fredenburgh J.C., Roberts R.S., Weitz J.I. Only high levels of dabigatran attenuate catheter thrombosis in vitro and in rabbits. Thromb Haemost. 2014;112:79–86. doi: 10.1160/TH13-12-1047. [DOI] [PubMed] [Google Scholar]
- 28.Davies G.A., Lazo-Langner A., Gandara E., Rodger M., Tagalakis V., Louzada M., et al. A prospective study of rivaroxaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients (Catheter 2) Thromb Res. 2018;162:88–92. doi: 10.1016/j.thromres.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 29.Kovacs M.J., Wells P.S., Rodger M.A., Carrier M., Yeo E., Kovacs J.A., et al. A prospective study of apixaban for central venous catheter associated upper extremity deep vein thrombosis in cancer patients: catheter 3. Blood. 2022;140:1245–1246. [Google Scholar]
- 30.Yaghi S., Shu L., Bakradze E., Salehi Omran S., Giles J.A., Amar J.Y., et al. Direct oral anticoagulants versus warfarin in the treatment of cerebral venous thrombosis (ACTION-CVT): a multicenter international study. Stroke. 2022;53:728–738. doi: 10.1161/STROKEAHA.121.037541. [DOI] [PubMed] [Google Scholar]
- 31.Ferro J.M., Coutinho J.M., Dentali F., Kobayashi A., Alasheev A., Canhão P., et al. Safety and efficacy of dabigatran etexilate vs dose-adjusted warfarin in patients with cerebral venous thrombosis: a randomized clinical trial. JAMA Neurol. 2019;76:1457–1465. doi: 10.1001/jamaneurol.2019.2764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ageno W., Beyer Westendorf J., Contino L., Bucherini E., Sartori M.T., Senzolo M., et al. Rivaroxaban for the treatment of noncirrhotic splanchnic vein thrombosis: an interventional prospective cohort study. Blood Adv. 2022;6:3569–3578. doi: 10.1182/bloodadvances.2022007397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bikdeli B., Zahedi Tajrishi F., Sadeghipour P., Talasaz A.H., Fanikos J., Lippi G., et al. Efficacy and safety considerations with dose-reduced direct oral anticoagulants: a review. JAMA Cardiol. 2022;7:747–759. doi: 10.1001/jamacardio.2022.1292. [DOI] [PubMed] [Google Scholar]
- 34.Di Minno M.N., Lupoli R., Di Minno A., Ambrosino P., Scalera A., Dentali F. Effect of body weight on efficacy and safety of direct oral anticoagulants in the treatment of patients with acute venous thromboembolism: a meta-analysis of randomized controlled trials. Ann Med. 2015;47:61–68. doi: 10.3109/07853890.2014.982064. [DOI] [PubMed] [Google Scholar]
- 35.Di Nisio M., Vedovati M.C., Riera-Mestre A., Prins M.H., Mueller K., Cohen A.T., et al. Treatment of venous thromboembolism with rivaroxaban in relation to body weight. A sub-analysis of the EINSTEIN DVT/PE studies. Thromb Haemost. 2016;116:739–746. doi: 10.1160/TH16-02-0087. [DOI] [PubMed] [Google Scholar]
- 36.Leong R., Chu D.K., Crowther M.A., Mithoowani S. Direct oral anticoagulants after bariatric surgery-what is the evidence? J Thromb Haemost. 2022;20:1988–2000. doi: 10.1111/jth.15823. [DOI] [PubMed] [Google Scholar]
- 37.Martin K.A., Beyer-Westendorf J., Davidson B.L., Huisman M.V., Sandset P.M., Moll S. Use of direct oral anticoagulants in patients with obesity for treatment and prevention of venous thromboembolism: updated communication from the ISTH SSC Subcommittee on Control of Anticoagulation. J Thromb Haemost. 2021;19:1874–1882. doi: 10.1111/jth.15358. [DOI] [PubMed] [Google Scholar]
- 38.Kröll D., Nett P.C., Rommers N., Borbély Y., Deichsel F., Nocito A., et al. Efficacy and safety of rivaroxaban for postoperative thromboprophylaxis in patients after bariatric surgery: a randomized clinical trial. JAMA Netw Open. 2023;6 doi: 10.1001/jamanetworkopen.2023.15241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamashita Y., Morimoto T., Toyota T., Shiomi H., Makiyama T., Ono K., et al. Asian patients versus non-Asian patients in the efficacy and safety of direct oral anticoagulants relative to vitamin K antagonist for venous thromboembolism: a systemic review and meta-analysis. Thromb Res. 2018;166:37–42. doi: 10.1016/j.thromres.2018.04.008. [DOI] [PubMed] [Google Scholar]
- 40.Yamada N., Hirayama A., Maeda H., Sakagami S., Shikata H., Prins M.H., et al. Oral rivaroxaban for Japanese patients with symptomatic venous thromboembolism – the J-EINSTEIN DVT and PE program. Thromb J. 2015;13:2. doi: 10.1186/s12959-015-0035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura M., Wang Y.Q., Wang C., Oh D., Yin W.H., Kimura T., et al. Efficacy and safety of edoxaban for treatment of venous thromboembolism: a subanalysis of East Asian patients in the Hokusai-VTE trial. J Thromb Haemost. 2015;13:1606–1614. doi: 10.1111/jth.13055. [DOI] [PubMed] [Google Scholar]
- 42.Bapat P., Kedar R., Lubetsky A., Matlow J.N., Aleksa K., Berger H., et al. Transfer of dabigatran and dabigatran etexilate mesylate across the dually perfused human placenta. Obstet Gynecol. 2014;123:1256–1261. doi: 10.1097/AOG.0000000000000277. [DOI] [PubMed] [Google Scholar]
- 43.Bapat P., Pinto L.S., Lubetsky A., Berger H., Koren G. Rivaroxaban transfer across the dually perfused isolated human placental cotyledon. Am J Obstet Gynecol. 2015;213:710.e1–710.e6. doi: 10.1016/j.ajog.2015.06.065. [DOI] [PubMed] [Google Scholar]
- 44.Beyer-Westendorf J., Tittl L., Bistervels I., Middeldorp S., Schaefer C., Paulus W., et al. Safety of direct oral anticoagulant exposure during pregnancy: a retrospective cohort study. Lancet Haematol. 2020;7:e884–e891. doi: 10.1016/S2352-3026(20)30327-6. [DOI] [PubMed] [Google Scholar]
- 45.Quenby S., Gallos I.D., Dhillon-Smith R.K., Podesek M., Stephenson M.D., Fisher J., et al. Miscarriage matters: the epidemiological, physical, psychological, and economic costs of early pregnancy loss. Lancet. 2021;397:1658–1667. doi: 10.1016/S0140-6736(21)00682-6. [DOI] [PubMed] [Google Scholar]
- 46.Zhao Y., Arya R., Couchman L., Patel J.P. Are apixaban and rivaroxaban distributed into human breast milk to clinically relevant concentrations? Blood. 2020;136:1783–1785. doi: 10.1182/blood.2020006231. [DOI] [PubMed] [Google Scholar]
- 47.Ayuk P., Kampouraki E., Truemann A., Sidgwick F., McDonald L., Bingham J., et al. Investigation of dabigatran secretion into breast milk: implications for oral thromboprophylaxis in post-partum women. Am J Hematol. 2020;95:E10–E13. doi: 10.1002/ajh.25652. [DOI] [PubMed] [Google Scholar]
- 48.Konstantinides S.V., Meyer G., Becattini C., Bueno H., Geersing G.J., Harjola V.P., et al. 2019 ESC Guidelines for the diagnosis and management of acute pulmonary embolism developed in collaboration with the European Respiratory Society (ERS) Eur Heart J. 2020;41:543–603. doi: 10.1093/eurheartj/ehz405. [DOI] [PubMed] [Google Scholar]
- 49.Lyman G.H., Carrier M., Ay C., Di Nisio M., Hicks L.K., Khorana A.A., et al. American Society of Hematology 2021 guidelines for management of venous thromboembolism: prevention and treatment in patients with cancer. Blood Adv. 2021;5:927–974. doi: 10.1182/bloodadvances.2020003442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Khorana A.A., Noble S., Lee A.Y., Soff G., Meyer G., O’Connell C., et al. Role of direct oral anticoagulants in the treatment of cancer-associated venous thromboembolism: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16:1891–1894. doi: 10.1111/jth.14219. [DOI] [PubMed] [Google Scholar]
- 51.Lyon A.R., López-Fernández T., Couch L.S., Asteggiano R., Aznar M.C., Bergler-Klein J., et al. 2022 ESC Guidelines on cardio-oncology developed in collaboration with the European Hematology Association (EHA), the European Society for Therapeutic Radiology and Oncology (ESTRO) and the International Cardio-Oncology Society (IC-OS) Eur Heart J. 2022;43:4229–4361. doi: 10.1093/eurheartj/ehac244. [DOI] [PubMed] [Google Scholar]
- 52.Di Nisio M., Valeriani E., Riva N., Schulman S., Beyer-Westendorf J., Ageno W. Anticoagulant therapy for splanchnic vein thrombosis: ISTH SSC Subcommittee Control of Anticoagulation. J Thromb Haemost. 2020;18:1562–1568. doi: 10.1111/jth.14836. [DOI] [PubMed] [Google Scholar]
- 53.Saposnik G., Barinagarrementeria F., Brown R.D., Jr., Bushnell C.D., Cucchiara B., Cushman M., et al. Diagnosis and management of cerebral venous thrombosis: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2011;42:1158–1192. doi: 10.1161/STR.0b013e31820a8364. [DOI] [PubMed] [Google Scholar]
- 54.University College London Rivaroxaban for stroke patients with antiphospholipid syndrome (RISAPS) 2023. https://www.clinicaltrials.gov/ct2/show/NCT03684564
- 55.McBane R.D., 2nd, Loprinzi C.L., Ashrani A., Lenz C.J., Houghton D., Zemla T., et al. Extending venous thromboembolism secondary prevention with apixaban in cancer patients: the EVE trial. Eur J Haematol. 2020;104:88–96. doi: 10.1111/ejh.13338. [DOI] [PubMed] [Google Scholar]
- 56.Bikdeli B., Hogan H., Morrison R.B., Fanikos J., Campia U., Barns B.M., et al. Extended-duration low-intensity apixaban to prevent recurrence in patients with provoked venous thromboembolism and enduring risk factors: rationale and design of the HI-PRO trial. Thromb Haemost. 2022;122:1061–1070. doi: 10.1055/a-1646-2244. [DOI] [PubMed] [Google Scholar]
- 57.Anthos Therapeutics Inc A study comparing abelacimab to dalteparin in the treatment of gastrointestinal/genitourinary cancer and associated VTE (MAGNOLIA) 2024. https://clinicaltrials.gov/ct2/show/NCT05171075