Abstract
Objective
Despite the worsening of the opioid epidemic, access to quality treatment for opioid use disorder (OUD) including buprenorphine remains a challenge. With the onset of the COVID-19 public health emergency, temporary regulatory changes and expanded reimbursement for telehealth services allowed for the rapid expansion of remote treatment for OUD and increased access to buprenorphine, but limited research exists to support this revolutionary shift in care delivery. This study evaluates the feasibility and acceptability of a novel digital therapeutic intervention for OUD combining buprenorphine and behavioral therapy.
Methods
Adults (n = 27) with OUD received treatment with daily sublingual buprenorphine and psychosocial treatment delivered digitally via a smartphone app over 12 weeks. Participants were evaluated monthly for continued opioid use, medication adherence, anxiety and depression indicators, abstinence self-efficacy, craving, and overall well-being, as well as a one-time measure of treatment acceptability.
Results
Participants reported increased opioid abstinence days from baseline (M = 8.2, SD = 8.6) to 12 weeks per 30 days (M = 24.9, SD = 10.1), t(20) = −6.5, p < .000, with strong medication adherence across study waves (96.2%). Anxiety and depression indicators, and opioid craving significantly decreased, and abstinence self-efficacy and overall well-being significantly increased following the intervention. Participants also demonstrated high rates of treatment engagement.
Conclusions
As current public health emergency regulatory changes are reviewed for permanency, this feasibility and acceptability study of a novel digital therapeutic intervention for OUD including buprenorphine adds to the growing evidence that supports maintaining telehealth access for quality OUD treatment.
Keywords: Buprenorphine, opioid use disorder, telemedicine, feasibility studies, mobile application
Introduction
The opioid epidemic continues to worsen year after year, with over 80,000 lives lost in the United States in 2021 to an overdose involving an opioid 1 and despite a declared public health emergency in 2017, opioid overdose rates have only increased. 2 Between 2020 and 2021, the opioid epidemic clashed with the devastating arrival of the COVID-19 pandemic, and more than nine million people reportedly misused opioids, with nearly 2.7 million becoming diagnosed with an opioid use disorder (OUD). 3
The gold standard for treating OUD is a combination of psychotherapy and pharmacology, personalized to the patient's needs. 4 Three medications for OUD (MOUD) are currently FDA-approved: naltrexone, methadone, and buprenorphine. While all three medications have a high degree of effectiveness in reducing opioid cravings and relapse, and preventing future overdose episodes, buprenorphine remains the most optimal for use in outpatient settings given its pharmacological properties and relaxed service delivery guidelines compared to more regulated medications. 5
Traditionally, buprenorphine has been offered in person at established outpatient clinics and other office-based settings. In-person office-based buprenorphine settings have largely produced positive clinical outcomes among patients, 6 and greatly expanded the availability of MOUDs beyond traditional opioid treatment programs that solely leveraged methadone. These programs not only initially improved access to MOUDs, but also have the potential to provide a positive therapeutic milieu for patients.7–9 However, despite the demonstrated effectiveness of in-person buprenorphine treatment programs, numerous barriers have limited its availability and associated treatment retention, 10 and resulted in a minority of individuals with OUD (10.3%) being able to access the medication. 11 Historically, provider unwillingness to prescribe buprenorphine and lack of trained providers, especially in rural areas, has resulted in patients being placed on long waiting lists before they are able to initiate treatment; unfortunately, this delay significantly impacts treatment initiation and engagement.12,13
In 2017, buprenorphine was available in just 27% of treatment programs, extended-release naltrexone in just 24%, and methadone in as few as 9%. Contingencies for treatment fueled additional barriers to care, even for those programs that do offer medication: many require an extensive admissions process, clinic-based dosing for at least the first prescription, abstinence from all substances (both prior to initiation and throughout treatment), and required engagement in counseling or community support groups. 12 Patients struggle with overcoming these barriers, often managing competing priorities and lacking adequate transportation to get to and from appointments, sufficient childcare during appointment times and extensive group requirements, and inflexible treatment schedules. 13 Geographic region can also impact access to treatment, with patients in rural settings required to travel farther to acquire buprenorphine, as well as the often greater economic distress characteristic of these areas with disproportionately higher rates of OUD and overdose.14,15
Telehealth, or the delivery of healthcare via technology, has been underutilized as a method of accessing care until the COVID-19 pandemic dramatically expanded access through a variety of legislative means. 16 Opioid use and associated overdoses accelerated during the COVID-19 pandemic beginning in 2020, a shift that has been attributed to increased isolation from peer support and treatment resources, and reduced access to emergency response and life-saving medications. 17 As stay-at-home pandemic policies exacerbated many of the barriers with which patients were already struggling, the number of opioid overdoses increased by 31% between 2019 and 2020, and public health leaders began to scramble for a solution to improve access to life-saving OUD treatment. 1 These policy adjustments led to a significant shift in treatment delivery: prior to the COVID-19 pandemic 1 in 800 opioid patients engaged in telehealth care; during the pandemic, that number rose to one in eight patients. 18
Complementing MOUD with evidence-based psychotherapies like cognitive behavioral therapy (CBT) and motivational interviewing (MI) provides a holistic approach to care. While MOUDs reduce physiological cravings and urge to use opioids, clinical interventions promote the use of skills for managing negative emotions, working through ambivalence, and reframing thoughts around using substances. 19 For example, skills gained through CBT allow patients to change their behaviors by bringing mindfulness to unhelpful thought and response patterns, and MI techniques stimulate a patient's ability, willingness, and readiness for change. Both CBT and MI have been demonstrated to positively impact treatment outcomes for those with OUD. 20 Unfortunately, even though many providers prescribing buprenorphine recommend pairing MOUD with behavioral therapies, only 36% report having sufficient local resources for counseling referrals. Telehealth and digital programs may ease access to care and offer advantages like convenient peer support, the ability to track progress, and on-demand CBT-based education. 21
Studies conducted since the pandemic onset support the use of telehealth for expanding access to OUD treatment, documenting improved treatment retention, reduced odds of medically treated or fatal overdose events, and comparable clinical outcomes to in-person care.18,22 Within this ever-changing context, providing access to buprenorphine, evidence-based therapeutic approaches, and strong patient support services through digital treatment and interventions presents a promising solution to many of the historical access barriers that patients face initiating and engaging in sustainable care. This pilot study evaluated the feasibility, acceptability, and preliminary outcomes of an integrated digital therapeutic intervention combining buprenorphine with psychosocial treatment for OUD.
Methods
Design
This observational study evaluated the feasibility and acceptability of providing treatment for OUD including buprenorphine via a novel digital therapeutic intervention. All psychosocial and medical visits were conducted over a video platform within a mobile application, and all participants had access to digital therapeutic content and use tracking within the application.
After study enrollment, eligible participants met virtually with a research counselor and completed a baseline diagnostic assessment based on the American Society of Addiction Medicine's guidelines. 23 Following this assessment, participants downloaded the mobile application and met virtually, via the app, with a study clinician to complete a biopsychosocial assessment to confirm eligibility. Participants were inducted onto buprenorphine and scheduled to meet with the research counselor weekly, and study provider monthly for pharmacotherapy, for 12 weeks. At counseling visits, participants received manualized CBT and motivational enhancement therapy (MET) content, as well as personal therapeutic support. At provider visits, a study clinician assessed treatment progress and provided medication management. Survey research assessments were completed online by participants monthly to track progress, and research interviews were conducted monthly to collect retrospective opioid use and medication adherence data. Medication adherence was attained monthly via unannounced film counts conducted by phone with study staff. Although the intervention period consisted of 12 weeks of care with continued data collection, participants were provided optional continued treatment services for 12 additional weeks to ensure stabilization and appropriate referrals to ongoing care.
Participants
Potential participants were recruited via social media advertisements and directed to a study website with eligibility criteria and a comprehensive study description. If potential participants were interested, their emails would be sent to research staff and added to a recruitment list. These interested individuals were emailed a survey link that helped determine their eligibility. If eligibility criteria were met, research staff sent a study requirement comprehension quiz to each potential participant. The onboarding process started with individuals who passed the comprehension quiz.
Inclusion criteria for study participation included: (1) ≥18 years of age; (2) reside in the United States; (3) own a smartphone with video call functionality to utilize the study mobile application; (4) have a Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-5) diagnosis of moderate to severe OUD; (5) be able and willing to participate in study procedures, including taking study medication (buprenorphine/naloxone); (6) be in good general health; and (7) have Medicaid, Medicare, or commercial insurance. Exclusion criteria included: (1) had a known sensitivity to buprenorphine/naloxone formulation; (2) received a MOUD including naltrexone, methadone, or buprenorphine for OUD within the past 30 days; (3) had a serious medical condition that would make participation hazardous; (4) required medical detoxification from any substances; (5) lacked English proficiency; or (6) had clinically significant psychiatric symptoms that would make study participation difficult. Study participants were recruited from April to November 2022.
To complete eligibility and study onboarding, study staff verified insurance coverage and U.S. residency through photo identification. Informed consent was obtained through an electronic signature website while completing a phone call with study staff to thoroughly describe study requirements and answer questions. Participants agreed to (1) 12 weekly 45-min video-based counselor-delivered CBT sessions; (2) virtual medical pharmacotherapy visits monthly with a study provider; (3) monthly urine drug screen tests; (4) monthly online self-report assessments with $40 compensation for the baseline, and $25 for each of the monthly assessments and each film count call. Oversight for this study was provided by the Ethical and Independent Review Services’ Institutional Review Board.
Intervention
Pelago-Opioid (Pel-O) is a commercially available virtual outpatient treatment program that combines pharmacotherapy with CBT and MET content, and community reinforcement. The Pel-O program also provides patients access to a mobile application that allows them access to virtual meetings with their counselor and medical provider over video; ongoing treatment progress tracking, including streaks of opioid abstinence; interactive digital CBT and MET training modules; craving-management tools; information about OUD and buprenorphine; message and chat functions that allow patients to communicate directly with their counselor outside of scheduled visits; cravings exercises; and, mindfulness-based guided meditation audio. The app is Health Insurance Portability and Accountability Act compliant and has video capabilities certified by the Health Information Trust Alliance.
The digital CBT/MET content is delivered at a sixth-grade reading level and can be guided by audio if needed. The app prompts participants to self-report any opioid use daily, as well as provides reminders to patients about upcoming appointments. Pel-O delivers personalized, behavioral support to individuals who are seeking to abstain from using opioids and prevent future use, and includes CBT-based content in the form of videos, in-app text, audio recordings, and quizzes. The application's therapeutic content utilizes a combination of text, motivational content, bidirectional messaging, and gamification elements (e.g. progress monitoring, rewards, achievements, badges, and stories) to engage users and motivate them to abstain from opioid use. The digital CBT content delivered through the app, although separate from the virtual CBT counseling delivered live via video, is complementary and mutually reinforcing to the therapeutic programming delivered by counselors. During the course of counseling appointments, counselors direct participants to the specific app content and modules that work to reinforce the subject areas covered during counseling sessions. The Pel-O app also contained helpful information and frequently asked questions related to buprenorphine.
Counseling is provided by a licensed drug and alcohol counselor who has training in delivering evidence-based therapeutic techniques such as CBT, MET, and MI. Counselors received standardized training and clinical supervision weekly from the Principal Investigator. Urine drug screens (UDS) were collected remotely through the delivery of point-of-care test cups to the participants’ home addresses. During the intervention period, counselors periodically requested participants to complete remote urine screens during appointments. Participants provided urine samples off camera, then presented the results through video as they appeared on the point-of-care cup. UDS cups were equipped with a dual scale temperature test strip to ensure the urine sample validity.
Medical provider visits
After obtaining informed consent and determining eligibility, study participants were immediately scheduled to meet with a medical provider (either a nurse practitioner or physician) licensed in their state of residence virtually to determine their appropriateness for buprenorphine treatment. Study providers completed a DSM-V checklist for OUD to determine diagnostic severity, a medical assessment, substance use history, and clinical impressions. Participants were inducted onto buprenorphine/naloxone film formulation according to standard clinical practice, after the full clinical assessment. Buprenorphine was titrated to between 16 mg and 24 mg per day. After standard titration, dosage and frequency of buprenorphine prescribing varied between participants to adapt treatment to each participant's unique needs and levels of craving. Participants attended monthly, 30-min video conferencing medical management sessions with their provider, which included discussion of dose adjustments, side effects, and limited counseling as commonly provided to patients in outpatient settings. Initial buprenorphine prescriptions were issued to the participants’ local pharmacy to facilitate immediate induction onto the medication. Future prescriptions were mailed directly via by-mail pharmacy to participants’ residences for maximum convenience and continuity.
Four weeks prior to completing treatment, the study team referred each participant to a provider or program for continued treatment locally in their community. Study staff coordinated the referral, including all necessary documentation to ensure continuity of pharmacological care for each participant.
Measures
Study staff gathered self-report opioid use and medication adherence data via telephone interview at baseline, 4-, 8-, and 12-weeks. All remaining survey measures were collected via self-report questionnaires sent electronically, also at baseline, 4-, 8-, and 12-weeks.
Primary outcome
Opioid use over the past 30 days was recorded at baseline and monthly follow-up using the Timeline Follow Back (TLFB) method, a phone interview assisted by calendar. 24 The primary outcome, the number of abstinent days from opioids in the last 30, was confirmed by subsample biochemical testing (UDS).
Secondary outcomes
Medication adherence was determined by conducting three unannounced telephone-based film counts over the 12-week study period by a validated method.25,26 The first film count was completed one month after the date of buprenorphine stabilization (determined by the study clinician), and each count allowed for an adherence score to be calculated by the ratio of films remaining relative to films prescribed, taking into account the number of films dispensed since the last count. Medication adherence scores were calculated using the established method developed by Kalichman et al. 25
Opioid abstinence self-efficacy was assessed using the Drug Taking Confidence Questionnaire, 27 a self-reported total score measure of confidence to not use opioids in the course of both positive and negative regularly occurring life events. Opioid craving was assessed via the Visual Analogue Scale instrument, constituting a 10-point visualized scale rating from “not want it (0)” to “I could not resist taking it (100).”
Depression indicators were assessed using the Patient Health Questionnaire-9, 28 featuring nine items, each of which is scored 0–3 providing a 0–27 severity score. Anxiety indicators were assessed using the Generalized Anxiety Disorder-7, 29 featuring seven items, each of which is scored 0–3 providing a 0–21 severity score. Well-being was assessed using the World Health Organization Well-Being Index, 30 which indexes a total transformed score from 0 to 100 measuring the overall physical, mental, and emotional well-being of participants.
Treatment engagement metrics included the number of CBT digital app modules completed (27 total possible), number of digital app activities completed during study participation (including chat messaging, daily abstinence check-ins, CBT modules, meditation audio, and cravings exercises), number of days during study participation with app activities completed, number of days during study participation with counselor and provider interactions completed (including digital chat messages and virtual appointments), total number of digital chat messages sent and received between the participant and their counselor, and total number of attended appointments during study participation.
Treatment acceptability was measured at a four-week follow-up assessing participants’ perceptions of the quality of services received, the degree to which the program met their individual needs, their likelihood of recommending the program to others, helpfulness of the information provided in the program, program effectiveness to manage their opioid use, and whether they would return to the program. Treatment acceptability was measured using a 4-point Likert scale.
Statistical analysis
Illicit opioid use in the past 30 days was indicated by the proportion of self-reported days abstinent in the last 30 days. Independent samples t-tests were used to analyze baseline to posttreatment differences in opioid use and secondary outcomes between baseline and 12 weeks posttreatment initiation. Participants self-reported opioid use was confirmed with subsample biochemical testing (UDS).
Results
A total of 40 participants consented to participate in the study, five participants were found to be ineligible for the study after consenting, and eight consented participants were lost to follow-up and never engaged in care. Of the final sample (n = 27), 51.9% were female, 48.2% employed, and 85.2% identified as white (Table 1). Thirty-seven percent of the sample resided in an area classified as rural, and a vast majority of participants lived in areas determined to have health professional shortages in primary care (77.8%) and mental health (81.4%) by the Health Resources and Services Administration (HRSA). Fentanyl was the leading opioid of choice among the sample (51.9%), followed by heroin (37.0%) and prescription opioids (33.3%) at relatively similar rates. The preferred route of opioid administration was snorting/intranasal (44.4), followed by oral (22.2%) and smoking (18.5%).
Table 1.
Sample demographic characteristics.
| Demographics | N = 27 |
|---|---|
| n (%) | |
| Gender | |
| Female | 14 (51.9) |
| Employed | 13 (48.2) |
| Education | |
| High school | 18 (66.7) |
| Undergraduate degree | 5 (18.5) |
| Other | 4 (14.8) |
| Location | |
| Rural | 10 (37.0) |
| Primary care shortage area | 22 (77.8) |
| Mental health shortage area | 21 (81.4) |
| Race/ethnicity | |
| White | 13 (85.2) |
| African American | 1 (3.7) |
| Mixed | 1 (3.7) |
| Other | 1 (3.7) |
| Prefer not to answer | 1 (3.7) |
| Opioid of choice a | |
| Prescription opioid | 9 (33.3) |
| Heroin | 10 (37.0) |
| Fentanyl | 14 (51.9) |
| Preferred route of administration | |
| Oral | 6 (22.2) |
| Snorting/intranasal | 12 (44.4) |
| Injection | 3 (11.1) |
| Smoking | 5 (18.5) |
| Other | 1 (3.7) |
| M (SD) | |
| Age | 37.7 (7.0) |
Not mutually exclusive categories.
Primary outcome: Opioid use
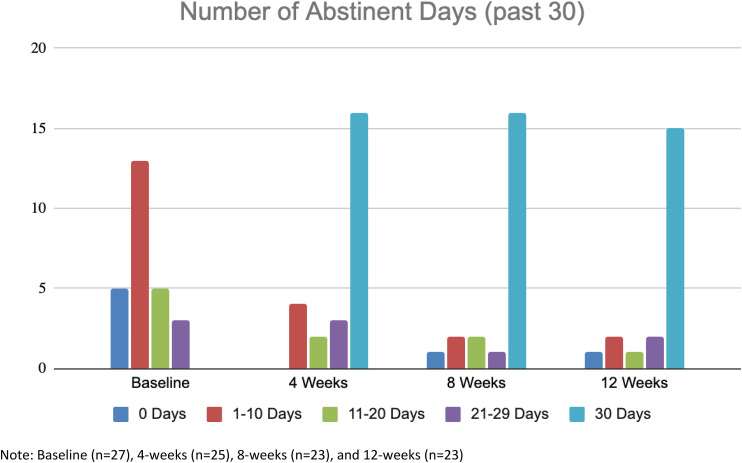
Over the 12-week intervention period, participants in this sample (n = 27) significantly increased the number of days of opioid abstinence from baseline (M = 8.2, SD = 8.6) to 12 weeks per 30 days (M = 24.9, SD = 10.1), t(20) = −6.5, p < .000 (Table 2 and Figure 1). Of those participants who completed 12-week TLFB data collection (n = 21, 78%), 71% (n = 15) reported full 30-day opioid abstinence over the last four weeks of the trial, and 67% (n = 14) reported full 90-day opioid abstinence over the full 12-week intervention period of the study. Of those urine screens collected from these participants, 94.5% of all screens collected during the intervention and follow-up period were negative for opioids. There were no reported return to use or overdose events by participants retained and engaged in the Pel-O program.
Table 2.
Primary and secondary outcomes by follow-up timepoint.
| Measure | Baseline M (SD) n = 27 | 4-Week M (SD) n = 25 | 8-Week M (SD) n = 23 | 12-Week M (SD) n = 23 | p |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Days abstinent a | 8.2 (8.6) | 24.7 (9.3) | 25 (9.4) | 24.9 (10.1) | .000 |
| Secondary outcomes | |||||
| Opioid abstinence self-efficacy | 50.7 (26.5) | 68.6 (26.2) | 74.8 (22.4) | 74.5 (23.9) | .001 |
| Opioid craving | 65.8 (22.4) | 35.3 (27.0) | 37.4 (31.6) | 28.7 (29.2) | .000 |
| Depression indicators | 12.0 (6.4) | 7.4 (4.8) | 7.3 (5.2) | 7.7 (4.7) | .003 |
| Anxiety indicators | 11.8 (6.5) | 7.8 (4.9) | 8.3 (5.7) | 7.5 (5.1) | .005 |
| Overall well-being | 50.6 (16.9) | 68.8 (22.1) | 69.7 (21.6) | 72.0 (25.4) | .002 |
Baseline (n = 27), eight-week (n = 22), 12-week (n = 21).
Note: Significance tests reference changes from baseline to 12-week follow-up.
Figure 1.
Total number of self-reported opioid abstinent days for study participants completing each respective wave of data collection at baseline, 4-, 8-, and 12-weeks posttreatment entry.
Secondary outcomes
Participants who were successfully inducted onto buprenorphine following study enrollment, and who were maintained on buprenorphine through the intervention period, completed 89.7% of all virtual film count meetings with research staff. At one-month follow-up, participants reported an average of 95.2% adherence, 95.4% adherence at two months, and 98.1% adherence at three months. This indicates an overall average of 96.2% adherence across study waves. Using >80% as the cut-off for adherent scores, i.e. the minimum level of adherence to achieve stable remission for OUD according to prior studies, 28 only one participant at one time point indicated a less than adherent score (75%).
Participants in this sample reported a significant increase in opioid abstinence self-efficacy throughout the intervention period from baseline (M = 50.7, SD = 26.5) through 12 weeks (M = 74.5, SD = 23.9), t(22) = 4.0, p = .001, as well as a significant decrease in opioid craving during the same period (M = 65.8, SD = 22.4 vs. M = 28.7, SD = 29.2), t(22) = −5.1, p < .000. Regarding mental health indicators, participants reported a significant decrease in both indicators of depression from baseline (M = 12.0, SD = 6.4) through 12 weeks (M = 7.7, SD = 4.7), t(22) = −3.3, p < .003, as well as indicators of anxiety during the same period (M = 11.8, SD = 6.5 vs. M = 7.5, SD = 5.1), t(22) = −3.2, p = .005. Self-reported overall well-being also increased significantly during the treatment period (M = 50.6, SD = 16.9 vs. M = 72.0, SD = 25.4), t(22) = 3.6, p = .002.
Treatment retention and engagement
For those participants who were retained in treatment for the full 12-week intervention (n = 19; 70%), all remained engaged through 24 weeks. Throughout the intervention period, participants completed an average of 16 (59%) CBT content modules in the mobile application, with five participants completing all 27 available modules (Table 3). Participants also completed an average of approximately 187 digital app activities, many of which included daily prompts to report opioid abstinence. On average, participants actively engaged with app activities or counselors and providers approximately one out of every three days (about 31 and 36 days, respectively), with five participants interacting with app activities during every day of the intervention period.
Table 3.
Treatment engagement metrics collected during study participation (n = 27 participants).
| Measure (No) | M (SD) |
|---|---|
| CBT modules completed (27 possible) | 15.95 (9.5) |
| Digital app activities completed | 186.89 (181.0) |
| Days with completed app activities | 35.89 (30.8) |
| Days with counselor and provider interactions | 31.3 (24.6) |
| Digital chat messages with counselor sent and received | 66.33 (63.5) |
| Attended counselor and provider appointments | 35.3 (25.1) |
CBT: cognitive behavioral therapy.
Treatment acceptability
The vast majority of participants in the study found the Pel-O program to be highly acceptable (Table 4), with nearly all study participants indicating that the quality of services they received during the program was either good or excellent (92.3%), met their individual needs (84.6%), provided helpful information (100%), and was effective in helping to manage their opioid use (92.3%).
Table 4.
Treatment acceptability outcomes.
| Measure | N = 26 |
|---|---|
| n (%) | |
| Quality of services | |
| Excellent or good | 24 (92.3) |
| Met individual needs | |
| All or most of my needs were met | 22 (84.6) |
| Recommend program to others | |
| Yes | 23 (88.5) |
| Helpfulness of information provided | |
| Very helpful or helpful | 26 (100) |
| Program effectiveness to manage opioid use | |
| Very helpful or helpful | 24 (92.3) |
| Return to program in the future if needed | |
| Yes | 24 (92.3) |
Discussion
This is the first study to test the feasibility and acceptability of a digital therapeutic intervention for OUD that also included the delivery of buprenorphine and virtual CBT therapy. Initial findings from this pilot study indicate that the delivery of virtual CBT services in combination with buprenorphine is not only feasible, but acceptable to patients, and these early pilot results also indicate promising outcomes for strong treatment engagement, medication adherence, and reducing opioid use. Similarly, although the Pel-O program did not directly address psychiatric symptoms, reductions in depression and anxiety indicators, as well as overall well-being, are encouraging.
The initial results from this study indicate that OUD participants found the combination of virtual care delivery of medical and psychotherapy treatment, mobile app-based content, and remote delivery of buprenorphine services offered through the Pel-O program to be more than satisfactory, with high ratings for quality of service, meeting individual needs, helpful information delivery, and program effectiveness. These elements together are likely reflective of the high degree of engagement and retention measured through rates of app activities and CBT-content completion, digital chat messaging, and virtual care appointment attendance.
In addition to promoting improved initial access to care, telehealth treatment has consistently demonstrated comparable or improved rates of engagement and retention for patients with OUD.31–33 One review was unable to find any studies demonstrating reduced treatment retention with telehealth treatment, and another study found retention rates to significantly improve under the expanded telehealth policies during the COVID-19 pandemic.34,35 The results of the current study support these existing findings and contribute additional evidence to the feasibility and acceptability of telehealth combined with buprenorphine for the successful treatment of OUD.
Positive outcomes related to program satisfaction, engagement, and retention were likely related to the significant reduction in opioid use reported by study participants, as well as the absence of a return to use or overdose events during the intervention period. Similarly, high rates of buprenorphine adherence by study participants who were retained and engaged in the Pel-O program were also likely a contributing factor to significantly greater abstinence rates reported by participants at follow-up time points. These factors combined indicate that the feasibility and acceptability of the Pel-O program for OUD participants could have a positive influence on reduced opioid use in a future randomized trial.
This feasibility and acceptability study had greater rates of overall treatment retention and engagement than in-person studies in which patients were evaluated at 12 weeks, 36 as well as greater rates of medication adherence. 37 Overall these supportive findings from the current study bolster the ongoing emergent evidence that treatment retention and engagement is higher among individuals with OUD who are enrolled in telehealth as compared to in-person programs. 38 Likely associated with this improved engagement and retention, telehealth OUD has also been associated with lower rates of overdose events compared to in-person care, 39 a finding that this pilot study also supports.
Studies now indicate OUD telehealth treatment outcomes are similar to those engaged with in-person care regarding retention rates, therapeutic alliance, and opioid use outcomes.18,40–42 One study found patients utilizing a telehealth program designed for those living in rural areas to reduce their opioid use by a third—comparable with in-person programs in similar communities. 36 Among individuals participating in this study, the vast majority lived in an area designated by HRSA as a Health Professional Shortage area for primary care and mental health. Overall, just under half lived in an area designated by HRSA as a medically underserved area/population, and just over a third lived in an area designated as rural. This study demonstrated equivalent feasibility and clinical outcomes in treating individuals in both rural and urban areas, specifically those with reduced medical capacity and resources.
Beyond rural communities, other studies have demonstrated that telehealth services for OUD have similar loss-to-follow-up rates as in-person care, as well as low rates of adverse clinical outcomes, ultimately concluding telehealth-based treatment to be a safe and feasible method for providing MOUD. 43 The use of telehealth services has been linked with a reduction in the number of patients who experience a medically treated overdose, and patients engaged in an OUD telehealth program were 33% less likely to experience a fatal overdose.18,44 The results from this feasibility and acceptability pilot support these existing findings, and contribute additional evidence that virtual delivery of OUD care combined with complementary digital support information and services through a mobile application can reduce opioid use and positively impact the lives of those living with OUD.
Among study participants in the Pel-O program, this feasibility study also demonstrated a significant reduction in depression and anxiety indicators, largely as an ancillary outcome to positive treatment engagement and retention, as well as subsequent reductions in opioid use. Individuals with OUD are also more likely to experience mental disorders, including anxiety and depression, with almost two-thirds of adults diagnosed with OUD also meeting the criteria for a mental health disorder.18,45,46 As a result of its accompanying social isolation, financial impact, and reduced access to care, the prevalence of these mental disorders has only worsened among this population since the COVID-19 pandemic. 40 Findings from this study support existing evidence that patients can receive quality telehealth services for OUD treatment that also positively impact mental health-related outcomes.
Future activities
Findings from this study will be used to inform a future randomized trial that will test the effectiveness of the Pel-O program against treatment as usual. The use of feasibility and acceptability pilot studies for this purpose focuses on elements of both processes (recruitment and sample characteristics, procedures and measures, intervention acceptability, and preliminary evaluation of participant responses), and a focus on preliminary outcomes. 47 Outcomes from this study support the procedures and measures that were utilized (validity and reliability of the measures for the intended study outcomes were sufficient), intervention acceptability (demonstrated by various engagement and retention metrics), and preliminary evaluation of participant responses (demonstrated by treatment satisfaction measures). With regard to preliminary outcomes, this observational study analyzed sample differences from baseline across follow-up time points to discern statistically significant differences, indicating the potential for hypothesis testing and determining effect size between groups in a randomized trial.
Recruitment and sample, however, will need to be revisited by the study team during trial development. The current recruitment strategy of social media advertising campaigns yielded a racially and ethnically homogenous sample, with low recruitment rates for those who use opioids intravenously. In order to capture a sample with greater generalizability within a larger sample size, the study team will need to consider improving this approach in the future.
Limitations
This study has several limitations. First, this study was executed as a feasibility and acceptability pilot and therefore was designed to recruit a limited sample that is not generalizable to the larger population, which limits the conclusions that can be drawn with respect to overall acceptability. Generalizability is further limited by the characteristics of the recruited sample with all participants required to have access to a smartphone, the vast majority of the sample identifying as white, and a small minority of the sample self-reporting intravenous opioid use. Given that the purpose of this study was to determine the feasibility and acceptability of the intervention, and not to detect effect sizes with regard to hypothesis testing, there was no need to calculate a predetermined, sufficient sample size.
Participants were also recruited from online and social media advertising campaigns, therefore limiting the catchment population to only those with access to these sites which also may have had an impact on the overall characteristics of the sample. This recruitment method will likely be reconsidered or refined in the future randomized trial design. This study did not contain a control group and did not randomize participants to study conditions and therefore cannot compare the effectiveness of the Pel-O program to any other treatment delivery condition.
Conclusion
Telehealth for the treatment of OUD is supported by a growing body of literature that demonstrates quality remote and virtual care can produce comparable clinical outcomes to traditional in-person services, especially when combined with buprenorphine. In addition to these outcomes, telehealth as a modality also has the ability to overcome barriers associated with in-person treatment especially for populations that face significant health disparities and reduced treatment capacity access. Findings from this feasibility study further support the existing literature that telehealth treatment for OUD is safe and effective and contributes additional evidence to the acceptability and patient-centeredness of the model.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076241258400 for Feasibility and acceptability of a novel digital therapeutic combining behavioral and pharmacological treatment for opioid use disorder by Laura B. Monico, Megan Eastlick, Darcy Michero, Peyton Pielsticker and Suzette Glasner in DIGITAL HEALTH
Footnotes
All authors of this article and staff responsible for the conduct of this research are employees of the study funder, Digital Therapeutics, Inc.
Funding: Funding for this study was provided by Digital Therapeutics, Inc.
ORCID iD: Laura B. Monico https://orcid.org/0000-0002-1504-4968
Supplemental material: Supplemental material for this article is available online.
References
- 1.Anonymous Understanding the Opioid Overdose Epidemic, https://www.cdc.gov/opioids/basics/epidemic.html (2022, accessed February 21, 2023).
- 2.Chandler RK, Villani J, Clarke T, et al. Addressing opioid overdose deaths: the vision for the HEALing communities study. Drug Alcohol Depend 2020 Dec; 217. DOI: 10.1016/j.drugalcdep.2020.108329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahmoud H, Naal H, Whaibeh E, et al. Telehealth-based delivery of medication-assisted treatment for opioid use disorder: a critical review of recent developments. Curr Psychiatry Rep 2022. July 27;24(9):375-386. DOI: 10.1007/s11920-022-01346-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sofuoglu M, DeVito EE, Carroll KM. Pharmacological and behavioral treatment of opioid use disorder. Psychiatr Res Clin Pract 2019. April 1;1(1):4-15. DOI: 10.1176/appi.prcp.20180006. [DOI] [Google Scholar]
- 5.Cioe K, Biondi BE, Easly R, et al. A systematic review of patients’ and providers’ perspectives of medications for treatment of opioid use disorder. J Subst Abuse Treat 2020 Dec; 119. DOI: 10.1016/j.jsat.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duncan LG, Mendoza S, Hansen H. Buprenorphine maintenance for opioid dependence in public sector healthcare: benefits and barriers. J Addict Med Ther Sci 2015. Aug;1(2):31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Filteau MR, Kim FL, Green B. “It’s more than just a job to them”: a qualitative examination of patient and provider perspectives on medication-assisted treatment for opioid use disorder. Community Ment Health J 2022;58(2):321-327. DOI: 10.1007/s10597-021-00824-7. [DOI] [PubMed] [Google Scholar]
- 8.Beharie N, Kaplan-Dobbs M, Urmanche A, et al. “I didn't feel like a number”: the impact of nurse care managers on the provision of buprenorphine treatment in primary care settings. J Subst Abuse Treat 2022. Jan;132. DOI: 10.1016/j.jsat.2021.108633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Poulsen MN, Santoro W, Scotti R, et al. Implementation of telemedicine delivery of medications for opioid use disorder in Pennsylvania treatment programs during COVID-19. J Addict Med 2023 Mar-April;17(2):e110-e118. DOI: 10.1097/ADM.0000000000001079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tofighi B, Williams AR, Chemi C, et al. Patient barriers and facilitators to medications for opioid use disorder in primary care. Subst Use Misuse 2019 Aug;54(14):2409-2419. DOI: 10.1080/10826084.2019.1653324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCurry MK, Avery-Desmarais S, Schuler M, et al. Perceived stigma, barriers, and facilitators experienced by members of the opioid use disorder community when seeking healthcare. J Nurs Scholarsh 2023 May;55(3):701-710. DOI: 10.1111/jnu.12837. [DOI] [PubMed] [Google Scholar]
- 12.Roy PJ, Choi S, Bernstein E, et al. Appointment wait-times and arrival for patients at a low-barrier access addiction clinic. J Subst Abuse Treat 2020. Jul;114. DOI: 10.1016/j.jsat.2020.108011. [DOI] [PubMed] [Google Scholar]
- 13.Madras BK, Ahmad NJ, Wen J, et al. The prevention, treatment, and recovery working group of the action collaborative on countering the US opioid epidemic. Improving access to evidence-based medical treatment for opioid use disorder: strategies to address key barriers within the treatment system. NAM Perspect Discus 2020. DOI: 10.31478/202004b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cambron C, Gouripeddi R, Facelli JC. Healthcare provider reports on social determinants of health in opioid treatment. Psych 2023 Jan;5(1):60-69. DOI: 10.3390/psych5010007. [DOI] [Google Scholar]
- 15.Gregory HM, Hill VM, Parker RW, et al. Implications of increased access to buprenorphine for medical providers in rural areas: a review of the literature and future directions. Cureus 2021 Nov;13(11):e19870 DOI: 10.7759/cureus.19870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oesterle TS, Kolla B, Risma CJ, et al. Substance use disorders and telehealth in the COVID-19 pandemic era: a new outlook. Mayo Clinic Proceedings Anonymous 2020 Dec;95(12):2709–2718: DOI: 10.1016/j.mayocp.2020.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Linas BP, Savinkina A, Barbosa C, et al. A clash of epidemics: impact of the COVID-19 pandemic response on opioid overdose. J Subst Abuse Treat 2021 Jan;120. DOI: 10.1016/j.jsat.2020.108158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones CM, Shoff C, Hodges K, et al. Receipt of telehealth services, receipt and retention of medications for opioid use disorder, and medically treated overdose among Medicare beneficiaries before and during the COVID-19 pandemic. JAMA Psychiatry 2022. Oct;79(10):981-992. DOI: 10.1001/jamapsychiatry.2022.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meyer B, Utter G, Hillman C. A personalized, interactive, cognitive behavioral therapy–based digital therapeutic (MODIA) for adjunctive treatment of opioid use disorder: development study. JMIR Ment Health 2021 Oct;8(10):e31173. DOI: 10.2196/31173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Potts C, Campbell MH, Kirwan C, et al. Development of a novel behavioral intervention for opioid use disorders. Online J Issues Nurs 2020 Sep;25(3). [Google Scholar]
- 21.Miller-Rosales C, Morden NE, Brunette MF, et al. Provision of digital health technologies for opioid use disorder treatment by US health care organizations. JAMA Network Open 2023. Jul;6(7):e2323741. DOI: 10.1001/jamanetworkopen.2023.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hailu R, Mehrotra A, Huskamp HA, et al. Telemedicine use and quality of opioid use disorder treatment in the US during the COVID-19 pandemic. JAMA Network Open 2023 Jan;6(1):e2252381. DOI: 10.1001/jamanetworkopen.2022.52381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stallvik M, Gastfriend DR, Nordahl HM. Matching patients with substance use disorder to optimal level of care with the ASAM criteria software. J Subst Use 2015;20(6). DOI: 10.3109/14659891.2014.934305. [DOI] [Google Scholar]
- 24.Hjorthøj CR, Hjorthøj AR, Nordentoft M. Validity of timeline follow-back for self-reported use of cannabis and other illicit substances—systematic review and meta-analysis. Addict Behav 2012 Mac;37(3):225-33. DOI: 10.1016/j.addbeh.2011.11.025. [DOI] [PubMed] [Google Scholar]
- 25.Kalichman SC, Amaral CM, Stearns H, et al. Adherence to antiretroviral therapy assessed by unannounced pill counts conducted by telephone. J Gen Intern Med 2007. Mar;22:1003-1006. DOI: 10.1007/s11606-007-0171-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kalichman SC, Pellowski J, Kegler C, et al. Medication adherence in people dually treated for HIV infection and mental health conditions: test of the medications beliefs framework. J Behav Med 2015;38(4):632-641. DOI: 10.1007/s10865-015-9633-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sklar SM, Turner NE. A brief measure for the assessment of coping self-efficacy among alcohol and other drug users. Addiction 1999 May;94(5):723-9. DOI: 10.1046/j.1360-0443.1999.94572310.x. [DOI] [PubMed] [Google Scholar]
- 28.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001. Sep;16(9):606-13. DOI: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spitzer RL, Kroenke K, Williams JBet al. et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006 May;166(10):1092-7. DOI: 10.1001/archinte.166.10.1092. [DOI] [PubMed] [Google Scholar]
- 30.Topp CW, Østergaard SD, Søndergaard Set al. et al. The WHO-5 well-being index: a systematic review of the literature. Psychother Psychosom 2015. Mar;84(3):167-76. DOI: 10.1159/000376585. [DOI] [PubMed] [Google Scholar]
- 31.Kaur J, Mania I, Tirupathi R, et al. Impact of telemedicine on retention in medications for opioid use disorder (MOUD) treatment with buprenorphine in the times of COVID-19 pandemic: a retrospective chart review. J Rural Ment Health 2022;46(2):75-81. DOI: 10.1037/rmh0000206. [DOI] [Google Scholar]
- 32.Lin LA, Fortney JC, Bohnert AS, et al. Comparing telemedicine to in-person buprenorphine treatment in US veterans with opioid use disorder. J Subst Abuse Treat 2022 Feb;133. DOI: 10.1016/j.jsat.2021.108492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Weintraub E, Seneviratne C, Anane J, et al. Mobile telemedicine for buprenorphine treatment in rural populations with opioid use disorder. JAMA Network Open 2021;4(8):e2118487. DOI: 10.1001/jamanetworkopen.2021.18487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cunningham CO, Khalid L, Deng Y, et al. A comparison of office-based buprenorphine treatment outcomes in Bronx community clinics before versus during the COVID-19 pandemic. J Subst Abuse Treat 2022 April;135:108641. DOI: 10.1016/j.jsat.2021.108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guillen AG, Reddy M, Saadat S, et al. Utilization of telehealth solutions for patients with opioid use disorder using buprenorphine: a scoping review. Telemed E-Health 2022. Jun;28(6):761-767. DOI: 10.1089/tmj.2021.0308. [DOI] [PubMed] [Google Scholar]
- 36.Weintraub E, Greenblatt AD, Chang J, et al. Outcomes for patients receiving telemedicine-delivered medication-based treatment for opioid use disorder: a retrospective chart review. Heroin Addict Relat Clin Probl 2021;23(2):5-12. [PMC free article] [PubMed] [Google Scholar]
- 37.Bhatraju EP, Radick AC, Leroux BG, et al. Buprenorphine adherence and illicit opioid use among patients in treatment for opioid use disorder. Am J Drug Alcohol Abuse 2023 Jul;49(4):511-518. DOI: 10.1080/00952990.2023.2220876. [DOI] [PubMed] [Google Scholar]
- 38.Frost MC, Zhang L, Kim HM, et al. Use of and retention on video, telephone, and in-person buprenorphine treatment for opioid use disorder during the COVID-19 pandemic. JAMA Network Open 2022;5(10):e2236298. DOI: 10.1001/jamanetworkopen.2022.36298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen B, Zhao C, Bailly E, et al. Telehealth initiation of buprenorphine for opioid use disorder: patient characteristics and outcomes. J Gen Intern Med 2023. Jan;39(1):95-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edinoff AN, Kaufman SE, Chauncy TM, et al. Addiction and COVID: issues, challenges, and new telehealth approaches. Psychiatry Int 2022;3(2):169-180. DOI: 10.3390/psychiatryint3020014. [DOI] [Google Scholar]
- 41.Hser Y, Mooney LJ. Integrating telemedicine for medication treatment for opioid use disorder in rural primary care: beyond the COVID pandemic. J Rural Health 2021 Winter;37(1):246-248. DOI: 10.1111/jrh.12489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang YT, Weintraub E, Haffajee RL. Telemedicine's role in addressing the opioid epidemic. Mayo Clinic proceedings 2018 Sep;93(9):1177–1180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tofighi B, McNeely J, Walzer D, et al. A telemedicine buprenorphine clinic to serve New York City: initial evaluation of the NYC public hospital system's initiative to expand treatment access during the COVID-19 pandemic. J Addict Med 2022. Jan-Feb;16(1):e40-e43. DOI: 10.1097/ADM.0000000000000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jones CM, Shoff C, Blanco C, et al. Association of receipt of opioid use disorder–related telehealth services and medications for opioid use disorder with fatal drug overdoses among Medicare beneficiaries before and during the COVID-19 pandemic. JAMA Psychiatry 2023 May;80(5):508-514. DOI: 10.1001/jamapsychiatry.2023.0310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Namchuk AB, Lucki I, Browne CA. Buprenorphine as a treatment for major depression and opioid use disorder. Adv. Drug Alcohol Res 2022 Feb;2:10254 DOI: 10.3389/adar.2022.10254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Snell-Rood C, Pollini RA, Willging C. Barriers to integrated medication-assisted treatment for rural patients with co-occurring disorders: The gap in managing addiction. Psychiatr Serv 2021 Aug;72(8):935-942. DOI: 10.1176/appi.ps.202000312. [DOI] [PubMed] [Google Scholar]
- 47.Orsmond GI, Cohn ES. The distinctive features of a feasibility study: objectives and guiding questions. OTJR Occup Particip Health 2015. Jul;35(3):169-77. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076241258400 for Feasibility and acceptability of a novel digital therapeutic combining behavioral and pharmacological treatment for opioid use disorder by Laura B. Monico, Megan Eastlick, Darcy Michero, Peyton Pielsticker and Suzette Glasner in DIGITAL HEALTH