Abstract
Background:
Gastrointestinal (GI) angiodysplasias is a potential cause of life-threatening bleeding. Thalidomide may have a certain effect on the treatment.
Objectives:
We aim to evaluate the efficacy and safety of thalidomide and used trial sequential analysis (TSA) to assess the need for further randomized controlled trials (RCTs).
Design:
Meta-analysis of RCTs.
Data sources and methods:
We systematically searched Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Embase, WanFang, and China National Knowledge Infrastructure databases for RCTs evaluating thalidomide in GI angiodysplasias without language restrictions. We used a random-effects model to obtain pool data and followed Grading of Recommendations Assessment, Development and Evaluation framework. TSA was employed to control the risk of random errors and to evaluate the validity of our conclusions.
Results:
Three RCTs were included involving 279 patients with the proportion of small intestinal angiodysplasias of 87.1%. Thalidomide led to improved mean change of hemoglobin level [mean difference (MD): 3.06, 95% confidence interval: 2.66–3.46] without severe adverse effects occurring. Other secondary endpoints, including effective response rate, cessation of bleeding after treatment, hospitalization rate because of bleeding, change in duration of hospital stays for bleeding, transfused red cell requirements, and overall adverse effects, also showed significantly better outcomes in the thalidomide group compared to the control group. TSA for all outcomes exceeded required information sizes, and cumulative Z curve all traverse trial sequential monitoring boundary.
Conclusion:
Almost all of the evidence was of moderate quality, suggesting that thalidomide holds promise for treating GI angiodysplasias, with favorable safety profiles. TSA suggests that conducting large-scale real-world research is recommended over relying solely on RCTs conducted within the same population and trial design.
Trial registration:
This meta-analysis protocol was registered on PROSPERO (CRD42023480621).
Keywords: efficacy, gastrointestinal angiodysplasias, meta-analysis, safety, thalidomide
Introduction
Gastrointestinal (GI) angiodysplasias are important causes of GI bleeding,1,2 resulting in 5–10% of all GI bleeding cases. 3 And the small bowel is the most common site of GI angiodysplasias, accounting for approximately 80% of cases, followed by colon (44%) and the stomach (32%).4,5 GI angiodysplasias contribute to about 60% of small bowel bleeding cases. 6 Bleeding tends to be recurrent, can pose a life-threatening risk due to the need for large transfusions and potential emergent hemostatic interventions.
Currently, there is insufficient evidence to provide treatment recommendations in the guidelines. The treatment mainly includes endoscopic therapy, surgery, and pharmacologic treatment, in which endoscopic therapy is the superior option for initial treatment with high recurrence bleeding rate. 1 However, endoscopic therapy has limitations for treating specific lesions, multiple lesions. The degree of evidence supporting pharmacologic treatments is low.1,7,8
The dilemma was partially mitigated when the randomized controlled trial (RCT) was published to evaluate the effect of thalidomide on GI angiodysplasias, 9 which was also demonstrated in other RCTs, 10 case reports,11,12 and observational studies.13,14 Based on these results, thalidomide is a reasonable option for patients with recurrent or refractory bleeding of GI angiodysplasia who have failed other treatments.
Meta-analysis of RCTs is considered the highest level of evidence. 15 However, currently there is a lack of such meta-analyses. Considering the scarcity of RCTs, trial sequential analysis (TSA) can be used to determine the needed sample size to assess the need for more RCTs, control the risk of random errors, and evaluate the conclusions drawn from the meta-analysis. 16 Therefore, we aimed to evaluate the efficacy and safety of thalidomide on GI angiodysplasias. And most importantly, given the excellent treatment effects confirmed in previous high-quality RCT, our aim was to further explore whether the minimum required sample size was reached using TSA, indicating whether the further RCTs are essential in the future.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement (Supplemental Material 1). 17 This study protocol was registered on PROSPERO (CRD42023480621).
Literature search strategy
We searched databases of Cochrane Central Register of Controlled Trials (CENTRAL), Medical Literature Analysis and Retrieval System Online (MEDLINE), Embase, WanFang Data (China), and China National Knowledge Infrastructure (CNKI) for relevant studies on thalidomide and GI angiodysplasia until 9 February 2024. The keywords used for the search included ‘angiodysplasia’, ‘arteriovenous malformation’, ‘angiodysplastic’, ‘vascular malformation’, and ‘angioectasia’, ‘thalidomide’, and ‘randomized controlled trial’. There were no limitations on publication time or language. In addition, the reference lists of all primary studies and review articles were manually searched. Detailed search strategies are listed in Supplemental Material 2.
Inclusion and exclusion criteria
The inclusion criteria were as follows: (a) RCTs published as full text in any language; (b) adult participants aged 18 years or older; (c) diagnosis of GI angiodysplasia confirmed by endoscopy; (d) patients experiencing recurrent GI bleeding attributed to GI angiodysplasia; (e) all studies must have a minimum 1-year follow-up period and include a control group. The control group should receive one (or a combination) of the following: placebo, iron supplementation, or general supportive therapy.
The exclusion criteria were as follows: (a) studies of non-RCTs such as observational cohort studies, case–control studies, case series, case reports, editorials, narrative reviews, and conference abstracts; (b) studies lacking clear definition of drug usage; (c) studies without sufficient patient information or duplicate publications; (d) studies involving pregnant women or children.
Outcome measures
Primary outcomes included the mean change in hemoglobin level after treatment as an efficacy outcome. Secondary outcomes included effective response rate, cessation of bleeding after treatment, hospitalization rate because of bleeding, change in duration of hospital stays for bleeding, transfused red cell requirements, and overall adverse effects. Safety outcomes were adverse effects and severe adverse effects. Among the outcomes, effective response was defined as a reduction of at least a 50% in the number of bleeding episodes after treatment compared to the number occurring during the 1-year observation period before treatment.9,10
Study selection and data collection
Two reviewers (XY and KP) independently screened the titles and abstracts of relevant studies and included potentially eligible articles. Subsequently, the full texts of the included articles were reviewed based on inclusion and exclusion criteria. Then, two reviewers (KS and KH) extracted all relevant data independently. Disagreements among reviewers were resolved by discussion. And a third author was consulted if necessary. The specific details extracted included:
(1) General information: year of publication and author’s name.
(2) Methods: study design, study date, total duration of the study and run-in period, number and location of study centers, randomization methodology, allocation concealment, blinding, withdrawals, and follow-up.
(3) Participants: inclusion and exclusion criteria, number of participants, age, gender, co-morbidities, diagnostic techniques, location of angiodysplasia, single or multiple lesions, bleeding episodes, and blood transfusions.
(4) Interventions: description of the interventions, control groups, concomitant medications used, and any excluded medications.
(5) Outcomes: identification and collection of primary and secondary outcomes, as well as the time points at which they were reported.
Statistical analysis
Measures of treatment effect
We conducted a meta-analysis using RevMan (Review Manager software, version 5.4.1, Cochrane Collaboration, UK). A random-effects model was used to pool the data. For continuous data, we extracted the mean value and standard deviation (SD) of the changes observed in each arm of the trial. Effect sizes were calculated using mean differences (MDs) with 95% confidence intervals (CIs). In cases where the data were reported as medians, minimum and maximum values, and/or first and third quartiles, we employed a data transformation method developed by Wan et al. 18 to convert the data into mean values and SDs, ensuring consistency in pooling the results. For dichotomous data, effect sizes were calculated as risk ratios (RRs) with 95% CIs. Besides, as a random-effects model was employed for pooling, prediction intervals were computed using the approach proposed by Higgins et al. 19
Assessment of risk of bias
For each study, two review authors (CL and DY) independently assessed the risk of bias using criteria outlined in the Cochrane Collaboration Risk of Bias 2 tool. 20 The risk of bias for each study was assessed through discussions among the reviewers. Each potential source of bias was categorized as high risk, low risk, or some concerns. To evaluate the certainty of the evidence for the primary outcomes, we used the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) framework. GRADE Pro-version 3.6 software, McMaster University, Hamilton, ON, Canada (http://gradepro.org/) was used.
Assessment of heterogeneity and subgroup analysis
We utilized the I2 statistic to assess the level of heterogeneity among the RCTs included. An I2 statistic of 50% or higher was considered indicative of moderate to substantial heterogeneity. In cases where the I2 statistic exceeded 75%, we presented the results using forest plots without pooled estimates to reflect the heterogeneity. 21
We planned to perform subgroup analyses for potential sources of heterogeneity when the I2 statistic exceeded 60%. Specifically, we aimed to analyze varying thalidomide doses and angiodysplasia locations as subgroups.
Assessment of publication biases
If more than 10 studies were included in the analysis of an outcome parameter, funnel plots would be generated to assess their symmetry for potential publication bias. Otherwise, Egger’s test was performed to investigate potential publication bias.
Trial sequential analysis
TSA was used to control the risk of random errors caused by low sample sizes and repeated significance testing, and adjust the thresholds for statistical significance in the meta-analysis. 22 TSA was performed via TSA software version 0.9 beta (Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark). 16 The required sample size for important outcomes was conservatively calculated based on the incidence or MD in low risk of bias studies, with a type-1 error of 5%, power (1 − β) of 90%, and D2 as 50%. The statistical significance and the required information size of the meta-analysis were evaluated according to the position of cumulative Z curve with conventional boundary, trial sequential monitoring boundary (TSMB), and futility boundary in the figure. For example, a stable and firm conclusion was reached, and no further studies were needed if the cumulative Z curve crossed the TSMB or entered the futility area below the futility boundary. 23
Results
Study selection and study characteristics
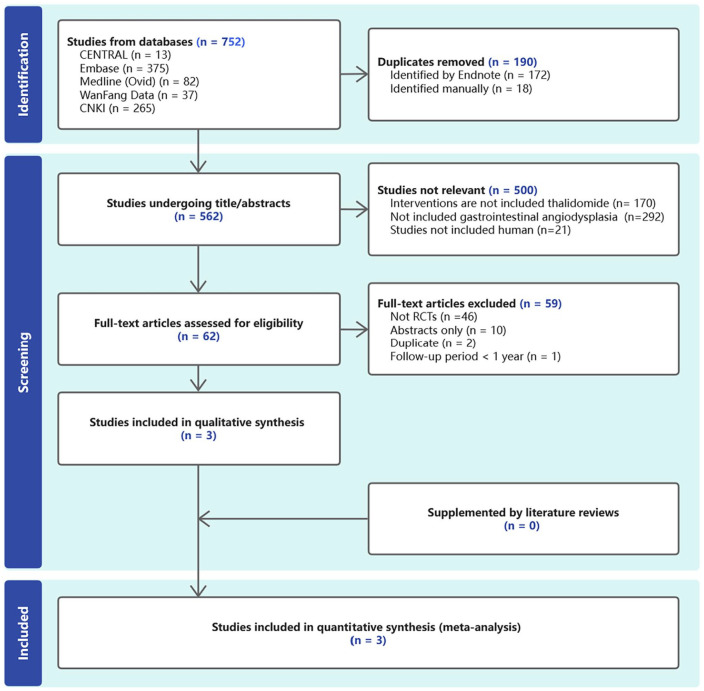
As shown in Figure 1, we identified 730 records, of which 187 duplicated studies were excluded. Another 540 articles were also excluded because they did not meet the selection criteria. Three RCTs were included in this meta-analysis. Table 1 shows the details of the included studies.
Figure 1.
PRISMA flowchart illustrates the different phases of the systematic review and meta-analysis.
PRISMA, preferred reporting items for systematic reviews and meta-analyses; RCTs, randomized controlled trials.
Table 1.
Characteristics of the included studies.
| First author (publication year) | Patients, n (Thalidomide/Control) | Age (years), mean ± SD (Thalidomide versus Control) | M/F, n (Thalidomide versus Control) | Thalidomide | Control | Follow-up period | Proportion of small intestine (Thalidomide versus Control) |
|---|---|---|---|---|---|---|---|
| Chen et al., 20239 | 100/50 | 60.6 ± 7.3 versus 61.8 ± 7.5 | 39/61 versus 22/28 | 25 mg orally four times daily for 51 patients for 4 months; or 25 mg orally two times daily for 49 patients for 4 months | One placebo tablet four times daily for 4 months | One year after the end of treatment | 100/100 versus 50/50 |
| Ge et al., 201110 | 28/27 | 58.8 ± 12.2 versus 59.0 ± 10.5 | 4/24 versus 5/22 | 25 mg orally four times daily for 4 months | 100 mg ferrous succinate tablets orally four times daily for 4 months | One year after the end of treatment | 26/28 versus 26/27 |
| Li and Qiu, 201524 | 38/36 | 43.8 ± 9.7 versus 42.2 ± 12.5 | 18/20 versus 17/19 | 25 mg orally four times daily for 4 months | Ordinary infusion therapy to maintain electrolyte balance | One year after the beginning of treatment | 21/38 versus 20/36 |
M/F, male/female; N, No; SD, standard deviation; Y, Yes.
A total of 279 participants were enrolled in this meta-analysis, with 166 participants in the thalidomide group and the remaining 113 participants in control group.9,10,24 The proportion of small intestinal angiodysplasias was 87.1%. The remaining lesions in the remaining patients are located in other parts of the GI tract. Multiple lesions were present in 34 (12.2%) patients.
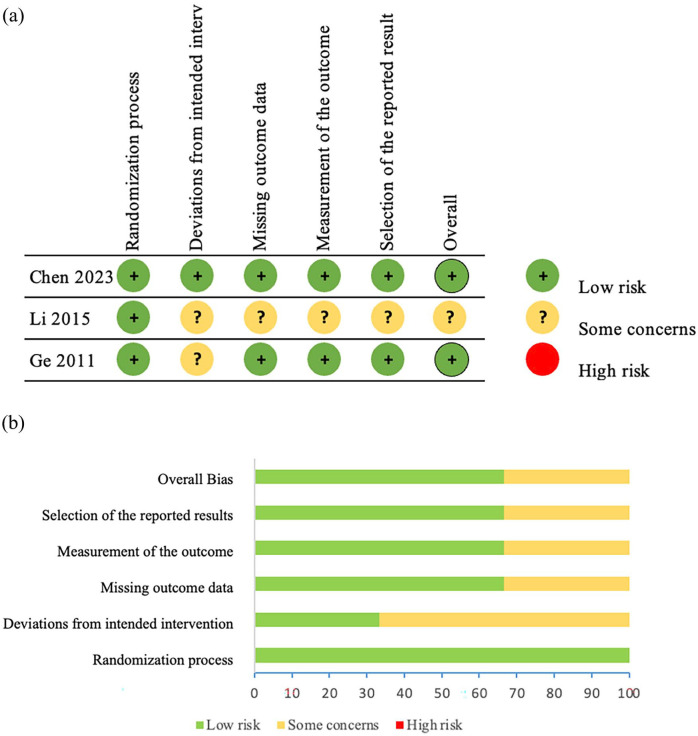
The risk of bias in eligible studies is shown in Figure 2. We assessed the quality of evidence for the main outcomes using the GRADE methodology which is shown in Table 2.
Figure 2.
Methodological quality of included studies according to the Cochrane risk of bias 2 tool for assessing the risk of bias. (a) Risk of bias summary. (b) Risk of bias graph.
Table 2.
Summary of main findings.
| Population: patients with gastrointestinal angiodysplasias | |||||
|---|---|---|---|---|---|
| Intervention: Thalidomide; Comparison: Control group | |||||
| Outcomes | Anticipated absolute effects a (95% CI) | Relative effect (95% CI) | Number (studies) | Quality of the evidence (GRADE) | |
| Risk with thalidomide | Risk with control | ||||
| Mean change in hemoglobin level | MD 3.06 g/dl higher (2.66–3.46) | −0.10 g/dl | RR: 3.06 (2.66–3.46) | 279 (3 RCTs) | ⨁⨁⨁◯ Moderate b |
| Effective response | 778/1000 (159–1000) | 117/1000 | RR: 6.66 (1.36–32.69) | 205 (2 RCTs) | ⨁⨁◯◯ Low c |
| Cessation of bleeding | 326/1000 (95–1000) | 26/1000 | RR: 12.57 (3.66–43.16) | 205 (2 RCTs) | ⨁⨁⨁◯ Moderate b |
| Hospitalization rates | 341/1000 (266–449) | 831/1000 | RR: 0.41 (0.32–0.54) | 205 (2 RCTs) | ⨁⨁⨁◯ Moderate b |
| Adverse effects | 654/1000 (454–941) | 299/1000 | RR: 2.19 (1.52–3.15) |
205 (2 RCTs) | ⨁⨁⨁◯ Moderate b |
| Severe adverse effects | 0/1000 (0–0) | 0/1000 | Not estimable | 279 (3 RCTs) | ⨁⨁⨁◯ Moderate b |
GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect; Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate; Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate; Very low quality: We are very uncertain about the estimate.
The basis for the assumed risk is the average control group proportion across all comparison.
Downgraded one level for small sample size.
Downgraded two levels for small sample size and heterogeneity.
CI, confidence interval; GRADE, Grading of Recommendations Assessment, Development and Evaluation; MD, mean difference; RCTs, randomized controlled trials; RR, relative risk.
Efficacy of thalidomide on outcomes
Primary outcomes
Mean change in hemoglobin level after treatment
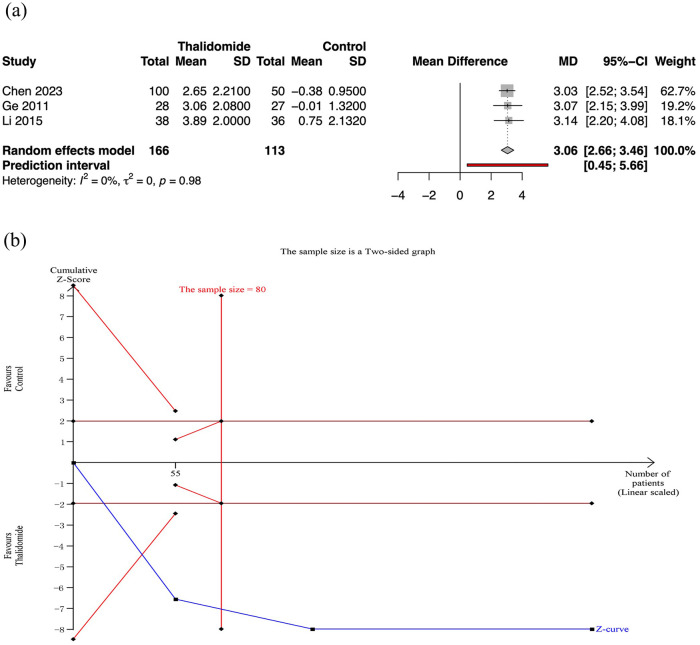
Three RCTs assessed the mean change in hemoglobin level after treatment. Meta-analysis revealed that thalidomide led to a significant higher mean change of hemoglobin level than control group (MD 3.06, 95% CI: 2.66–3.46; p < 0.01; I2 = 0%; n = 279; RCTs = 3; moderate quality of evidence) [Figure 3(a)].
Figure 3.
Mean change in hemoglobin level after treatment in thalidomide comparing to control group. (a) Forest plot of primary outcome of different treatment groups. (b) TSA for thalidomide group.
CI, confidence interval; SD, standard deviation; TSA, trial sequential analyses.
TSA for primary outcomes
For the mean change in hemoglobin level, TSA revealed that the required information sizes was 80. And the cumulative Z curves for the primary outcomes crossed the conventional boundary and the futility boundary, suggesting that previous studies meet the need for conclusive results [Figure 3(b)].
Secondary outcomes
Effective response rate
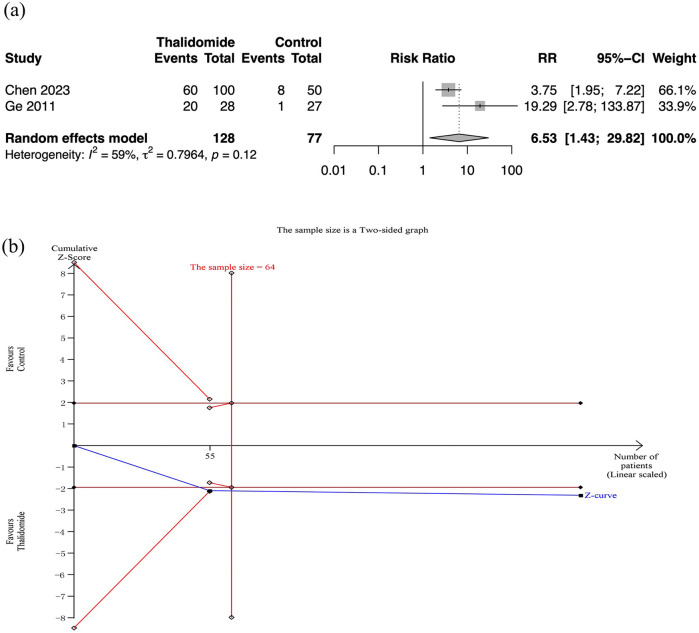
Only two RCT reported the effective response rate, demonstrating a significant difference between the thalidomide group and the control group (RR: 6.53, 95% CI: 1.43–29.82; p = 0.02; I2 = 59%; n = 205; RCTs = 2). The quality of evidence was low [Figure 4(a)]. TSA indicated that the required information size was reached and the cumulative Z curves crossed the TSMB [Figure 4(b)].
Figure 4.
Effective response rate in thalidomide comparing to control group. (a) Forest plot of effective response rate of different treatment groups. (b) TSA for thalidomide group.
CI, confidence interval; SD, standard deviation; TSA, trial sequential analyses.
Cessation of bleeding
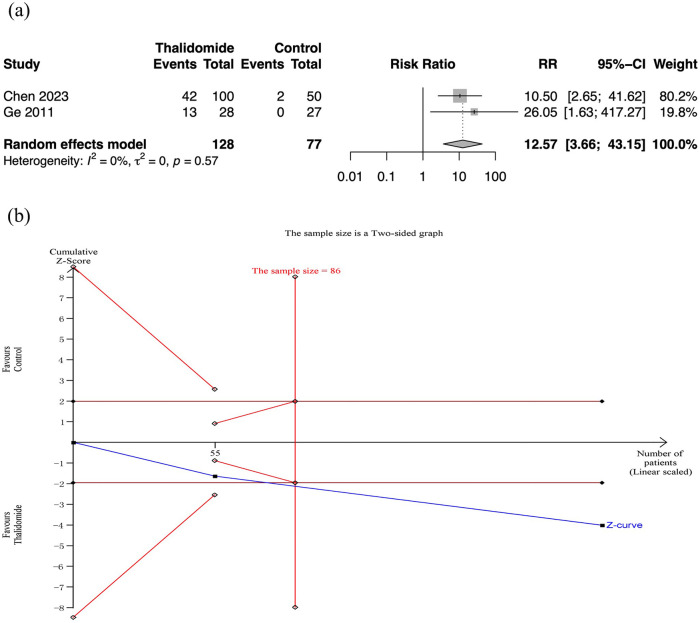
Two RCTs assessed cessation of bleeding. Meta-analysis revealed that thalidomide contributed to higher rate of cessation of bleeding than the control group (RR: 12.57, 95% CI: 3.66–43.15; p < 0.01; I2 = 0%; n = 205; RCTs = 2; moderate quality of evidence) [Figure 5(a)]. And the TSA also showed reliable evidence of thalidomide improving cessation rate of bleeding [Figure 5(b)].
Figure 5.
Cessation rate of bleeding in thalidomide comparing to control group. (a) Forest plot of effective response rate of different treatment groups. (b) TSA for thalidomide group.
CI, confidence interval; SD, standard deviation; TSA, trial sequential analyses.
Other secondary outcomes
Meta-analysis revealed significant differences between the thalidomide and control groups in hospitalization rates for bleeding (RR: 0.41, 95% CI: 0.32–0.54; p < 0.01; I2 = 0%; n = 205; RCTs = 2) (Supplemental Figure 1), median change in duration of hospital stays for bleeding (MD: −4.84, 95% CI: −5.71 to −3.97; p < 0.01; I2 = 0%; n = 205; RCTs = 2) (Supplemental Figure 2), and transfused red cell requirements (RR: 0.32, 95% CI: 0.21–0.48; p < 0.01; I2 = 0%; n = 205; RCTs = 2) (Supplemental Figure 3). TSA for all other secondary outcomes demonstrated that the required information size was reached and the cumulative Z curves crossed the TSMB.
Safety outcomes of thalidomide
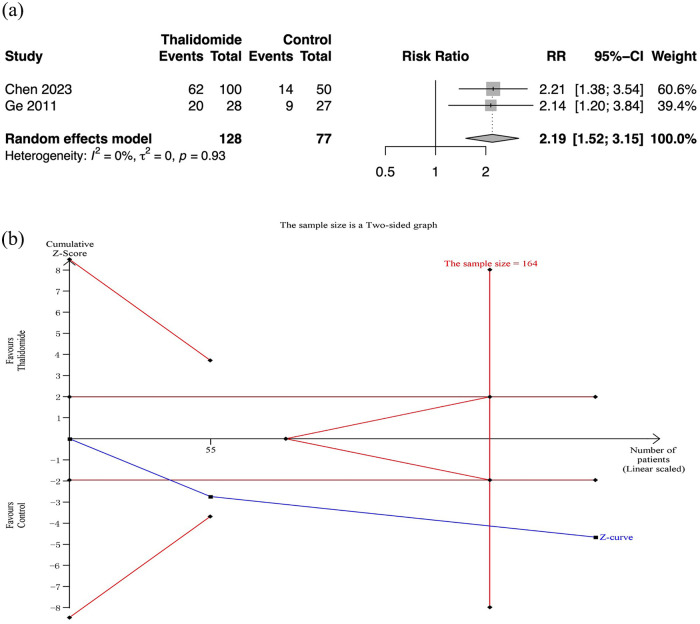
No severe adverse effects were observed in both thalidomide and control groups (moderate quality of evidence). And there were two RCTs with 205 participants that reported detailed adverse effects.9,10 The results showed that thalidomide was related to a higher rate of adverse effect than placebos (RR: 2.19, 95% CI: 1.52–3.15; p < 0.01; I2 = 0%; n = 205; RCTs = 2) (Figure 6). The quality of evidence was moderate.
Figure 6.
Adverse effects in thalidomide comparing to control group. (a) Forest plot of effective response rate of different treatment groups. (b) TSA for thalidomide group.
CI, confidence interval; SD, standard deviation; TSA, trial sequential analyses.
Publication bias
Egger’s test revealed no publication bias for our primary outcome. The publication bias of secondary outcomes and safety outcome could not be assessed because of the limited number of studies (less than 3). The result of mean change in hemoglobin level after treatment (p = 0.704 > 0.05) suggested the publication bias was unobvious.
Subgroup analysis
In the secondary endpoint of effective response rate, we observed an I2 near 60%. However, due to the inclusion of only two studies in this endpoint, further subgroup analysis was not feasible. Similarly, concerning the efficacy of different doses of thalidomide, the study by Chen et al. 9 is the only one that explored various doses, rendering the study number insufficient for further quantitative analysis. Likewise, the lack of data from studies on angiodysplasias in different locations does not support further subgroup analysis.
Discussion
Our meta-analysis represents the first comprehensive evaluation of RCTs on thalidomide for GI angiodysplasias, demonstrating a significant effect of thalidomide on GI angiodysplasias. The average change in hemoglobin levels was significantly better compared to the control group, with moderate-quality evidence. Moreover, low to moderate-quality evidence indicated that thalidomide led to higher rates of cessation of bleeding and effective response after treatment, and the hospitalization rate and blood transfusion rate were significantly lower after thalidomide treatment. Importantly, the included studies demonstrated zero incidence of severe adverse effects, further highlighting the safety profile of thalidomide.9,10,24
Additionally, we utilized TSA and demonstrated that the required information size was attained for all significant outcomes, with cumulative Z curves consistently crossing the TSMB. This provides firm and conclusive evidence supporting the efficacy of thalidomide. 25 Most of the study endpoints were assigned moderate-quality evidence using the GRADE approach, which further strengthens the reliability of our meta-analysis results. We firmly advocate for the use of thalidomide in managing small intestinal angiodysplasias. The necessity for further RCTs specifically targeting small intestinal angiodysplasias may be relatively low. 22 Instead, conducting head-to-head studies comparing different effective drugs could yield more meaningful insights. Additionally, given the relatively small proportion of patients with gastric/colonic angiodysplasias in our included RCTs, conducting trials focusing on these GI tract segments would be beneficial to assess the applicability of thalidomide. Moreover, large-scale observational studies in real-world settings are imperative to further clarify the optimal dosage of thalidomide.
Besides the well-known severe teratogenic effects on fetuses, the most commonly reported side effect of thalidomide is somnolence with an incidence ranging from 34% to 43%, followed by peripheral neuropathy. 26 And the side effects of thalidomide are dose-dependent.27,28 However, peripheral neuropathy induced by thalidomide is usually reversible upon dosage reduction or discontinuation. 29 In a previous RCT of thalidomide for the treatment of multiple myeloma, 42% (65/155) of patients discontinued treatment due to side effects. 30 However, the dosage of thalidomide administered (200–400 mg, orally on days 1–28, followed by sequential maintenance therapy of 50 mg orally daily) was significantly higher compared to the RCTs included in our article (the highest dose was 100 mg daily). In another study on the treatment of chemotherapy-induced vomiting, a dosage of 100 mg of thalidomide twice daily on days 1–5 was used, and all adverse effects were mild to moderate. 31 In the articles we included, all adverse events resolved upon discontinuation of treatment (within 8 weeks), with no reports of additional therapeutic measures being taken, and demonstrating potential dose-dependent adverse reactions (in the study by Chen et al., the incidence of adverse events was 68.6% in the 100-mg thalidomide group and 55.1% in the 50-mg thalidomide group).9,10,24 The incidence of adverse events ranged from 20% to 68.6%, and all adverse events were grade 1 or 2, with the most common being constipation or fatigue.9,10,24 Further research are needed to investigate the long-term safety of thalidomide in the treatment of GI angiodysplasias. Additionally, due to thalidome’s slow onset of efficacy, the course of treatment with thalidomide typically extends beyond 3 months.32,33 It is necessary to monitor adverse events during the course of medication. For sustained treatment, routine monitoring of complete blood count and comprehensive metabolic panel is recommended, with consideration given to nerve conduction velocity measures to detect asymptomatic neuropathy. 33 And it is important to emphasize that, although adverse reactions were not severe in the RCTs we included, strict contraception measures are required in women of childbearing age. 34
Our findings are consistent with previous meta-analyses that included both RCTs and observational studies. 5 Pharmacologic treatment of GI angiodysplasias showed a significant reduction in the incidence of rebleeding [odds ratio (OR) = 0.08, 95% CI: 0.04–0.18, I2 = 50%], and improvement in hemoglobin levels (MD = 3.21 g/dl, 95% CI: 2.42–3.99). Moreover, no participants discontinued treatment due to adverse effects. Within the thalidomide group, the primarily included cohort studies allowed for longer follow-up periods, providing a more comprehensive understanding of the long-term effects of thalidomide (with a maximum median follow-up time of 42.6 months). 35 However, apart from the primary endpoint rebleeding rate, high heterogeneity was observed in other endpoints, with I2 values of 91% for transfusion rate, 90% for MD in hemoglobin levels, and 64% for rate of side effects. The strength of our study lies in the inclusion of only RCTs with better consistency, which provides a higher level of research evidence. Additionally, focusing on the efficacy and safety of thalidomide provides valuable insights and enhances the quality of information provided. Additionally, all RCTs have a follow-up period of at least 1 year, offering a more extensive assessment of the long-term effects of thalidomide.
Our study has several limitations. Firstly, due to the lack of studies specifically targeting different doses of thalidomide, we were unable to explore the appropriate dosage. Additionally, the included RCTs had a relatively small proportion of patients with gastric and colonic angiodysplasias. Further research is necessary to investigate the effects of thalidomide on the stomach and colon. Furthermore, although all RCTs include a control group, the variability in the control group’s implementation across different studies introduces potential biases. And as all included studies are from the China and the number of studies is limited, RCTs involving populations from other regions may help complement the evidence.
Conclusion
Almost all of the evidence was of moderate quality, supporting the consistent efficacy of thalidomide in treating GI angiodysplasias, particularly those affecting the small intestine, with a favorable safety profile. TSA indicates that large-scale real-world studies, rather than RCTs, are warranted for patients with small intestinal angiodysplasias. Further investigation is necessary to determine the optimal dosage of thalidomide and its efficacy in treating angiodysplasias in different GI locations.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848241255295 for Efficacy and safety of thalidomide in gastrointestinal angiodysplasias: systematic review and meta-analysis with trial sequential analysis of randomized controlled trials by Kai Song, Kun He, Xiaxiao Yan, Ke Pang, Rou Tang, Chengzhen Lyu, Daiyu Yang, Yuelun Zhang and Dong Wu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848241255295 for Efficacy and safety of thalidomide in gastrointestinal angiodysplasias: systematic review and meta-analysis with trial sequential analysis of randomized controlled trials by Kai Song, Kun He, Xiaxiao Yan, Ke Pang, Rou Tang, Chengzhen Lyu, Daiyu Yang, Yuelun Zhang and Dong Wu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_17562848241255295 for Efficacy and safety of thalidomide in gastrointestinal angiodysplasias: systematic review and meta-analysis with trial sequential analysis of randomized controlled trials by Kai Song, Kun He, Xiaxiao Yan, Ke Pang, Rou Tang, Chengzhen Lyu, Daiyu Yang, Yuelun Zhang and Dong Wu in Therapeutic Advances in Gastroenterology
Acknowledgments
We would like to express our appreciation to Dr Hanyuan Xu (Department of Clinical Nutrition, Peking Union Medical College Hospital) for her kind help in data visualization.
Footnotes
ORCID iD: Dong Wu  https://orcid.org/0000-0001-9430-9874
https://orcid.org/0000-0001-9430-9874
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Kai Song, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Kun He, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Xiaxiao Yan, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Ke Pang, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Rou Tang, Beijing Key Laboratory of Drug Delivery Technology and Novel Formulation, Institute of Materia Medica, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Chengzhen Lyu, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Daiyu Yang, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, Beijing, China.
Yuelun Zhang, Center for Prevention and Early Intervention, National Infrastructures for Translational Medicine, Institute of Clinical Medicine, Peking Union Medical College Hospital, Chinese Academy of Medical Science and Peking Union Medical College, Beijing, China.
Dong Wu, State Key Laboratory of Complex Severe and Rare Diseases, Department of Gastroenterology, Peking Union Medical College Hospital, Chinese Academy of Medical Sciences and Peking Union Medical College, No.1 Shuaifuyuan, Dongcheng District, Beijing 100730, China.
Declarations
Ethics approval and consent to participate: Not applicable.
Consent for publication: Not applicable.
Author contributions: Kai Song: Conceptualization; Data curation; Formal analysis; Methodology; Software; Validation; Writing – original draft; Writing – review & editing.
Kun He: Conceptualization; Data curation; Formal analysis; Resources; Software; Writing – original draft.
Xiaxiao Yan: Conceptualization; Data curation; Formal analysis; Resources; Writing – original draft.
Ke Pang: Conceptualization; Data curation; Formal analysis; Resources; Writing – original draft.
Rou Tang: Methodology; Visualization; Writing – original draft.
Chengzhen Lyu: Methodology; Visualization; Writing – original draft.
Daiyu Yang: Methodology; Visualization; Writing – original draft.
Yuelun Zhang: Methodology; Validation; Visualization; Writing – original draft.
Dong Wu: Conceptualization; Data curation; Funding acquisition; Validation; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research received financial support from the National High Level Hospital Clinical Research Funding (2022-PUMCH-A-020 and 2022-PUMCH-B-023), the National Key Clinical Specialty Construction Project (ZK108000), and Beijing Natural Science Foundation (L232016 and 7232123), Chinese Academy of Medical Sciences Innovation Fund for Medical Sciences (2022-I2M-1-003).
The authors declare that there is no conflict of interest.
Availability of data and materials: All articles in this manuscript are publicly available from Cochrane Central Register of Controlled trials (CENTRAL), MEDLINE, Embase, WanFang, and CNKI databases.
References
- 1. Jackson CS, Gerson LB. Management of gastrointestinal angiodysplastic lesions (GIADs): a systematic review and meta-analysis. Am J Gastroenterol 2014; 109: 474–483; quiz 484. [DOI] [PubMed] [Google Scholar]
- 2. Raju GS, Gerson L, Das A, et al. American Gastroenterological Association (AGA) Institute medical position statement on obscure gastrointestinal bleeding. Gastroenterology 2007; 133: 1694–1696. [DOI] [PubMed] [Google Scholar]
- 3. Garcia-Compean D, Del Cueto-Aguilera AN, Jimenez-Rodriguez AR, et al. Diagnostic and therapeutic challenges of gastrointestinal angiodysplasias: a critical review and view points. World J Gastroenterol 2019; 25: 2549–2564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jackson CS, Strong R. Gastrointestinal angiodysplasia: diagnosis and management. Gastrointest Endosc Clin N Am 2017; 27: 51–62. [DOI] [PubMed] [Google Scholar]
- 5. Gkolfakis P, Fostier R, Tziatzios G, et al. Efficacy of pharmacologic treatment for treating gastrointestinal angiodysplasias-related bleeding: a systematic review and meta-analysis. Eur J Gastroenterol Hepatol 2022; 34: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 6. Goltstein L, Grooteman KV, Rocco A, et al. Effectiveness and predictors of response to somatostatin analogues in patients with gastrointestinal angiodysplasias: a systematic review and individual patient data meta-analysis. Lancet Gastroenterol Hepatol 2021; 6: 922–932. [DOI] [PubMed] [Google Scholar]
- 7. Barnert J, Messmann H. Diagnosis and management of lower gastrointestinal bleeding. Nat Rev Gastroenterol Hepatol 2009; 6: 637–646. [DOI] [PubMed] [Google Scholar]
- 8. Sami SS, Al-Araji SA, Ragunath K. Review article: gastrointestinal angiodysplasia – pathogenesis, diagnosis and management. Aliment Pharmacol Ther 2014; 39: 15–34. [DOI] [PubMed] [Google Scholar]
- 9. Chen H, Wu S, Tang M, et al. Thalidomide for recurrent bleeding due to small-intestinal angiodysplasia. N Engl J Med 2023; 389: 1649–1659. [DOI] [PubMed] [Google Scholar]
- 10. Ge ZZ, Chen HM, Gao YJ, et al. Efficacy of thalidomide for refractory gastrointestinal bleeding from vascular malformation. Gastroenterology 2011; 141: 1629–1637.e1-4. [DOI] [PubMed] [Google Scholar]
- 11. Mimidis K, Kaliontzidou M, Tzimas T, et al. Thalidomide for treatment of bleeding angiodysplasias during hemodialysis. Ren Fail 2008; 30: 1040–1041. [DOI] [PubMed] [Google Scholar]
- 12. Alberto SF, Felix J, de Deus J. Thalidomide for the treatment of severe intestinal bleeding. Endoscopy 2008; 40: 788; author reply 789. [DOI] [PubMed] [Google Scholar]
- 13. Garrido A, Sayago M, López J, et al. Thalidomide in refractory bleeding due to gastrointestinal angiodysplasias. Rev Esp Enferm Dig 2012; 104: 69–71. [DOI] [PubMed] [Google Scholar]
- 14. Bauditz J. Effective treatment of gastrointestinal bleeding with thalidomide – chances and limitations. World J Gastroenterol 2016; 22: 3158–3164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Norris SL, Atkins D. Challenges in using nonrandomized studies in systematic reviews of treatment interventions. Ann Intern Med 2005; 142: 1112–1119. [DOI] [PubMed] [Google Scholar]
- 16. Thorlund K, Wetterslev J, Brok J, et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit, 2017, pp. 1–119. [Google Scholar]
- 17. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol 2014; 14: 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Higgins JP, Thompson SG, Spiegelhalter DJ. A re-evaluation of random-effects meta-analysis. J R Stat Soc Ser A Stat Soc 2009; 172: 137–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ 2019; 366: l4898. [DOI] [PubMed] [Google Scholar]
- 21. Higgins JPT, Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.4. Cochrane. (2023). www.training.cochrane.org/handbook.
- 22. Brok J, Thorlund K, Wetterslev J, et al. Apparently conclusive meta-analyses may be inconclusive – trial sequential analysis adjustment of random error risk due to repetitive testing of accumulating data in apparently conclusive neonatal meta-analyses. Int J Epidemiol 2009; 38: 287–298. [DOI] [PubMed] [Google Scholar]
- 23. Wetterslev J, Jakobsen JC, Gluud C. Trial sequential analysis in systematic reviews with meta-analysis. BMC Med Res Methodol 2017; 17: 39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li KJ, Qiu XL. The intervention effect of thalidomide on patients with intestinal vascular malformation hemorrhage. Lab Med Clin 2015; 12: 1745–1747. [Google Scholar]
- 25. Takwoingi Y, Hopewell S, Tovey D, et al. A multicomponent decision tool for prioritising the updating of systematic reviews. BMJ 2013; 347: f7191. [DOI] [PubMed] [Google Scholar]
- 26. Franks ME, Macpherson GR, Figg WD. Thalidomide. Lancet 2004; 363: 1802–1811. [DOI] [PubMed] [Google Scholar]
- 27. Ghobrial IM, Rajkumar SV. Management of thalidomide toxicity. J Support Oncol 2003; 1: 194–205. [PMC free article] [PubMed] [Google Scholar]
- 28. Yang C, Singh P, Singh H, et al. Systematic review: thalidomide and thalidomide analogues for treatment of inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41: 1079–1093. [DOI] [PubMed] [Google Scholar]
- 29. Morawska M, Grzasko N, Kostyra M, et al. Therapy-related peripheral neuropathy in multiple myeloma patients. Hematol Oncol 2015; 33: 113–119. [DOI] [PubMed] [Google Scholar]
- 30. van de Donk NW, van der Holt B, Minnema MC, et al. Thalidomide before and after autologous stem cell transplantation in recently diagnosed multiple myeloma (HOVON-50): long-term results from the phase 3, randomised controlled trial. Lancet Haematol 2018; 5: e479–e492. [DOI] [PubMed] [Google Scholar]
- 31. Zhang L, Qu X, Teng Y, et al. Efficacy of thalidomide in preventing delayed nausea and vomiting induced by highly emetogenic chemotherapy: a randomized, multicenter, double-blind, placebo-controlled phase III trial (CLOG1302 study). J Clin Oncol 2017; 35: 3558–3565. [DOI] [PubMed] [Google Scholar]
- 32. Dunne KA, Hill J, Dillon JF. Treatment of chronic transfusion-dependent gastric antral vascular ectasia (watermelon stomach) with thalidomide. Eur J Gastroenterol Hepatol 2006; 18: 455–456. [DOI] [PubMed] [Google Scholar]
- 33. Peng M, Guo X, Yi F, et al. Pharmacotherapy for the treatment of gastric antral vascular ectasia: a narrative review. Adv Ther 2021; 38: 5065–5077. [DOI] [PubMed] [Google Scholar]
- 34. Mellin GW, Katzenstein M. The saga of thalidomide. Neuropathy to embryopathy, with case reports of congenital anomalies. N Engl J Med 1962; 267: 1284–92 contd. [DOI] [PubMed] [Google Scholar]
- 35. Chen H, Fu S, Feng N, et al. Bleeding recurrence in patients with gastrointestinal vascular malformation after thalidomide. Medicine (Baltimore) 2016; 95: e4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848241255295 for Efficacy and safety of thalidomide in gastrointestinal angiodysplasias: systematic review and meta-analysis with trial sequential analysis of randomized controlled trials by Kai Song, Kun He, Xiaxiao Yan, Ke Pang, Rou Tang, Chengzhen Lyu, Daiyu Yang, Yuelun Zhang and Dong Wu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848241255295 for Efficacy and safety of thalidomide in gastrointestinal angiodysplasias: systematic review and meta-analysis with trial sequential analysis of randomized controlled trials by Kai Song, Kun He, Xiaxiao Yan, Ke Pang, Rou Tang, Chengzhen Lyu, Daiyu Yang, Yuelun Zhang and Dong Wu in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-3-tag-10.1177_17562848241255295 for Efficacy and safety of thalidomide in gastrointestinal angiodysplasias: systematic review and meta-analysis with trial sequential analysis of randomized controlled trials by Kai Song, Kun He, Xiaxiao Yan, Ke Pang, Rou Tang, Chengzhen Lyu, Daiyu Yang, Yuelun Zhang and Dong Wu in Therapeutic Advances in Gastroenterology