Abstract
Urothelial carcinoma of the upper urinary tract (UTUC) presents a significant clinical challenge, often requiring aggressive surgical intervention for optimal management. We present a case of an 84-year-old woman with recurrent high-grade papillary UTUC of the left renal pelvis, refractory to prior endourologic interventions, who underwent neoadjuvant treatment with pembrolizumab and enfortumab vedotin (Pembro/EV) due to contraindications to cisplatin therapy. Following a favorable response to neoadjuvant therapy, the patient underwent laparoscopic left radical nephroureterectomy, achieving a pathologic complete response. We discuss the utility of Pembro/EV in the perioperative management of patients with UTUC, particularly in those ineligible for cisplatin-based therapy. In addition, we highlight the potential role of somatic mutation testing and the integration of novel therapeutic agents such as olaparib in personalized treatment strategies for UTUC. This case underscores the importance of exploring innovative treatment approaches and optimizing patient selection for kidney preservation strategies in the management of UTUC. Further research and clinical trials are warranted to elucidate the full therapeutic potential of Pembro/EV and other emerging therapies in this setting.
Keywords: pembrolizumab, enfortumab vedotin, neoadjuvant, upper tract urothelial carcinoma
Introduction
Urothelial carcinoma of the upper urinary tract (UTUC) involves the renal pelvis or ureter and tends to be multifocal. Among all urothelial carcinoma cases, about 10% to 15% involve the upper urinary tract while the remaining cases involve the bladder. 1 Primary tumors in the UTUC can manifest as urothelial/transitional cell carcinoma, or as adenocarcinoma, or squamous cell carcinoma. Like bladder cancer, more than 90% of UTUC tumors are of urothelial origin, exhibiting an identical histology. 2 Interestingly, UTUC occurs twice as frequently in men than in women and typically affects older individuals, with a median age at diagnosis of 73 years. 2 Cases of concurrent UTUC and bladder cancer are relatively rare, occurring in only about 17% of patients. 3
Clinical presentations of UTUC vary from hematuria to ureteral or uteropelvic junction obstruction and urinary tract symptoms. Occasionally, a palpable flank mass or flank pain may be present. 4 Radiologic imaging either with computed tomography or retrograde pyelography, ureteropyeloscopy with cystoscopy, and urine cytology are standard diagnostic tests for UTUC. 5 Management of localized UTUC typically involves nephroureterectomy with excision of the cuff of normal bladder and bladder mucosa, 6 along with adjuvant chemotherapy7,8 or immunotherapy 9 for high-risk disease. There is limited information on neoadjuvant chemotherapy or treatment for patients who are poor surgical candidates. Intracavitary administration of pyelocaliceal mitomycin is considered for low-grade non-invasive UTUC mainly and has a 59% response rate.10,11 Herein, we present a case of UTUC treated with neoadjuvant pembrolizumab (Merck, Rahway, New Jersey) and enfortumab vedotin (Astellas, Northbrook, Illinois; Pembro/EV) demonstrating a complete pathologic response followed by nephrectomy. This case highlights potential therapeutic options for this tumor subgroup in the perioperative setting and potential future kidney organ preservation.
Case Presentation
An 84-year-old White woman presented with a medical history notable for hypertension, coronary artery disease, and a previous diagnosis of high-grade papillary UTUC of the left renal pelvis at age 77 in 2016. For 7 years, the patient had opted to retain her kidney; she underwent multiple left ureteroscopic laser ablations, intracavitary mitomycin installation, ureteral dilation, and had a double-J left nephroureteral stent placed due to persistent hydronephrosis. Persistent atypical urothelial cells had been observed in cytology from left pelvis washings since 2016, with no subsequent administration of chemotherapy or immunotherapy. In 2023, she was referred from the urology department to our medical oncology clinic for evaluation of a growing 6-cm left renal mass accompanied by hematuria and left flank discomfort. The patient also reported an unintentional weight loss of 24 pounds during this period. A staging computed tomography (CT) scan of the chest, abdomen, and pelvis with IV contrast conducted in August 2023 revealed a 5.9 × 5.8 × 6.2 cm mixed cystic and solid mass in the left renal pelvis and inferior pole, along with newly enlarged left posterior hemi-diaphragmatic and left periaortic lymph nodes (Figure 1). In addition, a 6-mm new pulmonary nodule was noted. Biopsy of the left renal pelvis mass confirmed high-grade papillary urothelial carcinoma (Figure 2), with positive cytology findings from bladder and left renal pelvic washings, indicating high-grade urothelial carcinoma with significant degenerative changes and inflammation.
Figure 1.
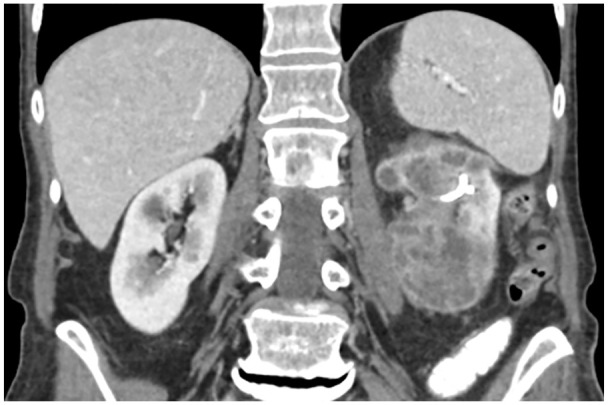
CT scan of the abdomen, and pelvis with IV contrast in August 2023 revealed a 5.9 × 5.8 × 6.2 cm mixed cystic and solid mass in the left renal pelvis and inferior pole.
Figure 2.

Pathologic examination of the biopsy sample showed high-grade papillary urothelial carcinoma with focal inverted growth pattern.
Based on the patient’s oncology history, biopsy results, and imaging findings, she was diagnosed with locally advanced or metastatic recurrence of UTUC in left renal pelvis. The pulmonary nodule was deemed too small for biopsy. Somatic mutation testing (Tempus, Chicago, Illinois) revealed positivity for FGFR3 (Gain of functio [GOF] 7.9%), PIK3CA (GOF 6.9%), BRCA1 (GOF 1.8%), and BRCA2 (GOF 0.3%), with negative germline mutation findings.
Due to her advanced age, frailty, and underlying chronic kidney disease with an estimated glomerular filtration rate (eGFR) of around 30 to 40, the patient was not considered a candidate for cisplatin therapy. Given promising results with Pembro/EV in bladder cancer, it was decided to initiate neoadjuvant Pembro/EV therapy: pembrolizumab 400 mg every 6 weeks plus enfortumab vedotin 1 mg/kg weekly on a schedule of 2 weeks on and 1 week off (a reduced dose of EV as a precaution due to her age). An interim restaging scan after 2 cycles of Pembro/EV demonstrated a favorable response, prompting the addition of olaparib (AstraZeneca, Wilmington, Delaware) 300 mg twice per day to further consolidate the response, as guided by the somatic mutation testing results. The patient tolerated the neoadjuvant triple regimen well, experiencing minimal side effects, including a resolving rash on her arm, hair loss, taste alterations, and further weight loss.
After 4 months of neoadjuvant Pembro/EV therapy, the patient underwent a laparoscopic left radical nephroureterectomy in February 2024 (Figure 3), which showed a pathologic complete response with no residual viable tumor identified in the kidney or ureter (Figure 4). Pathologic examination of the resected specimen revealed necrotic tissue with dense chronic inflammation involving the majority of the renal tissue surrounding the necrotic mass (Figure 5). Postoperatively, patient had the follow up in our clinic and last seen in March 2024, the patient is in excellent condition, with no planned adjuvant treatment. She is now on periodic surveillance for her UTUC.
Figure 3.

Repeat CT scan of the abdomen, and pelvis with IV contrast in February 2024 after 4 cycles of Pembro/EV revealed a resolution of the renal mass in the left renal pelvis and inferior pole.
Figure 4.
Surgical specimen of laparoscopic left radical nephroureterectomy with pathologic complete response showing no residual viable tumor identified in the kidney or ureter.
Figure 5.

Pathologic examination of the resected specimen revealed necrotic tissue with dense chronic inflammation involving the majority of the renal tissue surrounding the necrotic mass. No viable tumor is seen.
Discussion
The management of localized UTUC primarily revolves around surgical intervention, which represents the sole potential curative strategy for patients with this condition. Given its location in the renal pelvis, diagnosis and localized treatment can pose challenges. Ureteropyeloscopy, in conjunction with cystoscopy, facilitates biopsy for confirming the diagnosis and may aid in endourologic treatment.12,13 However, biopsy samples obtained during ureteropyeloscopy are typically small and could potentially underestimate the risk of more advanced disease. Assessment of the depth of invasion in UTUC is not possible because of the risk of perforation through the thinly walled renal pelvis or ureter. Imaging studies may be helpful in determining whether there is renal parenchyma invasion or large-volume extension beyond the urothelial lining. Nephroureterectomy, which entails removal of the entire kidney along with the ipsilateral ureter and excision of a cuff of normal bladder tissue, is regarded as the reference standard for managing high-risk UTUC. 6 This is primarily due to the high incidence of multiple ipsilateral lesions, with approximately 20% of patients experiencing tumor recurrence within the residual ureteral stump. 14 For individuals with low-grade lesions seeking a kidney-sparing approach or those with high-grade but low-volume disease, tumor ablation may present itself as an alternative option. However, ablation carries a high risk of recurrence.15,16 In a single-institution study, 83 patients with a normal contralateral upper tract were treated using an endoscopic approach. Over a median follow-up period of 4.6 years, 46 patients experienced 76 upper tract recurrences, and bladder recurrences were observed in 37 patients. 15
Cisplatin-based neoadjuvant treatment had shown to improve overall survival (OS) in muscle-invasive urothelial tumors of the bladder.17-19 The use of neoadjuvant carboplatin has historically demonstrated less benefit than cisplatin in urothelial cancer. 20 Postoperative loss of nephrons supports the use of cisplatin-based chemotherapy in the neoadjuvant setting. 21 Although there are no randomized phase III trials assessing the use of neoadjuvant platinum-based chemotherapy in patients with UTUC due to the rarity of UTUC resulting in low trial accruals, the American Urological Association recommends cisplatin-based neoadjuvant chemotherapy for individuals with high-grade UTUC who are undergoing definitive nephroureterectomy with excision of the bladder cuff, particularly if they have comorbidities that preclude them from receiving adjuvant platinum-based therapy or are expected to have decreased eGFR post-surgery. 22 This recommendation is based on 2 phase II trials investigating neoadjuvant platinum-based chemotherapy in high-grade UTUC prior to surgery.23,24
Margulis et al 23 studied 29 patients with pathologically confirmed high-grade UTUC who received 4 cycles of neoadjuvant accelerated methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) with baseline creatinine clearance rate of >50 mL/min, and for creatinine clearance rate of 30 to 50 mL/min due to poor accrual, only 6 patients enrolled to receive gemcitabine and carboplatin. Following nephroureterectomy, the pathologic complete response and downstaging rates were 14% and 62%, respectively. Coleman et al presented data from a prospective phase II open-label trial of neoadjuvant gemcitabine and cisplatin in 57 patients with high-grade UTUC in which 63% of patients achieved P < .001; 2-year OS 100% and 100% vs 80%, P < .001). 24 In addition, there is an ongoing randomized phase II/III trial of neoadjuvant platinum-based chemotherapy with or without durvalumab chemotherapy for 4 neoadjuvant cycles before nephroureterectomy in high-risk UTUC (clinical trials ID: NCT04628767).
Patients with advanced-stage UTUC who have undergone radical nephroureterectomy and did not receive neoadjuvant chemotherapy often receive adjuvant platinum-based therapy. This practice is supported by findings from the POUT trial, a phase III randomized trial conducted in the United Kingdom. In that trial, chemotherapy-naive patients with postoperative pathologic stage (≥pT2N any) were randomized to receive either platinum chemotherapy (cisplatin or carboplatin for individuals with a glomerular filtration rate <50 mL/min) with gemcitabine for 4 planned adjuvant cycles or to undergo observation without adjuvant chemotherapy. At a median follow-up of 30.3 months, individuals who received adjuvant chemotherapy had improved disease-free survival (hazard ratio [HR], 0.45) and a reduced risk of metastases or death (HR, 0.48) compared with those in the observation arm. However, the completion rate of 4 adjuvant cisplatin cycles in this dataset was low, standing at 58%, with 21% of patients initiating cisplatin but transitioning to carboplatin due to a decline in glomerular filtration rate post-allocation. 25
Despite the promising results from the adjuvant POUT (Peri-Operative chemotherapy versus sUrveillance in upper Tract urothelial cancer) trial and neoadjuvant trials, the use of cisplatin is limited by dose-dependent toxicity in patients with renal impairment. Stage 3 or greater chronic kidney disease is present in roughly 40% of patients with newly diagnosed UTUC and up to 85% of patients following nephroureterectomy.26,27 Patients who are ineligible for cisplatin and undergo non–cisplatin-based neoadjuvant therapy tend to exhibit lower response rates. Specifically, non-cisplatin chemotherapy demonstrates a pathologic complete response rate of 3%, whereas MVAC yields a pathologic complete response rate of 17% in a multicenter study. 28 Consequently, there are low completion rates and limited implementation of cisplatin in patients with UTUC compared with those with bladder cancer. Therefore, there is a pressing need for improved neoadjuvant approaches that do not rely on platinum in order to avoid additional renal toxicity in patients with UTUC. Pembro/EV emerges as a particularly exciting option, especially given its promising responses in urothelial cancer.
Enfortumab vedotin is an antibody-drug conjugate comprising a fully human monoclonal antibody targeting nectin-4, a cell-adhesion protein overexpressed in >95% of urothelial cancer cells, and the cytotoxic agent monomethyl auristatin E (MMAE), a microtubule-disrupting agent. Upon binding to the Nectin-4 antigen on cancer cell surfaces, EV is internalized, leading to intracellular release of MMAE through proteolytic cleavage. This targeted delivery of MMAE results in cell-cycle arrest and apoptosis. 29 The combination of Pembro/EV received Food and Drug Administration (FDA) approval in December 2023 as the preferred first-line treatment for locally advanced or metastatic urothelial cancer, especially in patients ineligible for cisplatin. This decision was based on promising results from EV-302, which demonstrated a 67% response rate, including 29% complete response rate and an OS of 31.5 months improvement over 16 months in patients receiving platinum-based chemotherapy. 30
Our patient exhibited a promising response following 4 months of neoadjuvant Pembro/EV treatment. Initially diagnosed with locally advanced UTUC at a clinical stage of T3N1 and potential pulmonary metastasis indicated by the presence of a new pulmonary nodule, she presented as frail, aged >80 years, and with chronic kidney disease as demonstrated by an eGFR ranging between 30 and 40, rendering her ineligible for platinum-based therapy, whether in the neoadjuvant or adjuvant setting. Remarkably, she achieved a pathologic complete response, characterized by ypT0N0 status. Throughout the treatment, she had excellent tolerance to Pembro/EV, experiencing minimal side effects. In light of her somatic mutation testing revealing BRCA somatic mutations, olaparib was additionally incorporated into her treatment regimen. Notably, she encountered no significant adverse effects necessitating dose interruptions. Presently, she is recovering well postoperatively, with ongoing surveillance.
Conclusion
This case report, illustrating a promising outcome in a patient with UTUC treated with neoadjuvant Pembro/EV, suggests the potential effectiveness and applications of Pembro/EV in the perioperative management of patients with UTUC. Our case underscores the importance of investigating new treatment approaches in patients with UTUC to target pathologic complete response, as studies have indicated that achieving a lower pathologic stage correlates with improved disease-free survival and OS. Our future aim is to integrate improved imaging techniques and ureteropyeloscopy to accurately define and predict pathologic complete response prior to nephroureterectomy. This approach aims to identify a subset of vulnerable patients, such as those who are poor surgical candidates or have a solitary kidney, who may opt for kidney preservation and avoid nephroureterectomy. Clinical trials and ongoing research play a critical role in comprehensively understanding the range of responses and optimizing the utilization of Pembro/EV in this context.
Acknowledgments
We express our sincere gratitude and appreciation to Ms Virginia Mohlere, ELS, who helped revise the grammar of our manuscript.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: P.C., MD, serves on an advisory board for Eisai, Merck, Taiho, Pfizer, Astellas, and Sirtex.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval for reporting individual cases or case series.
Informed Consent: Written, informed consent was obtained from the patient for her anonymized information to be published in this article.
ORCID iD: Kok Hoe Chan  https://orcid.org/0000-0002-2550-1122
https://orcid.org/0000-0002-2550-1122
References
- 1. Babjuk M, Burger M, Capoun O, et al. European Association of Urology Guidelines on non-muscle-invasive bladder cancer (Ta, T1, and Carcinoma in Situ). Eur Urol. 2022;81(1):75-94. doi: 10.1016/j.eururo.2021.08.010 [DOI] [PubMed] [Google Scholar]
- 2. Raman JD, Messer J, Sielatycki JA, Hollenbeak CS. Incidence and survival of patients with carcinoma of the ureter and renal pelvis in the USA, 1973-2005. BJU Int. 2011;107(7):1059-1064. doi: 10.1111/j.1464-410X.2010.09675.x [DOI] [PubMed] [Google Scholar]
- 3. Cosentino M, Palou J, Gaya JM, Breda A, Rodriguez-Faba O, Villavicencio-Mavrich H. Upper urinary tract urothelial cell carcinoma: location as a predictive factor for concomitant bladder carcinoma. World J Urol. 2013;31(1):141-145. doi: 10.1007/s00345-012-0877-2 [DOI] [PubMed] [Google Scholar]
- 4. Rouprêt M, Babjuk M, Compérat E, et al. European association of urology guidelines on upper urinary tract urothelial carcinoma: 2017 update. Eur Urol. 2018;73(1):111-122. doi: 10.1016/j.eururo.2017.07.036.0 [DOI] [PubMed] [Google Scholar]
- 5. Williams SK, Denton KJ, Minervini A, et al. Correlation of upper-tract cytology, retrograde pyelography, ureteroscopic appearance, and ureteroscopic biopsy with histologic examination of upper-tract transitional cell carcinoma. J Endourol. 2008;22(1):71-76. doi: 10.1089/end.2007.9853 [DOI] [PubMed] [Google Scholar]
- 6. Lughezzani G, Sun M, Perrotte P, et al. Should bladder cuff excision remain the standard of care at nephroureterectomy in patients with urothelial carcinoma of the renal pelvis? A population-based study. Eur Urol. 2010;57(6):956-962. doi: 10.1016/j.eururo.2009.12.001 [DOI] [PubMed] [Google Scholar]
- 7. Seisen T, Krasnow RE, Bellmunt J, et al. Effectiveness of adjuvant chemotherapy after radical nephroureterectomy for locally advanced and/or positive regional lymph node upper tract urothelial carcinoma. J Clin Oncol. 2017;35(8):852-860. doi: 10.1200/JCO.2016.69.4141 [DOI] [PubMed] [Google Scholar]
- 8. Leow JJ, Chong YL, Chang SL, Valderrama BP, Powles T, Bellmunt J. Neoadjuvant and adjuvant chemotherapy for upper tract urothelial carcinoma: a 2020 systematic review and meta-analysis, and future perspectives on systemic therapy. Eur Urol. 2021;79(5):635-654. doi: 10.1016/j.eururo.2020.07.003 [DOI] [PubMed] [Google Scholar]
- 9. Bajorin DF, Witjes JA, Gschwend JE, et al. Adjuvant nivolumab versus placebo in muscle-invasive urothelial carcinoma [published correction appears in N Engl J Med 2021;385(9):864]. N Engl J Med. 2021;384(22):2102-2114. doi: 10.1056/NEJMoa2034442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kleinmann N, Matin SF, Pierorazio PM, et al. Primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel (OLYMPUS): an open-label, single-arm, phase 3 trial. Lancet Oncol. 2020;21(6):776-785. doi: 10.1016/S1470-2045(20)30147-9 [DOI] [PubMed] [Google Scholar]
- 11. Matin SF, Pierorazio PM, Kleinmann N, et al. Durability of response to primary chemoablation of low-grade upper tract urothelial carcinoma using UGN-101, a mitomycin-containing reverse thermal gel: OLYMPUS Trial final report. J Urol. 2022;207(4):779-788. doi: 10.1097/JU.0000000000002350 [DOI] [PubMed] [Google Scholar]
- 12. Blute ML, Segura JW, Patterson DE, et al. Impact of endourology on diagnosis and management of upper urinary tract urothelial cancer. J Urol. 1989;141(6):1298-1301. doi: 10.1016/s0022-5347(17)41286-9 [DOI] [PubMed] [Google Scholar]
- 13. Bagley DH, Rivas D. Upper urinary tract filling defects: flexible ureteroscopic diagnosis. J Urol. 1990;143(6):1196-1200. doi: 10.1016/s0022-5347(17)40223-0 [DOI] [PubMed] [Google Scholar]
- 14. Strong DW, Pearse HD. Recurrent urothelial tumors following surgery for transitional cell carcinoma of the upper urinary tract. Cancer. 1976;38(5):2173-2183. doi:10.1002/1097-0142(197611)38:5<2178::aid-cncr2820380548>3.0.co;2-1 [DOI] [PubMed] [Google Scholar]
- 15. Thompson RH, Krambeck AE, Lohse CM, et al. Endoscopic management of upper tract transitional cell carcinoma in patients with normal contralateral kidneys. Urology. 2008;71(4):713-717. doi: 10.1016/j.urology.2007.11.018 [DOI] [PubMed] [Google Scholar]
- 16. Krambeck AE, Thompson RH, Lohse CM, Patterson DE, Elliott DS, Blute ML. Imperative indications for conservative management of upper tract transitional cell carcinoma. J Urol. 2007;178(3, pt 1):792-796. doi: 10.1016/j.juro.2007.05.056 [DOI] [PubMed] [Google Scholar]
- 17. International Collaboration of Trialists; Medical Research Council Advanced Bladder Cancer Working Party (now the National Cancer Research Institute Bladder Cancer Clinical Studies Group); European Organisation for Research and Treatment of Cancer Genito-Urinary Tract Cancer Group. International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol. 2011;29(16):2171-2177. doi: 10.1200/JCO.2010.32.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sherif A, Holmberg L, Rintala E, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45(3):297-303. doi: 10.1016/j.eururo.2003.09.019 [DOI] [PubMed] [Google Scholar]
- 19. Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer [published correction appears in N Engl J Med 2003;349(19):1880]. N Engl J Med. 2003;349(9):859-866. doi: 10.1056/NEJMoa02214 [DOI] [PubMed] [Google Scholar]
- 20. Galsky MD, Chen GJ, Oh WK, et al. Comparative effectiveness of cisplatin-based and carboplatin-based chemotherapy for treatment of advanced urothelial carcinoma. Ann Oncol. 2012;23(2):406-410. doi: 10.1093/annonc/mdr156 [DOI] [PubMed] [Google Scholar]
- 21. Ho MD, Black AJ, Zargar H, et al. The effect of cisplatin-based neoadjuvant chemotherapy on the renal function of patients undergoing radical cystectomy. Can Urol Assoc J. 2023;17(10):301-309. doi: 10.5489/cuaj.8570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Coleman JA, Clark PE, Bixler BR, et al. Diagnosis and management of non-metastatic upper tract urothelial carcinoma: AUA/SUO guideline. J Urol. 2023;209(6):1071-1081. [DOI] [PubMed] [Google Scholar]
- 23. Margulis V, Puligandla M, Trabulsi EJ, et al. Phase II trial of neoadjuvant systemic chemotherapy followed by extirpative surgery in patients with high grade upper tract urothelial carcinoma. J Urol. 2020;203:690-698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Coleman JA, Yip W, Wong NC, et al. Multicenter phase II clinical trial of gemcitabine and cisplatin as neoadjuvant chemotherapy for patients with high-grade upper tract urothelial carcinoma. J Clin Oncol. 2023;41(8):1618-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birtle AJ, Jones R, Chester J, et al. Improved disease-free survival with adjuvant chemotherapy after nephroureterectomy for upper tract urothelial cancer: final results of the POUT trial. J Clin Oncol. 2024;42(13):1466-1471. doi: 10.1200/JCO.23.01659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Shao IH, Lin YH, Hou CP, et al. Risk factors associated with ineligibility of adjuvant cisplatin-based chemotherapy after nephroureterectomy. Drug Des Devel Ther. 2014;8:1985-1990. doi: 10.2147/DDDT.S72197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Raman JD, Lin YK, Kaag M, et al. High rates of advanced disease, complications, and decline of renal function after radical nephroureterectomy. Urol Oncol. 2014;32(1):47.e9-14. doi: 10.1016/j.urolonc.2013.06.015 [DOI] [PubMed] [Google Scholar]
- 28. Grossmann NC, Pradere B, D’Andrea D, et al. Neoadjuvant chemotherapy in elderly patients with upper tract urothelial cancer: oncologic outcomes from a multicenter study. Clin Genitourin Cancer. 2022;20(3):227-236. doi: 10.1016/j.clgc.2022.01.004 [DOI] [PubMed] [Google Scholar]
- 29. Maiorano BA, Catalano M, Maiello E, Roviello G. Enfortumab vedotin in metastatic urothelial carcinoma: the solution EVentually. Front Oncol. 2023;13:1254906. doi: 10.3389/fonc.2023.1254906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Powles T, Valderrama BP, Gupta S, et al. Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer. N Engl J Med. 2024;390(10):875-888. doi: 10.1056/NEJMoa2312117 [DOI] [PubMed] [Google Scholar]



