Abstract
Fibrinogen concentrate treatment is recommended for acute bleeding episodes in adult and pediatric patients with congenital and acquired fibrinogen deficiency. Previous studies have reported a low risk of thromboembolic events (TEEs) with fibrinogen concentrate use; however, the post-treatment TEE risk remains a concern. A retrospective evaluation of RiaSTAP®/Haemocomplettan® P (CSL Behring, Marburg, Germany) post-marketing data was performed (January 1986–June 2022), complemented by a literature review of published studies. Approximately 7.45 million grams of fibrinogen concentrate was administered during the review period. Adverse drug reactions (ADRs) were reported in 337 patients, and 81 (24.0%) of these patients experienced possible TEEs, including 14/81 (17.3%) who experienced fatal outcomes. Risk factors and the administration of other coagulation products existed in most cases, providing alternative explanations. The literature review identified 52 high-ranking studies with fibrinogen concentrate across various clinical areas, including 26 randomized controlled trials. Overall, a higher number of comparative studies showed lower rates of ADRs and/or TEEs in the fibrinogen group versus the comparison group(s) compared with those that reported higher rates or no differences between groups. Post-marketing data and clinical studies demonstrate a low rate of ADRs, including TEEs, with fibrinogen concentrate treatment. These findings suggest a favorable safety profile of fibrinogen concentrate, placing it among the first-line treatments effective for managing intraoperative hemostatic bleeding.
Keywords: fibrinogen concentrate, safety, post-marketing, adverse drug reaction
Introduction
Reduced levels of fibrinogen can occur in patients with either congenital or acquired fibrinogen deficiencies. 1 As a key component of blood clots, deficiencies of fibrinogen affect the ability of the blood to form clots during hemorrhage, resulting in bleeding episodes of varying severity. Low levels of fibrinogen have been shown to be associated with an increased risk of bleeding in cardiac surgery and postpartum hemorrhage.2–4 However, treatment of hemorrhage with only red blood cell transfusions can lead to dilutional coagulopathy. 5
Fibrinogen replacement therapy is currently recommended for both congenital6,7 and acquired fibrinogen deficiencies caused by various clinical conditions, including major bleeding and cardiac surgery.8–10 Transfusion of fresh frozen plasma (FFP), cryoprecipitate, or human fibrinogen concentrate is performed with the aim of replacing fibrinogen.11,12 However, cryoprecipitate is not recommended or available in some European countries due to the risk of infectious disease transmission, 13 and FFP is associated with the risk of viral transmission, allergic reactions, transfusion-related acute lung injury, and transmission-associated circulatory overload due to the large volume required to raise factor levels.14–17 Fibrinogen concentrate offers advantages over alternatives, including standardized fibrinogen concentration, low infusion volume, no requirement for cross-matching, ease of reconstitution, and viral inactivation steps during production. 18 A favorable safety profile of fibrinogen concentrate has been demonstrated across a range of indications, 19 and a recent international consensus statement reported that fibrinogen concentrate does not appear to increase adverse outcomes in cardiac surgery, including thromboembolic events (TEEs), noting that conclusive evidence is lacking. 20
RiaSTAP® and Haemocomplettan® P (CSL Behring, Marburg, Germany) are lyophilized, highly purified, virus-inactivated fibrinogen concentrate products manufactured from screened human plasma. RiaSTAP® and Haemocomplettan® P are identical in form and preparation and are both indicated for the treatment of acute bleeding episodes in adult and pediatric patients with congenital fibrinogen deficiency. Haemocomplettan® P is also indicated for the treatment of bleeding in acquired fibrinogen deficiency in adult and pediatric patients. Haemocomplettan® P entered the European market in 1986 and is currently licensed in 22 countries. RiaSTAP® has been approved for the treatment of congenital fibrinogen deficiency in the United States by the Food and Drug Administration in 2009 and is currently licensed in 25 countries.
The aim of the present evaluation is to provide an up-to-date overview of available data on the safety of fibrinogen concentrate. For this purpose, post-marketing surveillance data on RiaSTAP®/Haemocomplettan® P from the CSL Behring Global Safety Database were evaluated, complemented by a literature search using the PubMed database to identify published studies reporting safety data for RiaSTAP®/Haemocomplettan® P.
Methods
Post-Marketing Data
A retrospective review of post-marketing surveillance data from the CSL Behring Global Safety Database from January 1, 1986 to June 30, 2022 was performed. The database includes spontaneous reports, reports from post-marketing studies, reports from regulatory agencies, and cases identified from a review of worldwide scientific literature. All adverse events (AEs) assessed as related/possibly related to the study drug were considered to be adverse drug reactions (ADRs). Spontaneous AE reports were considered ADRs unless they were assessed as unrelated to the drug by the reporter and the company. CSL Behring uses a causality assessment based on three causality categories of related, not related, and unassessable. All reported ADRs for RiaSTAP®/Haemocomplettan® P were included in the analysis.
ADRs were coded using the Medical Dictionary for Regulatory Activities (MedDRA) Version 25, and events were classified as serious versus non-serious according to regulatory definition (ie, those that resulted in death, were life-threatening, required hospitalization, resulted in persistent or significant disability/incapacity, were a congenital anomaly/birth defect, or were medically important were considered serious events). For each ADR report, the year, country of origin, patient age and sex, indication, concomitant products, and manifestations/outcome of the ADR were recorded if available. The cumulative quantity of RiaSTAP®/Haemocomplettan® P distributed was established from CSL Behring commercial records. As the actual total number of patients who received fibrinogen concentrate is not known, patient exposure is presented as the number of estimated 4 g standard doses based on the units distributed in line with the recommended dose in the European guideline on the management of major bleeding and coagulopathy following trauma. 21
Standardized MedDRA Queries (SMQs), High Level Group Terms (HLGT), High Level Terms (HLT), and Preferred Terms (PT) within the MedDRA dictionary were used to identify events of special interest (important identified and potential risks) for analysis. Anaphylaxis and hypersensitivity/allergic reactions were identified using the narrow SMQs ‘Anaphylactic reaction’ and ‘Hypersensitivity,’ respectively, and thromboembolic complications were identified using the SMQ ‘Embolic and thrombotic events.’ Suspicion of virus transmission was identified using terms from the HLGT ‘Viral infectious disorders,’ a selection of terms related to viral infections from the HLGT ‘Ancillary infectious topics,’ the HLT ‘Sepsis, bacteremia, viremia and fungemia NEC,’ a selection of terms associated with investigations related to viral infection from the HLT ‘Virus identification and serology,’ the HLT ‘Microbiology and serology tests NEC,’ and a selection of terms associated with ‘Procedures relating to viral infection’ from the HLT ‘Anti-infective therapies.’
Literature Review
An updated literature search was conducted using MEDLINE (PubMed) with the objective of identifying original English language articles reporting clinical studies of RiaSTAP®/Haemocomplettan® P in bleeding patients. Updated literature searches were performed on July 12, 2022 (see Supplemental Materials for further details). Clinical studies reporting AEs from January 1, 2014 to June 30, 2022 were included and reviewed. In addition, studies using RiaSTAP®/Haemocomplettan® P that were published before 2014 and included in the 2015 study by Solomon et al 19 were also included and summarized in this manuscript in context of the updated findings.
The following search terms were used: fibrinogen concentrate or concentrates; fibrinogen supplementation; Haemocomplettan® P; RiaSTAP®; and the MeSH terms ‘fibrinogen/therapeutic use’ or ‘fibrinogen/administration and dosage.’ The clinical study subjects, authors, methods, and time periods were examined to avoid inclusion of redundant data from multiple reports. The following data were extracted from the study reports: number and age of study patients, type of fibrinogen deficiency, indication for fibrinogen concentrate infusion, treatment regimen, and occurrence of AEs. Only studies reporting safety data were included, and relevant AEs reported in the clinical studies were included, regardless of whether or not a relationship to fibrinogen concentrate was established. In the context of the literature review, AEs refer to any AE reported in the literature, while the term ADR here refers specifically to AEs extracted from the CSL Behring safety database that were either assessed as related/possible related to the study drug or were spontaneous AE reports (unless assessed as unrelated to the drug).
The literature search inclusion criteria were randomized controlled trials, non-randomized prospective studies, and retrospective studies in patients receiving RiaSTAP®/Haemocomplettan® P. Studies that used fibrinogen from other manufacturers or from more than one source or did not specify the source of fibrinogen used as study treatment, case reports/case series, guidelines, meta-analyses, systematic reviews, reviews articles, conference abstracts or proceedings, opinion pieces, editorials, commentaries, and articles not reporting original data were excluded.
Results
Post-Marketing Data
Baseline Characteristics
Commercial data indicated that 7,449,797 g of RiaSTAP®/Haemocomplettan® P was distributed over the post-marketing surveillance period, corresponding to 1,862,449 standard doses of 4 g each, across a range of clinical settings and indications in 25 countries across Australia, Europe, Asia, and America, including the US, Canada, and South America.
Nature and Rate of Adverse Drug Reactions
Between January 1, 1986 and June 30, 2022, a total of 806 ADRs were retrieved from the safety database among 337 patients, equating to ∼1 patient per 22 106 g or 5527 standard doses. Of these, 540 ADRs were reported among 192 patients for Haemocomplettan® P and 266 ADRs among 145 patients for RiaSTAP®. ADRs classified as serious according to regulatory definition were experienced by 243 patients, equating to ∼1 patient per 30 658 g distributed or 7664 standard doses administered. Table 1 provides an overview of the seriousness and outcome of all ADRs retrieved from the database, as well as for the most commonly reported ADRs (TEEs, hypersensitivity, and transmissions of infectious agents).
Table 1.
Seriousness and Outcome of the Reported Adverse Drug Reactions from the CSL Behring Global Safety Database.
| All ADRs (n = 806) | TEEs (n = 114) | Hypersensitivity reaction events (n = 79) | Transmissions of infectious agents (n = 51) | |
|---|---|---|---|---|
| Seriousness | ||||
| Non-serious | 249 | 0 | 16 | 3 |
| Serious | 557 | 114 | 63 | 48 |
| Outcome | ||||
| Fatal | 76 | 20 | 7 | - |
| Not recovered/Not resolved | 42 | 5 | 2 | 7 |
| Not reported | 124 | 22 | 3 | 3 |
| Recovered/Resolved | 351 | 33 | 57 | 6 |
| Recovering/Resolving | 13 | 4 | 1 | - |
| Recovered/Resolved with sequelae | 7 | 3 | - | 3 |
| Recovered with treatment | 2 | 1 | - | - |
| Unknown | 191 | 26 | 9 | 32 |
N values represent adverse drug reactions.
Abbreviations: ADR, adverse drug reaction; TEE, thromboembolic event.
A total of 57 ADRs among 34 patients were excluded due to lack of a trade name. Age data were available for 247 patients, with a mean ± standard deviation (SD) age of 39.8 ± 22.3 years (minimum: 0.04, lower quartile: 23.0, median: 38.0, upper quartile: 59.0, maximum: 86.0). ADRs were reported in more females than males (170 vs 115, respectively), and 33 of the females who experienced ADRs were pregnant. The sex data for 52 patients were not available. A total of 35 fatal cases were possibly related to RiaSTAP®/Haemocomplettan® P treatment. Concomitant medications (eg, FFP and platelets) and/or underlying conditions (eg, severe trauma, major traumatic hemorrhage, surgical procedures, and sepsis) were considered confounding variables in most cases.
Thromboembolic Complications
Until June 30, 2022, 114 possible TEEs were retrieved from the safety database in 81 patients, equating to ∼1 patient per 91 973 g distributed. Table 1 provides a summary of the seriousness and outcome of these 114 TEEs. Of these, 38 were reported for Haemocomplettan® P and 43 for RiaSTAP®. Among the 58 patients with age data available, the mean ± SD age was 40.4 ± 20.0 years (minimum: 0.04, lower quartile: 25.3, median: 37.5, upper quartile: 54.3, maximum: 81.0). The majority were female (41 female vs 21 male patients; sex data were not available for 19 patients). As shown in Table 2, the most commonly reported TEEs were ‘Pulmonary embolism’ (18 patients), ‘Deep vein thrombosis’ (11 patients), ‘Thrombosis not otherwise specified’ (seven patients), ‘Myocardial infarction’ (five patients), and ‘Jugular vein thrombosis’ (five patients). All other TEEs were reported in fewer than five instances. In 62 of the 81 patients who experienced TEEs, fibrinogen concentrate was used without any further reported concomitant product. The use of tranexamic acid was reported in five patients; plasma was reported in four patients; and red blood cells (RBCs) were found in four patients (information on the use of concomitant products was not available for all patients).
Table 2.
Reported Cases Involving Thromboembolic Complications from the CSL Behring Global Safety Database.
| Details of TEEs reported as possibly related to RiaSTAP®/Haemocomplettan® P administration | |
|---|---|
| Number of possible TEEs, n | 81 |
| RiaSTAP® | 43 |
| Haemocomplettan® P | 38 |
| Median (range) age of patients, years a | 37.5 (0.04–81.0) |
| Female/male patient ratio a | 41:21 |
| Most common TEEs, n | |
| Pulmonary embolism | 18 |
| Deep vein thrombosis | 11 |
| Thrombosis not otherwise specified | 7 |
| Myocardial infarction | 5 |
| Jugular vein thrombosis | 5 |
| Fatal TEEs b , n | 14 |
Includes only cases where age and sex of patient were reported. N values represent patients. bAdditional risk factors existed in most of these patients, which provide alternative explanations.
Abbreviation: TEEs, thromboembolic events.
Fatal outcomes were reported in 14 of the 81 patients who experienced TEEs (11 with Haemocomplettan® P and three with RiaSTAP®). Eight of these reports were retrieved from five publications (Velik-Salchner et al, 22 Weiss et al, 23 Vidmar et al, 24 Taslimi et al, 25 and Bilecen et al, 26 ), reporting the MedDRA PTs ‘Acute myocardial infarction,’ ‘Cerebral infarction’ (each reported twice), ‘Brachiocephalic vein thrombosis,’ ‘Mesenteric vein thrombosis,’ ‘Monoparesis,’ ‘Myocardial infarction,’ ‘Portal vein thrombosis,’ ‘Pulmonary artery thrombosis,’ ‘Pulmonary embolism,’ ‘Subclavian artery thrombosis,’ and ‘Thrombotic stroke’ (each reported once). The remaining six reports were from spontaneous sources with pulmonary embolism and thrombosis (each reported twice), cardiac ventricular thrombosis, carotid artery thrombosis, graft thrombosis, and myocardial infarction (each reported once). In most cases, additional risk factors existed (eg, underlying concurrent conditions and/or concomitant treatments), which could provide alternative explanations. However, the possibility of a causal relationship with RiaSTAP®/Haemocomplettan® P administration cannot be excluded.
Anaphylaxis and Hypersensitivity/Allergic Reaction
A total of 79 possibly related hypersensitivity reaction events (retrieved from the safety database using the SMQ ‘Hypersensitivity’) were reported in 47 patients, equating to ∼1 patient per 158 506 g of administered fibrinogen concentrate. Table 1 provides a summary of the seriousness and outcome of the 79 hypersensitivity reaction events. Hypersensitivity events occurred in the same number of males and females (n = 22), and sex data were not available for three patients. Age was available for 40 cases, with a mean ± SD of 35.6 ± 26.1 years (minimum: 0.5, lower quartile: 10.0, median: 34.0, upper quartile: 59.8, maximum: 86.0). The most common hypersensitivity reaction was ‘Anaphylactic reaction’ (15 patients), followed by ‘Hypersensitivity’ and ‘Urticaria’ (nine patients each), ‘Shock’ and ‘Rash’ (seven patients each), and ‘Anaphylactic shock’ (six patients). In 33 of the 47 patients with hypersensitivity reaction events, fibrinogen was used without any further reported concomitant products, while plasma was used in six patients, RBCs in six patients, factor VII in four patients, factor X in four patients, and platelets in four patients (information on the use of concomitant products was not available for all patients).
Three cases of hypersensitivity reaction were fatal, all of which were reported for Haemocomplettan® P. Anaphylactic reaction was reported in one patient; circulatory collapse and shock were reported in another patient; and macular rash, shock, tongue edema, and type I hypersensitivity were reported in the remaining patient. Of note, all patients concomitantly received treatment with other medical products, such as protamine, hydroxyethyl starch, RBCs, and pooled plasma, which are considered to be co-suspected.
Suspicion of Virus Transmission
Until June 30, 2022, 51 possible events of infectious agent transmissions were retrieved from the safety database in 33 patients, equating to ∼1 patient per 225 751 g distributed or 56 438 standard doses. None had a fatal outcome. Table 1 provides a summary of the seriousness and outcome of the 51 transmissions of infectious agent events. Among the 26 patients with available age data, the mean ± SD age was 53.1 ± 16.9 years (minimum: 21.0, lower quartile: 38.0, median: 59.0, upper quartile: 66.3, maximum: 77.0). Fourteen were female, and 18 were male (sex was not available in one patient). In four of the 33 patients with possible events of infectious agent transmissions, fibrinogen concentrate was used without any further reported concomitant products. The use of plasma was reported in 14 patients; albumin was used in seven patients; four-factor prothrombin complex concentrate was used in seven patients; RBCs were used in nine patients; antithrombin was used in five patients; factor X was used in four patients; and platelets were used in four patients (information on the use of concomitant products was not available for all patients; some patients received more than one allogeneic product).
Of the 33 patients with possible events of infectious agent transmissions, one event of PT ‘Hepatitis A,’ 14 of ‘Hepatitis B,’ 21 of ‘Hepatitis C,’ four of ‘HIV,’ and one of ‘cytomegalovirus’ were reported. However, most were considered by the company as unlikely to have a causal relationship with the product due to alternative explanations and/or non-reactive polymerase chain reaction (PCR) tests for the concerned batches and/or related plasma pools for fractionation. The PCR data were only available in reports that had a lot number (27 patients). In the patient who experienced cytomegalovirus infection, it is likely that re-activation of a previous infection occurred due to immunosuppressant treatment following organ transplantation.
Other Reported Events
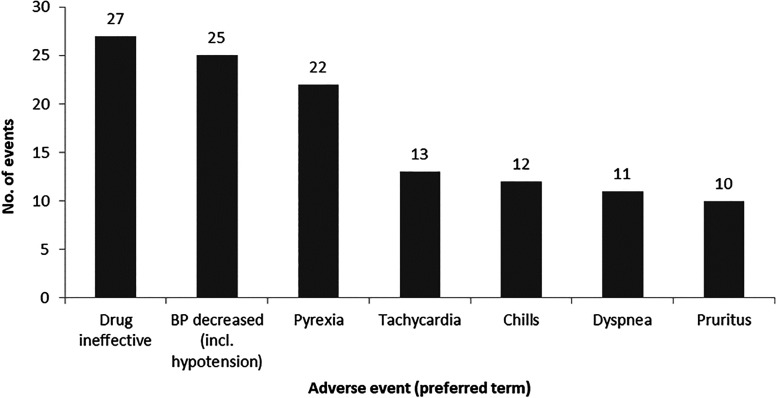
A total of 511 ADRs were not covered by the Standard MedDRA query of ‘Anaphylactic reaction’ or ‘Thromboembolic events’ or the Company MedDRA query of ‘Transmission of infectious agents.’ The most common types of events were in the MedDRA System Organ Class (SOC) ‘General disorders and administration site conditions’ (122 ADRs), followed by ‘Investigations’ (57 ADRs), ‘Respiratory, thoracic, and mediastinal disorders’ (51 ADRs), ‘Injury, poisoning, and procedural complications’ (46 ADRs), ‘Vascular disorders’ (42 ADRs), ‘Cardiac disorders’ (37 ADRs), and ‘Nervous system disorders (36 ADRs).’ The most common MedDRA PTs reported were ‘Drug ineffective’ (27 ADRs), ‘Blood pressure decreased, including hypotension’ (25 ADRs), ‘Pyrexia’ (22 ADRs), ‘Tachycardia’ (13 ADRs), ‘Chills’ (12 ADRs), ‘Dyspnea’ (11 ADRs), and ‘Pruritus’ (10 ADRs) (Figure 1). A lack of drug effect was reported in 41 patients, including 10 in whom a lack of effect in an unapproved indication was reported.
Figure 1.
Events reported with a frequency of 10 or more that were not classified as anaphylactic, allergic, thromboembolic, or virus-related.
BP, blood pressure.
Literature Review
A summary of studies reporting data on the safety of RiaSTAP®/Haemocomplettan® P is shown in Tables 3, 4 and Supplementary Table 3, including events of special interest (ie, important identified and potential risks used in the analysis of post-marketing data), as well as the studies previously summarized in the 2015 safety review. 19 Studies that described cases covered in the post-marketing results were excluded from the literature search results (Kreuz et al, 1 Weiss et al, 23 Gollop et al, 27 Velik-Salchner et al, 22 Vidmar et al, 24 Taslimi et al, 25 and Bilecen et al 26 ). In addition to the 17 studies previously described, 35 new studies were identified (52 in total).
Table 3.
Clinical Studies of RiaSTAP®/Haemocomplettan® P in Cardiac Surgery.
| Study | Design | Indication | N patients (FC group) | Dose | Patients with AEs (FC group) | Comparator product(s); n | Patients with AEs (comparator group) | Differences between groups |
|---|---|---|---|---|---|---|---|---|
| Karlsson et al (2009) 28 a | RCT | CABG | 10 | 2 g | None b | No FC; 10 | 1 MI | NR |
| Rahe-Meyer et al (2013) 29 a | RCT | Replacement of aorta with CPB | 29 | Median 8 g (IQR, 6–9) |
1 fatal MI; 4 reoperation; 24 TEAEs (10-day follow-up); 5 SAEs (45-day follow-up); 1 SAEs leading to death | No FC; 32 | 1 cardiorespiratory arrest; 1 cerebral hemorrhage; 1 cerebral infarction; 1 operative hemorrhage 4 reoperation; 27 TEAEs (10-day follow-up); 5 SAEs (45-day follow-up); 4 SAEs leading to death |
NS |
| Tanaka et al (2014) 30 a | RCT | Valve replacement | 10 | Weight-averaged 46.2 mg/kg (SD, 5.0) |
3 pulmonary edema; 1 re-exploration | Platelets; 10 | 5 pulmonary edema; 2 30-day readmission; 2 re-exploration; 1 acute MI | NR |
| Bilecen et al (2017) 26 | RCT | Elective high risk cardiac surgery | 60 | Mean 3.1 g (95% CI, 2.7–3.5) |
2 deaths (in-hospital); 4 stroke; 3 MI; 3 renal insufficiency/failure; 3 infection; 4 re-thoracotomy with 5 days | No FC; 60 | 1 stroke; 1 TIA; 1 MI; 2 renal insufficiency/failure; 2 infection; 5 re-thoracotomy with 5 days | NR |
| Downey et al (2020) 31 | RCT | Cardiac surgery with CPB (pediatric) | 29 | Median 107.8 mg/kg (IQR, 86.5–118.1) |
9 any AE; 5 arrhythmia; 1 thrombosis; 1 tamponade; 2 chest exploration; 1 death | Cryoprecipitate; 25 | 10 any AE; 6 arrhythmia; 1 infection within 14 days; 2 repeat surgery within 7 days; 1 chest exploration; 1 stroke | NS |
| Galas et al (2014) 32 | RCT | Cardiac surgery (pediatric) | 30 | 60 mg/kg | 1 AKI; 2 acute MI; 2 septic shock; 4 reoperation | Cryoprecipitate; 33 | 6 AKI; 5 acute MI; 5 septic shock; 6 reoperation | NS |
| Jeppsson et al (2016) 33 | RCT | Cardiac surgery | 24 | 2 g | None | No FC; 24 | 1 reoperation | NR |
| Rahe-Meyer et al (2016) 34 and Rahe-Meyer et al (2021) 35 | RCT | Complex cardiovascular surgery | 78 | Mean 6.29 g (SD, 1.99 |
6 TEEs; 1 death | No FC; 74 | 10 TEEs; 5 deaths | NR |
| Ranucci et al (2015) 36 | RCT | Complex cardiovascular surgery | 58 | Median 4 g (IQR, 3–6) |
1 death (operative); 5 AKI; 7 infection | No FC; 58 | 1 mesenteric infarction; 3 deaths (operative); 6 AKI; 11 infection | NS |
| Siemens et al (2020) 37 | RCT | Cardiac surgery (pediatric) | 60 | Median 114 mg/kg (range, 51–218) | 10 TEEs | No FC; 30 | 2 TEEs | NR |
| Tirotta et al (2022) 38 | RCT | CPB (pediatric) | 15 | 70 mg/kg | 4 thrombus; 3 respiratory failure | No FC; 15 | 2 thrombus; 3 respiratory failure | NS |
| Jahangirifard et al (2018) 39 | RCT | Heart transplant | 23 | 2 g | 7 AKI; 2 reoperation; 3 deaths (in-hospital) | No FC; 30 | 3 AKI; 6 reoperation; 2 sepsis; 4 deaths (in-hospital) | NS |
| Rahe-Meyer et al (2009) 40 a | Prospective | Replacement of ascending aorta and/or arch | 10 | Mean 5.7 g (SD, 0.7) |
1 postoperative atrial fibrillation | No FC; 5 | 1 postoperative atrial fibrillation; 1 re-exploration | NS |
| Rahe-Meyer et al (2009) 41 a | Prospective | Thoraco-abdominal aortic aneurysm operation | 6 | Mean 7.8 g (SD, 2.7) |
1 prolonged respiratory assistance | ABP; 12 | 5 prolonged respiratory assistance; 4 re-exploration; 1 postoperative atrial fibrillation; 2 renal failure; 2 major neurologic events; 2 deaths (30-day mortality) | NS |
| Solomon et al (2012) 42 a | Prospective | CABG | 10 | Median 6 g (range, 4–6) |
None | ABP; 19 | 1 thrombosis requiring CABG reoperation | NR |
| Bilecen et al (2013) 43 a | Prospective | Complex cardiac surgery | 264 | Median 2 g (IQR, 2–3) |
18 deaths (30-day mortality); 14 MI; 11 CVA/TIA; 13 renal insufficiency/failure; 29 infection; 52 prolonged respiratory assistance | No FC; 811 | 33 deaths (30-day mortality); 30 MI; 20 CVA/TIA; 38 renal insufficiency/failure; 74 infection; 45 prolonged respiratory assistance | Statistically significant difference for prolonged respiratory assistance NS other outcomes |
| Waldén et al (2020) 44 | Retrospective | Cardiac surgery | 487 | Median 3.6 g (IQR, 3.1–4.3) | 14 deaths (30-day mortality); 8 TEEs in 30 days; 31 deaths in 1 year; 16 TEEs in 1 year | No FC; 478 | 23 deaths (30-day mortality); 9 TEEs in 30 days; 36 deaths in 1 year; 17 TEEs in 1 year | NS |
| Hanna et al (2016) 45 | Prospective | Aortic reconstruction | 22 | 70 mg/kg | None | N/A | N/A | N/A |
| Solomon et al (2010) 46 a | Retrospective | CPB | 39 | Mean 78 mg/kg (SD, 20) |
None | N/A | N/A | N/A |
Studies reported in Solomon et al 19
Although no clinically detectable adverse events were observed, following a post-operative CT scan, one vein graft occlusion and one subclinical peripheral pulmonary embolus were reported.
Abbreviation: ABP, allogeneic blood product; AE, adverse event; AKI, acute kidney injury; CABG, coronary artery bypass grafting; CI, confidence interval; CPB, cardiopulmonary bypass; CVA, cerebrovascular accident; FC, fibrinogen concentrate; IQR, interquartile range; MI, myocardial infarction; N/A, not applicable; NR, not reported; NS, not significant; RCT, randomized controlled trial; SAE, serious adverse event; SD, standard deviation; TEAE, treatment-emergent adverse event; TEE, thromboembolic event; TIA, transient ischemic attack.
Table 4.
Clinical Studies of RiaSTAP®/Haemocomplettan® P in Trauma and Non-Cardiac Surgery.
| Study | Design | Indication | N patients (FC group) | Dose | Patients with AEs (FC group | Comparator product (s); n | Patients with AEs (comparator group) | Differences between groups |
|---|---|---|---|---|---|---|---|---|
| Trauma | ||||||||
| Akbari et al (2018) 47 | RCT | Severe blunt trauma | 30 | 2 g | 2 multiple organ failure; 5 sepsis; 3 deaths | FFP; 30 | 8 multiple organ failure; 16 sepsis; 11 deaths | Significant difference in mortality (FC vs FFP/No FC) and sepsis (FC vs FFP/No FC) NS other AEs |
| No FC; 30 | 7 multiple organ failure; 4 sepsis; 11 deaths | |||||||
| Curry et al (2018) 48 | RCT | Trauma active bleeding | 20 | 6 g | 3 TEEs; 4 sepsis; 4 multiple organ failure; 6 single organ failure; 1 new onset major bleeding; 8 deaths; 2 deaths due to bleeding | No FC; 19 | 2 TEEs; 6 sepsis; 1 multiple organ failure; 1 single organ failure; 3 new onset major bleeding; 3 deaths; 1 death due to bleeding | NR |
| Innerhofer et al (2017) 49 | RCT | Trauma | 50 | Median 8 g (IQR, 5–10) | 25 multiple organ failure; 4 venous thrombosis; 3 pulmonary embolism; 9 sepsis; 32 infection; 5 deaths (in-hospital) | FFP; 44 | 29 multiple organ failure; 8 venous thrombosis; 1 pulmonary embolism 7 sepsis; 32 infection; 2 deaths (in-hospital) |
NS |
| Nascimento et al (2016) 50 | RCT | Severe trauma | 21 | 6 g | 2 deaths (28-day mortality); 1 death by exsanguination; 2 DVT; 2 pulmonary embolism; 3 AKI; 2 multiple organ failure; 5 infection | No FC; 24 | 1 death (28-day mortality); 3 DVT; 1 pulmonary embolism; 2 acute lung injury; 2 acute respiratory distress syndrome; 2 AKI; 2 multiple organ failure; 8 infection | NS |
| Lucena et al (2021) 51 | RCT | Severe trauma | 16 | 50 mg/kg | None | No FC; 16 | None | N/A |
| Wafaisade et al (2013) 52 a | Retrospective | Trauma | 294 | NR | 20 TEE; 61 sepsis; 217 organ failure; 180 multiple organ failure; 82 deaths (30-day mortality) | No FC; 294 | 10 TEEs; 52 sepsis; 182 organ failure; 144 multiple organ failure; 73 deaths (30-day mortality) | Significant difference in organ failure and multiple organ failure NS other AEs |
| Almskog et al (20200 53 | Retrospective | Trauma | 108 | Median 2 g (IQR, 2-3) |
7 arterial thrombosis; 5 venous thrombosis; 1 multiple organ failure; 4 AKI; 23 deaths (30-day mortality) | No FC; 108 | 8 arterial thrombosis; 3 venous thrombosis; 1 multiple organ failure; 4 AKI; 11 deaths (30-day mortality) | Significant difference 30-day mortality NS other AEs |
| Barquero-Lopez et al (2022) 54 | Retrospective | Severe trauma | 42 | Median 6.5 g (IQR, 4–11) | 3 TEEs; 13 deaths; 5 deaths due to bleeding; 4 pneumonia; 4 AKI; 2 multiple organ failure | Plasma; 28 | 1 TEE; 10 deaths; 7 deaths due to bleeding; 7 pneumonia; 4 AKI; 12 multiple organ failure | Significant differences in pneumonia and multiorgan failure (FC versus other groups NS other AEs |
| Plasma and FC; 64 | 1 TEE; 20 deaths; 17 deaths due bleeding; 17 pneumonia; 9 AKI; 14 multiple organ failure | |||||||
| Non-cardiac surgery | ||||||||
| Fathi et al (2021) 55 | RCT | Radical cystectomy | 35 | 2 g | None | No FC; 35 | None | N/A |
| Najafi et al (2014) 56 | RCT | Hip arthroplasty | 15 | 30 mg/kg | None | No FC; 15 | None | N/A |
| Soleimani et al (2017) 57 | RCT | Transurethral resection of the prostate | 31 | 2 g | None | Saline; 29 | None | N/A |
| Craniosynostosis and scoliosis surgery | ||||||||
| Machotta et al (2021) 58 | RCT | Craniosynostosis (pediatric) | 56 | Mean 79 mg/kg | None | No FC; 55 | None | N/A |
| Haas et al (2015) 59 | RCT | Craniosynostosis and scoliosis (pediatric) | 49 (craniosynostosis 30; scoliosis 19) | Craniosynostosis: median 90 mg/kg (IQR, 60–90) conventional group and 90 mg/kg (IQR, 75–120) early substitution group Scoliosis: median 30 mg/kg (IQR, 30–60) conventional group and 60 mg/kg (IQR, 30–68) early substitution group |
None | N/A | N/A | N/A |
| Haas et al (2008) 60 a | Retrospective | Craniosynostosis (pediatric)n | 9 | Median 76 mg/kg (IQR, 67–100) | None | N/A | N/A | N/A |
| Liver transplantation | ||||||||
| Sabate et al (2016) 61 | RCT | Liver transplantation | 48 | Median 4.14 g (IQR, 3.38–4.92) | 1 TEE; 1 death (in-hospital); 1 renal replacement therapy; 33 any AE | Saline; 44 | 5 TEEs; 3 deaths (in-hospital); 5 renal replacement therapy; 33 any AE | NS |
| Surgery or trauma | ||||||||
| Sabouri et al (2022) 62 | RCT | Acute post-traumatic hypofibrinogenemia in isolated severe traumatic brain injury | 36 | >200 mg/dL | 2 mild allergic reactions (rash) | No FC; 35 | None | N/A |
| Leal-Noval et al (2014) 63 | Retrospective | Massive transfusion associated with cardiac surgery, liver transplant or GI bleeding | 71 | Median 2 g (IQR, 2–4) |
29 deaths (in-hospital) | No FC; 72 | 20 deaths (in-hospital) | NS |
| Vigstedt et al (2022) 64 | Retrospective | Trauma or surgery | 161 | Median 2 g (IQR, 2–3) |
14 TEEs within 30 days | PCC; 111 | 3 TEEs within 30 days | NS |
| rFVIIa; 15 | 4 TEEs within 30 days | |||||||
| Danés et al (2008) 65 a | Retrospective | Surgery/trauma; sepsis; upper GI tract hemorrhage; gynecological diseases; hematological malignancies; liver transplantation; hepatic insufficiency; other | 69 | Median 3.52 g (range, 0.5–8) | None | N/A | N/A | N/A |
| Weinkove and Rangarajan (2008) 66 a | Retrospective | Placental abruption; massive blood loss and transfusion; liver failure; postcardiac surgery; other | 30 | Median 6 g (range, 2–35) | 3 ischemic CVA; 1 MI | N/A | N/A | N/A |
| Fenger-Eriksen et al (2008) 67 a | Retrospective | Surgery and massive hemorrhage | 43 | Adults: median 2 g (range, 1–5) Children: median 0.35 g (range, 0.2–0.5) |
1 jitter and snoring respiration; 1 shivering; 1 death | N/A | N/A | N/A |
| Thorarinsdottir et al (2010) 68 a | Retrospective | Severe hemorrhage following surgery; GI hemorrhage | 37 | Median 2 g (range, 1–6) | None | N/A | N/A | N/A |
Studies reported in Solomon et al 19
Abbreviation: AE, adverse event; AKI, acute kidney injury; CVA, cerebrovascular accident; DVT, deep vein thrombosis; FC, fibrinogen concentrate; FFP, fresh frozen plasma; GI, gastrointestinal; IQR, interquartile range; MI, myocardial infarction; N/A, not applicable; NR, not reported; NS, not significant; PCC, prothrombin complex concentrate; rFVIIa, recombinant factor VIIa; RCT, randomized controlled trial; TEE, thromboembolic event.
Fibrinogen was administered to 3014 patients for a range of indications, covering both congenital and acquired fibrinogen deficiencies. Thirty-six of the studies were prospective,26,28–34,36–43,45,47–51,55–59,61,62,69–75 and 16 were retrospective.44,46,52–54,60,63–68,76–79 Seven pediatric studies were identified, and the remainder included adults. Seventeen of the studies reported that no AEs were observed with fibrinogen concentrate.20,23,30,32,38,43,45,47,50,52,55,57,59,62,66,73,74
Cardiac Surgery
The literature review included a total of 17 studies, in which fibrinogen concentrate was given versus a comparator medication/control group for coagulation therapy in cardiac surgery, including heart transplantation, and two studies that did not include a comparator group. Twelve studies were randomized controlled studies; five were prospective; and two were retrospective. The comparator was placebo in seven studies, control (no fibrinogen) in five studies, allogeneic blood products in two studies, cryoprecipitate in two studies, and platelets in one study. Taking together all comparative studies, a similar number of AEs was reported in the fibrinogen (310 AEs among 1194 patients corresponding to 0.26 AEs per patient) and comparison groups (464 AEs among 1726 corresponding to 0.27 AEs per patient; Table 3). Among 10 studies reporting statistical significance when comparing AEs in the fibrinogen groups versus the comparator, no significant difference was reported between groups in nine studies, with one study reporting a higher rate of prolonged mechanical ventilation with fibrinogen versus control. Overall, a similar number of TEEs was reported in the combined fibrinogen and comparison groups: 72 (corresponding to 0.06 TEEs per patient) versus 95 (0.06 TEEs per patient).26,28–33,35–46
Non-Cardiac Surgery and Trauma
Among 22 studies reporting AE data on fibrinogen concentrate in trauma and/or non-cardiac surgery, six studies did not include a comparator group (Table 4). Twelve studies were randomized controlled trials, and 10 studies were retrospective. The comparator(s) was/were placebo in seven studies (including saline), control (no fibrinogen or no early fibrinogen) in five studies, FFP in two studies, and plasma, prothrombin complex concentrate (PCC), and recombinant activated factor VII in one study each. Among all comparative studies, there was a similar number of AEs reported in the fibrinogen versus comparator groups (842 AEs among 1034 patients [0.81 AEs per patient] vs 849 among 1068 patients [0.79 AEs per patient]). Among 10 studies reporting statistical significance when comparing AEs in the fibrinogen groups versus the comparator, no significant difference was reported between groups in six studies. Pneumonia and multiorgan failure were significantly lower in the fibrinogen group versus other groups in one study, and death and sepsis were significantly lower in the fibrinogen group versus control in another study. There was significantly higher organ failure and multiple organ failure with fibrinogen versus the comparator in one study. The 30-day mortality was significantly higher in the fibrinogen group versus control in another study. Overall, similar TEEs were reported in the combined fibrinogen and comparison groups: 64 (corresponding to 0.06 TEEs per patient) versus 50 (0.05 TEEs per patient).47–68
A summary of studies in other settings is provided in Supplementary Table 3.
Discussion
Overall, the evaluation of over 35 years of RiaSTAP®/Haemocomplettan® P post-marketing data found 806 ADRs among 337 patients or one patient per 9243 g of fibrinogen concentrate administered, demonstrating a low rate of ADRs across multiple clinical settings. This was lower than the rate of ADRs found in the previous RiaSTAP®/Haemocomplettan® P safety review by Solomon et al (one patient per 5500 vs 6200 standard doses to the nearest 100). 19 With an additional 4,838,503 g of fibrinogen concentrate distributed (or nearly three times as much) and 22 additional randomized controlled trials retrieved from PubMed since the 2015 safety review by Solomon et al, the results of this analysis added weight to the findings of the previous review, demonstrating a robust safety profile of fibrinogen concentrate. The 2015 safety review described only two randomized controlled trials, in which 39 cardiac surgery patients were treated with fibrinogen concentrate. 19 The present study reports the data of 24 randomized controlled trials, in which a total of 946 patients were treated with fibrinogen concentrate across various surgical settings. 19
In line with the favorable safety profile demonstrated by this safety review, a study by Beyerle et al demonstrated a good safety profile and found no AEs and no influence on circulation, respiration, or hematological parameters in a range of animal models. 80 Findings from the literature review of a large up-to-date range of studies supported the findings of the post-marketing data.
Among the 337 patients with reported ADRs in the present evaluation, 81 (24.0%) experienced TEEs, equating to ∼1 patient per 22 993 of standard doses distributed. Fourteen (17.3%) of these patients experienced fatal TEEs. However, most had concomitant risk factors that provide alternative explanations. In the literature, the rate of ADRs, particularly TEEs, was generally low. The majority of studies reported no significant differences between the fibrinogen and control groups, including placebo, allogeneic blood products, cryoprecipitate, FFP, and PCC, where available. Nonetheless, a prospective non-randomized study reported a significantly higher rate of prolonged mechanical ventilation with fibrinogen versus control, which the authors attributed to the fibrinogen group being a ‘high-risk’ group, and a retrospective database study that reported a significantly higher organ failure and multiple organ failure in the fibrinogen group versus the control group, but significantly lower 6 h mortality with fibrinogen versus control.43,52 One study found a significantly lower mortality rate in the fibrinogen group versus FFP and control and a significantly lower rate of sepsis in the fibrinogen and control groups versus FFP. 47
Since the publication of the previous RiaSTAP®/Haemocomplettan® P safety review, the findings of several major clinical studies have been published, including the REPLACE, FiiRST, and FIBCON trials, each finding that RiaSTAP®/Haemocomplettan® P was associated with a low rate of ADRs.34,35,37,50 The REPLACE phase III, multinational, multi-center, randomized, double-blind, placebo-controlled study of elective aortic surgery patients requiring cardiopulmonary bypass found that the incidences of AEs in the fibrinogen concentrate and placebo groups were comparable, including treatment-emergent AEs and the type, severity, and outcome of TEEs.34,35 In the FiiRST single-center, randomized, controlled, double-blind feasibility trial, no statistically significant differences in the rate of deep vein thrombosis, pulmonary embolism, acute lung injury, acute respiratory distress syndrome, acute kidney injury, multiple organ failure/sepsis, and infection were reported in adult patients with severe trauma treated with fibrinogen concentrate versus cryoprecipitate. 50 The FIBCON study was a single-center, phase Ib/IIa, randomized controlled trial in infants undergoing cardiopulmonary bypass surgery. 37 Although more instances of possible thrombosis were reported in the fibrinogen concentrate group versus the placebo group (10 vs two events), potential alternative explanations were identified in all participants. 37 Furthermore, a 2020 meta-analysis of 13 randomized trials in postoperative blood loss in adult surgical patients reported no notable differences in AEs, including TEEs, with fibrinogen concentrate versus placebo. 81
The present safety review suggests a favorable safety profile of fibrinogen concentrate when compared to the safety of allogeneic blood products, such as FFP, cryoprecipitate, or platelets. Fibrinogen concentrate has a lower rate of viral transmission events versus cryoprecipitate, which is likely a result of several viral inactivation steps in the manufacturing of fibrinogen concentrate. 82 This is supported by the findings of the present safety review, which found no events of viral transmission likely to be related to fibrinogen concentrate using post-marketing surveillance data from the safety database. In a 2010 review, platelets were shown to have a less favorable safety profile than FFP, particularly in transfusion-associated acute lung injury (1/500 vs 1/60,000, respectively). 83 Furthermore, platelet transfusion has been shown to be associated with an increased risk of allergic reactions, transfusion-associated cardiac overload, acute respiratory distress syndrome, bacterial contamination, deep venous thromboembolism, and febrile reactions, none of which were reported as with fibrinogen concentrate in the present safety review, except for two reports of mild allergic reaction (rash) in a study included in the systematic literature review.84–86
The limitations of this analysis include the potential underreporting of ADRs as this is a voluntary process, and the provision of details required for assessment is not mandatory. Additionally, as ADRs are AEs that are considered to be possibly related to the study drug, they do not confirm a cause-and-effect relationship with treatment. This study only assesses post-marketing surveillance data and published literature on one fibrinogen concentrate (RiaSTAP®/Haemocomplettan® P); therefore, the findings might not be generalizable to other fibrinogen concentrates. Finally, the true incidence of TEE is not clear as the total number of patients who received fibrinogen concentrate is unknown. The subclinical incidence of TEE is also unknown due to most institutions not screening for these.
Conclusion
The results of the review of post-marketing surveillance data and data from a literature review demonstrate a low rate of ADRs, including TEEs, with fibrinogen concentrate treatment. This comprehensive review of safety data over 35 years validates the promising safety profile reported by Solomon et al in 2015.
Supplemental Material
Supplemental material, sj-docx-1-cat-10.1177_10760296241254106 for Long-Term Safety Analysis of a Fibrinogen Concentrate (RiaSTAP®/Haemocomplettan® P) by Niels Rahe-Meyer, Gabriele Neumann, Dirk S Schmidt and Laura A Downey in Clinical and Applied Thrombosis/Hemostasis
Acknowledgments
The authors would like to thank Joseph Whitten and Nataliya Doliba for their input during the development of this manuscript. All authors have read and approved the final manuscript and declare that all sources of financial support and possible conflicts of interest have been reported accurately. Medical writing support was provided by Bioscript Group (Macclesfield, UK) in accordance with Good Publication Practice guidelines and was funded by CSL Behring. The manuscript was also submitted by Bioscript Group, which was authorized by all authors.
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article. NRM has acted as a consultant for CSL Behring and Biotest and a principal investigator in a study by Biotest. GN and DSS are employees of CSL Behring. LAD received research support from the International Anesthesia Research Society.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by CSL Behring.
Supplemental Material: Supplemental material for this article is available online.
References
- 1.Kreuz W, Meili E, Peter-Salonen K, et al. Efficacy and tolerability of a pasteurised human fibrinogen concentrate in patients with congenital fibrinogen deficiency. Transfus Apher Sci. 2005;32(3):247‐253. [DOI] [PubMed] [Google Scholar]
- 2.Blome M, Isgro F, Kiessling AH, et al. Relationship between factor XIII activity, fibrinogen, haemostasis screening tests and postoperative bleeding in cardiopulmonary bypass surgery. Thromb Haemost. 2005;93(6):1101‐1107. [DOI] [PubMed] [Google Scholar]
- 3.Essa Y, Zeynalov N, Sandhaus T, Hofmann M, Lehmann T, Doenst T. Low fibrinogen is associated with increased bleeding-related re-exploration after cardiac surgery. Thorac Cardiovasc Surg. 2018;66(8):622‐628. [DOI] [PubMed] [Google Scholar]
- 4.Cortet M, Deneux-Tharaux C, Dupont C, et al. Association between fibrinogen level and severity of postpartum haemorrhage: secondary analysis of a prospective trial. Br J Anaesth. 2012;108(6):984‐989. [DOI] [PubMed] [Google Scholar]
- 5.Lier H, Krep H, Schroeder S, Stuber F. Preconditions of hemostasis in trauma: a review. The influence of acidosis, hypocalcemia, anemia, and hypothermia on functional hemostasis in trauma. J Trauma. 2008;65(4):951‐960. [DOI] [PubMed] [Google Scholar]
- 6.Peyvandi F. Epidemiology and treatment of congenital fibrinogen deficiency. Thromb Res. 2012;130(Suppl 2):S7‐11. [DOI] [PubMed] [Google Scholar]
- 7.Tziomalos K, Vakalopoulou S, Perifanis V, Garipidou V. Treatment of congenital fibrinogen deficiency: overview and recent findings. Vasc Health Risk Manag. 2009;5(5):843‐848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faraoni D, Meier J, New HV, Van der Linden PJ, Hunt BJ. Patient blood management for neonates and children undergoing cardiac surgery: 2019 NATA Guidelines. J Cardiothorac Vasc Anesth. 2019;33(12):3249‐3263. [DOI] [PubMed] [Google Scholar]
- 9.Rossaint R, Afshari A, Bouillon B, et al. The European guideline on management of major bleeding and coagulopathy following trauma: sixth edition. Crit Care. 2023;27(1):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kietaibl S, Ahmed A, Afshari A, et al. Management of severe peri-operative bleeding: guidelines from the European Society of Anaesthesiology and Intensive Care: second update 2022. Eur J Anaesthesiol. 2023;40(4):226‐304. doi: 10.1097/eja.0000000000001803 [DOI] [PubMed] [Google Scholar]
- 11.de Moerloose P, Neerman-Arbez M. Treatment of congenital fibrinogen disorders. Expert Opin Biol Ther. 2008;8(7):979‐992. [DOI] [PubMed] [Google Scholar]
- 12.O'Shaughnessy DF, Atterbury C, Bolton Maggs P, et al. Guidelines for the use of fresh-frozen plasma, cryoprecipitate and cryosupernatant. Br J Haematol. 2004;126(1):11‐28. [DOI] [PubMed] [Google Scholar]
- 13.Yang L, Stanworth S, Baglin T. Cryoprecipitate: an outmoded treatment? Transfus Med. 2012;22(5):315‐320. [DOI] [PubMed] [Google Scholar]
- 14.Kozek-Langenecker S, Sørensen B, Hess JR, Spahn DR. Clinical effectiveness of fresh frozen plasma compared with fibrinogen concentrate: a systematic review. Crit Care. 2011;15(5):R239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Narick C, Triulzi DJ, Yazer MH. Transfusion-associated circulatory overload after plasma transfusion. Transfusion. 2012;52(1):160‐165. [DOI] [PubMed] [Google Scholar]
- 16.Murad MH, Stubbs JR, Gandhi MJ, et al. The effect of plasma transfusion on morbidity and mortality: a systematic review and meta-analysis. Transfusion. 2010;50(6):1370‐1383. [DOI] [PubMed] [Google Scholar]
- 17.Khan H, Belsher J, Yilmaz M, et al. Fresh-frozen plasma and platelet transfusions are associated with development of acute lung injury in critically ill medical patients. Chest. 2007;131(5):1308‐1314. [DOI] [PubMed] [Google Scholar]
- 18.Franchini M, Lippi G. Fibrinogen replacement therapy: a critical review of the literature. Blood Transfus. 2012;10(1):23‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Solomon C, Gröner A, Ye J, Pendrak I. Safety of fibrinogen concentrate: analysis of more than 27 years of pharmacovigilance data. Thromb Haemost. 2015;113(4):759‐771. [DOI] [PubMed] [Google Scholar]
- 20.Erdoes G, Koster A, Meesters MI, et al. The role of fibrinogen and fibrinogen concentrate in cardiac surgery: an international consensus statement from the Haemostasis and Transfusion Scientific Subcommittee of the European Association of Cardiothoracic Anaesthesiology. Anaesthesia. 2019;74(12):1589‐1600. [DOI] [PubMed] [Google Scholar]
- 21.Spahn DR, Bouillon B, Cerny V, et al. The European guideline on management of major bleeding and coagulopathy following trauma: fifth edition. Crit Care. 2019;23(1):98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velik-Salchner C, Sergi C, Fries D, Moser P, Streif W, Kolbitsch C. Use of recombinant factor VIIa (Novoseven) in combination with other coagulation products led to a thrombotic occlusion of the truncus brachiocephalicus in a neonate supported by extracorporal membrane oxygenation. Anesth Analg. 2005;101(3):924. [DOI] [PubMed] [Google Scholar]
- 23.Weiss G, Lison S, Glaser M, et al. Observational study of fibrinogen concentrate in massive hemorrhage: evaluation of a multicenter register. Blood Coagul Fibrinolysis. 2011;22(8):727‐734. [DOI] [PubMed] [Google Scholar]
- 24.Vidmar J, Serša I, Kralj E, Popovič P. Unsuccessful percutaneous mechanical thrombectomy in fibrin-rich high-risk pulmonary thromboembolism. Thromb J. 2015;13(8):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taslimi R, Golshani K. Thrombotic and hemorrhagic presentation of congenital hypo/afibrinogenemia. Am J Emerg Med. 2011;29(5):573.e3‐5. [DOI] [PubMed] [Google Scholar]
- 26.Bilecen S, de Groot JA, Kalkman CJ, et al. Effect of fibrinogen concentrate on intraoperative blood loss among patients with intraoperative bleeding during high-risk cardiac surgery: a randomized clinical trial. JAMA. 2017;317(7):738‐747. [DOI] [PubMed] [Google Scholar]
- 27.Gollop ND, Chilcott J, Benton A, Rayment R, Jones J, Collins PW. National audit of the use of fibrinogen concentrate to correct hypofibrinogenaemia. Transfus Med. 2012;22(5):350‐355. [DOI] [PubMed] [Google Scholar]
- 28.Karlsson M, Ternström L, Hyllner M, et al. Prophylactic fibrinogen infusion reduces bleeding after coronary artery bypass surgery. A prospective randomised pilot study. Thromb Haemost. 2009;102(1):137‐144. [DOI] [PubMed] [Google Scholar]
- 29.Rahe-Meyer N, Solomon C, Hanke A, et al. Effects of fibrinogen concentrate as first-line therapy during major aortic replacement surgery: a randomized, placebo-controlled trial. Anesthesiology. 2013;118(1):40‐50. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka KA, Egan K, Szlam F, et al. Transfusion and hematologic variables after fibrinogen or platelet transfusion in valve replacement surgery: preliminary data of purified lyophilized human fibrinogen concentrate versus conventional transfusion. Transfusion. 2014;54(1):109‐118. [DOI] [PubMed] [Google Scholar]
- 31.Downey LA, Andrews J, Hedlin H, et al. Fibrinogen concentrate as an alternative to cryoprecipitate in a postcardiopulmonary transfusion algorithm in infants undergoing cardiac surgery: a prospective randomized controlled trial. Anesth Analg. 2020;130(3):740‐751. [DOI] [PubMed] [Google Scholar]
- 32.Galas FR, de Almeida JP, Fukushima JT, et al. Hemostatic effects of fibrinogen concentrate compared with cryoprecipitate in children after cardiac surgery: a randomized pilot trial. J Thorac Cardiovasc Surg. 2014;148(4):1647‐1655. [DOI] [PubMed] [Google Scholar]
- 33.Jeppsson A, Waldén K, Roman-Emanuel C, Thimour-Bergström L, Karlsson M. Preoperative supplementation with fibrinogen concentrate in cardiac surgery: a randomized controlled study. Br J Anaesth. 2016;116(2):208‐214. [DOI] [PubMed] [Google Scholar]
- 34.Rahe-Meyer N, Levy JH, Mazer CD, et al. Randomized evaluation of fibrinogen vs placebo in complex cardiovascular surgery (REPLACE): a double-blind phase III study of haemostatic therapy. Br J Anaesth. 2016;117(1):41‐51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rahe-Meyer N, Levy JH, Ueda Y, Schmidt DS, Gill R. Viscoelastic testing to assess the effects of rapid fibrinogen concentrate administration after cardiopulmonary bypass: insights from the REPLACE study. Blood Coagul Fibrinolysis. 2021;32(6):359‐365. [DOI] [PubMed] [Google Scholar]
- 36.Ranucci M, Baryshnikova E, Crapelli GB, Rahe-Meyer N, Menicanti L, Frigiola A. Randomized, double-blinded, placebo-controlled trial of fibrinogen concentrate supplementation after complex cardiac surgery. J Am Heart Assoc. 2015;4(6):e002066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Siemens K, Hunt BJ, Harris J, Nyman AG, Parmar K, Tibby SM. Individualized, intraoperative dosing of fibrinogen concentrate for the prevention of bleeding in neonatal and infant cardiac surgery using cardiopulmonary bypass (FIBCON): a phase 1b/2a randomized controlled trial. Circ Cardiovasc Interv. 2020;13(12):e009465. [DOI] [PubMed] [Google Scholar]
- 38.Tirotta CF, Lagueruela RG, Gupta A, et al. A randomized pilot trial assessing the role of human fibrinogen concentrate in decreasing cryoprecipitate use and blood loss in infants undergoing cardiopulmonary bypass. Pediatr Cardiol. 2022;43(7):1444‐1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jahangirifard A, Ahmadi ZH, Naghashzadeh F, et al. Prophylactic fibrinogen decreases postoperative bleeding but not acute kidney injury in patients undergoing heart transplantation. Clin Appl Thromb Hemost. 2018;24(6):998‐1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rahe-Meyer N, Pichlmaier M, Haverich A, et al. Bleeding management with fibrinogen concentrate targeting a high-normal plasma fibrinogen level: a pilot study. Br J Anaesth. 2009;102(6):785‐792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rahe-Meyer N, Solomon C, Winterhalter M, et al. Thromboelastometry-guided administration of fibrinogen concentrate for the treatment of excessive intraoperative bleeding in thoracoabdominal aortic aneurysm surgery. J Thorac Cardiovasc Surg. 2009;138(3):694‐702. [DOI] [PubMed] [Google Scholar]
- 42.Solomon C, Schöchl H, Hanke A, et al. Haemostatic therapy in coronary artery bypass graft patients with decreased platelet function: comparison of fibrinogen concentrate with allogeneic blood products. Scand J Clin Lab Invest. 2012;72(2):121‐128. [DOI] [PubMed] [Google Scholar]
- 43.Bilecen S, Peelen LM, Kalkman CJ, Spanjersberg AJ, Moons KG, Nierich AP. Fibrinogen concentrate therapy in complex cardiac surgery. J Cardiothorac Vasc Anesth. 2013;27(1):12‐17. [DOI] [PubMed] [Google Scholar]
- 44.Waldén K, Jeppsson A, Nasic S, Karlsson M. Fibrinogen concentrate to cardiac surgery patients with ongoing bleeding does not increase the risk of thromboembolic complications or death. Thromb Haemost. 2020;120(3):384‐391. [DOI] [PubMed] [Google Scholar]
- 45.Hanna JM, Keenan JE, Wang H, et al. Use of human fibrinogen concentrate during proximal aortic reconstruction with deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2016;151(2):376‐382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Solomon C, Pichlmaier U, Schoechl H, et al. Recovery of fibrinogen after administration of fibrinogen concentrate to patients with severe bleeding after cardiopulmonary bypass surgery. Br J Anaesth. 2010;104(5):555‐562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Akbari E, Safari S, Hatamabadi H. The effect of fibrinogen concentrate and fresh frozen plasma on the outcome of patients with acute traumatic coagulopathy: a quasi-experimental study. Am J Emerg Med. 2018;36(11):1947‐1950. [DOI] [PubMed] [Google Scholar]
- 48.Curry N, Foley C, Wong H, et al. Early fibrinogen concentrate therapy for major haemorrhage in trauma (E-FIT 1): results from a UK multi-centre, randomised, double blind, placebo-controlled pilot trial. Crit Care. 2018;22(1):164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Innerhofer P, Fries D, Mittermayr M, et al. Reversal of trauma-induced coagulopathy using first-line coagulation factor concentrates or fresh frozen plasma (RETIC): a single-centre, parallel-group, open-label, randomised trial. Lancet Haematol. 2017;4(6):e258‐e271. [DOI] [PubMed] [Google Scholar]
- 50.Nascimento B, Callum J, Tien H, et al. Fibrinogen in the initial resuscitation of severe trauma (FiiRST): a randomized feasibility trial. Br J Anaesth. 2016;117(6):775‐782. [DOI] [PubMed] [Google Scholar]
- 51.Lucena LS, Rodrigues RDR, Carmona MJC, et al. Early administration of fibrinogen concentrate in patients with polytrauma with thromboelastometry suggestive of hypofibrinogenemia: a randomized feasibility trial. Clinics (Sao Paulo). 2021;76:e3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wafaisade A, Lefering R, Maegele M, et al. Administration of fibrinogen concentrate in exsanguinating trauma patients is associated with improved survival at 6 h but not at discharge. J Trauma Acute Care Surg. 2013;74(2):387‐383. discussion 393-5. [DOI] [PubMed] [Google Scholar]
- 53.Almskog LM, Hammar U, Wikman A, et al. A retrospective register study comparing fibrinogen treated trauma patients with an injury severity score matched control group. Scand J Trauma Resusc Emerg Med. 2020;28(1):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Barquero López M, Martínez Cabañero J, Muñoz Valencia A, et al. Dynamic use of fibrinogen under viscoelastic assessment results in reduced need for plasma and diminished overall transfusion requirements in severe trauma. J Trauma Acute Care Surg. 2022;93(2):166‐175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fathi M, Lashay A, Massoudi N, Nooraei N, Nik MA. Fibrinogen prophylaxis for reducing perioperative bleeding in patients undergoing radical cystectomy: a double-blind placebo-controlled randomized trial. J Clin Anesth. 2021;73:110373. [DOI] [PubMed] [Google Scholar]
- 56.Najafi A, Shariat Moharari R, Orandi AA, et al. Prophylactic administration of fibrinogen concentrate in perioperative period of total hip arthroplasty: a randomized clinical trial study. Acta Med Iran. 2014;52(11):804‐810. [PubMed] [Google Scholar]
- 57.Soleimani M, Masoumi N, Nooraei N, Lashay A, Safarinejad MR. The effect of fibrinogen concentrate on perioperative bleeding in transurethral resection of the prostate: a double-blind placebo-controlled and randomized study. J Thromb Haemost. 2017;15(2):255‐262. [DOI] [PubMed] [Google Scholar]
- 58.Machotta A, Huisman EJ, Appel IM, et al. Prophylactic fibrinogen concentrate administration in surgical correction of paediatric craniosynostosis: a double-blind placebo-controlled trial. Eur J Anaesthesiol. 2021;38(9):908‐915. [DOI] [PubMed] [Google Scholar]
- 59.Haas T, Spielmann N, Restin T, et al. Higher fibrinogen concentrations for reduction of transfusion requirements during major paediatric surgery: a prospective randomised controlled trial. Br J Anaesth. 2015;115(2):234‐243. [DOI] [PubMed] [Google Scholar]
- 60.Haas T, Fries D, Velik-Salchner C, Oswald E, Innerhofer P. Fibrinogen in craniosynostosis surgery. Anesth Analg. 2008;106(3):725‐731. [DOI] [PubMed] [Google Scholar]
- 61.Sabate A, Gutierrez R, Beltran J, et al. Impact of preemptive fibrinogen concentrate on transfusion requirements in liver transplantation: a multicenter, randomized, double-blind, placebo-controlled trial. Am J Transplant. 2016;16(8):2421‐2429. [DOI] [PubMed] [Google Scholar]
- 62.Sabouri M, Vahidian M, Sourani A, Mahdavi SB, Tehrani DS, Shafiei E. Efficacy and safety of fibrinogen administration in acute post-traumatic hypofibrinogenemia in isolated severe traumatic brain injury: a randomized clinical trial. J Clin Neurosci. 2022;101:204‐211. [DOI] [PubMed] [Google Scholar]
- 63.Leal-Noval SR, Casado M, Arellano-Orden V, et al. Administration of fibrinogen concentrate for refractory bleeding in massively transfused, non-trauma patients with coagulopathy: a retrospective study with comparator group. BMC Anesthesiol. 2014;14:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vigstedt M, Henriksen HH, Chaachouh HW, Stensballe J, Johansson PI. Real-life experiences with goal-directed prohemostatic therapy with fibrinogen concentrate, prothrombin complex concentrate, and recombinant factor VIIa: a retrospective study of 287 consecutive patients. Scand J Clin Lab Invest. 2022;82(2):156‐161. [DOI] [PubMed] [Google Scholar]
- 65.Danés AF, Cuenca LG, Bueno SR, Mendarte Barrenechea L, Ronsano JB. Efficacy and tolerability of human fibrinogen concentrate administration to patients with acquired fibrinogen deficiency and active or in high-risk severe bleeding. Vox Sang. 2008;94(3):221‐226. [DOI] [PubMed] [Google Scholar]
- 66.Weinkove R, Rangarajan S. Fibrinogen concentrate for acquired hypofibrinogenaemic states. Transfus Med. 2008;18(3):151‐157. [DOI] [PubMed] [Google Scholar]
- 67.Fenger-Eriksen C, Lindberg-Larsen M, Christensen AQ, Ingerslev J, Sørensen B. Fibrinogen concentrate substitution therapy in patients with massive haemorrhage and low plasma fibrinogen concentrations. Br J Anaesth. 2008;101(6):769‐773. [DOI] [PubMed] [Google Scholar]
- 68.Thorarinsdottir HR, Sigurbjornsson FT, Hreinsson K, Onundarson PT, Gudbjartsson T, Sigurdsson GH. Effects of fibrinogen concentrate administration during severe hemorrhage. Acta Anaesthesiol Scand. 2010;54(9):1077‐1082. [DOI] [PubMed] [Google Scholar]
- 69.Ahmed S, Harrity C, Johnson S, et al. The efficacy of fibrinogen concentrate compared with cryoprecipitate in major obstetric haemorrhage—an observational study. Transfus Med. 2012;22(5):344‐349. [DOI] [PubMed] [Google Scholar]
- 70.Kreuz W, Meili E, Peter-Salonen K, et al. Pharmacokinetic properties of a pasteurised fibrinogen concentrate. Transfus Apher Sci. 2005;32(3):239‐246. [DOI] [PubMed] [Google Scholar]
- 71.Manco-Johnson MJ, Dimichele D, Castaman G, et al. Pharmacokinetics and safety of fibrinogen concentrate. J Thromb Haemost. 2009;7(12):2064‐2069. [DOI] [PubMed] [Google Scholar]
- 72.Ross C, Rangarajan S, Karimi M, et al. Pharmacokinetics, clot strength and safety of a new fibrinogen concentrate: randomized comparison with active control in congenital fibrinogen deficiency. J Thromb Haemost. 2018;16(2):253‐261. [DOI] [PubMed] [Google Scholar]
- 73.Collins PW, Cannings-John R, Bruynseels D, et al. Viscoelastometric-guided early fibrinogen concentrate replacement during postpartum haemorrhage: OBS2, a double-blind randomized controlled trial. Br J Anaesth. 2017;119(3):411‐421. [DOI] [PubMed] [Google Scholar]
- 74.Vandelli L, Marietta M, Trenti T, et al. Fibrinogen concentrate replacement in ischemic stroke patients after recombinant tissue plasminogen activator treatment. Adv Clin Exp Med. 2019;28(2):219‐222. [DOI] [PubMed] [Google Scholar]
- 75.Wikkelsø AJ, Edwards HM, Afshari A, et al. Pre-emptive treatment with fibrinogen concentrate for postpartum haemorrhage: randomized controlled trial. Br J Anaesth. 2015;114(4):623‐633. [DOI] [PubMed] [Google Scholar]
- 76.Lasky J, Teitel J, Wang M, Dalton D, Schmidt DS, Brainsky A. Fibrinogen concentrate for bleeding in patients with congenital fibrinogen deficiency: observational study of efficacy and safety for prophylaxis and treatment. Res Pract Thromb Haemost. 2020;4(8):1313‐1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Barra ME, Feske SK, Sylvester KW, et al. Fibrinogen concentrate for the treatment of thrombolysis-associated hemorrhage in adult ischemic stroke patients. Clin Appl Thromb Hemost. 2020;26:1076029620951867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Giordano P, Grassi M, Saracco P, et al. Human fibrinogen concentrate and fresh frozen plasma in the management of severe acquired hypofibrinogenemia in children with acute lymphoblastic leukemia: results of a retrospective survey. J Pediatr Hematol Oncol. 2019;41(4):275‐279. [DOI] [PubMed] [Google Scholar]
- 79.Giordano P, Luciani M, Grassi M, De Leonardis F, Coletti V, Santoro N. Supplementation of fibrinogen concentrate in children with severe acquired hypofibrinogenaemia during chemotherapy for acute lymphoblastic leukaemia: our experience. Blood Transfus. 2014;12(Suppl 1):s156‐s157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Beyerle A, Nolte MW, Solomon C, Herzog E, Dickneite G. Analysis of the safety and pharmacodynamics of human fibrinogen concentrate in animals. Toxicol Appl Pharmacol. 2014;280(1):70‐77. [DOI] [PubMed] [Google Scholar]
- 81.Ng KT, Yap JLL, Kwok PE. The effect of fibrinogen concentrate on postoperative blood loss: a systematic review and meta-analysis of randomized controlled trials. J Clin Anesth. 2020;63:109782. [DOI] [PubMed] [Google Scholar]
- 82.Nascimento B, Goodnough LT, Levy JH. Cryoprecipitate therapy. Br J Anaesth. 2014;113(6):922‐934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Holcomb JB, Spinella PC. Optimal use of blood in trauma patients. Biologicals. 2010;38(1):72‐77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gajic O, Dzik WH, Toy P. Fresh frozen plasma and platelet transfusion for nonbleeding patients in the intensive care unit: benefit or harm? Crit Care Med. 2006;34(5 Suppl):S170‐S173. [DOI] [PubMed] [Google Scholar]
- 85.Silliman CC, Ambruso DR, Boshkov LK. Transfusion-related acute lung injury. Blood. 2005;105(6):2266‐2273. [DOI] [PubMed] [Google Scholar]
- 86.MacLennan S, Williamson LM. Risks of fresh frozen plasma and platelets. J Trauma. 2006;60(6 Suppl):S46‐S50. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-cat-10.1177_10760296241254106 for Long-Term Safety Analysis of a Fibrinogen Concentrate (RiaSTAP®/Haemocomplettan® P) by Niels Rahe-Meyer, Gabriele Neumann, Dirk S Schmidt and Laura A Downey in Clinical and Applied Thrombosis/Hemostasis