Abstract
The heat resistance of spores of Bacillus subtilis formed at 30°C was enhanced by pretreatment at 48°C for 30 min, 60 min into sporulation, for all four strains examined. High-resolution two-dimensional gel electrophoresis showed the generation and/or overexpression of 60 proteins, 11 of which were specific to heat shock, concurrent to this acquired thermotolerance. The greatest number of new proteins was observed between 30 and 60 min after heat shock, and the longer the time between exponential growth and heat treatment, the fewer differences were observed on corresponding protein profiles. The time at which heating produced the maximum increase in spore resistance and the most new proteins on two-dimensional gels occurred before alkaline phosphatase and dipicolinic acid production and corresponded to stage I or II of sporulation. The stress proteins formed disappeared later in sporulation, suggesting that heat shock proteins increase spore heat resistance by altering spore structure rather than by repairing heat damage during germination and outgrowth.
Soil bacteria, such as the gram-positive bacterium Bacillus subtilis, are frequently faced with various adverse environmental conditions and have developed a complex regulatory network to respond rapidly to environmental changes in temperature, humidity, or nutrient source availability. The adaptational network of B. subtilis involves the induction of stress proteins (2, 8) and the production of small acid-soluble proteins (SASP) (4). The stress proteins can be divided, according to their induction pattern, into general stress proteins and stress-specific proteins (2, 8). The former are induced in response to a wide range of stimuli and provide a nonspecific protective function regardless of the stress type, whereas the latter provide a protective response against a single stress or a group of related stresses. SASP are synthesized as sporulating cells become heat resistant and constitute 10 to 20% of the total spore protein. The two main types of SASP are α and β, and they are thought to confer a high degree of resistance to spores by binding to spore DNA and altering its configuration from the B-type helix to the more stable A-type helix (19). Spores that are deficient in SASP are more sensitive to a variety of adverse stimuli and have decreased longevity compared to wild-type spores (5).
Heat inducible thermotolerance, a phenomenon whereby cell survival at lethal temperatures is significantly enhanced by a short pretreatment at sublethal temperatures, has been described for several eukaryotic and prokaryotic organisms (6). Many genes are turned on in response to heat shock, which increases the cell's ability to deal with an increase in the amount of denatured proteins by either refolding or degrading them (13). In a preliminary study Todd et al. (20) showed that heat shocking sporulating cells of B. subtilis produced stress response proteins. More-extensive studies with Bacillus megaterium and Clostridium perfringens showed that by applying heat shock it was possible to increase the heat resistance of the spores formed subsequently (9, 18) and that, for B. megaterium, heat shock proteins were induced in parallel. If there is a positive correlation between the induction of heat shock proteins in sporulating cells and the heat resistance of the spores formed subsequently, identifying these proteins would allow ascertainment of the sites at which these proteins act and would allow a better understanding of the mechanisms of heat resistance of cells. In this study the heat resistance of wild-type B. subtilis spores, as well as SASP− spores, which are significantly more heat sensitive than wild-type spores, was investigated and compared to that of a sigB mutant which lacks a number of general stress proteins (21). The induction of stress proteins in vegetative cells of B. subtilis has been extensively studied (2, 21); this is the first study where the induction of stress proteins in sporulating cells of B. subtilis has been assessed and correlated with the heat resistance of spores formed subsequently.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The bacterial strains used were wild-type B. subtilis 168 (trpC2) (1) and its isogenic sigB mutant strain ML6 (trpC2 sigB::ΔHindIII-EcoRV::cat) carrying chloramphenicol resistance as described previously (10), as well as B. subtilis PS 346 (sspA::lacZ trpC2) and PS 361 (sspA::lacZ ΔsspA ΔsspB trpC2) (3). The latter two strains are also derivatives of B. subtilis 168 carrying chloramphenicol resistance. Strain PS 346 (wild-type; α+β+) has the ability to produce α- and β-SASP. Strain PS 361 (α−β−) is a mutant of strain PS 346 and lacks the ability to produce α- and β-SASP (12).
All strains were grown at 30°C in 2× SG medium (11) supplemented with 3 μg of chloramphenicol/ml. Growth was monitored by determination of optical density at 600 nm. Sporulation was induced by nutrient exhaustion in 2× SG sporulation medium. Spores were harvested after 24 to 48 h of growth and were purified by repeated centrifugation and washing with reverse osmosis water (12). All spore preparations used were refractile and free (>95%) of sporulating cells, cell debris, and germinated spores, as determined with a phase-contrast microscope, and were stored at 4°C in reverse osmosis water.
To identify the growth stage of each culture, the notation Tn was used, where n is the time in hours elapsed after the end of the exponential phase.
Stress induction.
For protein analysis, samples were taken during sporulation immediately prior to imposition of stress or at the time indicated in the relevant figures. The remaining part of the culture was incubated at 30°C until sporulation was completed and spores were released. Different stress conditions were imposed for 30 min according to the following procedures: for heat shock the culture was transferred from 30 to 48°C; for cold shock the culture was transferred from 30 to 10°C; for glucose starvation the culture was transferred to 2× SG medium containing 0.05% (wt/vol) glucose.
Assessment of spore heat resistance.
Spore wet heat resistance was assessed by incubating purified spores in water in thin-walled capillary tubes (Sigma C-6148) at temperatures of 85 to 100°C and plating serial dilutions of unheated and heated spores on 2× SG plates (1.5% [wt/vol] agar) at 30°C for 24 h. Spores were heated at 60°C for 10 min before plating out to kill any remaining vegetative cells. Spore heat resistance was expressed as percent survivors because stressed cells produced spore populations with extended “shoulders” and “tails” or as a D value. The D value is the time needed at a given temperature for a 10-fold reduction in spore viability. D values were calculated as the negative reciprocal of the slope of the regression line plotted with the values of the straight portions of thermal death curves (log survivors versus heating time).
Preparation of protein samples.
Samples were taken at intervals after heat shock, and crude cell extracts were prepared using the protocol of Schmid et al. (16). Cells were harvested by centrifugation (4°C, 18,000 × g, 10 min) and washed three times in 0.1 M Tris-HCl–1 mM EDTA, pH 7.5, and the pellet was resuspended in 1 ml of disruption buffer (10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 2 mM phenylmethylsulfonyl fluoride). Cells were disrupted using a mini-bead beater and 0.1-mm Zirconia beads (both Biospec Products) for up to 10 cycles of 1 min each on ice, and the cell debris was removed by centrifugation (4°C, 42,000 × g, 10 min).
Two-dimensional gel electrophoresis.
The proteins present in the sporulating B. subtilis cells were resolved on two-dimensional gels using the products and protocols of Amersham Pharmacia Biotech (Uppsala, Sweden). Proteins were resolved by isoelectric focusing on a precast Immobiline DryStrip with a linear pH gradient (pH 3 to 10 or pH 4 to 7) followed by sodium dodecyl sulfate polyacrylamide gel electrophoresis on 12.5% acrylamide gels for the second dimension. For analytical gels 100 μg of crude cell extract was applied to gels and proteins were stained with a Pharmacia Biotech silver stain kit. For preparative two-dimensional protein gel electrophoresis 500 μg of the crude protein extract was separated and proteins were visualized using Coomassie blue R-350 (Phast Gel BlueR; Amersham Pharmacia Biotech).
Computer-aided analysis of the two-dimensional gels.
Images of the gels were captured using a Sharp JX-330 flat-bed scanner, and image analysis of the protein profiles was performed using Amersham Pharmacia Biotech ImageMaster 2-D Elite software. The relative amount of each protein spot was calculated and expressed by the software as the percentage of the spot volume and represented the intensity of each individual spot compared to the intensity of the whole gel.
Western blotting and N-terminal amino acid sequencing.
For the analysis of the N-terminal protein sequences Coomassie-stained protein spots were excised from the preparative two-dimensional gels, pooled, concentrated according to the protocol of Rider et al. (14), and transferred onto a polyvinylidene difluoride membrane (Immobilon P; Millipore) by semidry, discontinuous horizontal electroblotting for 1 h at 0.8 mA/cm2. The proteins were stained with Coomassie blue R-350 and sequenced on an Applied Biosystems A473a protein sequencer.
Miscellaneous assays.
Protein was quantified by the Bradford method using the Bio-Rad protein assay kit and bovine serum albumin as a standard. Alkaline phosphatase activity was measured using the hydrolysis of 4-nitrophenyl phosphate at pH 8.5 and 37°C at 405 nm. Spores and sporulating cells were extracted and analyzed for dipicolinic acid (DPA) colorimetrically at 440 nm as described previously (15).
RESULTS
Spore heat resistance.
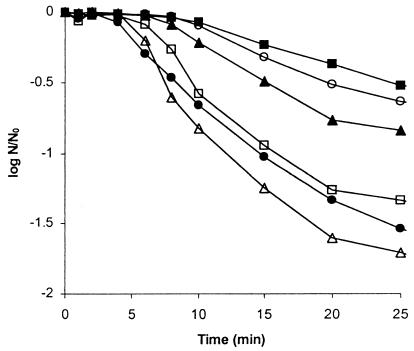
The effect of sublethal heating of sporulating cells of B. subtilis on the heat resistance of wild-type and mutant spores formed subsequently was assessed. The culture was treated by a heat shock of 48°C for 30 min applied at T0, T0.5, T1, T1.5, and T2.0, i.e., immediately after the end of the exponential phase and at 30-min intervals thereafter. The heat-shocked cells were transferred to 30°C and incubated until sporulation was completed and free spores were released. If heat shock was applied immediately after the cessation of exponential growth, i.e., at T0, there was no increase in the heat resistance of spores formed subsequently. However, spores that developed in cells treated by heat shock at T0.5, T1, T1.5, and T2.0 showed increased heat resistance compared to that of the control spores, with the greatest difference observed in cells treated by heat shock at T1 (Fig. 1). Similar results were obtained for all four strains. Increased heat resistance was also shown by comparison of D values, although heat-shocked cells also produced spores with extended shoulders and tails in their kill curves (data not shown). In all ensuing experiments, heat shock was applied 1 h after the cessation of exponential growth, i.e., at T1.
FIG. 1.
Heat resistance of spores of B. subtilis PS 346 at 90°C. B. subtilis strain PS 346 cultures were untreated control cells (●) or cells treated by 30 min of heat shock applied at T0 (□), T0.5 (○), T1 (■), T1.5 (▴), and T2.0 (▵). The heat-shocked cells were transferred to 30°C and incubated until sporulation was completed and free spores were released. The spores were then assessed for heat resistance at 90°C for various periods of time, and the viability of the spores was expressed as N/N0 where N is the number of viable cells at time t and N0 is the number of viable cells at time zero. Error bars have been omitted for clarity, but the standard deviation was always ≤10% of the mean.
When B. subtilis cells were subjected to a pretreatment heat shock (from 30 to 48°C for 30 min 60 min into sporulation), the spores formed subsequently acquired a significant increase in thermotolerance to 85, 90, 95, and 100°C compared to control spores that had not been produced as a result of heat shock (Table 1). Heating sporulating cells of the SASP− mutant or sigB mutant increased resistance to heat at 100°C by threefold, whereas heating the more resistant wild-type strain 168 or SASP+ strain PS 346 spores at 100°C resulted in a twofold increase in D value.
TABLE 1.
Comparison of D values for heat-shocked spores of B. subtilisa
| Temp (°C) | Mean Db (min) ± SD for:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| Wild-type PS 346
(SASP+)
|
Mutant PS 361
(SASP−)
|
Wild-type
168
|
sigB mutant
|
|||||
| Control | Heat shocked | Control | Heat shocked | Control | Heat shocked | Control | Heat shocked | |
| 85 | 25 ± 4 | 36 ± 5 | 8 ± 1 | 16 ± 2 | 20 ± 2 | 22 ± 2 | 18 ± 1 | 21 ± 2 |
| 90 | 15 ± 2 | 33 ± 4 | 4 ± 1 | 12 ± 1 | 11 ± 1 | 15 ± 1 | 9 ± 0.6 | 12 ± 1 |
| 95 | 9 ± 1 | 20 ± 3 | 3 ± 0.5 | 10 ± 0.7 | 6 ± 0.5 | 10 ± 0.8 | 5 ± 0.6 | 8 ± 0.6 |
| 100 | 5 ± 0.3 | 11 ± 2 | 2 ± 0.3 | 8 ± 0.9 | 4 ± 0.4 | 8 ± 1 | 3 ± 0.5 | 10 ± 1 |
The effect of sublethal heat pretreatment (heat shock) of sporulating cells of B. subtilis on the heat resistance of wild-type and mutant spores formed subsequently was assessed at 85, 90, 95, and 100°C. The heat resistance of spores formed from pretreated cells was compared to that of spores formed from control unpretreated cells. The results are from two independent experiments.
D (decimal reduction time), time at a given temperature needed to kill 90% of a cell or spore population.
D values were obtained by calculating the reciprocal gradient of the straight-line portion of the graph, ignoring the shoulder and the tail of the graph. Heating spores produced by heat-shocked cells at lower temperatures (85, 90, and 95°C) resulted in an elevation in D values smaller than that for control spores.
Generation of heat stress proteins.
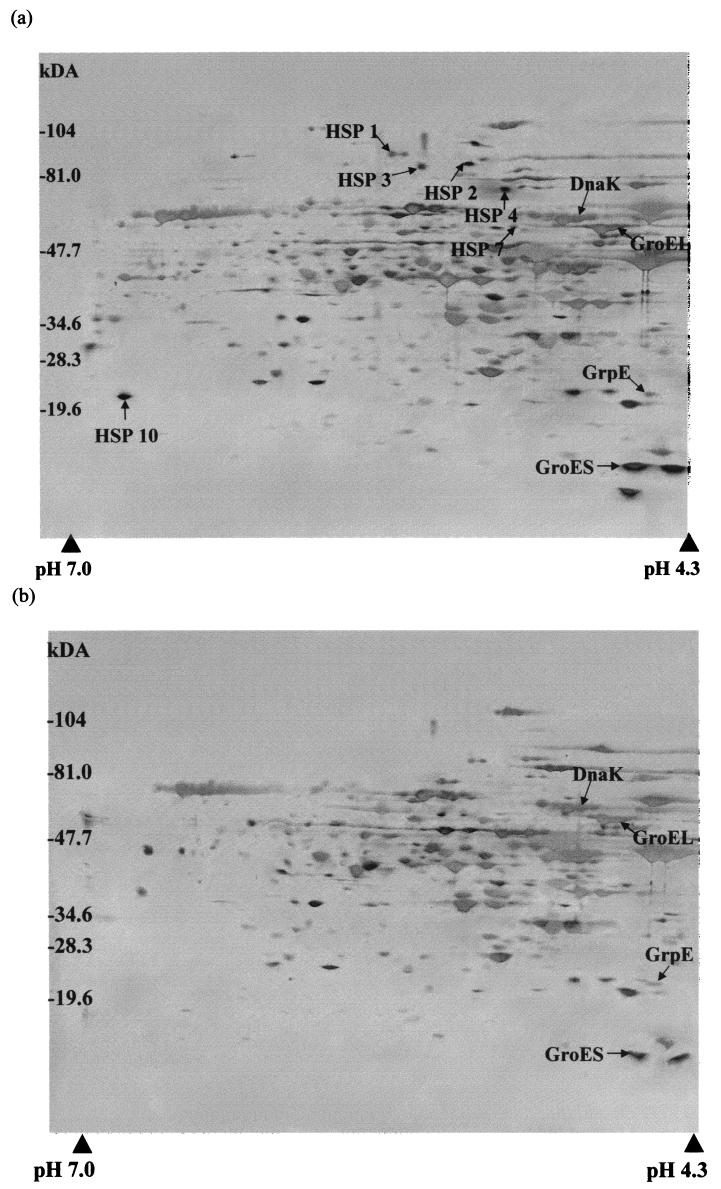
The effect of heat shock on the pattern of protein synthesis in sporulating cells of B. subtilis was investigated using high-resolution two-dimensional gel electrophoresis. Approximately 60 proteins were present in increased amounts or were newly synthesized after heat shock (Fig. 2). Of these, 11 were designated heat-specific stress proteins because they were either synthesized de novo (proteins 1 to 4, 7, 8, and 10) or overexpressed (proteins 5, 6, 9, and 11) in sporulating B. subtilis cells of all four strains specifically in response to heat shock but not cold shock or glucose starvation. None of these proteins were sigB dependent as they were also produced in the sigB mutant in response to heat shock. Five of the proteins have been identified by microsequence analysis and comparison with protein databases to be DnaK, GroEL, DnaJ, GrpE, and GroES. The same heat-specific proteins were induced in response to heat shock in all the strains tested, but the level of induction varied with the strain and was most pronounced with strain PS 346. The magnitude of the induction of heat shock proteins varied by up to threefold in these different strains.
FIG. 2.
Two-dimensional protein profile of the proteins in wild-type B. subtilis PS 346 cells. Bacteria were grown, and protein samples were taken from heat-shocked sporulating cells 30 min after application of heat shock (a) or from untreated control sporulating cells (b). Molecular masses are shown on the left. Arrows, heat-specific stress proteins. The identities of the proteins were determined by comparison of the N-terminal sequences with those in protein databases (SWISS-PROT and SubtiList). The protein samples were resolved on a pH 4 to 7 linear pH gradient in the first dimension. DnaJ (pI 7.2) was identified as a heat-specific stress protein on two-dimensional gels with a pH 3 to 10 linear pH gradient in the first dimension.
Temporal correlation of stress proteins to sporulation phase.
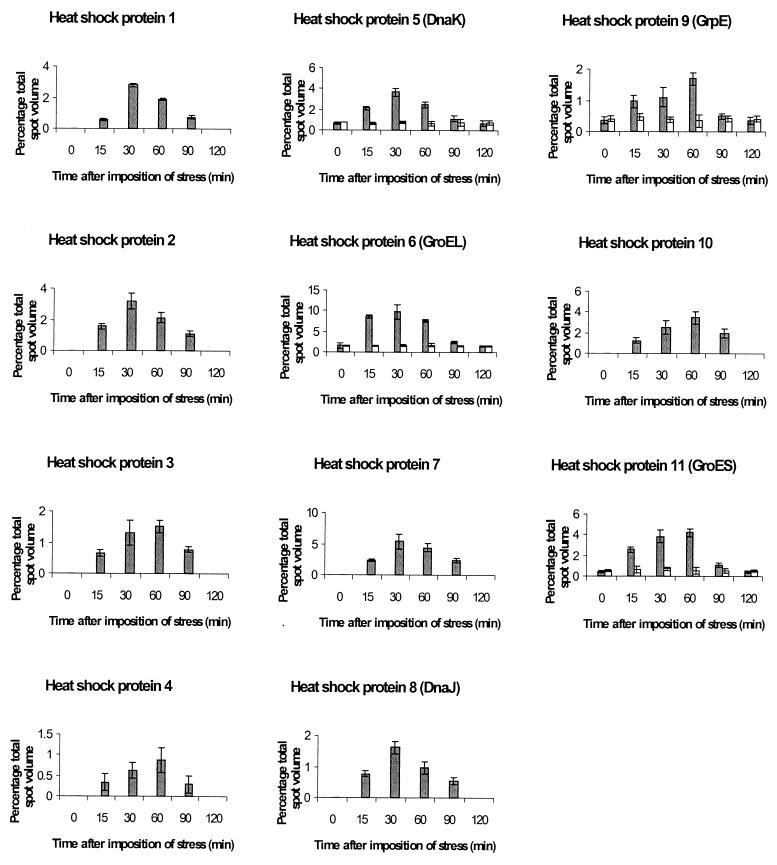
Time course studies showed that the heat shock proteins were induced within 15 min after heat shock and that the biochemical response was maintained for at least 60 min, with the maximum increase in new proteins apparent 30 to 60 min after heat shock (Fig. 3). By 120 min after heating the heat shock proteins had disappeared or returned to their original levels. Alkaline phosphatase, DPA, and transmission electron microscopy studies were performed on sporulating cells of B. subtilis in order to correlate the time of synthesis or overexpression of stress proteins to sporulation stage. According to electron-microscopical analysis of ultrathin sections, the cells at T1 and T2 had not yet developed forespores. By T3 clear septum formation was apparent in the majority of the cells, corresponding to stage II of sporulation, and forespore engulfment was observed in some cells. At T5 cells had developed fully engulfed forespores and alkaline phosphatase activity had reached a peak, corresponding to stage III of sporulation. Maximum DPA content was detected 9 h after the end of exponential phase, coinciding with the appearance of the first refractile spores and corresponding to stage V of sporulation. The period of maximum increase in resistance as a result of heat shock and maximum new proteins appearing on two-dimensional gels was 30 to 60 min after application of heat shock, T1.5 to T2, corresponding to stage I or II of sporulation. This was before the alkaline phosphatase increase and DPA accumulation and also before SASP production would be expected.
FIG. 3.
Time course of induction of heat-specific stress proteins in wild-type sporulating B. subtilis PS 346. Protein samples were taken at intervals after imposition of heat stress, 1 h into sporulation (░⃞), and applied to two-dimensional gels. Control (□) samples were from untreated cells. Error bars represent the standard deviations of two independent experiments.
DISCUSSION
This is the first detailed study of the stress response of sporulating B. subtilis cells; earlier studies had dealt with vegetative cells (2, 21). We have shown that heat shock treatment of B. subtilis cells during sporulation increases the heat resistance of spores formed subsequently. This is true for two wild-type strains of B. subtilis as well as SASP− and sigB mutants which lack a number of stress response proteins. SASP are specific to the spore stage of the bacterium and are associated with increased resistance of spores. Spores that are deficient in SASP are considerably more sensitive to hydrogen peroxide, UV radiation, and heat, as well as freeze-drying, dessication, and extremes of pH, and have decreased longevity compared to wild-type spores. The alternative sigma factor ςB is required for the induction of the majority of general stress proteins of B. subtilis during heat shock and other stress conditions in vegetative cells (21). ςB mutants exhibit normal vegetative growth and sporulation but are not able to induce ςB-dependent general stress proteins. However, none of the stress-specific proteins identified in this study is sigB dependent. Sublethal heat shock was followed by an increase in heat shock proteins and also increased heat resistance in all four strains. Furthermore the level of induction of heat-specific stress proteins showed a positive correlation with the heat resistance of the strains studied, indicating a causal relationship of the two events. The fact that heat shock was accompanied by an increase in heat shock proteins and increased heat resistance in all four strains irrespective of the presence of SASP or the sigB regulon indicates a causal relationship between the two events.
We have shown that spores developed in cells treated by heat shock 60 min after exponential growth stops had increased heat resistance compared with that of the control spores or spores from cells treated by heat shock at 0, 30, 90, or 120 min after the end of exponential growth. Similarly Sedlák et al. (18) found that heat shocking B. megaterium sporulating cells at T2 increased the heat resistance of spores formed subsequently compared to that of spores from cells treated by heat shock at T0 or T4. Furthermore Heredia et al. (9) found that heat shocking sporulating cells of C. perfringens 1 h after initiation of sporulation produced more heat-tolerant spores than were produced by the control. Little or no difference in spore heat resistance was observed when heat shock was applied 2 h into sporulation, and heat shock applied at 3 h resulted in the production of spores that were more heat sensitive than those of the control. In all three spore-forming species, imposition of sublethal heat shock early in sporulation resulted in increased heat resistance of spores. This suggests that the time of imposition of sublethal stress is critical in the subsequent development of heat resistance. If there is a positive causal relationship between acquired thermotolerance and the synthesis of heat shock proteins, then a determining factor that triggers the latter may be the stage of sporulation at which the stress is imposed.
Two-dimensional gel electrophoresis indicated the generation and/or overexpression of over 60 heat shock proteins in sporulating B. subtilis cells concurrent to acquired thermotolerance in spores. Of these, 11 were designated heat-specific shock proteins because they were either synthesized de novo or overexpressed in sporulating cells specifically in response to heat shock but not cold shock or glucose starvation. Five of these proteins were determined by sequence analysis to be analogous to those in vegetative cells reported by previous workers (2, 21). However, the remaining six, which still require identification, appear to be different from vegetative heat shock proteins on the basis of a comparison of molecular weight and isoelectric point measurements. All five heat-specific stress proteins are chaperonins whose expression is controlled by the concerted action of the CIRCE element (24) and the negative regulator HrcA (17, 22, 23). The stress-specific proteins induced by heat may act by counteracting the damage, adapting to the stress action, or repairing damage induced by the stress. For example, heat-specific chaperones, such as the GroES and DnaK machines, are able to assist proper protein folding.
In this study we found that the same heat-specific proteins were induced in response to heat shock in all the strains tested but that the level of induction varied with the strain and was most pronounced in the most heat-resistant strain. Similarly Bernhardt et al. (2) found that the extent of the ςB-dependent stress response in vegetative cells of B. subtilis varied with the strain. However, they did not determine whether this was also correlated with heat resistance.
Völker et al. (21) reported that the induction of stress proteins was one of the earliest responses of the vegetative cell to growth-restricting conditions and was detectable as early as 2 min after stress imposition. We have also found that the induction of stress proteins occurs very rapidly in sporulating cells of B. subtilis. The heat-specific stress proteins identified in this study were induced within 15 min after heat shock and reached maximum levels 30 to 60 min after heat shock. Heat shock proteins were either not detectable or had returned to basal preshock levels in extracts of sporulating cells by 2 h after the application of heat stress. Similarly, Völker et al. (21) reported that for all the genes and the stress conditions tested, the induction was transient, reaching a maximum between 6 and 12 min after the bacteria were exposed to the stimuli. It is important to recognize that if heat shock proteins disappear later on in sporulation, their activity cannot be to repair heat-damaged spores but only to alter spore structure during sporulation in ways which increase heat resistance.
The control mechanisms operating in vegetative and sporulating cells may well be different, given the differences in the sigma factors active in the two types of cells (7), as well as the differences between the mother cell and forespore. It would be possible to investigate whether these sigma factors play a role in the induction of enzymes involved in the removal of the stress proteins.
Future work will be directed at determining the function and temporal, spatial, and genetic control of the stress response proteins identified in this study.
ACKNOWLEDGMENTS
This work was supported by European Union Project Grant FAIR-CT97-3159.
We thank M. Hecker for donation of the 168 wild-type and sigB mutant strains and P. Setlow for donation of SASP+ and SASP− strains.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernhardt J, Völker U, Völker A, Antelmann H, Schmid R, Mach H, Hecker M. Specific and general stress proteins in Bacillus subtilis—a two-dimensional electrophoresis study. Microbiology. 1997;143:999–1017. doi: 10.1099/00221287-143-3-999. [DOI] [PubMed] [Google Scholar]
- 3.Connors M J, Mason J M, Setlow P. Cloning and nucleotide sequencing of genes for three small, acid-soluble proteins from Bacillus subtilisspores. J Bacteriol. 1986;166:417–425. doi: 10.1128/jb.166.2.417-425.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fairhead H, Setlow B, Setlow P. Prevention of DNA damage in spores and in vitro by small, acid-soluble proteins from Bacillusspecies. J Bacteriol. 1993;175:1367–1374. doi: 10.1128/jb.175.5.1367-1374.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Griffith J, Makhov A, Santiago-Lara L, Setlow P. Electron microscopic studies of the interaction between a Bacillusα/β-type small, acid-soluble spore protein with DNA: protein binding is cooperative, stiffens the DNA and induces negative supercoiling. Proc Natl Acad Sci USA. 1994;91:8224–8228. doi: 10.1073/pnas.91.17.8224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hahn G M, Li G C. Thermotolerance, thermoresistance and thermosensitisation. In: Morimoto R I, Tissières A, Georgopoulos C, editors. Stress proteins in biology and medicine. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1990. pp. 191–221. [Google Scholar]
- 7.Haldenwang W. The sigma factors of Bacillus subtilis. Microbiol Rev. 1995;59:1–30. doi: 10.1128/mr.59.1.1-30.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hecker M, Schumann W, Völker U. Heat shock and general stress response in Bacillus subtilis. Mol Microbiol. 1996;19:417–428. doi: 10.1046/j.1365-2958.1996.396932.x. [DOI] [PubMed] [Google Scholar]
- 9.Heredia N L, Garcia G A, Luevanos R, Labbe R G, Santos Garcia-Alvarado J S. Evaluation of the heat resistance of vegetative cells and spores of Clostridium perfringenstype A by sublethal heat shock. J Food Protect. 1997;60:998–1000. doi: 10.4315/0362-028X-60.8.998. [DOI] [PubMed] [Google Scholar]
- 10.Igo M, Lampe M, Ray C, Schafer W, Moran C P, Jr, Losick R. Genetic studies of a secondary RNA polymerase sigma factor in Bacillus subtilis. J Bacteriol. 1987;169:3464–3469. doi: 10.1128/jb.169.8.3464-3469.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leighton T J, Doi R H. The stability of messenger ribonucleic acid during sporulation in Bacillus subtilis. J Biol Chem. 1971;246:3189–3195. [PubMed] [Google Scholar]
- 12.Mason J M, Setlow P. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilisspores to UV light. J Bacteriol. 1986;167:174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polissi A, Goffin L, Georgopoulos C. The Escherichia coliheat shock response and bacteriophage lambda development. FEMS Microbiol Rev. 1995;17:159–169. doi: 10.1111/j.1574-6976.1995.tb00198.x. [DOI] [PubMed] [Google Scholar]
- 14.Rider M H, Puype M, VanDamme J, Gevaert K, DeBoeck S, D'Alayer J, Rasmussen H H, Celis J H, Vanderkerckhove J. An agarose-based gel-concentration system for micro-sequence and mass spectrometric characterisation of proteins previously purified in polyacrylamide gels starting at low picomole levels. Eur J Biochem. 1995;230:258–266. doi: 10.1111/j.1432-1033.1995.0258i.x. [DOI] [PubMed] [Google Scholar]
- 15.Rotman Y, Fields M L. A modified reagent for dipicolinic acid analysis. Anal Biochem. 1967;22:168. doi: 10.1016/0003-2697(68)90272-8. [DOI] [PubMed] [Google Scholar]
- 16.Schmid R, Bernhardt J, Antelmann H, Völker A, Mach H, Völker U, Hecker M. Identification of vegetative proteins for a two-dimensional protein index of Bacillus subtilis. Microbiology. 1997;143:991–998. doi: 10.1099/00221287-143-3-991. [DOI] [PubMed] [Google Scholar]
- 17.Schulz A, Tzschaschel B, Schumann W. Isolation and analysis of mutants of the dnaK operon of Bacillus subtilis. Mol Microbiol. 1995;15:421–429. doi: 10.1111/j.1365-2958.1995.tb02256.x. [DOI] [PubMed] [Google Scholar]
- 18.Sedlák M, Vinter V, Adamec J, Vohradsky J, Voburka Z, Chaloupka J. Heat shock applied early in sporulation affects heat resistance of Bacillus megateriumspores. J Bacteriol. 1993;175:8049–8052. doi: 10.1128/jb.175.24.8049-8052.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Setlow B L, Setlow P, Sun D. Studies of the interactions between DNA and alpha and beta type SASP, a new class of DNA binding protein. J Bacteriol. 1992;174:2312–2322. doi: 10.1128/jb.174.7.2312-2322.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Todd J A, Hubbard T J P, Travers A A, Ellar D J. Heat shock proteins during growth and sporulation of Bacillus subtilis. FEBS Lett. 1985;188:209–214. doi: 10.1016/0014-5793(85)80373-2. [DOI] [PubMed] [Google Scholar]
- 21.Völker U, Engelmann S, Maul B, Riethdorf S, Völker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 22.Yuan G, Wong S L. Regulation of groE expression in Bacillus subtilis: the involvement of the ςA-like promoter and the roles of the inverted repeat sequence (CIRCE) J Bacteriol. 1995;177:5427–5433. doi: 10.1128/jb.177.19.5427-5433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yuan G, Wong S L. Isolation and characterization of Bacillus subtilis groE regulatory mutants: evidence for orf39 in the dnaK operon as a repressor gene in regulating the expression of both groE and dnaK. J Bacteriol. 1995;177:6462–6468. doi: 10.1128/jb.177.22.6462-6468.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]