Abstract
Background:
Although immune checkpoint inhibitor treatment for advanced thymic carcinoma exhibits promising efficacy, factors that affect the efficacy and prognosis, including metastases sites, remain uncertain.
Objectives:
Our study aimed to investigate the determinants of survival among patients with advanced thymic carcinoma who underwent immunotherapy in real-world settings, with implications for clinical practice.
Designs:
Different therapy regimens of immunotherapy were produced to analyze the influence of liver metastases on survival and prognosis for advanced thymic carcinoma patients.
Methods:
Data for advanced thymic carcinoma patients receiving immunotherapy and their metastases sites were collected for analysis from seven different hospitals between January 2015 and January 2023. Progression-free survival (PFS) and overall survival (OS) analyses were performed using the Kaplan–Meier method. Cox analysis was used to evaluate factors influencing survival.
Results:
The present study analyzed 136 advanced thymic carcinoma patients from seven different hospitals.
The PFS for all patients receiving immunotherapy was 6.4 months, while the OS was 24.0 months. The objective response rate was different for patients with liver and non-liver metastases (11.9% versus 37.2%, p = 0.003). The disease control rate values were also different between the two groups (47.6% versus 80.9%, p = 0.037). The PFS for patients with liver metastases demonstrated poor immunotherapy efficacy compared to patients with non-liver metastases (3.0 versus 8.0 months, p < 0.0001). The OS was also significantly different between these two patient groups (16.1 versus 29.1 months, p = 0.009).
Conclusion:
Immunotherapy had poor efficacy in advanced thymic carcinoma patients with liver metastases.
Keywords: efficacy, immunotherapy, liver metastases, thymic carcinoma
Introduction
Thymic epithelial tumors have a thymic epithelial origin. They comprise three major types, including thymic carcinoma, thymoma, and thymic neuroendocrine tumors. 1 Thymic carcinoma is a malignant thymic epithelial tumor that exhibits significant heterogeneity and aggressive behavior compared to other tumor subtypes.2,3 Thymic squamous carcinoma accounts for the majority of thymic carcinomas and some rare pathological types, such as basaloid carcinoma and lymphoepithelioma-like carcinoma, which can also be considered a subtype of thymic carcinoma. 4 Systemic therapy can be explored as an option for advanced thymic carcinoma patients who lack the opportunity to receive surgical treatment.5,6 At present, only the combination of carboplatin and paclitaxel is regarded as the first-line standard treatment for advanced thymic carcinoma. 7
As the cornerstone of solid tumor treatment, immunotherapy has transformed the management of numerous malignancies. Given the unique immunological characteristics of the thymus, the efficacy of immunotherapy in advanced thymic carcinoma patients remains uncertain, warranting further investigation.8–11 Thymus-related immunotherapy has been under progressive development.12–14 At an open-label phase II trial, pembrolizumab has shown efficacy in thymic carcinoma with a partial response (PR) of 19.2%. 15 Pembrolizumab has achieved an objective response rate (ORR) of 22.5% in recurrent thymic carcinoma. 16 Therefore, immunotherapy has preliminarily demonstrated efficacy and promise in the treatment of thymic carcinoma.
However, due to the complexity of immune-microenvironment, different sites of metastases could manifest the different efficacy of immunotherapy in advanced non-small cell lung cancer patients, which is known as organ-specific efficacy.17–20 Some studies have found that immunotherapy had poor efficacy in advanced non-small cell lung cancer patients with liver metastases.21–23 However, whether a similar outcome can be observed in advanced thymic carcinoma patients is uncertain.
Therefore, the present study aimed to explore whether liver metastases can influence the efficacy of immunotherapy in advanced thymic carcinoma patients.
Methods
Patient eligibility
Patients with advanced thymic carcinoma who received immunotherapy as the first-line or further treatment were recruited at the Zhejiang Cancer Hospital, Zhejiang Provincial People’s Hospital, The First Affiliated Hospital of Zhejiang University, Fujian Cancer Hospital, The Second Affiliated Hospital of Guilin Medical University, Lishui Municipal Central Hospital, and Hunan Cancer Hospital. At the same time, the patients were also diagnosed with liver metastases. The histological diagnosis for patients with thymic carcinoma was performed in accordance with the criteria outlined by the National Comprehensive Cancer Network (NCCN) guidelines. The conditions for thymic carcinoma patients were confirmed using computed tomography or magnetic resonance imaging. Bone scans were carried out if bone metastases were present. The performance status of patients who were able to tolerate immunotherapy without appearing severely hepatic or experiencing renal dysfunction could be accepted. Patients with severe immune deficiency and autoimmune diseases who were unable to receive the systematic treatment were excluded. In addition, patients with other malignancies and those who received prior immunotherapy treatments were not incorporated into the study. The current study was approved by the institutional review board of the Zhejiang Cancer Hospital and each investigation site (IRB-2022-63). The study was performed in accordance with the Declaration of Helsinki. Individual consent was waived due to the nature of this retrospective study. The reporting of this study conforms to the statement of ESMO Guidance for Reporting Oncology real-world evidence. 24
Treatment methods
Patients diagnosed with thymic carcinoma underwent immunotherapy treatment that included different programmed death receptor 1 (PD-1) inhibitors, such as camrelizumab, nivolumab, pembrolizumab, sintilimab, tislelizumab, and toripalimab. Different PD-1 inhibitor doses were in accordance with the NCCN guidelines. Some drug dosage recommendations were obtained from prior clinical trials. The immune-combination therapy with other schemes, such as chemotherapy and antiangiogenic therapy, was also included in the analysis. Combined chemotherapy regimens utilized several agents. The nab-paclitaxel dose was 130 mg/m2 on days 1 and 8 or 260 mg/m2 on day 1, followed by carboplatin with an area under the curve (AUC) of 5 mg/mL/min (per Calvert formula) on day 1 with a dose of 75 mg/m2 every 3 weeks and 200–225 mg/m2 paclitaxel plus carboplatin with AUC of 5 mg/mL/min or 75 mg/m2 carboplatin on day 1. The DP regimen included docetaxel (70 mg/m2) in addition to cisplatin (75 mg/m2) every 3 weeks. The EP regimen included administration of etoposide (100 mg/m2) on days 1–3 in addition to one cycle of cisplatin (75 mg/m2) every 3 weeks. The gemcitabine dose was 1.5 g/m2 on days 1 and 8. The tegafur gimeracil dose was 40–60 mg twice a day. Antiangiogenic therapy schemes consisted of anlotinib and apatinib administered at respective doses of 10 and 250 mg daily. Other drug doses were in accordance with the NCCN guidelines.
PD-L1 assessment by immunohistochemistry
The PD-L1 (programmed death ligand 1) expression was assessed using PD-L1 22C3 pharmDx (Agilent Technologies, Santa Clara, CA, USA). The intensity of membrane staining reflects the degree of PD-L1 expression, which defines the tumor proportion score >0%. The PD-L1 expression data were obtained from the pathology reports. The immunohistochemistry results were confirmed by a professional pathologist.
Response
The tumor response was assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1). 25 The response assessment was carried out every two cycles. The response condition was also evaluated if significant signs of disease progression were present or in cases of toxicity intolerance. The ORR consisted of complete response (CR) and PR. The disease control rate (DCR) included CR, PR, and stable disease (SD).
Follow-up
All of the patients were evaluated in terms of their response, progression-free survival (PFS), and overall survival (OS). PFS was defined as the time from the first day of immunotherapy treatment to verified disease progression or death for any reason. OS was calculated from the first immunotherapy dose administration to death or the last follow-up. The last follow-up time was 31 March 2023.
Statistical analysis
Kaplan–Meier analysis was used to conduct the survival analysis and to generate the survival curves. Difference comparison between groups was performed using the log-rank test. The Statistical Package for Social Sciences software (version 25; IBM, Armonk, NY, USA) and GraphPad Prism software (Version 9; GraphPad Software, San Diego, CA, USA) were used to analyze the statistical data. Cox proportional hazards model was used to evaluate the factors influencing PFS and OS by calculating the hazard ratios (HRs) and 95% confidence intervals (CIs). Two-sided p-values of <0.05 were regarded as a sign of statistical significance.
Results
Patient characteristics
A total of 136 patients from 7 different hospitals were included in the study. Specific patient characteristics are listed in Table 1. The performance status for all patients ranged from 0 to 1. Male and female patients accounted for 65.4% (n = 89) and 34.6% (n = 47) of the cohort, respectively. The median age of all patients was 57 years (range: 19–71 years). Histological analysis showed that the majority of patients had squamous carcinoma (79.4%; n = 108). Fifty-seven patients had a history of smoking. A total of 50 patients received immunotherapy as the first-line treatment, while 63.2% (n = 86) of patients received latter-line therapy. In the first-line treatment group, 5 patients received mono-immunotherapy, and 45 patients received combination therapy. In the latter-line treatment group, 41 patients received mono-immunotherapy, and 45 patients received combination therapy. Overall, 20 patients expressed PD-L1, and only 3 patients did not. In addition, 113 patients did not undergo PD-L1 expression detection tests. Among all advanced thymic carcinoma patients, 42 (30.9%) experienced liver metastases before the initial immunotherapy treatment. The remaining patients did not have liver metastases (n = 94). Detailed information about patients with liver and non-liver metastases is provided in Table 2. Baseline-level sex, age, smoking history, histology results, previous therapy conditions, and PD-L1 expression data were not different between the two groups. The therapy line was different between the liver and non-liver metastases groups. The subgroups were formed based on different therapy lines for subsequent survival analyses.
Table 1.
Baseline characteristics for all thymic carcinoma patients.
| Characteristics | Thymic carcinoma patients (n = 136) | |
|---|---|---|
| No. | % | |
| Sex | ||
| Male | 89 | 65.4 |
| Female | 47 | 34.6 |
| Age | ||
| Median | 57 | |
| Range | 19–71 | |
| <65 | 111 | 81.6 |
| ⩾65 | 25 | 18.4 |
| Smoking history | ||
| Former | 57 | 41.9 |
| Never | 79 | 58.1 |
| Histology | ||
| Squamous carcinoma | 108 | 79.4 |
| Non-squamous carcinoma | 28 | 20.6 |
| ECOG PS | ||
| 0–1 | 136 | 100 |
| Previous surgery | ||
| Yes | 42 | 30.9 |
| No | 94 | 69.1 |
| Previous radiotherapy | ||
| Yes | 54 | 39.7 |
| No | 82 | 60.3 |
| Previous chemotherapy | ||
| Yes | 92 | 67.6 |
| No | 44 | 32.4 |
| Immunotherapy mode | ||
| Monotherapy | 46 | 33.8 |
| Combination therapy | 90 | 66.2 |
| Therapy line | ||
| First-line therapy | 50 | 36.8 |
| Latter-line therapy | 86 | 63.2 |
| Extrathoracic metastases | ||
| Yes | 73 | 53.7 |
| No | 63 | 46.3 |
| Liver metastases | ||
| Yes | 42 | 30.9 |
| No | 94 | 69.1 |
| PD-L1 expression | ||
| Yes | 20 | 14.7 |
| No | 3 | 2.2 |
| Unknown | 113 | 83.1 |
ECOG PS, Eastern Cooperative Oncology Group performance status.
Table 2.
Characteristics of advanced thymic carcinoma patients with liver metastases or not.
| Characteristics | Liver metastases (n = 42) | Not liver metastases (n = 94) | p-Value | ||
|---|---|---|---|---|---|
| No. | % | No. | % | ||
| Sex | 0.562 | ||||
| Male | 26 | 61.9 | 63 | 67.0 | |
| Female | 16 | 38.1 | 31 | 33.0 | |
| Age | 0.540 | ||||
| <65 | 33 | 78.6 | 78 | 83.0 | |
| ⩾65 | 9 | 21.4 | 16 | 17.0 | |
| Smoking history | 0.821 | ||||
| Former | 17 | 40.5 | 40 | 42.6 | |
| Never | 25 | 59.5 | 54 | 57.4 | |
| Histology | 0.124 | ||||
| Squamous carcinoma | 30 | 71.4 | 78 | 83.0 | |
| Non-squamous carcinoma | 12 | 28.6 | 16 | 17.0 | |
| Previous surgery | 0.697 | ||||
| Yes | 12 | 28.6 | 30 | 31.9 | |
| No | 30 | 71.4 | 64 | 68.1 | |
| Immunotherapy mode | 0.137 | ||||
| Monotherapy | 18 | 42.9 | 28 | 29.8 | |
| Combination therapy | 24 | 57.1 | 66 | 70.2 | |
| PD-L1 expression | 0.538 | ||||
| Yes | 5 | 11.9 | 15 | 16.0 | |
| No | 1 | 2.4 | 2 | 2.1 | |
| Unknown | 36 | 85.7 | 77 | 81.9 | |
| Therapy line | 0.013 | ||||
| First-line therapy | 9 | 21.4 | 41 | 43.6 | |
| Latter-line therapy | 33 | 78.6 | 53 | 56.4 | |
Clinical efficacy
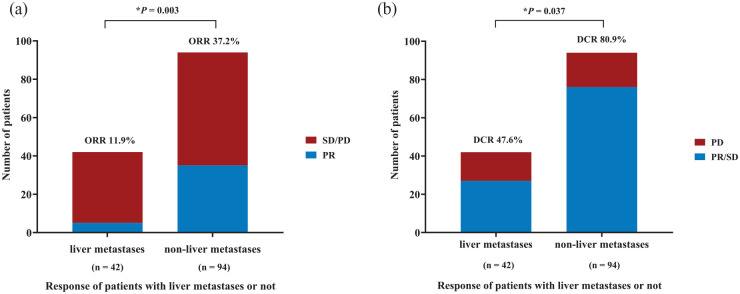
The ORR values were significantly different between patients with liver and non-liver metastases [11.9% versus 37.2%, p = 0.003; Figure 1(a)]. The DCR values were also significantly different between advanced thymic carcinoma patients with liver and non-liver metastases [47.6% versus 80.9%, p = 0.037; Figure 1(b)]. In the liver metastases group, only 5 patients achieved a PR, and 22 patients remained at SD. PD occurred in 15 patients in the liver metastases group. A total of 35 patients experienced a PR and 41 patients remained at SD in the non-liver metastases group, while 18 patients had a PD. In the first-line therapy group, the ORR was not significantly different between groups of patients with liver and non-liver metastases (33.3% versus 48.8%, p = 0.636). The DCR also did not show significant differences in these groups (66.7% versus 85.4%, p = 0.399). In the latter-line therapy, the ORR was significantly different between thymic carcinoma patients with liver and non-liver metastases (6.1% versus 28.3%. p = 0.012). The distinction in DCR between patients with liver and non-liver metastases was not obvious (63.6% versus 77.4%, p = 0.168; Table 3).
Figure 1.
Response of advanced thymic carcinoma patients with liver metastases and non-liver metastases to immunotherapy treatment. Outcomes were statistically different for (a) ORR between liver metastases and non-liver metastases (11.9% versus 37.2%, p = 0.003). (b) DCR between liver metastases and non-liver metastases (47.6% versus 80.9%, p = 0.037).
DCR, disease control rate; ORR, objective response rate; PD, progressive disease; PR, partial response; SD, stable disease.
Table 3.
Response rates for the intent-to-treat thymic carcinoma population with different therapy lines.
| Therapy line | Response rates | Liver metastases | Non-liver metastases | p-Value | ||
|---|---|---|---|---|---|---|
| No. | % | No. | % | |||
| First-line therapy | Overall response | 3 | 33.3 | 20 | 48.8 | 0.636 |
| Complete response | 0 | 0 | 0 | 0 | ||
| Partial response | 3 | 33.3 | 20 | 48.8 | ||
| Stable disease | 3 | 33.3 | 15 | 36.6 | ||
| Progressive disease | 3 | 33.3 | 6 | 14.6 | ||
| Latter-line therapy | Overall response | 2 | 6.0 | 15 | 28.3 | 0.012 |
| Complete response | 0 | 0 | 0 | 0 | ||
| Partial response | 2 | 6.0 | 15 | 28.3 | ||
| Stable disease | 19 | 57.6 | 26 | 49.1 | ||
| Progressive disease | 12 | 36.4 | 12 | 22.6 | ||
Survival
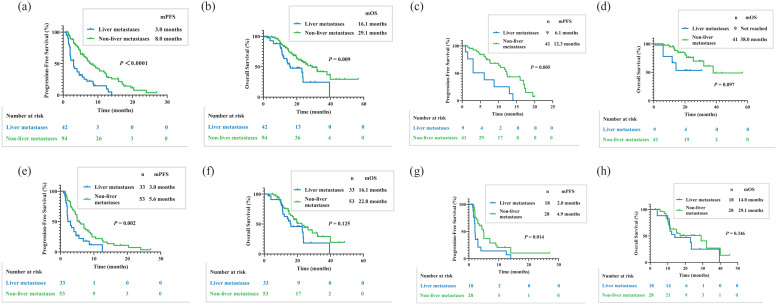
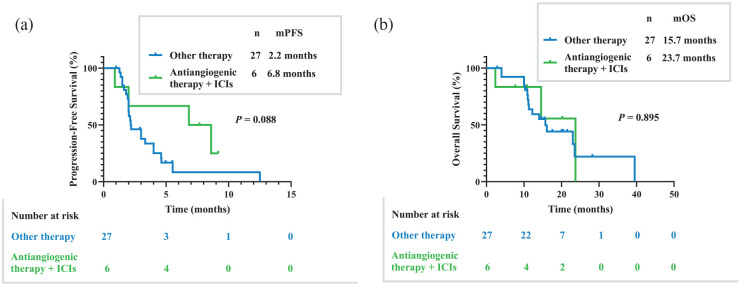
The PFS for all cohorts was 6.4 months, while the OS was 24.0 months. The PFS for patients with liver metastases revealed poor immunotherapy efficacy compared to those with non-liver metastases [3.0 versus 8.0 months, p < 0.0001; Figure 2(a)]. The OS was also significantly different between patients with liver and non-liver metastases [16.1 versus 29.1 months, p = 0.009; Figure 2(b)]. In the first-line therapy group, there was an obvious difference in PFS for immunotherapy patients with liver and non-liver metastases [6.1 versus 12.3 months, p = 0.005; Figure 2(c)]. The OS was not different among the first-line immunotherapy patients [not reached versus 38.0 months, p = 0.097; Figure 2(d)]. In the latter-line therapy, similar PFS results were achieved, showing an obvious difference in PFS for immunotherapy patients with liver and non-liver metastases [3.0 versus 5.6 months, p = 0.002; Figure 2(e)]. The OS for patients receiving immunotherapy was not different in the latter-line therapy group of liver and non-liver metastases [16.1 versus 22.8 months, p = 0.125; Figure 2(f)]. For mono-immunotherapy, PFS manifested differences in patients with liver metastases and non-liver metastases [2.0 versus 4.9 months, p = 0.014; Figure 2(g)]. For mono-immunotherapy, OS did not manifest the differences in patients with liver metastases and non-liver metastases [14.0 versus 29.1 months, p = 0.346; Figure 2(h)]. For patients with liver metastases in the latter-line therapy group, PFS was different between the other therapy group and immunotherapy combined with antiangiogenic therapy group [2.2 versus 6.8 months, p = 0.088; Figure 3(a)]. The OS for patients with liver metastases in the latter-line therapy group was not different between the other therapy group and immunotherapy combined with antiangiogenic therapy group [15.7 versus 23.7 months, p = 0.895; Figure 3(b)].
Figure 2.
Kaplan–Meier estimates of PFS and OS. (a) PFS differences in advanced thymic carcinoma patients with liver metastases and non-liver metastases (3.0 versus 8.0 months, p < 0.0001). (b) OS differences in advanced thymic carcinoma patients with liver metastases and non-liver metastases (16.1 versus 29.1 months, p = 0.009). (c) PFS differences in patients with liver metastases and non-liver metastases in first-line therapy group (6.1 versus 12.3 months, p = 0.005). (d) OS differences in patients with liver metastases and non-liver metastases in first-line therapy group (not reached versus 38.0 months, p = 0.097). (e) PFS differences in patients with liver metastases and non-liver metastases in latter-line therapy group (3.0 versus 5.6 months, p = 0.002). (f) OS differences in patients with liver metastases and non-liver metastases in latter-line therapy group (16.1 versus 22.8 months, p = 0.125). (g) PFS differences in patients with liver metastases and non-liver metastases for mono-immunotherapy (2.0 versus 4.9 months, p = 0.014). (h) OS differences in patients with liver metastases and non-liver metastases for mono-immunotherapy (14.0 versus 29.1 months, p = 0.346).
OS, overall survival; PFS, progression-free survival.
Figure 3.
Kaplan–Meier estimates of PFS and OS. (a) PFS differences in advanced thymic carcinoma patients treated using other therapy and immunotherapy combined with antiangiogenic therapy in latter-line therapy group (2.2 versus 6.8 months, p = 0.088). (b) OS differences in advanced thymic carcinoma patients treated using other therapy and immunotherapy combined with antiangiogenic therapy in latter-line therapy group (15.7 versus 23.7 months, p = 0.895).
ICIs, immune checkpoint inhibitors, OS, overall survival; PFS, progression-free survival.
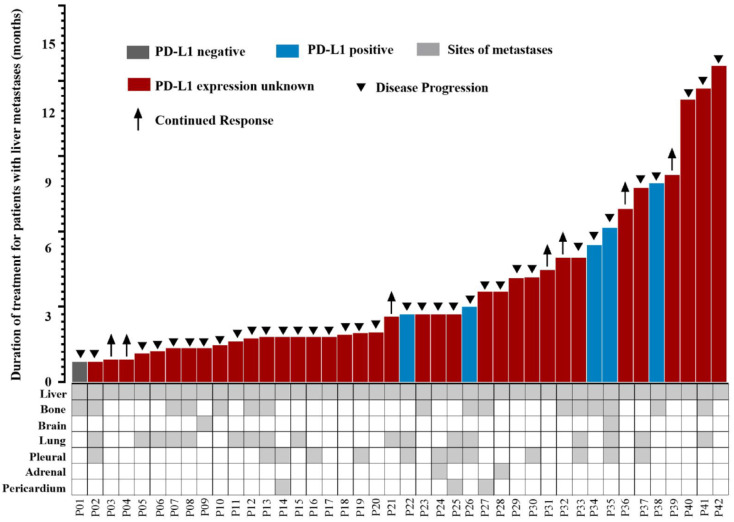
The PFS data for all patients with liver metastases are represented in Figure 4. Different PD-L1 expression conditions for advanced thymic carcinoma patients with liver metastases are also shown in Figure 4, as well as the condition of combined metastasis sites. PFS for advanced thymic carcinoma patients with, without, and with unknown PD-L1 expression was different (6.1 versus 2.2 months, p = 0.600; Supplemental Figure S1). PFS was different for advanced thymic carcinoma patients in the combined other sites of metastases group and only liver metastases group (3.0 versus 4.6 months, p = 0.029; Supplemental Figure S2).
Figure 4.
Swimmer’s plot showing PFS for all advanced thymic carcinoma patients with liver metastases receiving immunotherapy and details of other combined sites of liver metastases.
PFS, progression-free survival.
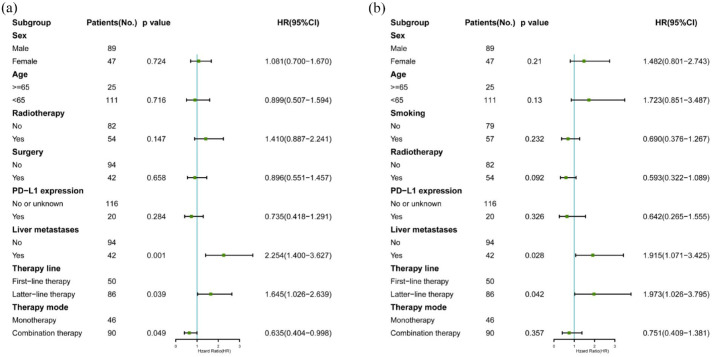
Subsequent multivariate PFS analysis evaluated sex, age, radiotherapy, surgery, PD-L1 expression, liver metastases, therapy line, and therapy mode data. The results showed that liver metastases, therapy lines, and therapy mode influenced the PFS. Liver metastases also influenced the PFS (p = 0.001, HR, 2.254; 95% CI, 1.400–3.627). Multivariate OS analysis explored sex, age, smoking, radiotherapy, PD-L1 expression, liver metastases, therapy line, and therapy mode data. The results showed that liver metastases and therapy lines influenced the OS. The HRs for liver metastases and therapy lines were 1.915 (95% CI, 1.071–3.425; p = 0.028) and 1.973 (95% CI, 1.026–3.795; p = 0.042), respectively. Multivariate analysis results were also represented as forest maps in Figure 5(a) and (b).
Figure 5.
Forest plots (a) and (b) of potential factors affecting PFS and OS in advanced thymic carcinoma patients receiving immunotherapy and results of multivariable analysis using a Cox proportional hazards model.
Discussion
The present study, with the largest sample size, explored the efficacy of immunotherapy in advanced thymic carcinoma patients with liver metastases. The study results showed a significant difference in PFS and OS in advanced thymic carcinoma patients with liver and non-liver metastases. The present study first explored the survival differences in advanced thymic carcinoma patients with specific organ metastases who underwent immunotherapy.
There are few reports on thymus-related immune response and antitumor treatment that explored the efficacy of immunotherapy in advanced thymic epithelial tumors. Some research studies have demonstrated the efficacy of immunotherapy in thymic carcinoma and thymoma patients.15,16,26,27 The PFS values for these cohorts of thymic carcinoma patients were 6.1, 4.2, and 3.8 months, respectively. Giaccone et al. 16 and Cho et al. 15 have described 15 (38%) and 10 (38.5%) thymic carcinoma patients with liver metastases, respectively. However, these studies did not analyze whether there was a difference in immunotherapy efficacy in thymic carcinoma patients with and without liver metastases. Some studies in non-small cell lung cancer or melanoma patients receiving immunotherapy have demonstrated poor efficacy in individuals with liver metastasis, which may have been associated with the lack of T cell infiltration, especially CD8+ T cells.18,28,29 The PFS for immunotherapy in non-small cell lung cancer patients with liver metastasis was only 1.8 months, which was similar to that in melanoma patients.18,30 Our previous study has shown a difference in OS for chemotherapy in 61 thymic carcinoma patients with and without liver metastasis (12.4 versus 24.8 months, p = 0.118). 31 Subsequently, our team performed a study with the largest sample size in China to analyze the efficacy of immunotherapy in advanced thymic carcinoma patients, which confirmed that liver metastasis was an independent prognostic factor for PFS. 32 The study involved 18 advanced thymic carcinoma patients (23.4%) with liver metastasis, which showed poor immunotherapy efficacy and a PFS of 1.8 months. 32 In order to confirm whether liver metastasis influences the survival of advanced thymic carcinoma patients receiving immunotherapy, the present study involved patients into analysis. Patients with liver metastases accounted for 30.9% (n = 42) of the cohort, which was the largest sample size compared to other research studies in relevant fields. PFS for patients with liver metastasis was only 3.0 months. Thus, the present study results further confirmed that liver metastases were associated with poor immunotherapy efficacy in advanced thymic carcinoma patients.
According to previous research, liver sustains the immune-suppression microenvironment, which may produce immune tolerance and influence the efficacy of immunotherapy. 33 The microenvironment can be heterogeneous in different metastases sites in the same patient receiving homogenous systematic treatment. 34 Tissue differences can also affect immunotherapy, which is the most important factor influencing its efficacy. 35 However, specific molecular mechanisms causing the differences in survival based on liver metastasis remain uncertain and should be explored in the future.
Our previous study has shown that antiangiogenic therapy exhibited promising efficacy in advanced thymic carcinoma patients. 36 Specifically, apatinib showed a median PFS of 9.0 months in the latter-line therapy for patients with recurrent or metastatic thymic epithelial tumors. 36 Related research studies have assumed that immunotherapy combined with antiangiogenic therapy promotes the antitumor activity and modulates the immune-microenvironment in patients with liver metastases. 37 Our team has performed a retrospective study using 10 patients with thymic epithelial tumors receiving immunotherapy combined with antiangiogenic therapy. 38 The results showed promising antitumor activity and a PFS of 6.7 months. 38 Similarly, the present study demonstrated a PFS of 6.8 months for immunotherapy combined with antiangiogenic therapy in the latter-line therapy group of advanced thymic carcinoma patients with liver metastases. Although advanced thymic carcinoma patients with liver metastases showed poor immunotherapy efficacy, the combination therapy with antiangiogenic therapy may still present a new opportunity for the latter-line therapy group patients with liver metastases. More prospective studies are needed to provide more therapy choices for patients.
There were some limitations in the present study. First, the retrospective nature of the study was unavoidable and was necessary to verify therapy outcomes. Second, the specific molecular mechanism for how liver metastases may influence the immunotherapy efficacy in advanced thymic carcinoma was not analyzed. The results also lacked the support of basic experiments and translational research. Finally, there was heterogeneity among patients in the study. Different gene mutation conditions that may influence the efficacy and survival of immunotherapy in thymic carcinoma patients should be explored in further research studies.
Conclusion
Immunotherapy demonstrated poor efficacy in advanced thymic carcinoma patients with liver metastases. Further exploration of this subject in a prospective study should be considered.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359241253127 for Poor efficacy of immune checkpoint inhibitor treatment in advanced thymic carcinoma patients with liver metastases by Yue Hao, Manyi Xu, Xiaohong Zeng, Yina Wang, Wenxian Wang, Gen Lin, Bihui Li, Jianhui Huang, Chunwei Xu, Yongchang Zhang and Zhengbo Song in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241253127 for Poor efficacy of immune checkpoint inhibitor treatment in advanced thymic carcinoma patients with liver metastases by Yue Hao, Manyi Xu, Xiaohong Zeng, Yina Wang, Wenxian Wang, Gen Lin, Bihui Li, Jianhui Huang, Chunwei Xu, Yongchang Zhang and Zhengbo Song in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors would like to appreciate all patients and their families for their cooperation and participation. Additionally, we are thankful to all research staff and co-investigators involved in this investigation.
Footnotes
ORCID iDs: Yue Hao  https://orcid.org/0000-0002-4725-6084
https://orcid.org/0000-0002-4725-6084
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yue Hao, Department of Clinical Trial, Zhejiang Cancer Hospital, Hangzhou, China; The Second Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, China.
Manyi Xu, Department of Clinical Trial, Zhejiang Cancer Hospital, Hangzhou, China; The Second Clinical Medical College of Zhejiang Chinese Medical University, Hangzhou, China.
Xiaohong Zeng, Postgraduate training base Alliance of Wenzhou Medical University (Zhejiang Cancer Hospital), Hangzhou, China.
Yina Wang, Department of Oncology, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, China.
Wenxian Wang, Department of Medical Oncology, Zhejiang Cancer Hospital, Hangzhou, China.
Gen Lin, Department of Medical Oncology, Fujian Cancer Hospital, Fujian Medical University Cancer Hospital, Fuzhou, China.
Bihui Li, Department of Oncology, The Second Affiliated Hospital of Guilin Medical University, Guilin, China.
Jianhui Huang, Department of Oncology, Lishui Municipal Central Hospital, Lishui, China.
Chunwei Xu, Institute of Cancer and Basic Medicine, Chinese Academy of Sciences, Hangzhou, China.
Yongchang Zhang, Lung Cancer and Gastrointestinal Unit, Department of Medical Oncology, Hunan Cancer Hospital, The Affiliated Cancer Hospital of Xiangya School of Medicine, Central South University, Changsha, China.
Zhengbo Song, Department of Clinical Trial, Zhejiang Cancer Hospital, No. 1 East Banshan Road, Gongshu, Hangzhou 310022, China.
Declarations
Ethics approval and consent to participate: Approval of the study protocol was obtained from Zhejiang Cancer Hospital Institutional Review Board Committee (approval number: IRB-2022-63). All patient’s individual consent for this retrospective analysis was waived.
Consent for publication: Not applicable.
Author contributions: Yue Hao: Data curation; Methodology; Resources; Software; Validation; Visualization; Writing – original draft.
Manyi Xu: Data curation; Investigation; Software; Writing – original draft.
Xiaohong Zeng: Data curation; Software; Visualization; Writing – original draft.
Yina Wang: Data curation.
Wenxian Wang: Writing – original draft.
Gen Lin: Data curation.
Bihui Li: Data curation.
Jianhui Huang: Data curation; Investigation.
Chunwei Xu: Conceptualization.
Yongchang Zhang: Conceptualization; Data curation.
Zhengbo Song: Conceptualization; Funding acquisition; Resources; Supervision; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The study was granted by the Foundation of CSCO-Shiyao (Y-SY201901-0068, to Zhengbo Song). This study was sponsored by a grant from the Zhejiang provincial program for the Cultivation of High-level Innovative Health talents (to Zhengbo Song).
The authors declare that there is no conflict of interest.
Availability of data and materials: The datasets used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
- 1. Falkson CB, Vella ET, Ellis PM, et al. Surgical, radiation, and systemic treatments of patients with thymic epithelial tumours: a clinical practice guideline. J Thorac Oncol 2022; 17: 1258–1275. [DOI] [PubMed] [Google Scholar]
- 2. Merveilleux du, Vignaux C, Maury JM, Girard N. Novel agents in the treatment of thymic malignancies. Curr Treat Options Oncol 2017; 18: 52. [DOI] [PubMed] [Google Scholar]
- 3. Marx A, Ströbel P, Badve SS, et al. ITMIG consensus statement on the use of the WHO histological classification of thymoma and thymic carcinoma: refined definitions, histological criteria, and reporting. J Thorac Oncol 2014; 9: 596–611. [DOI] [PubMed] [Google Scholar]
- 4. Multidisciplinary Committee of Oncology, Chinese Physicians Association. [Chinese guideline for clinical diagnosis and treatment of thymic epithelial tumors (2021 Edition)]. Zhonghua Zhong Liu Za Zhi 2021; 4: 395–404. [DOI] [PubMed] [Google Scholar]
- 5. Scorsetti M, Leo F, Trama A, et al. Thymoma and thymic carcinomas. Crit Rev Oncol Hematol 2016; 99: 332–350. [DOI] [PubMed] [Google Scholar]
- 6. Roden AC, Ahmad U, Cardillo G, et al. Thymic carcinomas – a concise multidisciplinary update on recent developments from the Thymic Carcinoma Working Group of the International Thymic Malignancy Interest Group. J Thorac Oncol 2022; 17: 637–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hirai F, Yamanaka T, Taguchi K, et al. A multicenter phase II study of carboplatin and paclitaxel for advanced thymic carcinoma: WJOG4207L. Ann Oncol 2015; 26: 363–368. [DOI] [PubMed] [Google Scholar]
- 8. Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. BMJ 2021; 375: n2363. [DOI] [PubMed] [Google Scholar]
- 9. Chaft JE, Shyr Y, Sepesi B, et al. Preoperative and postoperative systemic therapy for operable non-small-cell lung cancer. J Clin Oncol 2022; 40: 546–555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Boussageon M, Swalduz A, Chouaïd C, et al. First-line treatment of advanced non-small-cell lung cancer with immune-checkpoint inhibitors: new combinations and long-term data. BioDrugs 2022; 36: 137–151. [DOI] [PubMed] [Google Scholar]
- 11. Remon J, Passiglia F, Ahn MJ, et al. Immune checkpoint inhibitors in thoracic malignancies: review of the existing evidence by an IASLC expert panel and recommendations. J Thorac Oncol 2020; 15: 914–947. [DOI] [PubMed] [Google Scholar]
- 12. Kaira K, Imai H, Kagamu H. Perspective of immune checkpoint inhibitors in thymic carcinoma. Cancers (Basel) 2021; 13: 1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. He Y, Ramesh A, Gusev Y, et al. Molecular predictors of response to pembrolizumab in thymic carcinoma. Cell Rep Med 2021; 2: 100392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Weissferdt A, Fujimoto J, Kalhor N, et al. Expression of PD-1 and PD-L1 in thymic epithelial neoplasms. Mod Pathol 2017; 30: 826–833. [DOI] [PubMed] [Google Scholar]
- 15. Cho J, Kim HS, Ku BM, et al. Pembrolizumab for patients with refractory or relapsed thymic epithelial tumor: an open-label phase II trial. J Clin Oncol 2019; 37: 2162–2170. [DOI] [PubMed] [Google Scholar]
- 16. Giaccone G, Kim C, Thompson J, et al. Pembrolizumab in patients with thymic carcinoma: a single-arm, single-centre, phase 2 study. Lancet Oncol 2018; 19: 347–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hegde PS, Chen DS. Top 10 challenges in cancer immunotherapy. Immunity 2020; 52: 17–35. [DOI] [PubMed] [Google Scholar]
- 18. Tumeh PC, Hellmann MD, Hamid O, et al. Liver metastasis and treatment outcome with anti-PD-1 monoclonal antibody in patients with melanoma and NSCLC. Cancer Immunol Res 2017; 5: 417–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Tamiya M, Tamiya A, Inoue T, et al. Metastatic site as a predictor of nivolumab efficacy in patients with advanced non-small cell lung cancer: a retrospective multicenter trial. PLoS One 2018; 13: e0192227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Huang Y, Zhu L, Guo T, et al. Metastatic sites as predictors in advanced NSCLC treated with PD-1 inhibitors: a systematic review and meta-analysis. Hum Vaccin Immunother 2021; 17: 1278–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Deng J, Gao M, Gou Q, et al. Organ-specific efficacy in advanced non-small cell lung cancer patients treated with first-line single-agent immune checkpoint inhibitors. Chin Med J (Engl) 2022; 135: 1404–1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Deng JY, Gou Q, Yang L, et al. Immune suppressive microenvironment in liver metastases contributes to organ-specific response of immunotherapy in advanced non-small cell lung cancer. J Immunother Cancer 2023; 11: e007218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fang Y, Su C. Research progress on the microenvironment and immunotherapy of advanced non-small cell lung cancer with liver metastases. Front Oncol 2022; 12: 893716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Castelo-Branco L, Pellat A, Martins-Branco D, et al. ESMO guidance for reporting oncology real-world evidence (GROW). Ann Oncol 2023; 34: 1097–1112. [DOI] [PubMed] [Google Scholar]
- 25. Schwartz LH, Litière S, de Vries E, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 2016; 62: 132–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Rajan A, Heery CR, Thomas A, et al. Efficacy and tolerability of anti-programmed death-ligand 1 (PD-L1) antibody (Avelumab) treatment in advanced thymoma. J Immunother Cancer 2019; 7: 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Katsuya Y, Horinouchi H, Seto T, et al. Single-arm, multicentre, phase II trial of nivolumab for unresectable or recurrent thymic carcinoma: PRIMER study. Eur J Cancer 2019; 113: 78–86. [DOI] [PubMed] [Google Scholar]
- 28. Schmid S, Diem S, Li Q, et al. Organ-specific response to nivolumab in patients with non-small cell lung cancer (NSCLC). Cancer Immunol Immunother 2018; 67: 1825–1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ma SC, Bai X, Guo XJ, et al. Organ-specific metastatic landscape dissects PD-(L)1 blockade efficacy in advanced non-small cell lung cancer: applicability from clinical trials to real-world practice. BMC Med 2022; 20: 120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Funazo T, Nomizo T, Kim YH. Liver metastasis is associated with poor progression-free survival in patients with non-small cell lung cancer treated with nivolumab. J Thorac Oncol 2017; 12: e140–e141. [DOI] [PubMed] [Google Scholar]
- 31. Cheng G, Gu C, Song Z. Impact of metastasis site for survival of patients with advanced thymic epithelial tumors. Transl Cancer Res 2016; 5: 546–551. [Google Scholar]
- 32. Wang W, Lin G, Hao Y, et al. Treatment outcomes and prognosis of immune checkpoint inhibitors therapy in patients with advanced thymic carcinoma: a multicentre retrospective study. Eur J Cancer 2022; 174: 21–30. [DOI] [PubMed] [Google Scholar]
- 33. Li F, Tian Z. The liver works as a school to educate regulatory immune cells. Cell Mol Immunol 2013; 10: 292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Jiménez-Sánchez A, Memon D, Pourpe S, et al. Heterogeneous tumor-immune microenvironments among differentially growing metastases in an ovarian cancer patient. Cell 2017; 170: 927–938.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zhang C, Yu D. Suppressing immunotherapy by organ-specific tumor microenvironments: what is in the brain? Cell Biosci 2019; 9: 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Song Z, Lou G, Wang Y, et al. Apatinib in patients with recurrent or metastatic thymic epithelial tumor: a single-arm, multicenter, open-label, phase II trial. BMC Med 2022; 20: 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kitadai R, Okuma Y, Hakozaki T, et al. The efficacy of immune checkpoint inhibitors in advanced non-small-cell lung cancer with liver metastases. J Cancer Res Clin Oncol 2020; 146: 777–785. [DOI] [PubMed] [Google Scholar]
- 38. Xiang J, Si J, Hao Y, et al. Efficacy and safety of immune checkpoint inhibitors (ICIs) combined with antiangiogenic therapy for thymic epithelial tumors (TETs): a retrospective study. Transl Cancer Res 2023; 12: 550–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359241253127 for Poor efficacy of immune checkpoint inhibitor treatment in advanced thymic carcinoma patients with liver metastases by Yue Hao, Manyi Xu, Xiaohong Zeng, Yina Wang, Wenxian Wang, Gen Lin, Bihui Li, Jianhui Huang, Chunwei Xu, Yongchang Zhang and Zhengbo Song in Therapeutic Advances in Medical Oncology
Supplemental material, sj-docx-2-tam-10.1177_17588359241253127 for Poor efficacy of immune checkpoint inhibitor treatment in advanced thymic carcinoma patients with liver metastases by Yue Hao, Manyi Xu, Xiaohong Zeng, Yina Wang, Wenxian Wang, Gen Lin, Bihui Li, Jianhui Huang, Chunwei Xu, Yongchang Zhang and Zhengbo Song in Therapeutic Advances in Medical Oncology