Abstract
The outer membrane of gram-negative bacteria functions as a permeability barrier that protects cells against a large number of antibacterial agents. OprL protein of Pseudomonas putida has been shown to be crucial to maintain the stability of this cell component (J. J. Rodríguez-Herva, M.-I. Ramos-González, and J. L. Ramos. J. Bacteriol. 178:1699–1706, 1996). In the present study we cloned and mutagenized the orf1, tolQ, tolR, tolA, and tolB genes from P. putida KT2440, which were located upstream of the oprL gene. Polar and nonpolar mutations of the P. putida tolQ, tolR, tolA, and tolB genes were generated in vitro by using the Ω-Kmr interposon, which carries two transcriptional stop signals, or a promoterless xylE cassette, lacking any transcriptional stop signal, respectively. The mutant constructs were used to inactivate, by reverse genetics procedures, the corresponding chromosomal copies of the genes. The phenotype of each mutant strain was analyzed and compared with those of the wild-type strain and the previously characterized P. putida oprL::xylE mutant. All mutant strains exhibited a similar phenotype: altered cell morphology, bleb formation at the cell surface, release of periplasmic and outer membrane proteins to the extracellular medium, increased sensitivity to a variety of compounds (i.e., EDTA, sodium dodecyl sulfate, deoxycholate, and some antibiotics), filament formation, and severely reduced cell motility. Altogether, these results demonstrate the importance of the Tol-OprL system for the maintenance of outer membrane integrity in P. putida and suggest a possible role of these proteins in assembling outer membrane components.
Among other functions, the outer membrane of gram-negative bacteria plays a major role as an exclusion barrier against a number of potentially harmful compounds, as well as acting as a selective permeability barrier to other solutes (20, 47). Bacterial outer membrane consists of a lipid bilayer which significantly differs from most biological membranes because of its asymmetric structure and distinctive composition. While the inner leaflet of the outer membrane is composed of phospholipids (mainly with phosphatidylethanolamine as the head group), its outer monolayer consists of negatively charged lipopolysaccharide (LPS) molecules strongly associated with each other through divalent cation cross bridging (24, 50). All major outer membrane proteins studied so far have also been found to interact with LPS (21, 31). The stability of these associations constitutes the primary basis for the exclusion ability of the outer membrane (20, 45). In addition, the molecular sieving properties of this membrane are due to the presence of a number of proteins which form water-filled pores (43, 44).
Our current knowledge about the structure and functioning of the bacterial outer membrane is mainly based on studies with Escherichia coli (38, 45, 46), while the number of studies available for other bacteria is rather small. In E. coli, a number of mutants have been isolated which exhibit altered outer membrane organization. Among these mutants, just a few were found to be affected in structural genes involved in maintenance of outer membrane structure, namely, lpp mutants lacking the Braun lipoprotein (18), ompA mutants (47), and tol-pal mutants (5). Of these mutants, the tol-pal mutants exhibit the most severe alterations in outer membrane integrity. Their pleiotropic phenotype includes release of periplasmic proteins into the extracellular medium, hypersensitivity to some drugs and detergents, and formation of outer membrane vesicles (33). In E. coli, the Tol-PAL system consists of seven proteins: three inner membrane proteins (TolQ, TolR, and TolA), whose topologies have been extensively studied; two periplasmic proteins (TolB and Orf2), one outer membrane lipoprotein (PAL), and one cytoplasmic protein (Orf1). The genes encoding these proteins in E. coli are transcribed from two adjacent operons, one composed of the orf1, tolQ, tolR, and tolA genes and the other comprising tolB, pal, and orf2 (62). The Tol-PAL system is organized into two protein complexes: an inner membrane complex that consists of the TolQ, TolR, and TolA proteins, which interact with each other via their transmembrane domains, and another complex, associated with the outer membrane and composed of TolB and PAL, which also interact with Lpp, OmpA, and the peptidoglycan (for recent reviews, see references 33 and 34). Both orf1 and orf2 encode proteins of unknown function.
In addition to their structural role, TolQ, TolR, TolA, and TolB proteins are required for the uptake of most group A colicins and of single-stranded DNA from some filamentous phages (32, 33). Although it has been proposed that the Tol-PAL system could be involved in porin and/or LPS translocation or assembly, these hypotheses still lack solid experimental evidence (33).
The importance of the Tol-PAL system for cell architecture is supported by the fact that homologues of the tol-pal genes have been found in many gram-negative bacteria, such as Brucella abortus (60), Haemophilus influenzae (12, 56), Pseudomonas aeruginosa (13, 37), Pseudomonas putida (53), and many others. However, the effect of mutations in the tol-pal system has been studied only in E. coli and recently in Vibrio cholerae (23), since attempts to construct tol-pal mutants in other bacteria (including P. aeruginosa) have been unsuccessful (13, 57). This fact has considerably limited understanding of the Tol-PAL complex function in other gram-negative bacteria.
In a previous work, we constructed and characterized an oprL (pal) null mutant of P. putida (52). In the present study, the tol genes of P. putida, located upstream of the oprL gene, were cloned, sequenced, and mutagenized in vitro. Then, each tol mutation was transferred to the P. putida host chromosome, and the resulting tol mutants were characterized in detail. Our results revealed that these mutants show an altered cell morphology, exhibiting bleb formation at their cell surface and increased sensitivity to a number of drugs. The mutants also released periplasmic and outer membrane proteins to the extracellular medium and formed filaments, which showed reduced cell motility.
MATERIALS AND METHODS
Bacterial strains, plasmids, culture media, and growth conditions.
The bacterial strains and plasmids used are listed in Table 1. Bacterial strains were routinely grown in liquid Luria-Bertani (LB) medium (55) or in M9 minimal medium with benzoic acid (5 mM) as the sole carbon source (1). P. putida was usually incubated at 30°C, and E. coli strains were incubated at 37°C. When required, antibiotics were used at the following final concentrations (micrograms per milliliter): ampicillin, 100; chloramphenicol, 30; kanamycin, 25 or 50; and streptomycin, 50 or 100.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Relevant characteristic(s)a | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| CC118λpir | Δ(ara-leu) araD ΔlacX74 galE galK phoA20 thi-1 rpsE rpoB argE recA1, lysogenized with λpir | 25 |
| HB101 | supE44 hsdS20 recA13 ara-14 proA2 lacY1 galK2 rpsL20 xyl-5 mtl-1 | 9 |
| JM109 | recA1 supE44 endA1 hsdR17 gyrA96 relA1 thi Δ(lac-proAB) F′ [traD36 proAB+ lacIqlacZΔM15] | 55 |
| P. putida | ||
| KT2440 | hsdR1 | 17 |
| AX | KT2440 tolA::xylE (TolA shortened to 94 amino acids) | This study |
| AΩ | KT2440 tolA::ΩKm (insertion after codon 180) | This study |
| BX | KT2440 tolB::xylE (TolB shortened to 29 amino acids) | This study |
| BΩ | KT2440 tolB::ΩKm (insertion after codon 314) | This study |
| DOT-OX2 | KT2440 oprL::xylE (OprL shortened to 16 amino acids) | 52 |
| QX | KT2440 tolQ::xylE (TolQ shortened to 17 amino acids) | This study |
| QΩ | KT2440 tolQ::ΩKm (insertion after codon 129) | This study |
| RX | KT2440 tolR::xylE (TolR completely removed) | This study |
| RΩ | KT2440 tolR::ΩKm (insertion after codon 46) | This study |
| Plasmids | ||
| pHP45ΩKm | Apr, Kmr; oriColE1, source of the Ω-Kmr interposon | 15 |
| pJB3Km1 | Apr, Kmr; oriV RK2, trfA, oriTRK2, α-lacZ | 8 |
| pKNG101 | Smr; gene replacement vector, oriR6K, oriTRK2, sacB | 28 |
| pRK600 | Cmr; helper plasmid, oriColE1, mobRK2, traRK2 | 29 |
| pUC18 | Apr; cloning vector, oriColE1, rop mutant, α-lacZ | 48 |
| pUC18Not | Apr; identical to pUC18 but with NotI sites flanking the polylinker of pUC18 | 25 |
| pXYLE10 | Kmr; source of the promoterless xylE cassette | 58 |
| pKAΩKm | pKNG101 with the NotI insert from pNotAΩKm at the NotI site | This study |
| pKBΩKm | pKNG101 carrying, at the SmaI site, a 2.5-kb KpnI-Asp700 fragment from pTOL with an Ω-Kmr cassette inserted at the BglII site of tolB | This study |
| pKQΩKm | pKNG101 with the NotI insert from pNotQΩKm at the NotI site | This study |
| pKRΩKm | pKNG101 with the NotI insert from pNotRΩKm at the NotI site | This study |
| pKSmaIAxylE | pKNG101 carrying, at the SmaI site, a 2.6-kb XhoI-StuI fragment from pTOL with a xylE cassette replacing a 45-bp SfiI fragment internal to tolA | This study |
| pKSmaIBxylE | pKNG101 carrying, at the SmaI site, a 2.6-kb NcoI fragment from pTOL with a xylE cassette replacing a 633-bp BstEII fragment internal to tolB | This study |
| pKSmaIQxylE | pKNG101 carrying, at the SmaI site, a 2.1-kb SmaI-SfiI fragment from pTOL with a xylE cassette replacing a 294-bp BstXI fragment internal to tolQ | This study |
| pKSmaIRxylE | pKNG101 carrying, at the SmaI site, a 3.8-kb SmaI fragment from pTOL with a xylE cassette replacing a 393-bp NcoI fragment internal to tolR | This study |
| pNotAΩKm | pUC18Not carrying a 2.6-kb XhoI-StuI fragment from pTOL with the Ω-Kmr cassette in the NotI site of tolA | This study |
| pNotQΩKm | pUC18Not carrying a 2.7-kb SmaI-KpnI fragment from pTOL with the Ω-Kmr cassette in the XhoI site of tolQ | This study |
| pNotRΩKm | pUC18Not carrying a 2.7-kb SmaI-KpnI fragment from pTOL with the Ω-Kmr cassette in the EcoNI site of tolR | This study |
| pPRO200 | Apr; pUC18 with a 2.3-kb SphI insert from pPRO50 carrying the oprL and orf2 genes | 53 |
| pPRO50 | Tcr; pLAFR3 carrying a ∼29-kb chromosomal fragment from P. putida KT2440 with the oprL gene | 53 |
| pPRO6 | Tcr, Kmr; pLAFR3 carrying a ∼24-kb chromosomal fragment from P. putida 14G-3 with the oprL::phoA mutant gene | 53 |
| pTOL | pUC18 carrying a 5.7-kb SmaI-SphI chromosomal fragment from P. putida KT2440 (orf1 tolQ tolR tolA tolB oprL orf2) | This study |
Apr, Cmr, Kmr, Smr, and Tcr, resistance to ampicillin, chloramphenicol, kanamycin, streptomycin, and tetracycline, respectively.
Construction of the P. putida tol mutants.
The different tol mutant strains were constructed by reverse genetic procedures. All the plasmids used for allelic replacement were based on the pKNG101 suicide vector and are listed in Table 1. Plasmids pKQΩKm, pKRΩKm, pKAΩKm, and pKBΩKm were used to construct polar mutations in the tolQ, tolR, tolA, and tolB genes, respectively. For the construction of the tolQ, tolR, tolA, and tolB nonpolar mutant derivatives, plasmids pKSmaIQxylE, pKSmaIRxylE, pKSmaIAxylE, and pKSmaIBxylE were used, respectively. Each of these pKNG101 derivatives was transferred from E. coli CC118λpir to P. putida KT2440 by triparental mating using the helper strain E. coli HB101(pRK600), and the allelic exchange was carried out as previously described (52).
Sensitivity to different chemical compounds.
To determine the bacterial sensitivity to deoxycholate (DOC), sodium dodecyl sulfate (SDS), and EDTA, overnight cultures of each strain were diluted in fresh LB medium containing 2% (wt/vol) DOC, 0.5% (wt/vol) SDS, or 0.5 mM EDTA, respectively, to reach an optical density at 660 nm (OD660) of ∼0.1. After 4 h of incubation at 30°C with agitation, the numbers of CFU per milliliter in the different cultures were determined by spreading suitable dilutions on LB plates. As a control, CFU per milliliter in cultures without any added agent was also determined. Cell survival was calculated as the ratio of the CFU per milliliter in the cultures supplemented with the tested compound to the CFU per milliliter in the unsupplemented cultures. MICs of antibiotics were determined by the microtiter broth dilution method (3).
Microscopy studies.
P. putida cells grown in LB medium were harvested in the logarithmic growth phase and subjected to microscopy analysis. For transmission electron microscopy (TEM), samples were prepared and observed as previously described (53). For scanning electron microscopy (SEM) studies, cells were fixed with glutaraldehyde vapors for 24 h in a humid chamber at 4°C. Then the cells were rinsed with distilled water, dehydrated with a graded series of ethanol solutions, suspended in amyl acetate, critical point dried, and coated with gold. Samples were examined in a Zeiss DSM950 scanning electron microscope.
Leakage of proteins into the extracellular medium.
P. putida strains bearing the plasmid pJB3Km1, which encodes the periplasmic enzyme β-lactamase, were grown in LB medium to reach an OD660 of ∼0.5. Cultures were then centrifuged (15,000 × g, 7 min, 25°C), and the resulting supernatant fraction was centrifuged again under the same conditions. The pellet fraction from the first centrifugation was solubilized in Laemmli sample buffer (30) treated with Benzonase (Merck) for 10 min to degrade the DNA and heated for 5 min at 95°C. The resulting sample was designated whole-cell lysate. Part of the supernatant fraction from the second centrifugation step was precipitated by incubation for 30 min at 4°C with 10% (wt/vol) trichloroacetic acid. After 30 min of centrifugation (15,000 × g) at 4°C and washing with acetone, the pellet (designated the supernatant fraction) was suspended in Laemmli sample buffer and treated as mentioned above. Samples were separated by SDS-polyacrylamide gel electrophoresis (PAGE) and silver stained (63) or electrotransferred onto nitrocellulose and immunodetected with an anti-β-lactamase polyclonal antibody, with the monoclonal antibody MA7-2 raised against the P. aeruginosa OprF protein (39), or with the monoclonal antibody MA1-6 raised against the P. aeruginosa OprL protein (42). SDS-PAGE and Western immunoblotting analyses were performed as described previously (30, 61). The P. putida RpoS protein, used as a cytoplasmic marker, was detected with a polyclonal antibody raised against the E. coli RpoS protein.
Other methods.
Standard molecular biology techniques were used for DNA manipulations (55). Southern blot analyses, PCR amplifications, and nucleotide sequencing were performed as previously described (54). Amino acid sequence similarities were detected using the BLAST program (2) available at the National Center for Biology Information network server, with the default settings.
Nucleotide sequence accession number.
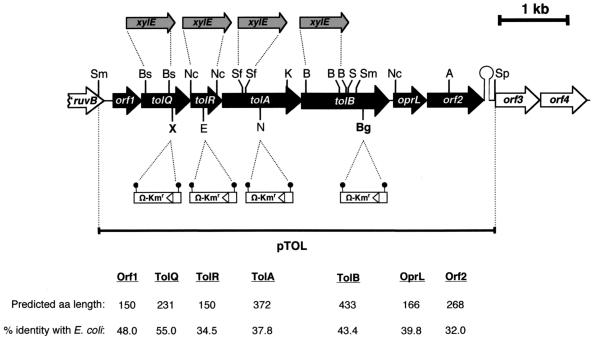
The nucleotide sequence corresponding to the P. putida chromosomal DNA fragment shown in Fig. 1 (7,577 bp) has been deposited in GenBank under accession number X74218. This sequence is identical to the P. putida KT2440 sequence deposited in The Institute for Genomic Research (TIGR) Microbial Database (http://www.tigr.org/tdb/mdb/mdb.html).
FIG. 1.
Schematic map of the 7,577-bp chromosomal DNA fragment containing the tol-oprL gene cluster of P. putida. The arrows indicate the different ORFs and their transcriptional direction. Closed arrows indicate the genes of the tol-oprL system; open arrows indicate adjacent ORFs (ruvB was only partially sequenced); shaded arrows represent the promoterless xylE cassette; and open rectangles represent the Ω-Kmr interposon (triangles in the open rectangles indicate the transcription direction, and the closed circles represent bacteriophage T4 transcriptional termination signals). A putative hairpin structure (ΔG° = −19.2 kcal) was found 25 bp downstream of the orf2 stop codon and proposed to function as a Rho-independent transcription termination signal (53). The predicted amino acid (aa) lengths of the P. putida Tol-OprL proteins and their percentages of identity to the E. coli Tol-PAL proteins are indicated below the chart. The long horizontal bar indicates the 5,745-bp SmaI-SphI insert carried by the pTOL plasmid. In vitro mutations were generated in the pTOL plasmid or its derivatives. For the P. putida QΩ, RΩ, and AΩ mutants, the Ω-Kmr cassette was excised from pHP45ΩKm as a 2,243-bp BamHI fragment, filled in with the Klenow fragment of E. coli DNA polymerase I, and inserted at the indicated sites, which had been blunt-ended before ligation. For P. putida BΩ, the BamHI Ω-Kmr cassette was cloned into the BglII site. The mutations were transferred to the host chromosome, and the position of the Ω-Kmr interposon in the different tol::ΩKm mutants is shown below the ORF map. For construction of the tol::xylE mutations, the xylE cassette was obtained from pXYLE10 as a 962-bp SmaI fragment. DNA fragments internal to the different tol genes were excised, and the resulting cohesive ends were blunt-ended by treatment with the Klenow fragment of E. coli DNA polymerase I or with T4 DNA polymerase. The xylE cassette was then cloned, replacing the deleted fragments. The position of the xylE cassette in the tol::xylE mutants is shown above the ORF map. Only relevant restriction sites are shown. Unique sites in the fragment are indicated in boldface type. The remaining sites are also present in other positions (not shown) in the fragment. Restriction sites are as follows: A, Asp700; B, BstEII; Bg, BglII; Bs, BstXI; E, EcoNI; K, KpnI; N, NotI; Nc, NcoI; S, StuI; Sf, SfiI; Sm, SmaI; Sp, SphI; X, XhoI.
RESULTS
Cloning and sequencing of the P. putida tol genes.
We previously reported the cloning and sequencing of the P. putida KT2440 oprL and orf2 genes (53) (note that, in the genus Pseudomonas, the pal gene is called oprL according to nomenclature recommendations for this genus [22]). These genes were located in the 2.3-kb SphI fragment of plasmid pPRO200 (Table 1). This fragment was used as a probe to search, in cosmids pPRO50 and pPRO6, for chromosomal regions flanking oprL and orf2. The different DNA fragments obtained from these cosmids were cloned and sequenced to complete a total of 7,577 bp. The sequenced region was predicted to contain nine complete open reading frames (ORFs), which included the oprL and orf2 genes, and one truncated ORF at the 5′ end (Fig. 1). This partial ORF was 516 bp, encoding the 171 carboxyl-terminal amino acids of a polypeptide which showed high similarity with the RuvB proteins from a number of microorganisms, such as P. aeruginosa, H. influenzae, and E. coli (89.4, 74.4, and 71.8% identity, respectively). Five ORFs were found between ′ruvB and oprL, whose predicted products exhibited high similarity with the orf1, tolQ, tolR, tolA, and tolB genes of the tol-pal system from various gram-negative bacteria (Fig. 1). The secondary structures predicted for these proteins were very similar to those determined for the E. coli Tol proteins. Separated from orf2 by 168 bp and from each other by 17 bp, two additional ORFs were found, called orf3 and orf4 (Fig. 1), whose hypothetical products showed similarity with the ExsD (28.6% identity) and ExsB (39.2% identity) proteins, respectively, from Rhizobium meliloti. In R. meliloti, the exsB gene is located in megaplasmid 2, adjacent to the exo genes, which are involved in the biosynthesis of the exopolysaccharide succinoglycan. ExsB has been proposed to function as a negative regulator of the synthesis of this polymer, although it does not act at the transcriptional level (4).
Construction of P. putida tol mutant strains.
To analyze the function of the Tol proteins in P. putida, different mutant strains bearing an inactivated chromosomal copy of the tolQ, tolR, tolA, or tolB gene were constructed by allelic exchange as described in Materials and Methods. Two types of mutant derivatives were designed: polar mutants, containing an Ω-Kmr interposon insertion in the corresponding tol gene (designated tol::ΩKm mutants) (Table 1; Fig. 1), and nonpolar mutants, in which different internal fragments of each tol gene were deleted and replaced by a promoterless xylE cassette lacking any transcriptional stop signals downstream of its stop codon (designated tol::xylE mutants) (Table 1; Fig. 1). These P. putida tol mutant strains were basically constructed as previously described for the P. putida oprL mutant (52), although with some modifications. First, the sucrose concentration in the selective media was increased to 10% (wt/vol) to improve the killing efficiency of the sacB gene encoded by the pKNG101 vector. Second, we observed that sacB-induced cell lysis of the P. putida clones bearing the pKNG101 cointegrate was more effective when bacteria were incubated at temperatures lower than 30°C. Consequently, to select for sucrose-resistant (Sucr) colonies, plates were incubated overnight at 22°C. In all cases the successful allelic exchange was checked by PCR and by Southern blot hybridization (data not shown). The complete excision of the pKNG101 vector from the host chromosome was also confirmed by Southern blot hybridization using the pKNG101 vector as a probe (data not shown). Figure 1 shows the insertion positions of the Ω-Kmr and xylE cassettes in each of the tol genes. All mutant strains were viable, although on LB plates, colonies of the mutants were more translucent than those of the parental strain. The viability of all P. putida tol mutants demonstrates that these genes are not essential for the survival of this microorganism, as was also the case for the P. putida oprL (pal) mutant and for the E. coli tol mutants (6, 52, 59), but in contrast with the essential role proposed for tolQ and tolA in P. aeruginosa (13).
Morphological and physiological characterization of the P. putida tol mutants.
P. putida KT2440 and the different tol mutant strains were grown in LB medium; the doubling times of all tol mutant strains in the exponential phase (40 ± 2 min, n = 6) were similar to that of the wild-type strain (doubling time, 37 ± 1 min; n = 4). Growth rates of the P. putida tolB::ΩKm and tolR mutants were slightly lower (doubling times around 44 and 48 min, respectively). The bacterial cultures exhibited high turbidities (OD660 > 2.5) when they reached the stationary phase, although all mutant strains showed a tendency to produce clumps at this phase, probably due to the adhesion of cell debris derived from lysed bacteria.
Cell cultures of the P. putida DOT-OX2 (oprL) and P. putida tol mutant strains were harvested in the exponential phase of growth and observed by phase-contrast microscopy. Cells of the wild-type strain presented the typical appearance of the members of the family Pseudomonaceae, whereas the tol-oprL mutant cells seemed to be shorter than the parental ones, and most of them grew by forming filaments frequently composed of 10 or more cell units (not shown). Motility of these cellular filaments seemed to be very reduced when compared with that of the individual cells. Swarm assays, carried out on LB plates containing 0.3% (wt/vol) agar, confirmed these results; while the wild type formed a 30- ± 2-mm-diameter growth halo after 16 h of incubation at 30°C, the halo of the mutant strains was basically restricted to the inoculation spot, indicating a severe deficiency in motility for the tol-oprL mutants.
Wild-type and tol mutant cells in the exponential phase of growth were also examined by SEM. Wild-type cells appeared as well-defined rod-shaped bacteria. Most of them were found in pairs, in different phases of the cell division process (Fig. 2A). However, all the P. putida tol-oprL mutants grew as chains, corroborating the optical-microscopy observations. In addition, cells within a chain were often shorter than wild-type cells (compare for instance wild-type cells [Fig. 2A] versus those of the different tol mutants [Fig. 2B to F]). Within chains, division septa were usually easily distinguishable, and many of them seemed to be in an incomplete but advanced stage of the cell division process (Fig. 2B to F). A difference found between P. putida tolQ, tolR, and tolA cells and P. putida BX or DOT-OX2 cells was the frequent presence of big blebs at the cell surface of the former (Fig. 2B to D), whereas blebs appeared only occasionally in the latter (Fig. 2E and F).
FIG. 2.
SEM of P. putida KT2440 and some of the P. putida tol mutants. Cells were grown on LB medium, harvested in the exponential phase of growth, and treated for SEM as described in Materials and Methods. (A) Strain KT2440. Magnification, ×15,000. (B) Strain QΩ. Magnification, ×10,000. (C) Strain RΩ. Magnification, ×10,000. (D) Strain AΩ. Magnification, ×10,000. (E) Strain BX. Magnification, ×5,000. (F) Strain DOT-OX2. Magnification, ×7,000.
Under TEM, the wild-type P. putida cells exhibited the typical appearance of bacteria of the genus Pseudomonas (Fig. 3A). The appearances of all P. putida tol strains harvested in the exponential phase of growth were similar to that of the parental strain except for the presence of filaments when several adjacent cells within a chain coincided with the plane of the section (Fig. 3B). A detailed examination of a number of cells from different filaments at their division sites confirmed that, in many cases, the division process was at a very advanced stage, as suggested by the previous SEM observations (Fig. 3C). Furthermore, outer and inner membranes, as well as the periplasm, of mutant cells appeared well defined with no appreciable structural alterations (Fig. 3C).
FIG. 3.
TEM of P. putida KT2440 and some of the P. putida tol::xylE mutants. Cells were grown on LB medium, harvested in the exponential phase of growth, and then processed for TEM as described in Materials and Methods. (A) Strain KT2440. Magnification, ×15,200. (B) Strain QX. Magnification, ×2,850. (C) Detail of the division septum between two DOT-OX2 cells which belong to a longer filament. Magnification, ×47,500.
The patterns of antibiotic resistance and sensitivity of the tol mutants were assayed by the MIC method with a number of antibiotics. The polar and nonpolar mutant strains were used in these assays. The results obtained with both types of mutants were similar, and Table 2 shows those obtained with the tol::xylE mutants. The tol::xylE strains were more sensitive than the KT2440 strain to the hydrophobic antibiotics fusidic acid, novobiocin, and, particularly, rifampin (Table 2). They also exhibited increased sensitivity to some aminoglycosides (such as gentamicin and streptomycin), some β-lactams (such as cefepime and piperacillin, but not imipenem), and to nalidixic acid. Mutants were also more sensitive to chloramphenicol and slightly more sensitive to tetracycline (both antibiotics are usually removed from the cell by active efflux mechanisms). In general, the most susceptible tol::xylE strains were P. putida BX and DOT-OX2.
TABLE 2.
MICs of antibiotics for P. putida KT2440 and the P. putida tol::xylE mutantsa
| Antibiotics | MIC (μg ml−1) for strain
|
|||||
|---|---|---|---|---|---|---|
| WTb | QX | RX | AX | BX | DOT- OX2 | |
| Cefepime | 2 | 0.5 | 0.25 | 0.5 | 0.12 | 0.12 |
| Chloramphenicol | 128 | 16 | 16 | 16 | 16 | 16 |
| Fusidic acid | 512 | 128 | 128 | 64 | 64 | 128 |
| Gentamicin | 2 | 0.25 | 0.5 | 0.5 | 0.12 | 0.12 |
| Imipenem | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 | 0.12 |
| Nalidixic acid | 32 | 8 | 8 | 8 | 4 | 4 |
| Novobiocin | 256 | 64 | 64 | 32 | 64 | 64 |
| Piperacillin | 16 | 16 | 4 | 4 | 4 | 2 |
| Rifampin | 8 | 2 | 1 | 4 | 0.5 | 0.5 |
| Streptomycin | 8 | 1 | 4 | 2 | 2 | 0.5 |
| Tetracycline | 2 | 1 | 0.5 | 1 | 1 | 2 |
Antibiotics were assayed following serial twofold dilutions. Data are the averages of three independent experiments.
WT, wild type.
The degrees of resistance or sensitivity of the mutant strains to the detergents DOC and SDS and to the chelating agent EDTA were also analyzed. Mutant cells were incubated for 4 h at 30°C in LB medium and in LB medium supplemented with 2% (wt/vol) DOC, 0.5% (wt/vol) SDS, or 0.5 mM EDTA, and the survival rates were determined as described in Materials and Methods. While survival of the wild-type cells was in all cases in the range of 70% to 99%, for the tol mutants, survival ranged from 0.1% to 0.6% for SDS and EDTA and from 0.004% to 0.1% for DOC (data not shown). Among the mutants, the P. putida tolR strains were the most sensitive, particularly to DOC (with a survival of 0.006%). P. putida DOT-OX2 was also very sensitive to this compound (survival of 0.004%).
In summary, all the above results clearly indicate that the permeability barrier functions of the outer membrane were significantly altered in the P. putida tol mutant strains.
Leakage of periplasmic β-lactamase.
Since many E. coli tol mutants were originally isolated as strains that released periplasmic proteins into the extracellular medium (16, 35), we decided to study whether the P. putida tol strains exhibited this phenotype. As a model protein we chose the periplasmic enzyme β-lactamase. First, plasmid pJB3Km1, which carries the bla gene encoding β-lactamase, was transferred to P. putida KT2440 and to the tol strains. Then the presence of β-lactamase in the supernatants of the different P. putida cultures was analyzed by Western blot. Samples were harvested from exponentially growing cultures to avoid possible interference with lysed cells, which could appear in a late growth phase. Analysis of the different culture supernatants by SDS-PAGE and silver staining showed the presence in the supernatant fractions of the tol mutants of numerous protein products (varying within a wide range of electrophoretic mobilities) that were absent from the P. putida wild-type extracellular fraction (Fig. 4A). The proteins were transferred onto a nitrocellulose membrane, and the presence of β-lactamase was analyzed by using a polyclonal antibody raised against this protein. The amounts of β-lactamase in the culture supernatant fractions of all mutant strains were similar (Fig. 4B). The periplasmic enzyme was not detected in the extracellular fraction of the parental strain. Immunodetection with an anti-RpoS (ς38) antibody did not show any detectable amounts of this protein, used as a cytoplasmic marker, in the culture supernatants (data not shown). However, this protein was present in considerable amounts in the whole-cell lysates of these strains, and it was also proven to be stable enough to be immunodetected after its release into the external medium (data not shown). On the other hand, it has been previously shown that, in addition to periplasmic proteins, the tol-pal mutants of E. coli released outer membrane vesicles into the extracellular medium which contained outer membrane proteins (5). Immunodetection of the supernatant fractions of the P. putida tol-oprL cultures with a monoclonal antibody raised against the P. aeruginosa OprF protein (cross-reacting with P. putida OprF) revealed the presence in these fractions of a product with an apparent molecular mass of 42 kDa, which would correspond to the P. putida OprF protein (Fig. 4C). However, in contrast with the E. coli tol-pal mutants, the OprL protein could not be immunodetected in the supernatant fractions of the P. putida tol-oprL mutants with the monoclonal antibody MA1-6, which cross-reacts with P. putida OprL (data not shown). From these results, it can be concluded that mutations in any of the P. putida tol genes lead to a significant leakage of periplasmic and outer membrane proteins.
FIG. 4.
Immunodetection of β-lactamase in the supernatant fractions (S) and whole-cell lysates (W) of P. putida KT2440 (WT) and the different tol::xylE mutants (QX, RX, AX, BX, and DOT-OX2), bearing the plasmid pJB3Km1. About 1 × 108 cells (3 × 106 for the silver staining) or the equivalent of the supernatant of 2 × 108 cells were loaded on the gel. The different fractions were prepared as described in Materials and Methods. Proteins were separated on SDS-polyacrylamide (12.5%, wt/vol) gel electrophoresis and silver stained (A), or they were transferred onto nitrocellulose and immunodetected with an anti-β-lactamase polyclonal antibody (B) or with the anti-OprF antibody MA7-2 (C). The Western blot was developed using the peroxidase colorimetric method (55). The arrows show the positions of the β-lactamase (B) or the OprF proteins (C). The positions of the molecular size markers are indicated on the left.
DISCUSSION
Sequence and functional similarities between the Tol-PAL (OprL) systems of E. coli and P. putida.
Previously we identified the oprL and orf2 genes of the P. putida tol-oprL system (53). In this study, we cloned and sequenced the remaining genes of the P. putida tol-oprL gene cluster. Among the different P. putida Tol-OprL proteins, TolQ and Orf1 were the best conserved (Fig. 1). The high degree of conservation of TolQ probably reflects its key role in the assembly of the TolQRA inner membrane complex, since TolQ is involved in a significant number of interactions within this protein complex (14, 19, 36). On the other hand, the conservation of Orf1 suggests that this protein could play an important role in the cell. However, E. coli orf1 chromosomal mutants did not show a Tol phenotype or any other differential phenotypes compared with the wild-type strain (11, 59), and consequently the function of Orf1 remains unknown. We are currently constructing P. putida orf1 mutants in order to elucidate the physiological role of this protein. P. putida TolR was one of the least conserved proteins with respect to the E. coli Tol proteins (Fig. 1). In spite of its relatively low degree of sequence similarity, P. putida tolR was able to complement the tolR mutant strain E. coli TPS300 (59) in terms of colicin A and colicin E3 tolerance and sensitivity, which demonstrated the existence of a high degree of functional similarity between both proteins in these bacteria (51). These results seem to contrast with those obtained by Dennis and coworkers (13) with the P. aeruginosa TolR protein (82% identical to P. putida TolR). These authors reported no complementation of the E. coli TPS300 strain with the P. aeruginosa tolR gene in terms of colicin E1 tolerance or sensitivity. However, the choice of colicin E1 to carry out these complementation studies is inappropriate since the E. coli TolR protein is not involved in the translocation of this colicin (27, 32). Nonetheless, we cannot discard the possibility that P. aeruginosa TolR could differ from P. putida TolR in some residue(s) critical for the complementation of the E. coli tolR mutation.
On the other hand, the existence of similarity between the E. coli TolQ-TolR-TolA and TonB-ExbB-ExbD protein complexes is well known (33). E. coli TolQ and TolR are in fact structurally and functionally homologous to ExbB and ExbD, respectively, and they are able to partially cross complement each other (10). As expected, P. putida TolQ and TolR were also very similar to P. putida ExbB and ExbD, respectively. Furthermore, in P. putida both systems were also similar in gene organization: tolQ-tolR-tolA (this work) and exbB-exbD-tonB (7). This supports the idea that these systems probably derive from a common ancestor. It has been reported that P. aeruginosa tolQ was able to complement an E. coli exbB mutant but not a P. putida exbB mutant (13). Perhaps this could be because P. putida ExbB (329 amino acids) is larger than E. coli ExbB protein (244 amino acids) and P. aeruginosa TolQ protein (231 amino acids). A relevant point concerning the possible role(s) of the Tol-PAL system is the recent finding that E. coli TolA could undergo conformational changes depending on TolQ, TolR, and the proton motive force (34). This would definitively confirm the relationship between the TolQ-TolR-TolA and the TonB-ExbB-ExbD complexes and could open new possibilities in the field of energy transduction between membranes.
Outer membrane integrity is altered in P. putida tol mutants.
In this study we constructed P. putida polar (by insertion of an interposon flanked by the transcriptional terminator of the phage T4 gene 32) and nonpolar mutations in each of the tol genes. The terminator activity of this T4 sequence has been previously demonstrated both in vivo and in vitro by other authors (49). We are currently studying the transcriptional organization of the P. putida tol-oprL gene cluster by different approaches (by primer extension analysis, measuring the catechol-2,3-dioxygenase activity in the tol::xylE mutants, and by Western blot analysis), and preliminary results strongly support the idea that, while the xylE cassette does not affect gene transcription, the Ω-Kmr interposon indeed acts as a transcriptional terminator in our system. On the other hand, we have also tried to complement the tol mutations by using different (medium- and low-copy-number) plasmids vectors bearing the whole gene cluster, the orf1, tolQ, and tolR genes, or the tolB gene alone, but we have been unable to obtain transconjugants (or transformants) which maintained these plasmids, even with the wild-type strain. These results suggest that even slight overexpression of the tol proteins in P. putida could be very toxic, and they are in agreement with other authors who suggest that the stoichiometry of the Tol complex is essential for its stability (5, 33). Our results could also explain why all our mutants (polar and nonpolar) exhibited similar phenotypes, since the lack of any component of the Tol system would cause an equivalent destabilization of this protein complex.
All mutant strains were viable although their survival during short-term storage (on LB plates at 4°C) and long-term storage (at −80°C) was reduced compared with that of the wild-type strain. Whereas on plates at 4°C the wild-type strain is viable for 3 months and for several years at −80°C, none of the tol mutants generated in this study survived longer than a month at 4°C on LB plates, and none were viable after 1 year at −80°C. This reduced viability could be related to alterations in the cell envelope of these mutants. Under SEM, mutant cells presented blebs at their cell surface. It should be noted that, when the mutant cells were visualized by TEM, the blebs were not present, which is probably due to the mechanical rupture of the blebs in the centrifugation steps used to collect and wash the cells during the fixation, as has been previously reported in the case of the E. coli lpo mutants (18). Blebbing was more frequent in the P. putida tolQ, tolR, and tolA than in the tolB and oprL mutants, which is in agreement with previous observations made in E. coli, where the tolQ, tolR, and tolA mutants presented a higher level of vesicle formation than the tolB and pal ones (5).
We analyzed the patterns of resistance and sensitivity of the different P. putida tol-oprL mutants to a variety of antibiotics and other chemical agents (SDS, DOC, and EDTA). All mutants were sensitive to a variety of compounds, although the P. putida BX (tolB) and P. putida DOT-OX2 (oprL) strains were the most susceptible to these drugs. Mills and Holloway (41) described a putative P. aeruginosa tol strain (selected by its tolerance to pyocin AP41) that showed specific hypersensitivity to aminoglycosides but not to other drugs. In P. putida, the tol-oprL mutations produced a wider antibiotic sensitivity pattern. The increased sensitivity to drugs of the P. putida tol-oprL mutants isolated in this study should not be specifically attributed to a defect in the hydrophobic barrier function of the outer membrane, to the inactivation of efflux pumps, or to any other particular deficiency in these strains, but rather it would seem that the tol-oprL mutants show a quite complex global permeability alteration.
Another striking phenotype exhibited by all the P. putida tol mutants was cell filamentation. This characteristic was also found in a presumed P. aeruginosa tol strain (selected as a spontaneous mutant tolerant to pyocin AP41) and in the V. cholerae tol mutants (23, 26). Meury and Devilliers (40) have reported that an E. coli tolA mutant showed cell filamentation when it was grown in conditions of low or high osmolarity. Within the filaments they observed the presence of oblique septa and numerous anucleate cells. Based on these results it was suggested that TolA could play a role in positioning the division sites. Our analysis of the P. putida tolA mutant cells under TEM did not reveal the above features. In fact, all P. putida tol strains formed relatively short filaments where the cells seemed to be in an advanced state of cell division. Hence, the P. putida Tol-OprL proteins could be directly or indirectly involved in the late stages of the cell division process, although they seemed not to be essential to complete this process.
The P. putida tol-oprL mutant strains also showed a leaky phenotype, releasing periplasmic and outer membrane proteins into the extracellular medium. In E. coli this phenotype was also observed with tol-pal strains (5). However, we found that P. putida did not release the OprL protein, while the homologous PAL protein was found in the outer membrane vesicles released by E. coli tol mutants (5). In short, our results show that strains with mutations in each of the tol-oprL genes of P. putida are viable although they exhibit severe defects in cell morphology and altered outer membrane structure and function.
ACKNOWLEDGMENTS
We thank Roland Lloubès and Danièle Cavard for giving us the anti-β-lactamase polyclonal antibody and Robert E. W. Hancock for providing monoclonal antibodies MA7-2 and MA1-6. We also express our appreciation to the Technical Services of University of Granada for their assistance with electron microscopic observations.
M. A. Llamas was the recipient of a fellowship from the Spanish Ministry of Education and Culture. This work was supported by grants from the Comisión Interministerial de Ciencia y Tecnología (FEDER IFD97-1437) and a grant from the European Commission (BIO4-CT97-2040).
REFERENCES
- 1.Abril M-A, Michán C, Timmis K N, Ramos J L. Regulator and enzyme specificities of the TOL plasmid-encoded upper pathway for degradation of aromatic hydrocarbons and expansion of the substrate range of the pathway. J Bacteriol. 1989;171:6782–6790. doi: 10.1128/jb.171.12.6782-6790.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 3.Amsterdam D. Susceptibility testing of antimicrobials in liquid media. In: Lorian V, editor. Antibiotics in laboratory medicine. Baltimore, Md: The Williams & Wilkins Co.; 1991. pp. 72–78. [Google Scholar]
- 4.Becker A, Kuester H, Niehaus K, Puehler A. Extension of the Rhizobium meliloti succinoglycan biosynthesis gene cluster: identification of the exsA gene encoding an ABC transporter protein, and the exsB gene which probably codes for a regulator of succinoglycan biosynthesis. Mol Gen Genet. 1995;249:487–497. doi: 10.1007/BF00290574. [DOI] [PubMed] [Google Scholar]
- 5.Bernadac A, Gavioli M, Lazzaroni J-C, Raina S, Lloubès R. Escherichia coli tol-pal mutants from outer membrane vesicles. J Bacteriol. 1998;180:4872–4878. doi: 10.1128/jb.180.18.4872-4878.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein A, Rolfe B, Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972;112:74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bitter W, Tommassen J, Weisbeek P J. Identification and characterization of the exbB, exbD and tonB genes of Pseudomonas putida WCS358: their involvement in ferric-pseudobactin transport. Mol Microbiol. 1993;7:117–130. doi: 10.1111/j.1365-2958.1993.tb01103.x. [DOI] [PubMed] [Google Scholar]
- 8.Blatny J M, Brautaset T, Winther-Larsen H C, Haugan K, Valla S. Construction and use of a versatile set of broad-host-range cloning and expression vectors based on the RK2 replicon. Appl Environ Microbiol. 1997;63:370–379. doi: 10.1128/aem.63.2.370-379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boyer H W, Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969;41:459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- 10.Braun V, Herrmann C. Evolutionary relationship of uptake systems for biopolymers in Escherichia coli: cross-complementation between the TonB-ExbB-ExbD and the TolA-TolQ-TolR proteins. Mol Microbiol. 1993;8:261–268. doi: 10.1111/j.1365-2958.1993.tb01570.x. [DOI] [PubMed] [Google Scholar]
- 11.Clavel T. Le système Tol-Pal d'Escherichia coli K12: organisation génétique et relation avec la synthèse de la capsule bactérienne. Ph.D. thesis. Lyon, France: University Claude Bernard; 1996. [Google Scholar]
- 12.Deich R A, Metcalf B J, Finn C W, Farley J E, Green B A. Cloning of genes encoding a 15,000-dalton peptidoglycan-associated outer membrane lipoprotein and an antigenically related 15,000-dalton protein from Haemophilus influenzae. J Bacteriol. 1988;170:489–498. doi: 10.1128/jb.170.2.489-498.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dennis J J, Lafontaine E R, Sokol P A. Identification and characterization of the tolQRA genes of Pseudomonas aeruginosa. J Bacteriol. 1996;178:7059–7068. doi: 10.1128/jb.178.24.7059-7068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Derouiché R, Bénédetti H, Lazzaroni J C, Lazdunski C, Lloubès R. Protein complex within Escherichia coli inner membrane. J Biol Chem. 1995;270:11078–11084. doi: 10.1074/jbc.270.19.11078. [DOI] [PubMed] [Google Scholar]
- 15.Fellay R, Frey J, Krisch H. Interposon mutagenesis of soil and water bacteria: a family of DNA fragments designed for in vitro insertional mutagenesis of Gram-negative bacteria. Gene. 1987;52:147–154. doi: 10.1016/0378-1119(87)90041-2. [DOI] [PubMed] [Google Scholar]
- 16.Fognini-Lefebvre N, Lazzaroni J C, Portalier R C. tolA, tolB, and excC, three cistrons involved in the control of pleiotropic release of periplasmic proteins by Escherichia coli K12. Mol Gen Genet. 1987;209:391–395. doi: 10.1007/BF00329670. [DOI] [PubMed] [Google Scholar]
- 17.Franklin F C H, Bagdasarian M, Bagdasarian M M, Timmis K N. Molecular and functional analysis of the TOL plasmid pWW0 from Pseudomonas putida and cloning of the genes for the entire regulated aromatic ring meta cleavage pathway. Proc Natl Acad Sci USA. 1981;78:7458–7462. doi: 10.1073/pnas.78.12.7458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fung J, MacAlister T J, Rothfield L I. Role of murein lipoprotein in morphogenesis of the bacterial division septum: phenotypic similarity of lkyD and lpo mutants. J Bacteriol. 1978;133:1467–1471. doi: 10.1128/jb.133.3.1467-1471.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Germon P, Clavel T, Vianney A, Portalier R, Lazzaroni J C. Mutational analysis of the Escherichia coli K-12 TolA N-terminal region and characterization of its TolQ-interacting domain by genetic suppression. J Bacteriol. 1998;180:6433–6439. doi: 10.1128/jb.180.24.6433-6439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hancock R E W. The bacterial outer membrane as a drug barrier. Trends Microbiol. 1997;5:37–42. doi: 10.1016/S0966-842X(97)81773-8. [DOI] [PubMed] [Google Scholar]
- 21.Hancock R E W, Karunaratne D N, Bernegger-Egli C. Molecular organization and structural role of outer membrane macromolecules. In: Ghuysen J-M, Hakenbeck R, editors. Bacterial cell wall. Amsterdam, The Netherlands: Elsevier; 1994. pp. 263–279. [Google Scholar]
- 22.Hancock R E W, Siehnel R, Martin N. Outer membrane proteins of Pseudomonas. Mol Microbiol. 1990;4:1069–1075. doi: 10.1111/j.1365-2958.1990.tb00680.x. [DOI] [PubMed] [Google Scholar]
- 23.Heilpern A J, Waldor M K. CTXΦ infection of Vibrio cholerae requires the tolQRA gene products. J Bacteriol. 2000;182:1739–1747. doi: 10.1128/jb.182.6.1739-1747.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heinrichs D E, Yethon J A, Whitfield C. Molecular basis for structural diversity in the core regions of the lipopolysaccharides of Escherichia coli and Salmonella enterica. Mol Microbiol. 1998;30:221–232. doi: 10.1046/j.1365-2958.1998.01063.x. [DOI] [PubMed] [Google Scholar]
- 25.Herrero M, de Lorenzo V, Timmis K N. Transposon vectors containing non-antibiotic resistance selection markers for cloning and stable chromosomal insertion of foreign genes in gram-negative bacteria. J Bacteriol. 1990;172:6557–6567. doi: 10.1128/jb.172.11.6557-6567.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holloway B W, Rossiter H, Burgess D, Dodge J. Aeruginocin tolerant mutants of Pseudomonas aeruginosa. Genet Res. 1973;22:239–253. doi: 10.1017/s0016672300013069. [DOI] [PubMed] [Google Scholar]
- 27.James R, Kleanthous C, Moore G R. The biology of E colicins: paradigms and paradoxes. Microbiology. 1996;142:1569–1580. doi: 10.1099/13500872-142-7-1569. [DOI] [PubMed] [Google Scholar]
- 28.Kaniga K, Delor I, Cornelis G R. A wide-host-range suicide vector for improving reverse genetics in Gram-negative bacteria: inactivation of the blaA gene of Yersinia enterocolitica. Gene. 1991;109:137–141. doi: 10.1016/0378-1119(91)90599-7. [DOI] [PubMed] [Google Scholar]
- 29.Kessler B, de Lorenzo V, Timmis K N. A general system to integrate lacZ fusions into the chromosomes of gram-negative eubacteria: regulation of the Pm promoter of the TOL plasmid studied with all controlling elements in monocopy. Mol Gen Genet. 1992;233:293–301. doi: 10.1007/BF00587591. [DOI] [PubMed] [Google Scholar]
- 30.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 31.Laird M W, Kloser A W, Misra R. Assembly of LamB and OmpF in deep rough lipopolysaccharide mutants of Escherichia coli K-12. J Bacteriol. 1994;176:2259–2264. doi: 10.1128/jb.176.8.2259-2264.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lazdunski C. Colicin import and pore formation: a system for studying protein transport across membranes? Mol Microbiol. 1995;16:1059–1066. doi: 10.1111/j.1365-2958.1995.tb02331.x. [DOI] [PubMed] [Google Scholar]
- 33.Lazdunski C J, Bouveret E, Rigal A, Journet L, Lloubès R, Bénédetti H. Colicin import into Escherichia coli cells. J Bacteriol. 1998;180:4993–5002. doi: 10.1128/jb.180.19.4993-5002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lazzaroni J C, Germon P, Ray M-C, Vianney A. The Tol proteins of Escherichia coli and their involvement in the uptake of biomolecules and outer membrane stability. FEMS Microbiol Lett. 1999;177:191–197. doi: 10.1111/j.1574-6968.1999.tb13731.x. [DOI] [PubMed] [Google Scholar]
- 35.Lazzaroni J C, Portalier R C. Genetic and biochemical characterization of periplasmic leaky mutants of Escherichia coli K-12. J Bacteriol. 1981;145:1351–1358. doi: 10.1128/jb.145.3.1351-1358.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lazzaroni J C, Vianney A, Popot J L, Bénédetti H, Samatey F, Lazdunski C, Portalier R C, Géli V. Transmembrane α-helix interactions are required for the functional assembly of the Escherichia coli Tol complex. J Mol Biol. 1995;246:1–7. doi: 10.1006/jmbi.1994.0058. [DOI] [PubMed] [Google Scholar]
- 37.Lim A, Jr, De Vos D, Brauns M, Mossialos D, Gaballa A, Qing D, Cornelis P. Molecular and immunological characterization of OprL, the 18 kDa outer-membrane peptidoglycan-associated lipoprotein (PAL) of Pseudomonas aeruginosa. Microbiology. 1997;143:1709–1716. doi: 10.1099/00221287-143-5-1709. [DOI] [PubMed] [Google Scholar]
- 38.Lugtenberg B, van Alphen L. Molecular architecture and functioning of the outer membrane of Escherichia coli and other gram-negative bacteria. Biochim Biophys Acta. 1983;737:51–115. doi: 10.1016/0304-4157(83)90014-x. [DOI] [PubMed] [Google Scholar]
- 39.Martin N L, Rawling E G, Wong R S Y, Rosok M, Hancock R E W. Conservation of surface epitopes in Pseudomonas aeruginosa outer membrane porin protein OprF. FEMS Microbiol Lett. 1993;113:261–266. doi: 10.1111/j.1574-6968.1993.tb06524.x. [DOI] [PubMed] [Google Scholar]
- 40.Meury J, Devilliers G. Impairment of cell division in tolA mutants of Escherichia coli at low and high medium osmolarities. Biol Cell. 1999;91:67–75. [PubMed] [Google Scholar]
- 41.Mills B J, Holloway B W. Mutants of Pseudomonas aeruginosa that show specific hypersensitivity to aminoglycosides. Antimicrob Agents Chemother. 1976;10:411–416. doi: 10.1128/aac.10.3.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mutharia L M, Hancock R E W. Monoclonal antibody for an outer membrane lipoprotein of the Pseudomonas fluorescens group of the family Pseudomonadaceae. Int J Syst Bacteriol. 1985;35:530–532. [Google Scholar]
- 43.Nikaido H. Porins and specific channels of bacterial outer membranes. Mol Microbiol. 1992;6:435–442. doi: 10.1111/j.1365-2958.1992.tb01487.x. [DOI] [PubMed] [Google Scholar]
- 44.Nikaido H. Transport across the bacterial outer membrane. J Bioenerg Biomembr. 1993;25:581–589. doi: 10.1007/BF00770245. [DOI] [PubMed] [Google Scholar]
- 45.Nikaido H. Outer membrane. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 29–47. [Google Scholar]
- 46.Nikaido H. Microdermatology: cell surface in the interaction of microbes with the external world. J Bacteriol. 1999;181:4–8. doi: 10.1128/jb.181.1.4-8.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nikaido H, Vaara M. Molecular basis of bacterial outer membrane permeability. Microbiol Rev. 1985;49:1–32. doi: 10.1128/mr.49.1.1-32.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norrander K, Kempe T, Messing K. Construction of improved M13 vectors using oligodeoxynucleotide-directed mutagenesis. Gene. 1983;26:101–106. doi: 10.1016/0378-1119(83)90040-9. [DOI] [PubMed] [Google Scholar]
- 49.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 50.Raetz C R H. Bacterial lipopolysaccharides: a remarkable family of bioactive macroamphiphiles. In: Neidhardt F C, Curtis III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1035–1063. [Google Scholar]
- 51.Rodríguez-Herva J J. Caracterización molecular del gen oprL de Pseudomonas putida. Ph.D. thesis. Granada, Spain: University of Granada; 1999. [Google Scholar]
- 52.Rodríguez-Herva J J, Ramos J L. Characterization of an OprL null mutant of Pseudomonas putida. J Bacteriol. 1996;178:5836–5840. doi: 10.1128/jb.178.19.5836-5840.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rodríguez-Herva J J, Ramos-González M I, Ramos J L. The Pseudomonas putida peptidoglycan-associated outer membrane lipoprotein is involved in maintenance of the integrity of the cell envelope. J Bacteriol. 1996;178:1699–1706. doi: 10.1128/jb.178.6.1699-1706.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rodríguez-Herva J J, Reniero D, Galli E, Ramos J L. Cell envelope mutants of Pseudomonas putida: physiological characterization and analysis of their ability to survive in soil. Environ Microbiol. 1999;1:479–488. doi: 10.1046/j.1462-2920.1999.00058.x. [DOI] [PubMed] [Google Scholar]
- 55.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 56.Sen K, Sikkema D J, Murphy T F. Isolation and characterization of the Haemophilus influenzae tolQ, tolR, tolA and tolB genes. Gene. 1996;178:75–81. doi: 10.1016/0378-1119(96)00338-1. [DOI] [PubMed] [Google Scholar]
- 57.Spinola S M, Hiltke T J, Fortney K, Shanks K L. The conserved 18,000-molecular-weight outer membrane protein of Haemophilus ducreyi has homology to PAL. Infect Immun. 1996;64:1950–1955. doi: 10.1128/iai.64.6.1950-1955.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Stein D C. Plasmids with easily excisable xylE cassettes. Gene. 1992;117:157–158. doi: 10.1016/0378-1119(92)90506-k. [DOI] [PubMed] [Google Scholar]
- 59.Sun T-P, Webster R E. Nucleotide sequence of a gene cluster involved in entry of E colicins and single-stranded DNA of infecting filamentous bacteriophages into Escherichia coli. J Bacteriol. 1987;169:2667–2674. doi: 10.1128/jb.169.6.2667-2674.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tibor A, Weynants V, Denoel P, Lichtfouse B, De Bolle X, Saman E, Limet J N, Letesson J J. Molecular cloning, nucleotide sequence, and occurrence of a 16.5-kilodalton outer membrane protein of Brucella abortus with similarity to PAL lipoproteins. Infect Immun. 1994;62:3633–3639. doi: 10.1128/iai.62.9.3633-3639.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Vianney A, Muller M M, Clavel T, Lazzaroni J C, Portalier R C, Webster R E. Characterization of the tol-pal region of Escherichia coli K-12: translational control of tolR expression by TolQ and identification of a new open reading frame downstream of pal encoding a periplasmic protein. J Bacteriol. 1996;178:4031–4038. doi: 10.1128/jb.178.14.4031-4038.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wray W, Boulikas T, Wray V P, Hancock R. Silver staining of proteins in polyacrylamide gels. Anal Biochem. 1981;118:197–203. doi: 10.1016/0003-2697(81)90179-2. [DOI] [PubMed] [Google Scholar]