Abstract
The corrinoids from the obligate anaerobe Clostridium cochlearium were extracted as a mixture of Coβ-cyano derivatives. From 50 g of frozen cells, approximately 2 mg (1.5 μmol) of B12 derivatives was obtained as a crystalline sample. Analysis of the corrinoid sample of C. cochlearium by a combination of high-pressure liquid chromatography and UV-Vis absorbance spectroscopy revealed the presence of three cyano corrinoids in a ratio of about 3:1:1. The spectroscopic data acquired for the sample indicated the main components to be pseudovitamin B12 (Coβ-cyano-7"-adeninylcobamide) (60%) and factor A (Coβ-cyano-7"-[2-methyl]adeninylcobamide) (20%). Authentic pseudovitamin B12 was prepared by guided biosynthesis from cobinamide and adenine. Both pseudovitamin B12 and its homologue, factor A, were subjected to complete spectroscopic analysis by UV-Vis, circular dichroism, mass spectrometry, and by one- and two-dimensional 1H, 13C-, and 15N nuclear magnetic resonance (NMR) spectroscopy. The third component was indicated by the mass spectra to be an isomer of factor A and is likely (according to NMR) to be 7"-[N6-methyl]-adeninylcobamide, a previously unknown corrinoid. C. cochlearium thus biosynthesizes as its native “complete” B12 cofactors the 7"-adeninylcobamides and two homologous corrinoids, in which the nucleotide base is a methylated adenine.
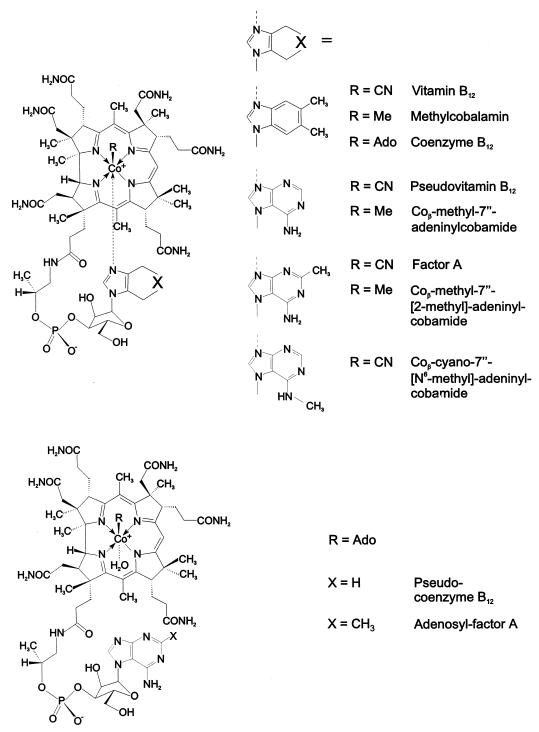
Besides vitamin B12 (cyanocobalamin) itself, a spectrum of corrinoids is provided in nature which differ from vitamin B12 by their cobalt-coordinating axial ligands (29). While the corrin moiety of the naturally occurring corrinoids is completely conserved structurally, a rationalized feature of (the catalytic moieties of) essential cofactors (24), the “complete” B12 derivatives contain a remarkable variety of aromatic nucleotide functions: benzimidazoles (such as 5-methylbenzimidazole, 5,6-dimethylbenzimidazole [DMB], 5-hydroxybenzimidazole, and 5-methoxybenzimidazole), purine bases (such as adenine or 2-methyladenine) (see Fig. 1) or phenols (phenol and p-cresol) (29, 43, 61, 67). In contrast to the situation in the other widespread adenine-containing dinucleotide cofactors, in pseudovitamin B12 (Coβ-cyano-7"-adeninylcobamide) and in factor A (Coβ-cyano-7"-[2-methyl]adeninylcobamide; Fig. 1), the ribose function is part of an unusual α-nucleotide moiety and binds to N7 of the purine ring. This latter property has been suggested by D. C. Hodgkin to be required in adeninylcobamides in order to enable the purine base to coordinate intramolecularly to the corrin-bound cobalt center via its N9 (30, 31, 41).
FIG. 1.
Structural formulae of vitamin B12 derivatives. The general formulae are given on the left; variation of the organic ligand R (Me, methyl; Ado, 5′-deoxy-5′-adenosyl) and/or of the nucleotide base give rise to the different cobamides. (Top) Base-on cobamides vitamin B12 (cyanocobalamin), methylcobalamin, coenzyme B12 (5′-deoxy-5′-adenosylcobalamin), pseudovitamin B12 (Coβ-cyano-7"-adeninylcobamide), Coβ-methyl-7"-adeninylcobamide, factor A (Coβ-cyano-7"-[2-methyl]adeninylcobamide), Coβ-methyl-7"-[2-methyl]adeninylcobamide, and Coβ-cyano-7"-[N6-methyl]adeninylcobamide. (Bottom) Base-off cobamides pseudocoenzyme B12 (5′-deoxy-5′-adenosyl-7"-adeninylcobamide) and adenosyl factor A (5′-deoxy-5′-adenosyl-7"-[2-methyl]-adeninylcobamide).
Three years after the discovery of vitamin B12 (62, 64), pseudovitamin B12 was isolated from an incompletely identified microorganism that was obtained from rumen contents (20). In 1958 the coenzyme form of pseudovitamin B12 (i.e., pseudocoenzyme B12) was discovered and identified by UV and visible (UV-Vis) spectroscopy to be the native cofactor of Clostridium tetanomorphum (2). Factor A was the main component of a mixture of corrinoids which were isolated in a crystalline form from pig manure, bovine gut contents, and feces (25, 26, 29). Pseudovitamin B12 and factor A often occur in anaerobically fermenting systems, such as ruminal (7) and intestinal (12) contents, feces, and sewage sludge (29). The crystal structure of factor A was determined in 1981 and confirmed the presence of an α-nucleotide and binding of N9 of the purine ring to the corrin-bound cobalt center (41). The different nucleotide bases in factor A and in vitamin B12 lead only to small differences in the axial bond lengths, in bond angles, and in the gross three-dimensional (3D) structures of vitamin B12 and factor A. The axial bond lengths of factor A [(Co—C) bond length = 1.86 Å and (Co—N) bond length = 2.12 Å] (41) differ only marginally from those of vitamin B12 [(Co—C) bond length = 1.86 Å and (Co—N) bond length = 2.01 Å] (45). The longer (Co—N) bond in factor A clearly is the consequence of a weaker coordinating adenine heterocycle compared to the more nucleophilic DMB base of vitamin B12.
Pseudovitamin B12 and factor A have been found specifically in a variety of microorganisms, e.g., in Clostridium (2, 28), Propionibacterium (29), and methanogenic (pseudovitamin B12) bacteria (66). Remarkably, Salmonella enterica serovar Typhimurium synthesizes cobalamins under microaerophilic conditions, while pseudovitamin B12 and factor A are found in serovar Typhimurium, when grown strictly anaerobically (39). Whereas aerobic microorganisms have developed a particularly efficient way to biosynthesize the DMB base of their cobalamins from flavine, the biosynthetic access to DMB is now known to be a complex task in obligate anaerobes (60).
Guided biosynthesis opens access to natural but commercially unavailable complete corrinoids and often is also applicable to the synthesis of complete cobamides, whose nucleotide base differs from the known ones, by supplementing the bacterial culture with a suitable nucleotide base precursor (see, for example, reference 29). Early on, the preparation by “guided biosynthesis” of pseudovitamin B12 and factor A was explored, e.g., by means of Escherichia coli, by adding both cobinamide and adenine or 2-methyladenine to the growth medium (27). A range of related fermentation methods for the production of these purinylcobamides were developed, based on other microorganisms, most notably Propionibacterium spp. (13). In line with this, guided biosynthesis has also been employed for the preparation of nonnatural cobamides, such as naphthimidazolyl- and imidazolylcobamides (22, 45, 59, 65), using cultures of Propionibacterium freudenreichii and P. shermanii.
The nature and the role of the nucleotide base in complete corrinoids has attained renewed interest lately (43): electron spin resonance (ESR) spectroscopic work by Stupperich et al. revealed the coordination of histidine to the protein-bound phenolyl-cobamides in the homoacetogenic Sporomusa ovata (67). On the other hand, the bound corrinoid in the corrinoid–iron-sulfur protein involved in the synthesis of acetyl-coenzyme A from Clostridium thermoaceticum was indicated by spectroscopic means to be “base-off,” i.e., to have the nucleotide base of the corrinoid decoordinated in the protein (57). A pioneering structure analysis by Drennan et al. of the B12-binding domain of a methionine synthase from E. coli that depends upon methylcobalamin as a cofactor (21, 51) uncovered the “base-off–His-on” form of the protein-bound complete methylcorrinoid, in which a proteinic histidine residue displaces the cobalt-coordinating DMB base of the corrinoid cofactor. The relevance of the base-off–His-on mode of binding of corrinoids in cobamide-dependent enzymes (46, 72) was supported further by two additional crystal structures, of methylmalonyl coenzyme A mutase (52) and glutamate mutase (58), which both depend upon an adenosylcobamide as a cofactor, such as coenzyme B12 (5′-deoxy-5′-adenosylcobalamin). In contrast, the crystal structure of a diol dehydratase showed the bound coenzyme B12 in its “classic” base-on constitution (63). Accordingly, questions concerning the effect of the nucleotide base on the binding the B12 cofactors by their apoproteins (35, 53, 69) and on the mechanisms of B12-dependent enzymatic reactions have received more attention (15, 43).
Ongoing studies in our laboratories concern adenosylcobamide-dependent glutamate mutase from C. cochlearium (9, 35), as well as from C. tetanomorphum (55, 69), which catalyzes the conversion of l-glutamate to (2S,3S)-3-methyl aspartate, a particularly intriguing mechanistic puzzle (9, 15). In connection with this work, the crystal structure of inactive recombinant glutamate mutase from C. cochlearium, reconstituted with vitamin B12 and methylcobalamin, was determined recently (58). Experiments concerning B12 binding and enzyme catalysis (6, 10, 17, 37, 54, 72) have been carried out with coenzyme B12, although pseudocoenzyme B12 is known to be the natural cofactor in C. tetanomorphum (2). The identity of the native cofactor(s) of the related C. cochlearium was therefore of interest.
We report here the identification of two of the three native corrinoids of C. cochlearium in their stable cyano-Co(III) form by means of a comparison of their UV-Vis, circular dichroism (CD), mass, and nuclear-magnetic-resonance (NMR) spectra with the spectra of authentic samples of pseudovitamin B12 and factor A. Authentic samples of pseudovitamin B12, prepared by guided biosynthesis using Propionibacterium acidi-propionici, and of factor A, were both subjected to detailed spectroscopic analysis. In particular, complete NMR signal assignment of both pseudovitamin B12 and factor A was carried out to achieve the first thorough spectroscopic analysis of purine analogues of vitamin B12. A third corrinoid compound was found, which was tentatively assigned the structure of the novel 7"-[N6-methyl]adeninylcobamide. In this way, the corrinoid sample from C. cochlearium was shown to contain three cobamides, pseudovitamin B12, factor A, and an unknown isomer of factor A, in a ratio of ca. 3:1:1.
MATERIALS AND METHODS
Strains and sources of organisms.
The strains used were P. acidi-propionici DSM 20273 and C. cochlearium DSM 1285.
Reagents and solvents.
Crystalline factor A was from the collection of the late W. Friedrich and presumably originated from sewage sludge (P. Renz, personal communication). Cobinamide was prepared by degradation of an aqueous solution of crystalline cyanocobalamin (Hoffmann-LaRoche, Basel, Switzerland) with cerous hydroxide by adaptation of the method described by Renz (59). Separation of cobinamide and α-d-ribazole formed in this reaction was achieved by preparative high-pressure liquid chromatography (HPLC) on an RP-18 column. XAD adsorbent resin (Serdolit AD-4; particle size, 0.1 to 0.2 mm; research grade [Serva, Heidelberg, Germany]) was used. Analytical HPLC was done on an ODS-Hypersil column (5 μm; 250 by 4.6 mm inner diameter, with Phenomenex Security Guard column protection containing two 4-mm C-18 cartridges). The pump used was a Gynkotek model M480G with a vacuum on-line degasser, with a methanol-water (20:80 [vol/vol]) eluent and a flow rate of 1 ml/min. The injection volume was 20 μl, and the detector was a Gynkotek diode array detector UVD340 with detection at 355 nm. All chromatograms were obtained at 18°C, and data were processed by the Gynkotek HPLC data system Gynkosoft 5.50. All other chemicals were of the highest purity and were purchased from Fluka (Neu-Ulm, Germany) Merck (Darmstadt, Germany), or Aldrich (Steinheim, Germany).
Preparation of pseudovitamin B12 by guided biosynthesis.
P. acidi-propionici was grown in a 100-liter fermentor which contained 1 kg of casein hydrolysate, 500 g of yeast extract, 170 g of NaH2PO4 · H2O, and 180 g of K3PO4 · H2O. The solution was sterilized at 120°C for 30 min. The medium was completed by adding autoclaved aqueous solutions of 750 g of glucose (1.5 liters), 60 g of MgCl2 · 6H2O, 860 mg of FeCl2 · 4H2O, and 1.7 g of CoCl2 · 6H2O (all 50 ml), as well as a sterile filtered solution of 400 mg of Ca-panthotenate, 30 mg of D-biotin, 1 g of cobinamide, and 10 g of adenine. The pH was adjusted to 6 to 7 by the addition of 400-ml batches of a solution containing 80 g of KHCO3 and 20 g of NaHCO3, and the temperature was kept at 37°C. The medium was inoculated with a 2-liter freshly grown culture of P. acidi-propionici. To prevent acidification of the batches during cell growth, the medium was further buffered with bicarbonate solution to keep the pH in the range of 6 to 7 and was supplied batchwise with aqueous glucose solution (250 g/500 ml). After 5 days of incubation, approximately 3 kg (wet weight) of cell material was harvested by centrifugation. The cells were resuspended in 5 liters of 0.1 M acetic acid (pH adjusted to 5.0 by NaOH) made 2 mM in cyanide by the addition of KCN and kept in closed half-filled, 1-liter bottles. The suspensions were incubated at 100°C for 20 min. A slightly red-colored cobamide-containing supernatant was obtained after centrifugation. The cell paste was reextracted once as described above. Portions of the pooled supernatants were run over a column of neutral aluminium oxide (3 by 10 cm) and loaded onto an XAD-4 column (3 by 10 cm; XAD grain diameter, 0.1 to 0.2 mm) to adsorb the cobamide. Prior to use, the XAD column was washed with 0.1 M KOH in methanol and then equilibrated with water. The cobamide-loaded column was rinsed with 10 bed volumes of water before eluting the cobamide with 80% methanol. The pooled methanol fractions were flash evaporated to dryness at 40°C. The residue was completely dissolved in approximately 20 ml of water. Further purification was performed by preparative HPLC (65) using an automated gradient controller and an RP-18 column (Nucleosil 120 C18; 1 by 25 cm [Marchery & Nagel]). Degassed methanol and 0.1% acetic acid were mixed as solvent components A and B and applied in two different systems at a flow rate of 3 ml/min. Solvent system I (23% A–77% B) led to the elution of pseudovitamin B12 after about 15 min; this was observed with a two-wavelength detector operating at 254 and 546 nm. Solvent system II (70% A–30% B, reached by a linear gradient within 10 min) was applied for approximately 30 min in order to elute the matrix. A linear program reversal reestablished the original conditions. The corrinoid-containing HPLC fractions were combined and flash evaporated to dryness. By crystallization from water-acetone, 230 mg (171 μmol) of pure pseudovitamin B12 (dried at 2 Pa, 6 h) was obtained. The sample of pseudovitamin B12 was subjected to UV-Vis, CD, mass, and NMR spectroscopic analysis as described below.
Isolation and purification of the corrinoids from C. cochlearium.
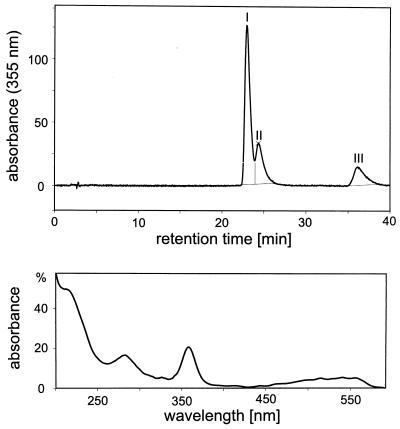
A culture of C. cochlearium was grown anaerobically as reported elsewhere (49). Cells were harvested aerobically and stored at −80°C. The isolation and purification of the corrinoids from C. cochlearium in the cyano form were carried out as described above for the isolation of pseudovitamin B12. The amounts of solvents used in the purification procedure were adapted to the amount of C. cochlearium cell material. From 50 g of frozen cells a sample of approximately 2 mg of crystalline corrinoids was obtained (corresponding to 30 nmol of corrinoids/g [wet weight]). This sample of corrinoids from C. cochlearium was subsequently analyzed by HPLC and by UV-Vis, mass, and NMR spectroscopy. HPLC chromatograms of the sample showed three fractions: I (retention time [RT] = 22.96 min, 63% relative integral area [RIA]), II (RT = 24.35 min, 22% RIA), and III (RT = 36.10 min, 15% RIA). All three components had highly similar UV-Vis spectra (200 to 600 nm) which showed no significant difference from those of pseudovitamin B12 and of factor A. By peak matching, fraction I was identified with pseudovitamin B12 and fraction III was identified with factor A (see Fig. 3).
FIG. 3.
(Top) HPLC chromatogram of the corrinoid sample isolated from C. cochlearium. (Bottom) UV-Vis spectra of the new corrinoid (fraction II of the HPLC chromatogram) (see Materials and Methods for technical details).
Spectroscopic data.
UV-Vis and CD spectra were measured on Hitachi U-3000 and on Jasco-J715 instruments, respectively. Fast-atom-bombardment (FAB) MS spectra were recorded on a Finnigan MAT95 spectrometer (nitrobenzyl alcohol matrix, Cs+ bombardment). NMR spectra were obtained on a Varian 500 Unity Plus spectrometer equipped with field gradient facilities, a 5-mm indirect detection and 5-mm broadband direct detection probe (499.887 MHz, 1H; 125.15 MHz, 13C; and 50.66 MHz, 15N). NMR solutions had a sample size of 0.7 ml at 26°C. The solvents included 90% 10 mM phosphate buffer (pH 5.2) and 10% D2O. 1H, 13C, and 15N signal assignments were from 1H NMR spectra with water presaturation; 1H-broad-band-decoupled 13C NMR spectra; 1H,13C gradient-enhanced heteronuclear single-quantum coherence experiments (PFG-HSQC) (19, 38); 1H,15N gradient-enhanced heteronuclear single-quantum coherence experiments (PFG-HSQC) (19, 38), 2D gradient-enhanced heteronuclear multiple-bond coherence experiments (PFG-HMBC) (5, 38), 2D total correlation spectroscopy (watergate-TOCSY) (3, 14, 18, 56), and 2D rotating frame Overhauser enhancement spectroscopy (watergate-ROESY) (4, 11, 56). All 2D NMR experiments were parametrized as described earlier (42). The 2D gradient-enhanced HSQC-TOCSY (PFG-HSQC-TOCSY) experiment (8, 34) was parametrized as described elsewhere (68).
Pseudovitamin B12.
For UV-Vis analyses (c = 6 · 10−4 M), with λmax expressed in nanometers and where s denotes the shoulders, the λmax (log ɛ) values were 276(4.26), 306(3.92), 321(3.89), 343s(4.11), 360(4.44), 410(3.53), 479s(3.67), 518(3.87), and 548(3.90). For CD analyses (c = 6 · 10−4 M), where the wavelengths of the extrema λmax and λmin and of the zero passages λ0 are given in nanometers, the molar decadic CD is indicated by Δɛ, and s denotes the shoulder the λmax/λmin(Δɛ) values were as follows: 578(1.48), 530s(−2.29), 488(−6.39), 429(14.13), 362(−11.21), 353(−7.97), 349(−8.38), 333(−4.77), 323(−6.82), 297(−0.35), 284s(−1.09), 269(−4.08), 258(−3.23), 247(−6.29), and 235(1.82). The λ0 values were 559, 462, 381, 238, and 232. For FAB-MS analyses, the positive-ion spectra, expressed as m/z (relative percent intensity) were 1,347.8(8), 1,346.8(29), 1,345.8(76), 1,344.8(100, MH+), 1,343.8(9), 1,342.8(10), 1,320.8(7), 1,319.8(15), 1,318.8(27, MH+-CN), 1,317.8(19),and 1,316.8(23). For NMR analyses (c = 10 mM; see Fig. 4 for a 500-MHz 1H NMR spectrum), a complete listing of assigned 1H, 13C, and 15N signals is given in Tables 1 and 2. For the atom numbering of B12 derivatives, see Fig. 2.
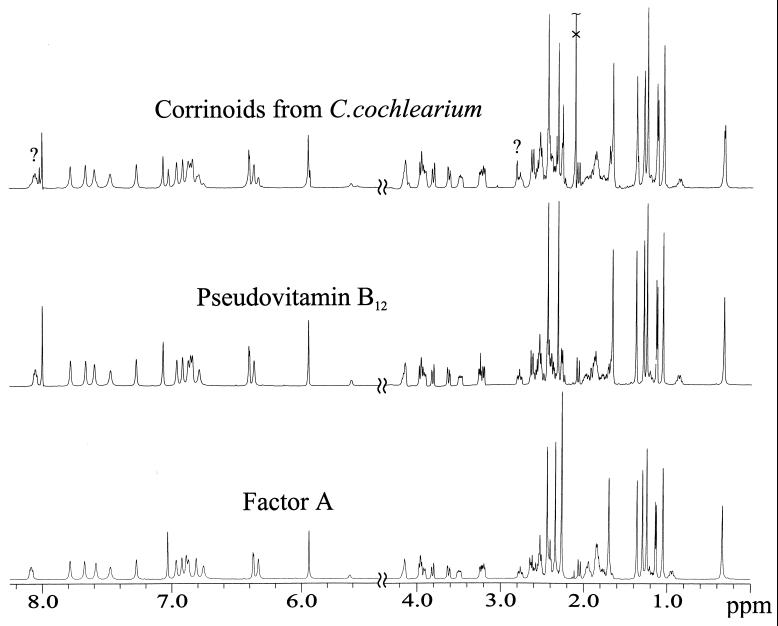
FIG. 4.
The 500-MHz 1H NMR spectra of aqueous solutions of pseudovitamin B12, factor A, and the corrinoid sample isolated from C. cochlearium are shown. Two signals, tentatively assigned to 7"-[N6-methyl]adeninylcobamide, are labeled with a question mark.
TABLE 1.
1H and 13C NMR chemical shifts and signal assignments for pseudovitamin B12, factor A, and vitamin B12a
| Assignment | Pseudovitamin B12a
|
Factor Aa
|
Vitamin B12b
|
|||
|---|---|---|---|---|---|---|
| δ(1H) (ppm) | δ(13C)c (ppm) | δ(1H) (ppm) | δ(13C) (ppm) | δ(1H) (ppm) | δ(13C) (ppm) | |
| β-CN | 124.2 | NM | ||||
| C1 | 87.9 | 88.5 | 87.9 | |||
| C1A | 0.33 | 22.2 | 0.34 | 22.8 | 0.34 | 22.2 |
| C2 | 50.4 | 50.9 | 50.1 | |||
| C21 | 2.27 | 45.9 | 2.27 | 46.2 | 2.29 | 45.6 |
| C22 | 179.5 | 180.0 | 178.6 | |||
| C2A | 1.29 | 19.8 | 1.29 | 20.0 | 1.29 | 19.6 |
| C3 | 3.94 | 59.5 | 3.96 | 60.2 | 4.06 | 59.2 |
| C31 | 1.86(Hsi) | 28.5 | 1.85(Hsi) | 29.3 | 1.84 | 28.8 |
| C31 | 1.98(Hre) | 28.5 | 1.95(Hre) | 29.3 | 28.8 | |
| C32 | 2.40 | 38.2 | 2.34(Hre) | 38.7 | 2.39 | 37.8 |
| C32 | 2.40 | 38.2 | 2.42(Hsi) | 38.7 | 2.47 | 37.8 |
| C33 | 182.1 | 182.1 | 180.7 | |||
| C4 | 183.2 | 183.7 | 182.8 | |||
| C5 | 111.7 | 112.4 | 110.3 | |||
| C51 | 2.31 | 18.9 | 2.34 | 19.0 | 2.43 | 18.2 |
| C6 | 167.6 | 167.9 | 168.1 | |||
| C7 | 53.8 | 54.6 | 54.2 | |||
| C71 | 2.08(Hre) | 46.5 | 2.05(Hre) | 47.1 | 2.09 | 45.9 |
| C71 | 2.44(Hsi) | 46.5 | 2.42(Hsi) | 47.1 | 2.48 | 45.9 |
| C72 | 179.4 | 179.5 | 177.9 | |||
| C7A | 1.66 | 22.1 | 1.70 | 22.4 | 1.76 | 21.9 |
| C8 | 3.25 | 59.5 | 3.24 | 59.9 | 3.31 | 58.5 |
| C81 | 0.87(Hre) | 29.1 | 0.95(Hre) | 29.3 | 28.8 | |
| C81 | 1.78(Hsi) | 29.1 | 1.85(Hsi) | 29.3 | 1.91 | 28.8 |
| C82 | 1.22(Hre) | 35.1 | 1.22(Hre) | 34.9 | 0.91 | 34.6 |
| C82 | 1.72(Hsi) | 35.1 | 1.79(Hsi) | 34.9 | 1.74 | 34.6 |
| C83 | 181.9 | 181.6 | 180.0 | |||
| C9 | 176.9 | 177.4 | 176.4 | |||
| C10 | 5.94 | 97.3 | 5.94 | 97.6 | 5.98 | 97.7 |
| C11 | 179.3 | 179.5 | 179.7 | |||
| C12 | 51.0 | 51.6 | 50.9 | |||
| C12A | 1.38 | 22.0 | 1.36 | 22.4 | 1.34 | 22.1 |
| C12B | 1.06 | 34.4 | 1.05 | 34.8 | 1.09 | 34.1 |
| C13 | 3.20 | 57.1 | 3.20 | 57.4 | 3.22 | 56.5 |
| C131 | 1.87 | 31.3 | 1.85 | 31.2 | 1.87 | 30.8 |
| C132 | 2.54 | 37.8 | 2.53 | 38.4 | 2.52 | 37.5 |
| C133 | 182.1 | 182.1 | 181.0 | |||
| C14 | 169.6 | 170.5 | 168.8 | |||
| C15 | 105.9 | 106.4 | 106.9 | |||
| C151 | 2.44 | 18.1 | 2.44 | 18.1 | 2.46 | 18.0 |
| C16 | 181.8 | 182.7 | 181.7 | |||
| C17 | 62.4 | 63.0 | 62.0 | |||
| C171 | 1.68(Hsi) | 36.0 | 1.69(Hsi) | 36.5 | 1.71 | 35.3 |
| C171 | 2.51(Hre) | 36.0 | 2.52(Hre) | 36.5 | 2.40 | 35.3 |
| C172 | 1.92(Hre) | 35.7 | 1.94(Hre) | 35.8 | 2.01 | 34.3 |
| C172 | 2.37(Hsi) | 35.7 | 2.40(Hsi) | 35.8 | 2.55 | 34.3 |
| C173 | 178.3 | 178.9 | 177.5 | |||
| C17B | 1.25 | 19.6 | 1.24 | 20.0 | 1.28 | 18.8 |
| C18 | 2.64 | 42.0 | 2.64 | 42.4 | 2.65 | 41.9 |
| C181 | 2.57 | 34.9 | 2.58 | 35.0 | 2.57 | 35.0 |
| C181 | 2.62 | 34.9 | 2.62 | 35.0 | 2.64 | 35.0 |
| C182 | 179.5 | 180.1 | 178.5 | |||
| C19 | 3.97 | 78.4 | 3.97 | 78.9 | 4.00 | 77.7 |
| C175 | 2.78 | 48.71 | 2.77 | 49.0 | 2.85 | 48.3 |
| C175 | 3.48 | 48.71 | 3.50 | 49.0 | 3.50 | 48.3 |
| C176 | 4.16 | 76.12 | 4.17 | 76.1 | 4.19 | 75.8 |
| C177 | 1.14 | 22.03 | 1.14 | 22.3 | 1.15 | 21.8 |
| C1R | 6.40 | 92.2 | 6.37 | 93.0 | 6.25 | 89.8 |
| C2R | 4.14 | 73.34 | 4.15 | 74.3 | 4.17 | 71.7 |
| C3R | 4.57 | 75.75 | 4.59 | 76.9 | 4.62 | 75.9 |
| C4R | 3.91 | 85.86 | 3.92 | 85.9 | 3.93 | 84.9 |
| C5R | 3.62 | 63.3 | 3.63 | 63.5 | 3.64 | 63.3 |
| C5R | 3.81 | 63.3 | 3.81 | 63.5 | 3.82 | 63.3 |
| O2R | 5.61 | 5.62 | ||||
| C2N | 8.00 | 156.6 | 166.3 | 135.8(DMB-C5N) | ||
| C4N | 113.1 | 111.8 | 139.5(DMB-C9N) | |||
| C5N | 160.7 | 161.5 | 132.8(DMB-C8N) | |||
| C6N | 155.1 | 155.7 | 114.3(DMB-C7N) | |||
| C8N | 7.06 | 147.7 | 7.03 | 147.7 | 7.10 | 144.7(DMB-C2N) |
| C21N | 2.26 | 28.4 | 2.16 | 22.8(DMB-C10N) | ||
1H NMR, δ with δ(H2O)int = 4.67 ppm; 13C NMR, δ with δ(TSP)ext = 0.0 ppm. NM, not measured.
All chemical shifts are from Calafat and Marzilli (16). 1H chemical shifts are referenced to H2O by subtracting 0.11 ppm to the reported values (relative to TSP).
31P-13C couplings: 1, JCP = 4.8 Hz; 2, JCP = 6.2 Hz; 3, JCP no information because of signal overlap; 4, JCP < 1 Hz; 5, JCP = 2.8 Hz; 6, JCP = 7.0 Hz.
TABLE 2.
Amide group 1H and 15N NMR chemical shifts for pseudovitamin B12 and factor A
| Assignment | Pseudovitamin B12a
|
Factor Aa [δ(1H)] (ppm) | |
|---|---|---|---|
| δ(1H) (ppm) | δ(15N) (ppm) | ||
| N23 | 6.96(HZ)/7.66(HE) | 110.8 | 6.97(HZ)/7.67(HE) |
| N34 | 6.79(HZ)/7.47(HE) | 104.7 | 6.76(HZ)/7.47(HE) |
| N73 | 6.84(HZ)/7.27(HE) | 109.5 | 6.89(HZ)/7.27(HE) |
| N84 | 6.36(HE)/6.85(HZ) | 102.6 | 6.33(HE)/6.81(HZ) |
| N134 | 6.87(HZ)/7.59(HE) | 105.3 | 6.87(HZ)/7.59(HE) |
| N174 | 8.05 | 113.4 | 8.09 |
| N183 | 6.92(HZ)/7.78(HE) | 106.3 | 6.92(HZ)/7.79(HE) |
1H NMR, δ with δ(H2O)int = 4.67 ppm; 15N NMR, δ with δ[NH3(l)]ext = 0.0 ppm.
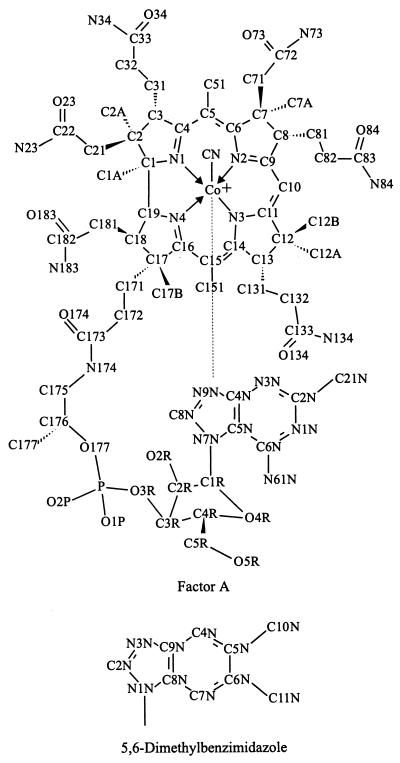
FIG. 2.
Atom numbering of pseudovitamin B12 (and factor A) used for the description of NMR results (44). The atom numbering for the DMB of vitamin B12 is also shown.
Factor A.
For UV-Vis analyses (c = 6 · 10−4 M), the λmax(log s(4.07), 361(4.41), 408(3.50), 480s(3.62), 517(3.84), and 548(3.87). For CD analyses (c = 6 · 10−4 M), the λmax/λmin(Δɛ) values were 580(0.62), 541(−2.60), 533(−2.44), 492(−5.84), 430(13.18), 387s(0.38), 362(−11.66), 352s(−7.86), 334(−4.38), 324(−6.78), 313s(−4.58), 296(−1.39), 292(−1.43), 284(−0.58), 271(−2.39), 258(−0.96), 247(−3.98), and 235(3.50). The λ0 values were 563, 461, 382, 241, and 230. For FAB-MS analyses, the m/z (relative percent intensity) values were 1,361.5(8), 1,360.6(25), 1,359.6(77), 1,358.5(100.0, MH+), 1,357.5 (7), 1,333.6(13), 1,332.6(28, MH+-CN), 1,331.6(16), and 1,330.6(16). For NMR analyses (c = 10 mM; see Fig. 4 for a 500-MHz 1H NMR spectrum), a complete listing of assigned 1H, 13C, and 15N signals is given in Tables 1 and 2. For the atom numbering of B12 derivatives, see Fig. 2.
Sample of cyano corrinoids from C. cochlearium.
For HPLC analyses, the three fractions were as follows: I, RT = 23 min (65%); II, RT = 24.4 min (20%); III, RT = 36.1 min (15%). For UV/Vis analyses, the λmax values were 276, 305s, 321, 360, 410, 517, and 547. For CD analyses, the values were 575, 429, 386s, and 236, the λmin values were 542, 534, 492, 364, 351, 347, 333, 323, 311s, 296, 290, 284, 268, 261, and 248, and the λ0 values were 563, 459, 384, 238, and 233. For FAB-MS analyses, 1,361.8(8), 1,360.8(22), 1,359.8(50), 1,358.8(65.5), 1,357.8(13), 1,356.8(15), 1,347.8(9), 1,346.8(30), 1,345.8(70), 1,344.8(100), 1,343.8(11), 1,342.8(14), 1,333.8(10), 1,332.8(16), 1,331.8(15), 1,330.8(19), 1,320.8(6), 1,319.8(14), 1,318.8(23), 1,317.8(19), and 1,316.8(25). For NMR analyses (c = 1.5 mM), see Fig. 4 for a 500-MHz 1H NMR spectrum.
RESULTS
Guided biosynthesis of pseudovitamin B12.
From about 3 kg of wet cells of a 100-liter P. acidi-propionici fermentation, supplemented with approximately 100 mg of adenine/liter of medium, 10 mg of cobinamide/liter, and 17 mg of CoCl2 · 6H2O/liter, a sample of about 230 mg (171 μmol, 20% yield relative to cobinamide) of bright-red crystalline pseudovitamin B12 was obtained and subjected to spectral analysis, as described below.
Spectroscopic analysis of pseudovitamin B12 and of factor A.
Aqueous solutions of pseudovitamin B12 and factor A exhibit practically identical UV-Vis and CD spectra. At wavelengths of >300 nm they are similar to the spectrum of vitamin B12 (29, 33). The UV-Vis spectra of both purinylcobamides in aqueous solution display typical maxima at 548, 518, and 360 nm (α, β, and γ bands) (33) and at 276 nm (band of the coordinating nucleotide), as described earlier (see, for example, reference 29). The spectra are consistent with the structures of complete base-on corrinoids, with one cyanide ligand bound to the cobalt center.
The FAB mass spectra of the cyano corrins pseudovitamin B12 and factor A displayed base peaks at m/z = 1,344.8 and 1,358.5, respectively, arising from the intact pseudomolecular ions (MH+). In addition prominent fragments (MH+-CN) due to loss of the cyanide ligand occurred at m/z = 1,318.8 for pseudovitamin B12 and at m/z = 1,332.6 for factor A. The FAB mass spectra confirmed the molecular formulae C59H83CoN17O14P for pseudovitamin B12 and C60H85CoN17O14P for factor A.
Very specific information on the structure of pseudovitamin B12 and factor A in aqueous solutions was obtained from thorough NMR spectroscopic investigations. Complete assignment of all but three exchange labile hydroxyl and amino protons of the nucleotide moiety and of all carbons was obtained for the spectra of pseudovitamin B12 and factor A. Assignment of 1H and 13C signals was obtained from heteronuclear (1H,13C PFG-HSQC, 1H,13C PFG-HMBC, and 1H,13C PFG-HSQC-TOCSY) and homonuclear correlations (watergate-TOCSY and watergate-ROESY). The 13C chemical shift of the axially bound cyanide group of pseudovitamin B12 could be identified by its broad signal in the 1D proton decoupled 13C spectrum. Assignments and chemical shifts of the signals in the 1H, 13C, and 15N spectra are listed in Tables 1 and 2; see Fig. 4 for the 500-MHz 1H NMR spectra.
With the exception of resonances originating from the constitutionally different nucleotide bases, the 1H and 13C chemical shifts of pseudovitamin B12 and factor A differ only slightly. The largest 1H shift difference is observed (Δδ = 0.08 ppm) for Hre(C81); the largest 13C shift difference is observed (Δδ = 1.2 ppm) for CR3. An unambiguous assignment (pro-R or pro-S) of the signals of all the magnetically nonequivalent methylene hydrogens of pseudovitamin B12 and factor A could be derived from analysis of nuclear Overhauser effects (NOEs) involving diastereotopic acetamide and propionamide side chain methylene protons. The 1H,15N PFG-HSQC spectra of pseudovitamin B12 in combination with watergate-ROESY spectra allowed the assignment of all seven side chain amide nitrogens at natural isotopic (15N) abundance, together with their directly bonded amide protons (Table 2). With the exception of the d-side chain, for each amide group the low-field proton signal was assigned to HE due to the ROESY cross-peaks displayed between the amide protons and side chain methylene protons, since only HE can be close in space to the α-methylene protons of the carboxamide functions. Such an inverse assignment concerning the d-side chain amide protons has already been reported, e.g., in NMR studies on methylcobalamin (68). This assignment is compatible with a specific shielding effect of the nearby cobalt-coordinated nucleotide base affecting both amide protons and causing highfield shifts of both amide signals. Analysis of the chemical shifts of the 1H, 15N, and 13C signals clearly confirmed the constitution and base-on nature of pseudovitamin B12 and factor A.
Notable chemical shift differences between the spectrum of vitamin B12 on one hand and those of pseudovitamin B12 and factor A on the other hand could be observed for signals that were assigned to the constitutionally different nucleotide bases. In the spectra of pseudovitamin B12 and factor A, additional 1H upfield or downfield shifts with |Δδ| > 0.1 ppm with respect to the signals in the spectrum of vitamin B12 are only observed for protons that experience the different anisotropic effects of the cobalt-coordinating nucleotide bases (Table 1): in the spectra of pseudovitamin B12 and factor A these are the resonances of H(C3), Hre(C31), Hre(C171), Hsi(C172), and H(CR1), and in the spectra of pseudovitamin B12 these are the resonances also of H(C51), H(C7A), and Hsi(C81). A comparison of the 13C NMR data for pseudovitamin B12 and factor A with those for vitamin B12 also show similar chemical shifts (|Δδ| < 2 ppm) of signals due to the constitutionally corresponding carbons, with the exception of the signal for C1R in the spectrum of pseudovitamin B12 and of the signals for C1R and C2R in the spectrum of factor A. The observed NOE intensities were compatible with relevant interproton distances from the crystal structures of vitamin B12 and factor A (41, 42). For the spectra of pseudovitamin B12 and factor A a strong NOE contact was observed between H(C8N) and the methylene protons H(C131) and the weaker ones of H(C8N) to the methyl groups H(C151) and H(C1A) as well as to the methylene proton Hre(C172). Furthermore, in the ROESY spectrum of pseudovitamin B12, weak contacts between H(C2N) and the methyl groups H(C51) and H(C7A), as well as to the methylene protons Hsi(C31), Hre(C31), and Hre(C81), exist. Likewise, the spectrum of factor A exhibits the corresponding contacts of H(C21N) and the methyl groups H(C51) and H(C7A), as well as the methylene protons Hsi(C31), Hre(C31), and Hre(C81). All of these NOE contacts indicate the nucleotide base to be cobalt coordinating and suggest a “north-south” orientation with respect to the cobalt-corrin portion, constitutional, and conformational properties of pseudovitamin B12 and factor A in aqueous solution, which are also indicated for vitamin B12 and factor A by their crystal structures.
Analysis of the corrinoids isolated from C. cochlearium.
The sample of crystalline cyano-corrinoids obtained from C. cochlearium was analyzed with respect to its composition by HPLC analysis and by UV-Vis, CD, mass, and NMR spectroscopy. These spectra could be examined by comparison with the spectra recorded for pseudovitamin B12 and factor A, two well-characterized, authentic reference compounds.
By a combination of careful HPLC and UV-Vis spectroscopic analysis, the sample was found to contain three corrinoid components, all having UV-Vis spectral features of Coβ-cyano-7"-adeninylcobamides (29, 66). (Fig. 3). The most polar, major fraction (RT = 23 min) and the least polar fraction (RT = 36.1 min) had retention times and UV-Vis spectra identical to those of pseudovitamin B12 and factor A, respectively. The fraction of intermediate polarity (RT = 23.4 min) had similar UV-Vis absorbance characteristics. The mass spectrum of the corrinoid sample from C. cochlearium was consistent with the superposition of the mass spectra of pseudovitamin B12 and factor A. The spectrum of the sample from C. cochlearium exhibits a base peak at m/z = 1,344.8 and the signal at m/z = 1,358.8 with a relative intensity of 65%. These signals are consistent with the pseudomolecular ions (MH+) of pseudovitamin B12 and factor A (or an isomer, thereof), respectively. In addition, signals at m/z = 1,318.8 and m/z = 1,332.8, due to the corresponding fragment ions (MH+-CN), have the same relative intensity ratio. Comparison of the signal pattern in the FAB mass spectrum with the HPLC trace suggests the sample from C. cochlearium to contain pseudovitamin B12 (representing ca. 60%), factor A, and an isomer of factor A (in total, ca. 40% of the sample).
The 1D 1H NMR spectrum of the corrinoid sample from C. cochlearium (Fig. 4) also can be deconvoluted mostly as a superposition of spectra of pseudovitamin B12 and of factor A. Table 1 and Fig. 4 show that most of the 1H (and 13C) NMR resonances of pseudovitamin B12 and factor A superimpose completely or in part. Only some signals experience observable shift differences of >0.01 ppm, due to the constitutionally differing nucleotide bases, and are consequently suitable for securing the existence of both corrinoids in aqueous solution. These signals are the intense methyl singlets of H(C51), H(C7A), H(C12A), and H(C1A) and the resonances of H(C8N), H(CR1), HZ(N34), HE(N84), HZ(N84), and H(N174) in the low field region. For all of these signals a doubling is observed, a finding consistent with an intensity ratio of approximately 3:2. In addition, the three protons of the methyl group C21N of the 2-methyladenine moiety of factor A have no counterpart in pseudovitamin B12, and their signal, a singlet at 2.26 ppm, is a feature of the spectrum of factor A. In the 1H NMR spectrum of the sample from C. cochlearium the corresponding singlet at 2.26 ppm is clearly identifiable. Due to the low total corrinoid concentration (ca. 1.5 mM) of the sample and lower signal resolution, the 2D 1H,13C-PFG-HSQC spectrum and the 1H,13C-PFG-HMBC spectrum of the corrinoid sample of C. cochlearium are less informative, but they are also consistent with the presence of pseudovitamin B12 and of factor A. A cross-peak with the 1H and 13C coordinates (2.26 and 28.4 ppm [HSQC]) and (2.26 and 166.3 ppm [HMBC]), respectively, can specifically be attributed to the methyl group H3(C21N) of the 2-methyladenine base of factor A. Two further singlets in the 1H NMR spectrum at chemical shifts of 8.02 and 2.81 ppm could not be explained by the spectrum of either factor A or pseudovitamin B12. Based on their chemical shifts, they were tentatively assigned to H(C2N) of a purinylcobamide and to a methyl group bound to an exocyclic amino function. These tentative assignments of the signals in the 1H NMR spectrum were supported by corresponding information from 1H,13C-PFG-HSQC and 1H,13C-PFG-HMBC spectra: the signal of 8.02 ppm [assigned to H(C2N)] in the proton dimension correlated with a 13C signal of 161.0 ppm (HBMC, assigned to C6N), and the one at 2.81 ppm corresponded with a signal at 31.5 ppm (HSQC, of H3C-N61N). In addition, the signal at 2.81 ppm in the 1H NMR spectrum produced an NOE to the one at 6.40 ppm, assigned to H(CR1). The data are consistent with the structure of an N6-methyladenine moiety bound via N7N to the ribose unit of the nucleotide function.
DISCUSSION
Authentic samples of pseudovitamin B12 (29), prepared by guided biosynthesis as described here, and of factor A were subjected to an extensive spectroscopic analysis. The most detailed spectroscopic and structural information was obtained from 1D and 2D homo- and heteronuclear NMR spectra in aqueous solution (40). From experiments with B12 derivatives (in aqueous medium with a mean deuterium content of 10% or less) signals for nearly all 1H, 13C, and 15N atoms were detected and could be assigned (a complete listing of assigned 1H, 13C, and 15N signals is given in Tables 1 and 2; see also Fig. 4 for 500 MHz 1H NMR spectra of pseudovitamin B12 and factor A). The close correspondence of the chemical shifts of most 1H, 15N, and 13C NMR signals in the spectra of the purinylcobamides pseudovitamin B12 and factor A on the one hand and of the DMB-cobamide vitamin B12 on the other (16) supports a constitutionally and configurationally identical buildup of their cyano-Co(III)-corrin segments. The spectra also indicate attachment at the ribose moiety of the purine base of the cobalt coordinating nucleotide via its N7 in the unique α-configuration. Notable chemical shift differences can be observed only for signals that have been assigned either to the constitutionally different nucleotide bases or to protons that experience the different anisotropic effects of the cobalt-coordinating nucleotide bases. Detailed information on the conformational properties of pseudovitamin B12 and factor A in aqueous solution was obtained from the ROESY data. By comparing the observed NOE intensities with the interproton distances from the crystal structures of vitamin B12 (45) and of factor A (41), the base-on nature of pseudovitamin B12 and factor A in aqueous solution and at pH 5.2 was confirmed, as was the north-south orientation of the cobalt-coordinating purine bases in pseudovitamin B12 and factor A.
The corrinoid sample from C. cochlearium thus represents a cocrystallisate of pseudovitamin B12, factor A, and a third cyanocobamide. The latter is indicated by the mass spectrum to have the same molecular mass as factor A and is tentatively assigned the structure of a Coβ-cyano-7"-[N6-methyl]adeninylcobamide, a previously unknown purinylcobamide. In view of the earlier isolation of pseudovitamin B12 and pseudocoenzyme B12 from C. tetanomorphum (2), the isolation of purinylcobamides from the related C. cochlearium is not unexpected. The corrinoids were isolated in their cyano-Co(III) forms, which are unlikely to have a direct cofactor function (23, 43). The presence of purinylcobamides in C. cochlearium (and the absence of benzimidazolylcobamides, when grown without the supplement of the corresponding base) can be explained primarily by the difficulty (or even incapacity) of this anaerobe to biosynthesize benzimidazoles. Such a situation is not untypical of anaerobes (see, for example, references 39, 60, and 66). The appearance, besides pseudovitamin B12, of its homologue factor A (and of 7"-[N6-methyl]adeninylcobamide), is in line with frequent observations on the coexistence of purinylcobamides in anaerobes (61). While the direct sources of the adeninyl bases are not established, their origin from degradation of oligo(deoxy)nucleotides has been suggested tentatively (28, 61). The appearance of 7"-[N6-methyl]adeninylcobamides would lend further support to this suggestion, since derivatives of N6-methyladenine are ubiquitous natural products of oligonucleotides (50). Clearly, additional methyl groups increase the hydrophobicity of the nucleotide appendage. A related situation is encountered with the naturally occurring benzimidazolylcobamides, which may also differ by the degree of their biosynthetic methylation at the nucleoside base (60).
The determination of two of the cobamides in C. cochlearium as the 7"-purinylcobamides pseudovitamin B12 and factor A points to the functional relevance in this anaerobe of the corresponding coenzyme forms, pseudocoenzyme B12 (Coβ-5′-deoxy-5′-adenosyl-7"-adeninylcobamide) and adenosyl factor A (Coβ-5′-deoxy-5′-adenosyl-7"-[2-methyl]adeninylcobamide). These two adenosylcobamides are known to exist in their base-off constitution mainly in neutral aqueous solution, in contrast to coenzyme B12 (47; W. Fieber, B. Hoffmann, H. Bothe, W. Buckel, R. Konrat, and B. Kräutler, unpublished data). It is also likely that the corresponding methyl-Co(III)-corrinoids (Coβ-methyl-7"- adeninylcobamide and Coβ-methyl-7"-[2-methyl]adeninylcobamide) have a functional role in biosynthetic methyl group transfer reactions (e.g., in some methanogens, such as in a variety of Methanococcales spp. [66]).
The different constitution of the nucleotide bases of coenzyme B12 and of the purinylcobamides could be considered to be of functional relevance in view of the recently established base-off–His-on mode of binding of the corrinoid cofactor to some adenosyl-dependent mutases (55, 58) (see above). The predominance of the base-off form of the purinylcobamides might therefore be considered to be a means in support of a proper preorganization of these “complete” corrinoids and to assist their incorporation in the base-off–His-on mode into the apo form of glutamate mutase. To test this notion, preliminary comparisons were carried out with the adenosylcobamides coenzyme B12, pseudocoenzymec B12, and adenosyl factor A as cofactors of glutamate mutase from C. cochlearium. In contrast to the simple expectations, Michaelis-Menten studies of glutamate mutase revealed coenzyme B12 to bind better than its base-off analogues pseudocoenzyme B12 and adenosyl factor A and the catalytic activity of the mutase to be similar, irrespective of the adenosyl-corrinoid cofactor used (Fieber et al., unpublished). Accordingly, a direct catalytic advantage for the adenosylcobamide-dependent glutamate mutase from C. cochlearium by the use of the purinylcobamides pseudocoenzyme B12 and adenosyl-factor A instead of the benzimidazolycobamide coenzyme B12 can be excluded.
The purinylcobamides carry a nucleotide base that is able to undergo H bonding with H donors and acceptors and that can become protonated (47), even when coordinated to the corrin-bound cobalt center. This situation is drastically different from that in the benzimidazolylcobamides, where H bonding and protonation of the nucleotide base cannot directly occur in the cobalt-coordinated state. A mechanism for the control of the organometallic reactivity of protein-bound corrinoid cofactors involves weakening of the cobalt coordination of the axial transligand, conveyed (in part) by H bonding it to other protein residues in “regulatory” diads or triads (21, 43, 51, 52). Only in their base-off–His-on form can bound benzimidazolylcobamides be set to participate directly in such H-bonded regulatory units via a coordinated histidine. A possible functional advantage of the presence of a purinyl base in complete corrinoid cofactors in their H-bonding base-on constitution may therefore not be dismissed. Indeed, diol-dehydratase and cobamide-dependent ribonucleotide reductase are two enzymes that depend upon adenosylcobamides and which have been discovered lately to carry their corrinoid cofactors in the base-on form (1, 48, 63).
While two of the native corrinoids of the obligate anaerobes C. tetanomorphum and C. cochlearium are widely occurring purinylcobamides, C. tetanomorphum has been shown to produce benzimidazolylcobamides when supplied with benzimidazoles (70), and coenzyme B12 can act as functional corrinoid cofactor of glutamate mutase in both of these clostridia (9, 37, 60). The incapacity of C. tetanomorphum and C. cochlearium to biosynthesize benzimidazoles parallels the situation encountered in several other anaerobes (29, 39, 66). The native cobamides from C. cochlearium are indicative of the biosynthesis of three relevant adeninylcobamides by this anaerobe. Two of these homologous corrinoids (pseudovitamin B12 and factor A) are known from other anaerobes also (61). It will be of interest to definitively clarify the structure of the third cobamide of C. cochlearium and to examine its occurrence in other anaerobic microorganisms.
ACKNOWLEDGMENTS
We thank Wolfgang Fieber and Wolfgang Schmidt for communicating their results on organometallic B12 derivatives and Iris Schall for her technical assistance. We are grateful to K. H. Ongania for measuring FAB mass spectra. We thank Paul Renz for providing us with authentic factor A and Hoffmann-LaRoche for a generous gift of vitamin B12.
This study was supported by the European Commission (TMR project FMRX.CT96.0018), by the Austrian National Science Foundation (FWF, projects P-13595 and P-12639), by the German Science Foundation (Deutsche Forschungsgemeinschaft), and by the Fonds der Chemischen Industrie.
REFERENCES
- 1.Abend A, Nitsche R, Banderian V, Stupperich E, Rétey J. Dioldehydratase binds coenzyme B12 in the “base-on” mode: ESR investigations on cob(II)alamin. Angew Chem Int Ed Engl. 1998;37:625–627. doi: 10.1002/(SICI)1521-3773(19980316)37:5<625::AID-ANIE625>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Barker H A, Weissbach H, Smith R D. A coenzyme containing pseudovitamin B12. Proc Natl Acad Sci USA. 1958;44:1093–1097. doi: 10.1073/pnas.44.11.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bax A, Davies D G. Practical aspects of two-dimensional transverse NOE spectroscopy. J Magn Reson. 1985;63:207–213. [Google Scholar]
- 4.Bax A, Davies D G. MLEV-17-based two-dimensional homonuclear magnetization transfer spectroscopy. J Magn Reson. 1985;65:355–360. [Google Scholar]
- 5.Bax A, Summers M F. 1H and 13C assignments from sensitivity-enhanced detection of heteronuclear multiple-bond connectivity by 2D multiple quantum NMR. J Am Chem Soc. 1986;108:2093–2094. [Google Scholar]
- 6.Beatrix B, Zelder O, Kroll F, Örlygsson G, Golding B T, Buckel W. Evidence for a mechanism involving transient fragmentation in coenzyme-B12-dependent carbon skeleton rearrangements. Angew Chem Int Ed Engl. 1995;34:2398–2401. [Google Scholar]
- 7.Bigger G W, Elliot J M, Rickard T R. Estimated ruminal production of pseudovitamin B12, factor A and factor B in sheep. J Anim Sci. 1976;43:1077–1081. doi: 10.2527/jas1976.4351077x. [DOI] [PubMed] [Google Scholar]
- 8.Bodenhausen G, Ruben D. Natural abundance nitrogen-15 NMR by enhanced spectroscopy. Chem Phys Lett. 1980;69:185–189. [Google Scholar]
- 9.Bothe H, Bröker G, Müller U, Schall I, Textor S, Golding B T, Buckel W. Mechanisms of coenzyme B12-dependent carbon-carbon and carbon-oxygen rearrangements. In: Kräutler B, Golding B T, Arigoni D, editors. Vitamin B12 and B12-proteins. Weinheim, Germany: Verlag Wiley-VCH; 1998. pp. 253–264. [Google Scholar]
- 10.Bothe H, Darley D J, Albracht S P, Gerfen G J, Golding B T, Buckel W. Identification of the 4-glutamyl radical as an intermediate in the carbon skeleton rearrangement catalyzed by coenzyme B12-dependent glutamate mutase from Clostridium cochlearium. Biochemistry. 1998;37:4105–4113. doi: 10.1021/bi971393q. [DOI] [PubMed] [Google Scholar]
- 11.Bothner-By A A, Stephens R L, Lee J, Warren C D, Jeanloz R W. Structure determination of a tetrasaccharide: transient nuclear Overhauser effects in the rotating frame. J Am Chem Soc. 1984;106:811–813. [Google Scholar]
- 12.Brandt L J, Bernstein L H, Wagle A. Production of vitamin B12 analogues in patients with small-bowel overgrowth. Ann Intern Med. 1977;87:546–551. doi: 10.7326/0003-4819-87-5-546. [DOI] [PubMed] [Google Scholar]
- 13.Brandt L J, Goldberg L, Bernstein L H, Greenberg G. The effect of bacterially produced vitamin B12 analogues (cobamides) on the in vitro absorption of cyanocobalamin. Am J Clin Nutr. 1979;32:1832–1836. doi: 10.1093/ajcn/32.9.1832. [DOI] [PubMed] [Google Scholar]
- 14.Braunschweiler L, Ernst R R. Coherence transfer by isotropic mixing: application to proton correlation spectroscopy. J Magn Reson. 1983;53:521–528. [Google Scholar]
- 15.Buckel W, Golding B T. Glutamate and 2-methyleneglutarate mutase: from microbial curiosities to paradigms for coenzyme B12-dependent enzymes. Chem Rev Soc. 1996;25:329–337. [Google Scholar]
- 16.Calafat A M, Marzilli L G. Investigation of B12 derivatives with inorganic ligands using 2D NMR spectroscopy. J Am Chem Soc. 1993;115:9182–9190. [Google Scholar]
- 17.Chen H P, Marsh E N G. How enzymes control the reactivity of adenosylcobalamin: effect on coenzyme binding and catalysis of mutations in the conserved histidine-aspartate pair of glutamate mutase. Biochemistry. 1997;36:7884–788932. doi: 10.1021/bi970169y. [DOI] [PubMed] [Google Scholar]
- 18.Davis D G, Bax A. Assignment of complex 1H NMR spectra via 2D-homonuclear Hartmann-Hahn spectroscopy. J Am Chem Soc. 1985;107:2820–2821. [Google Scholar]
- 19.Davis A L, Keeler J, Laue E D, Moskau D. Experiments for recording pure-absorption heteronuclear correlation spectra using pulsed field gradients. J Magn Reson. 1992;98:207–216. [Google Scholar]
- 20.Dion H W, Calkins D G, Pfiffner J J. Hydrolysis products of pseudovitamin B12. J Am Chem Soc. 1952;74:1108. [Google Scholar]
- 21.Drennan C L, Huang S, Drummond J T, Matthews R G, Ludwig M L. How a protein binds B12: a 3.0 Å X-ray structure of B12-binding domains of methionine synthase. Science. 1994;266:1669–1674. doi: 10.1126/science.7992050. [DOI] [PubMed] [Google Scholar]
- 22.Eberhard G, Schlayer H, Joseph H, Fridrich E, Utz B, Müller O. Untersuchungen zur biologischen Funktion der Nukleotidbase von Vitamin B12. Biol Chem Hoppe-Seyler. 1988;369:1091–1098. [PubMed] [Google Scholar]
- 23.Ellenbogen L, Cooper B A. Vitamin B12. In: Lawrence L, Machlin J, editors. Handbook of vitamins. New York, N.Y: Marcel Dekker, Inc.; 1991. pp. 491–536. [Google Scholar]
- 24.Eschenmoser A. Vitamin B12: Experimente zur Frage nach dem Ursprung seiner molekularen Struktur. Angew Chem Int Ed Engl. 1988;27:5–40. [Google Scholar]
- 25.Ford J E, Porter J W G. Some properties of vitamin B12-like factors from calf faeces. 1. Characteristics of different fractions. Biochem J. 1952;51:v–vi. [PubMed] [Google Scholar]
- 26.Ford J E, Holdsworth E S, Kon S K, Porter J W G. Occurrence of the various vitamin B12 active compounds. Nature. 1953;171:150. doi: 10.1038/171150a0. [DOI] [PubMed] [Google Scholar]
- 27.Ford J E, Holdsworth E S, Kon S K. The biosynthesis of vitamin B12-like compounds. Biochem J. 1955;59:86–93. [PMC free article] [PubMed] [Google Scholar]
- 28.Friedmann H C, Cagen L M. Microbial biosynthesis of B12-like compounds. Annu Rev Microbiol. 1970;24:159–208. doi: 10.1146/annurev.mi.24.100170.001111. [DOI] [PubMed] [Google Scholar]
- 29.Friedrich W. Vitamin B12 und verwandte Corrinoide. In: Ammon R, Dirscherl W, editors. Fermente, Hormone, und Vitamine. III/2. Stuttgart, Germany: Georg Thieme Verlag; 1975. p. 173. [Google Scholar]
- 30.Friedrich W, Bernhauer K. Beiträge zur Chemie und Biochemie der “Cobalamine.” II. Mitteilung: Über den Abbau der “Cobalamine” mit CerIII-hydroxid. 7α-[d-Ribofuranosido]-adenin, ein Abbauprodukt des Pseudovitamin B12. Chem Ber. 1956;89:2507–2512. [Google Scholar]
- 31.Friedrich W, Bernhauer K. Über den Abbau des Faktors A mit CerIII-hydroxyd. Chem Ber. 1957;90:465–470. [Google Scholar]
- 32.Friedrich W, Dellweg H, Gross G, Becher E, Spaude S, Bernhauer K. Vitamin B12-ähnliche Faktoren des Faulschlamms. In: Heinrich H C, editor. Vitamin B12 and intrinsic factor. Stuttgart, Germany: Enke; 1957. pp. 62–72. [Google Scholar]
- 33.Giannotti C. Electronic spectra of B12 and related systems. In: Dolphin D, editor. B12. Vol. 1. New York, N.Y: John Wiley & Sons; 1982. p. 393. [Google Scholar]
- 34.Gronenborn A M, Bax A, Wingfield P T, Clore G M. A powerful method of sequential proton resonance assignment in proteins using relayed 15N-1H multiple quantum coherence spectroscopy. FEBS Lett. 1989;243:93–98. doi: 10.1016/0014-5793(89)81224-4. [DOI] [PubMed] [Google Scholar]
- 35.Hoffmann B, Konrat R, Bothe H, Buckel W, Kräutler B. Structure and dynamics of the B12-binding subunit of glutamate mutase from Clostridium cochlearium. Eur J Biochem. 1999;263:178–188. doi: 10.1046/j.1432-1327.1999.00482.x. [DOI] [PubMed] [Google Scholar]
- 36.Hollenstein R, Stupperich E. Assignment of 15N-NMR resonances of vitamin B12 analogues by 2D-[15N,1H]long range correlation: fully [15N]-labelled co-β-cyano-5-hydroxybenzimidazolylcobamide (factor III) and derivatives. Helv Chim Acta. 1993;76:1258–1265. [Google Scholar]
- 37.Holloway D E, Marsh E N G. Adenosylcobalamin-dependent glutamate mutase from Clostridium tetanomorphum: over-expression in E. coli, purification and characterization of the recombinant enzyme. J Biol Chem. 1994;269:20425–20430. [PubMed] [Google Scholar]
- 38.Hurd R E, John B K. Gradient-enhanced proton-detected heteronuclear multiple-quantum coherence spectroscopy. J Magn Reson. 1991;91:648–653. [Google Scholar]
- 39.Keck B, Renz P. Salmonella typhimurium forms adenylcobamide and 2-methyladenylcobamide, but no detectable cobalamin during strictly anaerobic growth. Arch Microbiol. 2000;173:76–77. doi: 10.1007/s002030050011. [DOI] [PubMed] [Google Scholar]
- 40.Konrat R, Tollinger M, Kräutler B. New NMR structural and dynamical probes of organometallic B12 derivatives. In: Kräutler B, Golding B T, Arigoni D, editors. Vitamin B12 and B12-proteins. Weinheim, Germany: Verlag Wiley-VCH; 1998. pp. 349–368. [Google Scholar]
- 41.Kopf J, von Deuten K, Bieganowski R, Friedrich W. X-ray crystal structure analysis of factor A (2-methyladeninyl-cyanocobamide), a native vitamin B12-analogue. Z Naturforsch. 1981;36c:506–515. [Google Scholar]
- 42.Kratky C, Färber G, Gruber K, Wilson K, Dauter Z, Nolting H-F, Konrat R, Kräutler B. Accurate structural data demystify B12: high-resolution solid-state structure of aquocobalamin perchlorate and structure analysis of the aquocobalamin ion in solution. J Am Chem Soc. 1995;117:4654–4670. [Google Scholar]
- 43.Kräutler B. B12-coenzymes, the central theme. In: Kräutler B, Golding B T, Arigoni D, editors. Vitamin B12 and B12-proteins. Weinheim, Germany: Verlag Wiley-VCH; 1998. pp. 3–43. [Google Scholar]
- 44.Kräutler B. B12-nomenclature and a suggested atom-numbering. In: Kräutler B, Golding B T, Arigoni D, editors. Vitamin B12 and B12-proteins. Weinheim, Germany: Verlag Wiley-VCH; 1998. pp. 517–522. [Google Scholar]
- 45.Kräutler B, Konrat R, Stupperich E, Färber G, Gruber K, Kratky C. Direct evidence for the conformational deformation of the corrin ring by the nucleotide base in vitamin B12: synthesis and solution spectroscopic and crystal structure of Coβ-cyanoimidazolylcobamide. Inorganic Chem. 1994;33:4128–4139. [Google Scholar]
- 46.Kräutler B, Kratky C. Vitamin B12: the haze clears. Angew Chem. 1996;108:179–181. ; Angew. Chem. Int. Ed. Engl. 35:168–170. [Google Scholar]
- 47.Ladd J N, Hogenkamp H P C, Barker H A. Structure of cobamide coenzymes: influence of pH on absorption spectra and ionophoretic mobilities. J Biol Chem. 1961;236:2114–2118. [PubMed] [Google Scholar]
- 48.Lawrence C C, Gerfen G J, Samano V, Nitsche R, Robins M J, Rétey J, Stubbe J-A. Binding of cob(II)alamin to the adenosylcobalamin-dependent ribonucleotide reductase from Lactobacillus leichmanii. J Biol Chem. 1999;274:7039–7042. doi: 10.1074/jbc.274.11.7039. [DOI] [PubMed] [Google Scholar]
- 49.Leutbecher U, Böcher R, Linder D, Buckel W. Glutamate mutase from Clostridium cochlearium. Purification, cobamide content and stereospecific inhibitors. Eur J Biochem. 1992;205:759–765. doi: 10.1111/j.1432-1033.1992.tb16840.x. [DOI] [PubMed] [Google Scholar]
- 50.Limbach P A, Crain P F, McCloskey J A. The modified nucleosides of RNA. Nucleic Acids Res. 1994;22:2183–2196. doi: 10.1093/nar/22.12.2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ludwig M L, Matthews R G. Structure-based perspectives on B12-dependent enzymes. Annu Rev Biochem. 1997;66:269–313. doi: 10.1146/annurev.biochem.66.1.269. [DOI] [PubMed] [Google Scholar]
- 52.Mancia F, Keep N H, Nakagawa A, Leadlay P F, McSweeney S, Rasmussen B, Bösecke P, Diat O, Evans P R. How coenzyme B12 radicals are generated: the crystal structure of methylmalonyl-coenzyme A mutase at 2 Å resolution. Structure. 1996;4:339–350. doi: 10.1016/s0969-2126(96)00037-8. [DOI] [PubMed] [Google Scholar]
- 53.Marsh E N G, Holloway D E. Cloning and sequencing of glutamate mutase component S from Clostridium tetanomorphum. FEBS Lett. 1992;310:167–170. doi: 10.1016/0014-5793(92)81321-c. [DOI] [PubMed] [Google Scholar]
- 54.Marsh E N G, Ballou D P. Coupling of cobalt-carbon bond homolysis and hydrogen atom abstraction in adenosylcobalamin-dependent glutamate mutase. Biochemistry. 1998;37:11864–11872. doi: 10.1021/bi980512e. [DOI] [PubMed] [Google Scholar]
- 55.Marsh E N G, Holloway D E, Chen H P. Glutamate mutase. In: Kräutler B, Golding B T, Arigoni D, editors. Vitamin B12 and B12-proteins. Weinheim, Germany: Verlag Wiley-VCH; 1998. pp. 253–264. [Google Scholar]
- 56.Piotto M, Sautek V, Sklenar V. Gradient-tailored excitation for single-quantum NMR spectroscopy of aqueous solution. J Biomol NMR. 1992;2:661–665. doi: 10.1007/BF02192855. [DOI] [PubMed] [Google Scholar]
- 57.Ragsdale S W, Lindahl P A, Münck E. Mössbauer, EPR and optical studies of the corrinoid/iron-sulfur protein involved in the synthesis of acetyl coenzyme A by Clostridium thermoaceticum. J Biol Chem. 1987;262:14289–14297. [PubMed] [Google Scholar]
- 58.Reitzer R, Gruber K, Jogl G, Wagner U G, Bothe H, Buckel W, Kratky C. Glutamate mutase from Clostridium cochlearium: the structure of a coenzyme B12-dependent enzyme provides new mechanistic insights. Structure. 1999;7:891–902. doi: 10.1016/s0969-2126(99)80116-6. [DOI] [PubMed] [Google Scholar]
- 59.Renz P. Some intermediates in the biosynthesis of vitamin B12. Methods Enzymol. 1972;18:85. [Google Scholar]
- 60.Renz P. Investigations of the biosynthesis of the 5,6-dimethylbenzimidazole moiety of vitamin B12. In: Kräutler B, Golding B T, Arigoni D, editors. Vitamin B12 and B12-proteins. Weinheim, Germany: Verlag Wiley-VCH; 1998. pp. 119–130. [Google Scholar]
- 61.Renz P. Biosynthesis of the 5,6-dimethylbenzimidazole moiety of cobalamins and of other bases found in natural corrinoids. In: Banerjee R, editor. Chemistry and Biochemistry of B12. New York, N.Y: John Wiley & Sons; 1999. pp. 557–575. [Google Scholar]
- 62.Rickes E L, Brink N G, Koniuszy F R, Wood T R, Folkers K. Crystalline vitamin B12. Science. 1948;107:396–397. doi: 10.1126/science.107.2781.396. [DOI] [PubMed] [Google Scholar]
- 63.Shibata N, Masuda J, Tobimatsu T, Toraya T, Suto K, Morimoto Y, Yasuoka N. A new mode of B12 binding and the direct participation of a potassium ion in enzyme catalysis: X-ray structure of diol dehydratase. Structure. 1999;7:997–1008. doi: 10.1016/s0969-2126(99)80126-9. [DOI] [PubMed] [Google Scholar]
- 64.Smith E L, Parker L F J. Purification of anti-pernicious anaemia factor. Biochem J. 1948;43:VIII. [PubMed] [Google Scholar]
- 65.Stupperich E, Steiner I, Rühlemann M. Isolation and analysis of bacterial cobamides by high-performance liquid chromatography. Anal Biochem. 1986;155:365–370. doi: 10.1016/0003-2697(86)90447-1. [DOI] [PubMed] [Google Scholar]
- 66.Stupperich E, Kräutler B. Pseudovitamin B12 or 5-hydroxybenzimidazolyl-cobamide are the corrinoids found in methanogenic bacteria. Arch Microbiol. 1988;149:268–271. [Google Scholar]
- 67.Stupperich E, Eisinger H-J, Schurr S. Corrinoids in anaerobic bacteria. FEMS Microbiol Rev. 1990;87:355–360. [Google Scholar]
- 68.Tollinger M, Dérer T, Konrat R, Kräutler B. An efficient method for the preparation of methylcobalamin, nature's organometallic methyl transfer catalyst. J Mol Catal A. 1997;116:147–155. [Google Scholar]
- 69.Tollinger M, Konrat R, Hilbert B H, Marsh E N G, Kräutler B. How a protein prepares for B12 binding: structure and dynamics of the B12-binding subunit of glutamate mutase from Clostridium tetanomorphum. Structure. 1998;6:1021–1033. doi: 10.1016/s0969-2126(98)00103-8. [DOI] [PubMed] [Google Scholar]
- 70.Weissbach H, Toohey J, Barker H A. Isolation and properties of B12-coenzymes containing benzimidazole or dimethyl-benzimidazole. Proc Natl Acad Sci USA. 1959;45:521–525. doi: 10.1073/pnas.45.4.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zelder O, Beatrix B, Leutbecher U, Buckel W. Characterization of the coenzyme-B12-dependent glutamate mutase from Clostridium cochlearium produced in Escherichia coli. Eur J Biochem. 1994;226:577–585. doi: 10.1111/j.1432-1033.1994.tb20083.x. [DOI] [PubMed] [Google Scholar]
- 72.Zelder O, Beatrix B, Kroll F, Buckel W. Coordination of a histidine residue of the protein component S to the cobalt/Atom in coenzyme B12-dependent glutamate mutase from Clostridium cochlearium. FEBS Lett. 1995;369:252–254. doi: 10.1016/0014-5793(95)00762-x. [DOI] [PubMed] [Google Scholar]