Abstract
BACKGROUND
Intestinal flora disorder (IFD) poses a significant challenge after laparoscopic colonic surgery, and no standard criteria exists for its diagnosis and treatment.
AIM
To analyze the clinical features and risk factors of IFD.
METHODS
Patients with colon cancer receiving laparoscopic surgery were included using propensity-score-matching (PSM) methods. Based on the occurrence of IFD, patients were categorized into IFD and non-IFD groups. The clinical characteristics and treatment approaches for patients with IFD were analyzed. Multivariate regression analysis was performed to identify the risk factors of IFD.
RESULTS
The IFD incidence after laparoscopic surgery was 9.0% (97 of 1073 patients). After PSM, 97 and 194 patients were identified in the IFD and non-IFD groups, respectively. The most common symptoms of IFD were diarrhea and abdominal, typically occurring on post-operative days 3 and 4. All patients were managed conservatively, including modulation of the intestinal flora (90.7%), oral/intravenous application of vancomycin (74.2%), and insertion of a gastric/ileus tube for decompression (23.7%). Multivariate regression analysis identified that pre-operative intestinal obstruction [odds ratio (OR) = 2.79, 95%CI: 1.04–7.47, P = 0.041] and post-operative antibiotics (OR = 8.57, 95%CI: 3.31–23.49, P < 0.001) were independent risk factors for IFD, whereas pre-operative parenteral nutrition (OR = 0.12, 95%CI: 0.06–0.26, P < 0.001) emerged as a protective factor.
CONCLUSION
A stepwise approach of probiotics, vancomycin, and decompression could be an alternative treatment for IFD. Special attention is warranted post-operatively for patients with pre-operative obstruction or early use of antibiotics.
Keywords: Colon cancer, Laparoscopy; Intestinal flora disorder; Clinical characteristics; Risk factors
Core Tip: Intestinal flora disorders (IFD) pose challenges in laparoscopic colonectomy. This study provides a detailed analysis of IFD-related factors, offering valuable insights to improve clinical strategies. The results of this study have certain clinical practical significance. We summarized the clinical characteristics, management strategies, and prognosis of IFD in patients receiving laparoscopic colonectomy. The risk factors associated with IFD was also identified. This will help us focus on the subgroup of patients combined with risk factors during clinical practice and implement targeted preventive measures.
INTRODUCTION
Laparoscopic radical surgery has been established as the standard of care for colon cancer[1,2]. The safety and efficacy of laparoscopic surgery are well demonstrated in several randomised controlled trials[3,4]; however, post-operative complications (POCs) continue to pose a major problem in clinical practice[5,6]. These POCs prolong hospitalisation, increase healthcare costs, and adversely affect subsequent treatments, thereby compromising therapeutic outcomes[6,7].
Paralytic ileus is one of the common POCs in abdominal surgery[8,9]. We observed in clinical practice that some patients initially present with diarrheal symptoms, which later progress to abdominal distention, nausea and vomiting, fever, and cessation of gas and stool passage. In fact, the above mention symptoms are actually different from the classic features of paralytic ileus. Based on clinical practice and experience, we therefore defined these clinical syndromes as intestinal flora disorder (IFD). IFD might thus contribute to the development of paralytic ileus.
The composition and function of the intestinal flora may influence the development and progression of colon cancer[10,11]. Alterations in the abundance of certain bacteria in the intestine have shown a potential association with colon cancer, with a significant reduction in the population of probiotics such as Bifidobacteria and lactobacilli, and an increase in the abundance of harmful bacteria[11,12]. This imbalance in bacteria types and populations of probiotics and harmful flora, caused by the tumour itself, surgery, and the application of antibiotics, is defined as IFD.
As a result, it might be promising to detect the association of IFD with paralytic ileus in the perspective of the alterations in intestinal flora. The absence of specific diagnostic standards for IFD necessitates a comprehensive evaluation based on clinical manifestations, laboratory tests, and imaging examinations. Despite numerous studies focused on POCs after laparoscopic colon surgery, those specifically addressing IFD are scarce. This retrospective study used propensity score matching (PSM) to analyse the clinical features and risk factors associated with IFD after laparoscopic colonic surgery at our hospital, in an effort to offer valuable insights for the diagnosis and treatment of IFD.
MATERIALS AND METHODS
Study design and patients
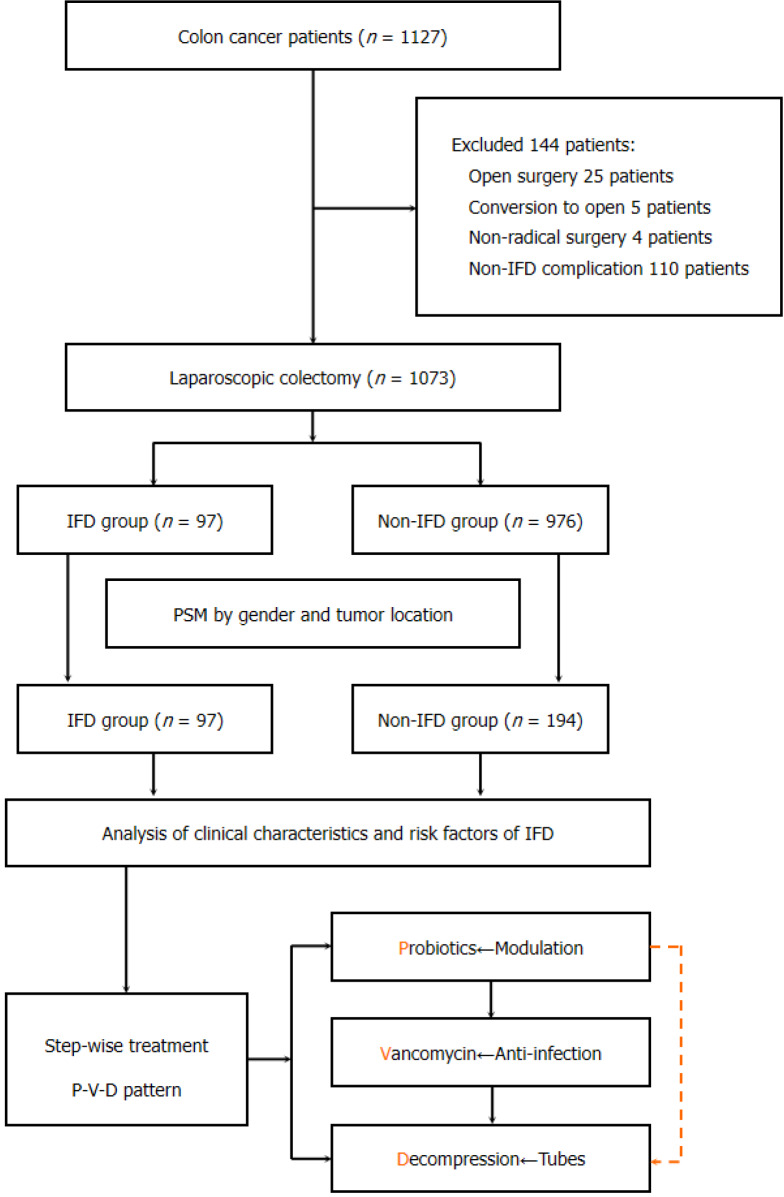
This retrospective PSM study included eligible patients with colon cancer admitted to the Department of General Surgery at Peking Union Medical College Hospital from January 2019 to October 2023. The inclusion criteria were: Histologically confirmed adenocarcinoma, including caecum, ascending, transverse, descending, or sigmoid colon cancer, and laparoscopic radical surgery. Exclusion criteria were: Simultaneous resection of hepatic metastatic lesions, concurrent occurrence of two or more POCs, open or conversion to open surgery, and incomplete clinical data. The patients were categorised into IFD and non-IFD groups based on the occurrence of POCs (Figure 1). This study was approved by the Ethics Committee of Peking Union Medical College Hospital (Approval No, I-23PJ157) and informed consent was obtained from all patients. The design and conduction of the study was strictly carried out according to the STROBE guidelines.
Figure 1.
The flowchart of this study. P-V-D: Probiotics-Vancomycin-Decompression; PSM: Propensity score matching; IFD: Intestinal flora disorder.
Clinical characteristics
Symptoms of intestinal obstruction were manifestations of incomplete ileus characterised by defecation issues, constipation, or abdominal pain observed during the patient’s initial medical consultation. Computed tomography (CT) confirmed intestinal obstruction, revealing classic radiological features, including bowel dilation, gas accumulation, and fluid retention. Pre-operative colonoscopy was performed on the day of surgery or the day before for tumour re-localization or endoscopic removal of colonic polyps. All patients underwent mechanical bowel preparation using one to three packages of polyethylene glycol electrolyte solution. During mechanical bowel preparation, fluid replacement was administered to all patients the day before surgery. Fluid replacement options included enteral nutrition, parenteral nutrition, and glucose-sodium chloride solution. The post-operative use of antibiotics was confined to the day of surgery until the occurrence of IFD; any incidences of the application of oral or intravenous vancomycin for IFD were excluded.
Surgery
All patients underwent laparoscopic radical surgery for colon cancer, including right hemicolectomy, transverse colectomy, left hemicolectomy, or sigmoid colon resection. The surgeries were performed by the chief attending physician and their specialised team, with quality control ensured through a review of the surgical videos by two doctors.
IFD
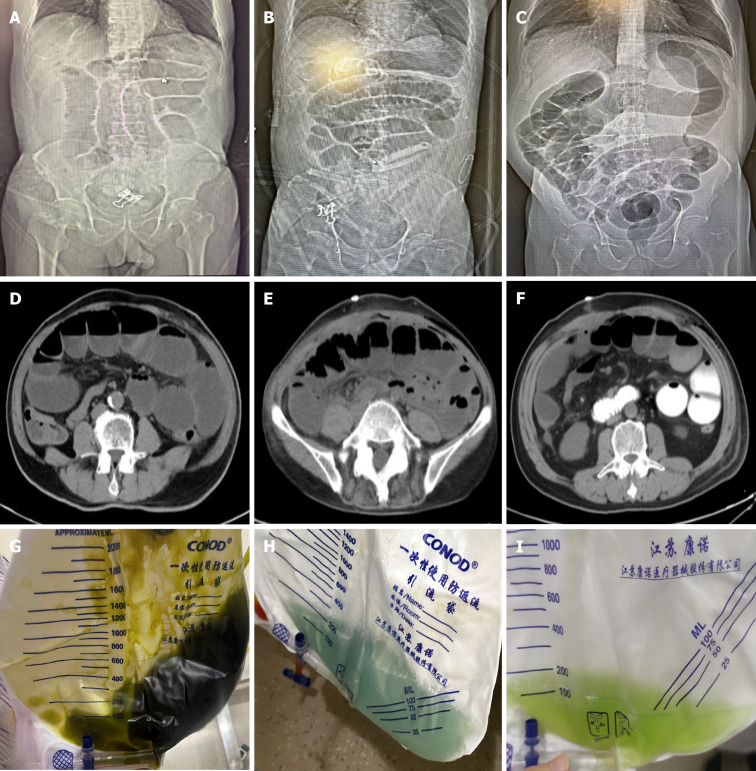
The diagnostic criteria for IFD were based on a previous study by our team: (1) clinical manifestations: Symptoms included abdominal distension, diarrhoea (or a significant increase in colostomy output, appearing as a large volume of dark green "seawater-like stool"), fever, abdominal pain, nausea, and vomiting (with vomitus characterised by dark green or azure gastric contents). Additionally, some patients with diarrhoea may develop paralytic ileus, leading to reduced gas and stool passage, aggravated abdominal distension, and, in severe cases, sepsis and septic shock; (2) laboratory examinations: The stool test may reveal increased white blood cells. Stool smears may show an inversion in the ratio of rod-shaped bacteria and testing for Clostridium difficile toxins (A and B) may be positive. Routine blood examinations may indicate increased neutrophils and their ratio, along with elevated procalcitonin and C-reactive protein levels; and (3) imaging examinations: Abdominal radiography or CT scans may reveal significant dilatation of the intestinal lumen, intestinal wall oedema, and other features, such as multiple air-fluid levels. Some patients may develop ascites. Radiologists reviewed all imaging findings of patients suspected of paralytic ileus owing to IFD, those with severe conditions of intestinal torsion or ischaemia were excluded. Typical images of patients with IFD are shown in Figure 2A-F[13].
Figure 2.
The typical images of patients with intestinal flora disorder. A-C: Depict typical abdominal X-ray findings of intestinal flora disorder patients, with extremely enlarged intestine; D-F: Represent classic computed tomography images, extensive intestinal gas and fluid accumulation are evident within the small bowel, accompanied by the presence of an air-fluid level; G-I: Represent the typical colour of the tube drainage, that are dark brown, cerulean blue and grass green.
IFD was graded using the Clavien–Dindo classification: Grade I: Requiring symptomatic treatment only; Grade II: Requiring antibiotics, blood transfusion, total parenteral nutrition, or placement of a gastric tube; Grade III: Requiring an intestinal obstruction tube. All patients had follow-up appointments within 1 month post-operatively to assess their recovery status upon discharge, conducted through outpatient visits and WeChat communication[14].
Treatment of IFD primarily involved conservative approaches[13]. Depending on the severity, therapeutic interventions included modulating the intestinal flora using probiotics, such as Bifidobacteria and Licheniformis bacillus, administration of oral or intravenous vancomycin for anti-infective treatment, or fasting, nutrition support, and gastrointestinal decompression. If symptoms persisted, interventional measures involves placing an ileus tube. The timing and duration of a gastric or ileus tube placement were documented.
Statistical analysis
Statistical analyses and graphical representations were conducted using SPSS 25.0 and R version 4.3.0. Categorical data were reported as counts and percentages n (%). Between-group comparisons were performed using the chi-squared test, as appropriate. Variables with a P-value less than 0.1 in the univariate analysis were included in the logistic regression analysis to calculate the odds ratio (OR) and 95%CI. A P value < 0.05 was considered statistically significant.
RESULTS
Baseline characteristics
Overall, 1127 patients with colon cancer were initially identified from our hospital’s colorectal disease database. After excluding 144 ineligible patients, 1073 patients who underwent laparoscopic surgery were included, comprising 97 patients (9.0%) in the IFD group and 976 patients (91.0%) in the non-IFD group. Final enrolment comprised of 97 patients in the IFD group and 194 patients in the non-IFD group. After PSM, no differences were observed regarding baseline characteristics between the two groups (Table 1).
Table 1.
The baseline characteristics of patients before and after propensity-score-matching, n (%)
| Factors |
Before PSM
|
After PSM
|
||||||
|
Non-IFD (n = 976)
|
IFD (n = 97)
|
χ²
|
P value
|
Non-IFD (n = 194)
|
IFD (n = 97)
|
χ² |
P value
|
|
| Gender | 6.831 | 0.009 | 0.000 | 1.000 | ||||
| Female | 426 (43.6) | 29 (29.9) | 58 (29.9) | 29 (29.9) | ||||
| Male | 550 (56.4) | 68 (70.1) | 136 (70.1) | 68 (70.1) | ||||
| Tumor locations | 8.131 | 0.043 | 0.000 | 1.000 | ||||
| Asending colon | 402 (41.2) | 51 (52.6) | 102 (52.6) | 51 (52.6) | ||||
| Transverse colon | 63 (6.5) | 7 (7.2) | 14 (7.2) | 7 (7.2) | ||||
| Descending colon | 395 (40.5) | 25 (25.8) | 50 (25.8) | 25 (25.8) | ||||
| Sigmoid colon | 116 (11.8) | 14 (14.4) | 28 (14.4) | 14 (14.4) | ||||
| Age (yr) | 2.060 | 0.151 | 0.063 | 0.801 | ||||
| < 65 | 499 (51.1) | 57 (58.7) | 111 (57.2) | 57 (58.7) | ||||
| ≥ 65 | 477 (48.9) | 40 (41.3) | 83 (42.8) | 40 (41.3) | ||||
| ASA grade | 1.336 | 0.248 | 0.248 | 0.619 | ||||
| I-II | 888 (84.2) | 86 (88.7) | 168 (86.6) | 86 (88.7) | ||||
| III-IV | 154 (15.8) | 11 (11.3) | 26 (13.4) | 11 (11.3) | ||||
| CEA | 1.212 | 0.271 | 1.457 | 0.227 | ||||
| < 5 ng/mL | 715 (73.3) | 66 (68.1) | 145 (77.7) | 66 (68.1) | ||||
| ≥ 5 ng/mL | 261 (26.7) | 31 (31.9) | 49 (22.3) | 31 (31.9) | ||||
| CA19-9 | 0.039 | 0.844 | 1.155 | 0.282 | ||||
| < 34 U/mL | 852 (87.3) | 84 (86.6) | 176 (90.8) | 84 (86.6) | ||||
| ≥ 34 U/mL | 124 (22.7) | 13 (13.5) | 18 (9.2) | 13 (13.5) | ||||
| Hypertension (yes) | 316 (32.4) | 33 (34.0) | 1.109 | 0.742 | 63 (32.5) | 33 (34.0) | 0.070 | 0.791 |
| DM (yes) | 198 (19.9) | 18 (18.6) | 0.098 | 0.755 | 26 (13.4) | 18 (18.6) | 1.339 | 0.247 |
| CHD (yes) | 98 (10.0) | 10 (10.3) | 0.007 | 0.933 | 10 (5.2) | 10 (10.3) | 2.685 | 0.101 |
| Antiplatelet (yes) | 89 (9.1) | 9 (9.3) | 0.003 | 0.956 | 10 (5.2) | 9 (9.3) | 1.802 | 0.179 |
| Abdominal surgery (yes) | 99 (10.1) | 12 (12.4) | 0.477 | 0.490 | 33 (17.1) | 12 (12.4) | 1.065 | 0.302 |
PSM: Propensity score matching; IFD: Intestinal flora disorder; ASA: American society of anesthesiologist; CEA: Carcino embryonic antigen; CA19-9: Carbohydrate antigen 19-9; DM: Diabetes mellitus; CHD: Coronary heart diseases.
Characteristics of IFD
Among the 97 patients with IFD, the common symptoms were diarrhoea (51.6%) and abdominal distension (39.1%). In total, 49.6% of patients experienced IFD on post-operative days 3 and 4. Clavien–Dindo grades for IFD were as follows: I, 10 patients (10.3%); II, 76 patients (78.4%); and III, 11 patients (11.3%). Among these, 32 patients (50.7%) had faecal white blood cell counts exceeding 5/HPF. The positivity rate for detecting C. difficile toxins was 29.4% (15/51). All patients recovered after conservative treatment, with no secondary surgeries performed. Treatment measures included intestinal microbiota modulation by probiotics (88/97, 90.7%), oral vancomycin (72/97, 74.2%), and intravenous vancomycin administration (33/97, 34.0%). The most common application duration of vancomycin was 1 to 4 d.
Among the patients, 23.7% (23 of 97 patients) continued to experience unimproved symptoms despite the aforementioned treatments, necessitating gastrointestinal decompression. Gastric tube placement most commonly occurred on post-operative days 4–6, constituting approximately 73.9% of cases (17 of 23 patients). Additionally, symptom improvement within 3 d of gastric tube insertion was observed in 69.6% of patients (16 of 23 patients). Ileus tubes were required in 11.3% of patients (11 of 97 patients), with the most frequent placement timing on post-operative days 7–12. Among these patients, 63.6% (7 of 11 patients) experienced smooth removal after approximately 4 d of adequate drainage (Table 2). The colour of the tube drainage varied among patients, with dark brown, cerulean, and grass-green drainages observed. The signs mentioned above might be a clinical indication to consider the possibility of IFD after laparoscopic surgery (Figure 2G-I).
Table 2.
The clinical features and treatment strategies of intestinal flora disorder, n (%)
|
Clinical features
|
Statistics
|
| The first symptoms at the diagnosis of IFD | |
| Abdominal distension | 38 (39.1) |
| Diarrhea | 50 (51.6) |
| Fever | 8 (8.3) |
| Septic shock | 1 (1.0) |
| The occurrence timepoint of IFD | |
| POD 1-2 | 15 (15.4) |
| POD 3-4 | 48 (49.6) |
| POD 5-6 | 34 (36.0) |
| The routin stool tests (Yes/No) | 63/34 |
| The results of stool test (WBC/HPF) | |
| 0-1 | 23 (36.6) |
| 2-5 | 8 (12.7) |
| 6-10 | 10 (15.9) |
| 11-15 | 5 (7.9) |
| > 15 | 17 (26.9) |
| The detection of Clostridium difficile (Yes/No) | 51/47 |
| The results of Clostridium test (Positive/Negative) | 15/36 |
| Intestinal flora treatment (Yes/No) | 88/9 |
| Oral norvancomycin (Yes/No) | 72/25 |
| The duration of oral rvancomycin | |
| 1-4 d | 41 (56.9) |
| 5-7 d | 21 (29.2) |
| > 7 d | 10 (13.9) |
| Venous vancomycin (Yes/No) | 33/64 |
| The duration of venous vancomycin | |
| 1-4 d | 15 (45.4) |
| 5-7 d | 13 (39.4) |
| > 7 d | 5 (15.2) |
| The insertion of gastric tube (Yes/No) | 23/74 |
| The timepoint of insertion | |
| POD 1-3 | 6 (26.1) |
| POD 4-6 | 17 (73.9) |
| The duration of gastric tube | |
| 1-3 d | 16 (69.6) |
| 4-7 d | 7 (30.4) |
| The insertion of ileus tube (Yes/No) | 11/86 |
| The timepoint of insertion | |
| POD 5-7 | 4 (36.4) |
| POD 7-12 | 7 (63.6) |
| The duration of ileus tube | |
| 4 d | 7 (63.6) |
| 6 d | 2 (18.2) |
| ≥ 7 d | 2 (18.2) |
IFD: Intestinal flora disorder; PSM: Propensity score matching; POD: Post-operative day; WBC: White blood cell; HPF: High power field.
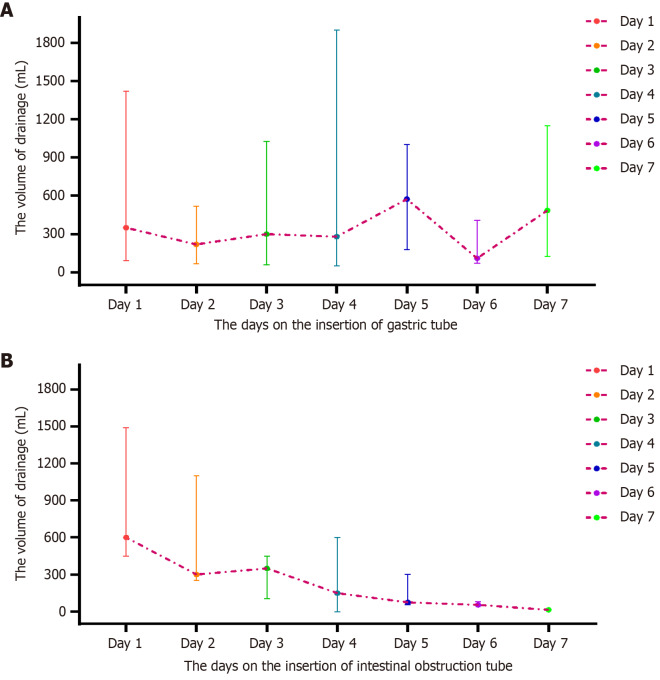
Additionally, we observed that the drainage volume gradually increased within the first 5 d following gastric tube placement, reaching its peak on the fifth day. Subsequently, the drainage volume in some patients gradually decreased to < 50 mL after symptom relief (Figure 3A). However, despite a reduction in drainage volume in a subset of patients, no apparent symptom alleviation was observed. In such cases, the placement of an ileus tube was deemed necessary. Following ileus tube placement, the drainage volume gradually decreased. For most patients, the drainage volume decreased to approximately 100 mL on the fourth to fifth days after the insertion of the ileus tube, prompting its removal (Figure 3B).
Figure 3.
The overall drainage volume of patients receiving decompression. A: Gastric tube; B: The intestinal obstruction tube.
Univariate analysis
The characteristics such as age, symptoms of intestinal obstruction, pre-operative colonoscopy, polyp removal, operative time, blood loss, and stoma had no significant impact on IFD after laparoscopic surgery (all P > 0.05; Table 3).
Table 3.
Uni-variate analysis after propensity score matching
|
Factors
|
IFD (n = 97)
|
Non-IFD (n = 194)
|
χ²
|
OR (95%CI)
|
P value
|
| Age (< 65/≥ 65 yr) | 57/40 | 110/84 | 0.112 | 1.09 (0.66-1.78) | 0.737 |
| BMI (< 24/≥ 24 kg/m2) | 51/46 | 105/89 | 0.062 | 0.94 (0.58-1.53) | 0.803 |
| The systom of obstruction (No/Yes) | 70/27 | 153/41 | 1.622 | 0.69 (0.40-1.22) | 0.203 |
| Total colonscopy (No/Yes) | 43/54 | 73/121 | 1.211 | 1.32 (0.80-2.16) | 0.271 |
| The CFR occupation (≥ 1/2/< 1/2) | 63/34 | 131/63 | 0.193 | 0.89 (0.53-1.49) | 0.660 |
| Neoadjuvant therapy (No/Yes) | 91/6 | 181/11 | 0.031 | 0.91 (0.33-2.54) | 0.860 |
| Colonscopy pre-surgery (No/Yes) | 71/26 | 151/43 | 0.769 | 0.78 (0.44-1.37) | 0.380 |
| Re-location pre-surgery (No/Yes) | 76/21 | 166/28 | 2.405 | 0.61 (0.33-1.14) | 0.121 |
| Polypectomy pre-surgery (No/Yes) | 83/14 | 172/22 | 0.571 | 0.76 (0.37-1.56) | 0.450 |
| Obstruction pre-surgery (No/Yes) | 83/14 | 185/9 | 8.521 | 0.29 (0.12-0.69) | 0.004 |
| PGEP (1-2/3 packages) | 63/34 | 139/55 | 1.368 | 1.36 (0.81-2.30) | 0.243 |
| Cephingosporins pre-surgery (No/Yes) | 87/10 | 159/35 | 2.957 | 1.92 (0.90-4.05) | 0.085 |
| Metronidazole pre-surgery (No/Yes) | 49/48 | 134/60 | 9.541 | 0.46 (0.28-0.75) | 0.002 |
| Operation duration (≥ 130/< 130 min) | 48/49 | 102/92 | 0.248 | 0.88 (0.54-1.44) | 0.619 |
| Total blood loss (≥ 20/< 20 mL) | 66/31 | 138/56 | 0.295 | 0.86 (0.51-1.46) | 0.587 |
| Stoma (No/Yes) | 93/4 | 192/2 | 1.723 | 0.24 (0.04-1.35) | 0.189 |
| Antibotics post-surgery (No/Yes) | 79/18 | 187/7 | 18.401 | 0.16 (0.07-0.41) | < 0.001 |
| Nutrition supply pre-surgery, n (%) | 33.880 | < 0.001 | |||
| EN | 42 (43.3) | 37 (19.1) | 1 (Refrence) | ||
| PN | 17 (17.5) | 99 (51.0) | 6.61(3.35-13.03) | ||
| GNS | 38 (39.2) | 58 (29.9) | 1.73 (0.95-3.16) |
IFD: Intestinal flora disorder; BMI: Body mass index; CFR: Circumferential range; PGEP: Polyethylene glycol electrolytes powder; EN: Enteral nutrition; PN: Parenteral nutrition; GNS: Glucose and sodium chloride injection.
Patients with pre-operative intestinal obstruction (OR = 0.29, 95%CI: 0.12–0.69, P = 0.004), pre-operative use of metronidazole (OR = 0.46, 95%CI: 0.28–0.75, P = 0.002), or post-operative antibiotics (OR = 0.16, 95%CI: 0.07–0.41, P < 0.001) were significantly associated with an increased risk of IFD. Compared to pre-operative enteral nutrition, initiating parenteral nutrition before surgery significantly reduced the risk of IFD (OR = 6.61, 95%CI: 3.35–13.03, P < 0.001).
Multivariate regression analysis
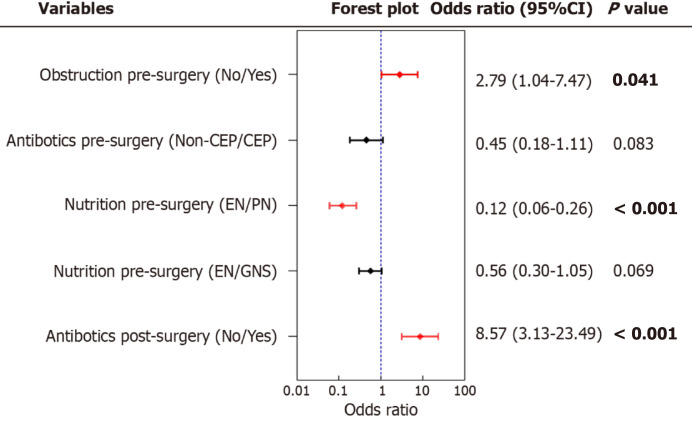
Multivariate analysis indicated that pre-operative intestinal obstruction (OR = 2.79, 95%CI: 1.04–7.47, P = 0.041) and post-operative antibiotics (OR = 8.57, 95%CI: 3.31–23.49, P < 0.001) were independent risk factors for IFD following laparoscopic colon surgery. Conversely, pre-operative parenteral nutrition support emerged as a protective factor against IFD after laparoscopic surgery (OR = 0.12, 95%CI: 0.06–0.26, P < 0.001; Figure 4).
Figure 4.
The multivariate analysis of factor affecting the incidence of intestinal flora disorder. CEP: Cephalosporin; EN: Enteral nutrition; PN: Parenteral nutrition; GNS: Glucose and sodium chloride injection.
DISCUSSION
IFD is a common complication of radical laparoscopic surgery for colon cancer. It manifests as a clinical syndrome with symptoms such as diarrhoea, abdominal distension, increased stoma output, intestinal obstruction, and fever[13,15,16]. The diagnosis of IFD lacks a gold standard, and relies on a comprehensive evaluation of clinical presentations, laboratory tests, and imaging examinations. IFD, particularly leading to paralytic ileus, prolongs hospitalisation, increases healthcare costs, and affects post-operative recovery and subsequent treatment[5,6]. Early identification, accurate diagnosis, and timely intervention are crucial for effective IFD management[17].
IFD predominantly emerges approximately the 3rd to 4th post-operative days, and it typically manifested within 1–2 wk[13]. Clinical presentations include abdominal distension, gas and stool passage cessation, reduced or absent of bowel sounds, and widespread intestinal dilation visible on abdominal CT scans with gas and fluid[18,19]. Given their significant role in post-operative paralytic ileus, early detection, accurate diagnosis, and prompt intervention are crucial for IFD management.
Currently, laboratory tests for diagnosing IFD lack high specificity. Common methods include routine stool examination and testing for C. difficile toxins; however, the overall detection rates and clinical relevance are limited[20,21]. In this study, the positivity rate for C. difficile toxins in patients was only 29.4%, slightly higher than rates reported in previous studies[22]. However, this positivity did not correlate significantly with disease severity, post-operative length of stay, or gastric or intestinal obstruction tube insertion. Besides, though the positivity rate of C. difficile toxins was as low as 30%, most of the patients could benefit from the administration of vancomycin, suggesting the important role of anti-infectious in the management of IFD. This emphasises the constraints associated with relying exclusively on these diagnostic measures.
IFD should primarily be managed conservatively to prevent progression to the point of surgical intervention[23]. Additionally, we observed that the drainage volume gradually increased within the first 5 d following gastric tube placement, reaching its peak on the fifth day. Our team developed a three-step treatment approach involving the modulation of intestinal flora through the application of Bifidobacteria and L. bacillus, anti-infectious therapy with oral or intravenous vancomycin, and decompression drainage with a nasal gastric tube or ileus tube[13,24]. Consequently, the aforementioned treatment measures can be summarised as “P (Probiotics)-V (Vancomycin)-D (Decompression)”. This stepwise approach of P-V-D, tailored to disease severity, has demonstrated satisfactory outcomes.
Early recognition of IFD risk factors is clinically significant[25]. Our study revealed that pre-operative intestinal obstruction and early post-operative antibiotics significantly increased the risk of IFD after laparoscopic surgery. Conversely, pre-operative parenteral nutritional support during mechanical bowel preparation significantly reduced the risk of post-operative IFD, compared to enteral nutrition.
Colon cancer, primarily presenting as a mass, often occupies more than half of the intestinal lumen at the time of diagnosis[26]. Patients with pre-operative intestinal obstruction resulting from tumour-induced luminal obstruction may experience bacterial overgrowth and increased intestinal pressure, potentially leading to bacterial translocation[27,28]. Surgical removal of the tumour can alleviate the obstruction, causing a sudden metabolic shift and disrupting the epithelial barrier, resulting in microbial dysbiosis. Alterations in the gut microbiota microenvironment during the early stages of disease contribute to IFD[29]. Previous studies have demonstrated an association between pre-operative intestinal obstruction and post-operative IFD[25,30]. A retrospective study involving 1366 patients with colorectal cancer reported a post-operative ileus rate of 5.1%, with multivariate analysis indicating that pre-operative intestinal obstruction was an independent risk factor for post-operative ileus[30].
Mechanical bowel preparation, which is effective in clearing faeces and cleaning the bowel, may alter the composition and quantity of the gut microbiota. Pre-operative parenteral nutrition during the bowel preparation period provides the intestinal mucosa with adequate nutrition, preventing mucosal malnutrition owing to prolonged fasting post-operatively[31,32]. This approach avoids disrupting the microbial colonisation of the mucosa. Perioperative antibiotics are recommended in the 0.5-2 h before surgery and are not routinely administered as prophylactic antimicrobial therapy. Multivariate analysis revealed that early post-operative application of antibiotics was an independent risk factor for IFD, possibly associated with the impact of broad-spectrum antibiotics on the distribution and composition of the gut microbiota.
The study also had certain limitations. First, as a retrospective study, despite ensuring an extent of baseline data consistency through PSM, we could not completely avoid potential inclusion bias. Further clarification is necessary through prospective cohort studies. Second, we were limited by the current lack of a gold standard for the clinical diagnosis of IFD, leading to a lack of uniform criteria among researchers when assessing IFD. Finally, establishing a clear causal relationship between IFD and postoperative paralytic ileus is challenging through specific examinations or tests.
CONCLUSION
In conclusion, patients with pre-operative intestinal obstruction, or those exposed to early post-operative antibiotics, should be monitored for potential IFD occurrence. Administering parenteral nutrition before surgery may reduce the risk of IFD. A stepwise and comprehensive “P-V-D” treatment approach of focusing on "modulating intestinal flora, anti-infectious therapy, decompression by applying a gastric or ileus tube", demonstrated clinical efficacy and can be applied to IFD management.
Footnotes
Institutional review board statement: The study was reviewed and approved by the Ethics Committee of Peking Union Medical College Hospital (Approval No: I-23PJ157).
Informed consent statement: Informed written consent was obtained from the patient for publication of this report and any accompanying images.
Conflict-of-interest statement: The authors declare that they have no conflict of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade B
P-Reviewer: Gupta A, India S-Editor: Liu H L-Editor: A P-Editor: Guo X
Contributor Information
Gan-Bin Li, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Chen-Tong Wang, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Xiao Zhang, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Xiao-Yuan Qiu, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Wei-Jie Chen, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Jun-Yang Lu, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Lai Xu, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Bin Wu, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Yi Xiao, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China.
Guo-Le Lin, Department of General Surgery, Peking Union Medical College Hospital, Peking Union Medical College, Beijing 100730, China. linguole@126.com.
Data sharing statement
No additional data are available.
References
- 1.Davidson KW, Barry MJ, Mangione CM, Cabana M, Caughey AB, Davis EM, Donahue KE, Doubeni CA, Krist AH, Kubik M, Li L, Ogedegbe G, Owens DK, Pbert L, Silverstein M, Stevermer J, Tseng CW, Wong JB US Preventive Services Task Force. Screening for Colorectal Cancer: US Preventive Services Task Force Recommendation Statement. JAMA. 2021;325:1965–1977. doi: 10.1001/jama.2021.6238. [DOI] [PubMed] [Google Scholar]
- 2.Ow ZGW, Sim W, Nistala KRY, Ng CH, Koh FH, Wong NW, Foo FJ, Tan KK, Chong CS. Comparing complete mesocolic excision versus conventional colectomy for colon cancer: A systematic review and meta-analysis. Eur J Surg Oncol. 2021;47:732–737. doi: 10.1016/j.ejso.2020.09.007. [DOI] [PubMed] [Google Scholar]
- 3.Bertelsen CA, Neuenschwander AU, Jansen JE, Tenma JR, Wilhelmsen M, Kirkegaard-Klitbo A, Iversen ER, Bols B, Ingeholm P, Rasmussen LA, Jepsen LV, Born PW, Kristensen B, Kleif J. 5-year outcome after complete mesocolic excision for right-sided colon cancer: a population-based cohort study. Lancet Oncol. 2019;20:1556–1565. doi: 10.1016/S1470-2045(19)30485-1. [DOI] [PubMed] [Google Scholar]
- 4.Xu L, Su X, He Z, Zhang C, Lu J, Zhang G, Sun Y, Du X, Chi P, Wang Z, Zhong M, Wu A, Zhu A, Li F, Xu J, Kang L, Suo J, Deng H, Ye Y, Ding K, Xu T, Zhang Z, Zheng M, Xiao Y RELARC Study Group. Short-term outcomes of complete mesocolic excision versus D2 dissection in patients undergoing laparoscopic colectomy for right colon cancer (RELARC): a randomised, controlled, phase 3, superiority trial. Lancet Oncol. 2021;22:391–401. doi: 10.1016/S1470-2045(20)30685-9. [DOI] [PubMed] [Google Scholar]
- 5.Diamantis A, Samara AΑ, Tzovaras G, Magouliotis D, Arnaoutoglou E, Volakakis G, Tepetes K. Use of preoperative EORTC quality-of-life questionnaires to predict postoperative complications after colorectal cancer surgery. Br J Surg. 2021;108:e402–e403. doi: 10.1093/bjs/znab343. [DOI] [PubMed] [Google Scholar]
- 6.Brown SR, Mathew R, Keding A, Marshall HC, Brown JM, Jayne DG. The impact of postoperative complications on long-term quality of life after curative colorectal cancer surgery. Ann Surg. 2014;259:916–923. doi: 10.1097/SLA.0000000000000407. [DOI] [PubMed] [Google Scholar]
- 7.Sánchez-Torralvo FJ, González-Poveda I, García-Olivares M, Porras N, Gonzalo-Marín M, Tapia MJ, Mera-Velasco S, Toval-Mata JA, Ruiz-López M, Carrasco-Campos J, Santoyo-Santoyo J, Olveira G. Poor Physical Performance Is Associated with Postoperative Complications and Mortality in Preoperative Patients with Colorectal Cancer. Nutrients. 2022;14 doi: 10.3390/nu14071484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greco CD, Petro CC, Thomas JD, Montelione K, Tu C, Fafaj A, Zolin S, Krpata D, Rosenblatt S, Rosen M, Beffa L, Prabhu A. Ileus rate after abdominal wall reconstruction: a retrospective analysis of two clinical trials. Hernia. 2022;26:1591–1598. doi: 10.1007/s10029-022-02687-7. [DOI] [PubMed] [Google Scholar]
- 9.Barry RE, Chow AW, Billesdon J. Role of intestinal microflora in colonic pseudoobstruction complicating jejunoileal bypass. Gut. 1977;18:356–359. doi: 10.1136/gut.18.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ren C, Yang M, Yang Z. The Role of Intestinal Flora and Ethnic Differences in Colorectal Cancer Risk. Gastroenterology. 2022;163:782–783. doi: 10.1053/j.gastro.2022.05.022. [DOI] [PubMed] [Google Scholar]
- 11.Zou Y, Wang S, Zhang H, Gu Y, Chen H, Huang Z, Yang F, Li W, Chen C, Men L, Tian Q, Xie T. The triangular relationship between traditional Chinese medicines, intestinal flora, and colorectal cancer. Med Res Rev. 2024;44:539–567. doi: 10.1002/med.21989. [DOI] [PubMed] [Google Scholar]
- 12.Jiang Y, Xu Y, Zheng C, Ye L, Jiang P, Malik S, Xu G, Zhou Q, Zhang M. Acetyltransferase from Akkermansia muciniphila blunts colorectal tumourigenesis by reprogramming tumour microenvironment. Gut. 2023;72:1308–1318. doi: 10.1136/gutjnl-2022-327853. [DOI] [PubMed] [Google Scholar]
- 13.Xue XQ, Bai XS, Lin GL, Li JH, Zhou JL, Qiu HZ. [Correlation between intestinal flora imbalance and anastomotic leakage after operation for middle to low rectal cancer] Zhongguo Shiyong Waike Zazhi. 2019;39:698–703. [Google Scholar]
- 14.Wang D, Zhang J, Bai Z, Yang Y, Wang T, Jin L, Wang J, Wu G, Kou T, Zhang Z. Associations of Postoperative Complications Assessed by Clavien-Dindo Classification and Comprehensive Complication Index with Long-Term Overall Survival in Elderly Patients after Radical CRC Resection. Clin Interv Aging. 2020;15:1939–1949. doi: 10.2147/CIA.S271969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen K, Man S, Wang H, Gao C, Li X, Liu L, Wang Y, Lu F. Dysregulation of intestinal flora: excess prepackaged soluble fibers damage the mucus layer and induce intestinal inflammation. Food Funct. 2022;13:8558–8571. doi: 10.1039/d2fo01884e. [DOI] [PubMed] [Google Scholar]
- 16.Jin Y, Geng R, Liu Y, Liu L, Jin X, Zhao F, Feng J, Wei Y. Prediction of Postoperative Ileus in Patients With Colorectal Cancer by Preoperative Gut Microbiota. Front Oncol. 2020;10:526009. doi: 10.3389/fonc.2020.526009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weng J, Wu H, Wang Z. Risk factors for postoperative ileus after colorectal cancer surgery: methodological issues. Colorectal Dis. 2018;20:351–352. doi: 10.1111/codi.14043. [DOI] [PubMed] [Google Scholar]
- 18.Dunbar BS, Skinner SM. Preparation of monoclonal antibodies. Methods Enzymol. 1990;182:670–679. doi: 10.1016/0076-6879(90)82052-4. [DOI] [PubMed] [Google Scholar]
- 19.Yang JW, Shao JK, Wang Y, Liu Q, Liang JW, Yan SY, Zhou SC, Yang NN, Wang LQ, Shi GX, Pei W, Liu CZ. Effect of acupuncture on postoperative ileus after laparoscopic elective colorectal surgery: A prospective, randomised, controlled trial. EClinicalMedicine. 2022;49:101472. doi: 10.1016/j.eclinm.2022.101472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xiao Q, Chen B, Zhu Z, Yang T, Tao E, Hu C, Zheng W, Tang W, Shu X, Jiang M. Alterations in the Fecal Microbiota Composition in Pediatric Acute Diarrhea: A Cross-Sectional and Comparative Study of Viral and Bacterial Enteritis. Infect Drug Resist. 2023;16:5473–5483. doi: 10.2147/IDR.S410720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paquet-Bolduc B, Gervais P, Roussy JF, Trottier S, Oughton M, Brukner I, Longtin J, Loo VG, Dascal A, Longtin Y. Detection and Isolation of Clostridium difficile Asymptomatic Carriers During Clostridium difficile Infection Outbreaks: An Exploratory Study. Clin Infect Dis. 2018;67:1781–1783. doi: 10.1093/cid/ciy425. [DOI] [PubMed] [Google Scholar]
- 22.Kamboj M, Brite J, Aslam A, Kennington J, Babady NE, Calfee D, Furuya Y, Chen D, Augenbraun M, Ostrowsky B, Patel G, Mircescu M, Kak V, Tuma R, Karre TA, Fry DA, Duhaney YP, Moyer A, Mitchell D, Cantu S, Hsieh C, Warren N, Martin S, Willson J, Dickman J, Knight J, Delahanty K, Flood A, Harrington J, Korenstein D, Eagan J, Sepkowitz K. Artificial Differences in Clostridium difficile Infection Rates Associated with Disparity in Testing. Emerg Infect Dis. 2018;24:584–587. doi: 10.3201/eid2403.170961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kobayashi T, Masaki T, Kogawa K, Matsuoka H, Sugiyama M. Efficacy of Gum Chewing on Bowel Movement After Open Colectomy for Left-Sided Colorectal Cancer: A Randomized Clinical Trial. Dis Colon Rectum. 2015;58:1058–1063. doi: 10.1097/DCR.0000000000000452. [DOI] [PubMed] [Google Scholar]
- 24.Van Hise NW, Bryant AM, Hennessey EK, Crannage AJ, Khoury JA, Manian FA. Efficacy of Oral Vancomycin in Preventing Recurrent Clostridium difficile Infection in Patients Treated With Systemic Antimicrobial Agents. Clin Infect Dis. 2016;63:651–653. doi: 10.1093/cid/ciw401. [DOI] [PubMed] [Google Scholar]
- 25.Rybakov EG, Shelygin YA, Khomyakov EA, Zarodniuk IV. Risk factors for postoperative ileus after colorectal cancer surgery. Colorectal Dis. 2017 doi: 10.1111/codi.13888. [DOI] [PubMed] [Google Scholar]
- 26.Wilson BE, Booth CM, Sullivan R, Aggarwal A, Sengar M, Jacob S, Bray F, Barton MB, Pearson SA. Global application of National Comprehensive Cancer Network resource-stratified guidelines for systemic treatment of colon cancer: a population-based, customisable model for cost, demand, and procurement. Lancet Oncol. 2023;24:682–690. doi: 10.1016/S1470-2045(23)00183-3. [DOI] [PubMed] [Google Scholar]
- 27.Jung SH, Kim JH. Comparative study of postoperative complications in patients with and without an obstruction who had left-sided colorectal cancer and underwent a single-stage operation after mechanical bowel preparation. Ann Coloproctol. 2014;30:251–258. doi: 10.3393/ac.2014.30.6.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cui JQ, Tian HL, Wang XJ. [Analysis of short-term efficacy of perioperative fecal microbiota transplantation combined with nutritional support in patients with radiation-induced enteritis complicated by intestinal obstruction] Zhonghua Weichang Waike Zazhi. 2023;26:955–962. doi: 10.3760/cma.j.cn441530-20230816-00052. [DOI] [PubMed] [Google Scholar]
- 29.Wong CC, Yu J. Gut microbiota in colorectal cancer development and therapy. Nat Rev Clin Oncol. 2023;20:429–452. doi: 10.1038/s41571-023-00766-x. [DOI] [PubMed] [Google Scholar]
- 30.Xu C, Chi P. [Relevant factor analysis on postoperative ileus following radical resection for colorectal cancer] Zhongguo Weichangbingxue Zazhi. 2014;17:361–364. [PubMed] [Google Scholar]
- 31.Okamoto K, Fukatsu K, Hashiguchi Y, Ueno H, Shinto E, Moriya T, Saitoh D, Yamamoto J, Hase K. Lack of preoperative enteral nutrition reduces gut-associated lymphoid cell numbers in colon cancer patients: a possible mechanism underlying increased postoperative infectious complications during parenteral nutrition. Ann Surg. 2013;258:1059–1064. doi: 10.1097/SLA.0b013e31827a0e05. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Li S, Xi H, Liu P, Liang W, Gao Y, Wang C, Wei B, Chen L, Tang Y, Qiao Z. Effect of preoperative nutrition therapy type and duration on short-time outcomes in gastric cancer patient with gastric outlet obstruction. Chin J Cancer Res. 2021;33:232–242. doi: 10.21147/j.issn.1000-9604.2021.02.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.