Abstract
BACKGROUND
Appendectomy is an acute abdominal surgery that is often accompanied by severe abdominal inflammation. Oral probiotics are one of the postoperative treatments for rapid rehabilitation. However, there is a lack of prospective studies on this topic after appendectomy.
AIM
To investigate whether the postoperative probiotics can modulate the inflammatory response and restore intestinal function in patients following appendectomy.
METHODS
This was a prospective, randomized trial. A total of 60 emergency patients were randomly divided into a control group (n = 30) and a probiotic group (n = 30). Patients in the control group started to drink some water the first day after surgery, and those in the probiotic group were given water supplemented with Bacillus licheniformis capsules for 5 consecutive days postsurgery. The indices of inflammation and postoperative conditions were recorded, and the data were analyzed with RStudio 4.3.2 software.
RESULTS
A total of 60 participants were included. Compared with those in the control group, the C-reactive protein (CRP), interleukin 6 and procalcitonin (PCT) levels were significantly lower in the probiotic group at 2 d after surgery (P = 2.224e-05, P = 0.037, and P = 0.002, respectively, all P < 0.05). This trend persisted at day 5 post-surgery, with CRP and PCT levels remaining significantly lower in the probiotic group (P = 0.001 and P = 0.043, both P < 0.05). Furthermore, probiotics resulted in a shorter time to first flatus and a greater percentage of gram-negative bacilli in the feces (P = 0.035, P = 0.028, both P < 0.05).
CONCLUSION
Postoperative oral administration of probiotics may modulate the gut microbiota, benefit the recovery of the early inflammatory response, and subsequently enhance recovery after appendectomy.
Keywords: Probiotics, Gut microbiota, Appendectomy, Inflammatory markers, Intestinal function, Enhanced recovery after surgery, Postsurgical infections
Core Tip: This research examines the impact of administering oral probiotics postoperatively on inflammation responses and intestinal function in patients undergoing appendectomy procedures. Our findings reveal that orally administered probiotics effectively decrease postoperative inflammatory indicators and enhance intestinal functionality, thereby resulting in reduced hospitalization durations. These insights highlight the potential contribution of probiotics in expediting post-surgical recovery and offer novel approaches for clinical application.
INTRODUCTION
Appendectomy is an acute abdominal surgery that is often accompanied by severe abdominal inflammation. Inflammation will continue even after surgery for almost a week. Severe inflammation can increase the translocation of endotoxin and pathogenic bacteria, leading to intestinal mucosal barrier dysfunction[1-3]. Therefore, inflammatory intestinal obstructions and abdominal abscesses often appear after surgery[2]. Oral probiotics are one of the postoperative treatments for rapid rehabilitation. Several studies have demonstrated that probiotics can reduce the inflammatory response and incidence of postoperative infections and promote the recovery of gastrointestinal function after gastrointestinal surgery[2-7]. Probiotics administered orally can modulate the intestinal mucosal barrier function, thereby enhancing the stability and balance of gut microbiota[8]. Thus, the role of probiotics after gastrointestinal surgery has attracted increasing amounts of attention[4,8-15]. However, there is a lack of prospective studies on this topic after appendectomy. In our study, whether the oral administration of postoperative probiotics can effectively modulate the inflammatory response and restore intestinal function in patients following appendectomy was investigated.
MATERIALS AND METHODS
We conducted a randomized controlled trial comprising 60 emergency adult patients who underwent laparoscopic appendectomy and were admitted to Zigong Fourth People's Hospital in Sichuan Province, China, between June 1st, 2023 and August 31st, 2023. This study was approved by the Institutional Ethics Committee and adhered to the ethical guidelines laid down by them.
Sixty adult patients were randomly divided into a control group (n = 30) and a probiotic group (n = 30). The process of assigning participants or experimental units into different groups has been carried out using the Random Number Table Method. In the control group, 15 patients were male, 15 were female, and the mean age was 44.5 ± 15.91 years. In the probiotics group, there were 13 males and 17 females aged 43.17 ± 15.73 years. All patients underwent computerized tomography and were diagnosed by an experienced attending doctor. The exclusion criteria for patients were as follows: Periappendiceal abscess, diabetes mellitus, severe organ dysfunction, or conversion to laparotomy. General data such as sex and age were comparable between the two groups. Before surgery, all patients or guardians signed informed consent.
Patients in the control groups started to drink some water the first day after surgery, and those in the probiotic group received Bacillus licheniformis capsules with water (Northeast Pharmaceutical Group Co., Ltd., Liaoning Province, China) for 5 consecutive day postsurgery. After surgery, the patients were treated with antibiotics (ceftazidime; Qilu Pharmaceutical Co., Ltd., Shandong Province, China).
Detection indices
The following indices were detected after admission to the hospital before surgery and at 2 and 5 d after surgery.
C-reactive protein (CRP) and interleukin 6 (IL-6) were detected using the enzyme-linked immunosorbent assay method. Procalcitonin (PCT) was detected by a Roche E411 automatic electrochemiluminescence analyzer.
The white blood cell (WBC) count and neutrophil percentage (NE%) were detected using a Labospect003 full-automatic biochemical analyzer (Hitachi).
Body temperature was measured every four hours with a mercury thermometer (axillary temperature < 37.4 °C), and the average heart rate was recorded by the electrocardiogram monitor and nurse. In addition, the time of the first anal exsufflation was recorded.
Statistical analysis
Based on the approximate means and standard deviations of various inflammation indicators, the sample size was determined. RStudio 4.3.2 software was used for statistical analysis. The quantitative data are presented as the mean ± SD. A t test was used to compare differences between two groups. The categorical data are presented as numbers and were compared by the chi-square test. P < 0.05 was considered to indicate statistical significance (aP < 0.05, bP < 0.01, cP < 0.001).
RESULTS
A comparison of WBC and NE% pre- and post-surgery among the two groups revealed that WBC levels in both groups were reduced following surgery and no significant difference was observed between the groups post-operatively (P > 0.05). The NE% was lower than that before surgery in both groups and was similar in both groups (P > 0.05). These findings indicate that the preoperative application of postoperative probiotics cannot significantly reduce the WBC and NE% after appendectomy (Table 1).
Table 1.
C-reactive protein/interleukin/procalcitonin and white blood cell count before and after surgery in both groups
|
Items
|
Groups
|
Before surgery
|
2 d after surgery
|
5 d after surgery
|
| WBC (× 109/L) | Control group | 13.09 ± 2.98 | 10.44 ± 2.17 | 7.82 ± 1.98 |
| Probiotics group | 12.72 ± 2.87 | 9.41 ± 2.19 | 7.69 ± 1.95 | |
| CRP (mg/L) | Control group | 32.83 ± 56.11 | 102.07 ± 73.49 | 33.03 ± 28.56 |
| Probiotics group | 30.89 ± 33.52 | 31.49 ± 26.57a | 13.46 ± 10.49a | |
| IL (pg/mL) | Control group | 24.73 ± 12.73 | 36.55 ± 45.58 | 9.32 ± 7.64 |
| Probiotics group | 30.31 ± 20.29 | 16.85 ± 18.2a | 8.71 ± 5.72 | |
| PCT (ng/mL) | Control group | 0.143 ± 0.2 | 0.895 ± 1.2 | 0.521 ± 1.19 |
| Probiotics group | 0.216 ± 0.29 | 0.155 ± 0.26a | 0.051 ± 0.07a |
P < 0.05 in the comparison between the two groups.
CRP: C-reactive protein; WBC: White blood cell count; IL: Interleukin; PCT: Procalcitonin.
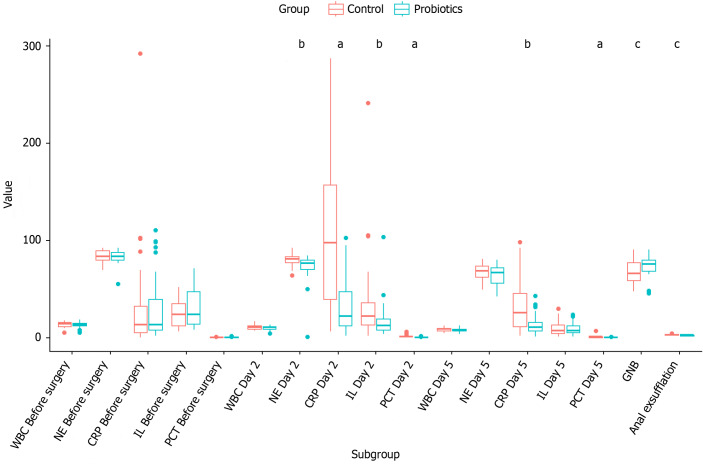
Comparison of CRP, IL, and PCT between two groups pre- and post-surgery. In the control group, CRP levels rose 2 d post-surgery, subsequently declining gradually towards the normal range by day 5, whereas in the probiotics group, this decrease was notably more pronounced. At 2 d post-surgery, the CRP increase was marginal in the probiotics group, resulting in statistically significant differences between the groups at both the 2-d (P = 2.224e-05) and 5-d (P = 0.001) marks, all with P values less than 0.05. The trend for PCT mirrored that of CRP: In the control group, it increased post-surgery at 2 d, later reducing gradually to normal levels by day 5, yet again experiencing a more evident decline within the probiotics group. The PCT levels also exhibited a slight decrease 2 d after surgery in the probiotics group, leading to statistically significant inter-group differences at both 2-d (P = 0.002) and 5-d (P = 0.043) assessments, all with P values below 0.05. As for IL levels, they followed a similar pattern in the control group, rising at 2 d post-surgery and gradually returning to normal levels by day 5. However, there was a statistically significant difference between the two groups solely at the 2-d post-surgical mark (P = 0.037, P < 0.05). These findings collectively suggest that the administration of probiotics significantly enhances the attenuation of the systemic inflammatory response following surgery (Table 1 and Figure 1).
Figure 1.
Various test indicator before and after surgery in both groups. aP < 0.05, bP < 0.01, cP < 0.001. NE: Neutrophil; GNB: Gram-negative bacilli; CRP: C-reactive protein; WBC: White blood cell count; IL: Interleukin; PCT: Procalcitonin.
Comparison of the percentage of gram-negative bacilli (GNB) in the feces after surgery between the two groups. The percentage of GNB in the feces was greater in the probiotics group than in the control group (P = 0.028, P < 0.05). These findings suggest that probiotics can modulate the gut microbiota (Table 2 and Figure 1).
Table 2.
Comparison of the postoperative general conditions between the two groups at 7 d after surgery
|
Groups
|
Fever (%)
|
Heart rate
|
First exhaust, time (d)
|
GNB%
|
| Control group | 16.6 | 74 ± 10.93 | 2.16 ± 0.69 | 67.2 ± 11.01 |
| Probiotics group | 13.3 | 76.4 ± 12.09 | 1.8 ± 0.6a | 73.3 ± 9.65a |
P < 0.05 in the comparison between the two groups.
GNB%: The percentage of gram-negative bacilli.
Comparison of the postoperative conditions between the two groups. The incidence rates of postoperative fever and average heart rate were similar in both groups (P > 0.05). The first exhaust time was shorter in the probiotic group than in the control group (P = 0.035, P < 0.05; Table 2 and Figure 1).
DISCUSSION
There is increasing interest in the relationship between the gut microbiota and human immunity[1,5,8]. In our study, we found that the CRP, IL-6 and PCT levels were significantly lower in the probiotic group after appendectomy. This outcome is consistent with findings reported in articles related to postoperative oral probiotics administration in colorectal cancer patients. Gastrointestinal surgery alters the gut microbiota due to surgical trauma and inflammation. Microbiota changes and intestinal barrier damage may cause systemic inflammation. According to previous studies[3,11,12,14,15], probiotics can be used to modulate the gut microbiota and have beneficial effects on humans. Perioperative probiotic administration during colorectal surgery can reduce infectious complications. Additionally, probiotics can reduce the levels of inflammatory markers and cytokines, such as CRP and IL[1,5,8,12].
In our study on appendicitis, it revealed that in the control group, CRP levels escalated 2 d post-surgery, thereafter gradually diminishing to reach normal levels by the fifth day post-procedure. Remarkably, this reduction was even more pronounced in the probiotics group. The inflammatory marker PCT was also similar to the CRP. Differences in the IL-6 concentration at 2 d after surgery were statistically significant between the two groups. This finding showed that postoperative probiotic administration may accelerate the duration of inflammation reduction after appendectomy. However, the WBC and NE% were lower after surgery than before surgery in both groups and were similar in both groups. This result was dissimilar to that obtained for inflammatory markers. This could be because inflammatory markers, such as CRP levels, are more sensitive than WBC counts are.
A comparison of the postoperative conditions between the two groups revealed that the time to first exhaust was shorter in the probiotics group than in the control group. These findings showed that postoperative probiotic administration after appendectomy may accelerate the recovery of intestinal function, contributing to enhanced recovery after surgery.
However, our study unfortunately did not include markers of intestinal mucosal barrier function. This will be our next step. Due to the wide fluctuations in CRP values, our study may suffer from inadequate sample size, and in future in-depth investigations, increasing the sample size should be taken into consideration.
CONCLUSION
In conclusion, postoperative oral administration of probiotics may modulate the gut microbiota, improve the recovery of the early inflammatory response, and subsequently enhance recovery after appendectomy.
Footnotes
Institutional review board statement: This study was approved by the Ethics Committee of Zigong Fourth People's Hospital and adhered to the ethical guidelines laid down by them (Approval No. 2023012).
Clinical trial registration statement: This study has already undergone retrospective registration for the clinical trial at https://www.chictr.org.cn/. The registration identification number is ChiCTR2400083131.
Informed consent statement: All study participants, or their legal guardian, provided informed written consent prior to study enrollment.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
CONSORT 2010 statement: The authors have read the CONSORT 2010 statement, and the manuscript was prepared and revised according to the CONSORT 2010 statement.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade A
Novelty: Grade A
Creativity or Innovation: Grade A
Scientific Significance: Grade A
P-Reviewer: Glumac S, Croatia S-Editor: Li L L-Editor: A P-Editor: Zheng XM
Contributor Information
Ke Lan, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Ke-Rui Zeng, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Fu-Rui Zhong, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Sheng-Jin Tu, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Jin-Long Luo, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Shi-Long Shu, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Xue-Feng Peng, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Hua Yang, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China.
Kai Lu, Department of General Surgery, Zigong Fourth People's Hospital, Zigong 643000, Sichuan Province, China. 597768288@qq.com.
Data sharing statement
Dataset available from the corresponding author at 597768288@qq.com.
References
- 1.Tang G, Huang W, Tao J, Wei Z. Prophylactic effects of probiotics or synbiotics on postoperative ileus after gastrointestinal cancer surgery: A meta-analysis of randomized controlled trials. PLoS One. 2022;17:e0264759. doi: 10.1371/journal.pone.0264759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Benson AB, Venook AP, Al-Hawary MM, Cederquist L, Chen YJ, Ciombor KK, Cohen S, Cooper HS, Deming D, Engstrom PF, Garrido-Laguna I, Grem JL, Grothey A, Hochster HS, Hoffe S, Hunt S, Kamel A, Kirilcuk N, Krishnamurthi S, Messersmith WA, Meyerhardt J, Miller ED, Mulcahy MF, Murphy JD, Nurkin S, Saltz L, Sharma S, Shibata D, Skibber JM, Sofocleous CT, Stoffel EM, Stotsky-Himelfarb E, Willett CG, Wuthrick E, Gregory KM, Freedman-Cass DA. NCCN Guidelines Insights: Colon Cancer, Version 2.2018. J Natl Compr Canc Netw. 2018;16:359–369. doi: 10.6004/jnccn.2018.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu ZH, Huang MJ, Zhang XW, Wang L, Huang NQ, Peng H, Lan P, Peng JS, Yang Z, Xia Y, Liu WJ, Yang J, Qin HL, Wang JP. The effects of perioperative probiotic treatment on serum zonulin concentration and subsequent postoperative infectious complications after colorectal cancer surgery: a double-center and double-blind randomized clinical trial. Am J Clin Nutr. 2013;97:117–126. doi: 10.3945/ajcn.112.040949. [DOI] [PubMed] [Google Scholar]
- 4.Liu PC, Yan YK, Ma YJ, Wang XW, Geng J, Wang MC, Wei FX, Zhang YW, Xu XD, Zhang YC. Probiotics Reduce Postoperative Infections in Patients Undergoing Colorectal Surgery: A Systematic Review and Meta-Analysis. Gastroenterol Res Pract. 2017;2017:6029075. doi: 10.1155/2017/6029075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Liang H, Lu J, He Y, Lai R. Probiotics Improve Postoperative Adaptive Immunity in Colorectal Cancer Patients: A Systematic Review and Meta-Analysis. Nutr Cancer. 2022;74:2975–2982. doi: 10.1080/01635581.2022.2056619. [DOI] [PubMed] [Google Scholar]
- 6.Gionchetti P, Amadini C, Rizzello F, Venturi A, Poggioli G, Campieri M. Probiotics for the treatment of postoperative complications following intestinal surgery. Best Pract Res Clin Gastroenterol. 2003;17:821–831. doi: 10.1016/s1521-6918(03)00071-4. [DOI] [PubMed] [Google Scholar]
- 7.Lytvyn L, Quach K, Banfield L, Johnston BC, Mertz D. Probiotics and synbiotics for the prevention of postoperative infections following abdominal surgery: a systematic review and meta-analysis of randomized controlled trials. J Hosp Infect. 2016;92:130–139. doi: 10.1016/j.jhin.2015.08.028. [DOI] [PubMed] [Google Scholar]
- 8.An S, Kim K, Kim MH, Jung JH, Kim Y. Perioperative Probiotics Application for Preventing Postoperative Complications in Patients with Colorectal Cancer: A Systematic Review and Meta-Analysis. Medicina (Kaunas) 2022;58 doi: 10.3390/medicina58111644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu C, Yang J, Dong W, Yuan J. Effects of probiotics on gastrointestinal complications and nutritional status of postoperative patients with esophageal cancer: A protocol of randomized controlled trial. Medicine (Baltimore) 2021;100:e25138. doi: 10.1097/MD.0000000000025138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen C, Wen T, Zhao Q. Probiotics Used for Postoperative Infections in Patients Undergoing Colorectal Cancer Surgery. Biomed Res Int. 2020;2020:5734718. doi: 10.1155/2020/5734718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu Q, Xu P, Cen Y, Li W. Effects of preoperative oral administration of glucose solution combined with postoperative probiotics on inflammation and intestinal barrier function in patients after colorectal cancer surgery. Oncol Lett. 2019;18:694–698. doi: 10.3892/ol.2019.10336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie H, Lu Q, Wang H, Zhu X, Guan Z. Effects of probiotics combined with enteral nutrition on immune function and inflammatory response in postoperative patients with gastric cancer. J BUON. 2018;23:678–683. [PubMed] [Google Scholar]
- 13.Ohigashi S, Hoshino Y, Ohde S, Onodera H. Functional outcome, quality of life, and efficacy of probiotics in postoperative patients with colorectal cancer. Surg Today. 2011;41:1200–1206. doi: 10.1007/s00595-010-4450-6. [DOI] [PubMed] [Google Scholar]
- 14.Pellino G, Sciaudone G, Candilio G, Camerlingo A, Marcellinaro R, De Fatico S, Rocco F, Canonico S, Riegler G, Selvaggi F. Early postoperative administration of probiotics versus placebo in elderly patients undergoing elective colorectal surgery: a double-blind randomized controlled trial. BMC Surg. 2013;13 Suppl 2:S57. doi: 10.1186/1471-2482-13-S2-S57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Li C, Huang M, Tong C, Zhang X, Wang L, Peng H, Lan P, Zhang P, Huang N, Peng J, Wu X, Luo Y, Qin H, Kang L, Wang J. Positive regulatory effects of perioperative probiotic treatment on postoperative liver complications after colorectal liver metastases surgery: a double-center and double-blind randomized clinical trial. BMC Gastroenterol. 2015;15:34. doi: 10.1186/s12876-015-0260-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Dataset available from the corresponding author at 597768288@qq.com.