Abstract
BACKGROUND
Pituitary gland metastasis is an unusual event, and pituitary metastasis from lung adenocarcinoma is extremely rare and associated with poor prognosis. To date, approximately 15 cases have been reported.
CASE SUMMARY
Here, we present the case of a 64-year-old woman with pituitary metastasis derived from lung adenocarcinoma, which was difficult to distinguish from other sellar tumors. The patient presented to the neurosurgery clinic with blurred vision and intermittent headache. During hospitalization, brain computed tomography (CT) and magnetic resonance imaging revealed a pituitary macroadenoma. Chest CT revealed irregular nodules in the basal segment of the lower lobe of the left lung, which were likely lung cancer. Positron emission tomography-CT revealed a carbohydrate metabolism tumor in the lungs and sellar region, which was considered malignant. Postoperative pathological examination of the sellar tumor revealed lung adenocarcinoma.
CONCLUSION
Excision of pituitary metastases combined with radiotherapy and chemotherapy should be a priority treatment for patients with pituitary metastasis.
Keywords: Pituitary metastasis, Lung adenocarcinoma, Prolactin, Sellar region tumors, Case report
Core Tip: Pituitary metastasis from lung adenocarcinoma is extremely rare, but physicians should consider it if it rapidly develops clinical symptoms or grows quickly, especially in patients with other tumors or those with a history of other systemic malignancies. Radiotherapy and chemotherapy are recommended as initial treatments. The blood supply to pituitary metastases is extremely rich; therefore, neurosurgeons must make adequate surgical preparations and plans.
INTRODUCTION
Pituitary metastasis is a rare condition with poor prognosis. The occurrence rate is approximately 1% for all sellar region tumors, with a median survival of less than 1 year[1]. Patients have non-specific clinical symptoms, such as visual field deficits, cranial nerve palsy, anterior pituitary dysfunction, ophthalmoplegia, and diabetes insipidus. Computed tomography (CT) and magnetic resonance imaging (MRI) reveal non-specific diagnostic characteristics. Lung cancer is the leading cause of death among all cancers worldwide[2]. It transfers to the nervous system, bone, liver, and respiratory system. It is important for clinicians to improve awareness to differentiate pituitary metastases in all sellar region tumors, especially in patients aged > 60 years. Here, we report the case of a 64-year-old woman with pituitary metastasis derived from lung adenocarcinoma.
CASE PRESENTATION
Chief complaints
A 64-year-old Asian woman was hospitalized for blurred vision and intermittent headache of 3 months.
History of present illness
Symptoms started 3 months before presentation with blurred vision and intermittent headache.
History of past illness
The patient had no history of hospitalization.
Personal and family history
The patient had no significant personal or family history.
Physical examination
The patient’s vital signs were unremarkable. Ophthalmological examination showed that the naked vision was 0.25 in the right eye and 0.8 in the left eye.
Laboratory examinations
Routine blood and urine analyses showed normal results. The endocrine examination results are presented in Table 1.
Table 1.
Endocrine examination result
|
Hormone
|
Level measured
|
Normal level
|
| ACTH (pg/mL) | 17.20 | 7.2-63.3 |
| PRL (high) (ng/mL) | > 200 | 2.18-26.53 |
| GH (ng/mL) | 0.32 | 0.02-4.77 |
| TSH (μIU/mL) | 1.987 | 0.35-4.94 |
| T3 (nmol/L) | 1.69 | 0.89-2.44 |
| TT4 (low) (nmol/L) | 61.22 | 62.70-150.80 |
| FT3 (pmol/L) | 4.26 | 2.43-6.01 |
| FT4 (pmol/L) | 9.96 | 9.00-19.00 |
| Tg (ng/mL) | 9.988 | 0.20-70.00 |
| LH (low) (pmol/L) | 0.03 | 5.16-61.99 |
| FSH (low) (mIU/mL) | 1.08 | 26.72-133.41 |
| E2 (pg/mL) | < 10 | < 10-28 |
| P4 (ng/mL) | < 0.1 | < 0.1-0.2 |
| CORT (8:00) (μg/dL) | 1.0 | 6.02-18.4 |
| CORT (16:00) (μg/dL) | 1.5 | 2.68-10.5 |
| CORT (24:00) (μg/dL) | 1.1 | 2.68-10.5 |
| TES (pmol/L) | < 12.98 | 10.83-56.94 |
ACTH: Adrenocorticotropic hormone; PRL: Prolactin hormone; GH: Growth hormone; TSH: Thyroid stimulating hormone; T3: Triiodothyronine; TT4: Total tetraiodothyronine; FT3: Free triiodothyronine; FT4: Free tetraiodothyronine; Tg: Thyroglobulin; LH: Luteinizing hormone; FSH: Follicle stimulating hormone; E2: Estrogen hormone; P4: Progestational hormone; CORT: Cortisol; TES: Testosterone.
Imaging examinations
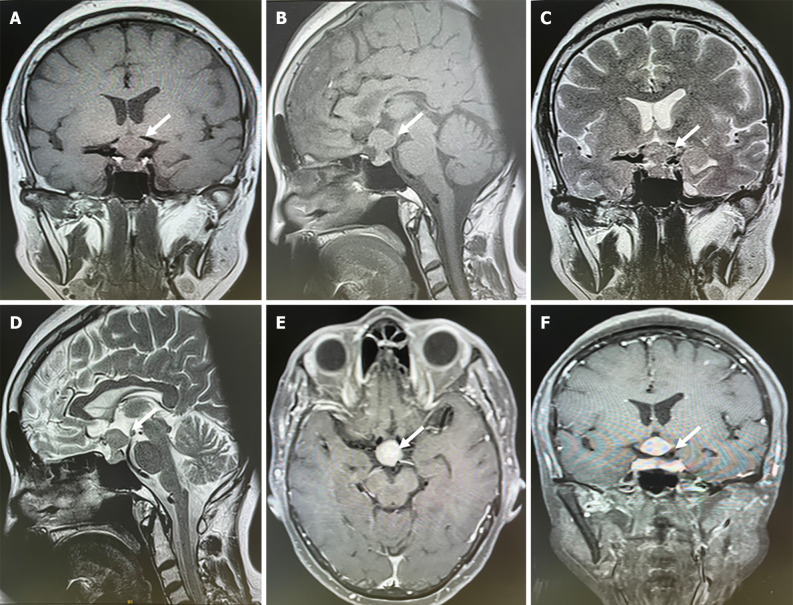
Brain CT showed that the sellar region tumor was related to the carotid artery and was considered a pituitary macroadenoma. Chest CT showed irregular nodules in the basal segment of the lower lobe of the left lung, measuring 2.0 cm × 1.7 cm, which were likely lung cancer. Brain MRI indicated sellar region tumors with isointense signals on T1- and T2-weighted images and an hourglass sign (Figure 1). The tumor was homogeneously enhanced on contrast MRI. The lesion was approximately 1.6 cm × 0.8 cm × 1.3 cm, which was considered pituitary macroadenoma. Positron emission tomography-CT revealed a carbohydrate metabolism tumor in the lungs and sellar region, which was considered malignant. Fiber bronchoscopy revealed a neoplasm in the left lung and hematoxylin and eosin (H&E) staining suggested lung adenocarcinoma.
Figure 1.
Sellar magnetic resonance imaging showed a sellar lesion related to carotid artery. A-D: The leision located in the sellar region presented with an isointense signal on T1 and T2-weighted images and could see hourglass sign; E and F: The leision was intensified homogenously enhanced after contrast magnetic resonance imaging.
Pathological examination
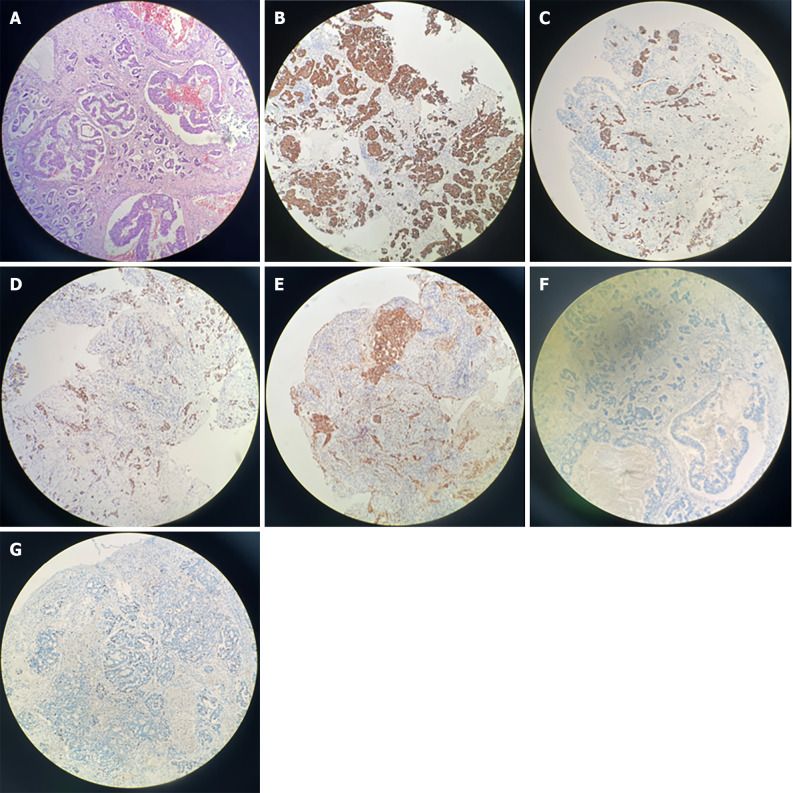
H&E staining of the sellar mass revealed a metastatic adenocarcinoma (Figure 2A). Immunohistochemistry showed positive results for CK7 (Figure 2B), CK18 (Figure 2C), TTF-1 (Figure 2D), Napsin A (+) (Figure 2E), and GATA3 (+) (Figure 2F) and negative results for CK20, CDX-2, GFAP, PAX-8, ER, and HER2. The Ki-67 index was 10%, indicating metastatic lung adenocarcinoma (Figure 2G). Next-generation sequencing confirmed somatic mutations in TSC2, POLD1, ARID1A, CTNNB1, ERBB2, and expressed PD-L1 (tumor proportion score = 1%) (Table 2).
Figure 2.
The histological features of the lesions revealed pituitary metastasis from lung adenocarcinoma. A: Hematoxylin and eosin staining found epithelial neoplasm and considered metastatic adenocarcinoma (× 100); B-F: Immunohistochemistry revealed positive for CK7, CK18, TTF-1, NapsinA and GATA3 (× 100); G: Immunohistochemical staining of Ki-67 (× 100).
Table 2.
Somatic gene mutations
|
Gene
|
Expressed region
|
Nucleotide
|
Amino acid
|
Mutation abundance (%)
|
| TSC2 | Exon 36 | c.4585C>T | p.R1529W | 5.1 |
| POLD1 | Exon19 | c.2312C>T | p.A771V | 3.8 |
| ARID1A | Exon 1 | c.1058C>T | p.A353V | 5.1 |
| ARID1A | Exon 1 | c.308C>T | p.S103L | 7.7 |
| CTNNB1 | Exon 4 | c.79G>A | p.G27R | 13.5 |
| ERBB2 | Exon 23 | c.2339C>T | p.A800V | 5.1 |
FINAL DIAGNOSIS
Based on the patient’s clinical symptoms and physical, imaging, and pathological examinations, the final diagnosis was pituitary metastasis.
TREATMENT
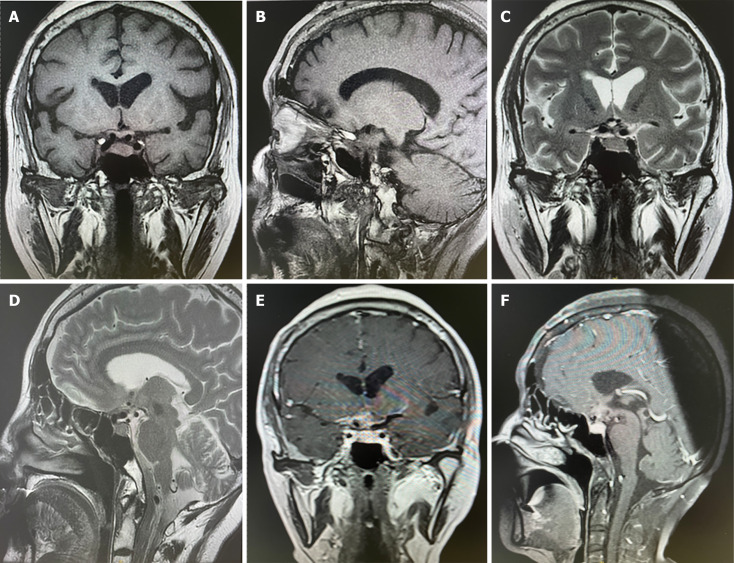
The patient was taken to the operating room for resection of the right coronary flap tumor of the sellar mass. During the operation, the tumor was gray, tough, aggressive, and rich in blood supply, infiltrating the sellar turcica, third ventricle of the cerebrum, and frontal lobe. The intraoperative bleeding volume was approximately 500 mL. Postoperatively, the patient recovered well and the vision was improved. The patient was advised to undergo immune and chemical therapies at a cancer hospital. Postoperative MRI revealed no tumor regrowth within 3 months (Figure 3).
Figure 3.
Sellar magnetic resonance imaging showed no neoplasm recurrence in 3 months after surgery. A-D: Postoperative changes of sellar region and there was no leision found in T1 and T2-weighted images; E and F: There was no intensified leision imaging after contrast magnetic resonance imaging.
OUTCOME AND FOLLOW-UP
The patient is still alive, with no recurrence noted 1 year after surgery.
DISCUSSION
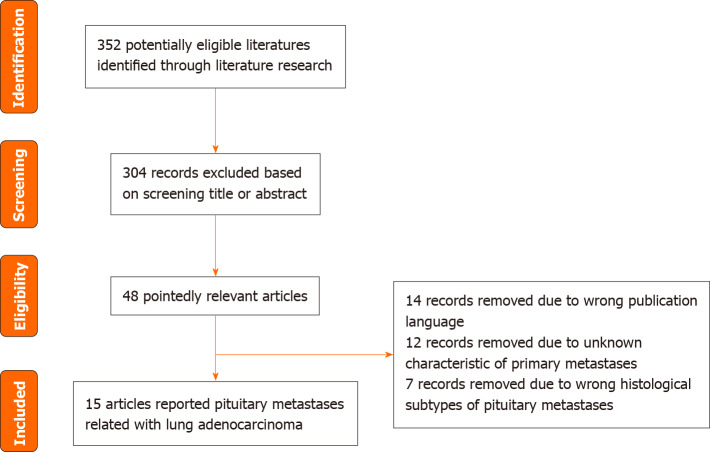
The literature was searched in PubMed till March 14, 2024. The medical subject heading search terms and strategies included pituitary metastasis and lung cancer. In total, 352 studies were screened using the PubMed database. The language was restricted to English and only published articles were included. We reviewed 15 articles that reported pituitary metastasis from lung adenocarcinoma. A flowchart of the literature selection process is shown in Figure 4. The characteristics of the included studies are presented in Table 3.
Figure 4.
Flowchart of the study selection.
Table 3.
Summary of previously reported cases of pituitary metastasis from lung adenocarcinoma
|
Ref.
|
Year
|
Age (yr)
|
Sex
|
Symptom
|
Hormone
|
Treatment
|
Tumor Supply
|
Survival
|
| Asim and Elashaal[13] | 2023 | 54 | F | None | ACTH (low), COTR (low), LH (low), FSH (low), TES (low), TSH (low), FT3 (low), FT4 (low) | RT, ST | ND | Alive, 3 months |
| Amaral et al[14] | 2022 | 43 | M | Headache, VFD | ACTH (low), CORT (low), FSH (low), FT4 (low), TES (low), LH (low) | ST, CT, RT, IT | R | Alive, 15 months |
| Wong et al[15] | 2022 | 53 | F | VFD | ND | ST, RT, CT | ND | Alive, 24 months |
| Han et al[16] | 2022 | 48 | F | Headache, DI | ACTH (low), Cort (low), PRL (high) | ST, CT | ND | Alive, 20 months |
| Lopes et al[17] | 2021 | 62 | F | Fatigue, Headache, Nocturia, Polydipsia, BV | FT4 (low), CORT (low), ACTH (low), FSH (low), PRL (high) | ST, CT | R | Alive, 24 months |
| Tanaka et al[9] | 2021 | 80 | F | Polyuria, Polydipsia | ADH (low) | RT, CT | ND | Alive, 7 months |
| Liu et al[3] | 2021 | 53 | M | BV, DI, VFD, Polyuria, Vomit, Fatigue | CORT (low), TSH (high), T4 (low), FSH (low), LH (low), PRL (high) | ST | R | Dead, 4 months |
| Watanabe et al[18] | 2020 | 70 | M | Anorexia | ADH (low), ACTH (low), CORT (low), FSH (low), LH (low), TSH (low) | RT, CT | ND | Dead, 5 months |
| Rajakumar et al[19] | 2020 | 49 | F | Headache, VFD | LH (low), FSH (low), CORT (low), TES (low), FT4 (low) | ST, RT, CT | NR | ND |
| Alhashem et al[11] | 2020 | 54 | M | Headache, Droop bilateral eyelid | ND | ST | NR | ND |
| Sheahan et al[5] | 2020 | 52 | F | MB | ACTH (low), CORT (low), FSH (low), TSH (low), PRL (high) | ST, CT | ND | ND |
| Yao et al[20] | 2019 | 67 | M | Headache, Vomit, ptosis | PRL (high), FSH (low), LH (low), ACTH (low), Cort (low) | ST, RT | ND | Dead, 3 months |
| Sirinvaravong et al[21] | 2019 | 72 | F | DI | PRL (high), FSH (low), LH (low), TSH (high) | ST | ND | Dead, 4 months |
| Gulati et al[12] | 2015 | 47 | F | Headache, DI | TSH (low), FSH (low) | ST | ND | ND |
| Arai et al[22] | 2010 | 61 | F | VFD | Normal | ST, RT | NR | Alive, 3 months |
F: Female; M: Male; R: Rich; CSI: Cavernous sinus invasion; VFD: Visual field defect; ND: Not described; NR: Not rich; BV: Blurred vision; MB: Monocular blindness; DI: Dizziness; ACTH: Adrenocorticotropic hormone; ADH: Vasopressin; CORT: Cortisol hormone; PRL: Prolactin hormone; TSH: Thyroid stimulating hormone; FT3: Free triiodothyronine; FT4: Free tetraiodothyronine; LH: Luteinizing hormone; FSH: Follicle stimulating hormone; Cort: Cortisol; TES: Testosterone; ST: Surgery treatment; CT: Chemotherapy; IT: Immunotherapy; RT: Radiotherapy.
Sellar tumors mostly originate from pituitary adenomas and rarely metastasize to other organs. Autopsies have revealed that pituitary metastases account for nearly 5% of all pituitary metastases in sellar tumors. In surgical sections, less than 1% of all sellar tumors are associated with pituitary metastasis[3]. Pituitary metastases always metastasize from the lungs and breasts, accounting for 39.7% and 23.7% of cases, respectively[4]. Small cell carcinomas are usually transferred to the liver and brain parenchyma, whereas adenocarcinomas often affect the bone and respiratory system[5]. A Swedish study reported that small cell lung cancer was the most frequent type of pituitary metastasis among all lung cancers. In contrast, adenocarcinoma rarely metastasizes to the pituitary gland but rather to the lungs and bones[6]. The present article reports the case of a patient with lung adenocarcinoma metastasizing to the pituitary gland without distant metastasis.
The clinical symptoms and radiographic findings of pituitary metastases are nonspecific. Patients present with visual field deficits, diabetes insipidus, cranial nerve palsy, or anterior pituitary dysfunction. Pituitary metastasis is a rare disease with poor prognosis. It is difficult to diagnose, especially in patients who are not diagnosed with a primary cancer. Physicians should consider these diseases, especially in older patients presenting with new-onset deficiencies in visual and anterior pituitary hormones. Pituitary adenoma often manifests hypointense on T1-weighted images and hyperintense on T2-weighted images, similar to pituitary metastasis. This is the main reason for misdiagnosis. However, pituitary metastasis often manifests as pituitary stalk thickening, invasion of the cavernous sinus, and coexistence of brain lesions, which have been observed in pituitary adenomas. Furthermore, if patients have other tumors detected on imaging or a history of other systemic malignancies, neurosurgeons should consider pituitary metastasis.
Pituitary metastases mostly located in posterior pituitary alone or combined with anterior pituitary accounting for 84.6%. Only 15.4% of cases have been found in the anterior pituitary alone[7]. It is mainly because the location and blood supply. The posterior pituitary has larger area to contact with the dura mater compared to anterior. The posterior pituitary receives blood supply from hypophyseal artery. on the contrary, the anterior pituitary only receives venous supply through hypophyseal portal system. The patient was admitted to the hospital due to blurred vision and headache accompanied by increased prolactin hormone and decreased cortisol, suggesting that the tumor had damaged both anterior and posterior pituitary glands. During operation, the tumor was tough, aggressive, and rich of blood which was different from the common sellar region tumors, such as pituitary adenoma, craniopharyngiomas and so on. According to the operation features and fiber bronchoscope, we suspected the tumor was likely pituitary metastases from lung. After operation, H&E and immunohistochemistry all confirmed pituitary metastasis from lung adenocarcinoma.
There are currently no standard protocols for the treatment of pituitary metastasis. The treatment for pituitary metastasis includes surgery, chemotherapy, and radiotherapy (RT). The choice of treatment depends on the primary tumor, clinical symptoms, and physical conditions. Surgical eradication includes transsphenoidal surgery and craniotomy. Total resection is difficult to achieve using either surgical method. Moreover, pituitary metastasis is highly aggressive and can destroy the sellar base and diaphragm and can adhere tightly to the pituitary gland. However, pituitary metastasis has a tough texture and abundant blood supply, which increases the difficulty of surgery. Although there is no consensus on whether surgery can extend the overall survival of patients with pituitary metastasis, it can relieve the symptoms and serve as a pathological diagnosis for treatment. RT and chemotherapy are recommended as initial treatments. RT can not only control local diseases with few morbidities but can also provide symptom relief. However, the 5-year radiation-associated hypopituitarism rate is 20% and 10-15 years radiation-associated hypopituitarism has been reported in up to 80% of cases[8,9]. Standard chemotherapy or immunotherapy crossing the blood-brain barrier indicates that patients with multiple organ involvement have longer survival advantages, although the evidence is limited to case reports[10]. The prognosis of pituitary metastasis is relatively poor. The median survival of pituitary metastasis was reported 17 months (range 0-240 months) while the longest survival of pituitary metastasis from lung adenocarcinoma was recorded 32 months[11]. It’s reported that the younger age, longer interval after pituitary metastasis, smaller pituitary lesion and utilization of RT and chemotherapy all correlated with improved survival[12].
CONCLUSION
Pituitary metastasis from lung adenocarcinoma is extremely rare, but physicians should consider it if rapidly developing clinical symptoms or growing quickly, especially for the patients found other tumors on images or had a history of other system malignancy. RT and chemotherapy are recommended as the initial treatments. The blood supply to pituitary metastases is extremely rich, so it is necessary for neurosurgeons to make adequate operation preparations and surgery plans.
Footnotes
Informed consent statement: Informed written consent was obtained from the patient and her families for publication of this report and any accompanying images.
Conflict-of-interest statement: All the authors report no relevant conflicts of interest for this article.
CARE Checklist (2016) statement: The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s classification
Scientific Quality: Grade C
Novelty: Grade B
Creativity or Innovation: Grade B
Scientific Significance: Grade C
P-Reviewer: Chien CR, Taiwan S-Editor: Zheng XM L-Editor: A P-Editor: Yu HG
Contributor Information
Qing Wang, Department of Critical Care Medicine, Chengdu First People’s Hospital, Chengdu 610000, Sichuan Province, China.
Xiao-Wei Liu, Department of Neurosurgery, Chengdu Second People's Hospital, Chengdu 610017, Sichuan Province, China; Department of the First Clinical Medical College, Lanzhou University, Lanzhou 730000, Gansu Province, China; Evidence Based Medicine Center, School of Basic Medical Sciences, Lanzhou University, Lanzhou 730000, Gansu Province, China.
Ke-Yu Chen, Department of Neurosurgery, Chengdu Second People's Hospital, Chengdu 610017, Sichuan Province, China. chenk8@qq.com.
References
- 1.Liu X, Wang R, Li M, Chen G. Pituitary Metastasis of Lung Neuroendocrine Carcinoma Mimicking Pituitary Adenoma:Case Report and Literature Review. Front Endocrinol (Lausanne) 2021;12:678947. doi: 10.3389/fendo.2021.678947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nooreldeen R, Bach H. Current and Future Development in Lung Cancer Diagnosis. Int J Mol Sci. 2021;22 doi: 10.3390/ijms22168661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu CY, Wang YB, Zhu HQ, You JL, Liu Z, Zhang XF. Hyperprolactinemia due to pituitary metastasis: A case report. World J Clin Cases. 2021;9:190–196. doi: 10.12998/wjcc.v9.i1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, Thalassinos NC. Tumors metastatic to the pituitary gland: case report and literature review. J Clin Endocrinol Metab. 2004;89:574–580. doi: 10.1210/jc.2003-030395. [DOI] [PubMed] [Google Scholar]
- 5.Sheahan KH, Huffman GC, DeWitt JC, Gilbert MP. Metastatic Lung Cancer Presenting as Monocular Blindness and Panhypopituitarism Secondary to a Pituitary Metastasis. Am J Case Rep. 2020;21:e920948. doi: 10.12659/AJCR.920948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Riihimäki M, Hemminki A, Fallah M, Thomsen H, Sundquist K, Sundquist J, Hemminki K. Metastatic sites and survival in lung cancer. Lung Cancer. 2014;86:78–84. doi: 10.1016/j.lungcan.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 7.Ng S, Fomekong F, Delabar V, Jacquesson T, Enachescu C, Raverot G, Manet R, Jouanneau E. Current status and treatment modalities in metastases to the pituitary: a systematic review. J Neurooncol. 2020;146:219–227. doi: 10.1007/s11060-020-03396-w. [DOI] [PubMed] [Google Scholar]
- 8.Gilard V, Alexandru C, Proust F, Derrey S, Hannequin P, Langlois O. Pituitary metastasis: is there still a place for neurosurgical treatment? J Neurooncol. 2016;126:219–224. doi: 10.1007/s11060-015-1967-y. [DOI] [PubMed] [Google Scholar]
- 9.Tanaka S, Hirabayashi T, Kimoto M, Hama M, Hachiya T, Gomi K. Gefitinib Treatment Was Unsuccessful for Central Diabetes Insipidus Due to Pituitary Metastasis of Lung Adenocarcinoma. Intern Med. 2021;60:1073–1076. doi: 10.2169/internalmedicine.5643-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Castle-Kirszbaum M, Goldschlager T, Ho B, Wang YY, King J. Twelve cases of pituitary metastasis: a case series and review of the literature. Pituitary. 2018;21:463–473. doi: 10.1007/s11102-018-0899-x. [DOI] [PubMed] [Google Scholar]
- 11.Alhashem A, Taha M, Almomen A. Pituitary metastasis of lung adenocarcinoma: Case report and literature review. Int J Surg Case Rep. 2020;67:98–101. doi: 10.1016/j.ijscr.2020.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gulati S, Kiefer C, Karim NA. Diabetes Insipidus: An Unusual Presentation of Adenocarcinoma of the Lung in a Patient with no Identifiable Lung Mass. N Am J Med Sci. 2015;7:476–479. doi: 10.4103/1947-2714.168677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asim SA, Elashaal AA. Metastasis of lung adenocarcinoma to the pituitary gland. Radiol Case Rep. 2023;18:3487–3491. doi: 10.1016/j.radcr.2023.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amaral S, Matias A, Bouça B, Manique I, Palha A, Cortez L, Cerqueira L, Forte D, Sagarribay A, Dutra E, Cristóvão M, Pontinha C, Mafra M, Silva-Nunes J. Pituitary metastasis as the first manifestation of lung carcinoma. Clin Case Rep. 2022;10:e6601. doi: 10.1002/ccr3.6601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wong PS, Rajoo S, Achmad Sankala H, Long Bidin MB. Unusual presentation of lung carcinoma with pituitary metastasis: a challenging diagnosis and sodium management dilemmas. Endocr Oncol. 2022;2:K15–K20. doi: 10.1530/EO-22-0064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han D, Zhao K, Yang Q, Zhang L, Fei S. Secondary mutant ALK-I1171s in pituitary metastases from a patient with ALK fusion-positive advanced lung adenocarcinoma: A case report and literature review. Front Oncol. 2022;12:1016320. doi: 10.3389/fonc.2022.1016320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lopes AM, Pereira J, Ribeiro I, Martins da Silva A, Queiroga H, Amaral C. Pituitary metastasis unveiling a lung adenocarcinoma. Endocrinol Diabetes Metab Case Rep. 2021;2021:20–0211. doi: 10.1530/EDM-20-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Watanabe M, Yasuda J, Ashida K, Matsuo Y, Nagayama A, Goto Y, Iwata S, Watanabe M, Sasaki J, Hoshino T, Nomura M. Masked Diabetes Insipidus Hidden by Severe Hyponatremia: A Case of Pituitary Metastasis of Lung Adenocarcinoma. Am J Case Rep. 2020;21:e928113. doi: 10.12659/AJCR.928113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rajakumar R, Rahmatullah IH, Rahim AA. Case Report of a Pituitary Metastasis from Lung Adenocarcinoma Masquerading as Pituitary Adenoma. J ASEAN Fed Endocr Soc. 2020;35:133–136. doi: 10.15605/jafes.035.01.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yao H, Rui W, Zhang Y, Liu Y, Lin S, Tang H, Zhao W, Wu Z. Prolactin-Secreting Lung Adenocarcinoma Metastatic to the Pituitary Mimicking a Prolactinoma: A Case Report. Neurosurgery. 2019;85:E773–E778. doi: 10.1093/neuros/nyy386. [DOI] [PubMed] [Google Scholar]
- 21.Sirinvaravong S, Vibhatavata P, Chunharojrith P, Cheunsuchon P, Sriussadaporn S. Diabetes insipidus and panhypopituitarism as a first presentation of silent adenocarcinoma of lung: a case report and literature review. BMC Endocr Disord. 2019;19:114. doi: 10.1186/s12902-019-0445-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arai A, Morishita A, Hanada Y, Aihara H. Solitary metastatic tumor within the optic chiasm--case report. Neurol Med Chir (Tokyo) 2010;50:158–161. doi: 10.2176/nmc.50.158. [DOI] [PubMed] [Google Scholar]