Abstract
A 6-month-old female spayed domestic shorthair cat was presented with an acute onset of vomiting and marked lethargy. The cat had undergone elective ovariohysterectomy via a small midline incision 2 weeks prior to presentation. Intestinal strangulation through a mesenteric rent was diagnosed via abdominal ultrasound and exploratory laparotomy. Intestinal resection and anastamosis resulted in a good clinical outcome despite excision of 60% of the small intestine.
Elective ovariohysterectomy is a procedure commonly performed in private practice. The potential complications described in the veterinary literature for cats have been limited 1,2 ; but have been described for other species such as dogs. 1–22 Reported complications of feline ovariohysterectomy range from 3.9% to at least 45%. 1,23
A 6-month-old female spayed domestic shorthair cat was presented with a 6 h history of persistent vomiting and marked lethargy. The cat had undergone elective ovariohysterectomy at another practice via a small midline incision 2 weeks prior to presentation using a Snook hook, and had reportedly made an unremarkable recovery. The night before presentation, the cat had been observed to be very active, running, climbing and swinging from indoor curtains.
On presentation, the cat had pale and tacky mucous membranes, a prolonged capillary refill time (2 s); tachycardia (heart rate 200 bpm); marked hypothermia (rectal temperature 33.6°C); increased respiratory effort on inspiration with normal lung sounds on auscultation (respiratory rate 36/min); and marked cranial abdominal pain with a palpable fluid wave, but no masses palpable within the abdomen.
Shock was suspected and the cat was admitted. Blood was collected prior to institution of treatment for haematology, biochemistry and retrovirus serology. Pending results, in-house serum blood glucose (24.8 mmol/l, reference range (RR) 3.36–7.28 mmol/l), urea (13.4 mmol/l, RR 5.35–12.13 mmol/l), packed cell volume (0.42) and total protein (42 g/l, RR 54–73 g/l) were performed.
Treatment was instituted with intravenous (IV) colloid (two separate boluses of 5 ml/kg of 6% hetastarch in 0.9% sodium chloride given over 5 min) and crystalloid fluids at 10 ml/kg/h. Active warming was commenced using a Bair hugger (Arizant Inc, 10393 West 70th Street Eden Prairie, MN 55344, USA). Analgesia was administered using methadone at 0.1 mg/kg intravenously.
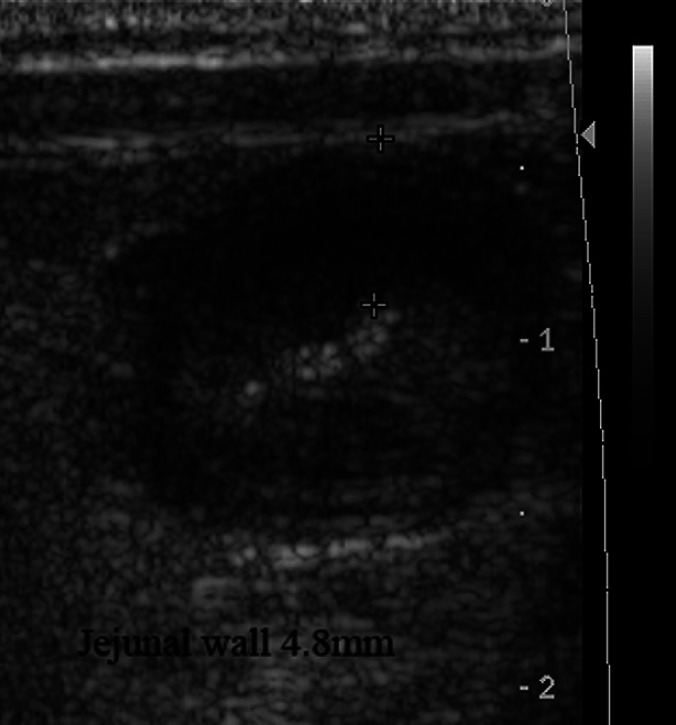
On abdominal ultrasonography there was a thickened section of jejunum (4.8 mm wall thickness) with loss of wall layering and there was free fluid within the abdomen (Fig. 1). The affected loops had fluid and gas present within them and there no sonographic evidence of peristalsis. However, there was another population of small intestinal loops of normal wall thickness and layering more caudally. The jejunal lymph nodes were rounded and hypoechoic. There were no signs of blood flow on colour flow Doppler in the affected region of jejunum and a segmental enteropathy was diagnosed. An ultrasound-guided abdominocentesis was performed.
Fig 1.
Ultrasonographic image of jejunum. Jejunal wall thickness is 4.8 mm (marked by +).
In addition to in-house laboratory findings of hyperglycaemia, hypoproteinaemia and mildly elevated urea, abnormalities on serum biochemistry included hypoglobulinaemia (globulin 14.5 g/l, RR 26–51 g/l), hypocholesterolaemia (cholesterol 1.69 mmol/l, RR 1.9–3.9 mmol/l), mild hyponatraemia (Na 145 mmol/l, RR 147–162 mmol/l), moderate increase in alkaline phosphotase (ALP; 156 U/l, RR<50 U/l). Haematology revealed a leukocytosis (leukocytes 22.9×109/l, RR 8–10×109/l), consisting of a mature neutrophilia (neutrophils 17.4×109/l, RR 3.76–10.8×109/l). All other results were within RRs. Serological tests for feline immunodeficiency virus and feline leukaemia virus were negative.
On analysis of the abdominal fluid, the total protein was 35 g/l, with a nucleated cell count 400×106/l, consisting of 53% neutrophils, 46% macrophages, 1% small lymphocytes and small sheets of mesothelial cells. No bacteria were visible.
Based on the clinical history, physical examination, abdominal fluid analysis (modified transudate) and ultrasonographic findings, a proximal enteropathy with intestinal ischaemia was suspected. Differential diagnoses considered included intestinal torsion or volvulus, mesenteric thrombosis, foreign-body obstruction, peracute enteritis and oedema, or intestinal entrapment in abdominal adhesions. Prior to general anaesthesia, the cat had an episode of haematamesis. She was blood typed as a type A (Rapid Vet-H [Feline], DMS and Agrolab products, Turino, Italy). Parenteral antimicrobial therapy was commenced (ticarcillin–clavulanate 30 mg/kg intravenously; and enrofloxacin 2.5 mg/kg subcutaneously) prior to anaesthesia induction.
On pre-anaesthetic examination, the cat had a heart rate of 160 bpm, and an equivalent pulse rate with poor pulse quality. She was pale, with a prolonged capillary refill time and was allocated a score of 5E on the American Society of Anesthesiologists physical status scale. Fresh whole blood transfusion of type A blood was commenced prior to induction of anaesthesia due to hypotension (systolic blood pressure 80 mmHg; Doppler method; RR 120–140 mmHg).
The cat was pre-medicated with midazolam (0.2 mg/kg) and methadone (0.1 mg/kg) IV, and she was preoxygenated via a mask. Alfaxalone was given intravenously to effect to induce anaesthesia, which was maintained with isoflurane and oxygen. A remifentanyl infusion (0.2 μg/kg/min) was used for intraoperative analgesia. Dopamine was administered at 5.1–7.2 μg/kg/minute as required to maintain systolic blood pressure.
A routine ventral midline celiotomy was performed. A small mesenteric rent, approximately 1 cm in length was identified just distal to the level of the duodenal flexure, with the two-thirds of the jejunum and all of the ileum, up to the level of the ileocaecal junction, passing through this rent. The rent was causing strangulation of the displaced jejunum and ileum (Fig. 2). Low molecular weight heparin 200 IU intravenously was administered prophylactically in case of thromboembolic event. Lahey forceps were used to widen the mesenteric rent, and the entrapped loops of small intestine were gently passed through the rent to release them. Once the loops were free they were left covered in moistened laparotomy sponges for 15 min to assess viability, by assessing mesenteric arterial flow. Due to lack of return of arterial flow, the devitalised segment of intestine was resected and an end-to-end anastamosis was performed between the proximal jejunum and the distal 1 cm of the ileum, removing approximately 60% of the small intestine, using 4/0 polydiaxanone in a single interrupted appositional pattern circumferentially. The abdomen was lavaged copiously prior to routine three-layer closure.
Fig 2.
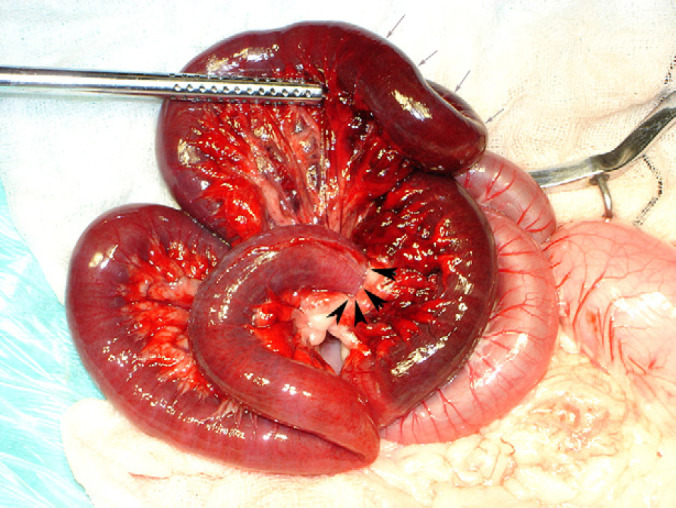
Intraoperative image of mesenteric rent, with margins of rent marked with black arrowheads. Note the dark discoloration of the strangulated bowel, marked with grey arrows.
Postoperatively, the cat was maintained on intravenous crystalloids (Hartmann's solution with 20 mmol/l potassium chloride supplementation, at 4 ml/kg/h) and a morphine–ketamine constant rate infusion for analgesia, and continued on parenteral antimicrobial therapy (ticarcillin–clavulanate 30 mg/kg IV q 6 h; and enrofloxacin 2.5 mg/kg subcutaneously (SC) q 12 h). The following day, the cat appeared bright, alert and responsive. Her analgesia was changed to buprenorphine boluses (0.01 mg/kg SC), and her intravenous crystalloid rate was reduced to 2.5 ml/kg/h. Small 2-hourly feeds were commenced 24 h postoperatively using a highly digestible canned food (Hill's Prescription Diet i/d Feline). The cat made an unremarkable recovery, and was discharged 6 days postoperatively. Histopathology of the resected section of bowel showed changes consistent with moderate to severe oedema, congestion, haemorrhage, and necrosis across all intestinal layers (Fig. 3).
Fig 3.
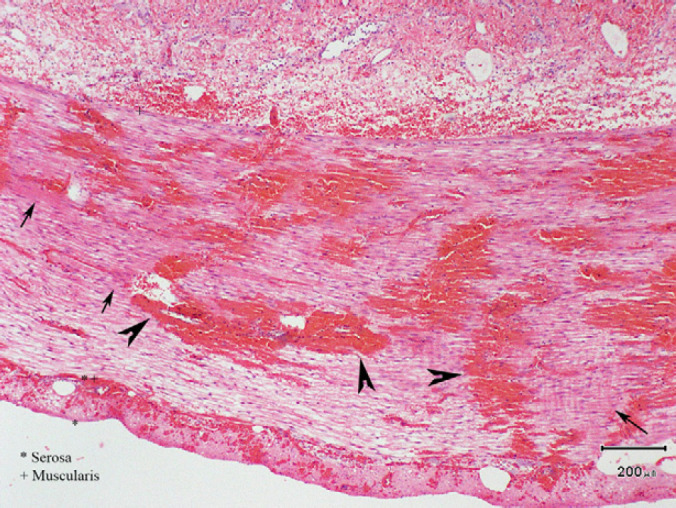
Histological image of ileum. Representative regions of haemorrhage and congestion are indicated with black arrowheads. Representative regions of congestion are indicated with black arrows.
Six months postoperatively, the cat remained polyphagic but had faeces of normal consistency and a body condition score of 3 out of 5. Nine months postoperatively, serum folate was normal and serum cobalamin was decreased (cobalamin 204 pmol/l, RR 250–1200 pmol/l) without parenteral supplementation. The cat was commenced on cobalamin supplementation, with weekly injections of 250 μg cobalamin subcutaneously.
This case describes a complication after elective ovariohysterectomy in a cat, which has not been previously described in this species. Intestinal entrapment in dogs after elective ovariohysterectomy has been recently described in five dogs 24 of which four developed an entrapment secondary to adhesions, while one developed an entrapment through a mesenteric rent. Partial extramural obstruction of the descending colon has also been described in a cat after elective ovariohysterectomy, due to the development of fibrous adhesions. 2
In our case, we suspect that the mesenteric rent was created during the use of the Snook hook, or inappropriate tissue handling during the ovariohysterectomy. The intestinal strangulation was thought to have occurred after vigorous activity the night before presentation. The risk of inadvertent trauma to abdominal tissue is theoretically increased when there is limited exposure due to a small abdominal incision; and when a Snook hook is used in a blind fashion. A variety of complications due to inadvertent ligation of structures, especially the ureter or urethra, have been reported previously. 1,8,10–12,17–22 It can be proposed that these complications could similarly have been avoided if there had been adequate surgical exposure or the use of video-assisted minimally invasive surgery to allow good visualisation of the abdominal viscera. 25 Laparoscopic ovariectomy is possible in cats and is a relatively simple method, despite the small size of the feline abdomen. The ovaries and the suspensory ligament are easy to visualise and manipulate even in obese animals because the ovarian ligaments contain minimal amounts of fat. There has been previous discussion regarding the effects of early age gonadectomy. 15,26 This is especially important in shelter animals, where the inclination to make smaller abdominal incisions may be present, thereby reducing surgical exposure. Additionally, the patients would be smaller in size, which may make identification of abdominal visceral more challenging.
Assessment of intestinal viability was made subjectively by assessing mesenteric arterial flow. Objective assessments have been described in the human and veterinary literature by the use of laser Doppler flowmetry, Doppler ultrasonography, electromyography, reflection densitometry, oximetry, and fluorescein assessment. 27–42 of practical application in veterinary medicine, Doppler ultrasonography and pulse oximetry would be of most use. These techniques were not used in this case due to the lack of sterile sleeves for the probes.
An interesting aspect of this case is the excellent recovery of the patient despite excision of approximately 60% of the small intestine. ‘Short bowel syndrome’ has previously been described in both cats and dogs 43 with intestinal adaptation occurring over time to resolve or improve the clinical signs of diarrhoea and weight loss. The decreased absorptive surface area of the small intestine due to extensive resection often results in inadequate digestion and absorption of nutrients and water as well as electrolyte imbalance and micro- and macroelement deficiencies. 44 However, a recent study showed no significant association between long term outcome and percentage of small bowel resected, based on owner assessments. 45
Absorption of dietary cobalamin from the gastrointestinal tract occurs solely in the ileum through specific receptors. On the other hand, folate can either be synthesised by intestinal bacteria, or be deconjugated from its polyglutamate form in the diet in the jejunum; and is subsequently absorbed in the proximal intestine. In this case, the findings of low serum cobalamin with normal serum folate is consistent with the surgical procedure, where the absorptive surface for cobalamin, but not folate, was markedly reduced.
Surgical exposure is vital to reduce the risk of inadvertent trauma to soft tissue in any surgery. This case highlights the need to have good surgical exposure or improved visualisation via laparoscopy during ovariohysterectomy, and demonstrates a previously unreported complication after ovariohysterectomy.
References
- 1.Berzon J. Complications of elective ovariohysterectomies in the dog and cat at a teaching institution: clinical review of 853 cases, Vet Surg 8, 1979, 89–91. [Google Scholar]
- 2.Coolman B.R., Marretta S.M., Dudley M.B., et al. Partial colonic obstruction following ovariohysterectomy: a report of three cases, J Am Anim Hosp Assoc 35, 1999, 169–172. [DOI] [PubMed] [Google Scholar]
- 3.Bradley K., Billet J., Barr F. Dysuria resulting from an encapsulated haematoma in a recently spayed bitch, J Small Anim Pract 41, 2000, 465–467. [DOI] [PubMed] [Google Scholar]
- 4.Grassi F., Romangnoli S., Camillo F., et al. Iatrogenic enterovaginal fistula following hysterectomy, J Small Anim Pract 35, 1994, 32–34. [Google Scholar]
- 5.Holt P. A color atlas and text of small animal urology, 1994, Mosby-Wolfe: London. [Google Scholar]
- 6.Holt P.E., Bohannon J., Day M.J., et al. Vaginoperitoneal fistula after ovariohysterectomy in three bitches, J Small Anim Pract 47, 2006, 744–746. [DOI] [PubMed] [Google Scholar]
- 7.Tidwell A., Ullman S., Schelling S. Urinoma (para-ureteral pseudocyst) in a dog, Vet Radiol Ultrasound 31, 1990, 203–206. [Google Scholar]
- 8.Furneaux R., Boysen B., Mero K. Complications of ovariohysterectomies, Can Vet J 14, 1973, 98–99. [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson H. The complications of ovariohysterectomy in the bitch, J Small Anim Pract 14, 1973, 257–266. [DOI] [PubMed] [Google Scholar]
- 10.Ewers R., Holt P. Urological complications following ovariohysterectomy in a bitch, J Small Anim Pract 33, 1992, 236–238. [Google Scholar]
- 11.Thun R., Smith C., Goodale R., et al. Iatrogenic hydronephrosis in a bitch, J Am Vet Med Assoc 167, 1975, 388–390. [PubMed] [Google Scholar]
- 12.Dorn A., Swist R. Complications of canine ovariohysterectomy, J Am Anim Hosp Assoc 13, 1977, 720–724. [Google Scholar]
- 13.Spackman C., Caywood D., Johnston G., et al. Granulomas of the uterine and ovarian stumps: a case report, J Am Anim Hosp Assoc 20, 1982, 449–453. [Google Scholar]
- 14.Maccoy D.M., Ogilvie G., Burke T., et al. Postovariohysterectomy ureterovaginal fistula in a dog, J Am Anim Hosp Assoc 24, 1988, 469–471. [Google Scholar]
- 15.Stocklin-Gautschi N.M., Hassig M., Reichler I.M., et al. The relationship of urinary incontinence to early spaying in bitches, J Reprod Fertil Suppl 57, 2001, 233–236. [PubMed] [Google Scholar]
- 16.Smith M., Daview N. Obstipation following ovariohysterectomy in a cat, Vet Rec 138, 1996, 163. [DOI] [PubMed] [Google Scholar]
- 17.Pearson H., Gibbs C. Urinary incontinence in the dog due to accidental vagino-ureteral fistulation during hysterectomy, J Small Anim Pract 21, 1980, 287–291. [DOI] [PubMed] [Google Scholar]
- 18.Burrow R., Batchelor D., Cripps P. Complications observed during and after ovariohysterectomy of 142 bitches at a veterinary teaching hospital, Vet Rec 157, 2005, 829–833. [DOI] [PubMed] [Google Scholar]
- 19.Kyles A., Douglass J., Rottman J. Pyelonephritis following inadvertent excision of the ureter during ovariohysterectomy in a bitch, Vet Rec 139, 1996, 471–472. [DOI] [PubMed] [Google Scholar]
- 20.Lamb C.R. Acquired ureterovaginal fistula secondary to ovariohysterectomy in a dog – diagnosis using ultrasound-guided nephropyelocentesis and antegrade ureterography, Vet Radiol Ultrasound 35, 1994, 201–203. [Google Scholar]
- 21.Neiger R., Lamb C.R. Uretrovaginal fistula as complication of a ovariohysterectomy: two cases, Schweizer Archiv Fur Tierheilkunde 142, 2000, 529–533. [Google Scholar]
- 22.Okkens A.C., Vandergaag I., Biewenga W.J., et al. Urological complications following ovariohysterectomy in dogs, Tijdschrift Voor Diergeneeskunde 106, 1981, 1189–1198. [PubMed] [Google Scholar]
- 23.Pollari F., Bonnett B., Bamsey S., et al. Postoperative complications of elective surgeries in dogs and cats determined by examining electronic and paper medical records, J Am Vet Med Assoc 208, 1996, 1882–1886. [PubMed] [Google Scholar]
- 24.Swift I. Ultrasonographic features of intestinal entrapment in dogs, Vet Radiol Ultrasound 50, 2009, 205–207. [DOI] [PubMed] [Google Scholar]
- 25.Van Nimwegen S., Kirpensteijn J. Laparoscopic ovariectomy in cats: comparison of laser and bipolar electrocoagulation, J Fel Med Surg 9, 2007, 397–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Olson P., Kustritz M. Root, Johnston S. Early-age neutering of dogs and cats in the United States (a review), J Reprod Fertil Suppl 57, 2001, 223–232. [PubMed] [Google Scholar]
- 27.Masayuki A., Masashi I., Zenro N., et al. Assessment of intestinal viability using a non-contact laser tissue blood flowmeter, Am J Surg 180, 2000, 176–180. [DOI] [PubMed] [Google Scholar]
- 28.Redaelli C.A., Schilling M.K., Buchler M.W. Intraoperative laser Doppler flowmetry: a predictor of ischemic injury in acute mesenteric infarction, Dig Surg 15, 1998, 55–59. [DOI] [PubMed] [Google Scholar]
- 29.Tateishi S., Arima S., Futami K. Assessment of blood flow in the small intestine by laser Doppler flowmetry: comparison of healthy small intestine and small intestine in Crohn's disease, J Gastroenterol 32, 1997, 457–463. [DOI] [PubMed] [Google Scholar]
- 30.Macdonald P.H., Dinda P.K., Beck I.T., et al. The use of oximetry in determining intestinal blood-flow, Surg Gynecol Obstet 176, 1993, 451–458. [PubMed] [Google Scholar]
- 31.Orland P.J., Cazi G.A., Semmlow J.L., et al. Determination of small-bowel viability using quantitative myoelectric and color analysis, J Surg Res 55, 1993, 581–587. [DOI] [PubMed] [Google Scholar]
- 32.Horgan P.G., Gorey T.F. Operative assessment of intestinal viability, Surg Clin North Am 72, 1992, 143–155. [DOI] [PubMed] [Google Scholar]
- 33.Schmotzer W.B., Riebold T.W., Rowe K.E., et al. Steady-state response characteristics of a pulse oximeter on equine intestine, Am J Vet Res 52, 1991, 619–625. [PubMed] [Google Scholar]
- 34.Wolfman E.F. Determination of intestinal viability, Vet Clin N Am Equine Pract 5, 1989, 295–307. [DOI] [PubMed] [Google Scholar]
- 35.Ferrara J.J., Dyess D.L., Lasecki M., et al. Surface oximetry – a new method to evaluate intestinal perfusion, Am Surg 54, 1988, 10–14. [PubMed] [Google Scholar]
- 36.Johansson K. Gastrointestinal application of laser Doppler flowmetry. An experimental and clinical study in cat and man, Acta Chir Scand Suppl 545, 1988, 1–64. [PubMed] [Google Scholar]
- 37.Johansson K., Ahn H., Lindhagen J. Assessment of small-bowel ischemia by laser Doppler flowmetry – some case-reports, Scand J Gastroenterol 21, 1986, 1147–1152. [DOI] [PubMed] [Google Scholar]
- 38.Bass B.L., Schweitzer E.J., Harmon J.W., et al. Intraluminal Pco2-a reliable indicator of intestinal ischemia, J Surg Res 39, 1985, 351–360. [DOI] [PubMed] [Google Scholar]
- 39.Bulkley G.B., Zuidema G.D., Hamilton S.R., et al. Intra-operative determination of small intestinal viability following ischemic-injury – a prospective, controlled trial of 2 adjuvant methods (Doppler and fluorescein) compared with standard clinical judgment, Ann Surg 193, 1981, 628–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cooperman M., Martin E.W., Carey L.C. Evaluation of ischemic intestine by Doppler ultrasound, Am J Surg 139, 1980, 73–77. [DOI] [PubMed] [Google Scholar]
- 41.Cooperman M., Martin E.W., Keith L.M., et al. Use of Doppler ultrasound in intestinal surgery, Am J Surg 138, 1979, 856–859. [DOI] [PubMed] [Google Scholar]
- 42.Lee B.Y., Trainor F.S., Kavner D., et al. Intraoperative assessment of intestinal viability with Doppler ultrasound, Surg Gynecol Obstet 149, 1979, 671–675. [PubMed] [Google Scholar]
- 43.Brown D. Small intestines. Slatter D. Textbook of small animal surgery, 3rd edn, 2003, Saunders: Philadelphia, 644–664. [Google Scholar]
- 44.Kouti V., Papazoglou L., Rallis T. Short-bowel syndrome in dogs and cats, Comp Cont Edu Pract Vet 28, 2006, 182–195. [Google Scholar]
- 45.Gorman S., Freeman L., Mitchell S., et al. Extensive small bowel resection in dogs and cats: 20 cases (1998–2004), J Am Vet Med Assoc 228, 2006, 403–407. [DOI] [PubMed] [Google Scholar]