Abstract
Directed, effective therapies for feline sepsis are needed to reduce the high morbidity and mortality associated with this disease. We investigated the anti-endotoxin effects of polymyxin B (PMB) in a blinded, placebo controlled fashion, both ex vivo in a feline whole blood culture system and in vivo, using a low-dose endotoxin infusion in cats. Serial measures of systemic inflammation, and hemodynamic stability, were compared between groups. Ex vivo, PMB significantly decreased lipopolysaccharide-induced tumor necrosis factor (TNF) production from whole blood. PMB (1 mg/kg over 30 min) demonstrated anti-endotoxin effects in vivo, including decreased peak plasma TNF activity (P<0.001) and increased white blood cell count (P=0.019), with no adverse effects. Given the apparent safety and anti-endotoxin effects of PMB in this endotoxemia model, a carefully designed, randomized, blinded, placebo controlled clinical trial evaluating the use of PMB in naturally occurring Gram-negative feline sepsis should be considered.
Sepsis, defined as the systemic inflammatory response to infection, is a serious problem in feline patients causing substantial morbidity and mortality. 1,2 In cats, Gram-negative bacterial infections are a common cause of sepsis. 1,3–7 During Gram-negative sepsis, endotoxin or lipopolysaccharide (LPS), the glycolipid component of the cell wall of Gram-negative bacteria, is released into circulation resulting in a systemic inflammatory response that can lead to multiple organ dysfunction, and death. 8,9 Despite the potentially devastating nature of sepsis in cats, little research has focused on treatment and current therapy relies on control of the source of infection, appropriate antimicrobial coverage and aggressive supportive care. Specific directed therapies for sepsis in cats have not been developed.
Polymyxin B (PMB) is a cyclic cationic polypeptide antibiotic that binds to the lipid A subunit of endotoxin molecules with high affinity, preventing the interaction of endotoxin with humoral and cellular receptors and the resultant activation of inflammatory pathways. 10,11 PMB, by neutralizing LPS, inhibits activation of nuclear factor kappa B (NF-κB), 12 one of the primary cytokine transcription regulators during Gram-negative sepsis. 13,14 The anti-endotoxic effects of PMB are well established both ex vivo and in vivo in many species, including experimental models of sepsis in rabbits, 15,16 rodents, 17 dogs, 18 horses, 19–21 and humans, 22 as well as naturally occurring parvoviral gastroenteritis induced sepsis in dogs. 23 Treatment with high-dose intravenous PMB in a feline high-dose endotoxemia model has been associated with a dramatic improvement in survival (from 12.5% in the placebo group to 100% in the PMB treated group). 24 Although compelling, the administration of PMB prior to endotoxemia, high total dose of PMB, severe nature of the endotoxin insult, extreme instrumentation, and use of anesthesia in that study limits the clinical application of these data. Nevertheless, the dramatic improvement in survival with PMB treatment cannot be ignored. Based on these studies, treatment with PMB may be beneficial in cats with naturally developing Gram-negative sepsis, although evaluation of the anti-endotoxic effects of PMB in a clinically applicable experimental model of feline sepsis was thought to be a logical first step prior to clinical use.
PMB is readily available, inexpensive, easily administered and has endotoxin neutralizing effects that long surpass its circulating pharmacological half-life making it an attractive drug for the treatment of Gram-negative sepsis. 19,21 We hypothesized that PMB would ameliorate endotoxin-induced systemic inflammation and hemodynamic derangement with minimal adverse effects. In this study we investigated the anti-endotoxin effects of PMB using a previously characterized, low-dose endotoxin infusion model of Gram-negative feline sepsis in minimally instrumented, conscious cats. 25
Materials and methods
Ex vivo preliminary data
Feline whole blood culture (Cwb) was used to investigate if PMB blunted LPS-induced whole blood tumor necrosis factor (TNF) production in cats, ex vivo. Adult male cats, which belong to a colony maintained at the University of Missouri, were used for this part of the study which was approved by the Animal Care and Use Committee at the University of Missouri. All cats were known to be healthy on the basis of a normal physical examination and complete blood count (CBC) and plasma biochemical analysis. Cats were cared for according to the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. A 12 ml sample of blood was collected from each cat into sodium heparin blood tubes. The cats have previously had vascular access ports (Norfolk Vet Products, Skokie, IL) surgically implanted in their right jugular vein; blood samples were taken from these ports using a three-syringe technique. Vascular access ports are maintained long-term in these cats; their patency is achieved by ‘locking’ them with heparin after each use. Within 1 h of blood collection, the blood samples were mixed to achieve a 1:2 dilution with modified Roswell Park Memorial Institute (RPMI) culture media (RPMI, 200 U penicillin/ml, 200 μg streptomycin/ml and 200 mM l-glutamine). The Cwb was stimulated with either LPS (1000 ng/ml) or a control solution (phosphate buffered saline [PBS]) with and without PMB (1, 5, 10, 25 μg/ml) in 12-well plates as previously described. 26,27,33 Plates were incubated at 37°C with 5% CO2 for 24 h before centrifugation (1000×g for10 min), and collection of the supernatant. Samples were then frozen at −80°C until analysis. A TNF assay was performed on cell culture supernatant.
TNF activity
TNF activity was evaluated in Cwb using a previously described cytotoxicity bioassay. 25 Briefly, cells from mouse fibroblast cell (L929) were cultured on 96-well plates. After 12 h, samples were added to the wells in triplicate. After a 20 h incubation with minimum essential medium (MEM) plus horse serum and actinomycin D (Sigma-Aldrich, St Louis, MO), 3-[4,5-dimethylthiazol-2-yl]-2,5,-di-phenyl tetrazolium bromide (MTT) (Sigma-Aldrich, St Louis, MO) was added and the cells were incubated for an additional 2.5 h to allow for formazen crystal formation. The formazen crystals were then solubilized in dimethylformanide and sodium dodecyl sulphate (SDS) (Sigma-Aldrich, St Louis, MO). Color development after 1 h was measured at 630 nm. Feline recombinant tumor necrosis factor (rTNF) (Endogen, Rockford, IL) was used to construct a standard curve to determine the concentration of TNF activity in the test wells. The lower limit of detection for this assay is 0.5 ng/ml.
In vivo study
Animals
Following the ex vivo pilot study, 12 adult, sexually intact, specific pathogen-free (SPF) male cats were purchased (Liberty Research, Waverly, NY) and used in a randomized, blinded, placebo controlled in vivo study. The study was approved by the Animal Care and Use Committee at the University of Missouri. Animals were cared for according to the principles outlined in the NIH Guide for the Care and Use of Laboratory Animals. The health of the cats was confirmed based on physical examination and assessment of renal function (ie, normal plasma creatinine concentration). The cats were 1 year old with a mean weight of 4.9 kg. The cats were maintained on commercial adult cat food and water ad libitum, but fasted each day prior to procedures.
Treatments
On day 1, the cats were instrumented with a 4 Fr single lumen jugular catheter (Cook Veterinary Products, Bloomington, IN) and a 22 ga cephalic catheter (Abbott, Abbott Park, IL) during brief sedation with a combination of intravenous medetomidine (15 μg/kg Domitor; Pfizer Animal Health) and butorphanol (0.2 mg/kg Torbugesic; Fort Dodge). On day 2, all cats received a 4 h, 2 μg/kg/h intravenous IV Escherichia coli 0127:B8 LPS infusion (Sigma-Aldrich, St Louis, MO) starting at baseline. Thirty minutes after the initiation of the LPS infusion, cats received a 30 min intravenous infusion of either 10 ml 0.9% NaCl (placebo) or 1 mg/kg PMB diluted to 10 ml with 0.9% NaCl (PMB for Injection, Bedford Laboratories, Bedford, OH). Cats were randomized to treatment groups using a table of random numbers. Crystalloid fluid (0.9% NaCl) was administered to any cat that developed severe hypotension (Doppler systolic blood pressure [BP]<60 mmHg) in 10 ml/kg increments to achieve resolution of severe hypotension (BP>60 mmHg).
Sample collection
Rectal temperature (T), respiratory rate (RR) and character, heart rate (HR), Doppler (Parks Medical Electronics, Las Vegas, NV) systolic arterial BP and neurologic examination (assessment of mentation, posture and cranial nerve function) were evaluated at baseline and then every 30 min for 6 h after initiation of LPS infusion. Cats were also evaluated for signs of anaphylaxis (including angioedema, urticaria, pruritus, respiratory distress, vomiting and hypotension) and development of gastrointestinal signs (vomiting, diarrhea, salivation). Blood was collected at baseline from the jugular catheter and at predetermined time points thereafter (0.5, 1, 1.5, 2, 3, 4 and 6 h) into potassium EDTA anticoagulated tubes for CBCs, and lithium heparin tubes (1, 1.5, 3 and 4 h) for plasma TNF activity. No more than 4 ml/kg of whole blood was drawn in total. For each volume of blood collected, an equal volume of 0.9% saline was administered intravenously. In addition, urine was collected via cystocentesis at 6 h. Plasma and urine supernatant were harvested immediately using a centrifuge (300×g for 6 min), and banked at −20°C until analysis.
Assays
CBC
CBCs were performed using a Coulter Counter (Z1 Particle Counter, Coulter Electronics, Hialeah, FL).
Urine biochemistry
Urine γ-glutamyl transferase (GGT) was measured 6 h after placebo or PMB treatment. Urinalysis (including dipstick [Multistix, Bayer, Pittsburgh, PA], urine specific gravity [USG] by refractometry and sediment examination) was also performed on the urine sample collected at 6 h.
TNF activity
Plasma TNF activity was evaluated at baseline, 1, 1.5, 3 and 4 h after initiation of LPS infusion using a cytotoxicity bioassay, described above. These time points were chosen based on the expected peak plasma concentration after low-dose endotoxin infusion in cats. 25
Data analysis
Statistical analyses were performed using commercially available software (SigmaStat, Systat Software, Chicago, IL). The Kolmogorov–Smirnov statistical test for normality was used to determine if data were normally distributed. Data that were not normally distributed were transformed using a natural logarithm prior to analysis. Treatments were compared using a repeated measures analysis of variance with post-hoc Tukey multiple comparison procedure. A P-value of <0.05 was considered statistically significant. Data are expressed as mean±Standard error (SE) unless otherwise noted.
Results
Ex vivo LPS-induced TNF production from feline Cwb
Whole blood stimulated with LPS produced significantly more TNF activity than control (P<0.001) in the absence of PMB (Fig. 1). Supernatant TNF activity was significantly less from LPS-stimulated whole blood treated with 1, 5, 10 and 25 μg/ml of PMB compared to blood not treated with PMB (P≤0.004). However, although LPS-induced TNF production was blunted by PMB, it was still significantly greater (P≤0.01) than TNF production from control at all concentrations tested with the exception of 25 μg/ml. At 25 μg/ml of PMB, LPS-induced TNF production was not significantly different from control (P=0.17).
Fig 1.
TNF concentrations in feline whole blood culture after stimulation with control (□) or LPS (▪) and treatment with PMB, in vitro. Data are expressed as mean±SE. *Supernatant TNF activity was significantly less from LPS-stimulated whole blood treated with 1, 5, 10 and 25 μg/ml of PMB compared to blood not treated with PMB (P≤0.004).
In vivo response to PMB treatment
Physiologic parameters
After LPS administration, rectal temperature increased significantly over the duration of the study (P<0.001) with a peak at 4–5 h (Fig. 2). None of the cats developed hypothermia. There was no significant temperature difference between treatment groups at any time point (P=0.109).
Fig 2.
Comparison of rectal temperature between cats treated with PMB (□) or placebo (▪). Endotoxin infusion was initiated at time 0. Data are expressed as mean±SE. Temperature in both groups increase significantly over time compared to baseline. There was no significant difference between treatments.
RR was variable in all cats, ranging from 18 to 120 breaths/min. There was no pattern of change over time or difference between treatment groups (data not shown). Of importance, despite the variability, none of the cats developed respiratory distress, open mouth breathing or cyanosis.
There was no significant difference in HR over time associated with LPS infusion or between treatment groups (P=0.718) at any time point (data not shown). No cat developed bradycardia (HR<140 beats/min). LPS infusion did, however, induce a significant decrease in BP over the duration of the study compared to baseline (P<0.001), with no significant difference between treatment groups (P=0.071) (Fig. 3). None of the cats developed severe hypotension (BP<60 mmHg).
Fig 3.

Comparison of systolic arterial BP between cats treated with PMB (□) or placebo (▪). Endotoxin infusion was initiated at time 0. BP decreased significantly in both groups compared to baseline. There was no significant difference between treatments. Data are expressed as mean±SE.
The demeanor of all cats was considered bright, alert and responsive at the commencement of the study. LPS infusion resulted in a change in the demeanor of all cats, regardless of treatment group, varying from quiet, alert and responsive to lethargic. Neurologic evaluation remained normal in all cats throughout the study period regardless of treatment. All cats completely recovered normal demeanor by the following day. None of the cats developed clinical signs attributable to anaphylaxis. One cat in the placebo group developed diarrhea during the study period; experiencing five episodes between time 1.5 and 5.5 h. None of the cats experienced vomiting.
CBC
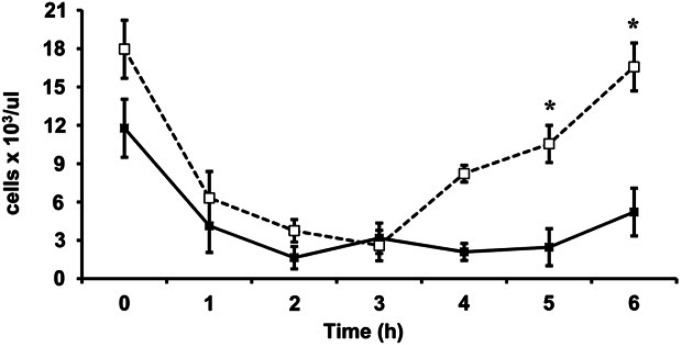
The white blood cell count (WBCC) significantly decreased (P<0.001) starting at 1 h, reaching a nadir at 2–3 h with recovery by 6 h after LPS administration. The WBCC was significantly higher at 4 and 6 h in the PMB treated cats compared to the placebo group (P=0.019) (Fig. 4).
Fig 4.
Comparison of WBCC between cats treated with PMB (□) or placebo (▪). Endotoxin infusion was initiated at time 0. Data are expressed as mean±SE. WBCCs in both groups decreased significantly compared to baseline. *The WBCC was significantly higher at 4 and 6 h in the PMB treated cats compared to the placebo group (P=0.019).
Urine biochemistry
At 6 h urine GGT was below the lower limit of detection (3 U/l) in all cats regardless of treatment group. There was no evidence of proteinuria, glucosuria, bilirubinuria or ketonuria in any cat regardless of treatment. Sediment examination was similarly unremarkable; all cats had an inactive sediment with no identifiable casts. The urine of all cats was isosthenuric (specific gravity 1.008–1.012).
TNF activity
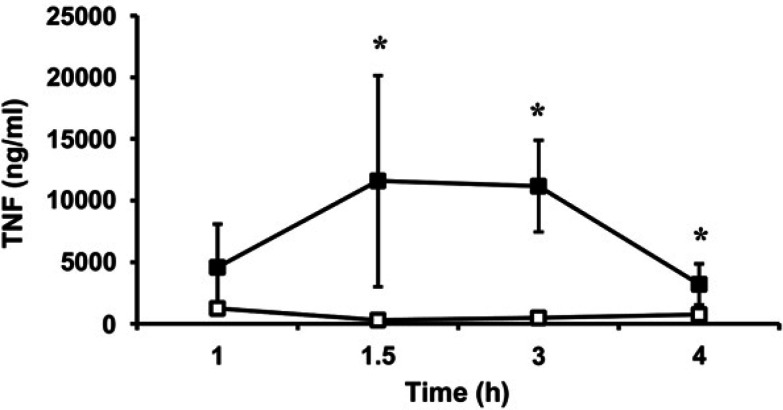
Plasma TNF activity was not detectable at baseline in any of the cats, regardless of treatment; therefore, this time point was removed from statistical analysis. Plasma TNF activity increased after initiation of endotoxin infusion in all cats with a peak at 1 h in the placebo group and 3 h in the PMB treatment group. The TNF activity was significantly lower in the PMB treated cats compared to the placebo group (P<0.001) (Fig. 5).
Fig 5.
Comparison of plasma TNF activity between cats treated with PMB (□) or placebo (▪). Endotoxin infusion was initiated at time 0. Data are expressed as mean±SE. *The TNF activity was significantly lower in the PMB treated cats compared to the placebo group (P<0.001).
Discussion
In this study, PMB was found to ameliorate pro-inflammatory sequelae of endotoxin challenge both ex vivo and in vivo in cats. Ex vivo, Cwb supernatant TNF activity was significantly less from LPS-stimulated whole blood treated with 1, 5, 10 and 25 μg/ml of PMB compared to blood not treated with PMB. In vivo, we evaluated the safety and anti-endotoxin effects of PMB in a clinically applicable, survival model of low-dose endotoxin infusion. As expected, 25 LPS administration induced significant systemic inflammation and hemodynamic derangement as indicated by the development of fever, leukopenia, increased TNF activity and relative hypotension. PMB administration in this model appeared safe and was found to blunt the LPS-induced leukopenia and plasma TNF activity. However, there was no significant difference between the placebo and PMB treatment groups in regards to rectal temperature, HR or BP.
The objective of the ex vivo study reported here was to investigate the pharmacodynamic properties of PMB using an LPS-stimulated whole blood culture system from adult cats to determine if and at which doses PMB could blunt TNF activity. Whole blood cultures have been validated in healthy humans as low-cost, surrogate measures of monocytic cytokine production. 27 TNF-α is a prototypic, early phase pro-inflammatory mediator that has earned a position of prominence at the head of the inflammatory cytokine cascade because it can increase the expression of a wide variety of other pro-inflammatory mediators (eg, nitric oxide and cyclo-oxygenase 2 of the arachidonic acid cascade), other cytokines (eg, IL-1β, IL-6), chemokines (eg, CXCL-8) and adhesion molecules resulting in a cytokine storm. 28 In addition, TNF is involved in the hemodynamic derangement that accompanies sepsis. Infusion of TNF in experimental models results in hypotension, negative inotropy and clinical signs of shock. TNF also stimulates neutrophil release from bone marrow; the resultant early neutrophilia is one of the clinical criteria used in the diagnosis of sepsis. Another important consequence of TNF production includes resetting of the hypothalamic set point resulting in fever. 29,30 Some studies have documented a correlation between plasma TNF concentrations and mortality in septic people. 31,32
TNF production from LPS-stimulated feline Cwb has been previously reported 26,33 ; however, to the knowledge of the authors, this is the first study to evaluate the effect of PMB on TNF production from endotoxin-stimulated feline Cwb. In our study, TNF production was significantly less from LPS-stimulated whole blood treated with PMB compared to blood not treated with PMB but remained greater than production from control except at 25 μg/ml PMB. These findings are consistent with what has been documented in an ex vivo model of endotoxemia in horses where PMB caused a significant dose-dependent decrease in endotoxin-induced TNF activity. 21 Similarly, treatment with low-dose intramuscular PMB in a cecal ligation and puncture model of sepsis in rats attenuated TNF production from isolated Kupffer cells, ex vivo. 17 It should be noted that, while not significant (P=0.42), whole blood treated with 25 μg/ml of PMB in the absence of LPS had more than twice the TNF production of whole blood treated with the other concentrations of PMB. Therefore, it is possible that the lack of difference between TNF production from LPS stimulated and control whole blood treated with PMB at 25 μg/ml was due to an increase in TNF production from the control blood as opposed to a lack of a true treatment effect from PMB. PMB stimulation of TNF production has been previously documented at higher concentrations of PMB (>20 μg/ml) 34 but not at 10 μg/ml. 12,34 Documentation of a reduction in LPS-induced TNF activity by PMB subsequently prompted an in vivo study evaluating the anti-endotoxin effects of PMB. While there is no literature documenting a study which has correlated intravenous dose of PMB with concentrations in whole blood cultures, a dose of 1 mg/kg of PMB was chosen to achieve a plasma concentration around 1 μg/ml in a 4.9 kg cat with an estimated blood volume of 240 ml. 35 Higher doses were avoided to reduce the risk of toxicity and given the aforementioned stimulation of TNF production by high concentrations of PMB. 34
In vivo, we chose to evaluate PMB in a low-dose endotoxin infusion model to avoid several disadvantages of high-dose endotoxin bolus models of sepsis, such as that used by Hughes et al notably the induction of massive pro-inflammatory cytokine production leading to overwhelming inflammation, acute circulatory collapse and rapid mortality. 24,36 Unlike the aforementioned model, the pre-clinical feline model using low-dose endotoxin not only has less severe clinical manifestations but as a result also facilitates use of minimally instrumented conscious cats thereby preventing anesthesia induced interference with the neuro-endocrine axis and cardiovascular function. Compared with models of overwhelming sepsis resulting in rapid mortality, this model is more likely to provide clinically useful data pertaining to novel sepsis treatments prior to evaluation in pet cats.
Altered body temperature is a common finding in cats with sepsis. 1,3 Endotoxin infusion induces a febrile response in cats as was found in our study. 25 The etiology of fever in sepsis is likely associated with endogenous pyrogen production. In this study, PMB did not ameliorate the endotoxin-induced fever; in contrast to PMB administration in studies of experimental sepsis in horses 19 and naturally developing sepsis in humans. 22 The lack of a treatment effect in this study may be related to timing of administration in that body temperature had already increased by the time that PMB treatment was commenced (ie, 30 min after the start of the endotoxin infusion). Barton et al documented that pretreatment with PMB before the initiation of endotoxin infusion prevented LPS-induced fever, whereas horses receiving PMB after the onset of endotoxemia still developed fever, albeit less severe at peak, consistent with their overall conclusions that PMB was most beneficial to horses when given before the onset of endotoxemia. 19 Nonetheless, fever in isolation is not considered a deleterious manifestation of sepsis, and may actually be protective in the presence of bacterial infection. 37,38
PMB has been previously reported to have adverse effects on respiration in cats secondary to neuromuscular blockade, with respiratory depression occurring at doses of 3 mg/kg intravenously (18,000 U/kg) progressing to respiratory arrest at 5 mg/kg intravenously (30,000 U/kg). 39 RR was highly variable in all cats of this study (treated and untreated), but no cat developed evidence of respiratory distress, change in respiratory pattern or cyanosis. RR is likely influenced by a variety of factors in the setting of endotoxemia and sepsis in addition to the severity of pulmonary inflammation, including the presence of fever, discomfort and anxiety. Of important clinical relevance is that no adverse effects on respiration were observed in our study with the use of lower doses of PMB (1 mg/kg IV).
In this study PMB did not have an effect on the hemodynamic status of endotoxemic cats with no significant difference evident in HR or BP between treatment groups at any time point. While there was no change in HR over time, these cats failed to develop compensatory tachycardia in the face of systemic hypotension which is suggestive of hemodynamic derangement. The inability of PMB to prevent acute phase systemic hypotension seen in our study is consistent with findings in dogs and cats treated with PMB (5 mg/kg IV over 30 min beginning 1 h after IV bolus injection of LPS, and 5 mg/kg IV bolus 1 min prior to endotoxin administration followed by 30 min infusion of an additional 5 mg/kg). 18,24 Interestingly however, the second/late phase of LPS-induced hypotension, which is a preterminal event in these lethal models of endotoxemia, was ameliorated in both the feline study 24 and a subgroup of dogs treated with PMB-modified LPS. 18 Similarly, treatment of rabbits with PMB 1 h after intraperitoneal E coli injection resulted in higher BP compared to placebo treated animals. 16 These studies taken together, suggest that the ability of PMB to ameliorate LPS-induced hemodynamic derangement, as well as other biologic effects, may be related to the timing of administration. Pretreatment of the LPS with PMB (ie, mixing the two compounds together) prior to administration and pretreatment of the patient with PMB prior to LPS administration is likely to dramatically reduce the ability of LPS to activate the innate immune response by preventing lipid A interaction with CD14 and TLR4, and thus preventing much of the inflammatory cascade and resultant hemodynamic instability that occurs in endotoxemia. While the aforementioned rabbit study did not pretreat with PMB, 16 it is likely that intraperitoneal bacterial injection rather than intravenous endotoxin dosing may result in a more gradual onset of endotoxemia, thus ensuring greater anti-endotoxin efficacy of PMB in regards to prevention of downstream pro-inflammatory signaling. Unfortunately, prophylactic administration of PMB prior to the onset of endotoxemia and sepsis is not practical in naturally occurring sepsis in cats, reducing the clinical applicability of such data.
While an earlier report documented that high-dose PMB has dramatic and beneficial effects on late phase hypotension, metabolic acidosis and survival in feline endotoxemia 24 the dose used is high enough to induce neurotoxicosis, nephrotoxicosis, respiratory arrest, cardiovascular depression and histamine-mediated hypersensitivity. 11,40–42 Concerns about the toxic effects of PMB have previously limited its clinical use, both as an antibiotic and for its anti-endotoxin effects, in non-equine species. The toxicity of PMB results from its ability to bind to phospholipid membranes with the potential to disrupt cellular function in multiple organ systems. 40 Given the potential for severe side effects, the authors felt that it was vital to evaluate the safety of a low-dose PMB treatment protocol before use in naturally occurring feline sepsis. Importantly, our study showed that low-dose PMB (1 mg/kg IV) is safe with no differences in neurologic examination, RR and character, HR and BP between placebo and PMB treated endotoxic cats; also, while urine GGT:creatinine ratios could not be performed because the urine GGT was below the limits of detection, the cats did not develop overt evidence of renal failure. Additionally, we did not observe signs of anaphylaxis or gastrointestinal disturbance. Our findings are consistent with the dose-dependent nature of PMB toxicity and results of previous studies in which low doses of PMB (1–2 mg/kg) maintain considerable anti-endotoxin activity while avoiding adverse effects. 20,22,23 The dose of PMB (1 mg/kg IV over 30 min) was chosen for use in this study as it was below the toxic threshold for many species, and indeed the same appears to be true in cats.
The beneficial effects of PMB treatment in maintaining higher WBCC in our study are additional evidence of the anti-endotoxin effects of PMB, but interestingly are in contrast to other studies in other species evaluating the anti-endotoxin effects of PMB. For example, in experimental Pasteurella multicoda sepsis in rabbits, PMB treatment improved survival but did not modify the leukopenia. 15 Similarly, leukocyte numbers were unaltered by PMB treatment in both experimental canine endotoxemia 18 and naturally occurring endotoxic shock in dogs. 23 CBCs were not reported in the previous study evaluating the anti-endotoxin effects of PMB in feline endotoxemia. 24
TNF is a prototypic pro-inflammatory cytokine and early phase mediator of systemic inflammation in sepsis. Only two other veterinary studies have used in vivo TNF concentrations to evaluate the anti-endotoxin effects of PMB. 19,23 Similar to our study, these studies have documented reduced TNF production in septic patients treated with PMB. Administration of polymyxin E (2 mg/kg IM q 12 h for two doses) to dogs with naturally occurring, parvoviral-induced, endotoxic shock resulted in a significant reduction in plasma TNF concentrations compared to control dogs. 23 This finding is especially important as rising plasma TNF concentrations are associated with mortality in dogs with naturally occurring parvovirus-induced sepsis. 43 In an equine model of endotoxemia, treatment with PMB, both before and after administration of endotoxin, significantly reduced serum TNF concentrations, compared to horses receiving saline placebo. 19
Conclusion
These data indicate that administration of PMB after the initiation of endotoxin infusion is safe and results in an amelioration of the severity of endotoxin-induced leukopenia and TNF activity in this experimental model of feline sepsis. While PMB was safe and effective in blunting systemic inflammatory sequelae of LPS in this endotoxemia model, its clinical utility in cats with established sepsis is not known. A carefully designed, randomized, blinded, placebo controlled clinical trial, with close adverse event monitoring, evaluating the use of PMB in naturally occurring Gram-negative feline sepsis would be the next step in evaluation of this therapy for clinical use.
Acknowledgements
The authors would like to thank Chee-Hoon Chang for technical assistance with this study. This study was funded by a grant from the University of Missouri, Research Council.
References
- 1.Brady C.A., Otto C.M., Van Winkle T.J., King L.G. Severe sepsis in cats: 29 cases (1986–1998), J Am Vet Med Assoc 217, 2000, 531–535. [DOI] [PubMed] [Google Scholar]
- 2.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis, Crit Care Med 20, 1992, 864–874. [PubMed] [Google Scholar]
- 3.Costello M.F., Drobatz K.J., Aronson L.R., King L.G. Underlying cause, pathophysiologic abnormalities, and response to treatment in cats with septic peritonitis: 51 cases (1990–2001), J Am Vet Med Assoc 225, 2004, 897–902. [DOI] [PubMed] [Google Scholar]
- 4.Dow S.W., Curtis C.R., Jones R.L., Wingfield W.E. Bacterial culture of blood from critically ill dogs and cats: 100 cases (1985–1987), J Am Vet Med Assoc 195, 1989, 113–117. [PubMed] [Google Scholar]
- 5.Sergeeff J.S., Armstrong P.J., Bunch S.E. Hepatic abscesses in cats: 14 cases (1985–2002), J Vet Intern Med 18, 2004, 295–300. [DOI] [PubMed] [Google Scholar]
- 6.Walker A.L., Jang S.S., Hirsh D.C. Bacteria associated with pyothorax of dogs and cats: 98 cases (1989–1998), J Am Vet Med Assoc 216, 2000, 359–363. [DOI] [PubMed] [Google Scholar]
- 7.Greiner M., Wolf G., Hartmann K. Bacteraemia in 66 cats and antimicrobial susceptibility of the isolates (1995–2004), J Feline Med Surg 9, 2007, 404–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hodgson J.C. Endotoxin and mammalian host responses during experimental disease, J Comp Pathol 135, 2006, 157–175. [DOI] [PubMed] [Google Scholar]
- 9.Munford R.S. Detoxifying endotoxin: time, place and person, J Endotoxin Res 11, 2005, 69–84. [DOI] [PubMed] [Google Scholar]
- 10.Rustici A., Velucchi M., Faggioni R., et al. Molecular mapping and detoxification of the lipid A binding site by synthetic peptides, Science 259, 1993, 361–365. [DOI] [PubMed] [Google Scholar]
- 11.Hermsen E.D., Sullivan C.J., Rotschafer J.C. Polymyxins: pharmacology, pharmacokinetics, pharmacodynamics, and clinical applications, Infect Dis Clin North Am 17, 2003, 545–562. [DOI] [PubMed] [Google Scholar]
- 12.Tsuzuki H., Tani T., Ueyama H., Kodama M. Lipopolysaccharide: neutralization by polymyxin B shuts down the signaling pathway of nuclear factor kappaB in peripheral blood mononuclear cells, even during activation, J Surg Res 100, 2001, 127–134. [DOI] [PubMed] [Google Scholar]
- 13.Brown M.A., Jones W.K. NF-kappa B action in sepsis: the innate immune system and the heart, Front Biosci 9, 2004, 1201–1217. [DOI] [PubMed] [Google Scholar]
- 14.Liu S.F., Malik A.B. NF-kappa B activation as a pathological mechanism of septic shock and inflammation, Am J Physiol Lung Cell Mol Physiol 290, 2006, L622–L645. [DOI] [PubMed] [Google Scholar]
- 15.Corrigan J.J., Jr., Kiernat J.F. Effect of polymyxin B sulfate on endotoxin activity in a Gram-negative septicemia model, Pediatr Res 13, 1979, 48–51. [DOI] [PubMed] [Google Scholar]
- 16.Flynn P.M., Shenep J.L., Stokes D.C., et al. Polymyxin B moderates acidosis and hypotension in established, experimental Gram-negative septicemia, J Infect Dis 156, 1987, 706–712. [DOI] [PubMed] [Google Scholar]
- 17.Mayumi T., Takezawa J., Takahashi H., et al. Low-dose intramuscular polymyxin B improves survival of septic rats, Shock 11, 1999, 82–86. [DOI] [PubMed] [Google Scholar]
- 18.From A.H., Fong J.S., Good R.A. Polymyxin B sulfate modification of bacterial endotoxin: effects on the development of endotoxin shock in dogs, Infect Immun 23, 1979, 660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barton M.H., Parviainen A., Norton N. Polymyxin B protects horses against induced endotoxaemia in vivo, Equine Vet J 36, 2004, 397–401. [DOI] [PubMed] [Google Scholar]
- 20.Morresey P.R., Mackay R.J. Endotoxin-neutralizing activity of polymyxin B in blood after IV administration in horses, Am J Vet Res 67, 2006, 642–647. [DOI] [PubMed] [Google Scholar]
- 21.Parviainen A.K., Barton M.H., Norton N.N. Evaluation of polymyxin B in an ex vivo model of endotoxemia in horses, Am J Vet Res 62, 2001, 72–76. [DOI] [PubMed] [Google Scholar]
- 22.Endo S., Inada K., Kikuchi M., et al. Clinical effects of intramuscular administration of a small dose of polymyxin B to patients with endotoxemia, Res Commun Chem Pathol Pharmacol 83, 1994, 223–235. [PubMed] [Google Scholar]
- 23.Senturk S. Evaluation of the anti-endotoxic effects of polymyxin-E (colistin) in dogs with naturally occurred endotoxic shock, J Vet Pharmacol Ther 28, 2005, 57–63. [DOI] [PubMed] [Google Scholar]
- 24.Hughes B., Madan B.R., Parratt J.R. Polymyxin B sulphate protects cats against the haemodynamic and metabolic effects of E coli endotoxin, Br J Pharmacol 74, 1981, 701–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DeClue A.E., Williams K.J., Sharp C., et al. Systemic response to low dose endotoxin infusion in cats, Vet Immunol Immunopath 132 (2–4), 2009, 167–174. [DOI] [PubMed] [Google Scholar]
- 26.Stich A., DeClue A.E. Pathogen associated molecular pattern-induced TNF, IL-1β, IL-6 and CXCL-8 production from feline whole blood culture, J Vet Emergency Crit Care 19, 2009, A1–10. [DOI] [PubMed] [Google Scholar]
- 27.Damsgaard C.T., Lauritzen L., Calder P.C., et al. Whole-blood culture is a valid low-cost method to measure monocytic cytokines – a comparison of cytokine production in cultures of human whole-blood, mononuclear cells and monocytes, J Immunol Methods 340, 2009, 95–101. [DOI] [PubMed] [Google Scholar]
- 28.Gosain A., Gamelli R.L. A primer in cytokines, J Burn Care Rehabil 26, 2005, 7–12. [DOI] [PubMed] [Google Scholar]
- 29.Tracey K.J., Lowry S.F., Fahey T.J., 3rd, et al. Cachectin/tumor necrosis factor induces lethal shock and stress hormone responses in the dog, Surg Gynecol Obstet 164, 1987, 415–422. [PubMed] [Google Scholar]
- 30.Kreil E.A., Greene E., Fitzgibbon C., et al. Effects of recombinant human tumor necrosis factor alpha, lymphotoxin, and Escherichia coli lipopolysaccharide on hemodynamics, lung microvascular permeability, and eicosanoid synthesis in anesthetized sheep, Circ Res 65, 1989, 502–514. [DOI] [PubMed] [Google Scholar]
- 31.Casey L.C., Balk R.A., Bone R.C. Plasma cytokine and endotoxin levels correlate with survival in patients with the sepsis syndrome, Ann Intern Med 119, 1993, 771–778. [DOI] [PubMed] [Google Scholar]
- 32.Marano M.A., Fong Y., Moldawer L.L., et al. Serum cachectin/tumor necrosis factor in critically ill patients with burns correlates with infection and mortality, Surg Gynecol Obstet 170, 1990, 32–38. [PubMed] [Google Scholar]
- 33.Otto C.M., Rawlings C.A. Tumor necrosis factor production in cats in response to lipopolysaccharide: an in vivo and in vitro study, Vet Immunol Immunopathol 49, 1995, 183–188. [DOI] [PubMed] [Google Scholar]
- 34.Jaber B.L., Sundaram S., Neto M. Cendoroglo, et al. Polymyxin-B stimulates tumor necrosis factor-alpha production by human peripheral blood mononuclear cells, Int J Artif Organs 21, 1998, 269–273. [PubMed] [Google Scholar]
- 35.Groom A.C., Rowlands S. The cardiac output and blood volume of the anaesthetized cat, Phys Med Biol 3, 1958, 138–156. [DOI] [PubMed] [Google Scholar]
- 36.Deitch E. Animal models of sepsis and shock: a review and lessons learned, Shock 9, 1998, 1–11. [DOI] [PubMed] [Google Scholar]
- 37.Laupland K.B. Fever in the critically ill medical patient, Crit Care Med 37 (suppl), 2009, S273–S278. [DOI] [PubMed] [Google Scholar]
- 38.Su F., Nguyen N.D., Wang Z., et al. Fever control in septic shock: beneficial or harmful?, Shock 23, 2005, 516–520. [PubMed] [Google Scholar]
- 39.Viswanath D.V., Jenkins H.J. Neuromuscular block of the polymyxin group of antibiotics, J Pharm Sci 67, 1978, 1275–1280. [DOI] [PubMed] [Google Scholar]
- 40.Sanders W.E., Jr., Sanders C.C. Toxicity of antibacterial agents: mechanism of action on mammalian cells, Annu Rev Pharmacol Toxicol 19, 1979, 53–83. [DOI] [PubMed] [Google Scholar]
- 41.Kunin C.M. Nephrotoxicity of antibiotics, J Am Med Assoc 202, 1967, 204–208. [PubMed] [Google Scholar]
- 42.Kunin C.M., Bugg A. Binding of polymyxin antibiotics to tissues: the major determinant of distribution and persistence in the body, J Infect Dis 124, 1971, 394–400. [DOI] [PubMed] [Google Scholar]
- 43.Otto C., Drobatz K., Soter C. Endotoxemia and tumor necrosis factor activity in dogs with naturally occurring parvoviral enteritis, J Vet Int Med 11, 1997, 65–70. [DOI] [PubMed] [Google Scholar]