Abstract
An 11-year-old cat presented for evaluation of intermittent vomiting, constipation and hyporexia of 3 weeks duration. Ultrasonographic and endoscopic examination revealed a soft tissue mass adjacent to the lower gastro-esophageal sphincter. Surgical excision of the mass was successfully performed resulting in a resolution of clinical signs. Histologically the mass was consistent with a smooth muscle hamartoma. At follow-up 7 months after surgery, the cat remained free from clinical signs.
An 11-year-old, male castrated, domestic longhair cat presented for evaluation of intermittent vomiting (clear liquid or bile colored), constipation and hyporexia of 3 weeks duration. Preceding referral, symptomatic treatment for constipation and vomiting had been unsuccessful. Orthogonal radiographic views of the abdomen, taken by the referring veterinarian, were reviewed and considered unremarkable.
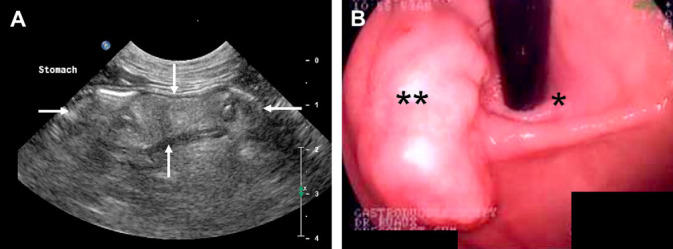
On physical examination the cat had a body condition score of 3/5, body weight 4.9 kg; was quiet, alert and reactive. Digital palpation of the abdomen revealed a cranial-to-mid abdominal soft tissue mass. Ultrasonographic examination of the abdomen revealed moderate sludge within the gallbladder without evidence of gallbladder wall thickening or extra-hepatic biliary distention and mild hypoechoic free peritoneal effusion. An approximately 2 cm diameter, irregular, hyperechoic gastric intramural mass with low vascularity was present at the level of the cardia (Fig. 1). The remaining gastric wall appeared within normal limits without evidence of gastric wall thickening or loss of wall layering. Cytological examination of ultrasound-guided fine needle aspirates (FNA) of the gastric mural mass showed very small amounts of blood and sparse cellular debris, and was considered non-diagnostic.
Fig 1.
(A) Ultrasound image at the level 1 cm aborad to the lower gastro-esophageal sphincter. A soft tissue lesion (arrows) of heterogeneous mixed echogenicity is seen within the stomach wall. (B) Endoscopic view of the gastro-esophageal sphincter, the endoscope is seen exiting the esophagus adjacent to the soft tissue mass.
Following general anesthesia, endoscopic examination of the upper alimentary tract revealed a soft tissue mass measuring 2–3 cm×3 cm×1.5 cm (Fig. 1), visualized immediately aborad, and medial to the lower esophageal sphincter at the level of the gastric cardia. The remaining gastric wall and proximal duodenum were grossly normal. Pinch biopsies were taken of the gastric mass, proximal duodenum and areas of grossly normal gastric wall. Cytological and histological examination of these samples showed normal gastric mucosa with mild granulomatous inflammation in the lamina propria; lymphocytes, plasma cells and a few eosinophils were present in the duodenum, consistent with lymphoplasmacytic duodenitis. Based on the ultrasonic and endoscopic images, an intramural gastric body mass was highly suspected. Ventro-dorsal, left and right lateral thoracic radiographs taken to assess for any potential metastatic disease were unremarkable. Complete blood count, blood smear evaluation and serum biochemistry were relatively unremarkable with values for cholesterol and lymphocytes only marginally outside of the reference range.
Despite these findings, and although gastric tumors are rare in cats, 1,2 a non-exfoliating soft tissue neoplasm of the muscularis and or serosal layers was considered to be the most likely cause of the gastric luminal mass, with leiomyosarcoma or leiomyoma the primary differential diagnoses before resection. Despite being the most common gastric tumor in cats 3,4 gastric lymphoma was considered unlikely based on the lack of round cells from the ultrasound-guided FNA. Gastric carcinoma or adenocarcinoma is very rare in cats 1,4 and were considered unlikely based on histological examination of the gastric mucosal biopsies. Other pre-surgical differential diagnoses for the mass included mast cell tumor, benign adenomatous polyp and plasmacytoma. 3–5 Due to the chronicity, ultrasonographic findings and lack of cytological evidence, gastric abscess, amyloidosis, hematoma, seroma and granuloma were considered unlikely.
Surgical exploration of the abdomen was performed for the purpose of assessing the extent of the gastric mass, for excision biopsy of the mass and potential marginal excision of the mass, if it could be performed without affecting lower gastro-esophageal function.
Cefazolin (Cefazolin, Orchid Healthcare, India) 22 mg/kg IV q 90 min was administered from the time of induction of general anesthesia until extubation. An abdominal exploratory examination through a ventral midline celiotomy allowed identification of a firm, approximately 2 cm diameter soft tissue mass located ventral, aborad and medial to the lower esophageal sphincter within the cardia of the stomach. The mass was sharply excised using a #10 scalpel blade approximately 1.5 cm from the gross margins and placed in 10% neutral buffered formalin. The gastric wall defect was closed routinely using a two layer closure technique (initial simple full-thickness continuous pattern followed by a continuous Cushing pattern) using 3/0 polydioxanone (PDS; Ethilon). Subjectively, the mesenteric lymph nodes looked mildly enlarged and excision biopsy of a single prominent node was performed. The remaining abdominal organs were considered grossly normal. An incision biopsy of the left lateral liver lobe was taken to assess for concurrent liver pathology. An incision line block was performed by local injection of 1 mg/kg diluted to 0.25% solution of bupivicaine (Bupivicaine HC; Hospira) into the subcutaneous tissue following routine abdominal closure.
Postoperative therapy included intravenous lactated Ringer's solution supplemented with 1 ml/l of vitamin B complex (Vitamin B complex Injection; Vedco) and 20 mEq KCl/l, at a rate of 2 ml/kg/h; buprenorphine (Buprenorphine HCL; Bedford Laboratories) 0.01 mg/kg IV q6-8 h for 24 h; and sucralfate (Sucralfate; Le Vista) 0.25 g PO tid for 5 days for gastric mucosal protection. The cat was discharged 3 days postoperatively with buprenorphine 0.01 mg/kg transmucosally q8 h, for 3 days; cyproheptadine (Cyproheptadine, Par Pharmaceuticals) 0.42 mg/kg PO bid, for 7 days; and famotidine (Famotidine; Par Pharmaceutical Companies) 0.5 mg/kg bid PO, for 7 days.
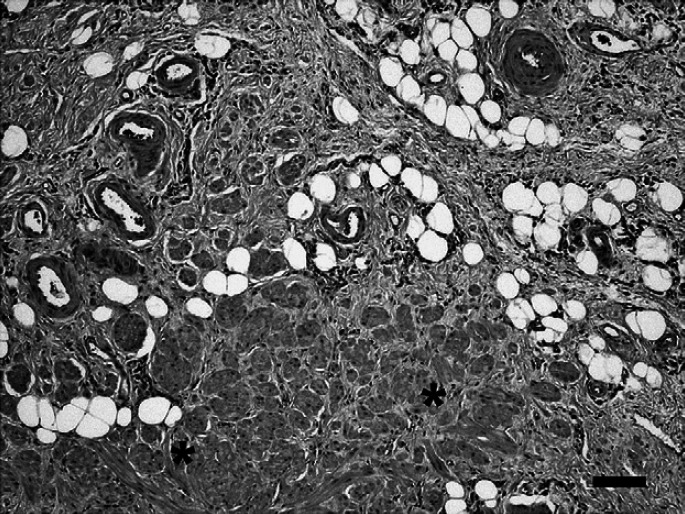
Histologically, the mass consisted of admixed yet distinct, well differentiated connective tissue elements native to the gastric submucosa and confined to that layer (Fig. 2). The submucosa was markedly thickened by abundant fibroadipose tissue within which were embedded widely separated and randomly oriented bundles of smooth muscle as well as numerous thick walled vessels of varying caliber. In some areas, the smooth muscle bundles approached and merged with the adjacent muscularis mucosae. The diagnosis of smooth muscle hamartoma was made based upon these findings. Mild hepatocellular swelling and mild lymphoid hyperplasia of mesenteric lymph node were also noted.
Fig 2.
Gastric smooth muscle hamartoma in a cat. Greatly thickened submucosa consisting of a fibroadipose matrix harboring well differentiated smooth muscle bundles* and numerous thick walled vessels. Bar=100 μm.
At 2 weeks postoperatively the cat was clinically normal and free from signs of vomiting. Follow-up at 7 months postoperatively by telephone communication with the owner revealed the cat was clinically normal. No vomiting had been noted, appetite was considered normal and the cat was maintaining a steady body mass of approximately 5.5 kg.
A hamartoma is a benign overgrowth of mature cells and tissues that are normally present in the area of the body where the growth occurs. Hamartomas do not reproduce the normal architecture of the surrounding tissue. 6,7 Hamartomas are most often focal in nature, grossly resemble neoplasia 8 and have been regarded as a link between neoplasm and malformations. 9 Typically, morbidity related to hamartomas is attributable to their infiltration into or mass effect on local surrounding tissues.
In the dog, case reports of hamartoma include involvement the spleen, 7 carpus, 10 spinal cord, 9 myocardium, 11 hepatic vasculature, 12 pulmonary vasculature, 13 kidney, 14 hypothalamus, 15 tongue, 16 cranial nerves, 17 respiratory tract, 18,19 and intestines 20 and cerebral vasculature. 21 Few case reports of hamartomas in the cat are available within the veterinary literature. Isolated case reports have described involvement of the lungs, 22 kidney, 23 gingiva 24 and central nervous system. 1,8 To the best of the authors' knowledge this is the first case report to describe the clinical signs, diagnostic findings, successful surgical treatment, histological features of a gastric smooth muscle hamartoma in a cat.
While there have been many reports of hamartomas of the stomach in people, we are aware of only one published description in other animals, that being a smooth muscle hamartoma of the abomasum of a bovine calf found at necropsy. 4 In both the calf and this cat, the connective tissue elements of both hamartomas were similar, although the smooth muscle bundles were more prominent in the calf. The prominence of the fibroadipose and vascular components in the current case suggest this may be more correctly termed an angio-myo-fibro-lipomatous hamartoma, but smooth muscle hamartoma has been retained for purposes of consistency.
In apparent contrast to animals, gastric hamartomas in people consist of prominent smooth muscle bundles that encircle diverse epithelial cell arrangements. 25–27 The epithelial cells are hypothesized to arise from displaced gastric stem cells or pancreatic anlage, or possibly from pancreatic metaplasia during embryogenesis. In people, gastric pain, nausea, vomiting, and pyloric obstruction have been observed 26 in patients with gastric hamartoma, and it is reasonable to presume the clinical impact of the lesion in this cat was due to its size and anatomic location. We postulate that its presence interfered with either function of the lower esophageal sphincter or gastric body wall. Clinical signs abated following simple excision of the mass, providing strong evidence that the mass was indeed the inciting cause of the hyporexia and vomiting. The hamartoma from a calf 4 did not present clinical signs that could be attributed to the hamartoma. As that animal was euthanased, no follow-up history is available.
Usually the term hamartoma implies the presence of the lesion at birth. Their growth was initially thought to be liked to maturation of the animal but subsequently thought to be independent which explains why clinical signs can be seen later in life. 27 The age of onset of clinical signs and diagnosis (11-years) is in contrast to previous reports of hamartoma in the cat which have a median age of diagnosis of 7 months, range 4–16 months. 1,8,22–24 We postulate that the hamartoma reported herewith may have been either acquired or present at birth. In the event of the latter subsequent growth of the hamartoma to a size sufficient to cause clinical signs was either very slow or more likely occurred later in life potentially in response to an unknown stimulus.
The prevalence of gastric hamartoma is unknown within the feline population, although from the absence of case reports from animals in general and cats in particular, we consider them extremely rare. The possibility that gastric hamartomas may exist within the feline population but do not cause clinical signs remains, particularly if the anatomical location is in an area of the stomach such as the fundus, where pyloric or lower gastro-esophageal function is not compromised. It is also possible that other cases of feline gastric hamartoma have occurred but that these cases have been euthanased (based on an assumed diagnosis of gastric neoplasia) or has not been submitted for histological examination.
Exploration of the abdominal cavity was considered justified as it allowed greater evaluation of the extent of the mass and could potentially allow surgical excision or at least incisional biopsy of the mass. Endoscopic excision of a gastric polyp has been reported in a dog 28 but was not performed in this case due to the close proximity of the mass to the gastro-esophageal sphincter.
In conclusion, gastric hamartoma in the cat should be considered as a potential differential diagnosis for presenting clinical signs of hyporexia and vomiting with the presence of a gastric mass. Provided surgical excision can be achieved without interference with anatomical or physiological sphincters, based on the experience of this single case report, surgical excision can be associated with an excellent prognosis in the short and medium term.
Acknowledgements
We acknowledge Dr Patricia Shea for referring this case; and Drs Kelvin Kow and Jana Gordon, all students, house officers and technicians at The College of Veterinary Medicine, Oregon State University for their help during the investigation and treatment of this case.
References
- 1.Parkes J.D., Kline K.L., Riesedel E.A., et al. A vascular harmartoma arising from the cervical spine of a cat, J Fel Med Surg 11, 2008, 724–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van der Gaag. Gastrointestinal tumors in dogs and cats: a retrospective study. Abstract. Proceedings of the 15th Meeting of the European Society of Veterinary Pathology, Sassari-Alghero, Italy.
- 3.Ogilvie G.K., Moore A.S. Managing the Veterinary Cancer Patient: A Practice Manual 355–357, 1995, Veterinary Learning Systems: Trenton, NJ, 280–290. [Google Scholar]
- 4.Yamaguchi M., Machida N., Mitsumori K., et al. Smooth muscle hamartoma of the abomasums of a calf, J Comp Path 130, 2004, 66–69. [DOI] [PubMed] [Google Scholar]
- 5.Zikes C.D., Spielman B., Shapiro W., et al. Gastric extramedullary plasmacytoma in a cat, J Vet Intern Med 12, 1998, 381–383. [DOI] [PubMed] [Google Scholar]
- 6.Kumar V, Cotran RS, Robbins SL. Neoplasia, genetic and pediatric disease. In Basic Pathology. 5th edn. Kumar V, Cotran RS, Robbins SL, eds. Philadelphia. WB Saunders, 1992: 167–168. [Google Scholar]
- 7.Matos A.J.F., Duarte S., Lopes C., et al. Splenic hamartomas in a dog, Vet Rec 161, 2007, 308–310. [DOI] [PubMed] [Google Scholar]
- 8.Stalin C.E., Granger N., Jeffery N.D. Cerebellar vascular hamartoma in a British Shorthair cat, J Fel Med Surg 10, 2008, 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanders S.G., Bagley R.S., Gavin P.R., et al. Surgical treatment of an intramedulary spinal cord hamartoma in a dog, J Am Vet Med Assoc 221, 2002, 659–661. [DOI] [PubMed] [Google Scholar]
- 10.Corzo-Menendez N., White R.N., Whitelock R.G., et al. Vascular hamartoma within the flexor muscles of the left carpus in a dog, J Sm Anim Pract 42, 2001, 399–402. [DOI] [PubMed] [Google Scholar]
- 11.Machinda N., Katsuda S., Yamamura H., et al. Myocardial hamartoma in the right atrium in a dog, J Comp Path 127, 2002, 297–300. [DOI] [PubMed] [Google Scholar]
- 12.Gavin M.D., Henry J. Canine hepatic vascular hamartoma associated with ascites, J Am Vet Med Assoc 160, 1972, 864–866. [PubMed] [Google Scholar]
- 13.Njoku C.O., Hanry J.D., Cook J.E., et al. Pulmonary vascular hamartoma in a dog, J Am Vet Med Asso 161, 1972, 378–381. [PubMed] [Google Scholar]
- 14.Splitter G.A., Rawlings C.A., Casey H.W. Renal hamartoma in a dog, Am J Vet Res 33, 1972, 273–275. [PubMed] [Google Scholar]
- 15.Cook R.W. Hypothalamic hamartoma in a dog, Vet Path 14, 1977, 138–145. [DOI] [PubMed] [Google Scholar]
- 16.Dennis M.M., Ehrhart N., Duncan C.G., et al. Frequency of and risk factors associated with lingual lesions in dogs: 1,196 cases (1995–2004), J Am Vet Med Assoc 228, 2006, 1533–1537. [DOI] [PubMed] [Google Scholar]
- 17.Saunders G.K. Cranial nerve hamartoma in a dog, Vet Pathol 44, 2007, 253–254. [DOI] [PubMed] [Google Scholar]
- 18.Takahashi K., Maeda K., Nakamura S., et al. Pulmonary microcystic hamartoma in an adult dog, Vet Pathol 37, 2000, 499–501. [DOI] [PubMed] [Google Scholar]
- 19.Pearson G.R., Lane J.G., Holt P.E., et al. Chondromatous hamartomas of the respiratory tract in the dog, J Small Anim Pract 28, 1987, 705–712. [Google Scholar]
- 20.Brown P.J., Adam S.M., Wooton P.R., et al. Harmartomatous polyps in the intestine of two dogs, J Comp Path 110, 1994, 97–102. [DOI] [PubMed] [Google Scholar]
- 21.Smith S.H., Van Winkle T. Cerebral vascular hamartomas in five dogs, Vet Path 38, 2001, 108–112. [DOI] [PubMed] [Google Scholar]
- 22.Drolet R., Phaneuf J.B. Pulmonary chondromatous hamartoma in a young cat, Vet Rec 3, 1983, 541–542. [PubMed] [Google Scholar]
- 23.Wang F., Liang S.L., Chen G.H., et al. Unilateral concurrence of pyelocaliceal diverticula and intracapsular angiomyolipoma in the kidney of a cat, J Vet Diagn Invest 13, 2001, 167–169. [DOI] [PubMed] [Google Scholar]
- 24.Padgett S.L., Tillson D.M., Henry C.J., et al. Gingival vascular hamartoma with associated paraneoplastic hyperglycemia in a kitten, J Am Vet Med Assoc 210, 1997, 914–915. [PubMed] [Google Scholar]
- 25.Iishi H., Tatsuta M., Okuda S. Clinicopathological features and natural history of gastric hamartomatous polyps, Digest Dis Sci 34, 1989, 890–894. [DOI] [PubMed] [Google Scholar]
- 26.Portale T.R., Mosca F., Vicari S., et al. Myoepithelial hamartoma of the stomach simulating a gastric carcinoma. A case report, Tumori 93, 2007, 220–222. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy P.C., Miller P.B. The female genital system. Jubb K.V.F., Kennedy P.C., Palmer N. Pathology of Domestic Animals, 5th edn, 1993, Saunders Harcourt Brace Jovanovich, Academic Press: San Diego, CA, 553–781. [Google Scholar]
- 28.Tappin S.W., Brissot H. Endoscopic excision of a gastric polyp causing intermittent pyloric obstruction in a dog, Vet Rec 165, 2009, 379–380. [DOI] [PubMed] [Google Scholar]