Abstract
One hundred and fifty-three cats undergoing surgery in seven veterinary practices in Great Britain were studied. They were randomly allocated to receive either 10–20 μg/kg buprenorphine or 0.4 mg/kg butorphanol with acepromazine before anaesthesia with propofol, Saffan or thiopentone and isoflurane or halothane. Routine monitoring was undertaken. Pain and sedation were assessed blind using a four point (0–3) simple descriptive scale (SDS) at 1, 2, 4, 8 and 24 h. Pain and sedation data were compared using non-parametric statistical tests and continuous data using t tests or analysis of variance (ANOVA). Anaesthesia and surgery were uneventful, and cardiorespiratory data were within normal limits. After surgery, overall, more cats had pain score 0 after buprenorphine and more had pain score 3 after butorphanol (P=0.0465). At individual time points, more cats had lower pain scores after buprenorphine at 2 (P=0.040) and 24 (P=0.036) h. At 24 h 83% after buprenorphine and 63% after butorphanol had pain score 0 (P<0.04). Buprenorphine provided better and longer lasting postoperative analgesia than butorphanol.
Cats are now one of the most popular pets; in the UK alone, the population was 7.2 million in 2008. 1 Most pet cats are neutered, hence, even without any other treatment, millions of cats undergo at least one surgical procedure. Unfortunately, cats still receive less treatment for perioperative pain compared with dogs, even after similar surgery. 2–8 Recently the need for analgesia in cats has become better acknowledged, and many clinical and research studies have been undertaken to address the deficiency. These have been reviewed in the last few years. 9–11
Mu (OP3) opioid receptor agonists are generally accepted as providing the best analgesia, and morphine is still regarded as the gold standard analgesic. 12 Myths about morphine-induced mania in cats have prevented wide use of morphine in this species, in spite of clear demonstration that, at doses used clinically in other species, morphine is both effective and does not cause mania. 13 However, in at least dogs and man, some of morphine's analgesic effect is due to production of morphine metabolites, particularly morphine 6 glucuronide 14 and, as cats appear to have limited ability to produce such metabolites 15 , this may result in less effective morphine analgesia in this species. In addition, morphine frequently causes vomiting 13,16 and, overall, alternative opioids may result in better analgesia in cats.
Buprenorphine, usually regarded as a partial mu (OP3) agonist opioid, 17 was originally developed in the 1970s for treatment of drug addicts as well as for analgesia. 18 It was first investigated in veterinary patients in the 1980s in a study of postoperative analgesia in dogs. 19 Buprenorphine has since become the most popular opioid used in small animal practice in Great Britain 4 and is also widely used in the rest of Europe, Australia and South Africa. 3,5 It now holds market authorisation for dogs and cats in Great Britain and it has been widely used in these and many other species for decades. 20 In laboratory animals buprenorphine has been shown to have a ‘bell shaped’ dose–response curve 21 indicating that higher doses decrease the analgesic effect. This has led to label restrictions on the dose and dosing interval, but serious clinical relevance has never been demonstrated. 9
Butorphanol is a kappa-opioid (OP2) receptor agonist that has held market authorisation for analgesia in cats for a number of years. It is also commonly used in combination with α2 agents and ketamine for anaesthesia in this species, 22 but there are no published data reporting analgesic performance of the triple combination.
Although butorphanol has been used for many years, its analgesic effect for many clinical procedures has often been challenged. 23–25 Laboratory investigations suggest that buprenorphine may provide better and longer lasting analgesia; for example, a much longer duration of thermal antinociception was reported after intramuscular (IM) buprenorphine (up to 12 h) compared with morphine (up to 6 h) and butorphanol (up to 30 min). 16 Lascelles and Robertson 26 reported 1–2 h thermal antinociception after 0.1–0.4 mg/kg butorphanol. Mechanical antinociception was also only of 90 min duration. 27 Visceral analgesia (0.1 mg/kg IV or 0.4 SC) lasted 5–6 h in a model producing pain by inflation of a rectal balloon. 28 In contrast, one investigation differs from the rest and reported butorphanol-induced thermal antinociception of 8 h, longer than that produced by buprenorphine. 29
There have been numerous clinical studies investigating postoperative analgesia induced by either buprenorphine or butorphanol, but no comparisons between them. Three early investigations reported that buprenorphine produced better analgesia than morphine 30 oxymorphone 31 and pethidine. 32 More recent studies have demonstrated that buprenorphine, although providing analgesia, did not perform as well as non-steroidal anti inflammatory drugs (NSAIDs). 33,34 Most clinical studies of butorphanol indicate postoperative pain relief of a few hours duration. 35–38 Those reporting longer periods included repeat dosing. 39–41 As with buprenorphine, NSAIDs provided better postoperative analgesia than butorphanol. 38,42
The aim of this investigation was to compare the effects of buprenorphine and butorphanol in providing postoperative analgesia in cats after routine surgery in general practice. A secondary aim was to examine the effect of any higher and repeated doses of buprenorphine to elucidate the clinical relevance of the bell shaped dose–response curve. The study was conducted under ATC number 00117/2004. Some of the data were presented at the spring conference of the Association of Veterinary Anaesthetists in 2006. 43
Materials and methods
Animals
Domestic cats admitted for surgery at seven veterinary practices in the UK were enrolled. Informed consent was obtained prior to entry of the cat into the trial. At admission, the cats were randomly allocated to receive buprenorphine (Vetergesic; Alstoe Animal Health) or butorphanol (Torbugesic; Fort Dodge Animal Health) at label or proposed label doses. Drinking water was available ad libitum prior to premedication and after recovery from anaesthesia. Food was withheld for at least 6 h prior to surgery. All cats underwent a full clinical examination to ascertain their health status. Biochemistry and haematology were undertaken only if the clinical examination indicated an abnormality that required further investigation. No cats were excluded on grounds of health.
Premedication and anaesthesia
Cats were premedicated according to normal practice routine with acepromazine (aim 10–50 μg/kg) IM with or without atropine (0.01–0.1 mg/kg) and either buprenorphine (aim 10–20 μg/kg IM) or butorphanol (aim 0.4 mg/kg IM) approximately 60 min prior to induction of anaesthesia. No other analgesics were given. Anaesthesia was induced with propofol, Saffan (Schering Plough Animal Health) or thiopentone given intravenously (IV) to effect. Where the expected duration of anaesthesia was more than a few minutes the cat's trachea was intubated and anaesthesia was maintained with isoflurane or halothane in oxygen, with or without nitrous oxide, using an appropriate breathing system. Routine physiological monitoring, always including heart and respiratory rate, took place during anaesthesia and in the immediate postoperative period. Where equipment was available pulse oximetry and indirect arterial blood pressure measurement using a Doppler signal were undertaken. The return of signal on slow release of the cuff pressure was taken as mean arterial pressure (MABP). 44 Standard antibiotic and intravenous glucose and electrolyte solutions were given during anaesthesia as required by the individual patient.
Assessment of pain and sedation
Pre- and postoperative pain and sedation were assessed by an individual who did not know which opioid had been administered. Assessment was carried out using a four point simple descriptive scale (SDS) based on a previous study 45 (see Table 1). Pain was assessed before anaesthesia, and both pain and sedation were assessed at 1, 2, 4, 8, and 20–24 h after the end of anaesthesia (when maintenance agent switched off). All assessments for a single cat were carried out by one trained individual, but there were one or two assessors at each clinic.
Table 1.
Simple descriptive scales for sedation and analgesia.
| Score | Description | Behaviour |
|---|---|---|
| Pain scores | ||
| 0 | No pain | Cat is in normal posture, no response to wound palpation |
| 1 | Mild pain | Cat looks normal but responds to firm wound pressure |
| 2 | Moderate pain | Cat may look slightly abnormal, eg, hunched posture/coat staring and responds to gentle wound pressure |
| 3 | Severe pain | Cat looks miserable and cannot bear wound to be touched |
| Sedation scores | ||
| 0 | No sedation | Cat is in normal posture, normal response to contact with assessor |
| 1 | Mild sedation | Cat looks normal but slower response to assessor than normal |
| 2 | Moderate sedation | Cat appears sleepy and minimal response to assessor contact |
| 3 | Extreme sedation | Cat is asleep. No response to assessor |
During the assessment period, repeat administration of the same dose of the same analgesic was given if required, followed by rescue analgesia with carprofen 4.0 mg/kg subcutaneously (SC) if the repeat dosing did not alleviate the pain.
Statistical analysis
Power calculations were based on best estimates from experience of use of the two opioids in cats. Using pain scores at the 2-h time point as the primary variable, with an expected distribution of 0 pain score 55:45 (buprenorphine: butorphanol), at 80% power, 160 cats per group (320 total) were proposed, allowing 10% withdrawal.
The data were analysed by techniques suited to parametric and non-parametric data as appropriate. Student's t test was used to compare single numerical normally distributed data such as body weight. Non-parametric data such as type of surgery were compared using the χ2 test. Numerical data collected over time, such as pulse and respiratory rates, were compared using two-way repeated measures ANOVA followed by Dunnett's test when a significant difference was detected. Summary overall comparison of all postoperative pain and sedation scores from each group (comprising five scores for each cat×number of cats in the group) were compared using the Mann–Whitney test. The χ2 test with Bonferroni correction for multiple comparisons was used to compare the groups at each data point. GraphPad Prism was used for all analyses. P<0.05 was considered significant. Numerical data are presented as mean±SD unless otherwise stated.
Results
Interim analysis of the pain assessment data after 156 cats had been enrolled indicated a significant difference between the groups; hence, data collection was stopped before the planned 160 per group had been included. Post induction data were not collected from three of the 156 cats as one proved too aggressive to allow any assessments, and two others became aggressive after premedication (butorphanol group). Hence 153 cats were studied, 83 given buprenorphine and 70 given butorphanol.
There were no differences between the groups with respect to age, sex, body weight, breed or type and duration of surgery (Table 2). Most cats were domestic shorthairs undergoing neutering, but a number of other procedures was also included (Table 3).
Table 2.
Age, body weight, breed and sex of 153 cats studied. No significant differences between groups.
| Group | Buprenorphine n=83 | Butorphanol n=70 |
|---|---|---|
| Age | 23±45 months | 18±33 months |
| Mean±SD (range) | (4–222) (max 18.5 years) | 65%<10 months |
| (3–156) (max 13 years) | 70%<10 months) | |
| Body weight | 3.0±0.8 kg | 2.9±0.6 kg |
| Mean±SD (range) | (1.4–5.4) | (1.7–4.8) |
| Sex % distribution | 34 male | 23 male |
| 62 female | 70 female | |
| 2 neutered male | 0 neutered male | |
| 2 neutered female | 7 neutered female | |
| Breed % distribution | 82 DSH | 86 DSH |
| 11 DLH | 7 DLH | |
| 7 pedigree | 7 pedigree |
DSH=domestic shorthair, DLH=domestic longhair.
Table 3.
Surgical procedures performed in 153 cats studied. No significant differences between groups.
| Group | Buprenorphine n=83 | Butorphanol n=70 |
|---|---|---|
| Surgical procedures % distribution | 58 ovarohysterectomy | 70 ovarohysterectomy |
| 31 castration | 22 castration | |
| 5 excision of superficial mass | 3 excision of superficial mass | |
| 4 dental surgery | 4 dental surgery | |
| 1 orthopaedic | 0 orthopaedic | |
| 1 thyroidectomy | 1 thyroidectomy |
All cats received acepromazine (54–56 μg/kg) as part of the premedication, and atropine was also given to 17 cats in the buprenorphine group and 14 in the butorphanol group. The mean dose of buprenorphine was 13 μg/kg, but doses ranged between 8 and 21 μg/kg. Eighteen cats received more than 18 μg/kg. The mean dose of butorphanol was 0.4 mg/kg, although doses ranged between 0.31 and 0.57 mg/kg. Most cats received propofol and isoflurane, but Saffan, thiopentone and halothane were also used in both groups. There were no significant differences between the groups in the dose or identity of the anaesthetics used (Table 4). No attempt was made to assess the degree of sedation provided before anaesthesia. However, a number of comments were recorded describing the good quality of sedation in both groups at the time of induction. Two cats became aggressive in the butorphanol group, leading to a difficult induction.
Table 4.
Anaesthetic agents used for premedication, induction and maintenance. No significant differences between groups.
| Group | Buprenorphine n=83 | Butorphanol n=70 |
|---|---|---|
| Premedication Mean dose±SD (range) | Buprenorphine 13±4 μg/kg (8–21) Acepromazine 54±20 μg/kg (26–124) Atropine 43±14 μg/kg (16–58) n=17 | Butorphanol 0.4±0.03 mg/kg (0.31–0.57) Acepromazine 56±20 μg/kg (24–120) Atropine 47±16 μg/kg (16–76) n=14 |
| Induction of anaesthesia Mean dose±SD (range) | Propofol 7.6±2.6 mg/kg (3.5–15.9) n=64 Saffan 0.56±0.15 ml/kg (0.29–0.78) n=14 Thiopentone 14.9±3.2 mg/kg (11.0–18.8) n=5 | Propofol 7.5±2.2 mg/kg (3.6–12.2) n=52 Saffan 0.51±0.16 ml/kg (0.25–0.74) n=9 Thiopentone 16.5±4.3 mg/kg (11.0–25.8) n=9 |
| Maintenance of anaesthesia % | 69 isoflurane | 75 isoflurane |
| 10 halothane | 7 halothane | |
| 20 none | 17 none | |
| 1 further IV induction agent | 1 further IV induction agent |
Surgery lasted 20±12 and 23±14 min in the buprenorphine and butorphanol groups respectively (P>0.05). The time elapsed between administration of premedication and the end of anaesthesia (time 0) was 95±62 and 87±41 min after buprenorphine and butorphanol respectively (P>0.05).
Heart rate before anaesthesia was 166±32 beats per min (bpm) after buprenorphine and 163±37 bpm after butorphanol. During anaesthesia, the rate ranged from 158±33 and 149±33 bpm 5 min after induction to 153±33 and 146±29 bpm at 15 min in the buprenorphine and butorphanol groups, respectively. Respiratory rate before anaesthesia was 44±16 breaths per min after buprenorphine and 46±15 per min after butorphanol. During anaesthesia it remained at 26±10 breaths per min in the buprenorphine group and ranged from 25±9 per min 5 min after induction to 23±7 per min at 15 min in the butorphanol group. There were no significant differences between the groups in heart or respiratory rates during anaesthesia and there were no reports of apnoea or bradycardia.
After anaesthesia, heart and respiratory rates remained within normal limits, and no life threatening cardiovascular or respiratory events occurred. Heart rate was higher than before anaesthesia in both groups, reaching a maximum at 30 min after anaesthesia of 180±32 bpm after buprenorphine and 173±41 bpm after butorphanol. At 2 h heart rate was higher (172±25 bpm) in the buprenorphine group compared with the butorphanol group (162±28 bpm) (P=0.0362). Respiratory rate was lower than before anaesthesia in both groups, ranging from 30±9 and 32±9 breaths per min at 15 min to 34±12 and 35±17 breaths per min at 4 h in the buprenorphine and butorphanol groups, respectively. Respiratory rate was lower after buprenorphine than after butorphanol at 1 h (31±8 vs 35±12 breaths per min, P=0.0301) and 2 h (31±9 vs 36±13 breaths per min, P=0.0129).
Pulse oximetry was carried out in 37 cats in the buprenorphine group and 32 in the butorphanol group. Oxygen haemoglobin saturation (SpO2) was generally above 95%; only one cat, after buprenorphine, had a transient reading below 90% (89%) when breathing air. Indirect blood pressure monitoring was undertaken in 16 cats in the buprenorphine group and 11 in the butorphanol group. MABP ranged between 87±16 and 85±16 mmHg immediately after induction to 94±20 and 88±22 mmHg at 30 min in the buprenorphine and butorphanol groups, respectively. There were no significant differences between the groups in either SpO2 or MABP.
All cats recovered normally from anaesthesia, and there was no significant difference between the groups in the waning of sedation. Around one third of the cats were still heavily sedated (score 2) 1 h postoperatively but less than 10% had this score at 2 h. By 4 h after surgery, only one cat, after butorphanol, was still moderately sedated, and around 80% were fully conscious.
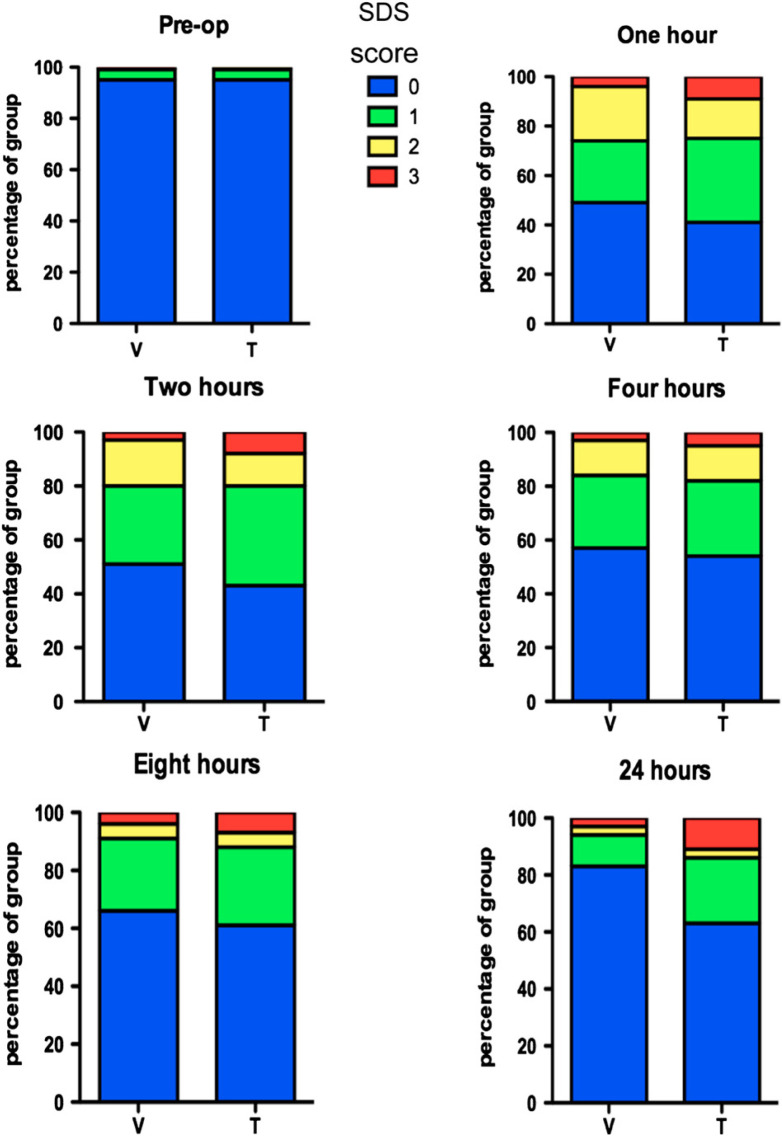
Preoperative pain scores in each group were not significantly different. Postoperatively, overall pain scores were significantly lower after buprenorphine than after butorphanol (Mann–Whitney test; P=0.0465). At individual time points, the difference between the groups was significant at 2 h (χ2 test; P=0.040) and 24 h (χ2 test; P=0.036) after surgery (Fig. 1). At all post surgical time points there were more cats in the buprenorphine with pain score 0 and more in the butorphanol group with pain score 3.
Fig 1.
Distribution of SDS pain scores in 153 cats before (pre-op) and after anaesthesia and surgery (1, 2, 4, 8 and 24 h) following premedication with buprenorphine (n=83) or butorphanol (n=70). Overall scores in the buprenorphine group were lower than in the butorphanol group (Mann–Whitney U, P=0.0465). Groups were significantly different at 2 h (χ2 test; P=0.040) and 24 h (χ2 test; P=0.036).
Further analgesia with either buprenorphine or butorphanol was required in 15% of the cats: 11 cats after buprenorphine and 12 after butorphanol (P>0.05). Repeat analgesia was required 6.4±6.3 (1–24) h after surgery in the buprenorphine group and 5.6±5.3 (1–14) h after surgery in the butorphanol group (P>0.05). The original dose of buprenorphine given to cats that required further analgesia was 0.013±0.004 (0.009–0.019) mg/kg. The time at which further analgesia was required was not related to the original dose given. Rescue analgesia with carprofen was required in only two cats, both in the butorphanol group, at 2 and 24 h after surgery.
Discussion
All the cats in the study were client-owned and highly representative of the normal pet cat population. They were typical of those normally admitted to general veterinary practice clinics for surgery in that the majority were young domestic shorthair cats undergoing neutering. However, a wide age range and some pedigree animals were also included, and a variety of other common types of surgery was undertaken in both treatment groups.
Anaesthetic and perioperative management was according to the normal practice in each clinic. Hence, a fair selection of anaesthetic protocols commonly used in feline practice were represented. Any drug with analgesic properties, such as the α2 adrenoceptor agonists, ketamine and any NSAID, was excluded so that the analgesic effects of the two opioids were not confounded by the co-administration of other classes of analgesics.
Pain assessment scales in cats have not yet been validated as in dogs, and a variety of systems have been used historically in clinical studies. Visual analogue scales (VAS) are arguably the most powerful tool, not least because parametric statistical analysis can be used. However, in a multi-centre study with several assessors, VAS scoring is inappropriate; it has been shown that variability among observers accounted for 32–36% of the total variability with VAS, but that agreement was fair if a SDS was used. 46 The SDS used in this study was able to detect a difference between the analgesic effects of buprenorphine and butorphanol in spite of the potential limitations of this scoring system.
The study demonstrated that IM buprenorphine (8–21 μg/kg) provided better analgesia than butorphanol (0.31–0.57 mg/kg) in the postoperative period. Although no placebo group was included that would demonstrate the extent of analgesia produced by either agent, buprenorphine produced pain relief over and above that of butorphanol, producing more pain free cats (score 0) and fewer maximum pain scores (score 3) than butorphanol. The significant difference at 24 h suggests that buprenorphine also provided pain relief of a considerably longer duration.
These clinical data are consistent with laboratory reports where most investigations demonstrate that buprenorphine produces antinociception for many hours. 16,47,48 There is no vomiting or dysphoria, and generally, cats become sedated, appearing happy and euphoric. In contrast, laboratory reports of butorphanol antinociception, with one notable exception, 29 describe short periods of antinociception; dysphoria is not uncommon 26–28 but this was seen in only two cats in this study.
A number of other clinical studies have investigated the postoperative effects of buprenorphine and butorphanol, and results are consistent with the laboratory data. Unfortunately, there are few placebo controlled studies and many include other analgesics such as ketamine, well known for its pre-emptive analgesic effect, making it difficult to assess the effect of the opioid. 36,38,49 Most reports indicate a relatively short duration of effect of butorphanol, with the period of analgesia lengthened by repeated dosing. 39–41 Clinical reports of buprenorphine suggest longer lasting analgesia 30 but this has not been rigorously examined under clinical conditions. The need for rescue analgesia might elucidate the duration of analgesia under clinical conditions. However, this study did not show any difference between the groups in the need for rescue analgesia. This was given at the discretion of the treating clinicians, when pain was considered severe, generally at scores 2 and 3, and did not highlight any differences between scores of 0 and 1. Hence, although there were more cats in the buprenorphine group with a score of 0, compared with the butorphanol group where more had score 1, none of these were given rescue.
The route of injection of buprenorphine appears influential, particularly when lower doses are used. Studies reporting that NSAIDs produced better pain relief than buprenorphine used either 10 μg/kg, the subcutaneous (SC) route, or both. 33,34 Smaller increases in thermal threshold have been reported after SC buprenorphine compared with IV and transmucosal dosing. 13,47 A buprenorphine patch maintained similar plasma concentrations to those reported after IV and buccal dosing, without the initial peak; however, thermal threshold did not increase. 50 This result was considered likely to arise from the hysteresis between plasma and effector site concentration. Presumably, adequate effector site concentration was not reached because there was never a sufficient concentration gradient to drive the drug to the effector site as would occur, for instance, after IV administration. The SC route is presumably intermediary between transdermal and IV in achieving a sufficient concentration gradient, resulting in less analgesic effect than IV.
One hour was allowed between premedication and induction of anaesthesia. This was within normal practice protocol, and allowed the full effects of acepromazine to develop. 51 As onset and duration of butorphanol appear to be sooner and shorter than of buprenorphine 16,26,47 this is likely to have influenced the postoperative analgesic effect. Repeat dosing of butorphanol may be more appropriate.
The dose of buprenorphine has commonly been restricted as a result of early studies in laboratory animals demonstrating a ‘bell shaped’ dose–response curve, where higher doses reduced the analgesic effect. 21 The doses in those reports were much higher than used in clinical pain treatment in dogs and cats, and there is no evidence that increasing the dose in cats several fold decreases the effect. Dose related increases in mechanical antinociception occurred when IV buprenorphine was increased from 10 to 40 μg/kg. 52 Thermal antinociception did not increase with increasing doses between 10 and 80 μg/kg, as many thresholds reached the safety cut off temperature, 52,53 but increasing doses did not decrease thermal antinociception. Data from the present study are consistent with this. Doses of at least 20 μg/kg were used successfully, and were not associated with higher pain scores or the need for rescue analgesia. Repeat analgesia with buprenorphine in the 11 cases which required it led to improved comfort, not a deterioration in pain relief. Overall data from a number of other investigations of buprenorphine analgesia in cats 30–34,54 suggest that doses greater than 10 μg/kg provide better analgesia than lower doses.
An analgesic given preoperatively for postoperative analgesia may affect the course of anaesthesia. Opioids commonly reduce volatile anaesthetic requirements and are usually beneficial in reducing the required dose of sedatives and injectable anaesthetics. They may however, exacerbate anaesthetic induced cardiopulmonary depression. Both buprenorphine and butorphanol have been widely used for premedication in cats and are suitable opioids for this purpose. Akkerdass et al 55 considered buprenorphine and acepromazine to be better than midazolam/ketamine or medetomidine prior to IV induction and isoflurane maintenance as cardiopulmonary characteristics were acceptable. Ilkiw et al 56 and Pypendop 57 reported that both butorphanol and buprenorphine reduced isoflurane MAC, but buprenorphine's effect was less than after butorphanol or morphine. Physiological data collected during anaesthesia and in the recovery period in the present study indicate that neither opioid had any deleterious effect on vital function and were safely used for premedication before general anaesthesia with a range of different anaesthetic agents. Heart and respiratory rates, MABP and SpO2 were well within the normal range for cats undergoing anaesthesia and surgery, and there were never any moments where adequate cardiovascular and respiratory function were in question. Only one cat, which was breathing air, had an oxygen haemoglobin saturation reading of 89% after buprenorphine. This was transient, and is only marginally below the accepted minimum of 90%. Anaesthesia will always cause some respiratory depression, and the decrease in respiratory rate seen in both groups was not unusual. There were minor but statistically significant differences between the two groups in both heart and respiratory rates postoperatively. However, all values were well within the normal range and no significant biological effect can be attributed to this statistical difference. Neither opioid appeared to result in unacceptably slow recovery, as most cats were only mildly sedated 2 h after the end of anaesthesia.
This investigation studied the effects of a single analgesic drug in order to elucidate their individual effects. However, although most cats were at least moderately comfortable, it is becoming widely accepted that use of more than one type of analgesic, multimodal analgesia, probably provides better postoperative pain control. A combination of buprenorphine and carprofen produced better pain relief after ovarohysterectomy in cats than either drug alone. 58 It is also well recognised that there is considerable individual response to opioid analgesics in cats 26,29,59 and each animal must be individually assessed to provide the best clinical pain management.
Conclusions
Buprenorphine 10–20 μg/kg provided better and longer lasting postoperative analgesia than butorphanol 0.4 mg/kg. Both opioids provided satisfactory premedication before anaesthesia using a range of commonly used agents.
Acknowledgements
To Alstoe Animal Health who funded the trial and approved the study design and manuscript, and to the veterinarians and nurses in the veterinary practices who collected the data.
References
- 1.Association P.F.M. http://www.pfma.org.uk/overall/pet-population-figures-2.htm2008.
- 2.Dohoo S., Dohoo I. Postoperative use of analgesics in dogs and cats by Canadian veterinarians, Can Vet J 37, 1996, 546–551. [PMC free article] [PubMed] [Google Scholar]
- 3.Watson A., Nicholson A., Church D. Use of anti-inflammatory and analgesic drugs in dogs and cats, Aust Vet J 74, 1996, 203–210. [DOI] [PubMed] [Google Scholar]
- 4.Lascelles B.D.X., Capner C.A., Waterman-Pearson A.E. Current British veterinary attitudes to perioperative analgesia for cats and small mammals, Vet Rec 145, 1999, 601–604. [DOI] [PubMed] [Google Scholar]
- 5.Joubert K.E. The use of analgesic drugs by South African veterinarians, J S Afr Vet Assoc 72, 2001, 57–60. [DOI] [PubMed] [Google Scholar]
- 6.Hugonnard M., Leblond A., Keroack S., Cadore J.L., Troncy E. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats, Vet Anaesth Analg 31, 2004, 154–163. [DOI] [PubMed] [Google Scholar]
- 7.Williams V.M., Lascelles B.D., Robson M.C. Current attitudes to, and use of, peri-operative analgesia in dogs and cats by veterinarians in New Zealand, N Z Vet J 53, 2005, 193–202. [DOI] [PubMed] [Google Scholar]
- 8.Joubert K.E. Anaesthesia and analgesia for dogs and cats in South Africa undergoing sterilisation and with osteoarthritis–an update from 2000, J S Afr Vet Assoc 77, 2006, 224–228. [DOI] [PubMed] [Google Scholar]
- 9.Robertson S.A., Taylor P.M. Pain management in cats – past, present and future. Part 2. Treatment of pain–clinical pharmacology, J Feline Med Surg 6, 2004, 321–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor P.M., Robertson S.A. Pain management in cats – past, present and future. Part 1. The cat is unique, J Feline Med Surg 6, 2004, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robertson S.A. Managing pain in feline patients, Vet Clin North Am Small Anim Pract 38, 2008, 1267–1290, vi. [DOI] [PubMed] [Google Scholar]
- 12.Gaynor J.S., Muir W.W. Handbook of veterinary pain management, 2002, Mosby: St Louis. [Google Scholar]
- 13.Steagall P.V., Carnicelli P., Taylor P.M., Luna S.P., Dixon M., Ferreira T.H. Effects of subcutaneous methadone, morphine, buprenorphine or saline on thermal and pressure thresholds in cats, J Vet Pharmacol Ther 29, 2006, 531–537. [DOI] [PubMed] [Google Scholar]
- 14.Smith T.W., Binning A.R., Dahan A. Efficacy and safety of morphine-6-glucuronide (M6G) for postoperative pain relief: a randomized, double-blind study, Eur J Pain 13, 2009, 293–299. [DOI] [PubMed] [Google Scholar]
- 15.Taylor P.M., Robertson S.A., Dixon M.J., et al. Morphine, pethidine and buprenorphine disposition in the cat, J Vet Pharmacol Ther 24, 2001, 391–398. [DOI] [PubMed] [Google Scholar]
- 16.Robertson S.A., Taylor P.M., Lascelles B.D., Dixon M.J. Changes in thermal threshold response in eight cats after administration of buprenorphine, butorphanol and morphine, Vet Rec 153, 2003, 462–465. [DOI] [PubMed] [Google Scholar]
- 17.Raffa R.B., Ding Z. Examination of the preclinical antinociceptive efficacy of buprenorphine and its designation as full- or partial-agonist, Acute Pain 9, 2007, 145–152. [Google Scholar]
- 18.Mello N.K., Mendelson J.H. Buprenorphine treatment of cocaine and heroin abuse. Cowan A., Lewis J.W. Buprenorphine, 1995, Wiley-Liss: New York, 241–287. [Google Scholar]
- 19.Taylor P.M., Houlton J.E.F. Post operative analgesia in the dog. A comparison of morphine, buprenorphine and pentazocine, J Small Animal Pract 25, 1984, 437–445. [Google Scholar]
- 20.Roughan J.V., Flecknell P.A. Buprenorphine: a reappraisal of its antinociceptive effects and therapeutic use in alleviating post-operative pain in animals, Lab Anim 36, 2002, 322–343. [DOI] [PubMed] [Google Scholar]
- 21.Cowan A., Lewis J.W., Macfarlane I.R. Agonist and antagonist properties of buprenorphine, a new antinociceptive agent, Br J Pharmacol 60, 1977, 537–545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brodbelt D.C. The confidential enquiry into small animal perioperative fatalities, 2006, University of London. http://www.rvc.ac.uk/AboutUs/Staff/dbrodbelt/Index.cfm. [Google Scholar]
- 23.Wagner A.E. Is butorphanol analgesic in dogs and cats?, Vet Med 94, 1999, 346–351. [Google Scholar]
- 24.Hornstein S.E., Stein R., Thompson D., Headly R., Dyer A. Questions analgesic protocols and conclusions of onychectomy study, J Am Vet Med Assoc 227, 2005, 707. [PubMed] [Google Scholar]
- 25.Bonagura J.D. Therapeutics for feline cardiomyopathy, Proc Eur Soc Feline Med Surg Birmingham 2007, April 2007, 1–4. [Google Scholar]
- 26.Lascelles B.D., Robertson S.A. Use of thermal threshold response to evaluate the antinociceptive effects of butorphanol in cats, Am J Vet Res 65, 2004, 1085–1089. [DOI] [PubMed] [Google Scholar]
- 27.Dixon M.J., Taylor P.M., Steagall P.V., Brondani J.T., Luna S.P. Development of a pressure nociceptive threshold testing device for evaluation of analgesics in cats, Res Vet Sci 82, 2007, 85–92. [DOI] [PubMed] [Google Scholar]
- 28.Sawyer D.C., Rech R.H. Analgesia and behavioral effects of butorphanol, nalbuphine, and pentazocine in the cat, J Am Anim Hosp Assoc 23, 1987, 438–446. [Google Scholar]
- 29.Johnson J.A., Robertson S.A., Pypendop B.H. Antinociceptive effects of butorphanol, buprenorphine, or both, administered intramuscularly in cats, Am J Vet Res 68, 2007, 699–703. [DOI] [PubMed] [Google Scholar]
- 30.Stanway G., Taylor P.M., Brodbelt D.C. A preliminary investigation comparing pre-operative morphine and buprenorphine for postoperative analgesia and sedation in cats, Vet Anaes Analg 29, 2002, 29–35. [DOI] [PubMed] [Google Scholar]
- 31.Dobbins S., Brown N.O., Shofer F.S. Comparison of the effects of buprenorphine, oxymorphone hydrochloride, and ketoprofen for postoperative analgesia after onychectomy or onychectomy and sterilization in cats, J Am Anim Hosp Assoc 38, 2002, 507–514. [DOI] [PubMed] [Google Scholar]
- 32.Slingsby L.S., Waterman-Pearson A.E. Comparison of pethidine, buprenorphine and ketoprofen for postoperative analgesia after ovariohysterectomy in the cat, Vet Rec 143, 1998, 185–189. [DOI] [PubMed] [Google Scholar]
- 33.Gassel A.D., Tobias K.M., Egger C.M., Rohrbach B.W. Comparison of oral and subcutaneous administration of buprenorphine and meloxicam for preemptive analgesia in cats undergoing ovariohysterectomy, J Am Vet Med Assoc 227, 2005, 1937–1944. [DOI] [PubMed] [Google Scholar]
- 34.Mollenhoff A., Nolte I., Kramer S. Anti-nociceptive efficacy of carprofen, levomethadone and buprenorphine for pain relief in cats following major orthopaedic surgery, J Vet Med A Physiol Pathol Clin Med 52, 2005, 186–198. [DOI] [PubMed] [Google Scholar]
- 35.Lin H.C., Benson G.J., Thurmon J.C., Tranquilli W.J., Olson W.A., Bevill R.F. Influence of anesthetic regimens on the perioperative catecholamine response associated with onychectomy in cats, Am J Vet Res 54, 1993, 1721–1724. [PubMed] [Google Scholar]
- 36.Smith J.D., Allen S.W., Quandt J.E., Tackett R.L. Indicators of post-operative pain in cats and correlation with clinical criteria, Am J Vet Res 57, 1996, 1674–1678. [PubMed] [Google Scholar]
- 37.Smith J.D., Allen S.W., Quandt J.E. Changes in cortisol concentration in response to stress and postoperative pain in client-owned cats and correlation with objective clinical variables, Am J Vet Res 60, 1999, 432–436. [PubMed] [Google Scholar]
- 38.Al-Gizawiy M.M., Rude E.P. Comparison of preoperative and postoperative butorphanol as postsurgical analgesics in cats undergoing ovariohysterectomy, Vet Anaes Analg 31, 2004, 164–174. [DOI] [PubMed] [Google Scholar]
- 39.Carroll G.L., Howe L.B., Slater M.R., et al. Evaluation of analgesia provided by postoperative administration of butorphanol to cats undergoing onychectomy, J Am Vet Med Assoc 213, 1998, 246–250. [PubMed] [Google Scholar]
- 40.Franks J.N., Boothe H.W., Taylor L., et al. Evaluation of transdermal fentanyl patches for analgesia in cats undergoing onychectomy, J Am Vet Med Assoc 217, 2000, 1013–1020. [DOI] [PubMed] [Google Scholar]
- 41.Gellasch K.L., Kruse-Elliott K.T., Osmond C.S., Shih A.N., Bjorling D.E. Comparison of transdermal administration of fentanyl versus intramuscular administration of butorphanol for analgesia after onychectomy in cats, J Am Vet Med Assoc 220, 2002, 1020–1024. [DOI] [PubMed] [Google Scholar]
- 42.Carroll G.L., Howe L.B., Peterson K.D. Analgesic efficacy of preoperative administration of meloxicam or butorphanol in onychectomized cats, J Am Vet Med Assoc 226, 2005, 913–919. [DOI] [PubMed] [Google Scholar]
- 43.Taylor P.M. A feline ABC: analgesia, buprenorphine, cat, Proc Assoc Vet Anaesth Liverpool, 2006, 126–130.
- 44.Grandy J.L., Dunlop C.I., Hodgson D.S., Curtis C.R., Chapman P.L. Evaluation of the Doppler ultrasonic method of measuring systolic arterial blood pressure in cats, Am J Vet Res 53, 1992, 1166–1169. [PubMed] [Google Scholar]
- 45.Slingsby L.S., Lane E.C., Mears E.R., Shanson M.C., Waterman-Pearson A.E. Postoperative pain after ovariohysterectomy in the cat: a comparison of two anaesthetic regimens, Vet Rec 143, 1998, 589–590. [DOI] [PubMed] [Google Scholar]
- 46.Holtan L.L., Scott E.M., Nolan A.M., Reid J., Welsh E., Flaherty D. Comparison of three methods used for assessment of pain in dogs, J Am Vet Med Assoc 212, 1998, 61–66. [PubMed] [Google Scholar]
- 47.Robertson S.A., Lascelles B.D., Taylor P.M., Sear J.W. PK-PD modeling of buprenorphine in cats: intravenous and oral transmucosal administration, J Vet Pharmacol Ther 28, 2005, 453–460. [DOI] [PubMed] [Google Scholar]
- 48.Steagall P.V., Taylor P.M., Brondani J.T., Luna S.P., Dixon M.J., Ferreira T.H. Effects of buprenorphine, carprofen and saline on thermal and mechanical nociceptive thresholds in cats, Vet Anaesth Analg 34, 2007, 344–350. [DOI] [PubMed] [Google Scholar]
- 49.Tobias K.M., Harvey R.C., Byarlay J.M. A comparison of four methods of analgesia in cats following ovariohysterectomy, Vet Anaesth Analg 33, 2006, 390–398. [DOI] [PubMed] [Google Scholar]
- 50.Murrell J.C., Robertson S.A., Taylor P.M., McCown J.L., Bloomfield M., Sear J.W. Use of a transdermal matrix patch of buprenorphine in cats: preliminary pharmacokinetic and pharmacodynamic data, Vet Rec 160, 2007, 578–583. [DOI] [PubMed] [Google Scholar]
- 51.Hall L.W., Clarke K.W. C.M.T. veterinary anaesthesia, 2001, WB Saunders: London. [Google Scholar]
- 52.Steagall P.V., Mantovani F.B., Taylor P.M., Dixon M.J., Luna S.P. Dose-related antinociceptive effects of intravenous buprenorphine in cats, Vet J, 2008. [DOI] [PubMed]
- 53.Slingsby LS, Taylor PM. Pilot dose response study for intravenous buprenorphine using thermal nociceptive threshold testing in cats. Spring Conference of the Association of Veterinary Anaesthetists. Bristol, 2008.
- 54.Curcio K., Bidwell L.A., Bohart G.V., Hauptman J.G. Evaluation of signs of postoperative pain and complications after forelimb onychectomy in cats receiving buprenorphine alone or with bupivacaine administered as a four-point regional nerve block, J Am Vet Med Assoc 228, 2006, 65–68. [DOI] [PubMed] [Google Scholar]
- 55.Akkerdass L.C., Sap R., Hellebrekers L.J. Cardiopulmonary effects of three different anaesthesia protocols in cats, Vet Q 23, 2001, 182–186. [DOI] [PubMed] [Google Scholar]
- 56.Ilkiw J.E., Pascoe P.J., Tripp L.D. Effects of morphine, butorphanol, buprenorphine, and U50488H on the minimum alveolar concentration of isoflurane in cats, Am J Vet Res 63, 2002, 1198–1202. [DOI] [PubMed] [Google Scholar]
- 57.Pypendop B.H., Pascoe P.J., Ilkiw J.E. Effects of epidural administration of morphine and buprenorphine on the minimum alveolar concentration of isoflurane in cats, Am J Vet Res 67, 2006, 1471–1475. [DOI] [PubMed] [Google Scholar]
- 58.Steagall P.V., Taylor P.M., Rodrigues L.C., Ferreira T.H., Minto B.W., Aguiar A.J. Analgesia for cats after ovariohysterectomy with either buprenorphine or carprofen alone or in combination, Vet Rec 164, 2009, 359–363. [DOI] [PubMed] [Google Scholar]
- 59.Taylor PM, Slingsby LS, Pypendop BH, Robertson SA. Variable response to opioid analgesia in cats. Proceedings of the Spring Conference of the Association of Veterinary Anaesthetists Conference. Paris, 2007.