Abstract
Objective
Carotid atherosclerosis is a chronic inflammatory disease, which is a major cause of ischemic stroke. The purpose of this study was to analyze the relationship between carotid atherosclerosis and novel inflammatory markers, including platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to neutrophil ratio (PNR), neutrophil to lymphocyte platelet ratio (NLPR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and aggregate index of systemic inflammation (AISI), in order to find the best inflammatory predictor of carotid atherosclerosis.
Method
We included 10015 patients who underwent routine physical examinations at the physical examination center of our hospital from January 2016 to December 2019, among whom 1910 were diagnosed with carotid atherosclerosis. The relationship between novel inflammatory markers and carotid atherosclerosis was analyzed by logistic regression, and the effectiveness of each factor in predicting carotid atherosclerosis was evaluated by receiver operating characteristic (ROC) curve and area under the curve (AUC).
Result
The level of PLR, LMR and PNR in the carotid atherosclerosis group were lower than those in the non-carotid atherosclerosis group, while NLR, NLPR, SII, SIRI and AISI in the carotid atherosclerosis group were significantly higher than those in the non-carotid atherosclerosis group. Logistic regression analysis showed that PLR, NLR, LMR, PNR, NLPR, SII, SIRI, AISI were all correlated with carotid atherosclerosis. The AUC value of NLPR was the highest, which was 0.67, the cut-off value was 0.78, the sensitivity was 65.8%, and the specificity was 57.3%. The prevalence rate of carotid atherosclerosis was 12.4% below the cut-off, 26.6% higher than the cut-off, and the prevalence rate increased by 114.5%.
Conclusion
New inflammatory markers were significantly correlated with carotid atherosclerosis, among which NLPR was the optimum inflammatory marker to predict the risk of carotid atherosclerosis.
Introduction
Cardiovascular was always the main cause of premature death and rising healthcare costs [1, 2]. From 1990 to 2019, the total number cardiovascular disease cases has nearly doubled globally, while the number of cases and deaths from peripheral artery disease tripled [3]. Atherosclerosis, a major pathological process in most cardiovascular diseases, which may occuar as early as childhood and remain latent in the body for a long time [4]. Early detection of arterial disease in seemingly healthy individuals focuses on the peripheral arteries, especially the carotid arteries [5]. In clinical practice, carotid atherosclerosis (CAS) is the earlier and most easily detected form of atherosclerosis. Besides, carotid atherosclerotic plaque is an independent risk factor for stroke and coronary heart disease [6]. Atherosclerosis is widely recognized as a chronic inflammatory disease of the blood vessels caused by the accumulation of low density lipoprotein cholesterol [7]. Chronic inflammation is a low-grade, non-infectious, systemic inflammatory state that is associated with age, psychology, environment, lifestyle, and the resolution of acute inflammation [8]. Chronic inflammation is associated with endothelial dysfunction, leukocyte recruitment, transformation of monocytes into macrophages and eventually into foam cells, smooth muscle cell migration and other processes [7]. Chronic inflammation is involved in the whole process of the occurrence and development of atherosclerosis and is the core of atherosclerosis.
In clinical practice, peripheral blood cell count is often used as a predictor and evaluation factor for the severity of inflammation and treatment outcome of acute inflammatory diseases, such as lung infection and sepsis. Platelet to lymphocyte ratio (PLR), neutrophil to lymphocyte ratio (NLR), lymphocyte to monocyte ratio (LMR), platelet to neutrophil ratio (PNR), neutrophil to lymphocyte platelet ratio (NLPR), systemic immune-inflammation index (SII), systemic inflammation response index (SIRI), and aggregate index of systemic inflammation (AISI) are commonly used blood cell count derived values in clinical practice, also known as novel inflammatory markers. These novel inflammatory markers have repeatedly demonstrated their potential value in the early prediction and prognosis of cardiovascular disease [9–14]. However, the relationship between these readily available inflammation markers and atherosclerosis, especially carotid atherosclerosis, has not yet been clear, which nedds further research to be confirmed.
Therefore, this study aimed to explore the correlation between novel inflammatory markers and CAS, in order to find a better early warning indicator of clinical carotid atherosclerosis.
Method
Study design
This is a retrospective case-control study. All methods were carried out in accordance with relevant guidelines and regulations and no important aspects of the study have been omitted. Patients’ personal information is being kept confidentially. The study complies with the Declaration of Helsinki and the ethical approval of the study was obtained from the Ethics Committee of Union Hospital, Tongji Medical College, Huazhong University of Science and Technology at Sep. 4th 2023 ([2023]ID:0611). The ethics committee exempted the need for informed consent.
Population
In this study, we included 14,115 patients who underwent routine physical examinations at our physical examination center from January 2016 to December 2019. The first time we accessed the data was on September 10, 2023. The following were the inclusion criteria for this study: (1) Age>18 years; (2) People who have underwent carotid vascular ultrasound. The exclusion criteria of participants were as follows: (1) Receiving or being receiving anti-inflammatory therapy within 6 months; (2) Critically ill patients with unstable vital signs; (3) Have received or are receiving glucocorticoid therapy within 6 months; (4) Incomplete clinical data or incomplete personal information. After screening, a total of 10015 patients were included and divided into CAS group and non-CAS group according to diagnosis.
Collected clinical data and laboratory indicators and definition of inflammatory markers
Age, gender, height, and weight of the patient at admission were obtained from the hospital electronic system, and BMI was calculated according to BMI = weight/height ^2. Proper amount of venous blood was extracted by professional nurses with nursing qualification during the fasting period and sent to the laboratory for analysis to obtain white blood cell count (WBC), neutrophil count (NC), lymphocyte count (LC), monocyte count (MC), platelet (PLT), neutrophil percentage (NP), lymphocyte percentage (LP), monocyte percentage (MP), PLR, NLR, LMR, PNR, NLPR, SII, SIRI, AISI and biochemical index, such as aspartate aminotransferase (AST), triglyceride (TG), total cholesterol (TC), high density lipoprotein cholesterol (HDL-C), low density lipoprotein cholesterol (LDL-C), uric acid (UA), urea nitrogen (BUN), creatinine. The estimated glomerular filtration rate (eGFR) was calculated based on the modified MDRD equation. SIRI was defined as neutrophil count*monocyte count/lymphocyte count. SII was calculated using the formula: NLR*platele. AISI was calculated using the formula: (neutrophil count*monocyte count*platelet)/lymphocyte count. NLPR was calculated using the formula: neutrophil count*100/lymphocyte count*platelet. Comorbidity, smoking history and drinking history were obtained from the patient’s personal history.
Diagnostic criteria for carotid atherosclerosis
Carotid atherosclerosis is diagnosed in one of the following situations: (1) A clear history of atherosclerosis or revascularization treatment; (2) Carotid artery ultrasound showed atherosclerotic plaque or carotid intima-media thickness of 1.0mm or more [5].
Statistical analysis
Shapiro-Wilkstest is used to test whether the continuous variables obey normal distribution, and the continuous variables with normal distribution are represented by mean ± standard deviation (SD). Continuous variables that are not normally distributed are expressed as medians with interquartile range (IQR). The categorical variable is represented by number with percentages (%). ANOVA tests (conforming to normal distribution) and Kruskal-Wallis tests (non-conforming to normal distribution) were used for the differences between of continuous data among CAS and non-CAS. Chi-square test was used to compare the difference of Categorical data between patients with CAS and non-CAS. Logistic regression analysis was used to evaluate the correlation between various inflammatory indicators and CAS after adjusting factors such as age, gender, BMI, serum biochemical indicators and clinical diagnosis and other factors. In Logistic regression analysis, forward selection method was used to identify CAS risk factors. Finally, the efficiency of each inflammatory markers to CAS was evaluated by receiver operating characteristic (ROC) curve. P<0.05 was considered to be statistically significant. All statistical analyses and diagrams were done using SPSS (version 23.0) or R language.
Result
Clinical baseline data
A total of 10015 patients aged 18–94 were enrolled in the study, including 1910 (19.1%) in the CAS group. Table 1 shows the baseline characteristics between the two groups. There were no significant differences in BMI, PLR and dyshepatia between the two groups (P>0.05). While age, gender, WBC, NC, LC, MC, PLT, NP, LP, MP, NLR, LMR, PNR, NLPR, SII, SIRI, AISI, AST, TG, TC, HDL_C, LDL_C, BUN, creatinine, eGFR, UA, smoking history, drinking history, renaldysfuncyion, hyperuricemia, fatty liver, dyslipidemia, hypertension, diabetes and osteoporosis were significantly different between the two groups(P<0.05).
Table 1. Baseline characteristics between CAS and non-CAS.
| Non-CAS | CAS | P value | |
|---|---|---|---|
| N(%) | 8105(80.9) | 1910(19.1) | |
| Age,year | 43.21 ± 12.65 | 66.12 ± 12.07 | 0.000 |
| Male, N(%) | 5105(63.0) | 1605(84.0) | 0.000 |
| BMI, Kg/m2 | 23.80 ± 3.30 | 24.53 ± 3.19 | 0.148 |
| WBC | 6.11 ± 1.46 | 6.29 ± 1.59 | 0.000 |
| NC | 3.50 ± 1.09 | 3.78 ± 1.25 | 0.000 |
| LC | 2.08 ± 0.55 | 1.91 ± 0.56 | 0.000 |
| MC | 0.36 ± 0.12 | 0.39 ± 0.13 | 0.000 |
| PLT | 235.57 ± 54.26 | 212.40 ± 52.94 | 0.000 |
| NP | 56.69 ± 7.44 | 59.51 ± 7.82 | 0.000 |
| LP | 34.48 ± 7.05 | 31.04 ± 7.44 | 0.000 |
| MP | 5.95 ± 1.53 | 6.35 ± 1.67 | 0.000 |
| PLR | 120.07 ± 38.23 | 118.90 ± 41.34 | 0.239 |
| NLR | 1.76 ± 0.61 | 2.12 ± 0.91 | 0.000 |
| LMR | 6.19 ± 2.10 | 5.24 ± 1.93 | 0.000 |
| PNR | 72.54 ± 24.68 | 60.64 ± 20.90 | 0.000 |
| NLPR | 0.78 ± 0.31 | 1.06 ± 0.54 | 0.000 |
| SII | 418.42 ± 188.35 | 452.74 ± 237.00 | 0.000 |
| SIRI | 0.65 ± 0.36 | 0.86 ± 0.56 | 0.000 |
| AISI | 156.07 ± 104.87 | 186.33 ± 137.93 | 0.000 |
| TP, g/L | 74.86 ± 4.12 | 74.35 ± 4.40 | 0.000 |
| Albumin, g/L | 47.27 ± 2.54 | 46.30 ± 2.68 | 0.000 |
| Globulin, g/L | 27.58 ± 3.31 | 28.05 ± 3.77 | 0.000 |
| AGR | 1.74 ± 0.23 | 1.68 ± 0.25 | 0.000 |
| AST, U/L | 25.80 ± 19.48 | 24.48 ± 18.33 | 0.008 |
| TG, mmol/L | 1.58 ± 1.23 | 1.65 ± 1.09 | 0.016 |
| TC, mmol/L | 4.75 ± 0.86 | 4.84 ± 1.01 | 0.000 |
| HDL_C, mmol/L | 1.40 ± 0.34 | 1.37 ± 0.33 | 0.000 |
| LDL_C, mmol/L | 2.74 ± 0.70 | 2.84 ± 0.83 | 0.000 |
| BUN, mmol/L | 4.76 ± 1.21 | 5.65 ± 1.54 | 0.000 |
| Creatinine, umol/L | 71.46 ± 14.61 | 79.76 ± 19.64 | 0.000 |
| eGFR, mL/min/1.73m2 | 109.06 ± 22.75 | 93.81 ± 22.49 | 0.000 |
| Uric acid, mmol/L | 363.82 ± 96.91 | 375.34 ± 88.49 | 0.000 |
| Smoking history,N(%) | 2642(32.6) | 830(43.5) | 0.000 |
| Drinking history,N(%) | 1901(23.5) | 591(30.9) | 0.000 |
| Renaldysfuncyion,N(%) | 31(0.4) | 103(5.4) | 0.000 |
| Dyshepatia,N(%) | 83(1.0) | 13(0.7) | 0.166 |
| Dyslipidemia,N(%) | 5231(64.5) | 1402(73.4) | 0.000 |
| Hyperuricemia,N(%) | 4044(49.9) | 1096(57.4) | 0.000 |
| Fatty liver,N(%) | 2484(30.6) | 718(37.6) | 0.000 |
| Diabetes,N(%) | 339(4.2) | 297(15.5) | 0.000 |
| Osteoporosis,N(%) | 745(9.2) | 446(23.4) | 0.000 |
| Hypertension,N(%) | 1581(19.5) | 835(43.7) | 0.000 |
Continuous variables are presented as mean ± S.D. Categorical data are presented as number (percentages).
CAS, carotid atherosclerosis; BMI, body mass index; WBC, white blood cell; NC, neutrophil count; LC, lymphocyte count; MC, monocyte count; PLT, platelet; NP, neutrophil percentage; LP, lymphocyte percentage; MP, monocyte percentage; PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PNR, platelet to neutrophil ratio; NLPR, neutrophil to lymphocyte platelet ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation; TP, total protein; AGR, albumin-globulin ratio; AST, aspartate aminotransferase; TG, triglyceride; TC, total cholesterol; HDL_C, high density lipoprotein cholesterol; LDL_C, low density lipoprotein cholesterol; BUN, blood urea nitrogen; eGFR, estimated glomerular filtration rate.
Relationship between novel inflammatory markers and CAS
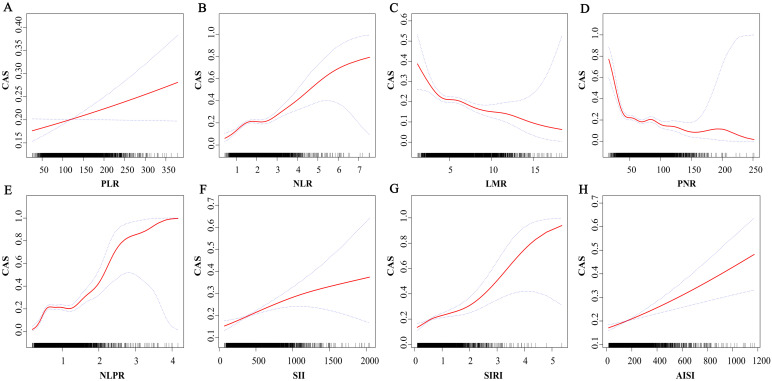
The relationship between novel inflammation markers and CAS was observed by spline smoothing plot, shown in Fig 1. LMR, PNR were negatively correlated with CAS, while PLR, NLR, NLPR, SII, SIRI, AISI, NLPR were positively correlated with CAS.
Fig 1. Spline smoothing plot between novel inflammation markers and carotid atherosclerosis.
A non- linear relationship between novel inflammation markers and carotid atherosclerosis after adjusting for age, gender, body mass index, aspartate aminotransferase, triglyceride, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, estimated glomerular filtration rate, uric acid, hypertension, diabetes, osteoporosis, fatty liver, smoking history, drinking history. CAS, carotid atherosclerosis; PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PNR, platelet to neutrophil ratio; NLPR, neutrophil to lymphocyte platelet ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation.
Logistic regression analysis of CAS
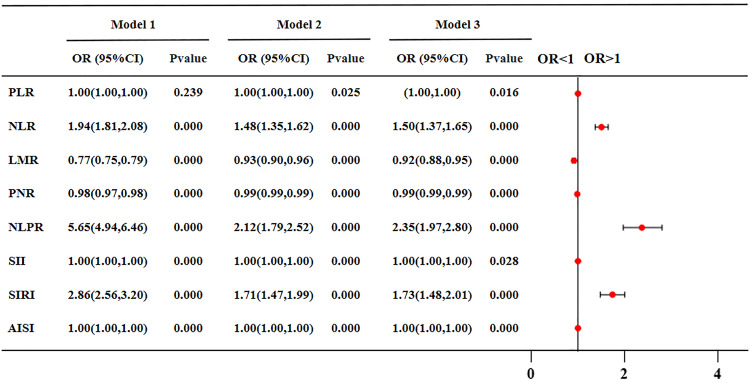
Univariate logistic regression analysis of the relationship between inflammatory markers and CAS showed the same difference as that between the two groups. Furthermore, after adjusting for many possible confounders such as age, sex, BMI, all inflammatory markers were statistically significant (P<0.05) with CAS. These infalmmatory markers have varying effects on CAS, and the risk value of NLPR is much higher than other markers (OR = 2.35). Their CAS risk value and forest plot were shown in Fig 2.
Fig 2. The ORs and 95% CI of novel inflammatory indicators to CAS risk by logistic regression.
Model 1 adjusted for none. Model 2 adjusted for age, sex, and body mass index. Model 3 adjusted for age, gender, body mass index, aspartate aminotransferase, triglyceride, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, estimated glomerular filtration rate, uric acid, hypertension, diabetes, osteoporosis, smoking history, drinking history. Forest plot of novel inflammatory markers for carotid atherosclerosis risk after adjusting for Model 3. PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PNR, platelet to neutrophil ratio; NLPR, neutrophil to lymphocyte platelet ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation.
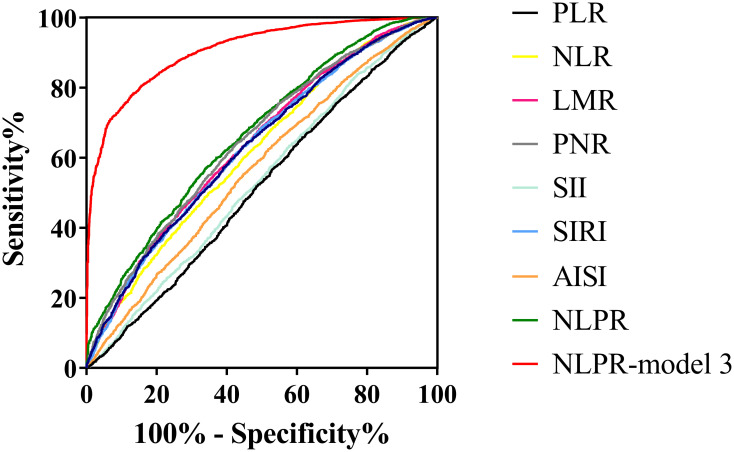
The efficacy of inflammatory markers to predict CAS was assessed by ROC curve. After adjusting for confounding factors, there were still statistical differences in all inflammatory markers. The ROC curve to evaluate the effectiveness of the above markers in CAS diagnosis is shown in Table 2 and Fig 3. The AUC values of NLR, SII, SIRI, AISI and NLPR were all in the range of 0.5–0.7. The highest AUC value for NLPR recognition of CAS is 0.67. And NLPR uses the adjusted predictive value of Model 3 to identify the AUC value of CAS as 0.91. The cut-off value calculated by Youden index was that NLPR was 0.78, that the sensitivity was 65.8%, and that the specificity was 57.3%. The prevalence rate of carotid atherosclerosis was 12.4% below the cut-off, 26.6% higher than the cut-off, and the prevalence rate increased by 114.5%.
Table 2. Evaluation of the predictive effect of the novel inflammatory markers on risk of CAS by ROC curves.
| AUC (95%CI) | Specificity | Sensitivity | Cut-off | Youden’s index | |
|---|---|---|---|---|---|
| PLR | 0.52 (0.50,0.53)a | 0.76 | 0.28 | 92.41 | 0.04 |
| NLR | 0.62(0.60,0.63)a | 0.69 | 0.47 | 1.96 | 0.16 |
| LMR | 0.64(0.62,0.65)a | 0.68 | 0.51 | 5.02 | 0.19 |
| PNR | 0.65(0.63,0.66)a | 0.64 | 0.57 | 61.24 | 0.21 |
| SII | 0.53(0.52,0.55)a | 0.84 | 0.23 | 571.56 | 0.07 |
| SIRI | 0.63(0.62,0.64)a | 0.69 | 0.50 | 0.72 | 0.19 |
| AISI | 0.57(0.55,0.58)a | 0.66 | 0.45 | 163.70 | 0.11 |
| NLPR | 0.67(0.65,0.68)a | 0.57 | 0.66 | 0.78 | 0.23 |
| Model 3-NLPR | 0.91(0.90,0.91)a | 0.80 | 0.85 | 0.18 | 0.65 |
CAS, carotid atherosclerosis; ROC, receiver operating curve; AUC, area under the curve; PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PNR, platelet to neutrophil ratio; NLPR, neutrophil to lymphocyte platelet ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation.
aP<0.05.
Fig 3. Receiver operating characteristic (ROC) curves of all inflammatory markers for identifying carotid atherosclerosis risk.
PLR, platelet to lymphocyte ratio; NLR, neutrophil to lymphocyte ratio; LMR, lymphocyte to monocyte ratio; PNR, platelet to neutrophil ratio; NLPR, neutrophil to lymphocyte platelet ratio; SII, systemic immune-inflammation index; SIRI, systemic inflammation response index; AISI, aggregate index of systemic inflammation.
Subgroup analyses between NLPR and CAS
In order to study the CAS risk and interaction of inflammatory markers in people with different clinicopathological characteristics. We further analyzed six subgroups of NLPR and CAS risk indicators (hyperuricemia, fatty liver, dyslipidemia, hypertension, diabetes, osteoporosis). The results showed that NLPR had the highest OR value in population without lipid metabolism abnormalities (Table 3). CAS were significantly associated with NLPR in all subgroups. The interaction among subgroups revealed that NLPR had an interaction with hyperuricemia, fatty liver, dyslipidemia, hypertension, diabetes and osteoporosis, and all of these disease subgroups significantly weakened the risk of NLPR for CAS.
Table 3. Association between NLPR and CAS in subgroups.
| N(%) | OR(95%CI) | P interaction | |
|---|---|---|---|
| Hyperuricemia | 0.000 | ||
| No | 4875 | 2.45(1.89,3.17) | |
| Yes | 5140 | 2.29(1.81,2.90) | |
| Fatty liver | 0.000 | ||
| No | 6813 | 2.59(2.07,3.25) | |
| Yes | 3202 | 1.98(1.49,2.63) | |
| Dyslipidemia | 0.000 | ||
| No | 3382 | 2.72(1.89,3.87) | |
| Yes | 6633 | 2.24(1.83,2.74) | |
| Hypertension | 0.000 | ||
| No | 7599 | 2.56(2.04,3.22) | |
| Yes | 2416 | 2.14(1.64,2.81) | |
| Diabetes | 0.003 | ||
| No | 9379 | 2.38(1.98,2.87) | |
| Yes | 636 | 2.26(1.37,3.73) | |
| Osteoporosis | 0.000 | ||
| No | 7755 | 2.55(2.08,3.13) | |
| Yes | 1191 | 1.91(1.31,2.76) |
Above subgroups were adjusted for age, gender, body mass index, aspartate aminotransferase, triglyceride, total cholesterol, high density lipoprotein cholesterol, low density lipoprotein cholesterol, estimated glomerular filtration rate, uric acid, hypertension, diabetes, osteoporosis, smoking history, drinking history.
CAS, carotid atherosclerosis; NLPR, neutrophil to lymphocyte platelet ratio.
Discussion
Novel inflammatory markers have been proven to be closely related to various diseases, and their easy availability has important clinical value. In this cross-sectional study, we analyzed the effect of multiple inflammatory markers on CAS, where NLR, SII, SIRI, AISI, NLPR remained significant after adjusting for multiple confounding factors. At the same time, this study further evaluated the value of various inflammatory markers in predicting CAS, among which NLPR had the largest AUC value in identifying CAS risk. Compared with other inflammatory markers, NLPR has a higher value for predicting CAS which may be the best inflammatory indicator for identifying atherosclerosis.
Chronic inflammation leads to atherosclerosis and cardiovascular disease. Neutrophils are the most numerous type of white blood cell and play a major role in inflammation. Although neutrophil count is primarily used as a biomarker for acute infection and inflammation, it has also been shown to accelerate chronic inflammation [15]. Monocyte-derived macrophages are important mediators in the atherosclerotic cascade and are associated with plaque formation through infiltration into the subendothelial layer. Subsequently, uptake of LDL-C complexes leads to the formation of foam cells. In addition to plaque formation, macrophages are involved in extracellular matrix remodeling by secreting pro-inflammatory cytokines and chemokines [16]. In our study, the number of neutrophils and monocytes increased in patients with CAS compared with non-CAS patients, indicating that the higher the number of neutrophils and monocytes, the higher the incidence of CAS. In contrast, lymphocytes slow the progression of atherosclerosis [14]. Our findings were the same, with a reduced number of lymphocytes in patients with CAS, suggesting that the higher the number of lymphocytes, the lower the incidence of CAS. Platelets play two main roles in atherosclerosis: platelets directly adhere to the blood vessel wall to promote plaque formation, and platelets then release inflammatory mediators and chemokines to promote leukocyte recruitment [17]. In our results, platelet counts were reduced in patients with CAS compared to non-CAS patients. The reason for this opposite situation may be that platelets have multiple roles in the formation of atherosclerosis, and in our study, the number of platelets alone was not significantly associated with the development of carotid atherosclerosis. In subsequent binary logistic regression analysis, there was no statistical difference between platelet count and CAS risk after adjusting for confounders.
In previous studies, we found that NLR, MLR, and PLR increase the risk of arterial stiffness [18]. In recent years, LMR, PNR, NLPR, SII, SIRI, AISI and other indicators have also proved their clinical significance in cardiovascular diseases. A small sample observational study showed that the novel inflammatory markers SIRI, NLR, and LMR were associated with CAS risk in middle-aged and older men [9]. PNR have also been shown to predict mortality in patients with ischemic stroke [10]. SII was also found to be a strong independent predictor of adverse outcomes in patients with acute coronary syndromes [12]. To find the best predictors of CAS, we included these markers in a larger population study at the same time. The results showed that the NLPR had the highest accuracy in identifying the risk of CAS. NLPR is the ratio of neutrophil to lymphocyte*platelet. Initially, NLPR was found to have predictive value in tumor prognosis [19, 20]. Subsequently, NLPR was also found to be associated with the prognosis of acute kidney injury, suppurative liver abscess, severe trauma, and COVID-19 [21–25]. The value of NLPR in cardiovascular disease is equally outstanding. Studies have shown that NLPR is an independent predictor of in-hospital mortality after acute type A aortic dissection [11]. A prospective cohort study found that NLPR was an independent predictor of adverse outcomes in patients with acute coronary syndrome, and patients with a higher NLRP had a higher incidence of major adverse cardiovascular events [26]. In this study, our results directly show that there is a significant correlation between NLPR and CAS, and the higher the level of NLPR, the higher the risk of CAS.
In statistical analyses of baseline characteristics, in addition to indicators of inflammation, we found a number of variables that were statistically different between the CAS and Non-CAS groups, including age, gender, AST, TG, TC, HDL_C, LDL_C, BUN, creatinine, eGFR, UA, smoking history, drinking history, renaldysfuncyion, hyperuricemia, fatty liver, dyslipidemia, hypertension, diabetes and osteoporosis. Undoubtedly, age is the number one risk factor for a wide range of chronic diseases and systemic chronic inflammatory states [8]. A significant increase in age in the CAS group was also found in our results. Some studies have shown that the incidence of atherosclerosis is higher in postmenopausal women compared to men [27]. Whereas, our results showed higher percentage of males in CAS group, which may be due to the uneven gender distribution in the total population of our medical examination, where males were significantly more than females. In addition to this, renaldysfuncyion, hyperuricaemia, fatty liver, dyslipidaemia, hypertension, diabetes, osteoporosis and history of smoking and alcohol consumption were all strongly associated with atherosclerosis [28–34]. And chronic inflammation can lead to cardiovascular disease, cancer, diabetes, chronic kidney disease, non-alcoholic fatty liver disease and many other diseases [8]. In our subsequent stratified analyses, we also analysed that there was a significant interaction between these diseases and NLPR, which together contributed to the development of CAS.
In this study, we evaluated the value of multiple inflammatory markers for the risk of CAS and found the best inflammatory marker that can be easily obtained to effectively identify the risk of CAS. In addition, the relatively large sample size ensures the accuracy and reliability of the results. Nevertheless, there are some limitations to this study. First, due to the cross-sectional design of the study, a causal relationship between inflammatory markers and CAS cannot be demonstrated, so more prospective studies are needed to confirm these findings. Second, because this study was a single-center study with a relatively narrow group of participants, the findings may not be well extrapolated to other populations. Finally, although we have carefully adjusted for potential confounding factors, it is difficult to rule out potential residual confounding.
Conclusion
Atherosclerosis is a chronic inflammatory disease of the walls of blood vessels. In this study, novel inflammatory markers have good predictive effects on carotid atherosclerosis, and NLPR has the highest predictive value. NLPR can be used as a potential predictor of carotid atherosclerosis.
Supporting information
(XLSX)
Data Availability
All relevant data are within the manuscript and its Supporting information files.
Funding Statement
The study was supported by the National Natural Science Foundation of China (Grant No.81571373, No.81601217, No.82001491), Natural Science Foundation of Hubei Province of China (Grant No. 2017CFB627), Health Commission of Hubei Province scientific research project (Grant No. WJ2021M247) and Scientific Research Fund of Wuhan Union Hospital (Grant No.2019). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Diseases GBD, Injuries C. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–22. doi: 10.1016/S0140-6736(20)30925-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mensah GA, Roth GA, Fuster V. The Global Burden of Cardiovascular Diseases and Risk Factors: 2020 and Beyond. Journal of the American College of Cardiology. 2019;74(20):2529–32. [DOI] [PubMed] [Google Scholar]
- 3.Roth GA, Mensah GA, Johnson CO, Addolorato G, Ammirati E, Baddour LM, et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. Journal of the American College of Cardiology. 2020;76(25):2982–3021. doi: 10.1016/j.jacc.2020.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McGill HC, McMahan CA, Herderick EE, Malcom GT, Tracy RE, Strong JP. Origin of atherosclerosis in childhood and adolescence. Am J Clin Nutr. 2000;72(5 Suppl):1307S–15S. doi: 10.1093/ajcn/72.5.1307s [DOI] [PubMed] [Google Scholar]
- 5.Piepoli MF, Hoes AW, Agewall S, Albus C, Brotons C, Catapano AL, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). European heart journal. 2016;37(29):2315–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bos D, Arshi B, van den Bouwhuijsen QJA, Ikram MK, Selwaness M, Vernooij MW, et al. Atherosclerotic Carotid Plaque Composition and Incident Stroke and Coronary Events. Journal of the American College of Cardiology. 2021;77(11):1426–35. doi: 10.1016/j.jacc.2021.01.038 [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MT, Fernando S, Schwarz N, Tan JT, Bursill CA, Psaltis PJ. Inflammation as a Therapeutic Target in Atherosclerosis. Journal of clinical medicine. 2019;8(8). doi: 10.3390/jcm8081109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, et al. Chronic inflammation in the etiology of disease across the life span. Nature medicine. 2019;25(12):1822–32. doi: 10.1038/s41591-019-0675-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Q, Che M, Shen W, Shao L, Yu H, Zhou J. Comparison of the Early Warning Effects of Novel Inflammatory Markers SIRI, NLR, and LMR in the Inhibition of Carotid Atherosclerosis by Testosterone in Middle-Aged and Elderly Han Chinese Men in the Real World: A Small Sample Clinical Observational Study. Am J Mens Health. 2023;17(3):15579883231171462. doi: 10.1177/15579883231171462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Geng L, Zuo L, Li H, Wang Y, Zhang Q, Ran J, et al. Association of platelet-to-neutrophil ratios with 1-year outcome and mortality in patients with acute ischemic stroke. Neurosci Lett. 2023;798:137016. doi: 10.1016/j.neulet.2022.137016 [DOI] [PubMed] [Google Scholar]
- 11.Guvenc O, Engin M. The role of neutrophil-lymphocyte platelet ratio in predicting in-hospital mortality after acute Type A aortic dissection operations. Eur Rev Med Pharmacol Sci. 2023;27(4):1534–9. doi: 10.26355/eurrev_202302_31396 [DOI] [PubMed] [Google Scholar]
- 12.Karadeniz F, Karadeniz Y, Altuntaş E. Systemic immune-inflammation index, and neutrophilto-lymphocyte and platelet-to-lymphocyte ratios can predict clinical outcomes in patients with acute coronary syndrome. Cardiovascular journal of Africa. 2023;34:1–7. doi: 10.5830/CVJA-2023-011 [DOI] [PubMed] [Google Scholar]
- 13.Aydin C, Engin M. The Value of Inflammation Indexes in Predicting Patency of Saphenous Vein Grafts in Patients With Coronary Artery Bypass Graft Surgery. Cureus. 2021;13(7):e16646. doi: 10.7759/cureus.16646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Song Y, Zhao Y, Shu Y, Zhang L, Cheng W, Wang L, et al. Combination model of neutrophil to high-density lipoprotein ratio and system inflammation response index is more valuable for predicting peripheral arterial disease in type 2 diabetic patients: A cross-sectional study. Frontiers in endocrinology. 2023;14:1100453. doi: 10.3389/fendo.2023.1100453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. 2013;13(3):159–75. doi: 10.1038/nri3399 [DOI] [PubMed] [Google Scholar]
- 16.Theofilis P, Oikonomou E, Tsioufis K, Tousoulis D. The Role of Macrophages in Atherosclerosis: Pathophysiologic Mechanisms and Treatment Considerations. Int J Mol Sci. 2023;24(11). doi: 10.3390/ijms24119568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nording HM, Seizer P, Langer HF. Platelets in inflammation and atherogenesis. Front Immunol. 2015;6:98. doi: 10.3389/fimmu.2015.00098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Wang R, Bai L, Liu Y, Liu L, He L, et al. The Leukocyte Subtype Counts and Ratios Can Effectively Predict the Risk of Arterial Stiffness Assessed by Cardio-Ankle Vascular Index: A Retrospective Study. Frontiers in cardiovascular medicine. 2021;8:671885. doi: 10.3389/fcvm.2021.671885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Albisinni S, Pretot D, Al Hajj Obeid W, Aoun F, Quackels T, Peltier A, et al. The impact of neutrophil-to-lymphocyte, platelet-to-lymphocyte and haemoglobin-to-platelet ratio on localised renal cell carcinoma oncologic outcomes. Prog Urol. 2019;29(8–9):423–31. doi: 10.1016/j.purol.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 20.Łochowski M, Łochowska B, Zawadzka I, Cieślik-Wolski B, Kozik D, Kozak J. Prognostic value of neutrophil-to-lymphocyte, platelet-to-lymphocyte and lymphocyte-to-monocyte ratio ratios in patients operated on due to non-small cell lung cancer. Journal of thoracic disease. 2019;11(8):3377–84. doi: 10.21037/jtd.2019.07.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang Z, Qiao Y, Wang D, Yan G, Tang C. Association Between Inflammatory Biomarkers and Contrast-induced Acute Kidney Injury in ACS Patients Undergoing Percutaneous Coronary Intervention: A Cross-sectional Study. Angiology. 2023:33197231185445. doi: 10.1177/00033197231185445 [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Zou Z, Zhang Y, Zhu B, Ning Y, Shen B, et al. Dynamics in perioperative neutrophil-to-lymphocyte*platelet ratio as a predictor of early acute kidney injury following cardiovascular surgery. Renal failure. 2021;43(1):1012–9. doi: 10.1080/0886022X.2021.1937220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li S, Yu S, Qin J, Peng M, Qian J, Zhou P. Prognostic value of platelet count-related ratios on admission in patients with pyogenic liver abscess. BMC Infect Dis. 2022;22(1):636. doi: 10.1186/s12879-022-07613-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chae YJ, Lee J, Park JH, Han DG, Ha E, Yi IK. Late Mortality Prediction of Neutrophil-to-Lymphocyte and Platelet Ratio in Patients With Trauma Who Underwent Emergency Surgery: A Retrospective Study. J Surg Res. 2021;267:755–61. doi: 10.1016/j.jss.2020.11.088 [DOI] [PubMed] [Google Scholar]
- 25.Fois AG, Paliogiannis P, Scano V, Cau S, Babudieri S, Perra R, et al. The Systemic Inflammation Index on Admission Predicts In-Hospital Mortality in COVID-19 Patients. Molecules. 2020;25(23). doi: 10.3390/molecules25235725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fan W, Wei C, Liu Y, Sun Q, Tian Y, Wang X, et al. The Prognostic Value of Hematologic Inflammatory Markers in Patients With Acute Coronary Syndrome Undergoing Percutaneous Coronary Intervention. Clinical and applied thrombosis/hemostasis: official journal of the International Academy of Clinical and Applied Thrombosis/Hemostasis. 2022;28:10760296221146183. doi: 10.1177/10760296221146183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu Y, Pechlaner R, Cai J, Yuan H, Huang Z, Yang G, et al. Trajectories of Age-Related Arterial Stiffness in Chinese Men and Women. Journal of the American College of Cardiology. 2020;75(8):870–80. doi: 10.1016/j.jacc.2019.12.039 [DOI] [PubMed] [Google Scholar]
- 28.Essop MR, Seedat F, Raal FJ. Dyslipidaemia in patients with chronic kidney disease—a neglected cardiovascular risk factor. South African medical journal = Suid-Afrikaanse tydskrif vir geneeskunde. 2023;113(11):35–40. doi: 10.7196/SAMJ.2023.v113i11.1089 [DOI] [PubMed] [Google Scholar]
- 29.Yan Z, Shao T. Chronic Inflammation in Chronic Kidney Disease. Nephron. 2024;148(3):143–51. doi: 10.1159/000534447 [DOI] [PubMed] [Google Scholar]
- 30.Wang Z, Xiao Y, Lu J, Zou C, Huang W, Zhang J, et al. Investigating linear and nonlinear associations of LDL cholesterol with incident chronic kidney disease, atherosclerotic cardiovascular disease and all-cause mortality: A prospective and Mendelian randomization study. Atherosclerosis. 2023;387:117394. doi: 10.1016/j.atherosclerosis.2023.117394 [DOI] [PubMed] [Google Scholar]
- 31.Zhang Y, Xia Z, Cai X, Su X, Jin A, Mei L, et al. Association of metabolic dysfunction-associated fatty liver disease with systemic atherosclerosis: a community-based cross-sectional study. Cardiovascular diabetology. 2023;22(1):342. doi: 10.1186/s12933-023-02083-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gu N, Liu Z, Wang Z, Shen C, Zhang W, Tian H, et al. Association Between Serum Uric Acid Levels and Neoatherosclerosis. International heart journal. 2024;65(1):4–12. doi: 10.1536/ihj.23-058 [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Yu Z. Mendelian randomization study on insulin resistance and risk of hypertension and cardiovascular disease. Scientific reports. 2024;14(1):6191. doi: 10.1038/s41598-023-46983-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Geng G, Li Z, Wang S, Yuan T, Quan G. Association between bone mineral density and coronary plaque burden in patients with coronary artery disease: a cross-sectional study using quantitative computed tomography. Coronary artery disease. 2024;35(2):105–13. doi: 10.1097/MCA.0000000000001316 [DOI] [PubMed] [Google Scholar]