Abstract
Growth of Escherichia coli is inhibited upon exposure to a large volume of a harmful solvent, and there is an inverse correlation between the degree of inhibition and the log POW of the solvent, where POW is the partition coefficient measured for the partition equilibrium established between the n-octanol and water phases. The AcrAB-TolC efflux pump system is involved in maintaining intrinsic solvent resistance. We inspected the solvent resistance of ΔacrAB and/or ΔtolC mutants in the presence of a large volume of solvent. Both mutants were hypersensitive to weakly harmful solvents, such as nonane (log POW = 5.5). The ΔtolC mutant was more sensitive to nonane than the ΔacrAB mutant. The solvent entered the E. coli cells rapidly. Entry of solvents with a log POW higher than 4.4 was retarded in the parent cells, and the intracellular levels of these solvents were maintained at low levels. The ΔtolC mutant accumulated n-nonane or decane (log POW = 6.0) more abundantly than the parent or the ΔacrAB mutant. The AcrAB-TolC complex likely extrudes solvents with a log POW in the range of 3.4 to 6.0 through a first-order reaction. The most favorable substrates for the efflux system were considered to be octane, heptane, and n-hexane.
There is increasing interest in culturing microorganisms in two-liquid-phase systems consisting of an aqueous medium and a hydrophobic organic solvent. This culture system is potentially advantageous for bioconversion of hydrophobic substrates with low solubility in water. Hydrophobic organic solvents can be toxic to microorganisms. When microbial cells are exposed to a large volume of an organic solvent with a log POW of 2 to 5, the solvent binds to the cells (2) and disturbs the structure of the cell membrane (3, 23). Here, log POW is the common logarithm of the partition coefficient (POW) measured for the partition equilibrium established between the n-octanol and water phases (10). It has been proposed that solvent toxicity is inversely correlated with the log POW of a solvent (6). The extent of the inhibition of growth of Escherichia coli cells by a solvent in a two-phase culture system seems to be inversely correlated with the log POW of the solvent (3).
On the other hand, it is reported that organic solvents with high log POW values are incorporated into erythrocytes and phospholipid liposomes more abundantly than those with low log POW values (15, 23). E. coli cells accumulate solvent in a manner positively dependent on the log POW and the concentration of the solvent in the medium. The empirical rule that there is an inverse correlation between the toxicity and the log POW of a solvent can be applied only to culture systems containing a large volume of solvent. The rule is based on the extent of growth inhibition, not on the toxicity strength of the solvent.
Several physiological and biochemical approaches have been applied in an effort to elucidate the mechanisms of microbial resistance to solvents (24). In recent years, it has been demonstrated that energy-dependent efflux is involved in organic solvent tolerance in gram-negative bacteria (7, 17, 18, 22). Cloned genes coding for components of efflux pumps belonging to the resistance/nodulation/cell division (RND) family (20) have been shown to serve to maintain solvent resistance in certain bacteria (4, 8, 11, 21, 25). Genetic evidence suggests that the AcrAB-TolC efflux pump, a member of the RND family (5, 14), is involved in solvent resistance of E. coli cells (4, 25). However, whether the AcrAB-TolC pump actually reduces the intracellular solvent concentration in E. coli cells exposed to a solvent remains to be demonstrated. In the present study, we examined solvent entry into acrAB and tolC mutants incubated in a two-phase culture system. We found that E. coli cells maintained a constant intracellular level of certain solvents. The specificity of solvent efflux by the AcrAB-TolC efflux pump was characterized by monitoring the release of solvents accumulated intracellularly.
In this report, we describe the characteristics of entry of solvent from the external milieu containing a large volume of the solvent, the release of intracellular solvent (AcrAB-TolC-dependent and -independent release), and solvent accumulation in E. coli.
MATERIALS AND METHODS
Bacterial strains and plasmids.
E. coli K-12 derivatives used to evaluate solvent resistance are summarized in Table 1. acrAB genes were cloned from strain W3110 [F− IN(rrnD-rrnE)1] (9). DH5α [supE44 ΔlacU169(φ80 lacZΔM15) hsdR17 recA1 endA1 gyrA96 thi-1 relA1] was used as the host for the construction of plasmids.
TABLE 1.
Bacterial strains and plasmids used
| Strain or plasmid | Genotype | Reference |
|---|---|---|
| E. coli strain | ||
| JA300 | F−thr leuB6 trpC1117 thi rpsL20 hsdS | 1 |
| JA300A | Same as JA300, but with ΔacrAB::cat | This study |
| JA300T | Same as JA300, but with ΔtolC | 4 |
| JA300TA | Same as JA300, but with ΔtolC ΔacrAB::cat | This study |
| JA300E | Same as JA300, but with emrB::kan | This study |
| JA300AE | Same as JA300, but with ΔacrAB::cat emrB::kan | This study |
| OLS111 | emrB::kan rpoS::tet | A. Xiong and A. Matin |
| Plasmid | ||
| pLTolC | 2.5-kb EcoRI-HindIII fragment containing the tolC gene in pMW119 oriented in the same direction as Plac | 4 |
| pLKAcrAB | 4.4-kb AvaI-EcoT22I fragment containing the acrAB genes in pMW219 oriented in the same direction as Plac | This study |
| pLKEmrAB | 3.2-kb PCR product containing the emrAB genes in pMW219 oriented in the same direction as Plac | This study |
| pLKYhiUV | 4.6-kb PCR product containing the emrAB genes in pMW218 oriented in the same direction as Plac | This study |
The low-copy-number vectors pMW119, pMW218, and pMW219 (GenBank accession no. AB005476 to 005478) (Nippon Gene, Tokyo, Japan), and the high-copy-number vector pBluescript II KS(+) (Toyobo Biochemical, Osaka, Japan) were used. Genes cat and kan were derived from plasmids pHSG399 (Takara Shyuzo, Osaka, Japan) and pUC4K (Pharmacia Biotech, Uppsala, Sweden), respectively. The temperature-sensitive plasmid pG+host4 (Appligene, Inc., Pleasanton, Calif.) was used to disrupt the acrAB genes in E. coli JA300.
Culture conditions.
The organisms were grown aerobically at 37°C in LBGMg medium consisting of 1% Bacto Tryptone (Difco Laboratories, Detroit, Mich.), 0.5% Bacto Yeast Extract (Difco), 1% NaCl, 0.1% glucose, and 10 mM MgSO4 (1). This medium was solidified with 1.5% (wt/vol) agar. Ampicillin (50 μg/ml) or kanamycin (50 μg/ml) was added to the medium when necessary.
Measurement of the organic solvent tolerance levels of E. coli.
Cultures of E. coli strains in LBGMg medium (optical density at 660 nm [OD660], 0.4 to 0.6) were diluted with 0.9% saline by serial 10-fold dilutions. Each suspension was plated on LBGMg agar. The surface of the agar was overlaid with a 3-mm-thick layer of an organic solvent. The approximate frequency at which the cells formed colonies on the agar was estimated after a 16-h incubation at 37°C.
Using the log POW calculation software, ClogP version 4.0 (Bio Byte Corporation, Claremont, Calif.), the log POW of each organic solvent was calculated by the addition rule (10).
Determination of the amount of solvent entering bacterial cells.
Solvent was added to a suspension of E. coli cells harvested during the late exponential phase of growth (OD660, 1.5 to 2.0). The suspension was centrifuged 30 min after addition of the solvent. The solvent layer, separated from the medium layer, was removed by aspiration. The medium was disposed of by decanting. The cell pellet was recovered and suspended in 1.5 ml of 0.9% NaCl–10 mM MgSO4. A 0.75-ml portion of the suspension was then transferred to an Eppendorf tube. The remaining portion was kept to measure the protein content. The 0.75-ml cell suspension was extracted with 0.75 ml of CHCl3 by vigorous shaking for 90 min at 20°C in a shaker (Handless shaker SHK-COCK; Asahi Technoglass, Tokyo, Japan).
The amount of solvent in the CHCl3 extract was measured using a gas-liquid chromatography apparatus (GC-9AM; Shimadzu, Kyoto, Japan). A sample of the CHCl3 extract was injected onto a column (3.2 mm by 3.1 m) of 25% polyethylene glycol 1500, Chromosorb W 60/80 AW · DMCS (Shimadzu), at 50°C or a column (3.2 mm by 2.1 m) of 15% Silicone D 200, Shimalite F 40/60 (Shimadzu), heated at 100°C. The column was eluted with N2 gas at a flow rate of 60 ml/min. The solvent was detected with a flame ionization detector.
Protein content.
Protein content was measured by the method of Lowry et al. (13).
DNA manipulation.
DNA manipulations, including preparation of plasmid DNA, agarose gel electrophoresis, restriction enzyme digestion, ligation, and transformation of E. coli cells, were carried out by standard methods.
Cloning of genes acrAB, emrAB, and yhiUV.
The region containing acrAB was amplified by PCR using W3110 chromosomal DNA as the template. The emrAB and yhiUV operons were cloned from JA300, also by PCR. The primers used were designed according to the sequence deposited in GenBank (accession numbers: acrAB, AE000152; emrAB, AE000353; and yhiUV, AE000427) as follows: a forward primer for acrAB, 5′-CGGTCATAACTTTCCAGACAGAGA-3′ (537 to 560 bp upstream of the initiation codon of acrA); a reverse primer for acrAB, 5′-AAAACTTACTGACCTGGACTTGCC-3′ (471 to 494 bp downstream of the stop codon of acrB); a forward primer for emrAB, 5′-AGCGGATCCGTCATCTCGCTCAA-3′ (110 to 132 bp upstream of the initiation codon of emrA); a reverse primer for emrAB, 5′-CAGGAATTCATATGAGTCTGATTGGTACG-3′ (298 to 326 bp downstream of the stop codon of emrB); a forward primer for yhiUV, 5′-AGTAGAATTCTTCGTTGCCCGAAT-3′ (189 to 210 bp upstream of the initiation codon of yhiU); and a reverse primer for yhiUV, 5′-ATTGGATCCTGAATGGTTAGCAGGAAA-3′ (46 to 72 bp downstream of the stop codon of yhiV). The underline shows the EcoRI or BamHI site introduced into each primer. A 4.4-kb XhoI-EcoT22I fragment containing the acrAB genes, a 3.2-kb BamHI-EcoRI fragment containing the emrAB genes, and a 4.6-kb BamHI-EcoRI fragment containing the yhiUV genes were obtained from the amplified products. The fragment containing the acrAB genes was inserted into the XhoI-PstI sites of vector pBluescript II KS(+). The cloned genes were recovered by XhoI-BamHI digestion of pAcrAB4 and inserted into the SalI-BamHI sites of vector pMW219. The BamHI-EcoRI fragment containing the emrAB genes and that containing the yhiUV genes were inserted into the BamHI-EcoRI sites of vector pMW219 and pMW218, respectively.
Disruption of the acrAB genes in JA300.
A Kanr cassette isolated by PstI digestion of pUC4K and vector pG+host4 digested with SacI were blunted and ligated. The resulting plasmid pGK619 is a pG+host4 derivative containing kan instead of erm. Plasmid pAcrAB4 was digested with BsmI and HpaI to obtain a truncated 2.6-kb fragment extending from the 156th codon of acrA to the 612th codon of acrB. This fragment was ligated with a cat gene isolated from pHSG399 digested with AccII. The resulting plasmid pΔAcrAB::cat is a pBluescript II KS(+) derivative containing the N-terminal region of acrA, a cat gene, and the C-terminal region of acrB tandemly. A part of this insert was amplified by PCR with pΔAcrAB::cat DNA as the template. The primers used were designed according to the deposited sequence as follows: a forward primer, 5′-GTGAATTCAAACAGGCCCAACAAG-3′ (corresponding to the 25th to 33rd codons of acrA), and a reverse primer, 5′-GGAAGGGCCCGTCATTGGTCAGGCCA-3′ (complementary to the 920th to 928th codons of acrB). The primer sequence was designed to contain an EcoRI or ApaI site, shown by the underlines. The amplified product was digested with EcoRI and ApaI and inserted into the sites of pGK619. The resulting plasmid pGK914 is a kanamycin-resistant pG+host4 derivative containing 385 bp of the central region of acrA, 1,057 bp of cat, and 937 bp of the central region of acrB.
JA300(pGK914) cells were resistant to chloramphenicol and kanamycin at 28°C but not at 42°C. Clones showing the resistance also at 42°C occurred at a low frequency in the JA300(pGK914) culture. These clones were candidates of cells in which pGK914 DNA was first inserted into the chromosome by a crossover event. Clones that were resistant to chloramphenicol (6.3 μg/ml) but sensitive to kanamycin (25 μg/ml) were obtained from one of the candidate clones.
The region containing acrAB was amplified by PCR using chromosomal DNA from one of the chloramphenicol-resistant and kanamycin-sensitive clones as the template. The primers used were the same as those used to construct pGK914 from pΔAcrAB::cat. Consistent with the expected values, the size of the amplified product from JA300 was 3.9 kb and those from JA300A and JA300TA were 2.4 kb. This acrAB disruptant was named JA300A. JA300TA, a tolC and acrAB disruptant, was constructed from JA300T(pGK914) in the same manner. It was confirmed that neither JA300A nor JA300TA produced a protein reactive with antiserum against AcrA (results not shown).
Materials.
The organic solvents used were of the highest quality available (Wako Pure Chemical Industries, Osaka, Japan). Antiserum against AcrA was a kind gift from H. Nikaido of the University of California, Berkeley. The emrB::kan disruptant OLS111 was kindly provided by A. Xiong and A. Matin of Stanford University.
RESULTS
Important contribution of acrAB and tolC to maintaining intrinsic solvent resistance of E. coli.
It has been reported that the acrAB genes are involved in the resistance of E. coli to n-hexane and cyclohexane (25). We found that tolC mutants are hypersensitive to various solvents, including n-hexane and cyclohexane (4). TolC protein functions as an outer membrane channel of efflux pumps, including the AcrAB system (5). To examine the role of the AcrAB-TolC efflux pump system in the solvent resistance mechanism of E. coli, the resistance of ΔacrAB and/or ΔtolC mutants was assessed by testing for growth on LBGMg agar overlaid with the individual solvents (Table 2).
TABLE 2.
Organic solvent resistance levels of acrAB and tolC mutants
| Straina | Plasmid | IPTG (1 mM) | Growth on agar overlaid withb:
|
|||||
|---|---|---|---|---|---|---|---|---|
| Decane (5.98) | Nonane (5.45) | Octane (4.93) | Heptane (4.40) | n-Hexane (3.87) | Cyclohexane (3.35) | |||
| JA300 | pMW219 | − | 100 | 100 | 100 | 100 | 50 | — |
| + | 100 | 100 | 100 | 100 | 50 | — | ||
| pLKAcrAB | − | 100 | 100 | 100 | 100 | 40 | — | |
| + | 100 | 100 | 100 | 100 | 100 | — | ||
| pLTolC | − | 100 | 100 | 100 | 100 | 30 | — | |
| + | 100 | 100 | 100 | 100 | 30 | — | ||
| JA300T | pMW219 | − | 100 | — | — | — | — | — |
| + | 100 | — | — | — | — | — | ||
| pLKAcrAB | − | 100 | — | — | — | — | — | |
| + | 100 | — | — | — | — | — | ||
| pLTolC | − | 100 | 100 | 100 | 100 | 50 | — | |
| + | 100 | 100 | 100 | 100 | 50 | — | ||
| JA300A | pMW219 | − | 100 | 50 | — | — | — | — |
| + | 100 | 50 | — | — | — | — | ||
| pLKAcrAB | − | 100 | 100 | 100 | 50 | 0.01 | — | |
| + | 100 | 100 | 100 | 100 | 30 | — | ||
| pLTolC | − | 100 | 0.01 | — | — | — | — | |
| + | 100 | — | — | — | — | — | ||
| JA300TA | pMW219 | − | 100 | — | — | — | — | — |
| + | 100 | — | — | — | — | — | ||
| pLKAcrAB | − | 100 | — | — | — | — | — | |
| + | 100 | — | — | — | — | — | ||
| pLTolC | − | 100 | 0.01 | — | — | — | — | |
| + | 100 | — | — | — | — | — | ||
| pLKAcrAB + pLTolC | − | 100 | 100 | 100 | 20 | 0.005 | — | |
| + | 100 | 100 | 100 | 100 | 30 | — | ||
Each strain carrying the plasmid was grown in LBGMg medium. Cells in the exponential phase of growth were plated on LBGMg agar. The agar was overlaid with the test solvent and incubated at 37°C overnight.
The log POW value of each solvent is shown in parentheses. The solvent resistance is represented by the frequency of colony formation, with that observed in the absence of any solvent taken as 100%. —, below 0.001%.
JA300 grew on the agar medium overlaid with any one of the solvents with a log POW value greater than or equal to 3.9. JA300T and JA300TA grew only on the agar overlaid with decane. JA300A grew in the presence of decane or nonane, although the number of colonies formed in the presence of nonane was low. Thus, the solvent resistance levels of these mutants were almost the same, but the ΔtolC mutants were slightly more sensitive to nonane than the ΔacrAB mutant.
The mutants were transformed with a plasmid containing acrAB or tolC under the control of Plac. JA300A became as resistant to solvents as the parent upon introduction of acrAB. Addition of IPTG (isopropyl-β-d-thiogalactopyranoside) improved the solvent resistance of JA300(pLKAcrAB) and that of JA300A(pLKAcrAB). On the other hand, the effect of tolC expression on solvent resistance was complicated. JA300T became as resistant to solvents as the parent by introduction of tolC. JA300T(pLTolC) was as resistant to solvent as JA300 in the presence and absence of IPTG. However, introduction of tolC into JA300A lowered the nonane resistance. JA300A(pLTolC) became sensitive to nonane in the presence of IPTG. These results suggest that the TolC channel overproduced in the absence of AcrAB allows entry of nonane from the external milieu. In the case of JA300TA, weak nonane resistance was restored by introduction of tolC but not acrAB. Overexpression of tolC in the presence of IPTG made JA300TA(pLTolC) sensitive to nonane. Thus, JA300TA(pLTolC) was as resistant to nonane as JA300A(pLTolC). The n-hexane resistance in JA300TA was restored only upon introduction of both genes, acrAB and tolC, although JA300TA(pLKAcrAB and pLTolC) was more sensitive to n-hexane or heptane than JA300. The resistance of this transformant to heptane or n-hexane was improved in the presence of IPTG. Thus, AcrAB and TolC are essential for E. coli to grow in the presence of a large volume of weakly harmful solvent, as reported previously (4, 25). It is evident that the AcrAB-TolC pump is the main system responsible for maintaining resistance to several solvents. However, it is likely that unidentified transporter systems depending on TolC confer weak nonane resistance on E. coli.
Putative contribution of genes encoding transporter systems to resistance to low-toxicity solvents in the acrAB mutant.
E. coli has several genes encoding putative or proven multidrug transporters (16, 20). Among them, the emrAB and yhiUV operons were cloned from the JA300 chromosome and inserted into a low-copy-number vector under the control of Plac. The nonane resistance of JA300A was improved by an increase in the copy number of emrAB or yhiUV (Table 3). In addition, JA300A acquired octane resistance upon introduction of these operons. The octane resistance was improved when expression of the operons contained in the plasmids was elevated in the presence of IPTG. The solvent resistance of JA300T was not improved upon introduction of the cloned operons (results not shown). These results indicate the operons conferred resistance to these weakly toxic solvents on E. coli, depending on TolC when overexpressed.
TABLE 3.
Improvement of the organic solvent tolerance level of an ΔacrAB mutant by an increase in the copy number of genes encoding transporters
| Plasmida | IPTG (1 mM) | Growth on agar overlaid withb:
|
||||
|---|---|---|---|---|---|---|
| Decane (5.98) | Nonane (5.45) | Octane (4.93) | Heptane (4.40) | n-Hexane (3.87) | ||
| pMW219 | − | 100 | 50 | — | — | — |
| pLKEmrAB | − | 100 | 100 | 1 | — | — |
| + | 100 | 100 | 100 | 0.001 | — | |
| pLKYhiUV | − | 100 | 100 | 0.001 | — | — |
| + | 100 | 100 | 1 | — | — | |
JA300A transformants carrying each plasmid were grown in LBGMg medium. The cells in the exponential phase of growth were plated on LBGMg agar. The agar was overlaid with the test solvent and incubated at 37°C overnight.
The log POW value of each solvent is shown in parentheses. The solvent resistance is represented by the frequency of colony formation, with that observed in the absence of any solvent taken as 100%. —, below 0.001%.
The Emr transporter system is known to extrude various drugs (12). To examine the contribution of the Emr transporter system encoded on the chromosome to solvent resistance, the emrB genes in JA300 and JA300A were disrupted by P1 transduction of emrB::kan from OLS111. The resulting emrB disruptants, JA300E and JA300AE, were as resistant to solvents as JA300 and JA300A, respectively (results not shown). These findings suggest that the nonane resistance observed in the case of the ΔacrAB mutant was not mediated only by the emr operon. Probably, several transporters are required to confer the nonane resistance on JA300A.
Entry of organic solvent into the E. coli tolC mutant in a two-phase culture system.
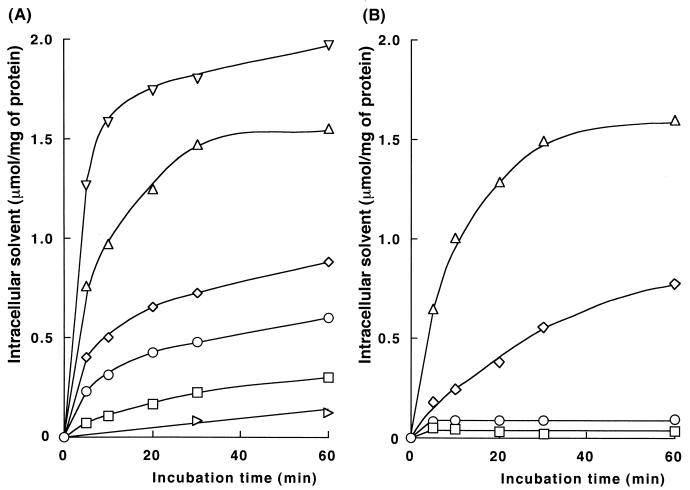
We previously observed that E. coli cells accumulate solvent in a two-phase culture system (2). E. coli cells possessing the AcrAB-TolC efflux pump system were used at that time. Results shown in Tables 2 and 3 suggest that solvent efflux from the cells occurs through TolC-dependent systems. In this study, we measured solvent entry into an E. coli ΔtolC mutant to avoid interference by the solvent efflux systems. The increase in the intracellular solvent level (Cc) was followed by measuring Cc periodically after the addition of each test solvent (10% [vol/vol]) to a culture of JA300 or JA300T in the late exponential phase of growth. Highly toxic solvents, such as toluene or p-xylene, rapidly entered the JA300T cells (Fig. 1). Weakly toxic solvents, such as nonane, entered the cells slowly. The initial rate of solvent entry and Cc after incubation with the solvent were correlated inversely with the log POW of the solvent in the two-phase culture system.
FIG. 1.
Entry of solvent into E. coli cells. JA300T (A) and JA300 (B) were each grown in 200 ml of LBGMg medium in a 2,000-ml Erlenmeyer flask rotated at 120 rpm. The cells in the late exponential phase (OD660, 1.5 to 2.0) were harvested by centrifugation (24°C, 4,000 × g, 6 min) and suspended in 25 ml of the medium. The suspension (5 ml) and 35 ml of the medium containing 4 ml of solvent were mixed in a 200-ml Erlenmeyer flask. The suspension was shaken at 160 rpm. Periodically, a portion of the culture was withdrawn and centrifuged (15°C, 6,000 × g, 1 min). The cells were suspended in 0.9% NaCl–10 mM MgSO4. The suspension was extracted with CHCl3, and the amount of solvent in the CHCl3 extract was measured. Each value shown is the mean value for two or three measurements. Symbols: ▿, p-xylene; ▵, cyclohexane; ◊, n-hexane; ○; heptane; □, octane; ▹, nonane.
Also in the case of JA300, the initial rate of solvent entry and Cc were correlated inversely with the log POW of the solvent. Cyclohexane entered the JA300 cells as rapidly as in the case of JA300T cells. n-Hexane, heptane, and octane entered the JA300 cells more slowly than in the case of JA300T cells. In particular, the Cc of heptane and that of octane were maintained at near-constant levels in JA300 cells, even after 60 min. These results indicate that a TolC-dependent transporter system contributed to keeping the Cc of heptane and octane at a low level, but not that of cyclohexane. These results were consistent with the observation that JA300 was resistant to heptane and sensitive to cyclohexane. The n-hexane resistance of JA300 depends on the culture conditions (3). It is clear that E. coli remains heptane resistant by keeping the Cc of heptane low.
Accumulation of organic solvent in E. coli incubated in a two-phase culture system.
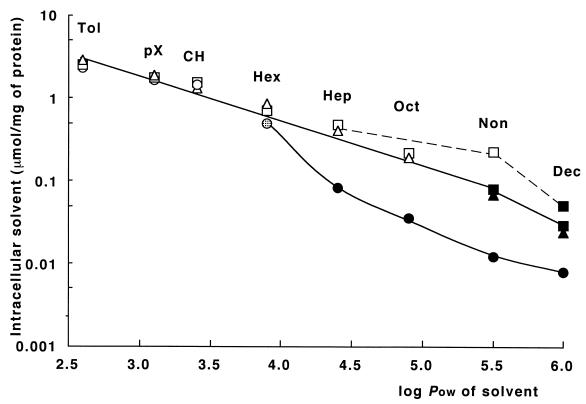
To assess the relative contribution of AcrAB and TolC to solvent resistance, we measured Cc in JA300, JA300A, and JA300T cells incubated in a two-phase system. Figure 1 shows that the Cc of each solvent was dependent on the incubation period. In particular, the entry of nonane was slow. Here, we show the Cc values obtained after exposure to each solvent for 30 min. Figure 2 shows the Cc of nonane or decane as measured 4 h after addition of the solvent. In addition, the figure shows the viability of the cells at the time when the Cc was determined.
FIG. 2.
Accumulation of solvents in E. coli cells in a two-phase culture system. JA300 (circles), JA300T (squares), and JA300A (triangles) were grown in LBGMg medium and exposed to solvent in the two-phase system, as described in the legend for Fig. 1. The solvents tested were toluene (log POW = 2.64) and p-xylene (log POW = 3.14) in addition to those shown in Table 2. The suspension was shaken at 160 rpm. A portion of the culture was centrifuged (15°C, 6,000 × g, 1 min) after 30 min. The amount of solvent accumulated in the cells was measured, as described in Fig. 1. A sample was also taken 4 h after the addition of n-nonane or n-decane in the case of JA300T (the result at 4 h is shown by a broken line). Each value shown is the mean value for three measurements. The viability of the cells was examined by plating a portion of the culture on LBGMg agar. The frequency of survivors is indicated by the following symbols: open symbol, less than 10−6; dotted symbol, 10−4; solid symbol, 1. The solubilities of these solvents in water, determined at 37°C, were 5.3, 1.4, 0.68, 0.21, 0.064, 0.034, and 0.0071 mM for toluene, p-xylene, cyclohexane, n-hexane, heptane, octane, and nonane, respectively. The solubility of decane was not determined because of its low solubility.
There was no difference in the Cc of toluene, p-xylene, or cyclohexane among the strains. All of the cells were killed within 30 min upon exposure to these solvents. When exposed to n-hexane, heptane, or octane, the Cc of each solvent was higher in JA300A and JA300T than that found in JA300. JA300A and JA300T accumulated the same Cc of each solvent. JA300A and JA300T were also killed in the presence of these solvents. When exposed to nonane or decane, the Cc of each of the two solvents was almost the same in JA300A and JA300T after 30 min. At this time, both strains were viable. The Cc of nonane or decane increased substantially in JA300T but only slightly in the other strains. After 4 h, nonane killed only JA300T, not the other strains. It can be concluded that the AcrAB-TolC pump is essential to reduce the Cc of n-hexane, heptane, or octane in E. coli cells exposed to these solvents. The Cc of nonane and that of decane are likely to be lowered partially by some TolC-dependent efflux system other than AcrAB-TolC. It is interesting that JA300A exposed to nonane or decane showed a lower Cc than that observed in JA300T. This is consistent with the finding that JA300T and JA300TA were more sensitive to nonane than JA300A (Table 2). These results support the view that in E. coli, efflux of nonane and decane also occurs via some TolC-dependent system other than AcrAB.
JA300T was killed by exposure to the solvents shown in Fig. 2, except for decane. Regardless of the difference among the strains, E. coli cells killed by exposure to the solvents showed a similar Cc for each solvent in the two-phase system. The Cc of solvents with a log POW in the range of 2.6 to 5.5 is given by the following equation: log Cc = 1.38 − 0.37 × log POW. This equation represents the solvent accumulation in E. coli cells killed upon exposure to a large volume of solvent in a two-phase system. On the other hand, almost all JA300 cells maintained viability in the two-phase system containing any solvent with a log POW in the range of 4.4 to 6.0. Cc corresponding to the linear part of the solvent accumulation curve for JA300 was lower than that found for JA300T exposed to such solvents. This part of the curve is given by the following equation: log Cc = 1.76 − 0.65 × log POW. This equation represents the solvent accumulation in viable JA300 cells growing in a two-phase system.
Release of intracellular organic solvents from E. coli cells.
Solvents probably diffuse into E. coli cells by partitioning between the cells and their external milieu. Therefore, the cells that had accumulated a solvent would release the solvent upon lowering the solvent concentration in the medium. It was supposed that the solvent would be released from the cells by passive diffusion and through the action of the solvent efflux pump.
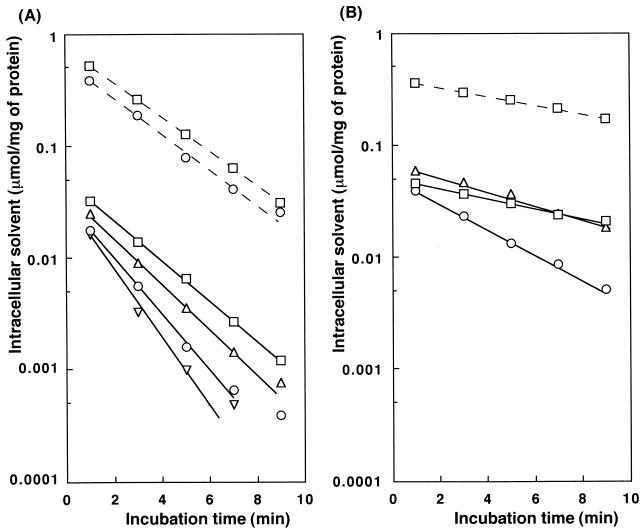
To observe the solvent efflux process, cells loaded with solvent were incubated in medium containing no solvent. Cell viability was maintained by loading the cells with the test solvent at a low Cc (0.01 to 0.1 μmol/mg of protein). It is reported that the Cc of a solvent is correlated with the concentration of the solvent in the medium (19). Cells loaded with solvent at a low Cc were prepared by incubation with a low concentration of the solvent. No strain was killed by the test solvent under these conditions. Release of the intracellular solvent from the cells was followed by periodic determination of Cc. Figure 3 shows typical examples of the release of n-hexane or heptane from JA300, JA300A, JA300T, and JA300(pLKAcrAB). It seems likely that each intracellular solvent was released from all strains through a first-order reaction, at least in the initial period. Assuming that the solvent is released from each cell by passive diffusion and active extrusion through a first-order reaction, the rate constants for the release of intracellular solvents were calculated for the viable cells (Table 4).
FIG. 3.
Release of intracellular solvent from E. coli cells. JA300 (○), JA300T (□), JA300A (▵), and JA300(pLKAcrAB) (▿) were loaded with n-hexane (A) or heptane (B), as described in the legend for Fig. 1. After incubation with the solvent for 30 min, the cell suspension (40 ml) was centrifuged (24°C, 6,000 × g, 1 min). The cells were suspended in 20 ml of LBGMg medium in a 50-ml Erlenmeyer flask and incubated at 37°C with shaking at 160 rpm. Time zero represents the time at which the cells were suspended in fresh medium. A portion of the cell suspension (2 ml) was withdrawn periodically and centrifuged (15°C, 6,000 × g, 1 min) at the times shown. The solvent in the cells was measured as described in the legend for Fig. 1. Solid and broken lines indicate the release of each solvent from cells in which Cc was low and high, respectively. The initial Cc was controlled by altering the volume of solvent added, as follows: for n-hexane, JA300, 0.2 or 4 ml; JA300T, 0.2 or 4 ml; JA300A, 0.2 ml; and JA300(pLKAcrAB), 0.25 ml; for heptane, JA300, 2 ml; JA300T, 0.1 or 2 ml; and JA300A, 2 ml. Each value shown is a typical example.
TABLE 4.
Rate constant for solvent release from E. colia
| Strain (plasmid) | Solvent release rate constant
|
||||
|---|---|---|---|---|---|
| Nonane | Octane | Heptane | Hexane | Cyclohexane | |
| JA300(pLKAcrAB) | NT | NT | NT | 0.696 | 0.755 |
| JA300 | 0.069 | 0.212 | 0.272 | 0.603 | 0.654 |
| JA300AB | 0.023 | 0.064 | 0.117 | 0.474 | NT |
| JA300T | 0.014 | 0.053 | 0.099 | 0.412 | 0.587 |
The rate constant for solvent release per minute was measured in the experiments shown in Fig. 3. NT, not tested.
The rate constant for each solvent was the highest in the case of JA300 and the lowest in the case of JA300T, among the cells loaded with solvent at low Cc. However, solvent release seemed to be retarded when the cells were loaded with solvent at high Cc. This retardation was clearly evident in the case of JA300 loaded with n-hexane loaded up to the level of 0.4 μmol/mg of protein, but not in the case of JA300T (Fig. 3A). Consequently, n-hexane was released at similar rates from JA300 and JA300T when the initial Cc of n-hexane was high. The membrane structure of JA300 is disordered under the conditions employed (3). Probably, the activity of RND family efflux pumps is lowered in the structurally disordered membrane. Therefore, as described below, we evaluated the relative contribution of AcrAB and TolC to solvent release on the basis of the results obtained with viable cells.
The rate constant for TolC-independent solvent release found in the case of JA300T was inversely dependent on the log POW of the solvent. The difference in the rate constants for release of the solvents among the strains is likely attributable to the difference in solvent efflux activity. The rate constants for solvent release via TolC were estimated by comparison of the rate constants found for JA300 and JA300T, as follows: nonane, 0.06/min; octane, 0.16/min; heptane, 0.17/min; hexane, 0.19/min; and cyclohexane, 0.07/min. The rate constants for solvent release via AcrAB were calculated as follows: nonane, 0.05/min; octane, 0.15/min; heptane, 0.16/min; and hexane, 0.13/min. Determining the solvent release rate revealed that AcrAB extrudes heptane most preferentially among the solvents tested. The rate constants for release of hexane and cyclohexane were improved by an increase in the copy number of acrAB, indicating that the AcrAB transporter indeed extrudes these solvents from E. coli cells.
DISCUSSION
Since a toluene-resistant strain of Pseudomonas putida was isolated (6), various mechanisms have been proposed to account for the solvent resistance of microbes. It has been shown that efflux pumps belonging to the RND family are important for solvent resistance in gram-negative bacteria (4, 8, 21, 25). The AcrAB-TolC efflux pump is involved in the solvent resistance of E. coli. We are interested in culturing microbes in a two-phase system containing a large volume of solvent. Our findings concerning solvent resistance, examined in the presence of a large volume of each solvent (Table 2), confirmed that the genes acrAB and tolC are essential to maintain the intrinsic level of resistance of E. coli to hydrophobic solvents, although it has been proposed that AcrAB functions preferentially in efflux of amphiphilic charged compounds (16). It is reported that tolC mutants are more sensitive to various antibiotics than acrAB mutants (5). Certain transporters other than AcrAB confer weak solvent resistance to E. coli (Tables 2 and 3), although we failed to identify the transporter.
Resistance to solvents with a log POW in the range of 3.9 to 5.5 is conferred on E. coli incubated in a two-phase culture system by the AcrAB-TolC pump encoded on the chromosome. Under these conditions, accumulation of solvents with a log POW in the range of 4.4 to 5.9 was reduced to a level one-sixth to one-tenth of that observed in the case of acrAB or tolC mutants (Fig. 1 and 2). n-Hexane (log POW, 3.9) is subtoxic to JA300 in the two-phase culture system.
The more polar solvents were more preferentially released by the TolC-independent process (Table 4), suggesting that this process involves passive diffusion of the solvent in response to the solubility of the solvent in the medium. Therefore, it is likely that the TolC-independent solvent release process does not occur substantially when the medium is saturated with the solvent in the presence of a large volume of the pure solvent. Alternatively, the solvent might be extruded by a TolC-independent process, although this possibility is not supported by the results of genetic analysis of solvent resistance (Table 2). The TolC-dependent process released octane, heptane, and n-hexane preferentially. These two solvent release processes differ in terms of solvent specificity. The AcrAB-dependent extrusion was responsible for the major portion of the TolC-dependent solvent release. A portion of the solvents is likely released through the TolC channel without any involvement of AcrAB. It is not likely that there is an AcrAB-dependent and TolC-independent process, considering that solvent accumulation was similar between JA300T and JA300TA.
In this study, we examined the features of solvent entry into E. coli cells containing no solvent and solvent release into the external milieu containing no solvent. It may be assumed that the influx and efflux of solvent are probably balanced in microbes growing in a two-phase culture system. We could not measure the rates of influx and efflux separately in the two-phase system. However, the Ccs of heptane, octane, nonane, and decane were maintained at low levels in JA300 (Fig. 2). We showed that the Cc of octane and that of heptane were constant (0.04 and 0.08 μmol/mg of protein, respectively) in JA300 for 60 min (Fig. 1), indicating that the influx and efflux of heptane or octane were balanced in JA300 incubated in the two-phase system. n-Hexane entered JA300 cells more slowly than it entered JA300T cells, although the rate increased gradually during the incubation period. The n-hexane resistance of JA300 is lowered at high cell density, probably because of a shortage of oxygen (17). It is supposed that influx and efflux of n-hexane are probably balanced in JA300 at low cell density in the two-phase system containing n-hexane (3). On the other hand, the activity of the AcrAB-TolC system in JA300 was insufficient to extrude cyclohexane from the cells in the two-phase system (Fig. 1). This is probably because of low activity, rather than the substrate specificity, because introduction of pLKAcrAB resulted in increased cyclohexane release from JA300 cells (Table 4).
It is reported that entry of toluene into P. putida S12 is lowered by some active efflux system (7). In that study, solvent entry was examined in a medium containing toluene below the saturation concentration. The reported Cc of toluene in P. putida S12 cells in which energy production was inhibited is one-tenth of that found in E. coli exposed to a large volume of toluene in this study. P. putida S12 might have an additional resistance mechanism to toluene other than the efflux system. Genes srpABC involved in toluene efflux have been cloned from P. putida S12. As a result of transformation with these genes, P. putida tolerant to 2.6 mM toluene became tolerant to 4.9 mM but not 5.6 mM toluene (8). The transformant cannot grow in a two-phase system containing toluene. Thus, the toxicity of the solvent is greatly dependent on the concentration. When JA300 was incubated in the presence of cyclohexane or p-xylene, the cell viability was dependent on the concentration of the solvent present (results not shown). Solvent entry is dependent on the concentration of the solvent in the medium (19). In this study, solvent entry was examined in E. coli cells incubated in a two-phase system. Therefore, the results described here show the features of solvent accumulation by E. coli cells in a two-phase system containing a large volume of solvent.
ACKNOWLEDGMENTS
This work was partially supported by a Grant-in-Aid for Scientific Research (no. 10450308) from the Ministry of Science, Education and Culture of Japan to R. Aono.
We thank H. Asako for his assistance. We thank H. Nikaido of the University of California, Berkeley, for the kind gift of antiserum against AcrA. We also thank A. Xiong and A. Matin of Stanford University for kindly providing the emrB::kan disruptant OLS111.
REFERENCES
- 1.Aono R, Aibe K, Inoue A, Horikoshi K. Preparation of organic solvent tolerant mutants from Escherichia coli K-12. Agric Biol Chem. 1991;55:1935–1938. [Google Scholar]
- 2.Aono R, Kobayashi H. Cell surface properties of organic solvent-tolerant mutants of Escherichia coli K-12. Appl Environ Microbiol. 1997;63:3637–3642. doi: 10.1128/aem.63.9.3637-3642.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aono R, Kobayashi H, Joblin K N, Horikoshi K. Effects of organic solvents on growth of Escherichia coli K-12. Biosci Biotechnol Biochem. 1994;58:2009–2014. doi: 10.1271/bbb.58.1231. [DOI] [PubMed] [Google Scholar]
- 4.Aono R, Tsukagoshi N, Yamamoto M. Involvement of outer membrane protein TolC, a possible member of the mar-sox regulon, in maintenance and improvement of organic solvent tolerance of Escherichia coli K-12. J Bacteriol. 1998;180:938–944. doi: 10.1128/jb.180.4.938-944.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fralick J A. Evidence that TolC is required for functioning of the Mar/AcrAB efflux pump of Escherichia coli. J Bacteriol. 1996;178:5803–5805. doi: 10.1128/jb.178.19.5803-5805.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Inoue A, Horikoshi K. A Pseudomonas thrives in high concentrations of toluene. Nature. 1989;338:264–265. [Google Scholar]
- 7.Isken S, de Bont J A M. Active efflux of toluene in a solvent-tolerant bacterium. J Bacteriol. 1996;178:6056–6058. doi: 10.1128/jb.178.20.6056-6058.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kieboom J, Dennis J J, de Bont J A M, Zylstra G J. Identification and molecular characterization of an efflux pump involved in Pseudomonas putida S12 solvent tolerance. J Biol Chem. 1998;273:85–91. doi: 10.1074/jbc.273.1.85. [DOI] [PubMed] [Google Scholar]
- 9.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 10.Leo A J. Calculating log POW from structures. Chem Rev. 1993;93:1281–1306. [Google Scholar]
- 11.Li X-Z, Li Z, Poole K. Role of the multidrug efflux systems of Pseudomonas aeruginosa in organic solvent tolerance. J Bacteriol. 1998;180:2987–2991. doi: 10.1128/jb.180.11.2987-2991.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lomovskaya O, Lewis K. emr, an Escherichia coli locus for multidrug resistance. Proc Natl Acad Sci USA. 1992;89:8938–8942. doi: 10.1073/pnas.89.19.8938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 14.Ma D, Cook D N, Alberti M, Pon N G, Nikaido H, Hearst J E. Genes acrA and acrB encode a stress-induced efflux system of Escherichia coli. Mol Microbiol. 1995;16:45–55. doi: 10.1111/j.1365-2958.1995.tb02390.x. [DOI] [PubMed] [Google Scholar]
- 15.Machleidt H, Roth S, Seeman P. The hydrophobic expansion of erythrocyte membranes by the phenol anesthetics. Biochim Biophys Acta. 1972;255:178–189. doi: 10.1016/0005-2736(72)90020-x. [DOI] [PubMed] [Google Scholar]
- 16.Nikaido H. Multidrug efflux pumps of gram-negative bacteria. J Bacteriol. 1996;178:5853–5859. doi: 10.1128/jb.178.20.5853-5859.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Noguchi K, Nakajima H, Aono R. Effects of oxygen and nitrate on growth of Escherichia coli and Pseudomonas aeruginosa in the presence of organic solvents. Extremophiles. 1997;1:193–198. doi: 10.1007/s007920050033. [DOI] [PubMed] [Google Scholar]
- 18.Noguchi K, Tsukagoshi N, Aono R. Deterioration of tolerance to hydrophobic organic solvents in a toluene-tolerant strain of Pseudomonas putida under the conditions lowering aerobic respiration. Biosci Biotechnol Biochem. 1999;63:1400–1406. doi: 10.1271/bbb.63.1400. [DOI] [PubMed] [Google Scholar]
- 19.Osborne S J, Leaver J, Turner M K, Dunnill P. Correlation of biocatalytic activity in an organic-aqueous two-liquid phase system with solvent concentration in the cell membrane. Enzyme Microb Technol. 1990;12:281–291. doi: 10.1016/0141-0229(90)90100-5. [DOI] [PubMed] [Google Scholar]
- 20.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ramos J L, Duque E, Godoy P, Segura A. Efflux pumps involved in toluene tolerance in Pseudomonas putida DOT-T1E. J Bacteriol. 1998;180:3323–3329. doi: 10.1128/jb.180.13.3323-3329.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ramos J L, Duque E, RodriguezHerva J J, Godoy P, Haidour A, Reyes F, FernandezBarrero A. Mechanisms for solvent tolerance in bacteria. J Biol Chem. 1997;272:3887–3890. doi: 10.1074/jbc.272.7.3887. [DOI] [PubMed] [Google Scholar]
- 23.Sikkema J, de Bont J A M, Poolman B. Intercalations of cyclic hydrocarbons with biological membranes. J Biol Chem. 1994;269:8022–8028. [PubMed] [Google Scholar]
- 24.Sikkema J, de Bont J A M, Poolman B. Mechanisms of membrane toxicity of hydrocarbons. Microbiol Rev. 1995;59:201–222. doi: 10.1128/mr.59.2.201-222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.White D G, Goldman J D, Demple B, Levy S B. Role of the acrAB locus in organic solvent tolerance mediated by expression of marA, soxS, or robA in Escherichia coli. J Bacteriol. 1997;179:6122–6126. doi: 10.1128/jb.179.19.6122-6126.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]