Abstract
Clinical importance:
Feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) infections are found in cats worldwide. Both infections are associated with a variety of clinical signs and can impact quality of life and longevity.
Scope:
This document is an update of the 2008 American Association of Feline Practitioners’ feline retrovirus management guidelines and represents current knowledge on pathogenesis, diagnosis, prevention and treatment of retrovirus infections in cats.
Testing and interpretation:
Although vaccines are available for FeLV in many countries and for FIV in some countries, identification of infected cats remains an important factor for preventing new infections. The retrovirus status of every cat at risk of infection should be known. Cats should be tested as soon as possible after they are acquired, following exposure to an infected cat or a cat of unknown infection status, prior to vaccination against FeLV or FIV, and whenever clinical illness occurs. It might not be possible to determine a cat’s infection status based on testing at a single point in time; repeat testing using different methods could be required. Although FeLV and FIV infections can be associated with clinical disease, some infected cats, especially those infected with FIV, can live for many years with good quality of life.
Management of infected cats:
There is a paucity of data evaluating treatments for infected cats, especially antiretroviral and immunomodulatory drugs. Management of infected cats is focused on effective preventive healthcare strategies, and prompt identification and treatment of illness, as well as limiting the spread of infection.
Keywords: Feline leukemia virus, feline immunodeficiency virus, FeLV, FIV, polymerase chain reaction, PCR, diagnostics, veterinary sciences
Introduction
Feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) are among the most common causes of infectious disease of cats and are found worldwide. A large observational study evaluated FeLV and FIV test results over a 9-year period from 2008 to 2016. 1 The data were collected from a referral laboratory database containing results from cats tested in the field using point-of-care (POC) tests. Almost 3 million test results from 68 countries grouped into seven global regions were analyzed (Table 1).
Table 1.
Prevalence of FeLV antigen and FIV antibody by region in samples submitted to a referral laboratory (2008–2016)
| Region (number of cats tested) | FeLV antigen prevalence (%) | FIV antibody prevalence
(%) |
|---|---|---|
| North America (2.5 million) | 4 | 5 |
| Caribbean (6882) | 9 | 13 |
| Latin America (9984) | 13 | 7 |
| Northern Europe (95,800) | 7 | 7 |
| Southern Europe (206,157) | 12 | 12 |
| Middle East/Africa (4787) | 14 | 14 |
| Asia-Pacific (81,201) | 6 | 13 |
From Buch J, Beall M, O’Connor T, et al 1
FeLV = feline leukemia virus; FIV= feline immunodeficiency virus
A 2006 survey of over 18,000 cats in the USA and Canada reported 2.3% of cats positive for FeLV antigen and 2.5% of cats positive for FIV antibody. 2 In 2009, a survey of over 11,000 cats in Canada reported prevalences of 3.4% for FeLV antigen and 4.3% for FIV antibody. 3 Another large study, in 2010, evaluated test results of over 62,000 cats from veterinary clinics and shelters in the USA and Canada for FeLV antigen and FIV antibody. 4 In that study, prevalence for FeLV antigen and FIV antibody was 3.1% and 3.6%, respectively. A prospective study in Europe that tested cats visiting a veterinary facility for FeLV RNA in saliva as a measure of antigenemia from September 2016 to March 2017 found an overall prevalence of 2.3%. 5 The highest prevalence was in Southern Europe (5.5%) and the lowest in Northern Europe (0.7%). These studies show that although guidelines for prevention of infection have been available for decades, there remains a need to improve adherence to testing and vaccination recommendations.


Pathogenesis and outcomes of infection
Feline leukemia virus infection
Transmission of feline leukemia virus
FeLV is transmitted through close contact among cats. Commonly, it is spread vertically and horizontally from infected queens to their kittens and horizontally among cats that live together or that fight. There is an age-related increase in resistance to FeLV infection; kittens have the highest risk of becoming progressively infected. 6 However, some studies have demonstrated efficient natural and experimental infection in adult cats. 7
Pathogenesis of feline leukemia virus
Progressively infected cats shed infectious virus in body fluids, including saliva, nasal secretions, milk, urine and feces.6,8 Cats typically acquire FeLV via the oronasal route but can also become infected through bite wounds. After virus exposure via the oronasal route, FeLV can be found first in the local lymphoid tissues; it then spreads via monocytes and lymphocytes (primary viremia; see ‘Glossary of terms on page 24) into the periphery. During this primary viremia, the virus can infect the bone marrow. 9 After bone marrow infection, a secondary viremia can occur, with FeLV-containing leukocytes and platelets appearing in the blood, resulting in virus being detectable by immunofluorescent antibody (IFA) test.
Outcomes following exposure to feline leukemia virus
Based on molecular methods, the possible outcomes of infection following FeLV exposure have been redefined.10–12 Outcomes of FeLV infection are now classified as abortive infection (comparable to the former ‘regressor cats’), regressive infection (comparable to the former ‘latent infection’, with or without previous ‘transient viremia’) and progressive infection (comparable to the former ‘persistent viremia’). The likelihood of each outcome depends on the infection pressure and the cat’s immune status, and has been described in experimental infections using specific pathogen-free cats.
In the past, exposure to FeLV has been described as resulting in abortive infection in 20–30% of cats, regressive infection in 30–40% of cats and progressive infection in 30–40% of cats. 13 However, large field studies testing simultaneously for p27 antigen, proviral DNA, viral RNA and virus-neutralizing antibodies have identified a higher proportion of cats that have presumed abortive infections based on a pattern of negative antigen and PCR tests in the presence of neutralizing antibodies. In a study of 495 owned pet cats in Germany, 4% were classified as having abortive infection, 2% as progressive and 1% as regressive. 14 In a study of 440 owned pet cats in Australia, 11% were classified as having presumptively abortive infection, 2% as presumptively regressive and 0.5% as presumptively progressive. 15 This suggests that abortive infection may be the most common outcome following exposure under typical conditions. In contrast, in two populations of cats in Australia (one group of 38 cats and one group of 51 cats) in which FeLV-infected and uninfected cats were co-mingled without separating healthy from clinically ill cats, 9% were classified as having abortive infection, 25% as regressive and 21% as progressive, suggesting that resistance to infection may be compromised by intense infectious pressure, comorbidities and a stressful environment. 15
Viral RNA is usually detectable in plasma by real-time reverse-transcriptase polymerase chain reaction (qRT-PCR) testing within 1 week of FeLV exposure, followed by proviral DNA detection by PCR within 2 weeks of exposure and finally by FeLV antigen detection, which usually occurs by 30 days but can be longer in some cats. 16 It is not only cats with progressive infection that undergo the early phases, but also some cats with regressive infection. These regressively infected cats have similar proviral and plasma viral RNA loads in their peripheral blood at the beginning of their infection; however, in contrast to cats with progressive infection, their viral load will decrease to undetectable levels over time.12,17
Progressive infection In cats with progressive infection, FeLV infection is not contained during the early stage, and extensive virus replication occurs first in the local lymphoid tissues, then in the bone marrow, and subsequently in mucosal and glandular epithelial tissues. 9 Mucosal and glandular infection is associated with excretion of infectious virus, mainly in saliva but also in other secretions. Progressive infection is characterized by insufficient FeLV-specific immunity and usually neutralizing antibodies are not detectable. Cats with progressive infection have a shorter survival time than cats with regressive FeLV infection and typically succumb to FeLV-associated diseases within several years after infection.11,18,19
-
Regressive infection Regressive infection is accompanied by an immune response that contains, but does not eliminate, virus replication. Viral shedding does not occur after the first antigenemic phase is over.8,20–23 However, FeLV proviral DNA can be detected in the blood by some PCR assays.10,17,24
FeLV is integrated into the cat’s genome and is unlikely to be completely cleared over time. 25 Regressively infected cats do not shed infectious virus. However, it has been demonstrated that proviral DNA is infectious via blood transfusion and can lead to viremia and FeLV-associated disease in susceptible recipient cats. 26 Cats with regressive infection demonstrate continuously high titers of virus-neutralizing antibodies 17 and are at low risk of developing FeLV-associated diseases.27,28 However, reactivation can occur in cats with regressive infection, particularly if they are immunosuppressed, so they become viremic and develop FeLV-associated disease. The risk of reactivation of viremia decreases with time (duration after the cat has cleared viremia) but it has been shown that the integrated provirus retains its replication capacity, so reactivation can still occur many years after the initial exposure to FeLV. 29 In some cats, regressive infection itself might be associated with clinical disease, such as lymphoma28,30 or bone marrow suppression. 27
Abortive infection Abortive infection has been observed following experimental FeLV inoculation and is characterized by negative test results for culturable virus, antigen, viral RNA and proviral DNA.10,31 The only indication of FeLV infection is the presence of antibodies. Although not common after experimental infection, abortive infection seems to be more common in the field, as cats with natural infections can show evidence of FeLV antibodies in the absence of detectable viral RNA, proviral DNA or antigen, and without having received FeLV vaccines.7,14,15,32
Feline immunodeficiency virus infection
Transmission of feline immunodeficiency virus
The major mode of FIV transmission is through bite wounds that introduce saliva containing virus and FIV-infected white blood cells. Transmission of FIV from infected queens to their kittens has been demonstrated experimentally,33,34 but appears to be uncommon in naturally infected cats.35,36 Transmission is also uncommon among cats living together in a household without fighting; however, a certain degree of risk remains. In one household of 26 cats that were not observed to fight, FIV infection was originally diagnosed in nine cats, but spread to six other cats during a 10-year observation period. 37 This household also included cats coinfected with FeLV, which might have predisposed some cats to FIV infection. However, in a sanctuary in which eight FIV-infected cats were housed with 130 uninfected cats, no transmission was documented over several years. 38 Sexual transmission, the most common mode of transmission of human immunodeficiency virus (HIV), appears to be unusual for FIV, even though the semen of infected cats frequently contains infectious virus and biting can occur during mating. 39
Pathogenesis of feline immunodeficiency virus
After experimental inoculation, acute FIV infection can be associated with transient fever, lymphadenopathy and lymphopenia, but this has not been reported in natural infection, perhaps because the early signs might not be noticed by cat owners. During this acute stage, FIV is detected in high concentrations in the blood by culture and PCR. Within the first few weeks of infection, both CD4+ (helper) and CD8+ (cytotoxic-suppressor) T lymphocyte concentrations decline.40,41 The initial phase is followed by an immune response characterized by the production of FIV antibodies, suppression of circulating virus leading to a decreasing viral load, and an increase in CD8+ T lymphocytes to higher than pre-infection levels. This results in an inversion of the CD4:CD8 ratio that can persist for the rest of the cat’s life. Over time, both CD4+ and CD8+ lymphocyte numbers continue to gradually decline.42,43
Following the primary phase, cats enter a long asymptomatic stage that can last for many years (Figure 1). During this stage, progressive dysfunction of the immune system can occur. Thus, FIV-infected cats are predisposed to chronic and recurrent infections. Neoplasia is about five times more common than in uninfected cats. 44 Although chronic inflammatory conditions and secondary infections are more common in cats with low CD4+ T lymphocyte counts, some cats with very low CD4+ counts remain healthy. Cell-mediated immunity is more profoundly affected than humoral immunity. Hyperglobulinemia, characteristic of nonspecific stimulation of humoral immunity, can also occur in cats with FIV infection. 45 Survival time for FIV-infected cats is highly variable among individuals, but can be similar to that of non-FIV-infected cats.37,45–48
Figure 1.
Outcomes of infection with feline immunodeficiency virus (FIV). Ab = antibody; PCR = polymerase chain reaction. Courtesy of IDEXX. Copyright © 2019, IDEXX Laboratories. All rights reserved. Used with permission
Diagnosis of retrovirus infections
The most important measure for the control of FeLV and FIV is the identification and segregation of infected cats. Thus, the American Association of Feline Practitioners (AAFP) recommends screening all cats for infection at the time they are first acquired, prior to initial vaccination against FeLV or FIV, following potential exposure to infected cats, or if clinical signs of illness are displayed.
POC tests based on ELISA or rapid immunomigration (RIM) methodologies are commonly used in veterinary practice to detect FeLV antigen and FIV antibodies in whole blood, serum or plasma. In addition, POC tests for the detection of FeLV antibodies 32 and in-house PCR tests detecting FeLV and FIV provirus49,50 are available in some countries, but only limited data evaluating these tests are available. Referral laboratories also offer various tests for FeLV and FIV detection.
Since a positive screening test result has potentially important clinical consequences, additional testing is recommended, especially in low-risk cats (eg, apparently healthy cats, indoor-only cats) where the likelihood of a false-positive result is greater than in higher risk cats (eg, sick, outdoor access). False-positive results might, among other things, arise from improperly conducted tests or test failure. Negative test results are generally reliable when highly sensitive POC tests are used, especially in apparently healthy cats with a low-risk lifestyle. The exception would be when the cat is in the early phase of infection before FeLV antigenemia (<30 days) or FIV antibodies (<60 days) have developed. In addition, false-negative test results can arise because changes in FIV isolates may occur over time; for example, through movement of cats geographically or between countries. Indeed, a study in Europe demonstrated an increasing number of cats testing negative for FIV antibody with POC tests but positive on Western blot between 1998 and 2019 compared with cats tested in earlier years, suggesting a reduction in the diagnostic efficiency of FIV POC tests in geographic areas where cats may be infected with imported isolates. 51
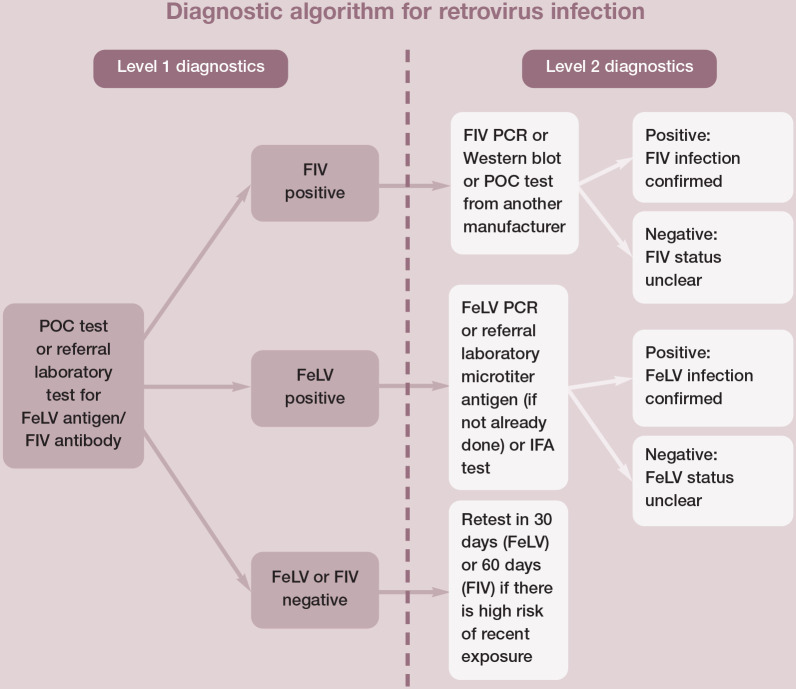
Cats are tested under various circumstances and for different reasons, so a single testing protocol is difficult to recommend for all cats. See Figure 2 for a testing protocol that can be adapted to different situations. A diagnostic tool developed in Europe for testing cats for FeLV is also available (abcdcatsvets.org). Note there are differences in test types and test performance in different countries.
Figure 2.
Level 1 diagnostics might be sufficient in circumstances where the test results are consistent with the patient’s signalment and clinical signs. Level 2 diagnostics can be appropriate to clarify infection status in some patients. This diagnostic algorithm will correctly identify the true infection status for most cats. Regressive feline leukemia virus (FeLV) infections, recent exposure, atypical responses and changes in the immune response over time complicate the interpretation and reliability of tests performed at a single point in time. The true status of cats with discordant results can be difficult to resolve. FIV = feline immunodeficiency virus; IFA = immunofluorescent antibody; POC = point-of-care; PCR = polymerase chain reaction
Several comparison studies of FeLV and FIV tests have been performed over the years.52–54 However, these studies are difficult to compare due to differences in study design, especially concerning the reference standards used. In addition, tests with similar names can differ among countries or might have undergone design changes over time. It is difficult to select an appropriate gold standard for FeLV diagnostic test comparison studies – there is no gold standard for antigen detection and PCR is of limited value (without concurrent results from antigen testing) since it detects not only progressively but also regressively infected cats (provirus carriers).
Feline leukemia virus infection
Diagnosis of FeLV infection is usually based on the detection of soluble FeLV p27 antigen using POC tests. Testing can be performed on serum, plasma or whole blood. FeLV antigen tests should not be performed on tears or saliva, as reported sensitivities are low.8,55,56 In one study, use of saliva was only able to detect 54% of infected cats. 56 Testing is not confounded by maternally acquired immunity or FeLV vaccination. Most cats will test positive within 30 days of exposure, although development of antigenemia can take longer in some cats. Since the consequences of a positive screening test for FeLV are significant for the cat’s future, additional testing is recommended, especially in low-risk and asymptomatic cats.17,52,57 Immediate retesting in the event of questionable or positive FeLV p27 antigen POC test results can be performed at a referral laboratory using either a microwell plate ELISA for p27 antigen or PCR detecting FeLV provirus. Alternatively, a POC p27 antigen test of a different brand can be used.
In one study, four different FeLV POC tests were compared using 146 FeLV-positive and 154 FeLV-negative serum or plasma samples. The results of two commercial ELISAs were used as the gold standard for the determination of true FeLV infection status. Sensitivity and specificity were 100% and 100% for IDEXX SNAP FIV/FeLV Combo, 89.0% and 95.5% for Witness FeLV-FIV, 91.8% and 95.5% for Anigen Rapid FIV Ab/FeLV Ag, and 85.6% and 85.7% for VetScan Feline FeLV/FIV Rapid test kits, respectively. 58 However, other studies investigating different cat populations and using different gold standards to determine infection status have revealed different results.56,59
Progressively infected cats can be identified using POC tests that detect soluble free FeLV p27 antigen in the blood, indicative of antigenemia; in general, antigenemia is equivalent to viremia, although exceptions have been reported. 60 Only viremic (antigen-positive) cats shed virus under natural circumstances and are infectious for other cats. This includes cats with progressive infection and cats with regressive infection in the early phase of transient viremia or after reactivation of infection.
Regressive infections are characterized by low levels of antigen and proviral DNA. At times, concentrations of one or the other can drop below the level of detection of some tests, leading to discordant results that may change over time. 61 Quantitative PCR assays for provi-ral DNA are becoming commercially available in more countries and they provide additional information to classify a cat’s status.15,61 Cats that initially test positive by both p27 antigen and PCR can transition to a regressive infection pattern, usually within 16 weeks of infection.
Although saliva is less sensitive than blood or serum for POC tests, it can be used for RT-PCR to detect FeLV RNA and, thus, FeLV shedding.8,56 Detection of viral RNA in saliva is a reliable parameter of antigenemia and shedding. 62 According to a European study, detection of viral RNA in saliva swabs can be useful if blood collection is not feasible in large groups of cats. Saliva swabs from several cats can be pooled for analysis (ideally from a maximum of 10 cats). However, if a pooled sample is positive for FeLV, individual testing must be performed to determine each cat’s status. 63 In an experimental setting, RT-PCR performed on saliva and blood can detect infection as early as 1–3 weeks post-exposure.8,16
IFA tests for blood or bone marrow smears are available from some commercial laboratories for the diagnosis of FeLV infection. These tests detect secondary viremia once bone marrow infection is established. Before bone marrow infection is established, cats will test negative using IFA. Most cats with regressive infections and those that resist bone marrow infection will also test negative. The subjective nature of IFA interpretation and differences in performance among laboratories can lead to both false-positive and false-negative results. False-negative results may also be observed in cats with leukopenia and regressive infections.
Discordant results between antigen tests and other techniques such as PCR and IFA can occur as these tests detect the cat’s stage of infection at a single point in time (Figure 3). Repeat testing over time might be needed to clarify the status of some cats. Cats with discordant test results should be considered potential sources of infection for other cats until their status is clarified.
Figure 3.
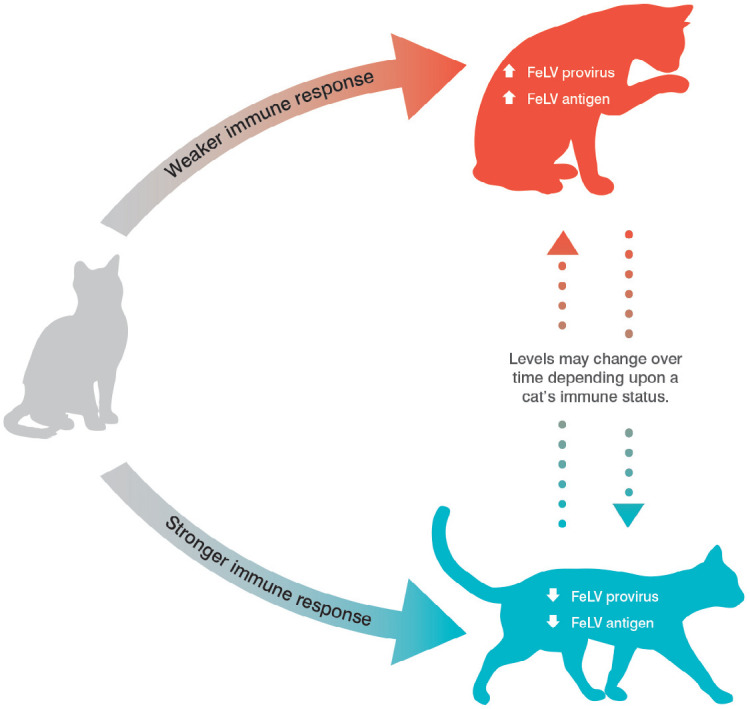
Feline leukemia provirus and antigen test results may vary depending on the cat’s immune status at the time of testing. High levels of provirus and antigen are most commonly associated with progressive infection, while low levels of provirus and antigen are most commonly associated with regressive infection. FeLV = feline leukemia virus. Courtesy of IDEXX. Copyright © 2019, IDEXX Laboratories. All rights reserved. Used with permission
Feline immunodeficiency virus infection
FIV infection is most commonly diagnosed through detection of FIV-specific antibodies using POC tests performed on whole blood, serum or plasma. Infected cats usually develop high concentrations of FIV-specific antibodies, and FIV produces a persistent infection from which cats do not recover. Thus, detection of antibodies is generally indicative of FIV infection. In veterinary practice, antibodies are usually identified using either ELISAs or RIM assays, which detect antibodies to various viral antigens. Different antibodies are detected by the available POC test kits. Most cats produce antibodies within 60 days of infection.
Most currently available POC tests for FIV have been shown to be highly sensitive and specific based on various comparison studies, despite differences in study design and reference standards.52–54 In a study comparing four different FIV POC tests in the USA using 94 FIV-positive and 97 FIV-negative serum or plasma samples, and comparing the results with virus isolation as the gold standard, sensitivity and specificity were 97.9% and 99.0% for IDEXX SNAP FIV/FeLV Combo, 94.7% and 100% for Witness FeLV-FIV, 96.8% and 99.0% for Anigen Rapid FIV Ab/FeLV Ag, and 91.5% and 99.0% for VetScan Feline FeLV/FIV Rapid test kits, respectively. 58 No significant differences in performance among the four tests were reported.
Several referral laboratory tests are available for additional testing after a positive POC test for FIV antibodies. However, establishing the true FIV infection status of cats can sometimes be difficult, even with extensive additional testing. Western blotting traditionally has been used as a gold standard diagnostic test for detection of FIV antibodies. While it did not perform as well as some POC tests in one North American study, 64 in a European study it was able to detect some cats that were antibody negative with POC tests. 51
Detection of FIV proviral DNA or viral RNA (or both) by PCR is commonly used as an additional test by commercial laboratories in North America. However, some infected cats are not detected by PCR, which is likely due to viral sequence variation or low virus loads.51,65–68 The primers used to amplify gene segments should be designed to bind to highly conserved regions such as the gag gene of FIV, since it has been shown that env recombinants occur commonly in naturally infected cats. 69 In addition, the accuracy of PCR results varies among different laboratories; 70 therefore, assays that have been independently validated should be used. In an independent study of 239 FIV-unvaccinated cats in Australia, the sensitivity and specificity of the FIV RealPCR test (IDEXX Laboratories) were 92% and 99%, respectively. 66 It is reasonable to further assess cats with a positive FIV POC test result by performing additional testing (Figure 2), especially in low-risk cats. However, some high-risk cats with positive FIV POC test results, such as free-roaming, aggressive male cats, may not require additional testing.
During the early phase of FIV infection, cats can test antibody negative. Therefore, when the results of antibody testing are negative, but recent infection cannot be ruled out, testing should be repeated no earlier than 60 days after the last potential exposure. Although most cats develop antibodies within 60 days of exposure to infection, antibody development can be delayed in some cats. Throughout the asymptomatic phase of infection, FIV-specific antibodies are readily detected in the blood of most cats. However, some cats entering the terminal phase of infection might test antibody negative because of high viral loads sequestering antibodies in antigen-antibody complexes. In addition, false-negative results are possible with any test. If a cat at high risk of FIV infection with typical clinical signs is antibody negative on a POC test, follow-up testing should be performed with another method, such as PCR or Western blot.
Although POC antibody tests are convenient and highly reliable in most situations, such tests should be interpreted carefully in kittens that test positive. Antibodies are passively transferred to kittens that nurse on naturally infected or vaccinated queens. This can lead to a positive POC antibody test result up to the age of 6 months if the queen was infected. In a study of 55 kittens born to FIV-vaccinated, uninfected queens, all kittens tested positive for FIV antibodies shortly after birth and for the first several weeks of life. 71 By 12 weeks of age, all kittens tested FIV antibody negative. Under natural circumstances, if a chronically FIV-infected queen is otherwise healthy, kittens born to that queen rarely acquire FIV infection in utero or post-natally. Consequently, most kittens that test antibody positive initially will test negative when maternal antibodies have waned. Therefore, FIV antibody-positive kittens can be retested immediately with a reliable PCR assay to clarify their status. Kittens persistently testing FIV antibody positive after 6 months of age are likely to be truly infected.
The use of the FIV vaccine (Fel-O-Vax FIV; Boehringer Ingelheim) in Canada, the USA, Australia, New Zealand and Japan has complicated the diagnosis of FIV infections based on antibody detection, since vaccinated cats produce antibodies that cannot be distinguished from antibodies induced by natural infection by some commercially available tests. Antibodies can usually be detected within a few weeks of vaccination and it has been shown that they can persist for more than 7 years in some cats. 66 Fel-O-Vax FIV was discontinued in Canada and the USA in 2015 but previously vaccinated cats testing FIV-antibody positive due to vaccination will remain in the cat population for some years to come. Also cats may travel from locations where the vaccine is still in use to Canada, the USA and other countries where the vaccine is not available.
Comparison of three commercially available POC antibody tests was performed in a population of 119 FIV-vaccinated and 239 FIV-unvaccinated Australian cats. 66 FIV infection status was determined by considering the results of all antibody tests together with results from PCR testing; virus isolation was used for rare discrepant cases. Two POC tests, Witness FeLV-FIV (sensitivity 100%, specificity 98%) and Anigen Rapid FIV Ab/FeLV Ag (sensitivity 100%, specificity 100%), demonstrated excellent sensitivity and specificity, and were shown to determine the true FIV infection status of cats irrespective of FIV vaccination history, if the primary vaccination had been administered at least 6 months previously. The IDEXX SNAP FIV/FeLV Combo test, however, detected antibodies induced by previous vaccination as well as those induced by FIV infection. In a follow-up study, the same research group evaluated the use of saliva (rather than blood) to diagnose FIV infection using the three POC tests and one PCR test. 72 Sensitivities were 44% (IDEXX SNAP FIV/FeLV Combo), 92% (Witness FeLV-FIV), 96% (Anigen Rapid FIV Ab/FeLV Ag) and 72% (RealPCR), whereas the specificity for all tests was similar at 98–100%. The researchers concluded that two POC test kits (Witness and Anigen) could accurately identify FIV infection using saliva, regardless of FIV vaccination history. Testing saliva could be useful in areas where FIV vaccination is available and when venipuncture without skilled restraint or sedation is not possible, such as in situations where large numbers of cats must be screened for FIV infection quickly and easily.
A study conducted in the USA also evaluated whether some POC tests could be used to differentiate between antibodies induced following FIV vaccination vs infection. 73 The study compared four tests: IDEXX SNAP FIV/FeLV Combo, Witness FeLV-FIV, Anigen Rapid FIV Ab/FeLV Ag and VetScan Feline FeLV/FIV Rapid test kits. In this study, 104 uninfected specific pathogen-free cats were vaccinated three times and plasma samples were collected 2–14 months after vaccination. Cats were confirmed to be FIV-free by virus culture. The IDEXX SNAP FIV/FeLV Combo and the VetScan Feline FeLV/FIV Rapid tests had positive results in 102/104 and 88/104 uninfected vaccinated cats, respectively. The Witness FeLV-FIV and the Anigen Rapid FIV Ab/FeLV Ag tests correctly identified nearly all vaccinated cats as uninfected. Specificity in FIV-vaccinated cats was 98.1% for Witness FeLV-FIV, 98.1% for Anigen Rapid FIV Ab/FeLV Ag, 21.2% for VetScan Feline FeLV/FIV Rapid and 1.9% for IDEXX SNAP FIV/FeLV Combo tests.
To determine the duration of interference of diagnostic tests by FIV vaccination, a longitudinal study of vaccinated cats was conduct-ed. 74 Kittens received a primary vaccination series according to the manufacturer’s recommendations and were periodically tested over 6 months using the IDEXX SNAP FIV/FeLV Combo, Anigen Rapid FIV Ab/FeLV Ag, Witness FeLV-FIV and VetScan Feline FeLV/FIV Rapid test kits. Some cats tested positive using all tests 4 weeks after the first vaccination. Subsequently, 100% of the cats remained positive with the IDEXX SNAP FIV/FeLV Combo and 83% remained positive with the VetScan Feline FeLV/FIV Rapid test for the duration of the study, while cats tested with the Anigen Rapid FIV Ab/FeLV Ag and Witness FeLV-FIV tests became negative by 6 months after the third vaccination for FIV. It was concluded that the Anigen Rapid FIV Ab/FeLV Ag and the Witness FeLV-FIV tests could be used for the diagnosis of FIV infection in vaccinated cats, providing that primary vaccination occurred more than 6 months previously.
Prevention of retrovirus infections
Maximizing prevention of retrovirus infection can be accomplished through a partnership between veterinarians and pet owners. Implementing testing and vaccination protocols, staff and owner education, owner vaccination reminder programs and environmental management can help contain the spread of these infections.
Traditionally, FeLV infection has primarily been viewed as a concern for cats that are ‘friendly’ or ‘social’ with other cats because close, intimate contact among cats facilitates salivary transmission. This type of contact occurs among cats through nursing, mutual grooming, and sharing of food, water and litter boxes. However, infection can also occur from inter-cat aggression and studies have shown cats exhibiting aggressive behavior to have an increased risk of FeLV infection.45,75 Less common sources of FeLV infection include contact with other body fluids (eg, tears, plasma, urine, feces), transplacental transmission, use of contaminated surgical and dental instruments, and blood transfusion.13,26,76 While susceptibility to infection is highest when cats are young, the cumulative lifetime risk of exposure results in a slighter higher prevalence of infection in older cats. 2
On the other hand, most natural FIV infections likely result from inter-cat aggression between ‘unfriendly’ cats because the major mode of transmission is through bite wounds.45,77,78 Transmission rarely occurs from queen to kittens in a natural environment.38,79
Risk factors for feline leukemia virus and feline immunodeficiency virus infections
Prevention strategies start with recognition of risk factors associated with FeLV and FIV infections. Avoidance or minimization of risk factors that are amenable to control (eg, lifestyle, vaccination) should be assessed for each cat (Figures 4 and 5). Patient characteristics associated with increased prevalence of retrovirus infection are listed in Table 2.
Figure 4.

Outdoor lifestyle is a risk factor for retrovirus infection; not all infected cats will appear ill. Courtesy of Janet Wolf
Figure 5.
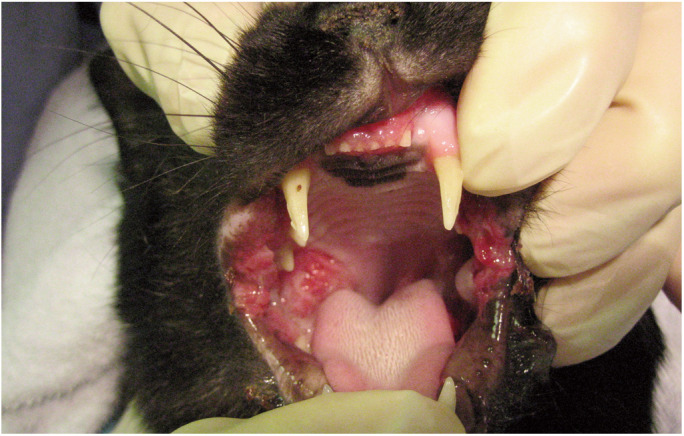
Inflammatory oral disease, such as gingivostomatitis, is associated with an increased risk of retrovirus infection. Courtesy of Susan Little
Table 2.
Patient characteristics associated with increased prevalence of retrovirus infection4,45,47,75,78,80,81
| Risk factor | FeLV | FIV |
|---|---|---|
| Increasing age | xx | xxx |
| Male sex | xx | xxx |
| Sexually intact status | xx | xxx |
| Outdoor access | xxx | xxx |
| Close contact with infected cats | xxx | xx |
| Inter-cat aggression | xx | xxx |
| Illness (especially oral disease, abscess, respiratory tract disease) | xxx | xxx |
| Kitten born to an infected queen | xxx | x |
‘xxx’ indicates a stronger risk association than ‘xx’ or ‘x’
FeLV = feline leukemia virus, FIV = feline immunodeficiency virus
Vaccination
Feline leukemia virus vaccination
While testing and identification of FeLV-infected cats is necessary for preventing FeLV infection, vaccination is also an important preventive tool. Combined use of testing and vaccination programs is likely the reason for the decrease in FeLV prevalence in Europe and North America in the initial decades after the virus was discovered.4,5,14,45,82,83 However, recent studies indicate that the prevalence of FeLV has plateaued in some countries, so increased efforts are necessary to further decrease the prevalence.4,64,84 In one study, a history of vaccination against FeLV was associated with a reduced risk of FeLV infection in cats treated for abscesses and bite wounds. 75 Unvaccinated cats with bite wounds were 7.5 times more likely to be infected with FeLV than vaccinated cats, suggesting that FeLV vaccination provides protection.
Several vaccines for FeLV are available, including adjuvanted inactivated whole virus vaccines, recombinant subunit vaccines and a genetically engineered subunit recombinant canarypox vector vaccine. Commercially available vaccines appear to provide protection against progressive infection and FeLV-associated diseases.11,85,86 Nevertheless, it remains difficult to assess vaccine efficacy for several reasons. Most of the published efficacy trials have been small studies conducted in research cats and have been performed or supported by the vaccine manufacturers.86–93 Other factors that hamper interpretation of vaccine efficacy studies include lack of standard challenge and testing protocols, as well as the difficulty of infecting control groups of adult cats without inducing immune suppression.
Although FeLV vaccines have been shown to protect some cats against progressive infection, vaccination will not always prevent proviral DNA integration after FeLV exposure. One study using inactivated vaccines found that, after challenge, vaccinated cats had no detectable viral antigen, viral RNA, proviral DNA or infectious virus. 94 Other studies showed that several current vaccines failed to consistently prevent proviral DNA integration following FeLV exposure.11,16 Therefore, it cannot be concluded that FeLV vaccination protects against all outcomes of FeLV infection. Nevertheless, several current vaccines are still of great clinical importance because they appear to be efficacious at preventing progressive infection and, thus, curtailing FeLV-associated diseases.12,86 Several early studies indicated that duration of immunity to FeLV persists for at least 12 months following vaccination95–97 and, in one study, most cats resisted infection when challenged 2 years after vaccination. 98
Vaccination against FeLV does not diminish the importance of testing to identify and isolate cats that are progressively infected. Vaccinated and unvaccinated cats that are progressively infected could be sources of infection for other cats. Vaccination against FeLV does not interfere with testing, as the available POC tests detect viral antigen. Therefore, the FeLV infection status of all cats, including vaccinated cats, should be determined. Administering FeLV vaccines to infected cats is of no therapeutic value and every unnecessary vaccination carries the risk of potential adverse reactions. 99 If a vaccinated cat’s status is unknown and the cat is later determined to have a progressive FeLV infection, vaccine efficacy would be questioned, and vaccine failure suspected. Cats should be tested for FeLV infection before initial vaccination.
The 2013 AAFP vaccination guidelines recommended FeLV vaccination for all kittens up to and including 1 year of age, and for at-risk adult cats. 100 Vaccination of all kittens is highly recommended (at least in areas with high prevalence of infection) because a kitten’s lifestyle and risk of exposure to FeLV frequently changes after acquisition. In addition, kittens are more susceptible to progressive infection, FeLV-associated disease and death if exposed to FeLV compared with adult cats.
When FeLV vaccination is determined to be appropriate, a two-dose primary series is recommended, with the first dose administered as early as 8 weeks of age followed by a second dose administered 3–4 weeks later. A single booster vaccination should be administered 1 year following completion of the initial series. Vaccination can be discontinued thereafter if there is no further risk based on lifestyle, environment and overall health status. The 2013 AAFP vaccination guidelines recommend revaccination every 2 years for cats at low risk of infection and annually for cats at higher risk, based on lifestyle, environment and overall health status. Since those vaccination guidelines were issued, FeLV vaccines with extended duration of immunity have become available. Where vaccines with a 3-year duration of immunity are available, their use can be considered. The 2013 AAFP Feline Vaccination Advisory Panel recommends administering subcutaneous FeLV vaccines in the left hindlimb distal to the stifle joint. The AAFP-recommended FeLV vaccination protocol is outlined in the box below.
Feline immunodeficiency virus vaccination
Multiple studies have shown that cats infected with FIV have low levels of morbidity and mortality with appropriate husbandry and disease management. 45 At the time of writing, only one FIV vaccine is commercially available (Fel-O-Vax FIV; Boehringer Ingelheim) and it is not available in Canada or the USA. Nevertheless, all veterinarians should be aware of this vaccine, because previously vaccinated cats are still present in Canada and the USA, and cats can relocate from other countries where the vaccine is available, such as Australia, New Zealand and Japan.
Fel-O-Vax FIV is a whole-virus, dual subtype (clades A and D), inactivated vaccine combined with an adjuvant, and is licensed for the vaccination of healthy cats 8 weeks of age or older. Variability in vaccine efficacy has been noted. One Australian study (the only field study published to date) found the vaccine had a protective rate of 56%. 101 A study using an FIV isolate in the UK found the vaccine failed to protect experimentally challenged cats. 102 A study of client-owned FIV-vaccinated cats in Australia found a lack of broadly neutralizing antibodies, suggesting cats might not be protected against some virulent recombinant strains in that country. 103
FIV vaccination is classified as ‘non-core’ according to the 2013 AAFP Feline Vaccination Advisory Panel 100 and is recommended for cats at high risk of exposure, such as cats with outdoor access or those living with FIV-infected cats. The 2013 AAFP vaccination guidelines recommend owners be informed of the difficulties in interpreting some FIV test results in vaccinated cats and the low protective rate of the vaccine. In addition, the AAFP recommends that all cats, including FIV-vaccinated cats, should carry both visual and permanent identification, such as a microchip and collar (see AAFP’s 2019 ‘Microchip Identification of Cats’ position statement; catvets.com/guidelines/position-statements/microchip-identification-cats-position-statement).
If the decision is made to vaccinate a cat at risk of infection (in a country where the vaccine is available), the cat should be tested for FIV immediately prior to vaccination. An initial series of three doses is administered subcutaneously 2–3 weeks apart. Annual revaccination is recommended if the risk of infection persists.
Limiting transmission in the veterinary practice
It is important that veterinarians familiarize themselves with guidelines, such as these, for management of retrovirus-infected cats, as these cats likely will survive for many years after diagnosis, especially FIV-infected cats.47,48
Retroviruses are unstable outside their host animals and are inactivated within a very short time on dry surfaces; therefore, they are considered to have little or no environmental persistence. Detergents and common hospital disinfectants quickly inactivate both FeLV and FIV, and there is little risk for transmission among cats by indirect exposure when simple precautions and routine cleaning procedures are followed.104,105 Hospitalized cats should not be allowed to have direct contact with one another. Isolation of hospitalized retrovirus-infected cats in an infectious disease ward is not required; they can be kept in the general hospital wards. Furthermore, since retrovirus-infected cats are potentially immunosup-pressed, they should not be placed in isolation wards with animals carrying contagious diseases, such as upper respiratory virus infection or panleukopenia, nor with dogs infected with feline-shared pathogens, such as canine parvovirus and Bordetella bronchiseptica.
Although casual transmission of the viruses via the environment is unlikely, both viruses are transmitted very efficiently via contaminated body fluids, especially blood. It is therefore imperative to institute and maintain appropriate clinical hygiene practices. Dental and surgical instruments, endotracheal tubes and other items potentially contaminated with body fluids should be thoroughly cleaned and sterilized between uses.106,107 Reused suture has been shown to be a source of FIV transmission. 106 Intravenous fluid lines and bags, as well as food, can become contaminated with body fluids (especially blood or saliva) and should not be shared among patients. Hypodermic needles should not be reused and oral dosing equipment such as syringes should not be shared among animals. Animal caretakers and other hospital staff members should wash their hands after handling animals and cleaning cages.
Both FeLV and FIV can be transmitted in blood transfusions. Therefore, all blood donors should be confirmed free of infection. Cats used for blood or tissue donation should be screened and confirmed to be negative for FeLV antigen and FeLV provirus by PCR as well as for FIV antibodies.26,108,109 PCR testing of donors with negative FeLV antigen tests is necessary because cats with regressive infections are capable of transmitting infection via blood transfusion. 26
Limiting transmission in the home
Ideally, retrovirus-infected cats should be confined indoors to prevent infection of other cats and to protect them against other infectious diseases. If a retrovirus-infected cat is identified in a household, the best method of preventing spread to other cats in the household is to prevent direct contact and interaction between the infected cat and its housemates, typically by isolation of infected cats from uninfected cats. Segregation of retrovirus-infected cats within a home can be difficult for owners to achieve and adherence to recommendations might be low. It is reasonable to counsel owners who are unwilling or unable to segregate infected cats on best practices to reduce the risk of disease transmission; for example, by meeting the environmental needs of all cats in the home to reduce conflict and stress, and by neutering all cats.48,110
Uninfected cats that reside in a household with FeLV-infected cats should be vaccinated against FeLV, even if the infected cats are isolated, because isolation and hygiene protocols might break down. Onset of protective immunity to FeLV typically takes 2–3 weeks after primary vaccination. Therefore, when a cat is vaccinated against FeLV for the first time, owners should be instructed to protect the cat from exposure to FeLV until at least 3 weeks after the final booster vaccination. 100 Owners should be informed that no FeLV vaccine is perfect and vaccination might not protect all cats against FeLV infection, especially in a high infection pressure situation. An infected queen can transmit FeLV to her kittens in utero or via infected milk.76,111–113 Therefore, infected queens should not be used for breeding and should be spayed if their condition is sufficiently stable to permit them to undergo surgery, thus eliminating the risk of vertical transmission and reducing stress from estrous cycles.
Generally, cats in households with stable social structures where housemates do not fight are at negligible risk of acquiring FIV infection. 38 One study did report a high rate of transmission within a household without observed fighting but this household also included cats coinfected with FeLV. 37 Vaccination of uninfected housemates might be considered in countries where an FIV vaccine is available. Owners should be informed that cats that cannot live peacefully with a housemate are more likely to fight and thus uninfected cats might be at higher risk of acquiring FIV infection. No new cats should be introduced into such households as this might lead to fighting, even among cats that did not interact aggressively before.
Experimentally, it has been shown that FIV can be vertically transmitted from infected queens to their kittens.34,114–116 Although this appears to be rare in nature,38,78 FIV-infected queens should not be used for breeding and should be spayed if their condition is sufficiently stable to permit them to undergo surgery, thus eliminating the risk of vertical transmission and reducing stress from estrous cycles.
Considerations for multi-cat environments
Breeding catteries
The prevalence of retrovirus infections in the controlled environments of catteries appears to be low, particularly since the advent of test and removal programs for FeLV that began in the 1970s. However, certain circumstances in catteries facilitate transmission of infectious diseases, including retrovirus infections, such as group living, mingling of kittens with older cats, close contact of cats during mating, the introduction of new cats and the practice of sending cats to other catteries for breeding. Therefore, ongoing vigilance is required to prevent introduction of FeLV or FIV into catteries.
Only healthy cats should be used for breeding and the retrovirus status of all cats in the cattery (whether breeding or non-breeding) should be known. When testing is performed in the cattery for the first time, all cats should be tested for both FeLV and FIV with a POC test. Cats with negative results should be retested for both FeLV and FIV no sooner than 60 days later to detect false-negative results due to recent infection. Infected cats should be removed from the cattery. All newly acquired kittens and cats should be placed in isolation and tested for FeLV and FIV on arrival. Ideally, they should remain isolated until a second negative test for both viruses is obtained 60 days later, particularly if they originate from a cattery with unknown retro-virus status.
Queens sent to another facility for breeding should be tested before leaving the home cattery and should only be exposed to other cats that have tested negative for FeLV antigen and FIV antibody. Upon return to the home cattery, the queen should be kept in isolation and retested for FeLV and FIV in 60 days.
Cat shows are not significant sources of retrovirus infection because cats on exhibition are housed separately and the viruses are susceptible to commonly used disinfectants. In addition, environmental contamination of surfaces is not a risk due to the fragile nature of retroviruses. Therefore, cats that have left the cattery solely to attend a cat show do not need to be retested for FeLV or FIV or isolated unless direct contact with another cat of unknown retrovirus status has occurred.
In catteries that follow testing guidelines and maintain retrovirus-negative status, vaccination against FeLV or FIV is not necessary if no cats have access to the outdoors or to cats with unknown retrovirus status. Time and resources should be focused on maintaining a retrovirus-negative cattery through testing. Some catteries do not maintain breeding toms and rely totally on breeding services from other catteries. In such circumstances, vaccination of queens against FeLV is recommended in addition to testing of queens that leave the cattery for breeding.
Cats in shelters
The sheltering industry, especially in North America, is in a state of flux as rising community demands to save healthy and treatable animals challenge traditional animal control paradigms that relied on euthanasia as a population control tool. However, the number of cats admitted to shelters, especially during kitten season, continues to outstrip the capacity of many shelters to provide optimal care and to ensure that each cat has an ideal outcome tailored to its unique circumstances. These increased expectations require shelter managers to continuously re-evaluate their protocols and resource allocations to achieve the best overall results for cats both inside and outside shelters.
Shelter management guidelines from the Association of Shelter Veterinarians (ASV) state that protection of the health and welfare of cats in shelters requires vaccination against acute life-threatening infections, parasite treatment, treatment of illness or injury, adequate nutrition, species-appropriate housing, enrichment and behavioral care. 117 Protocols regarding additional care, such as retrovirus management, should be devised based on the best allocation of available resources to support the shelter’s goals, and should be updated based on the most current evidence-based medicine. These decisions must consider the financial and personnel investment associated with testing for infections that generally have a low prevalence, the predictive value of single point-in-time testing, the practicality of additional testing, the outcomes for cats testing positive and the consequences of releasing cats that might have retroviral infections.
Long-term institutionalization creates several physical and emotional threats, especially for cats. Shelter operations and animal welfare are generally best served by investing resources in supporting alternatives to shelter admission altogether or quickly transitioning shelter cats to a permanent home or return to the commu-nity. 118 This transition should include a smooth transfer of care and medical history from the shelter to a primary care veterinarian in the community, who will work with the adopter to complete any necessary preventive healthcare procedures and establish ongoing care.
The ASV recommends that cats eligible for adoption or relocation be screened for FeLV and FIV (sheltervet.org/assets/docs/position-statements/felvfivtesting.pdf). This screening is provided pre-adoption in some shelters. However, in many situations, limited shelter resources do not permit routine testing of all cats prior to adoption. In such cases, if cats are housed individually, shelters might prioritize testing higher-risk cats such as sick cats, cats with bite wounds and cats from high-risk situations such as hoarding cases. However, if cats are not tested for retrovirus infection in the shelter, a recommendation for post-adoption testing should be clearly explained to the adopter and documented in the cat’s file. Arrangements should be made by the adopter to have the new cat examined and tested by a veterinarian as soon as possible. The new cat should be kept separate from other cats until the test result is known. Although most sheltered cats are free of infection, post-adoption testing is likely to result in some new pet owners confronting difficult decisions about what to do with a newly adopted cat that is subsequently diagnosed with a retroviral infection. If one cat in a litter or group is later reported to be infected, the adopters of other cats with exposure to the infected cat should be notified so that in-contact cats can be monitored and tested.
Although the prevalence of FeLV and FIV in shelter cats in North America mirrors the low rates found in pet cats, thousands of infected cats are likely to pass through shelters each year. 4 Therefore, all cats entering shelters should be considered potentially infected, regardless of the environment from which they originated. Group-housing of untested cats should be strictly avoided. Retroviruses are efficiently transmitted by contaminated body fluids, particularly blood and saliva.26,106 For this reason, surgical and dentistry instruments, needles, endotracheal tubes and other potentially contaminated equipment should be thoroughly disinfected before use on the next patient, even cats from the same litter. 107
Both FeLV and FIV infection differ from other infectious diseases of importance in shelters, such as feline panleukopenia virus, feline calicivirus, feline herpesvirus and feline corona-virus, because retroviruses are easily inactivated with routine disinfection and are not spread by aerosol or indirect contact. Because of the low risk of transmission if cats are housed separately (Figure 6), testing for FeLV and FIV is optional for individually housed cats, and vaccination against FeLV or FIV is not recommended. However, in facilities in which cats are group-housed, FeLV and FIV testing is essential before cats enter the group. Cats entering foster homes should be tested if resident cats are present. For cats that are group-housed for extended periods of time or that live in sanctuaries, FeLV and FIV testing and FeLV vaccination are recommended. Long-term group housing increases the chance of exposure to infected cats inadvertently admitted with negative intake screening tests due to recent infection or regressive infection. Vaccination against FIV is not recommended in shelters because transmission of FIV among co-housed cats that do not fight appears to be uncom-mon, 38 the level of vaccine-induced immunity is variable, 101 and vaccine-induced positive antibody test results can complicate future determination of the true FIV infection status of vaccinated cats.
Figure 6.
Cats of unknown retrovirus status should be housed individually in shelters
The recommendations for FeLV and FIV testing and vaccination for shelters are summarized in Table 3.
Table 3.
Feline leukemia virus (FeLV) and feline immunodeficiency virus (FIV) testing and vaccination recommendations for healthy cats in animal shelters and free-roaming populations in North America
| FeLV/FIV testing | FeLV vaccination | FIV vaccination | |
|---|---|---|---|
| Individually housed cats | Optional | Not recommended | Not recommended |
| Short-term group-housed cats | Recommended | Not recommended | Not recommended |
| Foster cats | Recommended | Optional | Not recommended |
| Long-term group housing and sanctuaries | Recommended | Recommended | Not recommended |
| Trap-neuter-return cats | Not recommended | Not recommended | Not recommended |
Costs of testing can be minimized by enrolling in vendor shelter discount programs or using reference laboratories for multiple samples submitted at a time. However, some tests and laboratories are more accurate than others, so cost should not be the only consideration when selecting tests for use in shelters. 58 The presence of infection can vary within individual litters, community cat colonies and households. Therefore, it is not appropriate to conserve costs by testing one cat as a proxy for others. Practices such as testing a queen and not her kittens, or testing only a few members of a litter, colony or household, are both unreliable and a poor use of resources. Shelter medical records should individually identify each cat and accurately reflect the actual testing procedures and test brand utilized. In addition, test procedures must be performed as indicated by the manufacturer to maintain accuracy. Pooling multiple blood samples for use in a single POC test will reduce test sensitivity and should not be performed.
Although screening tests can be used in shelters, confirmation of infection poses a greater challenge because many shelters have rapid turnover of large numbers of cats and limited resources. Increased costs, delays and difficulty in interpreting discordant results are reasons why many shelters in North America do not pursue additional testing for positive POC results or avoid testing altogether. A simplified ‘one and done’ testing protocol with a reliable screening test will identify most infected cats (Figure 2, level 1 diagnostics). Exceptions include cats recently exposed to infection, kittens tested as unweaned neonates, and kittens with colostral antibodies against FIV. Anti-coagulated whole blood is the most convenient sample for testing cats in shelters. Secondary tests such as PCR (Figure 2, level 2 diagnostics) are valuable when they corroborate screening test results, but do not always clarify the status of cats when results are discordant.
Although these Guidelines broadly recommend testing all cats for retroviral infection, an exception exists for free-roaming stray and feral community cats in trap–neuter–return (TNR) programs. An overarching objective of TNR is to sterilize and vaccinate a sufficient proportion of free-roaming cats in order to reduce the population. The success of TNR programs hinges on deploying adequate financial and personnel resources to sterilize cats faster than they can reproduce. In studies in North America, the prevalence of FeLV infection is similar in outdoor owned pet cats and unowned community cats. 4 In some countries, the prevalence of FIV has been reported as higher in feral cats compared with owned cats. 4 Sterilization reduces the two most important modes of transmission: transmission from queen to kitten for FeLV and fighting among males for both FeLV and FIV.75,119,120 Because population control of community cats requires a commitment to sterilizing the largest number of cats possible, it is recommended that resources in TNR programs be focused on maximizing the number of cats sterilized and that retroviral testing not be incorporated as a routine practice. 121
Like the AAFP, the ASV does not recommend euthanasia of cats solely based on retrovirus infection (sheltervet.org/assets/docs/position-statements/managementofcatswhotestpositive.pdf). In response to goals to save all healthy and treatable cats, a growing number of shelters have expanded their adoption programs to include cats with FeLV and FIV infections. These cats should be held in single-cat housing or group accommodations that segregate them from uninfected cats pending adoption. There are no medical reasons to exclude retrovirus-infected cats from public adoption rooms in shelters, off-site adoption events, or satellite adoption centers such as those at pet stores if they are housed separately and properly documented. Similarly, legislation in the USA aimed at excluding retrovirus-infected cats from shelter adoption and interstate transport programs is not supported by current medical evidence.
Some shelters have developed specific marketing and education programs to ensure that these cats do not linger unnecessarily in shelter confinement and receive the post-adoption care they require, and to minimize the risk of spreading the infection to other cats in their new homes. One report demonstrated lack of transmission between FIV-infected and uninfected co-mingled cats in a shelter, suggesting that FIV-infected cats could cohabit with compatible FIV-negative cats with little risk under some circumstances. 38 Recent studies investigating the risk of FeLV transmission in the home have not been reported, but transmission of FeLV within a home appears to be more common. This suggests that FeLV-infected cats should be adopted into homes only with other FeLV-infected cats or as single cats. Cats with FIV have been shown to survive longer in normal home environments than in a high-density cat sanctuary. 48 Since stress can exacerbate the clinical course of both FeLV and FIV infection, adoption into a home-like setting is likely to result in better long-term outcomes.
Management of retrovirus-infected cats
Longevity
Cats infected with FIV have been shown to have variable lifespans, with some infected cats living as long as uninfected cats. Long-term monitoring of a 26-cat household with endemic FeLV and FIV infections revealed that all progressively FeLV-infected cats died within 5 years of diagnosis, but FIV infection did not affect survival over the same period. 37
A large study compared the survival of more than 1000 FIV-infected cats with more than 8000 age- and sex-matched uninfected control cats. 122 The median age of cats in the study was 5 years. Of cats not euthanized near the time of diagnosis, the median survival time after the first test was 4.9 years for FIV-positive cats and 6.0 years for negative controls. The study also compared more than 800 FeLV-infected cats with 7000 matched controls. The median age of cats in the study was 2 years. Of cats not euthanized near the time of diagnosis, the median survival time after diagnosis was 2.4 years for progressively infected FeLV cats and 6.3 years for negative controls. A high rate of euthanasia in the first year after diagnosis in the case of both retro-viruses was likely due to disease conditions that prompted the veterinary visit and subsequent diagnosis of FeLV or FIV, or to euthanasia of healthy retrovirus-infected cats for the purposes of infection control.
As part of a large study of FIV and FeLV prevalence in owned cats in Germany, a subset of 100 cats (19 FIV positive, 18 FeLV positive, 63 uninfected) was evaluated for survival times. 45 There was no significant difference in the mean survival time of FIV-infected cats (785 days) compared with uninfected cats (620 days). However, the mean survival time of progressively infected FeLV cats (312 days) was significantly shorter compared with uninfected cats (732 days).
A retrospective case-control study used Kaplan-Meier curves to compare survival times of 76 FIV-infected and 444 uninfected owned cats in Australia. 46 Survival of FIV-infected cats was not significantly different from that of uninfected cats. Another retrospective study evaluated survival times in 58 FIV-infected cats compared with 58 age- and sex-matched uninfected cats. 47 The median survival time of FIV-infected cats after diagnosis (3.9 years) was not significantly different from that of uninfected cats (5.9 years). In an assessment of lifetime medical records for shelter cats classified as FIV infected (n = 63), progressively FeLV infected (n = 22), coinfect-ed (n = 4) or uninfected (n = 11), longevity was similar in FIV-infected cats compared with non-infected cats. 123 Cats with progressive FeLV infection and cats coinfected with FeLV and FIV had significantly shorter lifespans as well as a higher incidence of lymphoma.
These studies demonstrate that retrovirus-infected cats, especially FIV-infected cats, may experience normal longevity with appropriate husbandry and disease management. Diagnosis of a retrovirus infection should not be the sole criterion for euthanasia. Owners should be educated in detail about options for care of infected cats. Furthermore, owners should be made aware of the potential for false-positive test results and the clinician should offer additional testing whenever possible and feasible. Provision of an accurate prognosis and careful monitoring of each cat will assist owners in the care of the retrovirus-infected cat.
Retrovirus-infected cats are subject to the same diseases that befall cats free of those infections. A disease diagnosed in a retrovirus-infected cat might or might not be related to the retrovirus infection. 120 However, knowledge of current FIV and FeLV status in such cats is important because the presence of a retrovirus infection impacts long-term management.
Housing and environment
There are benefits to housing retrovirus-infected cats indoors and allowing access to the outdoors only within secure enclosures. Benefits include reduced exposure to other infectious diseases, reduced risk of trauma and injury, and limited ability to transmit retro-virus infection to other cats. Good nutrition and husbandry, and an enriched lifestyle if confined indoors, are essential to maintain good health. 48
Each case must be evaluated individually as some outdoor-living cats will not readily adapt to an indoor-only lifestyle. The stress of an enforced lifestyle change might have detrimental medical and behavioral effects. In some circumstances, it might be less stressful to allow retrovirus-infected cats access to the outdoors, preferably within a secure enclosure such as a ‘catio’. Cats that do not exhibit high-risk behaviours (eg, breeding, reproduction, fighting) pose little risk of disease transmission to other cats.
With proper care and environmental management, FIV-infected cats can live for many years. In a 22-month study, FIV-infected cats living in homes alone or with one other cat were compared with FIV-infected cats living in a population-dense multi-cat sanctuary. 48 The latter group of cats were more likely to display clinical signs related to their disease, with 51% of these cats dying during the study period. Lymphoma was the most common cause of mortality in these cases. The FIV-infected cats living in low-population households did not display clinical signs during the study period and only one death, owing to hypertrophic cardiomyopathy (presumably unrelated), was observed. The conclusion from this study was that management and housing conditions impact the development of clinical signs, disease progression and survival time in FIV-infected cats.
Housing conditions also appear to affect outcomes for FeLV-infected cats. In a study of cats in two rescue sanctuaries that group-housed FeLV-infected cats with uninfected cats without separating clinically ill cats from healthy cats, the prevalence of FeLV was more than 20-fold higher than in the general pet cat population. 15 Not only were cats more likely to be infected in the sanctuaries, but they were also more likely to develop the progressive form of infection, leading to poorer long-term outcomes.
The apparent benefit of low-density housing can be attributed to reduced levels of environmental stress, infectious pressure and coinfec-tions. Careful management of resources in multi-cat households might assist in reducing these stressors, leading to better clinical outcomes. Where possible, retrovirus-infected cats should be housed in low-density environments where stressors are reduced, resources are ample, and caregivers can observe patient health status carefully. Environmental needs of indoor cats have been detailed elsewhere. 110
Healthcare
Preventive healthcare
Cats infected with FeLV or FIV should receive preventive healthcare checkups at least every 6 months for prompt detection of changes in their health status. Veterinarians should obtain a detailed history to help identify changes requiring more intensive investigation and should perform a thorough physical examination at each visit. Special attention should be paid to the oral cavity because dental and oral diseases are more common in retrovirus-infected cats.48,123,124 Lymph nodes should be evaluated for changes in size and shape. All cats should undergo a thorough examination of the anterior and posterior segments of the eye. 125 The skin should be examined closely for evidence of external parasite infestations, fungal disease and neoplastic changes.
Retrovirus-infected cats should be prescribed appropriate prophylaxis for internal and external parasites. In areas where heart-worm is prevalent, cats should be on monthly chemoprophylaxis. Use of routine, consistent parasite control according to the Companion Animal Parasite Council recommendations (capcvet.org) will reduce the risk of secondary infection and disease in these potentially immunosuppressed cats.
Nutritional support is key to maintaining good health in these patients. A nutritionally balanced and complete feline diet appropriate to the cat’s life stage should be fed. Raw meat and raw dairy products should be avoided because the risk of food-borne bacterial and parasitic diseases is likely greater in these potentially immunosuppressed cats. Periodic nutritional assessments should evaluate food intake, body condition score (BCS), muscle condition score (MCS) and quality of nutrition to improve health and alert the clinician to early problems. Unexpected downward trends in body weight or reductions in BCS or MCS should prompt the clinician to investigate further. In any cat, changes in body weight can precede other signs of clinical disease by months or even years.126,127
A complete blood count should be performed annually for FIV-infected cats and at least every 6 months for FeLV-infected cats because of the greater frequency of virus-related hematologic disorders in FeLV-infected cats. A serum biochemical analysis and complete urinalysis (urine specific gravity, urine chemistries and sediment examination) should be performed annually for FeLV- and FIV-infected cats. Urine samples should be collected by cystocentesis so that bacterial cultures can be performed if indicated. Fecal examinations should be performed as needed.
Vaccine selection and immunization intervals for healthy cats with FeLV or FIV infection should be based on individual risk assessments using the AAFP vaccination guidelines developed for cats in general. 100 Vaccination should not be avoided in cats with retroviral infection because they can develop more severe clinical disease related to panleukope-nia virus and upper respiratory tract infections after natural exposure compared with unin-fected cats.128–131 Vaccination for rabies should follow local regulations. There is little evidence to suggest modified-live virus vaccines are a risk in retrovirus-infected cats and the response of asymptomatic retrovirus-infected cats can be similar to uninfected cats. 132
Sexually intact male and female cats should be neutered to reduce stress associated with estrus and mating behaviors. Neutered animals are also less likely to roam away from home and interact aggressively with other cats.
Surgical management and perioperative care
In otherwise healthy, retrovirus-infected cats, surgical procedures should be used as required to maintain health and manage disease. Retrovirus-infected cats should receive the same quality of anesthetic, analgesic, surgical and perioperative care as given to all feline patients. Preoperative evaluation, including laboratory testing, should follow the same standard of care as for uninfected cats.
As for all cats, the use of perioperative antibiotics should be reserved for those individuals with clear evidence of immunosup-pression and/or those undergoing surgeries where the risk of bacterial contamination is moderate to high. 133 Multimodal analgesia plans should be used in all cats when indicated, especially if they have concurrent painful conditions such as gingivostomatitis.
Management of clinical illness
Treatment of secondary diseases
Medical care of the clinically ill retrovirus-infected cat should be based on a complete review of the patient’s clinical status, the owner’s goals, and available therapeutics and their relative safety or toxicity. The patient should first be evaluated to determine whether the illness is unrelated to the retrovirus infection, secondary to immunosuppression from retrovirus infection or a direct cause of the retrovirus infection (see box below). Patients experiencing illness unrelated to retrovirus infection should be managed according to standard protocols for the specific health condi-tion(s). More vigilant and frequent monitoring of retrovirus-infected patients might be indicated depending on their health condition.
Retroviruses can contribute to any illness either as a direct effect of the viral infection or a secondary effect through mechanisms such as immunosuppression. A detailed review of the clinical aspects of retrovirus infections in cats has been published and should be con-sulted. 7 Careful assessment of each patient will assist the clinician in determining the etiology of the problem and the type of care required.
Cats infected with FeLV or FIV are at increased risk of developing neoplasia (primarily lymphoma), bone marrow suppression, neurologic disease and infections secondary to immunosuppression. An increased risk of inflammatory oral disease has also been associated with retroviral infection in cats.48,123,124 Retrovirus-infected patients with severe gingivostomatitis are most likely to benefit long term from full mouth extraction, with complete extraction of all tooth roots, rather than medical management. Anemia in cats infected with FeLV can be due to various causes including the direct effect of the virus on bone marrow (non-regenerative anemia), secondary infections (eg, infections with Mycoplasma species) and other mechanisms. An attempt should always be made to identify and treat underlying causes, especially for regenerative anemia. For a full discussion of the diagnosis and management of health conditions in retrovirus-infected cats, the reader is referred to the resources listed in the box above.
While disease status in human patients with HIV infection is assessed with various markers such as the CD4:CD8 ratio, these markers have not proven reliable in cats with natural retroviral infections. Weight loss can be indirectly related to retrovirus infection, 48 but it is also associated with many other diseases. Quality of life parameters can include the use of scoring systems, such as a modified Karnofsky score, which allows for assessment by both clinician and owner to detect diminishing quality of life. 134
Targeted therapeutics
Highly active combination antiretroviral therapies (‘drug cocktails’) are the mainstay of treatment in HIV-infected patients and result in longer survival times and improved quality of life. Unfortunately, few large long-term controlled studies in naturally infected cats have shown long-lasting benefits of using antiviral drugs. Drugs available to treat retrovirus-infected cats are limited and tend to show lower efficacy in feline patients compared with human patients. Many of these drugs require impractical long-term use, are costly and often come with mild to severe toxic side effects that limit their utility.
Zidovudine (azidothymidine; AZT) is a nucleoside analog and one of the few antiviral compounds used in both FeLV and FIV infections. The drug can reduce viral load and improve immunologic and clinical status, particularly in cats with neurologic signs or stom-atitis. 135 In cases where clinical illness is thought to be attributable to retroviral infection, AZT can be given at 5–10 mg/kg PO q12h. The higher dose should be used carefully in FeLV-infected cats because adverse effects, particularly non-regenerative anemia, can develop. 136
Interferons (human and feline) are often used in retrovirus-infected cats as antivirals and immunomodulators in the hope that viral load can be reduced and recovery from associated clinical syndromes can be facilitated. Unfortunately, well designed clinical trials of these drugs in retrovirus-infected cats are lacking or have failed to confirm therapeutic benefits. Feline interferon omega (Virbagen Omega; Virbac Animal Health) is available in some countries. A study using parenteral feline interferon omega showed a higher survival rate after 9 months in interferon-treated FeLV-infected cats when compared with a placebo-treated FeLV-infected control group. 137 Other studies provided some evidence of clinical improvement in FIV- or FeLV-infected cats,138,139 but those beneficial effects might not have been attributed to treatment of the retrovirus infection but rather to treatment of secondary infections. No controlled studies using oral feline interferon omega in FIV- or FeLV-infected cats have been published to date. For more information on antiretroviral chemotherapy for retrovirus-infected cats, the reader is referred to published reviews. 135
Summary Points
Retrovirus infections remain common and important diseases of cats worldwide.
Ongoing research into viral pathogenesis and improvements in diagnostic testing continue to refine our state of knowledge about these viruses.
Veterinary practitioners are advised to take advantage of current peer-reviewed published reviews and recommendations for testing and management of cats in different populations.
Additional resources for interested veterinary professionals are found in the box on page 23.
Supplemental Material
Feline Retrovirus Testing Client Brochure
Appendix: Client brochure
Footnotes
The Panelists have no conflicts of interest to declare.
Funding: The Panelists received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this article.
Ethical approval: This work did not involve the use of animals and therefore ethical approval was not required.
Informed consent: This work did not involve the use of animals and therefore informed consent was not required. For any animals individually identifiable within this publication, informed consent for their use in the publication (either verbal or written) was obtained from the people involved.
Contributor Information
Susan Little, Bytown Cat Hospital, Ottawa, ON, Canada.
Julie Levy, Maddie’s Shelter Medicine Program, Department of Small Animal Clinical Sciences, College of Veterinary Medicine, University of Florida, Gainesville, FL, USA.
Katrin Hartmann, Clinic of Small Animal Medicine, Centre for Clinical Veterinary Medicine, LMU Munich, Munich, Germany.
Regina Hofmann-Lehmann, Clinical Laboratory, Department of Clinical Diagnostics and Services, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland.
Margaret Hosie, MRC-University of Glasgow Centre for Virus Research, Glasgow, UK.
Glenn Olah, Albuquerque Cat Clinic, Albuquerque, NM, USA.
References
- 1. Buch J, Beall M, o’Connor T, et al. Worldwide clinic-based serologic survey of FIV antibody and FeLV antigen in cats. ACVIM Forum, National Harbor, Md, 8–10 June 2017. [Google Scholar]
- 2. Levy J K, Scott HM, Lachtara JL, et al. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in North America and risk factors for seropositivity. J Am Vet Med Assoc 2006; 228: 371–376. [DOI] [PubMed] [Google Scholar]
- 3. Little S, Sears W, Lachtara J, et al. Seroprevalence of feline leukemia virus and feline immunodeficiency virus infection among cats in Canada. Can Vet J 2009; 50: 644–648. [PMC free article] [PubMed] [Google Scholar]
- 4. Burling AN, Levy JK, Scott HM, et al. Seroprevalences of feline leukemia virus and feline immunodeficiency virus infection in cats in the United States and Canada and risk factors for seropositivity. J Am Vet Med Assoc 2017; 251: 187–194. [DOI] [PubMed] [Google Scholar]
- 5. Studer N, Lutz H, Saegerman C, et al. Pan-European study on the prevalence of the feline leukaemia virus infection -reported by the European Advisory Board on Cat Diseases (ABCD Europe). Viruses 2019; 11. DOI: 10.3390/v11110993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hartmann K, Levy JK. Feline leukemia virus infection. In: Ettinger SJ, Feldman EC, Cote E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier Saunders, 2017, pp 2442–2455. [Google Scholar]
- 7. Hartmann K. Clinical aspects of feline immunodeficiency and feline leukemia virus infection. Vet Immunol Immunopathol 2012; 143: 190–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cattori V, Tandon R, Riond B, et al. The kinetics of feline leukaemia virus shedding in experimentally infected cats are associated with infection outcome. Vet Microbiol 2009; 133: 292–296. [DOI] [PubMed] [Google Scholar]
- 9. Rojko JL, Hoover EA, Mathes LE, et al. Pathogenesis of experimental feline leukemia virus infection. J Natl Cancer Inst 1979; 63: 759–768. [DOI] [PubMed] [Google Scholar]
- 10. Torres AN, Mathiason CK, Hoover EA. Re-examination of feline leukemia virus:host relationships using real-time PCR. Virology 2005; 332: 272–283. [DOI] [PubMed] [Google Scholar]
- 11. Hofmann-Lehmann R, Cattori V, Tandon R, et al. Vaccination against the feline leukaemia virus: outcome and response categories and long-term follow-up. Vaccine 2007; 25: 5531–5539. [DOI] [PubMed] [Google Scholar]
- 12. Hofmann-Lehmann R, Cattori V, Tandon R, et al. How molecular methods change our views of FeLV infection and vaccination. Vet Immunol Immunopathol 2008; 123: 119–123. [DOI] [PubMed] [Google Scholar]
- 13. Lutz H, Addie D, Belak S, et al. Feline leukaemia: ABCD guidelines on prevention and management. J Feline Med Surg 2009; 11: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Englert T, Lutz H, Sauter-Louis C, et al. Survey of the feline leukemia virus infection status of cats in Southern Germany. J Feline Med Surg 2012; 14: 392–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Westman M, Norris J, Malik R, et al. The diagnosis of feline leukaemia virus (FeLV) infection in owned and group-housed rescue cats in Australia. Viruses 2019; 11: 503. DOI: 10.3390/v11060503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hofmann-Lehmann R, Tandon R, Boretti FS, et al. Re -assessment of feline leukaemia virus (FeLV) vaccines with novel sensitive molecular assays. Vaccine 2006; 24: 1087–1094. [DOI] [PubMed] [Google Scholar]
- 17. Hofmann-Lehmann R, Huder JB, Gruber S, et al. Feline leukaemia provirus load during the course of experimental infection and in naturally infected cats. J Gen Virol 2001; 82: 1589–1596. [DOI] [PubMed] [Google Scholar]
- 18. McClelland AJ, Hardy WD, Zuckerman EE. Prognosis of healthy feline leukemia virus infected cats. In: Hardy WD, Essex M, McClelland AJ. (eds). Development in cancer research. Amsterdam: Elsevier Scientific Publishing, 1980, pp 121–126. [Google Scholar]
- 19. Helfer-Hungerbuehler AK, Widmer S, Kessler Y, et al. Long-term follow up of feline leukemia virus infection and characterization of viral RNA loads using molecular methods in tissues of cats with different infection outcomes. Virus Res 2015; 197: 137–150. [DOI] [PubMed] [Google Scholar]
- 20. Pedersen NC, Theilen G, Keane MA, et al. Studies of naturally transmitted feline leukemia virus infection. Am J Vet Res 1977; 38: 1523–1531. [PubMed] [Google Scholar]
- 21. Lutz H, Pedersen NC, Theilen GH. Course of feline leukemia virus infection and its detection by enzyme linked immunosorbent assay and monoclonal antibodies. Am J Vet Res 1983; 44: 2054–2059. [PubMed] [Google Scholar]
- 22. Flynn JN, Hanlon L, Jarrett O. Feline leukaemia virus: protective immunity is mediated by virus-specific cytotoxic T lymphocytes. Immunology 2000; 101: 120–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flynn JN, Dunham SP, Watson V, et al. Longitudinal analysis of feline leukemia virus-specific cytotoxic T lymphocytes: correlation with recovery from infection. J Virol 2002; 76: 2306–2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pepin AC, Tandon R, Cattori V, et al. Cellular segregation of feline leukemia provirus and viral RNA in leukocyte subsets of long-term experimentally infected cats. Virus Res 2007; 127: 9–16. [DOI] [PubMed] [Google Scholar]
- 25. Cattori V, Tandon R, Pepin A, et al. Rapid detection of feline leukemia virus provirus integration into feline genomic DNA. Mol Cell Probes 2006; 20: 172–181. [DOI] [PubMed] [Google Scholar]
- 26. Nesina S, Helfer-Hungerbuehler AK, Riond B, et al. Retroviral DNA – the silent winner: blood transfusion containing latent feline leukemia provirus causes infection and disease in naive recipient cats. Retrovirology 2015; 12. DOI: 10.1186/s12977-015-0231-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stutzer B, Muller F, Majzoub M, et al. Role of latent feline leukemia virus infection in nonregenerative cytopenias of cats. J Vet Intern Med 2010; 24: 192–197. [DOI] [PubMed] [Google Scholar]
- 28. Stutzer B, Simon K, Lutz H, et al. Incidence of persistent viraemia and latent feline leukaemia virus infection in cats with lymphoma. J Feline Med Surg 2011; 13: 81–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Helfer-Hungerbuehler AK, Cattori V, Boretti FS, et al. Dominance of highly divergent feline leukemia virus A progeny variants in a cat with recurrent viremia and fatal lymphoma. Retrovirology 2010; 7. DOI: 10.1186/1742-4690-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. McLuckie A, Barrs V, Lindsay S, et al. Molecular diagnosis of Felis catus gammaherpesvirus 1 (FcaGHVl) infection in cats of known retrovirus status with and without lymphoma. Viruses 2018; 10. DOI: 10.3390/v10030128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Major A, Cattori V, Boenzli E, et al. Exposure of cats to low doses of FeLV: seroconversion as the sole parameter of infection. Vet Res 2010; 41. DOI: 10.1051/vetres/2009065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Boenzli E, Hadorn M, Hartnack S, et al. Detection of antibodies to the feline leukemia virus (FeLV) transmembrane protein p15E: an alternative approach for serological FeLV diagnosis based on antibodies to p15E. J Clin Microbiol 2014; 52: 2046–2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. O’Neil LL, Burkhard MJ, Diehl LJ, et al. Vertical transmission of feline immunodeficiency virus. Semin Vet Med Surg (Small Anim) 1995; 10: 266–278. [PubMed] [Google Scholar]
- 34. Allison RW, Hoover EA. Covert vertical transmission of feline immunodeficiency virus. AIDS Res Human Retrovir 2003; 19; 421–434. [DOI] [PubMed] [Google Scholar]
- 35. Ueland K, Nesse LL. No evidence of vertical transmission of naturally acquired feline immunodeficiency virus infection. Vet Immunol Immunopathol 1992; 33; 301–308. [DOI] [PubMed] [Google Scholar]
- 36. Pu R, Okada S, Little ER, et al. Protection of neonatal kittens against feline immunodeficiency virus infection with passive maternal antiviral antibodies. AIDS 1995; 9: 235–242. [PubMed] [Google Scholar]
- 37. Addie DD, Dennis JM, Toth S, et al. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet Rec 2000; 146: 419–424. [DOI] [PubMed] [Google Scholar]
- 38. Litster AL. Transmission of feline immunodeficiency virus (FIV) among cohabiting cats in two cat rescue shelters. Vet J 2014; 201: 184–188. [DOI] [PubMed] [Google Scholar]
- 39. Jordan HL, Howard J, Barr MC, et al. Feline immunodeficiency virus is shed in semen from experimentally and naturally infected cats. AIDS Res Human Retrovir 1998; 14: 1087–1092. [DOI] [PubMed] [Google Scholar]
- 40. Egberink H, Horzinek MC. Animal immunodeficiency viruses. Vet Microbiol 1992; 33: 311–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Yamamoto JK, Pu R, Sato E, et al. Feline immunodeficiency virus pathogenesis and development of a dual-subtype feline-immunodeficiency-virus vaccine. AIDS 2007; 21; 547–563. [DOI] [PubMed] [Google Scholar]
- 42. Hoffmann-Fezer G, Thum I, Herbold M, et al. Decline in CD4+ cell numbers in cats with naturally acquired feline immunodeficiency virus infection. J Virol 1992; 66: 1484–1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hoffmann-Fezer G, Mortelbauer W, Hartmann K, et al. Comparison of T-cell subpopulations in cats naturally infected with feline leukaemia virus or feline immunodeficiency virus. Res Vet Sci 1996; 61: 222–226. [DOI] [PubMed] [Google Scholar]
- 44. Hartmann K. Role of retroviruses in feline lymphoma. Eur J Comp Anim Pract 2015; 25: 4–15. [Google Scholar]
- 45. Gleich SE, Krieger S, Hartmann K. Prevalence of feline immunodeficiency virus and feline leukaemia virus among client-owned cats and risk factors for infection in Germany. J Feline Med Surg 2009; 11: 985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liem BP, Dhand NK, Pepper AE, et al. Clinical findings and survival in cats naturally infected with feline immunodeficiency virus. J Vet Intern Med 2013; 27: 798–805. [DOI] [PubMed] [Google Scholar]
- 47. Ravi M, Wobeser GA, Taylor SM, et al. Naturally acquired feline immunodeficiency virus (FIV) infection in cats from western Canada: prevalence, disease associations, and survival analysis. Can Vet J 2010; 51: 271–276. [PMC free article] [PubMed] [Google Scholar]
- 48. Beczkowski PM, Litster A, Lin TL, et al. Contrasting clinical outcomes in two cohorts of cats naturally infected with feline immunodeficiency virus (FIV). Vet Microbiol 2015; 176: 50–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Wilkes RP, Anis E, Dunbar D, et al. Rapid and sensitive insulated isothermal PCR for point-of-need feline leukaemia virus detection. J Feline Med Surg 2018; 20: 362–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wilkes RP, Kania SA, Tsai YL, et al. Rapid and sensitive detection of feline immunodeficiency virus using an insulated isothermal PCR-based assay with a point-of-need PCR detection platform. J Vet Diagn Invest 2015; 27: 510–515. [DOI] [PubMed] [Google Scholar]
- 51. Frankenfeld J, Meili T, Meli ML, et al. Decreased sensitivity of the serological detection of feline immunodeficiency virus infection potentially due to imported genetic variants. Viruses 2019, 11. DOI: 10.3390/v11080697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hartmann K, Griessmayr P, Schulz B, et al. Quality of different in-clinic test systems for feline immunodeficiency virus and feline leukaemia virus infection. J Feline Med Surg 2007; 9: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pinches MD, Diesel G, Helps CR, et al. An update on FIV and FeLV test performance using a Bayesian statistical approach. Vet Clin Pathol 2007; 36: 141–147. [DOI] [PubMed] [Google Scholar]
- 54. Sand C, Englert T, Egberink H, et al. Evaluation of a new in-clinic test system to detect feline immunodeficiency virus and feline leukemia virus infection. Vet Clin Pathol 2010; 39: 210–214. [DOI] [PubMed] [Google Scholar]
- 55. Hawkins EC. Saliva and tear tests for feline leukemia virus. J Am Vet Med Assoc 1991; 199: 1382–1385. [PubMed] [Google Scholar]
- 56. Westman ME, Malik R, Hall E, et al. Comparison of three feline leukaemia virus (FeLV) point-of-care antigen test kits using blood and saliva. Comp Immunol Microbiol Infect Dis 2017; 50: 88–96. [DOI] [PubMed] [Google Scholar]
- 57. Tandon R, Cattori V, Gomes-Keller MA, et al. Quantitation of feline leukaemia virus viral and proviral loads by TaqMan real-time polymerase chain reaction. J Virol Methods 2005; 130: 124–132. [DOI] [PubMed] [Google Scholar]
- 58. Levy JK, Crawford PC, Tucker SJ. Performance of 4 point-of-care screening tests for feline leukemia virus and feline immunodeficiency virus. J Vet Intern Med 2017; 31: 521–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Krecic MR, Velineni S, Meeus P, et al. Diagnostic performances of two rapid tests for detection of feline leukemia virus antigen in sera of experimentally feline leukemia virus-infected cats. JFMS Open Rep 2018; 4. DOI: 10.1177/2055116917748117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Jarrett O, Golder MC, Stewart MF. Detection of transient and persistent feline leukaemia virus infections. Vet Rec 1982; 110: 225–228. [DOI] [PubMed] [Google Scholar]
- 61. Beall MJ, Buch J, Cahill RJ, et al. Evaluation of a quantitative enzyme-linked immunosorbent assay for feline leukemia virus p27 antigen and comparison to proviral DNA loads by real-time polymerase chain reaction. Comp Immunol Microbiol Infect Dis 2019; 67. DOI: 10.1016/j.cimid.2019.101348. [DOI] [PubMed] [Google Scholar]
- 62. Gomes-Keller MA, Tandon R, Gonczi E, et al. Shedding of feline leukemia virus RNA in saliva is a consistent feature in viraemic cats. Vet Microbiol 2006; 112: 11–21. [DOI] [PubMed] [Google Scholar]
- 63. Gomes-Keller MA, Gonczi E, Tandon R, et al. Detection of feline leukemia virus RNA in saliva from naturally infected cats and correlation of PCR results with those of current diagnostic methods. J Clin Microbiol 2006; 44: 916–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Levy JK, Crawford PC, Slater MR. Effect of vaccination against feline immunodeficiency virus on results of serologic testing in cats. J Am Vet Med Assoc 2004; 25: 1558–1561. [DOI] [PubMed] [Google Scholar]
- 65. Ammersbach M, Little S, Bienzle D. Preliminary evaluation of a quantitative polymerase chain reaction assay for diagnosis of feline immunodeficiency virus infection. J Feline Med Surg 2013; 15: 725–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Westman ME, Malik R, Hall E, et al. Determining the feline immunodeficiency virus (FIV) status of FIV-vaccinated cats using point-of-care antibody kits. Comp Immunol Microbiol Infect Dis 2015; 42: 43–52. [DOI] [PubMed] [Google Scholar]
- 67. Nichols J, Weng HY, Litster A, et al. Commercially available enzyme-linked immunosorbent assay and polymerase chain reaction tests for detection of feline immunodeficiency virus infection. J Vet Intern Med 2017; 31: 55–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Klein D, Leutenegger CM, Bahula C, et al. Influence of pre-assay and sequence variations on viral load determination by a multiplex real-time reverse transcriptase-polymerase chain reaction for feline immunodeficiency virus. J AIDS 2001; 26: 8–20. [DOI] [PubMed] [Google Scholar]
- 69. Beczkowski PM, Hughes J, Biek R, et al. Feline immunodeficiency (FIV) env recombinants are common in natural infections. Retrovirology 2014; 11. DOI: 10.1186/s12977-014-0080-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Crawford PC, Slater MR, Levy JK. Accuracy of poly-merase chain reaction assays for diagnosis of feline immunodeficiency virus infection in cats. J Am Vet Med Assoc 2005; 226: 1503–1507. [DOI] [PubMed] [Google Scholar]
- 71. MacDonald K, Levy JK, Tucker SJ, et al. Effects of passive transfer of immunity on results of diagnostic tests for antibodies against feline immunodeficiency virus in kittens born to vaccinated queens. J Am Vet Med Assoc 2004; 225: 1554–1557. [DOI] [PubMed] [Google Scholar]
- 72. Westman ME, Malik R, Hall E, et al. Diagnosing feline immunodeficiency virus (FIV) infection in FIV-vaccinated and FIV-unvaccinated cats using saliva. Comp Immunol Microbiol Infect Dis 2016; 46: 66–72. [DOI] [PubMed] [Google Scholar]
- 73. Crawford C. Does a DIVA test exist for differentiating FIV infection from FIV vaccination? J Vet Intern Med 2016; 30: 1475–1476. [Google Scholar]
- 74. Westman ME, Malik R, Hall E, et al. Duration of antibody response following vaccination against feline immunodeficiency virus. J Feline Med Surg 2017; 19: 1055–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Goldkamp CE, Levy JK, Edinboro CH, et al. Seroprevalences of feline leukemia virus and feline immunodeficiency virus in cats with abscesses or bite wounds and rate of veterinarian compliance with current guidelines for retro-virus testing. J Am Vet Med Assoc 2008; 232: 1152–1158. [DOI] [PubMed] [Google Scholar]
- 76. Pacitti AM, Jarrett O, Hay D. Transmission of feline leukaemia virus in the milk of a non-viraemic cat. Vet Rec 1986; 118: 381–384. [DOI] [PubMed] [Google Scholar]
- 77. Yamamoto J, Hansen H, Ho E, et al. Epidemiologic and clinical aspects of feline immunodeficiency virus infection in cats from the continental United States and Canada and possible mode of transmission. J Am Vet Med Assoc 1989; 194: 213–220. [PubMed] [Google Scholar]
- 78. Chhetri BK, Berke O, Pearl DL, et al. Comparison of risk factors for seropositivity to feline immunodeficiency virus and feline leukemia virus among cats: a case-case study. BMC Vet Res 2015; 11. DOI: 10.1186/s12917-015-0339-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. de Oliveira Medeiros S, Martins AN, Dias CG, et al. Natural transmission of feline immunodeficiency virus from infected queen to kitten. Virol J 2012; 9: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. De Almeida NR, Danelli MGM, da Silva LHP, et al. Prevalence of feline leukemia virus infection in domestic cats in Rio de Janeiro. J Feline Med Surg 2012; 14: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ramirez H, Autran M, Garcia MM, et al. Genotyping of feline leukemia virus in Mexican housecats. Arch Virol 2016; 161: 1039–1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Hosie MJ, Robertson C, Jarrett O. Prevalence of feline leukaemia virus and antibodies to feline immunodeficiency virus in cats in the United Kingdom. Vet Rec 1989; 125: 293–297. [DOI] [PubMed] [Google Scholar]
- 83. Little S, Bienzle D, Carioto L, et al. Feline leukemia virus and feline immunodeficiency virus in Canada: recommendations for testing and management. Can Vet J 2011; 52: 849–855. [PMC free article] [PubMed] [Google Scholar]
- 84. Hofmann-Lehmann R, Gonczi E, Riond B, et al. Feline leukemia virus infection: importance and current situation in Switzerland. Schweiz Arch Tierheilkd 2018; 160: 95–105. [DOI] [PubMed] [Google Scholar]
- 85. Grosenbaugh DA, Leard T, Pardo MC, et al. Comparison of the safety and efficacy of a recombinant feline leukemia virus (FeLV) vaccine delivered transdermally and an inactivated FeLV vaccine delivered subcutaneously. Vet Ther 2004; 5: 258–262. [PubMed] [Google Scholar]
- 86. Grosenbaugh DA, Frances-Duvert V, Abedi S, et al. Efficacy of a nonadjuvanted recombinant FeLV vaccine and two inactivated FeLV vaccines when subject to consistent virulent FeLV challenge conditions. Biologicals 2017; 49: 76–80. [DOI] [PubMed] [Google Scholar]
- 87. Sparkes AH. Feline leukaemia virus: a review of immunity and vaccination. J Small Anim Pract 1997; 38: 187–194. [DOI] [PubMed] [Google Scholar]
- 88. Sparkes AH. Feline leukaemia virus and vaccination. J Feline Med Surg 2003; 5: 97–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Stuke K, King V, Southwick K, et al. Efficacy of an inactivated FeLV vaccine compared to a recombinant FeLV vaccine in minimum age cats following virulent FeLV challenge. Vaccine 2014; 32: 2599–2603. [DOI] [PubMed] [Google Scholar]
- 90. Hofmann-Lehmann R, Levy LS, Willett BJ. Comparing the efficacy of FeLV vaccines: Comment on: Stuke, K. et al. Efficacy of an inactivated FeLV vaccine compared to a recombinant FeLV vaccine in minimum age cats following virulent FeLV challenge [letter]. Vaccine 2015; 33: 2737–2738. [DOI] [PubMed] [Google Scholar]
- 91. Patel M, Carritt K, Lane J, et al. Comparative efficacy of feline leukemia virus (FeLV) inactivated whole-virus vaccine and canarypox virus-vectored vaccine during virulent FeLV challenge and immunosuppression. Clin Vaccine Immunol 2015; 22: 798–805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Patel M, Carritt K, Lane J, et al. Reply to immunosuppression in a comparative study of feline leukemia virus vaccines [letter]. Clin Vaccine Immunol 2015; 22: 1296–1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Poulet H, Thibault JC, Masias A. Immunosuppression in a comparative study of feline leukemia virus vaccines [letter]. Clin Vaccine Immunol 2015; 22: 1294–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Torres AN, O’Halloran KP, Larson LJ, et al. Feline leukemia virus immunity induced by whole inactivated virus vaccination. Vet Immunol Immunopathol 2010; 134: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Hofmann-Lehmann R, Holznagel E, Aubert A, et al. Recombinant FeLV vaccine: long-term protection and effect on course and outcome of FIV infection. Vet Immunol Immunopathol 1995; 46: 127–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Hoover EA, Mullins JI, Chu HJ, et al. Efficacy of an inactivated feline leukemia virus vaccine. AIDS Res Hum Retroviruses 1996; 12: 379–383. [DOI] [PubMed] [Google Scholar]
- 97. Harbour DA, Gunn-Moore DA, Gruffydd-Jones TJ, et al. Protection against oronasal challenge with virulent feline leukaemia virus lasts for at least 12 months following a primary course of immunisation with Leukocell 2 vaccine. Vaccine 2002; 20: 2866–2872. [DOI] [PubMed] [Google Scholar]
- 98. Jirjis F, Davis T, Lane J, et al. Protection against feline leukemia virus challenge for at least 2 years after vaccination with an inactivated feline leukemia virus vaccine. Vet Ther 2010; 11: E1–E6. [PubMed] [Google Scholar]
- 99. Helfer-Hungerbuehler AK, Spiri AM, Riond B, et al. No benefit of therapeutic vaccination in clinically healthy cats persistently infected with feline leukemia virus. Vaccine 2015; 33: 1578–1585. [DOI] [PubMed] [Google Scholar]
- 100. Scherk MA, Ford RB, Gaskell RM, et al. AAFP Feline Vaccination Advisory Panel report. J Feline Med Surg 2013; 15: 785–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Westman ME, Malik R, Hall E, et al. The protective rate of the feline immunodeficiency virus vaccine: an Australian field study. Vaccine 2016; 34: 4752–4758. [DOI] [PubMed] [Google Scholar]
- 102. Dunham SP, Bruce J, MacKay S, et al. Limited efficacy of an inactivated feline immunodeficiency virus vaccine. Vet Rec 2006; 158: 561–562. [DOI] [PubMed] [Google Scholar]
- 103. Beczkowski PM, Harris M, Techakriengkrai N, et al. Neutralising antibody response in domestic cats immunised with a commercial feline immunodeficiency virus (FIV) vaccine. Vaccine 2015; 33: 977–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Kramer A, Schwebke I, Kampf G. How long do nosoco-mial pathogens persist on inanimate surfaces? A systematic review. BMC Infect Dis 2006; 6. DOI: 10.1186/1471-2334-6-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Terpstra FG, van den Blink AE, Bos LM, et al. Resistance of surface-dried virus to common disinfection procedures. J Hosp Infect 2007; 66: 332–338. [DOI] [PubMed] [Google Scholar]
- 106. Druce JD, Robinson WF, Locarnini SA, et al. Transmission of human and feline immunodeficiency viruses via reused suture material. J Med Virol 1997; 53: 13–18. [DOI] [PubMed] [Google Scholar]
- 107. Griffin B, Bushby PA, McCobb E, et al. The Association of Shelter Veterinarians’ 2016 Veterinary Medical Care Guidelines for Spay-Neuter Programs. J Am Vet Med Assoc 2016; 249: 165–188. [DOI] [PubMed] [Google Scholar]
- 108. Marenzoni ML, Lauzi S, Miglio A, et al. Comparison of three blood transfusion guidelines applied to 31 feline donors to minimise the risk of transfusion-transmissible infections. J Feline Med Surg 2018; 20: 663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Wardrop KJ, Birkenheuer A, Blais MC, et al. Update on canine and feline blood donor screening for blood-borne pathogens. J Vet Intern Med 2016; 30: 15–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Ellis SL, Rodan I, Carney HC, et al. AAFP and ISFM feline environmental needs guidelines. J Feline Med Surg 2013; 15: 219–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Hardy WD, Hess PW, MacEwen EG, et al. Biology of feline leukemia virus in the natural environment. Cancer Res 1976; 36: 582–588. [PubMed] [Google Scholar]
- 112. Levy JK. FeLV and non-neoplastic FeLV-related disease. In: Ettinger SJ, Feldman EC. (eds). Textbook of veterinary internal medicine. 5th ed. Philadelphia: WB Saunders, 2000, pp 424–432. [Google Scholar]
- 113. Mims CA. Vertical transmission of viruses. Microbiol Rev 1981; 43: 267–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Boudreaux CE, Lockett NN, Chemerys DN, et al. Maternal hematological and virological characteristics during early feline immunodeficiency virus (FIV) infection of cats as predictors of fetal infection and reproductive outcome at early gestation. Vet Immunol Immunopathol 2009; 131: 290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. O’Neil LL, Burkhard MJ, Diehl LJ, et al. Vertical transmission of feline immunodeficiency virus. AIDS Res Hum Retroviruses 1995; 11: 171–182. [DOI] [PubMed] [Google Scholar]
- 116. Weaver CC, Burgess SC, Nelson PD, et al. Placental immunopathology and pregnancy failure in the FIV-infected cat. Placenta 2005; 26: 138–147. [DOI] [PubMed] [Google Scholar]
- 117. Newbury S, Blin K, Bushby PA, et al. Guidelines for standards of care in animal shelters. Association of Shelter Veterinarians. https://www.sheltervet.org/assets/docs/shelter-standards-oct2011-wforward.pdf (2010, accessed December 11, 2018). [Google Scholar]
- 118. Mostl K, Egberink H, Addie D, et al. Prevention of infectious diseases in cat shelters: ABCD guidelines. J Feline Med Surg 2013; 15: 546–554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Levy JK. Feline immunodeficiency virus update. In: Bonagura J. (ed). Current veterinary therapy XIII. Philadelphia: WB Saunders, 2000, pp 284–291. [Google Scholar]
- 120. Levy JK, Crawford PC. Feline leukemia virus. In: Ettinger SJ, Feldman ED. (eds). Textbook of veterinary internal medicine. 6th ed. Philadelphia: WB Saunders, 2005, pp 653–659. [Google Scholar]
- 121. Wallace JL, Levy JK. Population characteristics of feral cats admitted to seven trap-neuter-return programs in the United States. J Feline Med Surg 2006; 8: 279–284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Levy JK, Lorentzen L, Shields J, et al. Long-term outcome of cats with natural FeLV and FIV infection. 8th International Feline Retrovirus Research Symposium; 2006. Oct 8-13, Washington, DC. [Google Scholar]
- 123. McCallum H, Dunham S, Tarlinton R. Lifetime outcomes of feline immunodeficiency virus infection [abstract]. 4th International Society for Companion Animal Infectious Diseases; 2016. Oct 16-19, Bristol, UK. [Google Scholar]
- 124. Kornya MR, Little SE, Scherk MA, et al. Association between oral health status and retrovirus test results in cats. J Am Vet Med Assoc 2014; 245: 916–922. [DOI] [PubMed] [Google Scholar]
- 125. Willis AM. Feline leukemia virus and feline immunodeficiency virus. Vet Clin North Am Small Anim Pract 2000; 30: 971–986. [DOI] [PubMed] [Google Scholar]
- 126. Cupp C, Perez-Camargo G, Patil A, et al. Long-term food consumption and body weight changes in a controlled population of geriatric cats [abstract]. Comp Contin Educ Pract Vet 2004; 26: 60. [Google Scholar]
- 127. Freeman LM, Lachaud MP, Matthews S, et al. Evaluation of weight loss over time in cats with chronic kidney disease. J Vet Intern Med 2016; 30: 1661–1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Dawson S, Smyth NR, Bennett M, et al. Effect of primary-stage feline immunodeficiency virus infection on subsequent feline calicivirus vaccination and challenge in cats. AIDS 1991; 5: 747–750. [DOI] [PubMed] [Google Scholar]
- 129. Lutz H, Castelli I, Ehrensperger F, et al. Panleukopenia-like syndrome of FeLV caused by co-infection with FeLV and feline panleukopenia virus. Vet Immunol Immunopathol 1995; 46: 21–33. [DOI] [PubMed] [Google Scholar]
- 130. Reubel GH, George JW, Barlough JE, et al. Interaction of acute feline herpesvirus-1 and chronic feline immunodeficiency virus infections in experimentally infected specific pathogen free cats. Vet Immunol Immunopathol 1992; 35: 95–119. [DOI] [PubMed] [Google Scholar]
- 131. Reubel GH, Dean GA, George JW, et al. Effects of incidental infections and immune activation on disease progression in experimentally feline immunodeficiency virus-infected cats. J Acquir Immune Defic Syndr 1994; 7: 1003–1015. [PubMed] [Google Scholar]
- 132. Bergmann M, Schwertler S, Reese S, et al. Antibody response to feline panleukopenia virus vaccination in healthy adult cats. J Feline Med Surg 2018; 20: 1087–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Weese JS, Giguere S, Guardabassi L, et al. ACVIM consensus statement on therapeutic antimicrobial use in animals and antimicrobial resistance. J Vet Intern Med 2015; 29: 487–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134. Taffin ERI, Paepe D, Campos M, et al. Evaluation of a modified Karnofsky score to assess physical and psychological wellbeing of cats in a hospital setting. J Feline Med Surg 2016; 18: 913–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135. Hartmann K. Efficacy of antiviral chemotherapy for retro-virus-infected cats: what does the current literature tell us? J Feline Med Surg 2015; 17: 925–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Hartmann K. FeLV treatment strategies and prognosis. Comp Cont Educ Pract 2005; 27 Suppl: 14–26. [Google Scholar]
- 137. De Mari K, Maynard L, Sanquer A, et al. Therapeutic effects of recombinant feline interferon-omega on feline leukemia virus (FeLV)-infected and FeLV/feline immunodeficiency virus (FIV)-coinfected symptomatic cats. J Vet Intern Med 2004; 18: 477–482. [DOI] [PubMed] [Google Scholar]
- 138. Gil S, Leal RO, Duate A, et al. Relevance of feline interferon omega for clinical improvement and reduction of concurrent viral excretion in retrovirus infected cats from a rescue shelter. Res Vet Sci 2013; 94: 753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139. Gil S, Leal RO, McGahie D, et al. Oral Recombinant Feline Interferon-Omega as an alternative immune modulation therapy in FIV positive cats: clinical and laboratory evaluation. Res Vet Sci 2014; 96: 79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Feline Retrovirus Testing Client Brochure