Abstract
Practical relevance:
There has been increasing identification of vector-borne pathogens in cats presented to veterinary clinics around the world for evaluation of fever and the associated secondary effects, such as signs of depression and loss of appetite.
Aim:
The aim of this article is to summarize the clinically relevant information concerning fever in cats that is associated with pathogens known or suspected to be vectored by fleas, with an emphasis on presenting clinical abnormalities and optimal diagnostic, treatment and prevention strategies. Fever in cats that is associated with pathogens vectored by ticks or sandflies is discussed in Part 2 of this article series.
Keywords: Bartonella, hemoplasmas, Rickettsia, Yersinia, flea, Ctenocephalides
Introduction
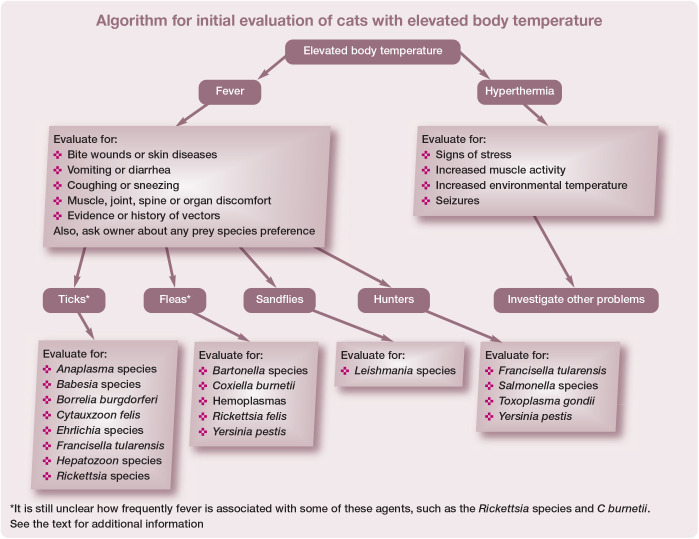
The two major differentials for elevated body temperature >39.2°C (>102.5°F) in cats are hyperthermia and fever (pyrexia). 1 Hyperthermia can result from increased muscle activity, increased environmental temperature, stress or increased metabolic rate (eg, hyperthyroidism). With fever, the thermoregulatory set point in the hypothalamus is increased, secondary to the release of pyrogens, resulting in increased body temperature from physiologic mechanisms inducing endogenous heat production or heat conservation. Fever develops when leukocytes, particularly mononuclear cells and neutrophils, are activated to release pyrogens such as interleukin. 1
Leukocytes are generally stimulated by contact with bacterial, viral, fungal and parasitic agents, as well as by neoplasia, tissue necrosis (eg, extensive trauma, pancreatitis), and primary immune-mediated diseases such as immune-mediated hemolytic anemia, immune-mediated thrombocytopenia and systemic lupus erythematosus (see the algorithm for initial evaluation of cats with elevated body temperature on page 32). A variety of soluble factors such as interleukin 1 and tumor necrosis factor, which are released by the activated cells, enter the central nervous system and change the thermoregulatory set point. 2 The thermoregulatory set point may also be altered by intracranial disease including trauma and neoplasia, or drugs such as tetracycline. Shivering and vasoconstriction are two of the most important physiologic responses to a thermoregulatory set point change, and result in generation and conservation of heat, respectively.


The differential list for fever in cats is long. In a recent review of 106 referral feline cases with a body temperature >39.2°C, infectious causes of fever were the most common category. 3 There are a number of infectious agents that are known causes of (or are considered reasonable differential diagnoses for) fever in cats that are vectored by several arthropods.
The following discussion provides veterinarians with an update on the flea-borne agents that could be associated with fever in cats. The particular focus is common clinical and laboratory findings, optimal diagnostic tests, treatments and strategies for prevention (Table 1). Here, as well as within the accompanying update on the tick- and sandfly-borne disease agents, 4 year-round flea and tick control in endemic areas is emphasized, as it is better to prevent these infections than have to treat clinically ill cats.
Table 1.
Concurrent findings and diagnostic plan suggestions for feline flea-borne pathogens associated with fever
| Diagnosis* |
||||
|---|---|---|---|---|
| Concurrent findings | Direct identification techniques | Serology | Comments | |
| Flea-associated pathogens | ||||
| Bartonella species | • Endocarditis • Hyperglobulinemia • Lymphadenopathy • Myocarditis • Osteomyelitis • Uveitis • Other |
• Silver stain on exudates or tissues • Culture of blood or tissues • PCR assay on blood or tissues |
• Several techniques available in some laboratories | • The combination of serology plus culture and PCR has the greatest diagnostic sensitivity |
| Coxiella burnetii | • Abortion • Stillbirth |
• PCR assay on blood | • Available in some laboratories | • Difficult to culture • The role that fleas play in the transmission of this agent is still being explored |
| Hemoplasmas | • Hemolytic anemia | • Blood smear cytology • PCR assay on blood |
• Not commercially available | • Organisms on erythrocyte surface • Cytology is falsely negative in many cases and does not allow speciation • PCR is preferred diagnostic method |
|
Rickettsia felis
Rickettsia typhi |
• Currently unknown | • PCR assay on blood | • Not commercially available | |
| Yersinia pestis | • Lymphadenopathy • Cough |
• Cytology • Fluorescent antibody staining • Culture • PCR assay |
• Available in some laboratories | • Lymph nodes, abscesses and airway wash samples used for direct identification techniques • Rising titers can be used to confirm recent infection if direct techniques are negative |
Results of direct tests (cytology ± staining techniques, culture, PCR assays) confirm infection when they are positive. However, for some agents, such as Bartonella species and the hemoplasmas, there is a carrier phase in many healthy cats and so positive test results do not confirm disease induced by the agent. Similarly, most positive antibody test results merely indicate past or current infection, but do not confirm current infection or disease
Flea-Borne Agents Associated with Fever
Bartonella species
A number of Bartonella species, including Bartonella henselae, Bartonella clarridgeiae, Bartonella koehlerae and Bartonella bovis, have been cultured, or their DNA has been amplified, from client-owned cats with fever.5–7 Fleas, in particular Ctenocephalides felis, are the known vectors for B henselae, B clarridgeiae and B koehlerae. 8 Bartonella quintana is associated with fever in humans, has been detected in cats and is transmitted among humans by lice. However, C felis has also been shown to acquire and excrete B quintana. 9 Evidence of exposure to Bartonella species has been found in cats of many countries, particularly in regions with high humidity and fleas.10–22
Fever following experimental inoculation with B henselae has been documented in a number of studies including one where 3/6 cats developed B henselae infection with associated fever that lasted at least 2 days after exposure to infected C felis. 23 The fever resolved in these three cats after enrofloxacin was administered. Several other studies have associated Bartonella species exposure or infection with fever in naturally exposed cats.5–7 However, most cats infected with a Bartonella species will remain clinically normal.
Whether fever will occur during Bartonella species infection is likely influenced by both host and organism factors in a complex interaction. Lymphadenopathy, endocarditis, myocarditis, hyperglobulinemia, osteomyelitis and uveitis are other well-documented manifestations of bartonellosis in cats. 24 The association with hyperglobulinemia and fever is notable because feline infectious peritonitis is also common in febrile cats with hyperglobulinemia. 3
As B henselae, B clarridgeiae and B koehlerae are transmitted by fleas, bacteremia and antibody-positive rates can be very high in flea-endemic areas. For example, serum antibodies were detected in 93% of cats housed in an animal shelter in North Carolina, USA. 25 The majority of these cats were thought to be normal, which emphasizes that fever from bartonellosis cannot be documented by test results alone. In one study of pair-matched cats with or without fever, serum Bartonella antibodies detected by ELISA or Western blot immunoassay were not correlated with the presence of fever. 6 In addition, serum antibody test results are negative in 3–15% of bacteremic cats, particularly in the acute phase of bacteremia. 23 Thus, if a cat with fever is to be evaluated for Bartonella species infection, the combination of blood culture and/or PCR assay on blood, and serologic testing will detect the greatest number of cats that are currently, or were previously, infected (Table 1; www.dlab.colostate.edu; www.galaxydx.com). 26 Laboratories performing solely serology, culture or PCR assays are likely misdiagnosing some cases of fever associated with bartonellosis. Febrile cats that are seronegative and negative for Bartonella species in blood by culture or PCR are unlikely to have this organism as the cause of fever.
If fever or other clinical signs from bartonellosis are suspected in a cat, administration of doxycycline or a fluoroquinolone is generally effective.5,7,23 The American Association of Feline Practitioners Panel Report recommends doxycycline at 10 mg/kg PO q24h for 7 days as the initial therapeutic trial. 26 If a positive response is achieved, treatment should be continued for 2 weeks past resolution of clinical signs or for a minimum of 28 days. 26 If the response achieved by day 7 is poor or doxycycline is not tolerated and bartonellosis is still considered a valid differential diagnosis, fluoroquinolones are an appropriate second choice. In experimental or field studies, administration of enrofloxacin or orbifloxacin has led to rapid resolution of fever in cats with presumed bartonellosis.7,23 Azithromycin is now considered contraindicated for treatment of bartonellosis in cats because of rapid selection for resistance. 27 The new veterinary fluoroquinolone, pradofloxacin, is the least likely to cause resistant strains of B henselae and so may be the preferred quinolone for the treatment of this pathogen. 27 However, some chronic cases of feline bartonellosis may require the administration of two drugs owing to the presence of coinfections, and quinolones are not effective for the treatment of potential pathogens such as Anaplasma species, Borrelia burgdorferi or Ehrlichia species. 28
B henselae is common in fleas and survives at least 9 days in flea frass, and so flea control is imperative to attempt to lessen infection of other cats, dogs or people.12–14,29–31 There have been experimental studies showing that the use of imidacloprid-containing compounds can block transmission of B henselae among cats by C felis.23,31 The flea and tick collar containing imidacloprid was well tolerated by cats owned by veterinary students. 32 Whether other flea control products are effective has not been studied. While several Bartonella species have been grown or amplified by PCR from ticks, the role ticks play in the transmission of Bartonella species among cats is unclear. 33 However, the presence of multiple tick-borne diseases in cats, as described in Part 2, 4 supports the use of acaricides in this species.
Coxiella burnetii
Coxiella burnetii is a rickettsial organism commonly carried by cats and many other mammals,21,34,35 and can be zoonotic to people, usually by airborne transmission.36,37 While long considered a tick-borne disease, C burnetii has also been detected in fleas in Cyprus. 38 In addition, the importance of tick transmission has been questioned. 39
While C burnetii is associated with fever in some people, additional studies will be required to determine the importance of this agent as a cause of vector-borne fever in cats.
Hemoplasmas
Hemolytic anemia, with or without fever, can occur following infection with pathogenic hemoplasmas, especially Mycoplasma haemofelis, but sometimes also Candidatus Mycoplasma haemominutum’ and ‘Candidatus Mycoplasma turicensis’.3,40–43 Like Bartonella species, the DNA of the hemoplasmas has been amplified from the blood of cats as well as fleas collected from cats in many regions of the world.13–17,42,44–46
Based on multiple studies of experimentally infected cats, M haemofelis is usually the most pathogenic hemoplasma species.47,48 Dual infection with hemoplasmas may potentiate pathogenesis of disease. In one study, cats with chronic ‘Candidatus M haemominutum’ infection had more severe and longer duration anemia when they were experimentally infected with M haemofelis compared with cats infected with M haemofelis alone. 47 Clinical signs of disease depend on the degree of anemia, the stage of infection and the immune status of infected cats. In one unpublished study, one of the authors (ML) detected an association between M haemofelis and fever in cats without anemia.
Direct transmission may occur with the hemoplasmas. Studies have found some of the agents in saliva and have also documented infection transmission following subcutaneous inoculation of hemoplasma-containing blood. Hence this pathogen should be on the differential list for cats with a history of fighting and fever, particularly if the fever does not respond to beta-lactam antibiotics.49,50
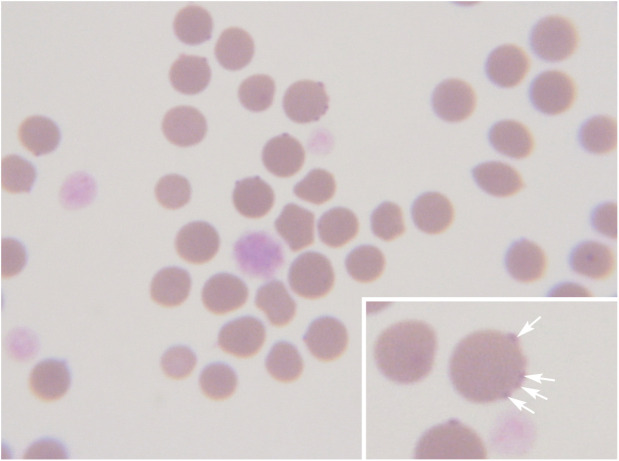
Diagnosis of hemoplasmosis can be based on demonstration of the organism on the surface of erythrocytes on examination of a thin blood film (Figure 1) or, far more reliably, on PCR assay results (Table 1). Organism numbers fluctuate and so blood film examination can be very insensitive for diagnosis (0–37.5%).47,51–53 Specificity is also likely to be an issue with blood film examination, especially when performed by someone inexperienced in clinical pathology. When properly designed and executed, PCR is far more sensitive and specific than cytology, and species-specific assays are now routinely used. 54 However, healthy cats can also be positive for haemoplasma DNA in blood and so PCR assay results do not always correlate with clinical illness, as is the case with the interpretation of many PCR assays.41,55,56
Figure 1.
Blood smear cytology for hemoplasma infection. Hemoplasmas are very difficult to find on cytology unless extremely high numbers of organisms are present in the blood at the time of sampling. Cytology of this blood smear (Wright’s stain, x 100) shows a number of erythrocytes with small purple-staining Mycoplasma haemofelis organisms epicellularly (arrows in inset). Large shift platelets are also visible
Doxycycline, often administered as a flavored suspension (to avoid esophagitis and esophageal strictures) at 10 mg/kg PO q24h or 5 mg/kg PO q12h, is generally effective for the treatment of clinical feline hemoplasmosis. The duration of recommended therapy varies, but is typically 2–4 weeks.57,58 In cats intolerant of doxycycline, fluoroquinolones have also been effective. Administration of marbofloxacin or orbifloxacin gave similar results to doxycycline in two studies.7,59 Pradofloxacin has shown promise in eliminating M haemofelis infection in some experimentally inoculated cats. 60 A recent study reported that to facilitate clearance of M haemofelis, when this is required, doxycycline treatment is administered for 28 days followed by monitoring of copy numbers in the blood by quantitative PCR. 61 If the cat remains PCR positive and clearance is needed, treatment should be switched to a fluoro-quinolone (marbofloxacin was used in the study) for 14 days, as this was associated with apparent clearance of infection. Owners should be informed that recurrences may occur with treatment, but are unusual. 43
Historically, enrofloxacin was also usually effective for the treatment of hemoplasmosis; however, there are safety concerns that need to be considered (see box). Azithromycin was not effective for the treatment of hemoplasmosis in one study. 47

While the hemoplasmas appear to be of low risk to people, there have been reports of infection with similar organisms and one described infection in a human with a hemoplasma species that could have originated in a cat.62,63
Feline hemoplasma DNA has been amplified from fleas and ticks.13,14,29,33,40,64,65 However, this does not equate with vector-mediated transmission, as the presence of hemoplasma DNA could merely reflect their hematophagous activity on infected hosts. Studies on the cat flea, C felis, showed only very transient M haemofelis infection transmission in cats experimentally infected via the hematophagous activity of fleas, and clinical and hematological signs of M haemofelis infection were not induced in the recipient cat. 66 However, the clustered geographical distribution of infection in some studies strongly supports the role of an arthropod vector in hemoplasma transmission and so hemoplasmas are often considered as vector-borne infections.22,67 Recently, Aedes aegypti were shown to ingest M haemofelis or ‘Candidatus M haemominutum’ but transmission to naive cats was not documented, suggesting this mosquito is not a biological vector. 68 Flea control and avoiding fights with other cats should lessen the risk of acquiring hemoplasmosis.
Rickettsia species
Rickettsia species are obligate intracellular Gram-negative bacteria that are divided into the spotted fever group and the typhus group. Rickettsia felis has been found in fleas that infest cats in almost all countries that have been studied.12,13,21,29,30,69,70 R felis DNA has been amplified from C felis, Ctenocephalides canis and Pulex irritans; these fleas have a worldwide distribution. C felis is a biological vector for R felis; this pathogen can be transmitted transovarially and transtadially within the flea. Rickettsia typhi DNA has been amplified from fleas in Spain and the USA.69,71–73 Some C felis fleas have been positive for both R felis and R typhi, suggesting cats could be exposed to both concurrently.69,72 In addition, DNA most consistent with Rickettsia asembonensis or ‘Candidatus Rickettsia senegalensis’ has been amplified from fleas collected from cats. 73
An attempt to associate fever with exposure to R felis was made in a small group of cats in the USA and, while seropositive cats were detected, an association with fever was not made. 74 To date, only small numbers of cats have been studied to attempt to make disease associations with R typhi, 69 and clinical illness has not been identified to the authors’ knowledge. Since flea-borne Rickettsia species are associated with fever in other species, it is possible that fever can also occur in cats, as has been suggested by others; 75 however, further data are needed to determine the significance of disease associations.
Currently, routine testing for cats is not available unless PCR panels using primers capable of amplifying R felis, R asembonensis, ‘Candidatus R senegalensis’ and R typhi are used.
Because clinical illness in cats has not been documented with the flea-associated Rickettsia species, optimal treatment is unknown. However, based on results in other species, doxycycline or a fluoroquinolone would be logical choices. As both R felis and R typhi are associated with disease in humans, it is imperative to provide flea control to cats all year round to potentially lessen the risk of exposure. 76
Yersinia pestis
Yersinia pestis is the cause of the plague in people and, when infection occurs, fever is common in both cats and people.77–80 However, transmission of this pathogen among cats or people by C felis is considered rare because of various flea-associated factors that include rapid bacterial clearance from the midgut of this flea species. 81 While it is possible that rodent fleas could transmit Y pestis to cats, ingestion of bacteremic rodents is likely the most common route of transmission. Y pestis should thus be considered on the differential list for fever of outdoor cats in endemic areas.
Bipolar staining rods are often seen on cytological evaluation of stained smears of lymph node aspirates. The diagnosis can be confirmed by culture (in laboratories with appropriate certification), fluorescent antibody staining or PCR assay; the latter techniques are available at many diagnostic laboratories, particularly in western USA (www.cdc.gov/plague/healthcare/veterinarians.html). In the USA, the state public health veterinarian should be contacted and sample submissions to the diagnostic laboratory clearly labeled as from a cat with possible plague so appropriate techniques can be used and precautions taken (nasphv.org).
Cats with Y pestis infection have responded to a number of antimicrobial drugs including aminoglycosides and doxycycline. Unpublished data of one of the authors (ML) recorded successful treatment of six cats with injectable enrofloxacin at 5 mg/kg SC q24h for 3–5 days (see box on page 35 for safety cautions when using enrofloxacin in cats) followed by doxycycline at 10 mg/kg PO q24h for 7–21 days. Cats should be provided with flea control all year round and hunting behavior should be minimized when possible, particularly for cats living in areas with proven cases of plague.
Key Points
Some flea-borne pathogens can be associated with fever in cats, and the history and concurrent clinical signs can trigger a diagnostic investigation and, ultimately, appropriate therapy.
It has been well documented that flea control can block the transmission of B henselae among cats.
Prevention of flea-borne infections is always preferred to treating clinically ill cats and so flea products should be used all year round.
Hands should be washed after handling cats with fleas.
Bites and scratches from cats should be avoided, particularly if fleas are present.
Acknowledgments
The authors thank Dr Susanne Siebert and Dr Annette Boegel for their support and encouragement as well as editorial comments.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This work did not involve the use of animals and therefore ethical approval was not required.
Informed Consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within the publication, and therefore additional consent for publication was not required.
References
- 1. Ramsey I, Tasker S. Fever. In: Ettinger SJ, Feldman EC, Cote E. (eds). Textbook of veterinary internal medicine. 8th ed. St Louis, MO: Elsevier, 2016, pp 195–203. [Google Scholar]
- 2. Hasday JD, Fairchild KD, Shanholtz C. The role of fever in the infected host. Microbes Infect 2000; 2: 1891–1904. [DOI] [PubMed] [Google Scholar]
- 3. Spencer SE, Knowles T, Ramsey IK, et al. Pyrexia in cats: retrospective analysis of signalment, clinical investigations, diagnosis and influence of prior treatment in 106 referred cases. J Feline Med Surg 2017; 19: 1123–1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lappin MR, Tasker S, Roura X. Role of vector-borne pathogens in the development of fever in cats. 2. Tick- and sandfly-associated diseases. J Feline Med Surg 2020; 22: 41-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Breitschwerdt EB, Broadhurst JJ, Cherry NA. Bartonella henselae as a cause of acute-onset febrile illness in cats. JFMS Open Rep 2015; 1. DOI: 10.1177/2055116915600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lappin MR, Breitschwerdt E, Brewer M, et al. Prevalence of Bartonella species DNA in the blood of cats with and without fever. J Feline Med Surg 2009; 11: 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lappin MR, Miller W, Sellins D. Effect of doxycycline or orbifloxacin administration on Bartonella spp. and Hemoplasma assay results in naturally exposed cats. Intern J Appl Res Vet Med 2012; 10: 225–233. [Google Scholar]
- 8. Breitschwerdt EB, Maggi RG, Chomel BB, et al. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care 2010; 20: 8–30. [DOI] [PubMed] [Google Scholar]
- 9. Kernif T, Leulmi H, Socolovschi C, et al. Acquisition and excretion of Bartonella quintana by the cat flea, Ctenocephalides felis felis. Mol Ecol 2014; 23: 1204–1212. [DOI] [PubMed] [Google Scholar]
- 10. Solano-Gallego L, Hegarty B, Espada Y, et al. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol 2006; 118: 274–277. [DOI] [PubMed] [Google Scholar]
- 11. Tabar MD, Altet L, Francino O, et al. Vector-borne infections in cats: molecular study in Barcelona area (Spain). Vet Parasitol 2008; 151: 332–336. [DOI] [PubMed] [Google Scholar]
- 12. Lappin MR, Hawley J. Presence of Bartonella species and Rickettsia species DNA in the blood, oral cavity, skin and claw beds of cats in the United States. Vet Dermatol 2009; 20: 509–514. [DOI] [PubMed] [Google Scholar]
- 13. Barrs VR, Beatty JA, Wilson BJ, et al. Prevalence of Bartonella species, Rickettsia felis, haemoplasmas and the Ehrlichia group in the blood of cats and fleas in eastern Australia. Aust Vet J 2010; 88: 160–165. [DOI] [PubMed] [Google Scholar]
- 14. Assarasakorn S, Veir JK, Hawley JR, et al. Prevalence of Bartonella species, hemoplasmas, and Rickettsia felis DNA in blood and fleas of cats in Bangkok, Thailand. Res Vet Sci 2012; 93: 1213–1216. [DOI] [PubMed] [Google Scholar]
- 15. Ayllon T, Diniz PP, Breitschwerdt EB, et al. Vector-borne diseases in client-owned and stray cats from Madrid, Spain. Vector Borne Zoonotic Dis 2012; 12: 143–150. [DOI] [PubMed] [Google Scholar]
- 16. Lobetti R, Lappin MR. Prevalence of Toxoplasma gondii, Bartonella species and haemoplasma infection in cats in South Africa. J Feline Med Surg 2012; 14: 857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Maia C, Ramos C, Coimbra M, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors 2014; 7: 115. DOI: 10.1186/1756-3305-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Gracia MJ, Marcen JM, Pinal R, et al. Prevalence of Rickettsia and Bartonella species in Spanish cats and their fleas. J Vector Ecol 2015; 40: 233–239. [DOI] [PubMed] [Google Scholar]
- 19. Bergmann M, Englert T, Stuetzer B, et al. Prevalence of Bartonella species infections in cats in Southern Germany. Vet Rec 2017; 180: 325. [DOI] [PubMed] [Google Scholar]
- 20. Alaman VM, Simon VC, Fuertes NH, et al. Molecular epidemiology of Bartonella henselae in stray and sheltered cats of Zaragoza, Spain [article in Spanish]. Rev Esp Salud Publica 2016; 90: e1–e11. [PubMed] [Google Scholar]
- 21. Bessas A, Leulmi H, Bitam I, et al. Molecular evidence of vector-borne pathogens in dogs and cats and their ectoparasites in Algiers, Algeria. Comp Immunol Microbiol Infect Dis 2016; 45: 23–28. [DOI] [PubMed] [Google Scholar]
- 22. Persichetti MF, Pennisi MG, Vullo A, et al. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasit Vectors 2018; 11: 136. DOI: 10.1186/s13071-018-2725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bradbury CA, Lappin MR. Evaluation of topical application of 10% imidacloprid-1% moxidectin to prevent Bartonella henselae transmission from cat fleas. J Am Vet Med Assoc 2010; 236: 869–873. [DOI] [PubMed] [Google Scholar]
- 24. Whittemore JC, Hawley JR, Radecki SV, et al. Bartonella species antibodies and hyperglobulinemia in privately owned cats. J Vet Intern Med 2012; 26: 639–644. [DOI] [PubMed] [Google Scholar]
- 25. Nutter FB, Dubey JP, Levine JF, et al. Seroprevalences of antibodies against Bartonella henselae and Toxoplasma gondii and fecal shedding of Cryptosporidium spp, Giardia spp, and Toxocara cati in feral and pet domestic cats. J Am Vet Med Assoc 2004; 225: 1394–1398. [DOI] [PubMed] [Google Scholar]
- 26. Brunt J, Guptill L, Kordick DL, et al. American Association of Feline Practitioners 2006 Panel Report on diagnosis, treatment, and prevention of Bartonella spp. infections. J Feline Med Surg 2006; 8: 213–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Biswas S, Maggi RG, Papich MG, et al. Comparative activity of pradofloxacin, enrofloxacin, and azithromycin against Bartonella henselae isolates collected from cats and a human. J Clin Microbiol 2010; 48: 617–618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Qurollo BAB, Balakrishnan N, Cannon CZ, et al. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J Feline Med Surg 2014; 16: 713–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Shaw SE, Kenny MJ, Tasker S, et al. Pathogen carriage by the cat flea Ctenocephalides felis (Bouche) in the United Kingdom. Vet Microbiol 2004; 102: 183–188. [DOI] [PubMed] [Google Scholar]
- 30. Slapeta J, Lawrence A, Reichel MP. Cat fleas (Ctenocephalides felis) carrying Rickettsia felis and Bartonella species in Hong Kong. Parasitol Int 2018; 67:209–212. [DOI] [PubMed] [Google Scholar]
- 31. Lappin MR, Davis WL, Hawley JR, et al. A flea and tick collar containing 10% imidacloprid and 4.5% flumethrin prevents flea transmission of Bartonella henselae in cats. Parasit Vectors 2013; 6: 26. DOI: 10.1186/1756-3305-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fink H, Wennogle S, Davis WL, et al. Field comparison of tolerance of a collar containing 10.0% imidacloprid/4.5% flumethrin (Seresto) and a placebo collar placed on cats. J Feline Med Surg 2016; 18: 1031–1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Duplan F, Davies S, Filler S, et al. Anaplasma phago-cytophilum, Bartonella spp., haemoplasma species and Hepatozoon spp. in ticks infesting cats: a large-scale survey. Parasit Vectors 2018; 11: 201. DOI: 10.1186/s13071-018-2789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Cairns K, Brewer M, Lappin MR. Prevalence of Coxiella burnetii DNA in vaginal and uterine samples from healthy cats of north-central Colorado. J Feline Med Surg 2007; 9:196–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Candela MG, Caballol A, Atance PM. Wide exposure to Coxiella burnetii in ruminant and feline species living in a natural environment: zoonoses in a human-livestock-wildlife interface. Epidemiol Infect 2017; 145: 478–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shapiro AJ, Norris JM, Bosward KL, et al. Q fever (Coxiella burnetii) knowledge and attitudes of Australian cat breeders and their husbandry practices. Zoonoses Public Health 2017; 64: 252–261. [DOI] [PubMed] [Google Scholar]
- 37. Mori M, Roest HJ. Farming, Q fever and public health: agricultural practices and beyond. Arch Public Health 2018; 76: 2. DOI: 10.1186/s13690-017-0248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Psaroulaki A, Chochlakis D, Ioannou I, et al. Presence of Coxiella burnetii in fleas in Cyprus. Vector Borne Zoonotic Dis 2014; 14: 685–687. [DOI] [PubMed] [Google Scholar]
- 39. Duron O, Sidi-Boumedine K, Rousset E, et al. The importance of ticks in Q Fever transmission: what has (and has not) been demonstrated? Trends Parasitol 2015; 31: 536–552. [DOI] [PubMed] [Google Scholar]
- 40. Willi B, Boretti FS, Tasker S, et al. From Haemobartonella to hemoplasma: molecular methods provide new insights. Vet Microbiol 2007; 125: 197–209. [DOI] [PubMed] [Google Scholar]
- 41. Roura X, Peters IR, Altet L, et al. Prevalence of hemotropic mycoplasmas in healthy and unhealthy cats and dogs in Spain. J Vet Diagn Invest 2010; 22: 270–274. [DOI] [PubMed] [Google Scholar]
- 42. Martinez-Diaz VL, Silvestre-Ferreira AC, Vilhena H, et al. Prevalence and co-infection of haemotropic mycoplasmas in Portuguese cats by real-time polymerase chain reaction. J Feline Med Surg 2013; 15: 879–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Weingart C, Tasker S, Kohn B. Infection with haemo-plasma species in 22 cats with anaemia. J Feline Med Surg 2016; 18: 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Andre MR, Filgueira KD, Calchi AC, et al. Co-infection with arthropod-borne pathogens in domestic cats. Rev Bras Parasitol Vet 2017; 26: 525–531. [DOI] [PubMed] [Google Scholar]
- 45. Ravagnan S, Carli E, Piseddu E, et al. Prevalence and molecular characterization of canine and feline hemotropic mycoplasmas (hemoplasmas) in northern Italy. Parasit Vectors 2017; 10: 132. DOI: 10.1186/s13071-017-2069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Diaz-Reganon D, Villaescusa A, Ayllon T, et al. Epidemiological study of hemotropic mycoplasmas (hemo-plasmas) in cats from central Spain. Parasit Vectors 2018; 11: 140. DOI: 10.1186/s13071-018-2740-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Westfall DS, Jensen WA, Reagan W, et al. Inoculation of two genotypes of Haemobartonella felis (California and Ohio variants) to induce infection in cats and response to treatment with azithromycin. Am J Vet Res 2001; 62: 687–691. [DOI] [PubMed] [Google Scholar]
- 48. Tasker S, Peters IR, Papasouliotis K, et al. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet Microbiol 2009; 139: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dean RS, Helps CR, Gruffydd Jones TJ, et al. Use of real-time PCR to detect Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’ in the saliva and salivary glands of haemoplasma-infected cats. J Feline Med Surg 2008; 10: 413–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Museux K, Boretti FS, Willi B, et al. In vivo transmission studies of ‘Candidatus Mycoplasma turicensis’ in the domestic cat. Vet Res 2009; 40: 45. DOI: 10.1051/vetres/2009028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jensen WA, Lappin MR, Kamkar S, et al. Use of a polymerase chain reaction assay to detect and differentiate two strains of Haemobartonella felis infection in naturally infected cats. Am J Vet Res 2001; 62: 604–608. [DOI] [PubMed] [Google Scholar]
- 52. Tasker S, Binns SH, Day MJ, et al. Use of a PCR assay to assess prevalence and risk factors for Mycoplasma haemo-felis and ‘Candidatus Mycoplasma haemominutum’ in cats in the United Kingdom. Vet Rec 2003; 152: 193–198. [DOI] [PubMed] [Google Scholar]
- 53. Ghazisaeedi F, Atyabi N, Zahrai Salehi T, et al. A molecular study of hemotropic mycoplasmas (hemoplasmas) in cats in Iran. Vet Clin Pathol 2014; 43: 381–386. [DOI] [PubMed] [Google Scholar]
- 54. Peters IR, Helps CR, Willi B, et al. The prevalence of three species of feline haemoplasmas in samples submitted to a diagnostics service as determined by three novel real-time duplex PCR assays. Vet Microbiol 2008; 126: 142–150. [DOI] [PubMed] [Google Scholar]
- 55. Willi B, Boretti FS, Baumgartner C, et al. Prevalence, risk factor analysis, and follow-up of infections caused by three feline hemoplasma species in cats in Switzerland. J Clin Microbiol 2006; 44: 961–969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Willi B, Boretti FS, Meli ML, et al. Real-time PCR investigation of potential vectors, reservoirs and shedding patterns of feline hemotropic mycoplasmas. Appl Environ Microbiol 2007; 73: 3798–3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Dowers KL, Olver CS, Radecki V, et al. Use of enrofloxacin for treatment of large-form Haemobartonella felis in experimentally infected cats. J Am Vet Med Assoc 2002; 221: 250–253. [DOI] [PubMed] [Google Scholar]
- 58. Tasker S, Hofmann-Lehmann R, Belak S, et al. Haemo -plasmosis in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg 2018; 20: 256–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Tasker S, Caney SM, Day MJ, et al. Effect of chronic FIV infection, and efficacy of marbofloxacin treatment, on Mycoplasma haemofelis infection. Vet Microbiol 2006; 117: 169–179. [DOI] [PubMed] [Google Scholar]
- 60. Dowers KL, Tasker S, Radecki SV, et al. Use of pradofloxacin to treat experimentally induced Mycoplasma hemofelis infection in cats. Am J Vet Res 2009; 70: 105–111. [DOI] [PubMed] [Google Scholar]
- 61. Novacco M, Sugiarto S, Willi B, et al. Consecutive antibiotic treatment with doxycycline and marbofloxacin clears bacteremia in Mycoplasma haemofelis-infected cats. Vet Microbiol 2018; 217: 112–120. [DOI] [PubMed] [Google Scholar]
- 62. Steer JA, Tasker S, Barker EN, et al. A novel hemotropic Mycoplasma (hemoplasma) in a patient with hemolytic anemia and pyrexia. Clin Infect Dis 2011; 53: e147–151. DOI: 10.1093/cid/cir666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Santos AP, Santos RP, Biondo AW, et al. Hemoplasma infection in HIV-positive patient, Brazil. Emerg Infect Dis 2008; 14: 1922–1924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Taroura S, Shimada Y, Sakata Y, et al. Detection of DNA of ‘Candidatus Mycoplasma haemominutum’ and Spiroplasma sp. in unfed ticks collected from vegetation in Japan. J Vet Med Sci 2005; 67: 1277–1279. [DOI] [PubMed] [Google Scholar]
- 65. Lappin MR, Griffin B, Brunt J, et al. Prevalence of Bartonella species, haemoplasma species, Ehrlichia species, Anaplasma phagocytophilum, and Neorickettsia risticii DNA in the blood of cats and their fleas in the United States. J Feline Med Surg 2006; 8: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Woods JE, Brewer MM, Hawley JR, et al. Evaluation of experimental transmission of ‘Candidatus Mycoplasma haemominutum’ and Mycoplasma haemofelis by Ctenocephalides felis to cats. Am J Vet Res 2005; 66: 1008–1012. [DOI] [PubMed] [Google Scholar]
- 67. Sykes JE, Drazenovich NL, Ball LM, et al. Use of conventional and real-time polymerase chain reaction to determine the epidemiology of hemoplasma infections in anemic and nonanemic cats. J Vet Intern Med 2007; 21: 685–693. [DOI] [PubMed] [Google Scholar]
- 68. Reagan KL, Clarke LL, Hawley JR, et al. Assessment of the ability of Aedes species mosquitoes to transmit feline Mycoplasma haemofelis and ‘Candidatus Mycoplasma haemominutum’. J Feline Med Surg 2017; 19: 798–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nogueras MM, Pons I, Ortuno A, et al. Molecular detection of Rickettsia typhi in cats and fleas. PLoS One 2013; 8: e71386. DOI: 10.1371/journal.pone.0071386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Teoh YT, Hii SF, Graves S, et al. The epidemiology of Rickettsia felis infecting fleas of companion animals in eastern Australia. Parasit Vectors 2018; 11: 138. DOI: 10.1186/s13071-018-2737-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Maina AN, Fogarty C, Krueger L, et al. Rickettsial infections among Ctenocephalides felis and host animals during a flea-borne rickettsioses outbreak in Orange County, California. PLoS One 2016; 11: e0160604. DOI: 10.1371/journal.pone.0160604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Noden BH, Davidson S, Smith JL, et al. First detection of Rickettsia typhi and Rickettsia felis in fleas collected from client-owned companion animals in the Southern Great Plains. J Med Entomol 2017; 54: 1093–1097. [DOI] [PubMed] [Google Scholar]
- 73. Blanton LS, Vohra RF, Fistein L, et al. Rickettsiae within the fleas of feral cats in Galveston, Texas. Vector Borne Zoonotic Dis 2019; 19: 647–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Bayliss DB, Morris AK, Horta MC, et al. Prevalence of Rickettsia species antibodies and Rickettsia species DNA in the blood of cats with and without fever. J Feline Med Surg 2009; 11: 266–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Pennisi MG, Hofmann-Lehmann R, Radford AD, et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg 2017; 19: 542–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Angelakis E, Mediannikov O, Parola P, et al. Rickettsia felis: the complex journey of an emergent human pathogen. Trends Parasitol 2016; 32: 554–564. [DOI] [PubMed] [Google Scholar]
- 77. Eidson M, Thilsted JP, Rollag OJ. Clinical, clinicopatho-logic, and pathologic features of plague in cats: 119 cases (1977-1988). J Am Vet Med Assoc 1991; 199: 1191–1197. [PubMed] [Google Scholar]
- 78. Gasper PW, Barnes AM, Quan TJ, et al. Plague (Yersinia pestis) in cats: description of experimentally induced disease. J Med Entomol 1993; 30: 20–26. [DOI] [PubMed] [Google Scholar]
- 79. Pennisi MG, Egberink H, Hartmann K, et al. Yersinia pestis infection in cats: ABCD guidelines on prevention and management. J Feline Med Surg 2013; 15: 582–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kassem AM, Tengelsen L, Atkins B, et al. Notes from the field: plague in domestic cats - Idaho, 2016. MMWR Morb Mortal Wkly Rep 2016; 65: 1378–1379. [DOI] [PubMed] [Google Scholar]
- 81. Bland DM, Hinnebusch BJ. Feeding behavior modulates biofilm-mediated transmission of Yersinia pestis by the cat flea, Ctenocephalides felis. PLoS Negl Trop Dis 2016; 10: e0004413. DOI: 10.1371/journal.pntd.0004413. [DOI] [PMC free article] [PubMed] [Google Scholar]