Abstract
Practical relevance:
There has been increasing identification of vector-borne pathogens in cats presented to veterinary clinics around the world for evaluation of fever and the associated secondary effects, such as signs of depression and loss of appetite.
Aim:
The aim of this article is to summarize the clinically relevant information concerning fever in cats that is associated with pathogens vectored by ticks or sandflies, with an emphasis on presenting clinical abnormalities and optimal diagnostic, treatment and prevention strategies. Fever in cats associated with pathogens known or suspected to be vectored by fleas was discussed within Part 1 of this two-part article series.
Keywords: Anaplasma, Bartonella, Borrelia, Ehrlichia, Leishmania, sandfly, tick
Introduction
In Part 1 of this two-part article series, the two major differentials for elevated body temperature (>39.2°C, 102.5°F) in cats – hyperthermia and fever (pyrexia) – were discussed and the infectious disease agents proven or likely to be vectored by fleas were summarized. 1 Here, in Part 2, which reviews the infectious disease agents vectored by ticks or sandflies, emphasis is placed on common clinical and laboratory findings, optimal diagnostic tests, treatments and strategies for prevention.

Tick-Borne Agents Associated With Fever
Anaplasma, Ehrlichia and Rickettsia species
Anaplasma phagocytophilum
Wild-caught adult Ixodes scapularis in the USA are commonly positive for Anaplasma phagocytophilum DNA and experimentally infested cats have been shown to be susceptible to A phagocytophilum infection.2,3 Cats do not develop permanent immunity to A phagocytophilum and repeated infections have been documented in experimentally infested cats. 4 DNA of A phagocytophilum or antibodies against the agent have been detected in naturally exposed cats in most countries with Ixodes species.5-13 Ixodes species also often carry Borrelia burgdorferi and coinfections with A phago-cytophilum are likely common. 12
A small number of cats experimentally infected with A phagocytophilum after infestation with I scapularis in two studies did not develop clinical signs of illness.2,4 Cats living in endemic areas are commonly seropositive but most do not have clinical signs of disease. However, when illness is recognized in naturally infected cats, fever, anorexia and lethargy are the most common clinical abnormalities, and lameness is likely to occur.4,6,9

Cats with fever, with or without thrombocytopenia, residing in Ixodes species-endemic areas should have blood smears examined cytologically to attempt to find morulae (Figure 1). 9 Many commercial laboratories offer PCR assays to amplify A phagocytophilum DNA from blood and these assays are more likely to be positive than serologic tests in acute illness (Table 1). 2 One commercial assay for the detection of A phagocytophilum antibodies in dog serum (SNAP 4DXPlus; IDEXX Laboratories) has been shown to detect these antibodies in feline serum. 2 Approximately 30% of cats with proven clinical infections induced by A phagocytophilum are seronegative when first assessed serologically, but most of the proven cases evaluated to date have ultimately seroconverted. 5
Figure 1.
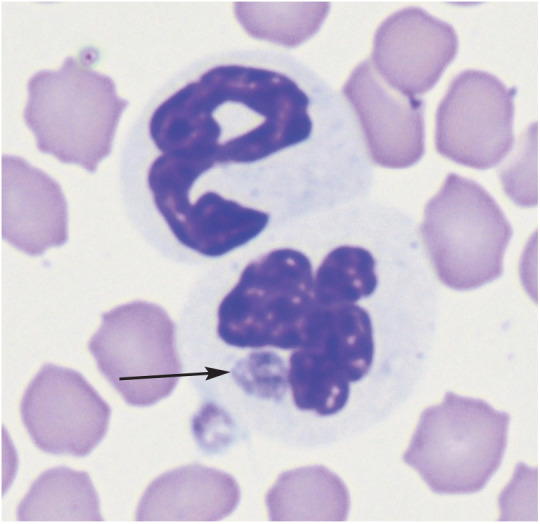
Anaplasma phagocytophilum morula (arrow) in a neutrophil of an experimentally infected cat
Table 1.
Concurrent findings and diagnostic plan suggestions for feline tick- or sandfly-borne pathogens associated with fever
|
Diagnosis
*
|
||||
|---|---|---|---|---|
| Concurrent findings | Direct identification techniques | Serology | Comments | |
| Tick-associated pathogens | ||||
| Anaplasma phagocytophilum | • Anorexia
• Lethargy • Lameness • Thrombocytopenia |
• Blood smear or joint fluid cytology
• PCR assay on blood |
• Several techniques
• Available in some laboratories • Canine SNAP 4DXPlus used in two experimental studies2,4 |
• Morulae in neutrophils |
| Anaplasma platys | • Lethargy
• Thrombocytopenia |
• Blood smear cytology
• PCR assay on blood |
• Not commercially available | • Whether canine serologic assays cross react with infected cats is unknown |
| Babesia species | • Hemolytic anemia | • Blood smear cytology
• PCR assay on blood |
• Not commercially available | • Cytology is likely falsely negative frequently |
| Borrelia burgdorferi | • Lethargy
• Lameness |
• PCR assay on skin affected by tick bites
• PCR assay on synovial fluid |
• Several techniques
• Available in some laboratories • Canine SNAP 4DXPlus used in two experimental studies2,4 |
• PCR assay on blood of affected dogs is generally negative; unknown in cats |
| Borrelia persica | • Anemia
• Thrombocytopenia |
• Blood smear cytology
• PCR assay on blood |
• Not commercially available | |
| Cytauxzoon species | • Anemia
• Dyspnea • Findings consistent with shock |
• Blood smear cytology
• PCR assay on blood |
• Not commercially available | |
| Ehrlichia species | • Anemia
• Thrombocytopenia • Pancytopenia • Hyperglobulinemia • Uveitis • Other |
• Blood smear cytology
• PCR assay on blood |
• Available in some US laboratories | • Cytology commonly falsely negative
• Morulae in mononuclear cells • Serum antibodies have been falsely negative in some cats • Most clinical findings in dogs have been described in cats |
| Francisella tularensis | • Lymphadenopathy | • Cytology
• Fluorescent antibody staining • Culture • PCR assay |
• Available in some laboratories | • Lymph nodes, abscesses, airway wash samples used for direct identification techniques
• Rising titers can be used to confirm recent infection if direct techniques are negative |
| Hepatozoon species | • Currently unknown | • Blood smear cytology
• PCR assay on blood |
• Not commercially available | |
| Rickettsia rickettsii Rickettsia conorii Rickettsia massiliae | • Currently unknown | • PCR assay on blood | • Not commercially available | •
Rickettsia rickettsii
is the
predominant species in the USA and others exist in Europe |
| Sandfly-associated pathogens | ||||
| Leishmania species (mainly Leishmania infantum) | • Skin lesions
• Hyperglobulinemia • Lymphadenopathy • Uveitis • Other |
• PCR assay on aspirates or imprints
• Cytology • Histopathology |
• Available in some laboratories | • In contrast to dogs, even a low antibody titer likely proves infection |
Results of direct tests (cytology ± staining techniques, culture, PCR assays) confirm infection when they are positive. However, for some agents, such as Ehrlichia species and Hepatozoon species, there can be a carrier phase in healthy cats and so positive test results do not confirm disease induced by the agent. Similarly, most positive antibody test results merely indicate past or current infection but do not confirm current infection or disease
Several antibiotics have been administered to naturally infected cats with suspected anaplasmosis. In two studies all cats became clinically normal within 24–48 h of initiation of tetracycline or doxycycline administration and recurrence was not reported.5,6,9 Two cats were still PCR positive 17 days and 90 days after treatment (of 21–30 days’ duration) while clinically normal, which suggests that treatment with tetracyclines for ⩽30 days may be inadequate for eliminating this pathogen from some cats. 5
The risk of infection in cats by A phagocytophilum should be reduced by appropriate use of an acaricide. However, in one study, purchase of a topical acaricide was not associated with a lessened risk of having antibodies against A phagocytophilum or B burgdorferi. 12 In that study, it appeared that owners of treated, seropositive cats did not administer the acaricide all year round. It is also possible that owners of cats with known exposure, and possibly previous infections, were more likely to purchase an acaracide.
Anaplasma platys
A number of cats have been shown to be infected by Anaplasma platys.14–17 This agent is suspected to be tick-borne, possibly by Rhipicephalus sanguineus. However, whether this agent induces fever in cats alone or as a co-infection has not been determined. Serologic tests have not been validated for this organism in cats. Thus, the diagnosis currently is based on the detection of morulae in platelets or amplification of specific DNA by PCR assay. Optimal treatments are unknown for cats, but doxycycline should be effective. Use of acaricides should lessen the risk of exposure.
Ehrlichia species
While canine ehrlichiosis is well characterized, less is known about the agents associated with disease in cats. It is likely that any country that has Ehrlichia canis infections in dogs also has E canis infections in cats. Naturally exposed cats in many countries have variously been shown to have Ehrlichia-like bodies or morulae in peripheral lymphocytes or monocytes, had DNA consistent with E canis amplified from the blood or tissues, or had antibodies that react to E canis morulae or peptides.10,13,18-30 There have been field cases that have been positive for DNA identical to E canis at two genes that never seroconverted. 21 Another study reported that cats at greater risk for R sanguineus infestation were more likely to have higher prevalence rates (9.4%) of PCR positivity for E canis DNA. 26 In Sicily, E canis DNA was amplified from ticks collected from cats. 31
Fever, lethargy and inappetence are commonly reported clinical abnormalities in cats with suspected ehrlichiosis and so testing may be indicated in cats showing these signs. Thrombocytopenia, anemia and monocytosis appear to be the most common clinical laboratory findings in naturally infected cats.19,20,25,30 Almost every abnormality noted in dogs with clinical ehrlichiosis has been detected in cats, including monoclonal gammopathy.
A validated serologic assay is not currently available and some cats with E canis-like DNA in blood have been seronegative. 21 Positive serologic test results occur in both healthy and clinically ill cats, and so a diagnosis of clinical ehrlichiosis should not be based on serologic test results alone. Ehrlichia species PCR and gene sequencing can be used in cats with suspicious clinical signs to confirm infection and should be considered the tests of choice at this time. However, not all cats that are PCR positive are clinically ill.
Clinical improvement after treatment with tetracycline, doxycycline or imidocarb dipropionate has been reported for most cats with suspected ehrlichiosis.19-21 The current recommendation of the American College of Veterinary Internal Medicine Infectious Disease Study Group is to administer doxycycline (10 mg/kg PO q24h or 5 mg/kg PO q12h for 28 days). Pancytopenia can occur in cats with ehrlichiosis; 21 in dogs, pancytopenia may not respond to treatment.
This pathogen is another example of why acaricides should be used to attempt to avoid infection with vector-borne disease agents. It has been shown in dogs that E canis can be transmitted within 3 h of tick attachment. 32 This short transmission time indicates the importance of using acaricides that either rapidly kill attached ticks or, preferably, prevent ticks from biting in the first place.
Rickettsia species
Rickettsia conorii and Rickettsia massiliae DNA was amplified or antibodies reacted to R conorii from the blood of cats in Spain and Italy.13,33 In one study of cats in the USA with fever, the Rickettsia rickettsii antibody prevalence rate was 6.6%, but DNA of the agent was not amplified from blood. 34 Similar results were seen in a study of cats in St Kitts, West Indies where 22/52 feral cats had R rickettsii antibodies in serum but were all negative for specific DNA in blood. 18 These results prove that cats are sometimes exposed to tick-borne spotted fever group organisms, but further data are needed to determine the significance of disease associations such as fever. Because clinical illness in cats has not been documented, optimal treatment is unknown. However, based on results in dogs, doxycycline or a fluoroquinolone would be logical choices. Use of acaricides should lessen the potential for transmission of these agents to cats.
Other bacteria
Borrelia species
Many cats exposed to Ixodes species in North America and parts of Europe develop antibodies against B burgdorferi. Some cats are also infected by Borrelia persica from infestation by Ornithodoros tholozani in some parts of the Middle East. 35 Fever can be associated with B burgdorferi or B persica infections in cats.12,35,36 However, since many cats have exposure to Ixodes species, it can be difficult to determine whether clinical illness is resulting from A phagocytophilum or B burgdorferi or both. 12
Cats with fever and suspected B persica infections should have peripheral blood smears evaluated for spirochetes and the infection can be confirmed by PCR assay. 35 While not approved for this use, serum from cats with fever and suspected B burgdorferi infection can be screened with a commercially available kit (SNAP 4DXPlus; IDEXX Laboratories) titrated for use with dog sera.2,4 However, a positive B burgdorferi antibody assay result proves only exposure, and not necessarily clinical borreliosis.
Cats with fever from suspected borreliosis generally respond to the administration of doxycycline at 5 mg/kg PO q12h or 10 mg/kg PO q24h.12,36 The effectiveness of different acaricides for the prevention of transmission of Borrelia species to cats has not been compared but, based on experiences in dogs, potentially all should be helpful in preventing borreliosis in cats if used appropriately. 37
Francisella tularensis
Cats in the USA can develop fever after infection by Francisella tularensis. 38 Cats are often infected by F tularensis through carnivorism but tick-borne transmission can occur. Infection can be proven by culture, amplification of specific DNA by PCR assay or rising antibody titers. 39 Clinically ill cats should respond to doxycycline or fluoroquinolones. Cats in endemic areas should be provided with tick control all year round and hunting behavior should be minimized if possible.
Protozoa
Babesia species
In South Africa and parts of Asia, there is a high number of Babesia species that infect cats. In Europe, Babesia vogeli or Babesia vulpes DNA have been amplified from ticks collected from cats and antibodies reactive to B vulpes detected in cats.13,31,37,40 In the Americas, B vogeli and Babesia gibsoni have been described in cats as both single and mixed infections.18,41 B vogeli and Babesia canis are likely more prevalent in areas with high infestation rates for R sanguineus. However, whether these agents induce fever in cats alone or as a coinfection has not been determined.
Serologic tests have not been validated for these agents in cats. Thus, the diagnosis currently is based on detection of piroplasms in erythrocytes or amplification of specific DNA by PCR assay. Optimal treatments are unknown for cats but options primarily extrapolated from canine babesiosis have been reviewed. 42 Use of acaricides should lessen the risk of exposure, except for B gibsoni which may be transmitted directly.
Cytauxzoon species
Cats in the USA, Brazil and Europe can be infected by Cytauxzoon species.10,43-49 It is apparent that Cytauxzoon felis infections in the USA (transmitted by Amblyomma americanum) can be very pathogenic when compared with the Cytauxzoon species infections occurring in cats in other countries. This may represent different species in different countries. 50 While fatal C felis infections are common in some regions of the USA, cats that survive or have subclinical infections are also common.51,52 These findings suggest that a wide range of clinical presentations may occur, with differences among cats perhaps related to inoculation dose, pre-existing immunity, strain variations, or as yet unidentified variations in immune response of the cat. A recent study showed that C felis could be transmitted after 36–48 h of tick attachment, and that ingestion of A americanum did not induce infections. 53
In the USA, clinical infections are recognized most commonly in the spring, summer and fall. Owners of clinically affected cats report nonspecific complaints, such as lethargy and anorexia, frequently. Cats have fever or hypothermia if presented in the final shock phase of infection. Common physical examination findings that might lead to consideration of this agent as a differential diagnosis include unresponsive fever, pale mucous membranes from shock or anemia, icterus, splenomegaly and hepatomegaly.10,45 Discomfort, clinical evidence of central nervous system disease including seizures, tachypnea with or without dyspnea, and sudden death on manipulation may all occur. Recently, ocular involvement of cytauxzoonosis has been described. 54
Cytauxzoon species can be seen on erythrocytes frequently, but cytology can be falsely negative in the acute stages of illness. The serious clinical signs of disease relate to the development of shizonts in the tissues. The syndrome can be diagnosed by cytological demonstration of piroplasms on erythrocytes or shizonts in spleen, liver or bone marrow samples, or by amplification of Cytauxzoon species DNA in blood or tissue aspirates by PCR. 45
To date, clinically affected cats have shown the best response to a combination of azithromycin at 10 mg/kg PO q24h and atovaquone at 15 mg/kg PO q8h, with approximately 60% of treated cats responding.55,56 This combination is superior to diminazene or imidocarb protocols. 57 Minimal restraint techniques should be used during the administration of supportive care to lessen the likelihood of sudden death.
The poor overall treatment response in clinical cytauxzoonosis cases is a perfect example of why tick control can be so important. Again, as referred to earlier in this article series, it is always better to prevent a vector-borne disease than attempt to treat it after illness has begun. Appropriate use of acaracides should lessen the risk of transmission of this agent. 58 One commercially available flea and tick collar containing flumethrin (Seresto; Bayer Animal Health) and a topical product containing sarolaner (Selamectin Plus; Zoetis) were shown to block transmission of C felis by A americanum.58,59
Hepatozoon species
A number of investigators have amplified Hepatozoon species DNA from cats and the agent has been detected in ticks collected from cats.3,28,47,60-62 In a recent report on cats in southern Italy, three different Hepatozoon species were detected. 61 In dogs, Hepatozoon americanum infection is induced by ingestion of ticks or ingestion of prey species such as rabbits. 63 Whether carnivorism results in infection of cats with Hepatozoon species has not been determined.
Fever associated with Hepatozoon species has not been studied extensively in cats; however, in dogs in the USA with H americanum infection, fever is common. In one study from Israel, fever was reported as one of the potential clinical manifestations of hepatozoonosis in cats. 60 PCR assays can be used to help confirm the diagnosis but optimal treatment protocols for cats with Hepatozoon species infections are unknown. Use of acaricides should lessen the potential for transmission of these agents among cats.
Sandfly-Borne Agents Associated With Fever
Leishmania species
Feline leishmaniosis has been reported globally.62,64-74 Traditionally skin lesions have been described as the most frequent clinical manifestation and sometimes were the only finding on physical examination. However, more recently cats have been described that do not have cutaneous manifestations but rather have other clinical signs, and in general these cases appear to be associated with a worse prognosis. A case of fever in a cat that may have related to leishmaniosis has been reported. 75
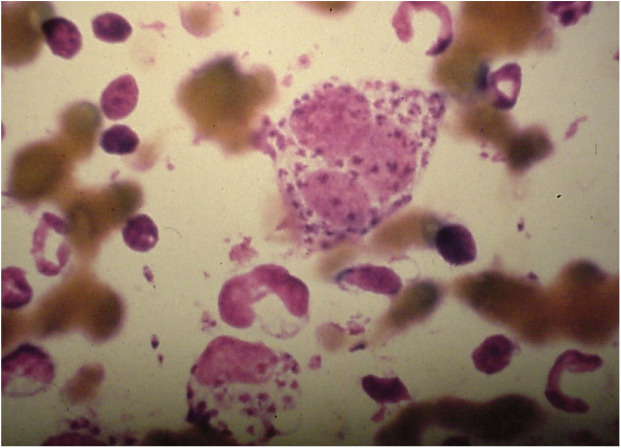
The diagnosis of leishmaniosis in cats, in the presence of suspected clinical signs, can be achieved using direct techniques such as cytology (Figure 2), histopathology and PCR assays. Alternatively, serologic techniques such as indirect fluorescent antibody assays or ELISAs that evaluate the immune response of the cat against Leishmania species can be used indirectly to support the diagnosis. In contrast to dogs that can have very high antibody levels even when subclinical carriers, a low positive titer in cats can be considered highly suggestive of a diagnosis of leishmaniosis. 76
Figure 2.
Cytology sample showing Leishmania species amastigotes in macrophages
Information about the best management of feline leishmaniosis is mostly based on single case reports. Long-term administration of allopurinol (10–20 mg/kg q12h or q24h) is usually clinically effective, but meglumine antimoniate has also been used successfully in a few cases. However, in the majority of cats the infection cannot be cleared, so the use of adequate prevention, including sandfly control, is recommended to reduce the number of cats infected with Leishmania species in endemic areas.77,78
Key Points
Some tick- and sandfly-associated pathogens can be associated with fever in cats and the history and concurrent clinical signs can trigger a diagnostic work-up and, ultimately, appropriate therapy.
Prevention of these infections is preferred and there is mounting evidence to show that consistent use of products that either rapidly kill vectors or, preferably, prevent vectors from biting a cat is desirable.
Acknowledgments
The authors thank Dr Susanne Siebert and Dr Annette Boegel for their support and encouragement as well as editorial comments.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: This work did not involve the use of animals and therefore ethical approval was not required.
Informed Consent: This work did not involve the use of animals and therefore informed consent was not required. No animals or humans are identifiable within the publication and therefore additional consent for publication was not required.
References
- 1. Lappin MR, Tasker S, Roura X. Role of vector-borne pathogens in the development of fever in cats. 1. Flea-associated diseases. J Feline Med Surg 2020; 22: 31-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lappin MR, Chandrashekar R, Stillman B, et al. Evidence of infection of cats by Borrelia burgdorferi and Anaplasma phagocytophilum after exposure to wild-caught adult Ixodes scapularis. J Vet Diagn Invest 2015; 27: 522-525. [DOI] [PubMed] [Google Scholar]
- 3. Duplan F, Davies S, Filler S, et al. Anaplasma phago-cytophilum, Bartonella spp., haemoplasma species and Hepatozoon spp. in ticks infesting cats: a large-scale survey. Parasit Vectors 2018; 11: 201. DOI: 10.1186/s13071-018-2789-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lappin M, Huesken R. Anaplasma phagocytophilum and Borrelia burgdorferi infections in cats exposed repeatedly to Ixodes scapularis [abstract]. J Vet Intern Med 2015; 29: 1201. [Google Scholar]
- 5. Lappin MR, Breitschwerdt EB, Jensen WA, et al. Molecular and serologic evidence of Anaplasma phagocytophilum infection in cats in North America. J Am Vet Med Assoc 2004; 225: 893-896. [DOI] [PubMed] [Google Scholar]
- 6. Bjoersdorff A, Svendenius L, Owens JH, et al. Feline granulocytic ehrlichiosis – a report of a new clinical entity and characterization of the new infectious agent. J Small Anim Pract 1999; 40: 20-24. [DOI] [PubMed] [Google Scholar]
- 7. Adaszek t, Gorna M, Skrzypczak M, et al. Three clinical cases of Anaplasma phagocytophilum infection in cats in Poland. J Feline Med Surg 2013; 15: 333-337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bergmann M, Englert T, Stuetzer B, et al. Prevalence of selected rickettsial infections in cats in Southern Germany. Comp Immunol Microbiol Infect Dis 2015; 42: 33-36. [DOI] [PubMed] [Google Scholar]
- 9. Savidge C, Ewing P, Andrews J, et al. Anaplasma phagocytophilum infection of domestic cats: 16 cases from the northeastern USA. J Feline Med Surg 2016; 18: 85-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Andre MR, Filgueira KD, Calchi AC, et al. Co-infection with arthropod-borne pathogens in domestic cats. Rev Bras Parasitol Vet 2017; 26: 525-531. [DOI] [PubMed] [Google Scholar]
- 11. Galemore ER, Labato MA, O’Neil E. Prevalence of Anaplasma phagocytophilum infection in feral cats in Massachusetts. JFMS Open Rep 2018; 4. DOI: 10.1177/2055116917753804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hoyt K, Chandrasheker R, Beall M, et al. Evidence for clinical borreliosis and anaplasmosis in cats in Maine. Top Companion Anim Med 2018; 33: 40-44. [DOI] [PubMed] [Google Scholar]
- 13. Persichetti MF, Pennisi MG, Vullo A, et al. Clinical evaluation of outdoor cats exposed to ectoparasites and associated risk for vector-borne infections in southern Italy. Parasit Vectors 2018; 11: 136. DOI: 10.1186/s13071-018-2725-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lima MLF, Soares PT, Ramos CAN, et al. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz J Microbiol 2010; 41: 381-385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Qurollo BAB, Balakrishnan N, Cannon CZ, et al. Co-infection with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’ in a cat diagnosed with splenic plasmacytosis and multiple myeloma. J Feline Med Surg 2014; 16: 713-720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Attipa C, Papasouliotis K, Solano-Gallego L, et al. Prevalence study and risk factor analysis of selected bacterial, protozoal and viral, including vector-borne, pathogens in cats from Cyprus. Parasit Vectors 2017; 10: 130. DOI: 10.1186/s13071-017-2063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zobba R, Anfossi AG, Visco S, et al. Cell tropism and molecular epidemiology of Anaplasma platys-like strains in cats. Ticks Tick Borne Dis 2015; 6: 272-280. [DOI] [PubMed] [Google Scholar]
- 18. Kelly PJ, Koster L, Li J, et al. Survey of vector-borne agents in feral cats and first report of Babesia gibsoni in cats on St Kitts, West Indies. BMC Vet Res 2017; 13: 331. DOI: 10.1186/s12917-017-1230-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beaufils JP, Marin-Granel J, Jumelle P, et al. Ehrlichiosis in cats. A retrospective study of 21 cases. Prat Med Chir Anim Cie 1999; 34: 587-596. [Google Scholar]
- 20. Bouloy RP, Lappin MR, Holland CH, et al. Clinical ehrlichiosis in a cat. J Am Vet Med Assoc 1994; 204: 1475-1478. [PubMed] [Google Scholar]
- 21. Breitschwerdt EB, Abrams-Ogg AC, Lappin MR, et al. Molecular evidence of Ehrlichia canis-like infection in cats. J Vet Intern Med 2002; 16: 642-649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Aguirre E, Tesouro MA, Amusategui I, et al. Assessment of feline ehrlichiosis in central Spain using serology and a polymerase chain reaction technique. Ann N Y Acad Sci 2004; 1026: 103-135. [DOI] [PubMed] [Google Scholar]
- 23. Solano-Gallego L, Hegarty B, Espada Y, et al. Serological and molecular evidence of exposure to arthropod-borne organisms in cats from northeastern Spain. Vet Microbiol 2006; 118: 274-277. [DOI] [PubMed] [Google Scholar]
- 24. Braga Mdo S, Andre MR, Freschi CR, et al. Molecular and serological detection of Ehrlichia spp. in cats on Sao Luis Island, Maranhao, Brazil. Rev Bras Parasitol Vet 2012; 21: 37-41. [DOI] [PubMed] [Google Scholar]
- 25. Braga IA, dos Santos LG, Melo AL, et al. Hematological values associated to the serological and molecular diagnostic in cats suspected of Ehrlichia canis infection. Rev Bras Parasitol Vet 2013; 22: 470-474. [DOI] [PubMed] [Google Scholar]
- 26. Braga IA, dos Santos L, de Souza Ramos D, et al. Detection of Ehrlichia canis in domestic cats in the central-western region of Brazil. Braz J Microbiol 2014; 45: 641-645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Maia C, Ramos C, Coimbra M, et al. Bacterial and protozoal agents of feline vector-borne diseases in domestic and stray cats from southern Portugal. Parasit Vectors 2014; 7: 115. DOI: 10.1186/1756-3305-7-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Oliveira AC, Luz MF, Granada S, et al. Molecular detection of Anaplasma bovis, Ehrlichia canis and Hepatozoon felis in cats from Luanda, Angola. Parasit Vectors 2018; 11: 167. DOI: 10.1186/s13071-018-2767-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pennisi MG, Hofmann-Lehmann R, Radford AD, et al. Anaplasma, Ehrlichia and Rickettsia species infections in cats: European guidelines from the ABCD on prevention and management. J Feline Med Surg 2017; 19: 542-548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Peavy GM, Holland CJ, Dutta SK, et al. Suspected Ehrlichia infection in a household of cats. J Am Vet Med Assoc 1997; 210: 231-234. [PubMed] [Google Scholar]
- 31. Pennisi MG, Persichetti MF, Serrano L, et al. Ticks and associated pathogens collected from cats in Sicily and Calabria (Italy). Parasit Vectors 2015; 8: 512. DOI: 10.1186/s13071-015-1128-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Jongejan F, Crafford D, Erasmus H, et al. Comparative efficacy of oral administrated afoxolaner (NexGard™) and fluralaner (Bravecto™) with topically applied permethrin/ imidacloprid (Advantix(®)) against transmission of Ehrlichia canis by infected Rhipicephalus sanguineus ticks to dogs. Parasit Vectors 2016; 9: 348. DOI: 10.1186/s13071-016-1636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Segura F, Pons I, Miret J, et al. The role of cats in the eco-epidemiology of spotted fever group diseases. Parasit Vectors 2014; 7: 353. DOI: 10.1186/1756-3305-7-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bayliss DB, Morris AK, Horta MC, et al. Prevalence of Rickettsia species antibodies and Rickettsia species DNA in the blood of cats with and without fever. J Feline Med Surg 2009; 11: 266-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Baneth G, Nachum-Biala Y, Halperin T, et al. Borrelia persica infection in dogs and cats: clinical manifestations, clinico-pathological findings and genetic characterization. Parasit Vectors 2016; 9: 244. DOI: 10.1186/s13071-016-1530-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pantchev N, Vrhovec MG, Pluta S, et al. Seropositivity of Borrelia burgdorferi in a cohort of symptomatic cats from Europe based on a C6-peptide assay with discussion of implications in disease aetiology. Berl Munch Tierarztl Wochenschr 2016; 129: 333-339. [PubMed] [Google Scholar]
- 37. Davies S, Abdullah S, Helps C, et al. Prevalence of ticks and tick-borne pathogens: Babesia and Borrelia species in ticks infesting cats of Great Britain. Vet Parasitol 2017; 244: 129-135. [DOI] [PubMed] [Google Scholar]
- 38. Spagnoli ST, Kuroki K, Schommer SK, et al. Pathology in practice. Francisella tularensis. J Am Vet Med Assoc 2011; 238: 1271-1273. [DOI] [PubMed] [Google Scholar]
- 39. Magnarelli L, Levy S, Koski R. Detection of antibodies to Francisella tularensis in cats. Res Vet Sci 2007; 82: 22-26. [DOI] [PubMed] [Google Scholar]
- 40. Solano-Gallego L, Sainz A, Roura X, et al. A review of canine babesiosis: the European perspective. Parasit Vectors 2016; 9: 336. DOI: 10.1186/s13071-016-1596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Malheiros J, Costa MM, do Amaral RB, et al. Identification of vector-borne pathogens in dogs and cats from Southern Brazil. Ticks Tick Borne Dis 2016; 7: 893-900. [DOI] [PubMed] [Google Scholar]
- 42. Ashley L, Ayoob AL, Prittie J, et al. Feline babesiosis. J Vet Emerg Crit Care 2010; 20: 90-97. [DOI] [PubMed] [Google Scholar]
- 43. Haber MD, Tucker MD, Marr HS, et al. The detection of Cytauxzoon felis in apparently healthy free-roaming cats in the USA. Vet Parasitol 2007; 146: 316-320. [DOI] [PubMed] [Google Scholar]
- 44. Carli E, Trotta M, Chinelli R, et al. Cytauxzoon sp. infection in the first endemic focus described in domestic cats in Europe. Vet Parasitol 2012; 183: 343-352. [DOI] [PubMed] [Google Scholar]
- 45. Sherrill MK, Cohn LA. Cytauxzoonosis: diagnosis and treatment of an emerging disease. J Feline Med Surg 2015; 17: 940-948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Alho AM, Silva J, Fonseca MJ, et al. First report of Cytauxzoon sp. infection in a domestic cat from Portugal. Parasit Vectors 2016; 9: 220. DOI: 10.1186/s13071-016-1506-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Diaz-Reganon D, Villaescusa A, Ayllon T, et al. Molecular detection of Hepatozoon spp. and Cytauxzoon sp. in domestic and stray cats from Madrid, Spain. Parasit Vectors 2017; 10: 112. DOI: 10.1186/s13071-017-2056-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Legroux JP, Halos L, Rene-Martellet M, et al. First clinical case report of Cytauxzoon sp. infection in a domestic cat in France. BMC Vet Res 2017; 13: 81. DOI: 10.1186/s12917-017-1009-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nentwig A, Meli ML, Schrack J, et al. First report of Cytauxzoon sp. infection in domestic cats in Switzerland: natural and transfusion-transmitted infections. Parasit Vectors 2018; 11: 292. DOI: 10.1186/s13071-018-2728-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Gallusova M, Jirsova D, Mihalca AD, et al. Cytauxzoon infections in wild felids from Carpathian-Danubian-Pontic Space: further evidence for a different Cytauxzoon species in European felids. J Parasitol 2016; 102: 377-380. [DOI] [PubMed] [Google Scholar]
- 51. Meinkoth J, Kocan AA, Whitworth L, et al. Cats surviving natural infection with Cytauxzoon felis: 18 cases (1997-1998). J Vet Intern Med 2000; 14: 521-525. [DOI] [PubMed] [Google Scholar]
- 52. Rizzi TE, Reichard MV, Cohn LA, et al. Prevalence of Cytauxzoon felis infection in healthy cats from enzootic areas in Arkansas, Missouri, and Oklahoma. Parasit Vectors 2015; 8: 13. DOI: 10.1186/s13071-014-0618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Thomas JE, Ohmes CM, Payton ME, et al. Minimum transmission time of Cytauxzoon felis by Amblyomma ameri-canum to domestic cats in relation to duration of infestation, and investigation of ingestion of infected ticks as a potential route of transmission. J Feline Med Surg 2018; 20: 67-72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Meekins J, Cino-Ozuna AG. Histologic identification of intraocular Cytauxzoon felis in three cats. JFMS Open Rep 2018; 4. DOI: 10.1177/2055116918813242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Cohn LA, Birkenheuer AJ, Brunker JD, et al. Efficacy of ato-vaquone and azithromycin or imidocarb dipropionate in cats with acute cytauxzoonosis. J Vet Intern Med 2011; 25: 55-60. [DOI] [PubMed] [Google Scholar]
- 56. Schreeg ME, Marr HS, Tarigo J, et al. Pharmacogenomics of Cytauxzoon felis cytochrome b: implications for atovaquone and azithromycin therapy in domestic cats with cytaux-zoonosis. J Clin Microbiol 2013; 51: 3066-3069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Lewis KM, Cohn LA, Marr HS, et al. Failure of efficacy and adverse events associated with dose-intense diminazene diaceturate treatment of chronic Cytauxzoon felis infection in five cats. J Feline Med Surg 2014; 16: 157-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Reichard MV, Thomas JE, Arther RG, et al. Efficacy of an imidacloprid 10%/flumethrin 4.5% collar (Seresto®, Bayer) for preventing the transmission of Cytauxzoon felis to domestic cats by Amblyomma americanum. Parasitol Res 2013; 112 Suppl 1: 11-20. [DOI] [PubMed] [Google Scholar]
- 59. Reichard MV, Rugg JJ, Thomas JE, et al. Efficacy of a topical formulation of selamectin plus sarolaner against induced infestations of Amblyomma americanum on cats and prevention of Cytauxzoon felis transmission. Vet Parasitol 2019; 270 Suppl 1: S31-S37. DOI: 10.1016/j.vetpar.2018.10.018. [DOI] [PubMed] [Google Scholar]
- 60. Baneth G, Aroch I, Tal N, et al. Hepatozoon species infection in domestic cats: a retrospective study. Vet Parasitol 1998; 79: 123-133. [DOI] [PubMed] [Google Scholar]
- 61. Giannelli A, Latrofa MS, Nachum-Biala Y, et al. Three different Hepatozoon species in domestic cats from southern Italy. Ticks Tick Borne Dis 2017; 8: 721-724. [DOI] [PubMed] [Google Scholar]
- 62. Attipa C, Neofytou K, Yiapanis C, et al. Follow-up monitoring in a cat with leishmaniosis and coinfections with Hepatozoon felis and ‘Candidatus Mycoplasma haemominutum’. JFMS Open Rep 2017; 3. DOI: 10.1177/2055116917740454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Johnson EM, Panciera RJ, Allen KE, et al. Alternate pathway of infection with Hepatozoon americanum and the epidemi-ologic importance of predation. J Vet Intern Med 2009; 23: 1315-1318. [DOI] [PubMed] [Google Scholar]
- 64. Hervas J, Chacon-M De, Lara F, Sanchez-Isarria MA, et al. Two cases of feline visceral and cutaneous leishmaniosis in Spain. J Feline Med Surg 1999; 1: 101-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Poli A, Abramo F, Barsotti P, et al. Feline leishmaniosis due to Leishmania infantum in Italy. Vet Parasitol 2002; 106: 181-191. [DOI] [PubMed] [Google Scholar]
- 66. Pennisi MG, Venza M, Reale S, et al. Case report of leishma-niasis in four cats. Vet Res Commun 2004; 28 Suppl 1: 363-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Leiva M, Lloret A, Pena T, et al. Therapy of ocular and visceral leishmaniasis in a cat. Vet Ophthalmol 2005; 8: 71-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Rufenacht S, Sager H, Miiller N, et al. Two cases of feline leishmaniosis in Switzerland. Vet Rec 2005; 156: 542-545. [DOI] [PubMed] [Google Scholar]
- 69. Navarro JA, Sanchez J, Penafiel-Verdu C, et al. Histopathological lesions in 15 cats with leishmaniosis. J Comp Pathol 2010; 143: 297-302. [DOI] [PubMed] [Google Scholar]
- 70. Rougeron V, Catzeflis F, Hide M, et al. First clinical case of cutaneous leishmaniasis due to Leishmania (Viannia) braziliensis in a domestic cat from French Guiana. Vet Parasitol 2011; 181: 325-328. [DOI] [PubMed] [Google Scholar]
- 71. Verneuil M. Ocular leishmaniasis in a cat: case report. J Fr Ophtalmol 2013; 36: 67-72. [DOI] [PubMed] [Google Scholar]
- 72. Richter M, Schaarschmidt-Kiener D, Krudewig C. Ocular signs, diagnosis and long-term treatment with allopurinol in a cat with leishmaniasis. Schweiz Arch Tierheilkd 2014; 156: 289-294. [DOI] [PubMed] [Google Scholar]
- 73. Pennisi MG, Persichetti MF. Feline leishmaniosis: is the cat a small dog? Vet Parasitol 2018; 251: 131-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Rivas AK, Alcover M, Martinez-Orellana P, et al. Clinical and diagnostic aspects of feline cutaneous leishmaniosis in Venezuela. Parasit Vectors 2018; 11: 141. DOI: 10.1186/s13071-018-2747-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Basso MA, Marques C, Santos M, et al. Successful treatment of feline leishmaniosis using a combination of allopurinol and N-methyl-glucamine antimoniate. JFMS Open Rep 2016; 2. DOI: 10.1177/2055116916630002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Persichetti MF, Solano-Gallego L, Vullo A, et al. Diagnostic performance of ELISA, IFAT and Western blot for the detection of anti-Leishmania infantum antibodies in cats using a Bayesian analysis without a gold standard. Parasit Vectors 2017; 10: 119. DOI: 10.1186/s13071-017-2046-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Brianti E, Falsone L, Napoli E, et al. Prevention of feline leishmaniosis with an imidacloprid 10%/flumethrin 4.5% polymer matrix collar. Parasit Vectors 2017; 10: 334. DOI: 10.1186/s13071-017-2258-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Otranto D, Dantas-Torres F, Napoli E, et al. Season-long control of flea and tick infestations in a population of cats in the Aeolian archipelago using a collar containing 10% imidacloprid and 4.5% flumethrin. Vet Parasitol 2017; 248: 80-83. [DOI] [PubMed] [Google Scholar]