ABSTRACT
Cholesterol is an essential structural component of the cell membrane, whereas excess cholesterol can be toxic and thus is stored in intracellular lipid droplets (LDs). Malignant tumor cells grow rapidly and require abundant cholesterol to build new membranes. How they maintain cholesterol homeostasis is largely unknown. We recently revealed that SREBF1/SREBP-1 (sterol regulatory element binding transcription factor 1), a key lipogenic transcription factor, plays a critical role in maintaining cholesterol homeostasis in tumor cells. We found that in addition to activation of de novo lipid synthesis and cholesterol uptake, SREBF1 also upregulates macroautophagy/autophagy to hydrolyze LDs, and increases the expression of NPC2, a lysosome cholesterol transporter, actively mobilizing LD-stored cholesterol and fatty acids to promote tumor growth. Our study demonstrates that SREBF1 controls the balance of lipid synthesis, uptake, storage and liberation to maintain lipid homeostasis for rapid tumor growth, while suggesting it as a very promising molecular target for cancer treatment.
KEYWORDS: Autophagy, cancer, cholesterol, glioblastoma, lipid droplets, lipophagy
Dysfunction in lipid metabolism has emerged as a hallmark of malignancies, and it has rapidly gained attention over the past 10 years. Among the various lipid nutrients, cholesterol plays a pivotal role in the maintenance of membrane integrity and function, though when in excess it can cause cellular toxicity. Sustaining cholesterol homeostasis is a challenging issue for rapidly dividing tumor cells. How cancer cells maintain proper cholesterol levels in the membrane to allow for extensive tumor growth, while preventing excess cholesterol accumulation from causing toxicity is poorly understood.
Under physiological conditions, cholesterol exists in two forms: unesterified and esterified. Unesterified cholesterol is considered as free cholesterol and is distributed to cellular membranes. Esterified cholesterol, also known as cholesteryl esters (CEs), is stored in lipid droplets (LDs) with the excess cholesterol, along with triglycerides (TGs), acting as a lipid core. Whereas LDs are characteristic of adipocytes that reside in the adipose tissue, they are also found in hepatocytes in cases of fatty liver disease and various other cell types. Our prior investigations have demonstrated that CE- and TG-laden LDs are abundant in the cancer tissue from patients with glioblastoma (GBM), as well as in xenograft tumors and in GBM cells in culture. Similar observations have been made in colon, prostate, liver cancer and clear-cell renal carcinoma. GBM is the grade IV primary brain tumor, and patients with GBM have a median survival of only 12–16 months after diagnosis despite extensive therapies. A deeper understanding of the lipid distribution in cancer cells, including in GBM, may identify tumor dependency and a vulnerability that allows for effective treatment.
Our group has had a long-term focus on understanding how tumor cells obtain sufficient lipids and how they regulate lipid distribution to maintain rapid growth. In solid tumors, cancer cells are exposed to a rapidly fluctuating nutrient environment. In an ample nutrient environment, our previous studies showed that GBM cells absorb and synthesize abundant fatty acids and cholesterol and store surplus levels in LDs by converting them to CEs and TGs. Thus, GBM cells inherently have a greater number of LDs and higher levels of these molecules stored there compared to the cells of normal brain tissue. After aggressive growth, a bulk of tumor cells will face a scarce nutrient environment as there is always a lag in angiogenesis and thus nutrient delivery. Under this circumstance, obtaining sufficient lipids to maintain tumor cell growth is a severe challenge for the cells.
Among the regulators implicated in lipid metabolism, members of the sterol regulatory element-binding protein (SREBP) family play the central role in controlling lipid uptake and de novo biosynthesis. SREBPs comprise three isoforms: SREBF1/SREBP-1a, SREBF1/SREBP-1c, and SREBF2/SREBP-2. SREBF1/SREBP-1a exhibits the highest transcriptional activity, regulating both fatty acid and cholesterol synthesis and uptake. SREBF1/SREBP-1c primarily governs fatty acid synthesis, whereas SREBF2 controls cholesterol synthesis and uptake. Unexpectedly, our recent study revealed that under conditions of lipid starvation, in addition to an increase in de novo lipid synthesis and uptake, SREBF1/SREBP-1a and SREBF1/SREBP-1c transcriptionally upregulates the expression of multiple autophagic genes; notably, ATG9B, ATG4A and LC3B, as well as the lysosome cholesterol transporter NPC2. Together this upregulation promotes autophagic hydrolysis of LDs and the release of their stored cholesterol and fatty acids to maintain GBM growth (Figure 1) [1]. These findings unveil a new role for SREBF1 in influencing intracellular lipid recycling in cancer cells, providing new insights into how cancer cells can survive during nutrient-restrictive conditions. Our data support the notion that under circumstances of nutrient deprivation, tumor cells can turn not only to cholesterol and fatty acid biosynthesis to supplement their pools, but also to the release of their storage in LDs via elevated autophagy, as well as an increase in the export of cholesterol from the lysosomes via elevated NPC2 expression, thus allowing for the continued survival and growth of the cancer cells (Figure 1).
Figure 1.
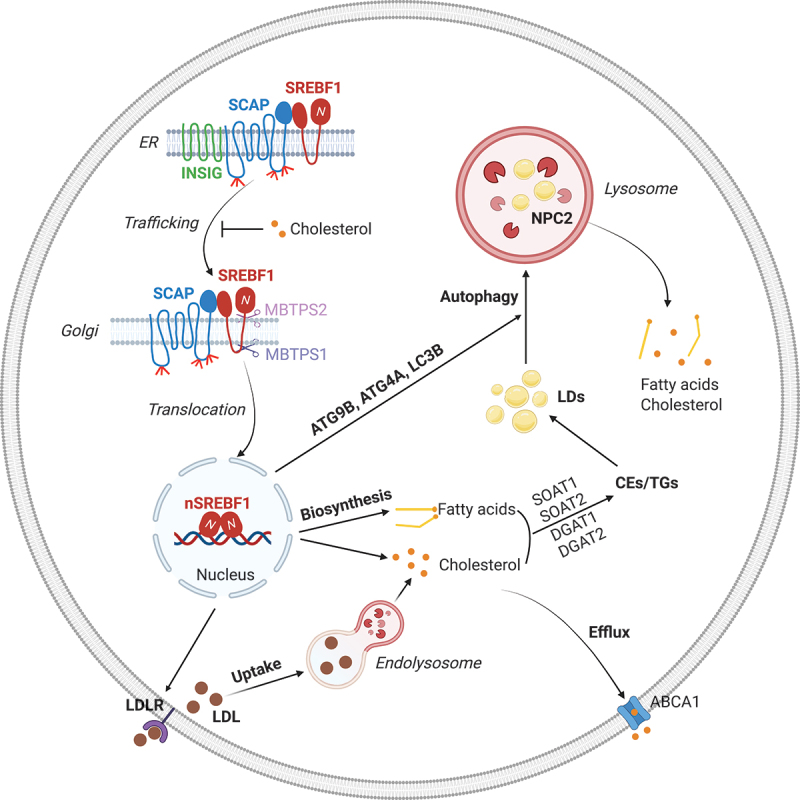
SREBF1 concurrently regulates lipid synthesis and lipophagy to maintain lipid homeostasis needed to promote tumor growth. After synthesis, SREBF1 is restrained in the endoplasmic reticulum (ER) by INSIG (insulin induced gene), which binds to SCAP (SREBF chaperone) to form the INSIG-SCAP-SREBF1 complex. Upon cholesterol reduction, SCAP dissociates from INSIG and transports SREBF1 from the ER to the Golgi, where SREBF1 is sequentially cleaved by MBTPS1/S1P and MBTPS2/S2P. This cleavage allows for the release of the SREBF1 N-terminal active form that then enters the nucleus to promote a diverse array of gene expression, including LDLR (low density lipoprotein receptor), cholesterol and fatty acid synthesis-related genes and autophagy-related genes ATG9B, ATG4A and LC3B, as well as NPC2 (NPC intracellular cholesterol transporter 2). LDLR mediates LDL entry into cells, which is then hydrolyzed in the lysosomes to release carried cholesterol and fatty acids to support tumor cell growth. Excess cholesterol and fatty acids are converted to cholesterol esters (CEs) and triglycerides (TGs), respectively, and then stored in lipid droplets (LDs). Excess cholesterol can also be exported outside of cells by ABCA1 (ATP binding cassette subfamily a member 1). Under conditions of nutrient deprivation, LDs are mobilized into lysosomes by an autophagic process (i.e., lipophagy) that releases the stored cholesterol and fatty acids to promote tumor cell growth. Thus, LDs serve as a critical reservoir to maintain proper cellular lipid levels, and they prevent excess free cholesterol and fatty acids from triggering cellular toxicity. The figure was created with BioRender.com.
During cancer treatment, various chemotherapeutic and radiation-based treatments trigger autophagy that has been considered a mechanism for tumor survival and drug resistance. Nevertheless, the specific contents hydrolyzed by autophagy to support tumor resistance have been unclear. Our study suggests that autophagic hydrolysis of LDs might be a major pro-survival activity that occurs during a variety of cancer treatments as it provides critical lipid-based cellular building blocks and energy resources to maintain tumor survival and to facilitate tumor resistance to such therapies. Therefore, targeting the interplay between LDs and autophagy may be a promising avenue to overcome tumor therapy resistance, which should be explored in future studies.
Funding Statement
This work was supported by NINDS and NCI of United States grants R01NS104332, R01NS112935, R01CA227874, and R01CA240726 to D.G. We also appreciate the support from the Urban and Shelly Meyer Foundation to D.G.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Reference
- [1].Geng F, Zhong Y, Su H, et al. SREBP-1 upregulates lipophagy to maintain cholesterol homeostasis in brain tumor cells. Cell Rep. 2023 Jul 25;42(7):112790. doi: 10.1016/j.celrep.2023.112790 [DOI] [PMC free article] [PubMed] [Google Scholar]


