Abstract
The phrenic neuromuscular system consists of the phrenic motor nucleus in the mid-cervical spinal cord, the phrenic nerve, and the diaphragm muscle. This motor system helps sustain breathing throughout life, while also contributing to posture, coughing, swallowing, and speaking. The phrenic nerve contains primarily efferent phrenic axons and afferent axons from diaphragm sensory receptors but is also a conduit for autonomic fibers. On a breath-by-breath basis, rhythmic (inspiratory) depolarization of phrenic motoneurons occurs due to excitatory bulbospinal synaptic pathways. Further, a complex propriospinal network innervates phrenic motoneurons, and may serve to coordinate postural, locomotor and respiratory movements. The phrenic neuromuscular system is impacted in a wide range of neuromuscular diseases and injuries. Contemporary research is focused on understanding how neuromuscular plasticity occurs in the phrenic neuromuscular system and using this information to optimize treatments and rehabilitation strategies to improve breathing and related behaviors.
Keywords: phrenic, motoneuron, diaphragm, breathing, spinal, interneuron
This chapter describes the “phrenic neuromuscular system”, including the diaphragm, phrenic motoneurons (PhrMN), and the associated neural circuitry (Figure 1). The phrenic neuromuscular system is essential for breathing since the diaphragm is the primary inspiratory muscle. In addition, the diaphragm is also activated during other behaviors such as swallowing, speaking, coughing and sneezing (Fogarty et al., 2018a), and also contributes to postural support (Hudson et al., 2011; Hodges and Gandevia, 2000). We have a strong foundational understanding of the anatomy and physiology of the phrenic neuromuscular system, although the neural regulation of PhrMNs, and particularly the mechanisms associated with neuroplastic changes in the phrenic motor system in health and disease, remain an active area of investigation (Fuller and Mitchell, 2017). This chapter begins with a brief overview of the diaphragm muscle, reviews diaphragm innervation via the phrenic nerve, and then describes the phrenic motor nucleus and its innervation from spinal, brainstem, and cortical neurons. The chapter concludes with a review of how PhrMNs and associated diaphragm myofibers (i.e., diaphragm motor units) are recruited during breathing and other behaviors. Basic principles related to the control of breathing such as rhythm generation, pulmonary afferent innervation and chemoreception are not covered here, nor is the mechanistic basis of plasticity in the phrenic neuromuscular system. These topics are reviewed elsewhere in this volume (Chapters X-X).
Figure 1. The phrenic neuromuscular system.
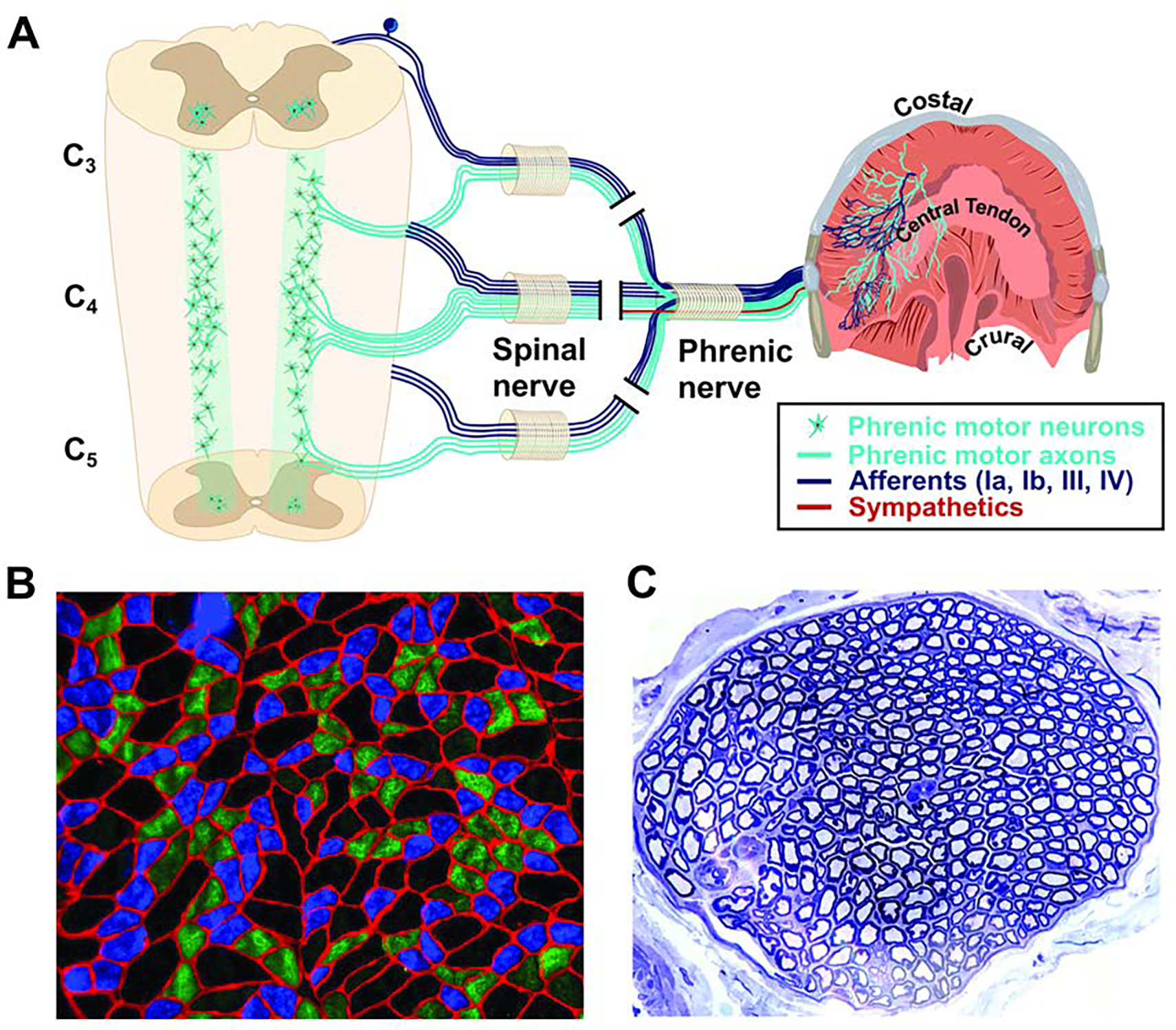
A. The diaphragm is innervated via the phrenic nerve. The phrenic nerve contains phrenic motoneuron axons which innervate diaphragm myofibers, sensory afferent fibers from the diaphragm, and sympathetic post-ganglionic fibers. The phrenic motor nucleus extends from C3-C5 in humans. B. Example histology from the costal diaphragm illustrating mixed myofiber type (Type 1, slow oxidative, blue; Type IIa, intermediate fibers, green, Type IIb/x, fast glycolytic, black). Red indicates the myofiber membrane (laminin stain). C. Histological cross section of the phrenic nerve stained with toluidine blue. Large myelinated axons are clearly visible. The examples in B and C were obtained from an adult rat. Scale bars. B, 130 μm; C, 300 μm.
1. The diaphragm.
A thin, dome-shaped skeletal muscle, the diaphragm forms a barrier between the thoracic and abdominal cavities. The diaphragm can be anatomically separated into the costal, sternal, and crural portions. The costal portion extends from the ribs to the central tendon, the sternal segment originates from the xiphoid process and inserts into the central tendon, and the crural diaphragm extends posteriorly from the central tendon, attaching to the vertebrae and surrounding the esophagus, abdominal aorta and inferior vena cava (Bains et al., 2021).
During inspiration the diaphragm contracts, flattening the domed shape to cause expansion of the thoracic cavity. The resultant drop in thoracic pressure creates a gradient, thereby causing the lungs to fill with air. As the primary inspiratory “pump muscle”, diaphragm contraction accounts for the majority of pressure changes required to produce a typical inspiratory tidal volume (Sant’ambrogio and Camporesi, 1973). During quiet breathing, the diaphragm rhythmically contracts 10–20 times per minute with a relatively high duty cycle (the ratio of inspiratory time to the total time for a breath) of 30–40% (Welch et al., 2018). Due to the high duty cycle, diaphragm myofibers must be fatigue resistant. Diaphragm myofiber characteristics associated with fatigue resistance include an abundance of capillaries as well as high aerobic oxidative enzyme activity (Polla et al., 2004). Fiber type distribution in the human costal diaphragm is relatively homogeneous, with estimates of 46–55% for slow (type I) fibers and 45–54% for fast (type IIa/b) fibers (Levine et al., 1997; Levine et al., 2008; Meznaric and Cvetko, 2016; Mizuno, 1991; Lieberman et al., 1973).
Despite being fatigue resistant, the diaphragm is highly susceptible to atrophy. This is particularly true during diseases such as chronic obstructive pulmonary disease or congestive heart failure (Mangner et al., 2021; Levine et al., 2013), during conditions associated with diaphragm inactivity such as mechanical ventilation or paralysis (Levine et al., 2008; Welvaart et al., 2011), and during aging (Greising et al., 2013). For example, prolonged mechanical ventilation, which is necessary when alveolar ventilation cannot be sustained by the patient, is associated with rapid diaphragm atrophy and contractile dysfunction. This condition is known as ventilator-induced diaphragm dysfunction (VIDD) and leads to weaning difficulties and prolonged time in intensive care units (ICU) (Powers et al., 2013c). VIDD occurs, at least in part, because ventilator support leads to diaphragm inactivity and associated myofiber proteolysis (Powers et al., 2013a; Powers et al., 2013b).
2. The phrenic nerve.
Innervation of the diaphragm occurs via the left and right phrenic nerves. The phrenic nerve is usually described as arising from the mid-cervical (C3-C5) ventral spinal roots. However, cadaveric studies indicate variability across individuals, with the phrenic nerve sometimes emerging from only the C3 and C4 ventral roots (Mendelsohn et al., 2011). An accessory phrenic nerve, which ultimately joins the main trunk of the phrenic, can provide diaphragm innervation, but with variable occurrence. A study of 80 cadavers identified an accessory phrenic nerve in 61% of cases (Loukas et al., 2006). When present, the accessory phrenic arises from the 5th cervical ventral ramus and joins the main phrenic nerve inside the thoracic cavity (Paraskevas et al., 2016; Mendelsohn et al., 2011). In the rat, the accessory phrenic nerve contains approximately 10% of all efferent phrenic motor axons (DeVries and Goshgarian, 1989).
The phrenic is a mixed sensory-motor nerve with sensory afferents representing approximately one-third of the total fibers (see Figure 1 for a histological cross section of the phrenic nerve). The afferent fibers in the phrenic nerve include large diameter myelinated (group Ia, Ib, II), small diameter myelinated (group III) and unmyelinated axons (group IV) (Duron et al., 1978; Landau et al., 1962; Langford and Schmidt, 1983). Compared to most skeletal muscles, the diaphragm has relatively few large diameter afferents from muscle spindles (Ia). Golgi tendon organs (Ib) are found in the central tendon and diaphragm margins (Nair et al., 2017). The majority of unmyelinated phrenic afferent axons have cell bodies in the mid-cervical dorsal root ganglia, but unmyelinated phrenic nerve afferents can also come from the cervical sympathetic chain (Langford and Schmidt, 1983; Balkowiec and Szulczyk, 1992). Unmyelinated preganglionic efferent (Langford and Schmidt, 1983) and sympathetic fibers (Balkowiec and Szulczyk, 1992) have also been reported in the phrenic nerve.
The precise physiological impact of activating diaphragm sensory afferents in the phrenic nerve is difficult to state, owing to the diversity of afferent types (Nair et al., 2017). Indeed, phrenic afferent neurons can trigger a range of physiological responses that include increased sympathetic neural outflow (Offner et al., 1992), increased arterial blood pressure (Hussain et al., 1991), inhibition of PhrMNs (Gill and Kuno, 1963; Marlot et al., 1987), increases in ventilation (Marlot et al., 1987; Revelette et al., 1988) and decreases in intercostal motor output (De Troyer, 1998). The particular response will depend on the type of receptor being activated, and the context in which the stimulation is provided. Phrenic afferents can also trigger activation of cortical somatosensory neurons, and therefore likely play a role in the perception of breathing as well as the affective responses to respiratory loads (Davenport and Vovk, 2009; Davenport et al., 2010). This may be particularly important to understanding dyspnea, which is the perception of difficult and/or labored breathing. Dyspnea is among the most common symptoms reported to medical providers, with a prevalence estimated at more than 20% in some populations (Gronseth et al., 2014).
The efferent fibers which make up approximately two-thirds of the phrenic nerve primarily represent the myelinated axons of the PhrMNs which innervate the diaphragm (see Section 3). However, the phrenic nerve is also a conduit for autonomic fibers and contains post-ganglionic fibers from the middle and stellate ganglion of the cervical sympathetic trunk (Verlinden et al., 2018). These fibers likely innervate diaphragm blood vessels and thereby serve to regulate the diameter of diaphragm vasculature (Balkowiec and Szulczyk, 1992). Interestingly, histological cadaveric studies demonstrate the presence of catecholaminergic axons in the right but not left phrenic nerve (Verlinden et al., 2018), and this finding emphasizes that the left and the right phrenic nerve are not identical in composition. Indeed, there are more total axons in the right nerve (Song et al., 1999). This asymmetry may indicate a larger overall innervation area of the diaphragm via the right phrenic nerve (Hebb et al., 1964; Laskowski et al., 1991), increases in the number of mechanoreceptor afferents in the right phrenic nerve (Kostreva and Pontus, 1993), or differences in post-ganglionic (likely the middle and/or stellate ganglia) efferent fibers (Verlinden et al., 2018).
The distribution of phrenic nerve branches in the diaphragm is depicted in Figure 2. Upon entering the diaphragm, the main trunk diverges to form distinct anterior and posterior branches, with lateral branches of varying number (An et al., 2012). The primary branches subdivide to form smaller branches, thereby creating a “net” which innervates the entire diaphragm. Awareness of phrenic nerve distribution is particularly important in the context of electrical diaphragm pacing. This method can be used when voluntary diaphragm control is dramatically impaired or impossible (e.g., after spinal cord injury). By placing a stimulating electrode at the primary “motor point” (roughly equivalent to the point that the main trunk of the phrenic nerve enters the diaphragm), electricity can be used to induce diaphragm contraction and enable functionally effective breathing (Onders et al., 2018; Onders et al., 2004). During surgical implantation of stimulating wires, the motor point can be determined by electrical stimulation, and this is used to guide surgical placement (Onders et al., 2004).
Figure 2. The distribution of phrenic nerve branches in the diaphragm.
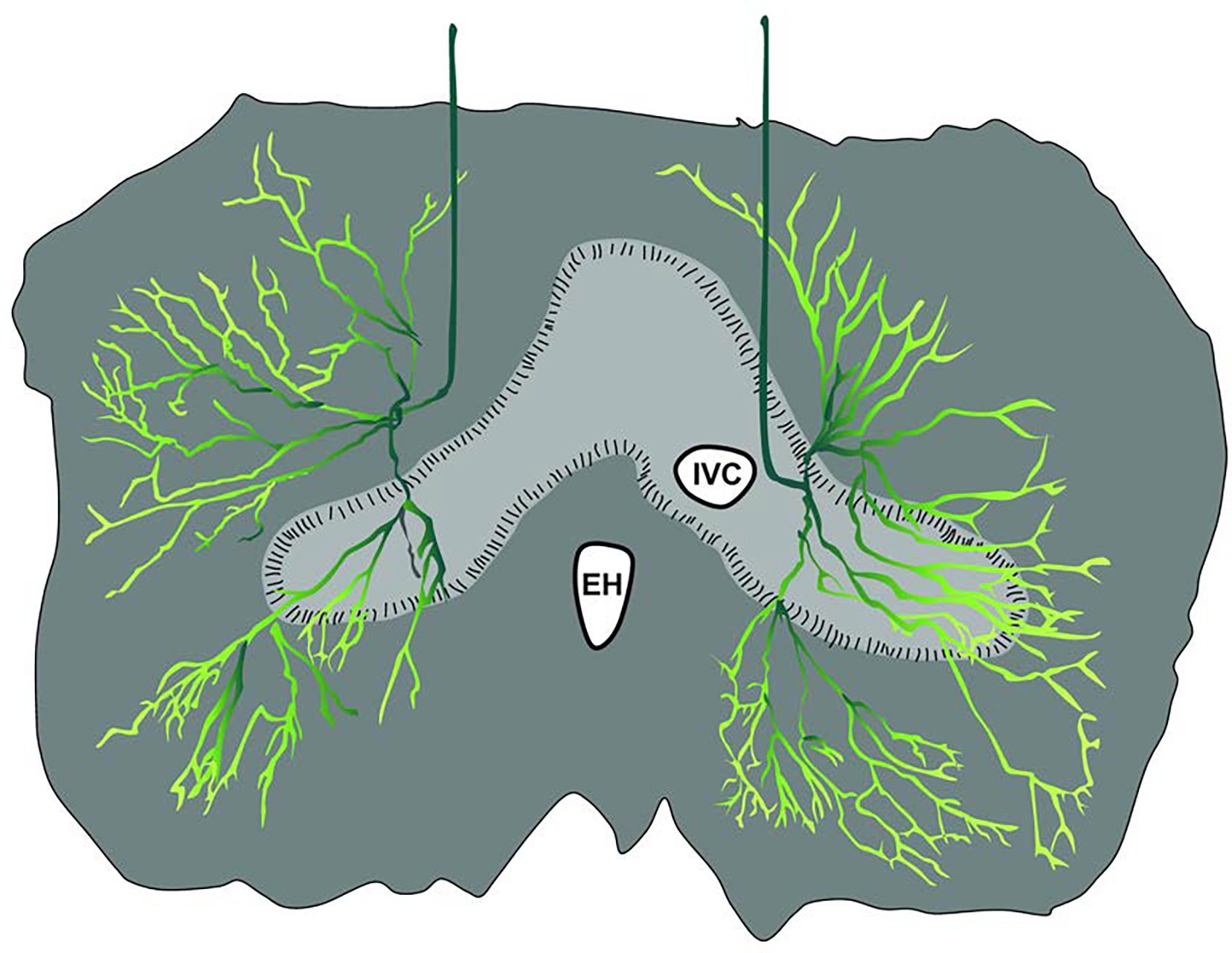
The drawing depicts the thoracic surface, and is based on histological evaluation of the human diaphragm (An et al., 2012). The main phrenic nerve trunk diverges to form distinct anterior and posterior branches, with lateral branches of varying number. The primary branches subdivide to form smaller branches, thereby creating a “net” which innervates the entire diaphragm. EH: esophageal hiatus; IVC: inferior vena cava
3. Phrenic motoneurons.
To borrow Sherrington’s phrase (Sherrington, 1906), PhrMNs are the “final common pathway” used by the central nervous system to control the diaphragm. In other words, all the different motor control circuits which impact the diaphragm, including cortical, brainstem, and spinal, ultimately act on PhrMNs. Purposeful contraction of the diaphragm occurs when the balance of excitatory and inhibitory synaptic inputs produces depolarization, to threshold, of a sufficient number of PhrMNs.
PhrMNs are located in the ventral horn of cervical spinal segments C3-C5 in humans (Hollinshead and Keswani, 1956; Routal and Pal, 1999). Phrenic dendrites typically form bundles with a distinct rostro-caudal orientation. The dendritic profile also shows extensive arborization in the ventral gray matter, largely confined to the ipsilateral spinal cord. PhrMN dendrites which cross the spinal midline are present early in development (Lindsay et al., 1991), but if present in the adult, appear to be extremely rare. To our knowledge, the total number of cells in the phrenic motor neuron pool (and therefore total diaphragm motor units) has not been quantified in humans. However, careful work in a rat model that permits retrograde labeling to identify individual PhrMNs indicates the total number is between 220–250 on each side of the spinal cord (Mantilla et al., 2009). Studies of the cat indicate a somatotopic organization of the phrenic pool, with more rostral cells innervating ventral costal and crural myofibers, and caudally located cells innervating the dorsal costal and dorsal crural myofibers (Fournier and Sieck, 1988).
Histological examples of the PhrMN pool are provided in Figure 3. Note the columnar organization of the motoneuron pool as seen in the longitudinal view, and the tight clustering of cells in the ventral horn as seen in the coronal view.
Figure 3. The phrenic motoneuron pool.
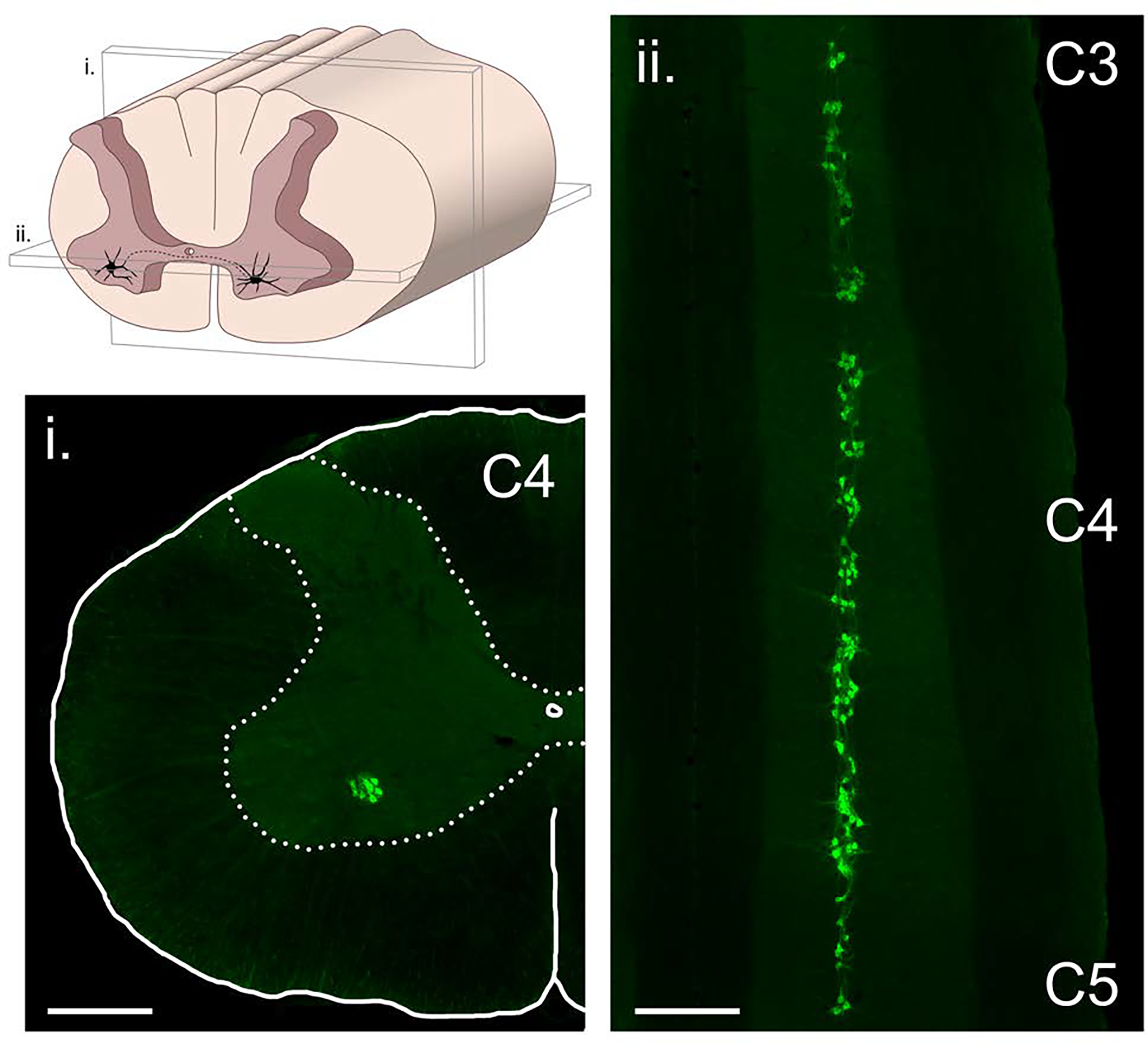
The column of phrenic motoneurons extends from approximately C3 to C5. The drawing in the upper left panel illustrates the location of phrenic motoneurons in the anterior horn, and also the plane of section that the two histological images were taken from. The histological images were obtained using a retrograde neuronal tracer (cholera toxin, ß-subunit) applied intrapleurally to the diaphragm muscle in an adult rat model. Scale bars indicate 500 μm.
Both morphological and neurophysiological data indicate heterogeneity across the motoneurons which comprise the phrenic motor pool. That is, a range of PhrMN soma size, neurophysiologic properties, and discharge patterns have been reported. Morphological evaluation of PhrMNs has been done most comprehensively in a rat model that enables definitive quantification (Rana et al., 2020b; Rana et al., 2020a; Rana et al., 2019); studies of the human PhrMN pool are few (Hollinshead and Keswani, 1956; Routal and Pal, 1999), and have not permitted the same rigorous evaluation as the animal studies. In the adult rat, there is a continuous (unimodal) distribution of PhrMN soma size, with values ranging from 2,000–8,000 μm2 (Rana et al., 2020a).
Neurophysiological studies in animal models which permit rigorous control of recording conditions reveal a range of PhrMN discharge patterns (Kong and Berger, 1986; Lee et al., 2009; St John and Bartlett, 1979; Hilaire et al., 1983) and membrane potentials (Berger, 1979; Hayashi and Fukuda, 1995). Further, a distinct recruitment order occurs in relation to the inspiratory cycle (Torikai et al., 1996; Hilaire et al., 1983; Hilaire et al., 1972). Thus, under anesthesia or following decerebration, a bimodal distribution of PhrMN bursting is observed during the inspiratory effort. This distribution typically shows a distinct population of PhrMNs being recruited at the onset of the inspiratory effort, and another population of neurons activated as the inspiration continues. It should be emphasized, however, that the majority of the phrenic motor pool is likely to be silent during quiet (i.e., “eupneic”) breathing (see Section 7 for discussion of PhrMN recruitment mechanisms). Studies of diaphragm motor unit activity (and thus PhrMN discharge) are technically challenging in humans. However, available data suggest that the bimodal distribution of early- and late-firing PhrMNs observed under controlled conditions in animal studies may not be present during spontaneous breathing in humans. When single diaphragm motor units were recorded using needle electrodes during spontaneous breathing in humans, units were progressively recruited as the inspiratory effort proceeded, producing the graded diaphragm contraction that typifies inspiration. However, there was no clear segmentation of diaphragm motor units as being early- vs. late-onset, indicating a unimodal distribution (Saboisky et al., 2007b). While this result could reflect a limited sampling of PhrMNs, another interpretation is that diaphragm activation during breathing reflects a progressive and ramping “neural drive” to PhrMNs (see section 7).
A diverse population of membrane receptors have been identified on PhrMNs (Table 1). The precise functional impact (i.e., impact on diaphragm activity and breathing) of each type of receptor is not definitively known, but this is an active area of research (Table 1). However, it is firmly established that inspiratory-related depolarization occurs due to release of glutamate onto PhrMNs, which then acts primarily on α-amino-3-hydroxy-methyl-4-isoxazolepropionic acid (AMPA) and N-methyl-D-aspartate (NMDA) receptors (Chitravanshi and Sapru, 1996). It is also clear that inhibitory inputs to PhrMNs act primarily via gamma aminobutyric acid (GABA) and glycine receptors (Chitravanshi and Sapru, 1999; Lalley, 1983; Marchenko and Rogers, 2009). Other receptors, including various serotonin subtypes and adenosine receptors, can trigger neuroplastic changes in PhrMNs, and this topic is reviewed in detail in Chapter X of this volume. Clinically, the membrane receptors expressed on PhrMNs have implications for pharmacological treatments that may impact breathing. For example, drugs which modulate the AMPA receptor can improve phrenic neural drive to the diaphragm after spinal cord injury (Wollman et al., 2020). Relatively few efforts have been made to modulate PhrMN excitability with pharmacologic approaches, but appropriate activation of the diverse array of membrane receptors (Table 1) may provide an opportunity to stimulate breathing in some cases of neurologic injury or disease (Table 2). This may be particularly effective when used as an adjunct to other rehabilitation approaches (Turner et al., 2018).
Table 1.
Membrane receptors expressed on phrenic motoneurons.
| Receptor Class | Subtypes/Isoforms | Function * | References |
|---|---|---|---|
| AMPA | Glur1 | Fast excitatory neurotransmission | (Robinson and Ellenberger, 1997) |
| Glur2 | Fast excitatory neurotransmission | (Robinson and Ellenberger, 1997) | |
| NMDA | NR1 | Excitatory neurotransmission; neuroplasticity | (Robinson and Ellenberger, 1997) |
| NR2A | Excitatory neurotransmission; neuroplasticity | (Alilain and Goshgarian, 2008) | |
| Kainate | Glur5–7 | Excitatory neurotransmission | (Robinson and Ellenberger, 1997) |
| mGluR | Group I-III | Modulates synaptic transmission | (Dong and Feldman, 1999) |
| Serotonin | 5-HT1a | Modulate PhrMN excitability | (Pecotic et al., 2009) |
| 5-HT1b | Modulate PhrMN excitability | (Bras et al., 2008) | |
| 5-HT2a | Modulate PhrMN excitability; Initiate phrenic motor plasticity | (Basura et al., 2001) | |
| 5-HT2c | Initiate phrenic motor plasticity | (Basura et al., 2001) | |
| 5-HT7 | Initiate phrenic motor plasticity | (Fields et al., 2015) | |
| Adenosine | A2A | Initiate phrenic motor plasticity | (Seven et al., 2018b) |
| 1 | Antagonism enhances phrenic recovery following spinal injury | (Nantwi et al., 2003) | |
| TrkB | TrkB.T1 / TrkB.T2 | Competitively inhibit Trk signaling | (Gransee et al., 2013) |
| TrkB.FL | Synaptic plasticity | (Mantilla et al., 2013) | |
| GABA | GABA-A | Inhibitory neurotransmission | (Chitravanshi and Sapru, 1999) |
| GABA-B | Inhibitory neurotransmission | (Lalley, 1983) | |
| Glycine | Glycine | Inhibitory neurotransmission | (Marchenko and Rogers, 2009) |
| nACHR | α7 | Nicotinic cholinergic neurotransmission | (Dehkordi et al., 2004) |
| ATP | P2X1 | Impacts PhrMN excitability | (Miles et al., 2002) |
| P2X2 | Impacts PhrMN excitability | (Miles et al., 2002) | |
| P2X5 | Impacts PhrMN excitability | (Miles et al., 2002) | |
| P2Y1 | Impacts PhrMN excitability | (Alvares et al., 2014) | |
| VEGFR | VEGFR2 | Evoke phrenic motor plasticity | (Dale-Nagle et al., 2011) |
| Erythropoietin | EPOR | Evoke phrenic motor plasticity | (Dale et al., 2012) |
| Peptide | NK-1 | Mediate substance p transmission | (Holtman et al., 1984) |
| Estrogen | alpha (ERa) | Modulate PhrMN output | (Behan and Thomas, 2005) |
| (ERβ) | Modulate PhrMN output | (Behan and Thomas, 2005) | |
| Androgen | AR | Modulate PhrMN output | (Behan and Thomas, 2005) |
| Adrenergic | α1 | Synaptic plasticity | (Snider and Gerald, 1982) |
regarding function: in some cases (e.g., AMPA, GABA), the precise function of the receptor is clear; in other cases the precise role can be speculated but not definitively stated (e.g., "modulate PhrMN output).
Abbreviations: α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA), Glutamate receptor 1 (Glurl), Glutamate receptor 2 (Glur2), N-methyl-D-aspartate (NMDA), N-methyl-D- aspartate receptor subunit 1 (NR1), N-methyl-D-aspartate receptor subunit 2A (NR2A), Glutamate receptor 5 (Glur5), Metabotropic glutamate receptors (mGlurR), 5-hydroxytryptamine receptor 1a (5-HTR1a), 5-hydroxytryptamine receptor 1b (5-HTR1b), 5-hydroxytryptamine receptor 2a (5-HTR2a), 5-hydroxytryptamine receptor 2c (5-HTR2c), Adenosine receptor 2A (A2A), Adenosine receptor 1 (A1), Tropomyosin receptor kinase B (TrkB), Truncated TrkB receptor 1 (TrkB.T1), Truncated TrkB receptor 2 (TrkB.T2), Full length TrkB receptor (TrkB.FL), Gamma aminobutyric acid receptor (GABA), Gamma aminobutyric acid receptor A (GABA-A), Gamma aminobutyric acid receptor B (GABA-B), Nicotinic acetylcholine receptors (nAChR), Alpha7 nicotinic acetylcholine receptor (nACHR-α7), Purinergic receptor 2X1 (P2X1), Purinergic receptor 2X2 (P2X2), Purinergic receptor 2X5 (P2X5), Purinergic receptor 2Y1 (P2Y1), Vascular endothelial growth factor receptor (VEGFR), Vascular endothelial growth factor receptor 2 (VEGFR2), Erythropoietin receptor (EPOR), Neurokinin-1 receptor (NK-1), Estrogen alpha receptor (ERα), Estrogen beta receptor (ERβ), Androgen receptor (AR), Adrenergic α1 receptor (α1)
Table 2. Diseases and injuries which can impact the function of the phrenic neuromuscular system.
Table partially based on (Howard, 2016)
| Premotor Neurons | Phrenic Neuromuscular Junction |
| Stroke | Myasthenia gravis |
| Transient ischemic attack Aneurysm | Lambert-Eaton myasthenia syndrome |
| Tumors | Botulism |
| Amyotrophic Lateral Sclerosis | Organophosphate poisoning |
| Pompe Disease | Aminoglycosides |
| Phrenic Motor Neurons | Prolonged neuromuscular blockade |
| Amyotrophic Lateral Sclerosis | Diaphragm Myopathy |
| Pompe Disease | Pompe Disease |
| Spinal cord injury | Duchenne Muscular Dystrophy |
| Cerebral Palsy | Limb-girdle muscular dystrophy |
| Poliomyelitis | Congenital diaphragmatic hernia Acquired diaphragmatic hernia |
| Peripheral Neuropathy | Hiatal Hernia |
| Trauma to phrenic nerve during thoracic and cardiac surgery or following Interscalene | Diaphragmatic tumor |
| block | Amyloidosis |
| Diabetes | Hypo/Hyper thyroidism |
| Multiple sclerosis | Mixed connective tissue disease |
| Guillain-Barre ’ syndrome | Systemic lupus erythematosus |
| Postviral phrenic neuropathy (HIV, West Nile virus, Herpes zoster) | Dermatomyositis |
| Bacterial Infection (Lyme Disease) | Inclusion body myositis |
| Compression by tumor | Mitochondrial disease |
| Birth trauma | Steroid myopathy |
| Nerve root compression Nerve root avulsion Cervical spondylosis |
Malnutrition |
Changes to PhrMNs occur across the age spectrum (Mantilla and Sieck, 2008) and this likely has implications for diaphragm motor control, particularly in old age (Khurram et al., 2018; Fogarty et al., 2018b). Description of the molecular regulation of PhrMN development is beyond the scope of this chapter but has been discussed previously (Vagnozzi et al., 2020). One striking observation during embryonic development is the loss of a substantial number of PhrMNs due to apoptosis (Allan and Greer, 1997). In addition, the dendritic architecture of PhrMNs changes from ventromedial and dorsolateral to the rostral-caudal orientation that is characteristic of the adult (Song et al., 2000). PhrMN loss also occurs on the other end of the aging spectrum. Fogarty et al. reported an approximately 20% reduction of PhrMNs in rats that were towards the end of their lifespan (Fogarty et al., 2018b). This motoneuron death occurred primarily in larger PhrMNs, indicating loss of larger, fast-twitch fibers which would impair the ability to generate high force diaphragm behaviors. The loss of motoneurons was also associated with neuromuscular junction alterations and diaphragm sarcopenia (Fogarty et al., 2019).
4. Bulbospinal innervation of the phrenic nucleus.
Figure 4 illustrates the direct, monosynaptic connection between brainstem respiratory centers and PhrMNs (Ellenberger et al., 1990; Dobbins and Feldman, 1994; Ellenberger and Feldman, 1988). These monosynaptic projections provide the primary “inspiratory drive” to activate PhrMNs during inspiration, but additional synaptic inputs from other sources can modulate PhrMN excitability, contribute to postural movements, etc. (these are discussed subsequently). Bulbospinal axons projecting to PhrMNs are found in cervical spinal white matter and are concentrated within the lateral and ventral cervical funiculi (Feldman et al., 1985; Fuller et al., 2009; Lipski et al., 1994). The cell bodies of these excitatory neurons can be found in the ventral and dorsal medulla (Bianchi et al., 1995; Duffin et al., 2000), and specifically in the ventral respiratory group (VRG) and dorsal respiratory group (DRG). The relative importance of bulbospinal projections from the VRG versus DRG is not firmly established and may be species specific. To illustrate this point, DRG neurons provide excitatory synaptic input to contralateral PhrMNs (axonal decussation in the medulla) in cats (Dick et al., 1988; Otake et al., 1989; Rikard-Bell et al., 1984) but apparently not in the rat (Tian and Duffin, 1998). PhrMNs also receive monosynaptic excitatory (glutamatergic) synaptic input from the Kolliker-Fuse nucleus in the pons (Yokota et al., 2004).
Figure 4. Direct, excitatory monosynaptic connection between the medulla and phrenic motoneurons.
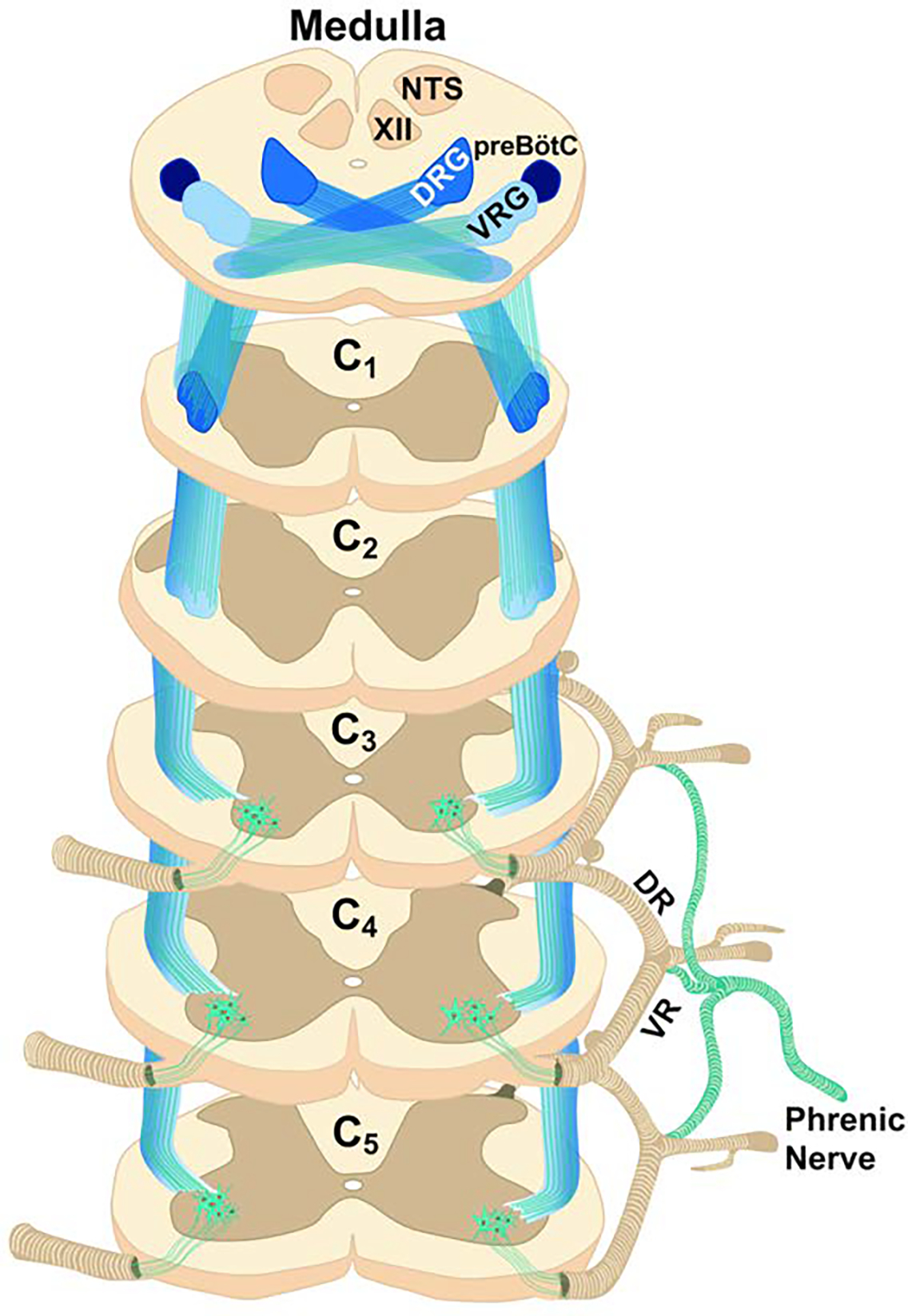
The illustrated synaptic projections provide the primary “inspiratory drive” which depolarize phrenic motoneurons during inspiration, and thereby trigger diaphragm contraction. The axons of these projections travel in lateral and ventral cervical funiculi (the image illustrates the ventro-lateral funiculus). As noted in the text, PhrMNs also receive monosynaptic inhibitory inputs from the Bötzinger complex in the medulla. VRG: ventral respiratory group; DRG: dorsal respiratory group; XII: hypoglossal motor nucleus; NTS: nucleus of the solitary tract; preBôtC: pre-Bötzinger complex
Bulbospinal synaptic inputs to PhrMNs are not exlusively excitatory. As originally reported by Berger, PhrMNs receive inhibitory synaptic input during the expiratory period of the respiratory cycle (Berger, 1979). Thus, the expiratory period is linked with an active inhibition of PhrMNs (Schreihofer et al., 1999; Tian et al., 1998; Merrill and Fedorko, 1984; Ellenberger and Feldman, 1988). The inhibitory input derives from a group of neurons in the rostral medulla known as the Bötzinger complex. The associated bulbospinal axons are found ipsilaterally and travel in the dorsolateral spinal cord (Tian et al., 1998).
As shown in Figure 5, the phrenic motor nucleus is also innervated by brainstem serotonergic (Pilowsky et al., 1990) and noradrenergic systems (Dobbins and Feldman, 1994). These inputs from neuromodulatory neurons are not primarily responsible for the phasic inspiratory activity observed during breathing. However, serotonergic and noradrenergic inputs can alter the excitability of PhrMNs, and serotonergic neurons in particular are fundamentally important for initiating plasticity in phrenic motor output (Fuller et al., 2001). Recent work also shows that adenosinergic input to PhrMNs can trigger neuroplastic changes in PhrMNs (Seven et al., 2018b). The role of these and other neuromodulators in initiating or maintaining phrenic motor plasticity is reviewed elsewhere in this volume (Chapter X).
Figure 5. Additional tracts innervating phrenic motor neurons.
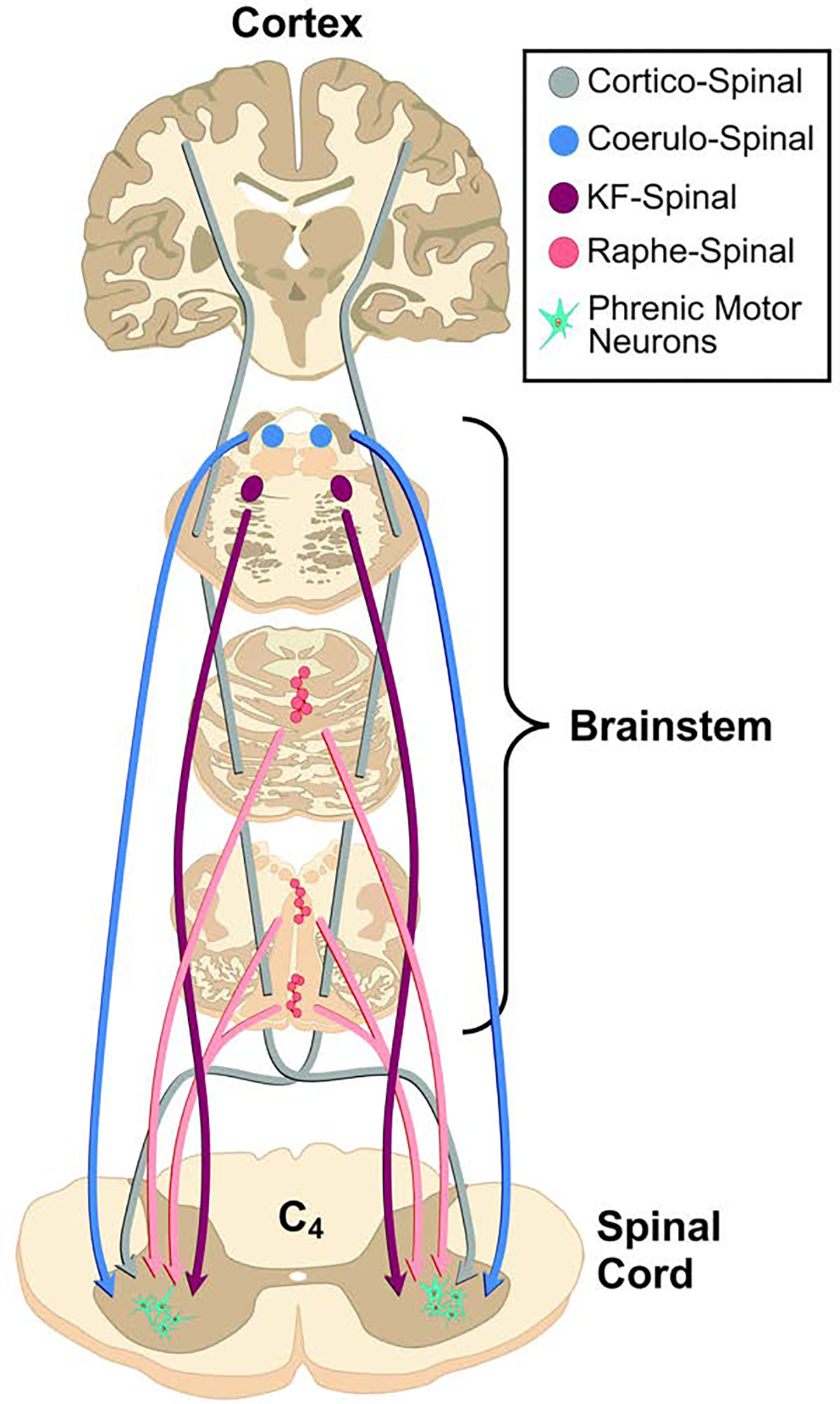
The phrenic motor nucleus is innervated by corticospinal pathways as well as brainstem serotonergic and noradrenergic neurons.
5. Corticospinal inputs to PhrMNs.
The diaphragm can be voluntarily controlled, and accordingly, there are corticospinal synaptic inputs to the phrenic motor pool (Gandevia and Rothwell, 1987) (Figure 5). While the respiratory rhythm is generated without conscious effort, the automatic control of the diaphragm can be “overridden” to enable swallowing, speech, voluntary cough, trunk movements, etc. (Butler, 2007). The diaphragm is relatively unique in this regard since routine motor control during wakefulness involves frequent interactions between voluntary (cortical) and involuntary (brainstem) neural circuits. However, precisely how the bulbospinal and corticospinal pathways controlling the phrenic motor nucleus interact has not been established (Hudson et al., 2020). For example, corticobulbar pathways by which respiratory rhythm generating pathways may be impacted by volitional thoughts have not been verified in humans (Hudson et al., 2020). Conversely, in animal models, there is clear anatomical evidence for corticospinal projections to PhrMNs (Rikard-Bell et al., 1986; Rikard-Bell et al., 1985).
6. Propriospinal neurons and the phrenic motor circuit.
Neurons located in the brainstem determine the fundamental respiratory rhythm in mammals (Feldman et al., 2003). However, spinal cord interneurons (Figure 6) also influence the output of PhrMNs (Streeter et al., 2017; Satkunendrarajah et al., 2018). The role of spinal cord interneurons in modulating respiratory motor output has been studied for decades (Pitts, 1946; Kirkwood et al., 1988), with renewed interest in recent years (Lane et al., 2008). In particular, spinal interneurons appear to be a prominent component of a neural substrate that can facilitate some degree of respiratory recovery after injuries to the cervical spinal cord (Zholudeva et al., 2018).
Figure 6. The phrenic motor nucleus receives synaptic inputs from spinal cord interneurons.
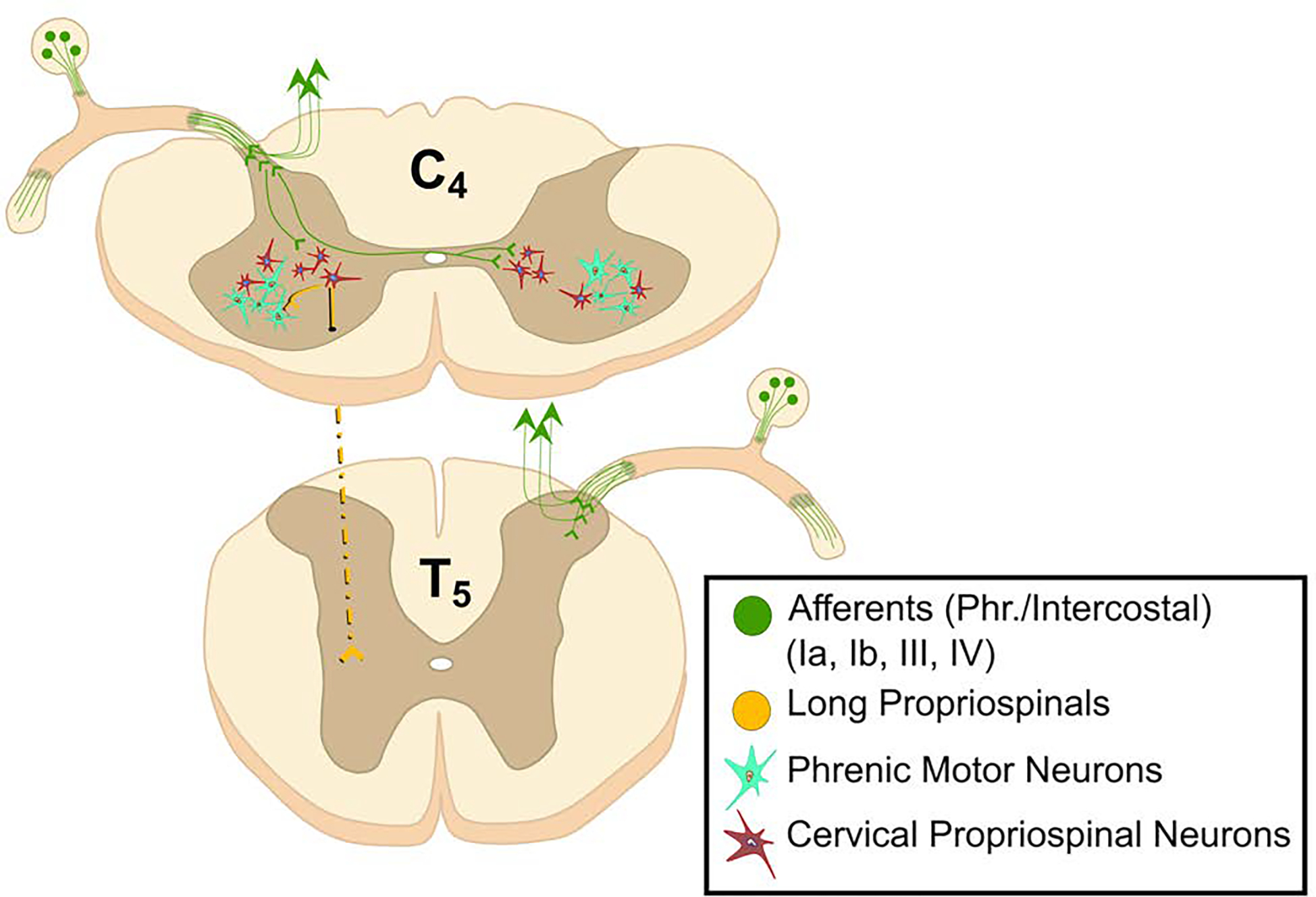
These propriospinal neurons do not produce the fundamental respiratory rhythm of breathing but play a role in modulating the excitability of phrenic motoneurons and coordinating postural, locomotor and respiratory movements. These cells may also provide a neural substrate for promoting diaphragm motor recovery after spinal cord injury.
Propriospinal neurons which are synaptically coupled to the phrenic motor nucleus are likely to represent a diverse population with both excitatory and inhibitory connections (Jensen et al., 2019). As such, it is difficult to ascribe a precise function to these neurons. Indeed, absolute classification of propriospinal neurons to functional categories such as “respiratory” or “locomotor” is not necessarily useful (or possible), due to the multifunctional nature of these cells (Jankowska, 2001) and the fact that bursting patterns will depend on the particular recording conditions. Based on the literature in this area (Sunshine et al., 2020), it appears that spinal cord interneurons can contribute to 1) shaping the respiratory pattern into the final efferent motor output that emerges from the phrenic motor pool (Monteau and Hilaire, 1991; Butler, 2007); 2) coordinating respiratory muscle activation across the spinal neuraxis (Bellingham, 1999; Decima et al., 1967); 3) coordinating postural, locomotor and respiratory movements (Hudson et al., 2011; Hodges and Gandevia, 2000; Hodges et al., 1997), and 4) enabling plasticity of respiratory motor output in health and disease (Streeter et al., 2017; Zholudeva et al., 2018).
The importance of propriospinal neurons for respiratory recovery after injuries to the spinal cord has become evident in recent years (Cregg et al., 2017; Hormigo et al., 2017; Satkunendrarajah et al., 2018; Zholudeva et al., 2018). The majority of cervical spinal cord injuries are anatomically and functionally incomplete, thus providing hope for at least some degree of functional recovery. This recovery process can be associated with formation of new spinal circuits that include interneurons, which are synaptically coupled to the phrenic motor nucleus (Zholudeva et al., 2017). Recent advances in chemogenetic technology have been leveraged to determine if activation (or inhibition) of spinal interneurons can impact breathing recovery after spinal injury (Jensen et al., 2019; Satkunendrarajah et al., 2018). For example, selective activation of glutamatergic spinal interneurons can robustly increase diaphragm electromyogram (EMG) activity following high cervical spinal injury (Satkunendrarajah et al., 2018). This work highlights the importance of pre-clinical basic science in understanding spinal circuits and also provides hope for new directions in therapeutics for spinal injury.
7. Recruitment of PhrMNs.
During quiet breathing, only a small portion of the PhrMN pool is active on any given breath. When diaphragm activity needs to increase, this occurs due to increased discharge frequency of active PhrMNs (i.e., “rate coding”) as well as recruitment of new PhrMNs which were previously silent (Lee et al., 2009) (Gesell et al., 1941; Kong and Berger, 1986; Pitts, 1942; Iscoe et al., 1976). Figure 7 depicts the seminal model of PhrMN recruitment developed by Sieck and colleagues (Fogarty et al., 2018a), and is based on a large body of experimental evidence from their laboratory (Sieck and Fournier, 1989; Jimenez-Ruiz et al., 2018; Seven et al., 2014). The model predicts that approximately 20% of PhrMNs are active during quiet breathing, and this increases to 40–45% during labored breathing such as during intense exercise. It is only during high intensity expulsive diaphragm activities, such as coughing or sneezing, that PhrMN recruitment approaches 100% of the available motor pool. Accordingly, in health there is a large “physiologic reserve” in regard to PhrMN recruitment during breathing. This reserve will diminish dramatically in neurologic conditions such as amyotrophic lateral sclerosis or cervical spinal cord injury (Seven and Mitchell, 2019; Seven et al., 2018a; Khurram et al., 2019). Depending on the phase of disease progression in these and related neurologic conditions, respiratory deficits may not be evident during quiet breathing, but the ability to increase diaphragm activation (e.g., during sigh or cough, as two examples) can be dramatically impaired. When the reserve is diminished, and maintaining alveolar ventilation during quiet breathing requires a large percentage of the phrenic motor pool to be active, respiratory failure may be imminent (Fuller et al., 2013).
Figure 7. A model depicting recruitment of phrenic motoneurons during progressively more intense diaphragm contraction.
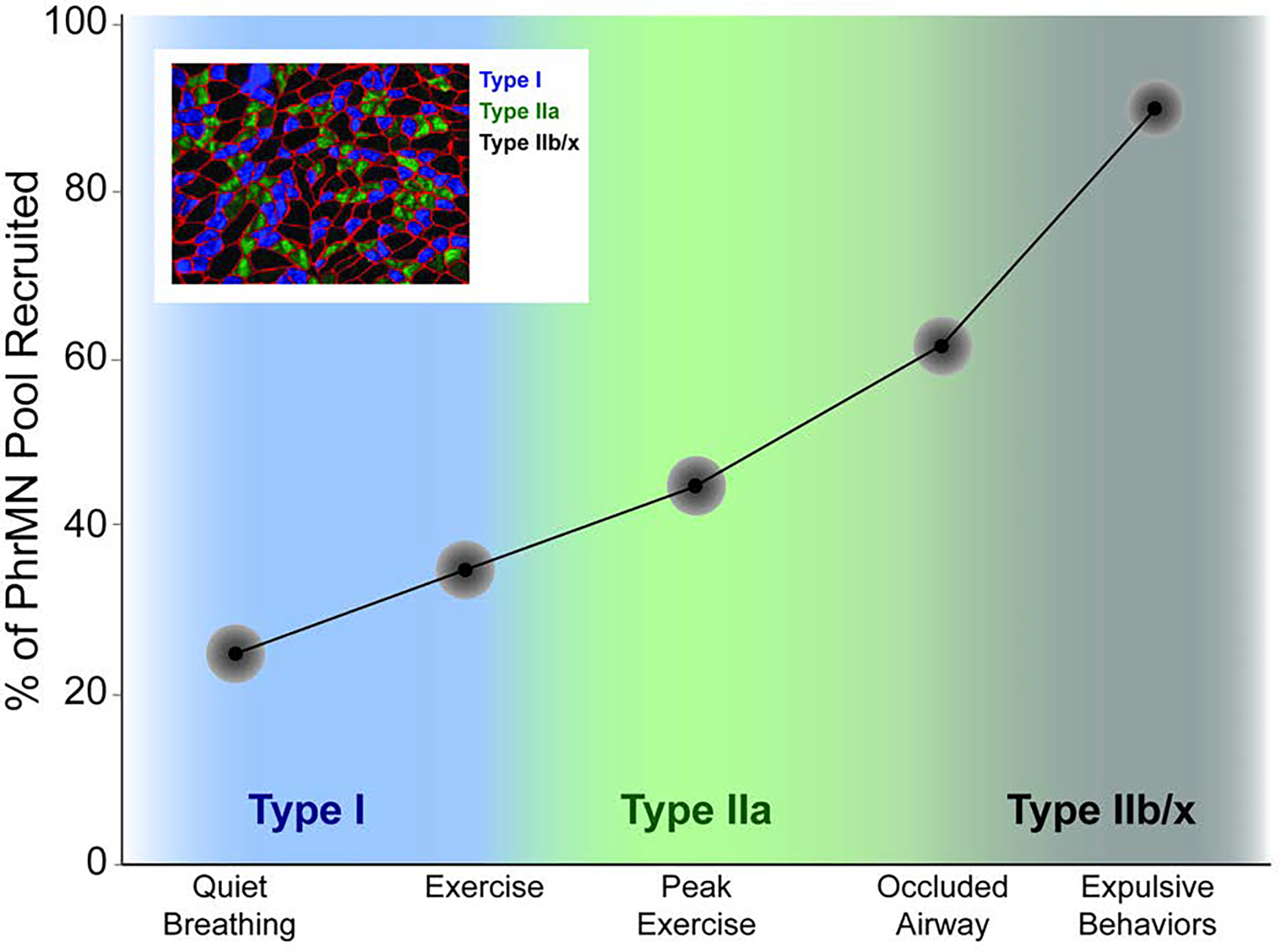
This model was developed by Sieck, Mantilla, and colleagues, and the image presented has been adapted from their work (Fogarty et al., 2018a). The model predicts that a relatively small amount of the total phrenic motoneuron pool (approximately 20%) is required for typical quiet breathing. This number increases during conditions such as intense exercise, but only approaches 100% during high force expulsive maneuvers.
What determines the recruitment of PhrMNs during breathing and other tasks? At the cellular level, the balance of excitatory and inhibitory synaptic inputs from brainstem, spinal, cortical, and other neurons (Figures 3–5) will determine if the PhrMN is depolarized to threshold. However, the precise mechanisms determining which of the approximately 250 total PhrMNs (per side) are recruited first (e.g., at the onset of a breath or sigh) have been debated. On the one hand, Henneman’s size principle of motoneuron recruitment predicts that smaller neurons with a greater electrical resistance will be the first to be activated for a given synaptic input (Henneman et al., 1965). Based on the fundamental electrical properties described by Ohm’s law [i.e., voltage (V) = current (I) * resistance (R)], for a given synaptic current (I), the resulting change in voltage across the membrane (ΔV), will be highest in cells with the greatest resistance (i.e., the small PhrMNs). The elegance of this hypothesis is that the “decision” for motoneuron recruitment does not require higher level processing, but rather the intrinsic motoneuron properties determine which cells are recruited first. Many investigations in animal models have provided support for this hypothesis (Dick et al., 1987; Webber and Pleschka, 1976; Hayashi and Fukuda, 1995). For example, intracellular PhrMN recordings in cats show that those cells recruited early during a breath have slower axonal conduction velocity and higher input resistance as compared to cells recruited later in the inspiratory effort (Berger, 1979).
Another possibility, however, is that an “organized central command” may exist in which the relative strength of pre-synaptic inputs determines which PhrMNs are recruited first during breathing (Monteau et al., 1985; Hilaire et al., 1983). That is, the nervous system may provide selective excitatory inputs to particular PhrMNs, and this contributes to the observed recruitment order. Studies of diaphragm motor unit discharge in humans have provided data consistent with that hypothesis. During voluntary and involuntary breathing tasks, diaphragm motor units show recruitment patterns that are not congruent with the size principle (Butler, 2007; Saboisky et al., 2007a). In particular, Saboisky et al. noted that diaphragm motor units show a unimodal recruitment distribution during spontaneous breathing in humans, and suggested that recruitment order of spinal respiratory motoneurons results from neural drive that is “unevenly distributed” across the motor pool (Saboisky et al., 2007a). A subsequent study of intercostal motor unit activity in humans reached a similar conclusion, speculating that recruitment order reflected “may be a result of different descending drives and afferent inputs, perhaps sculpted by spinal distribution networks of interneurones” (Hudson et al., 2017).
Recruitment order may also be impacted by the relative density of excitatory synaptic inputs on PhrMNs. When Rana and colleagues evaluated the density of glutamatergic presynaptic terminals on verified PhrMNs, they observed that the smallest cells had the greatest terminal density (Rana et al., 2019). Further, small PhrMNs have a higher expression of AMPA and NMDA receptor mRNA as compared to larger PhrMNs (Rana et al., 2020a). Since the smaller PhrMNs will innervate the smaller, “lower force” diaphragm motor units that are recruited first during breathing (Figure 7), the relative expression of excitatory pre-synaptic terminals and/or post-synaptic receptors could be a mechanism contributing to the orderly recruitment of PhrMNs during breathing.
Another interesting feature of the phrenic motor pool is illustrated by work from DiMarco and colleagues (DiMarco, 2009; DiMarco and Kowalski, 2009). Remarkably, delivery of trains of high frequency (300 Hz) electrical stimulation to the ventral surface of the thoracic spinal cord in dogs with high cervical spinal cord injury caused the previously silent (due to spinal injury) PhrMNs to burst at approximately 10 Hz. That discharge rate is similar to PhrMN discharge that occurs during “normal” breathing. Since the electrical signal could not have reached the brainstem (due to spinal cord injury), the results suggest that the phrenic motor pool (or “pre-phrenic” interneurons (Lane et al., 2008)) can integrate propriospinal inputs to produce orderly recruitment of diaphragm motor units. This method has also been successfully implemented in initial clinical trials in humans, and the positive outcomes suggest that the human spinal cord also has the ability to integrate synaptic inputs to produce diaphragm contraction (DiMarco et al., 2019).
In summary, the intrinsic properties of PhrMNs are likely to play a major role in determining which cells are activated by synaptic inputs from the brainstem and other sources. However, intrinsic motoneuron properties are unlikely to be the sole factor contributing to the recruitment order of PhrMNs during breathing and related behaviors. Thus, recruitment order and discharge patterns may reflect a balance of both intrinsic properties and some degree of “selectivity” of synaptic inputs (Hilaire et al., 1983; Saboisky et al., 2007a).
8. Summary and conclusions.
The phrenic neuromuscular system is, at first glance, a relatively simple motor system consisting of a single motor nucleus in the mid-cervical spinal cord (the phrenic motor nucleus), a single mixed nerve (the phrenic nerve), and one muscle (the diaphragm). However, in reality this is a highly complex motor system capable of sustaining breathing throughout life while also contributing to posture, coughing, swallowing, speaking and other motor behaviors. The diaphragm contracts every few seconds throughout life, and therefore must have a high fatigue resistance. It has approximately equal amounts of slow- (oxidative) and fast-twitch (glycolytic) myofibers, and only about 20% of diaphragm motor units are active during quiet (“eupneic”) breathing. This number increases during exercise, but it is only during high force expulsive behaviors that recruitment of the diaphragm motor units approaches 100%. The diaphragm is surprisingly susceptible to rapid atrophy and dysfunction during mechanical ventilator support, sepsis, or neurologic injury (e.g., spinal cord injury.). The innervation of the diaphragm is via the phrenic nerve, which contains primarily efferent phrenic axons and afferent axons from diaphragm sensory receptors. The phrenic nerve, however, is also a conduit for autonomic fibers. The fundamental anatomical organization and neurophysiological control of the PhrMNs which provide efferent motor innervation of the diaphragm are well established. On a breath-by-breath basis, rhythmic depolarization of PhrMNs (and thus diaphragm activation) occurs due to descending bulbospinal synaptic pathways. Brainstem rhythm generating neurons provide excitatory synaptic input to neurons in the dorsal and ventral respiratory groups, and these neurons, in turn, send excitatory synaptic inputs to PhrMNs. A complex interneuron network is synaptically connected to the phrenic motor pool. This propriospinal network likely functions to modulate inspiratory phrenic motor activity and coordinate postural, locomotor and respiratory movements. The phrenic motor nucleus and/or phrenic nerve is directly impacted in a wide range of neuromuscular diseases or injuries (Table 2). Accordingly, a fundamental understanding of phrenic anatomy and physiology is important for optimizing treatments and/or rehabilitation strategies designed to improve breathing and related behaviors. Indeed, much of the contemporary research on the phrenic neuromuscular system is focused on those aspects. As this field of research moves forward, important but unanswered questions include 1) the precise role of the spinal cord interneurons in regulating phrenic motor output, particularly after injuries to the phrenic motor nucleus; 2) the physiologic mechanisms that enable neuroplastic changes in phrenic motor control in health and disease; 3) how to leverage gene therapy / manipulation to improve phrenic motor output in genetic diseases or neurologic injury.
CITATIONS
- Alilain WJ & Goshgarian HG (2008). Glutamate receptor plasticity and activity-regulated cytoskeletal associated protein regulation in the phrenic motor nucleus may mediate spontaneous recovery of the hemidiaphragm following chronic cervical spinal cord injury. Exp Neurol 212: 348–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allan DW & Greer JJ (1997). Embryogenesis of the phrenic nerve and diaphragm in the fetal rat. J Comp Neurol 382: 459–468. [DOI] [PubMed] [Google Scholar]
- Alvares TS, Revill AL, Huxtable AG, et al. (2014). P2Y1 receptor-mediated potentiation of inspiratory motor output in neonatal rat in vitro. J Physiol 592: 3089–3111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An X, Yue B, Lee JH, et al. (2012). Intramuscular distribution of the phrenic nerve in human diaphragm as shown by Sihler staining. Muscle Nerve 45: 522–526. [DOI] [PubMed] [Google Scholar]
- Bains KNS, Kashyap S & Lappin SL (2021). Anatomy, Thorax, Diaphragm. StatPearls. Treasure Island (FL). [PubMed] [Google Scholar]
- Balkowiec A & Szulczyk P (1992). Properties of postganglionic sympathetic neurons with axons in phrenic nerve. Respir Physiol 88: 323–331. [DOI] [PubMed] [Google Scholar]
- Basura GJ, Zhou SY, Walker PD, et al. (2001). Distribution of serotonin 2A and 2C receptor mRNA expression in the cervical ventral horn and phrenic motoneurons following spinal cord hemisection. Exp Neurol 169: 255–263. [DOI] [PubMed] [Google Scholar]
- Behan M & Thomas CF (2005). Sex hormone receptors are expressed in identified respiratory motoneurons in male and female rats. Neuroscience 130: 725–734. [DOI] [PubMed] [Google Scholar]
- Bellingham MC (1999). Synaptic inhibition of cat phrenic motoneurons by internal intercostal nerve stimulation. J Neurophysiol 82: 1224–1232. [DOI] [PubMed] [Google Scholar]
- Berger AJ (1979). Phrenic motoneurons in the cat: subpopulations and nature of respiratory drive potentials. J Neurophysiol 42: 76–90. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M & Champagnat J (1995). Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev 75: 1–45. [DOI] [PubMed] [Google Scholar]
- Bras H, Gaytan SP, Portalier P, et al. (2008). Prenatal activation of 5-HT2A receptor induces expression of 5-HT1B receptor in phrenic motoneurons and alters the organization of their premotor network in newborn mice. Eur J Neurosci 28: 1097–1107. [DOI] [PubMed] [Google Scholar]
- Butler JE (2007). Drive to the human respiratory muscles. Respir Physiol Neurobiol 159: 115–126. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC & Sapru HN (1996). NMDA as well as non-NMDA receptors mediate the neurotransmission of inspiratory drive to phrenic motoneurons in the adult rat. Brain Res 715: 104–112. [DOI] [PubMed] [Google Scholar]
- Chitravanshi VC & Sapru HN (1999). GABA receptors in the phrenic nucleus of the rat. Am J Physiol 276: R420–428. [DOI] [PubMed] [Google Scholar]
- Dale-Nagle EA, Satriotomo I & Mitchell GS (2011). Spinal vascular endothelial growth factor induces phrenic motor facilitation via extracellular signal-regulated kinase and Akt signaling. J Neurosci 31: 7682–7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale EA, Satriotomo I & Mitchell GS (2012). Cervical spinal erythropoietin induces phrenic motor facilitation via extracellular signal-regulated protein kinase and Akt signaling. J Neurosci 32: 5973–5983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport PW, Reep RL & Thompson FJ (2010). Phrenic nerve afferent activation of neurons in the cat SI cerebral cortex. J Physiol 588: 873–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davenport PW & Vovk A (2009). Cortical and subcortical central neural pathways in respiratory sensations. Respir Physiol Neurobiol 167: 72–86. [DOI] [PubMed] [Google Scholar]
- De Troyer AD (1998). The canine phrenic-to-intercostal reflex. J Physiol 508 (Pt 3): 919–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decima EE, von Euler C & Thoden U (1967). Spinal intercostal-phrenic reflexes. Nature 214: 312–313. [DOI] [PubMed] [Google Scholar]
- Dehkordi O, Haxhiu MA, Millis RM, et al. (2004). Expression of alpha-7 nAChRs on spinal cord-brainstem neurons controlling inspiratory drive to the diaphragm. Respir Physiol Neurobiol 141: 21–34. [DOI] [PubMed] [Google Scholar]
- DeVries KL & Goshgarian HG (1989). Spinal cord localization and characterization of the neurons which give rise to the accessory phrenic nerve in the adult rat. Exp Neurol 104: 88–90. [DOI] [PubMed] [Google Scholar]
- Dick TE, Kong FJ & Berger AJ (1987). Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol 57: 245–259. [DOI] [PubMed] [Google Scholar]
- Dick TE, Viana F & Berger AJ (1988). Electrophysiological determination of the axonal projections of single dorsal respiratory group neurons to the cervical spinal cord of cat. Brain Res 454: 31–39. [DOI] [PubMed] [Google Scholar]
- DiMarco AF (2009). Phrenic nerve stimulation in patients with spinal cord injury. Respir Physiol Neurobiol 169: 200–209. [DOI] [PubMed] [Google Scholar]
- DiMarco AF, Geertman RT, Tabbaa K, et al. (2019). Complete Restoration of Respiratory Muscle Function in Three Subjects With Spinal Cord Injury: Pilot Interventional Clinical Trial. Am J Phys Med Rehabil 98: 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiMarco AF & Kowalski KE (2009). High-frequency spinal cord stimulation of inspiratory muscles in dogs: a new method of inspiratory muscle pacing. J Appl Physiol 107: 662–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins EG & Feldman JL (1994). Brainstem network controlling descending drive to phrenic motoneurons in rat. J Comp Neurol 347: 64–86. [DOI] [PubMed] [Google Scholar]
- Dong XW & Feldman JL (1999). Distinct subtypes of metabotropic glutamate receptors mediate differential actions on excitability of spinal respiratory motoneurons. J Neurosci 19: 5173–5184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duffin J, Tian GF & Peever JH (2000). Functional synaptic connections among respiratory neurons. Respir Physiol 122: 237–246. [DOI] [PubMed] [Google Scholar]
- Duron B, Jung-Caillol MC & Marlot D (1978). Myelinated nerve fiber supply and muscle spindles in the respiratory muscles of cat: quantitative study. Anat Embryol (Berl) 152: 171–192. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH & Feldman JL (1988). Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol 269: 47–57. [DOI] [PubMed] [Google Scholar]
- Ellenberger HH, Feldman JL & Goshgarian HG (1990). Ventral respiratory group projections to phrenic motoneurons: electron microscopic evidence for monosynaptic connections. J Comp Neurol 302: 707–714. [DOI] [PubMed] [Google Scholar]
- Feldman JL, Loewy AD & Speck DF (1985). Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci 5: 1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Mitchell GS & Nattie EE (2003). Breathing: rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci 26: 239–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields DP, Springborn SR & Mitchell GS (2015). Spinal 5-HT7 receptors induce phrenic motor facilitation via EPAC-mTORC1 signaling. J Neurophysiol 114: 2015–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Gonzalez Porras MA, Mantilla CB, et al. (2019). Diaphragm neuromuscular transmission failure in aged rats. J Neurophysiol 122: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Mantilla CB & Sieck GC (2018a). Breathing: Motor Control of Diaphragm Muscle. Physiology (Bethesda) 33: 113–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogarty MJ, Omar TS, Zhan WZ, et al. (2018b). Phrenic motor neuron loss in aged rats. J Neurophysiol 119: 1852–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fournier M & Sieck GC (1988). Somatotopy in the segmental innervation of the cat diaphragm. J Appl Physiol 64: 291–298. [DOI] [PubMed] [Google Scholar]
- Fuller DD, ElMallah MK, Smith BK, et al. (2013). The respiratory neuromuscular system in Pompe disease. Respir Physiol Neurobiol 189: 241–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD & Mitchell GS (2017). Respiratory neuroplasticity - Overview, significance and future directions. Exp Neurol 287: 144–152. [DOI] [PubMed] [Google Scholar]
- Fuller DD, Sandhu MS, Doperalski NJ, et al. (2009). Graded unilateral cervical spinal cord injury and respiratory motor recovery. Respir Physiol Neurobiol 165: 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller DD, Zabka AG, Baker TL, et al. (2001). Phrenic long-term facilitation requires 5-HT receptor activation during but not following episodic hypoxia. J Appl Physiol 90: 2001–2006; discussion 2000. [DOI] [PubMed] [Google Scholar]
- Gandevia SC & Rothwell JC (1987). Activation of the human diaphragm from the motor cortex. J Physiol 384: 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill PK & Kuno M (1963). Excitatory and Inhibitory Actions on Phrenic Motoneurones. J Physiol 168: 274–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gransee HM, Zhan WZ, Sieck GC, et al. (2013). Targeted delivery of TrkB receptor to phrenic motoneurons enhances functional recovery of rhythmic phrenic activity after cervical spinal hemisection. PLoS One 8: e64755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greising SM, Mantilla CB, Gorman BA, et al. (2013). Diaphragm muscle sarcopenia in aging mice. Exp Gerontol 48: 881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gronseth R, Vollmer WM, Hardie JA, et al. (2014). Predictors of dyspnoea prevalence: results from the BOLD study. Eur Respir J 43: 1610–1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi F & Fukuda Y (1995). Electrophysiological properties of phrenic motoneurons in adult rats. Jpn J Physiol 45: 69–83. [DOI] [PubMed] [Google Scholar]
- Hebb CO, Krnjevic K & Silver A (1964). Acetylcholine and choline acetyltransferase in the diaphragm of the rat. J Physiol 171: 504–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G & Carpenter DO (1965). Functional Significance of Cell Size in Spinal Motoneurons. J Neurophysiol 28: 560–580. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Gauthier P & Monteau R (1983). Central respiratory drive and recruitment order of phrenic and inspiratory laryngeal motoneurones. Respir Physiol 51: 341–359. [DOI] [PubMed] [Google Scholar]
- Hilaire G, Monteau R & Dussardier M (1972). [Pattern of recruitment of phrenic motor neurons]. J Physiol (Paris) 64: 457–478. [PubMed] [Google Scholar]
- Hodges PW, Butler JE, McKenzie DK, et al. (1997). Contraction of the human diaphragm during rapid postural adjustments. J Physiol 505 (Pt 2): 539–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodges PW & Gandevia SC (2000). Activation of the human diaphragm during a repetitive postural task. J Physiol 522 Pt 1: 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollinshead WH & Keswani NH (1956). Localization of the phrenic nucleus in the spinal cord of man. Anat Rec 125: 683–699. [DOI] [PubMed] [Google Scholar]
- Holtman JR Jr., Norman WP, Skirboll L, et al. (1984). Evidence for 5-hydroxytryptamine, substance P, and thyrotropin-releasing hormone in neurons innervating the phrenic motor nucleus. J Neurosci 4: 1064–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RS (2016). Respiratory failure because of neuromuscular disease. Curr Opin Neurol 29: 592–601. [DOI] [PubMed] [Google Scholar]
- Hudson AL, Butler JE, Gandevia SC, et al. (2011). Role of the diaphragm in trunk rotation in humans. J Neurophysiol 106: 1622–1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AL, Gandevia SC & Butler JE (2017). Task-dependent output of human parasternal intercostal motor units across spinal levels. J Physiol 595: 7081–7092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AL, Walsh LD, Gandevia SC, et al. (2020). Respiratory muscle activity in voluntary breathing tracking tasks: Implications for the assessment of respiratory motor control. Respir Physiol Neurobiol 274: 103353. [DOI] [PubMed] [Google Scholar]
- Hussain SN, Chatillon A, Comtois A, et al. (1991). Chemical activation of thin-fiber phrenic afferents. 2. Cardiovascular responses. Journal of Applied Physiology 70: 77–86. [DOI] [PubMed] [Google Scholar]
- Jensen VN, Seedle K, Turner SM, et al. (2019). V2a Neurons Constrain Extradiaphragmatic Respiratory Muscle Activity at Rest. eNeuro 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez-Ruiz F, Khurram OU, Zhan WZ, et al. (2018). Diaphragm muscle activity across respiratory motor behaviors in awake and lightly anesthetized rats. J Appl Physiol (1985) 124: 915–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurram OU, Fogarty MJ, Rana S, et al. (2019). Diaphragm muscle function following midcervical contusion injury in rats. J Appl Physiol (1985) 126: 221–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khurram OU, Fogarty MJ, Sarrafian TL, et al. (2018). Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol Rep 6: e13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood PA, Munson JB, Sears TA, et al. (1988). Respiratory interneurones in the thoracic spinal cord of the cat. J Physiol 395: 161–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong FJ & Berger AJ (1986). Firing properties and hypercapnic responses of single phrenic motor axons in the rat. J Appl Physiol 61: 1999–2004. [DOI] [PubMed] [Google Scholar]
- Kostreva DR & Pontus SP (1993). Hepatic vein, hepatic parenchymal, and inferior vena caval mechanoreceptors with phrenic afferents. Am J Physiol 265: G15–20. [DOI] [PubMed] [Google Scholar]
- Lalley PM (1983). Biphasic effects of baclofen on phrenic motoneurons: possible involvement of two types of gamma-aminobutyric acid (GABA) receptors. J Pharmacol Exp Ther 226: 616–624. [PubMed] [Google Scholar]
- Landau BR, Akert K & Roberts TS (1962). Studies on the innervation of the diaphragm. The Journal of Comparative Neurology 119: 1–10. [Google Scholar]
- Lane MA, White TE, Coutts MA, et al. (2008). Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol 511: 692–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford LA & Schmidt RF (1983). An electron microscopic analysis of the left phrenic nerve in the rat. Anat Rec 205: 207–213. [DOI] [PubMed] [Google Scholar]
- Laskowski MB, Norton AS & Berger PK (1991). Branching patterns of the rat phrenic nerve during development and reinnervation. Exp Neurol 113: 212–220. [DOI] [PubMed] [Google Scholar]
- Lee KZ, Reier PJ & Fuller DD (2009). Phrenic motoneuron discharge patterns during hypoxia-induced short-term potentiation in rats. J Neurophysiol 102: 2184–2193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Bashir MH, Clanton TL, et al. (2013). COPD elicits remodeling of the diaphragm and vastus lateralis muscles in humans. J Appl Physiol (1985) 114: 1235–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine S, Kaiser L, Leferovich J, et al. (1997). Cellular adaptations in the diaphragm in chronic obstructive pulmonary disease. N Engl J Med 337: 1799–1806. [DOI] [PubMed] [Google Scholar]
- Levine S, Nguyen T, Taylor N, et al. (2008). Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med 358: 1327–1335. [DOI] [PubMed] [Google Scholar]
- Lieberman DA, Faulkner JA, Craig AB, Jr., et al. (1973). Performance and histochemical composition of guinea pig and human diaphragm. J Appl Physiol 34: 233–237. [DOI] [PubMed] [Google Scholar]
- Lindsay AD, Greer JJ & Feldman JL (1991). Phrenic motoneuron morphology in the neonatal rat. J Comp Neurol 308: 169–179. [DOI] [PubMed] [Google Scholar]
- Lipski J, Zhang X, Kruszewska B, et al. (1994). Morphological study of long axonal projections of ventral medullary inspiratory neurons in the rat. Brain Res 640: 171–184. [DOI] [PubMed] [Google Scholar]
- Loukas M, Kinsella CR Jr., Louis RG Jr., et al. (2006). Surgical anatomy of the accessory phrenic nerve. Ann Thorac Surg 82: 1870–1875. [DOI] [PubMed] [Google Scholar]
- Mangner N, Garbade J, Heyne E, et al. (2021). Molecular Mechanisms of Diaphragm Myopathy in Humans With Severe Heart Failure. Circ Res 128: 706–719. [DOI] [PubMed] [Google Scholar]
- Mantilla CB, Gransee HM, Zhan WZ, et al. (2013). Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol 247C: 101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB & Sieck GC (2008). Key aspects of phrenic motoneuron and diaphragm muscle development during the perinatal period. J Appl Physiol (1985) 104: 1818–1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantilla CB, Zhan WZ & Sieck GC (2009). Retrograde labeling of phrenic motoneurons by intrapleural injection. J Neurosci Methods 182: 244–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchenko V & Rogers RF (2009). GABAAergic and glycinergic inhibition in the phrenic nucleus organizes and couples fast oscillations in motor output. J Neurophysiol 101: 2134–2145. [DOI] [PubMed] [Google Scholar]
- Marlot D, Macron JM & Duron B (1987). Inhibitory and excitatory effects on respiration by phrenic nerve afferent stimulation in cats. Respir Physiol 69: 321–333. [DOI] [PubMed] [Google Scholar]
- Mendelsohn AH, Deconde A, Lambert HW, et al. (2011). Cervical variations of the phrenic nerve. Laryngoscope 121: 1920–1923. [DOI] [PubMed] [Google Scholar]
- Merrill EG & Fedorko L (1984). Monosynaptic inhibition of phrenic motoneurons: a long descending projection from Botzinger neurons. J Neurosci 4: 2350–2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meznaric M & Cvetko E (2016). Size and Proportions of Slow-Twitch and Fast-Twitch Muscle Fibers in Human Costal Diaphragm. Biomed Res Int 2016: 5946520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miles GB, Parkis MA, Lipski J, et al. (2002). Modulation of phrenic motoneuron excitability by ATP: consequences for respiratory-related output in vitro. J Appl Physiol (1985) 92: 1899–1910. [DOI] [PubMed] [Google Scholar]
- Mizuno M (1991). Human respiratory muscles: fibre morphology and capillary supply. Eur Respir J 4: 587–601. [PubMed] [Google Scholar]
- Monteau R & Hilaire G (1991). Spinal respiratory motoneurons. Prog Neurobiol 37: 83–144. [DOI] [PubMed] [Google Scholar]
- Monteau R, Khatib M & Hilaire G (1985). Central determination of recruitment order: intracellular study of phrenic motoneurons. Neurosci Lett 56: 341–346. [DOI] [PubMed] [Google Scholar]
- Nair J, Streeter KA, Turner SMF, et al. (2017). Anatomy and physiology of phrenic afferent neurons. J Neurophysiol 118: 2975–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nantwi KD, Basura GJ & Goshgarian HG (2003). Adenosine A1 receptor mRNA expression and the effects of systemic theophylline administration on respiratory function 4 months after C2 hemisection. J Spinal Cord Med 26: 364–371. [DOI] [PubMed] [Google Scholar]
- Offner B, Dembowsky K & Czachurski J (1992). Characteristics of sympathetic reflexes evoked by electrical stimulation of phrenic nerve afferents. Journal of the Autonomic Nervous System 41: 103–111. [DOI] [PubMed] [Google Scholar]
- Onders RP, Dimarco AF, Ignagni AR, et al. (2004). Mapping the phrenic nerve motor point: the key to a successful laparoscopic diaphragm pacing system in the first human series. Surgery 136: 819–826. [DOI] [PubMed] [Google Scholar]
- Onders RP, Elmo M, Kaplan C, et al. (2018). Long-term experience with diaphragm pacing for traumatic spinal cord injury: Early implantation should be considered. Surgery 164: 705–711. [DOI] [PubMed] [Google Scholar]
- Otake K, Sasaki H, Ezure K, et al. (1989). Axonal trajectory and terminal distribution of inspiratory neurons of the dorsal respiratory group in the cat’s medulla. J Comp Neurol 286: 218–230. [DOI] [PubMed] [Google Scholar]
- Paraskevas G, Koutsouflianiotis K, Kitsoulis P, et al. (2016). Abnormal Origin and Course of the Accessory Phrenic Nerve: Case Report. Acta Medica (Hradec Kralove) 59: 70–71. [DOI] [PubMed] [Google Scholar]
- Pecotic R, Dogas Z, Valic Z, et al. (2009). Blockade of 5-HT(1A) receptors in the phrenic nucleus of the rat attenuated raphe induced activation of the phrenic nerve activity. J Physiol Pharmacol 60: 167–172. [PubMed] [Google Scholar]
- Pilowsky PM, de Castro D, Llewellyn-Smith I, et al. (1990). Serotonin immunoreactive boutons make synapses with feline phrenic motoneurons. J Neurosci 10: 1091–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitts RF (1946). Organization of the respiratory center. Physiol Rev 26: 609–630. [DOI] [PubMed] [Google Scholar]
- Polla B, D’Antona G, Bottinelli R, et al. (2004). Respiratory muscle fibres: specialisation and plasticity. Thorax 59: 808–817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Fuller D, et al. (2013a). CrossTalk proposal: Mechanical ventilation-induced diaphragm atrophy is primarily due to inactivity. J Physiol 591: 5255–5257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Smuder AJ, Fuller D, et al. (2013b). Rebuttal from Scott K. Powers, Ashley J. Smuder, David Fuller and Sanford Levine. J Physiol 591: 5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers SK, Wiggs MP, Sollanek KJ, et al. (2013c). Ventilator-induced diaphragm dysfunction: cause and effect. Am J Physiol Regul Integr Comp Physiol 305: R464–477. [DOI] [PubMed] [Google Scholar]
- Rana S, Mantilla CB & Sieck GC (2019). Glutamatergic input varies with phrenic motor neuron size. J Neurophysiol 122: 1518–1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Sieck GC & Mantilla CB (2020a). Heterogeneous glutamatergic receptor mRNA expression across phrenic motor neurons in rats. J Neurochem 153: 586–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rana S, Zhan WZ, Mantilla CB, et al. (2020b). Disproportionate loss of excitatory inputs to smaller phrenic motor neurons following cervical spinal hemisection. J Physiol 598: 4693–4711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Revelette WR, Jewell LA & Frazier DT (1988). Effect of diaphragm small-fiber afferent stimulation on ventilation in dogs. J Appl Physiol 65: 2097–2106. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK & Nail BS (1984). Brainstem projections to the phrenic nucleus: a HRP study in the cat. Brain Res Bull 12: 469–477. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Bystrzycka EK & Nail BS (1985). Cells of origin of corticospinal projections to phrenic and thoracic respiratory motoneurones in the cat as shown by retrograde transport of HRP. Brain Res Bull 14: 39–47. [DOI] [PubMed] [Google Scholar]
- Rikard-Bell GC, Tork I & Bystrzycka EK (1986). Distribution of corticospinal motor fibres within the cervical spinal cord with special reference to the phrenic nucleus: a WGA-HRP anterograde transport study in the cat. Brain Res 379: 75–83. [DOI] [PubMed] [Google Scholar]
- Robinson D & Ellenberger H (1997). Distribution of N-methyl-D-aspartate and non-N-methyl-D-aspartate glutamate receptor subunits on respiratory motor and premotor neurons in the rat. J Comp Neurol 389: 94–116. [DOI] [PubMed] [Google Scholar]
- Routal RV & Pal GP (1999). Location of the phrenic nucleus in the human spinal cord. J Anat 195 (Pt 4): 617–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, et al. (2007a). Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol 102: 772–780. [DOI] [PubMed] [Google Scholar]
- Saboisky JP, Gorman RB, De Troyer A, et al. (2007b). Differential activation among five human inspiratory motoneuron pools during tidal breathing. J Appl Physiol (1985) 102: 772–780. [DOI] [PubMed] [Google Scholar]
- Sant’ambrogio G & Camporesi E (1973). Contribution of various inspiratory muscles to ventilation and the immediate and distant effect of diaphragmatic paralysis. Acta Neurobiol Exp (Wars) 33: 401–409. [PubMed] [Google Scholar]
- Satkunendrarajah K, Karadimas SK, Laliberte AM, et al. (2018). Cervical excitatory neurons sustain breathing after spinal cord injury. Nature 562: 419–422. [DOI] [PubMed] [Google Scholar]
- Schreihofer AM, Stornetta RL & Guyenet PG (1999). Evidence for glycinergic respiratory neurons: Botzinger neurons express mRNA for glycinergic transporter 2. J Comp Neurol 407: 583–597. [DOI] [PubMed] [Google Scholar]
- Seven YB, Mantilla CB & Sieck GC (2014). Recruitment of rat diaphragm motor units across motor behaviors with different levels of diaphragm activation. J Appl Physiol (1985) 117: 1308–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB & Mitchell GS (2019). Mechanisms of compensatory plasticity for respiratory motor neuron death. Respir Physiol Neurobiol 265: 32–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Nichols NL, Kelly MN, et al. (2018a). Compensatory plasticity in diaphragm and intercostal muscle utilization in a rat model of ALS. Exp Neurol 299: 148–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seven YB, Perim RR, Hobson OR, et al. (2018b). Phrenic motor neuron adenosine 2A receptors elicit phrenic motor facilitation. J Physiol 596: 1501–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS (1906). The integrative actions of the nervous system., New Haven, CT. [Google Scholar]
- Sieck GC & Fournier M (1989). Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol 66: 2539–2545. [DOI] [PubMed] [Google Scholar]
- Snider RM & Gerald MC (1982). Noradrenergic-mediated potentiation of acetylcholine release from the phrenic nerve: evidence for presynaptic alpha 1-adrenoceptor involvement. Life Sci 31: 853–857. [DOI] [PubMed] [Google Scholar]
- Song A, Ashwell KW & Tracey DJ (2000). Development of the rat phrenic nucleus and its connections with brainstem respiratory nuclei. Anat Embryol (Berl) 202: 159–177. [DOI] [PubMed] [Google Scholar]
- Song A, Tracey DJ & Ashwell KW (1999). Development of the rat phrenic nerve and the terminal distribution of phrenic afferents in the cervical cord. Anat Embryol (Berl) 200: 625–643. [DOI] [PubMed] [Google Scholar]
- St John WM & Bartlett D, Jr. (1979). Comparison of phrenic motoneuron responses to hypercapnia and isocapnic hypoxia. J Appl Physiol 46: 1096–1102. [DOI] [PubMed] [Google Scholar]
- Streeter KA, Sunshine MD, Patel S, et al. (2017). Intermittent Hypoxia Enhances Functional Connectivity of Midcervical Spinal Interneurons. J Neurosci 37: 8349–8362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunshine MD, Sutor TW, Fox EJ, et al. (2020). Targeted activation of spinal respiratory neural circuits. Exp Neurol 328: 113256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian GF & Duffin J (1998). The role of dorsal respiratory group neurons studied with cross-correlation in the decerebrate rat. Exp Brain Res 121: 29–34. [DOI] [PubMed] [Google Scholar]
- Tian GF, Peever JH & Duffin J (1998). Botzinger-complex expiratory neurons monosynaptically inhibit phrenic motoneurons in the decerebrate rat. Exp Brain Res 122: 149–156. [DOI] [PubMed] [Google Scholar]
- Torikai H, Hayashi F, Tanaka K, et al. (1996). Recruitment order and dendritic morphology of rat phrenic motoneurons. J Comp Neurol 366: 231–243. [DOI] [PubMed] [Google Scholar]
- Turner S, Streeter KA, Greer J, et al. (2018). Pharmacological modulation of hypoxia-induced respiratory neuroplasticity. Respir Physiol Neurobiol 256: 4–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vagnozzi AN, Garg K, Dewitz C, et al. (2020). Phrenic-specific transcriptional programs shape respiratory motor output. Elife 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verlinden TJM, van Dijk P, Herrler A, et al. (2018). The human phrenic nerve serves as a morphological conduit for autonomic nerves and innervates the caval body of the diaphragm. Sci Rep 8: 11697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber CL Jr., & Pleschka K (1976). Structural and functional characteristics of individual phrenic motoneurons. Pflugers Arch 364: 113–121. [DOI] [PubMed] [Google Scholar]
- Welch JF, Archiza B, Guenette JA, et al. (2018). Sex differences in diaphragmatic fatigue: the cardiovascular response to inspiratory resistance. J Physiol 596: 4017–4032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welvaart WN, Paul MA, van Hees HW, et al. (2011). Diaphragm muscle fiber function and structure in humans with hemidiaphragm paralysis. Am J Physiol Lung Cell Mol Physiol 301: L228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman LB, Streeter KA, Fusco AF, et al. (2020). Ampakines stimulate phrenic motor output after cervical spinal cord injury. Exp Neurol 334: 113465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota S, Tsumori T, Ono K, et al. (2004). Glutamatergic pathways from the Kolliker-Fuse nucleus to the phrenic nucleus in the rat. Brain Res 995: 118–130. [DOI] [PubMed] [Google Scholar]
- Zholudeva LV, Karliner JS, Dougherty KJ, et al. (2017). Anatomical Recruitment of Spinal V2a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Interneurons into Phrenic Motor Circuitry after High Cervical Spinal Cord Injury. J Neurotrauma 34: 3058–3065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zholudeva LV, Qiang L, Marchenko V, et al. (2018). The Neuroplastic and Therapeutic Potential of Spinal Interneurons in the Injured Spinal Cord. Trends Neurosci 41: 625–639. [DOI] [PMC free article] [PubMed] [Google Scholar]


