Abstract
Introduction
Genital involvement is observed in approximately 60% of patients with psoriasis, presenting clinicians with formidable challenges in treatment. While new biologic drugs have emerged as safe and effective options for managing psoriasis, their efficacy in challenging-to-treat areas remains inadequately explored. Intriguingly, studies have shown that interleukin (IL)-17 inhibitors exhibit effectiveness in addressing genital psoriasis.
Objectives
We aimed to determine the effectiveness profile of bimekizumab in patients affected by moderate-to-severe plaque psoriasis with involvement of genitalia.
Methods
Bimekizumab, a dual inhibitor of both IL-17A and IL-17F, was the focus of our 16-week study, demonstrating highly favorable outcomes for patients with genital psoriasis. The effectiveness of bimekizumab was evaluated in terms of improvement in Static Physician Global Assessment of Genitalia (sPGA-G) and Psoriasis Area and Severity Index.
Results
Sixty-five adult patients were enrolled. Remarkably, 98.4% of our participants achieved a clear sPGA-G score (s-PGA-g = 0) within 16 weeks. Moreover, consistent improvements were observed in Psoriasis Area and Severity Index scores, accompanied by a significant reduction in the mean Dermatology Life Quality Index, signifying enhanced quality of life. Notably, none of the patients reported a severe impairment in their quality of life after 16 weeks of treatment. In our cohort of 65 patients, subgroup analyses unveiled that the effectiveness of bimekizumab remained unaffected by prior exposure to other biologics or by obesity.
Conclusions
Our initial findings suggest that bimekizumab may serve as a valuable treatment option for genital psoriasis. Nevertheless, further research with larger sample sizes and longer-term follow-up is imperative to conclusively validate these results.
Keywords: immonumodulatory therapies, Inflammatory Skin Diseases, psoriasis, psoriasis treatment
Introduction
Genital involvement occurs in over 60% of psoriasis patients throughout the course of their illness [1–3]. This condition is associated with a major impact to quality of life (QoL), since it causes intense burning and pruritus, and is associated with embarrassment in engaging sexual intercourses significantly impacting their quality of life [4–6]. Nevertheless, genital psoriasis is hardly diagnosed in clinical practice and its treatment poses challenges due to its classification as a ‘difficult-to-treat’ area [7]. Indeed, only 40% of patients reported to have had a previous examination of the genital area, and therefore treatment is often initiated late [3].
Topical treatments, predominantly corticosteroids, are the initial choice for mild-to-moderate genital psoriasis [5]. However, the long-term use of corticosteroids is associated with adverse effects, and limited data exist on the efficacy of immunomodulators or vitamin D derivatives in this context [8]. Systemic treatments are considered for moderate-to-severe cases, yet evidence for these therapies remains limited [5,7–10]. Monoclonal antibodies targeting key cytokines in psoriasis pathogenesis, primarily interleukin (IL)-23 and IL-17A, have been developed. While drugs like secukinumab and ixekizumab target IL-17A, brodalumab antagonizes the IL-17A receptor [11,12]. Ixekizumab has shown significant efficacy in treating genital psoriasis, providing consistent and lasting improvements[7,10,13–18]. Recent research has also emphasized the role of IL-17F, which exhibits overlapping pro-inflammatory functions with IL-17A and is more abundant in psoriatic lesions [19]. Bimekizumab, a humanized monoclonal antibody targeting both IL-17A and IL-17F, has gained approval for moderate-to-severe plaque psoriasis treatment following four successful phase-III clinical trials (BE READY, BE VIVID, BE SURE; BE RADIANT) demonstrating its superior efficacy compared to placebo, ustekinumab, adalimumab, and secukinumab [20–23].
Despite these achievements, real-world data on bimekizumab are limited to case reports and a recent retrospective multicenter study by Gargiulo et al [24–26]. However, no data are currently available regarding the effectiveness of bimekizumab on difficult-to-treat areas, and genitalia in particular. Given ixekizumab high efficacy in treating psoriatic lesions and the increased odds of genital psoriasis clearance with anti-IL-17A inhibitors, as demonstrated in a recent prospective observational study, coupled with the prevalence of IL-17F in psoriatic lesions, we undertook this study to explore the effectiveness of bimekizumab specifically in this challenging-to-treat body area [19,27].
Objectives
In this paper, we present the results of a retrospective observational multicenter study with a 16-week follow-up period, aiming to assess the effectiveness of bimekizumab in the treatment of genital psoriasis.
Methods
This was a retrospective, observational multicenter study conducted at 20 Italian Dermatology Clinics, from January 2023 to August 2023. Consecutive adult (≥18 years) patients with moderate-to-severe plaque psoriasis involving the genital area were eligible for inclusion in this study if they received treatment with bimekizumab. Patients eligibility for bimekizumab treatment was assessed in accordance with the Italian Guidelines as outlined by Gisondi et al in 2022 [28].
Definition of Genital Involvement
To differentiate genital psoriasis from inverse psoriasis, we defined genital involvement based on specific anatomical regions. For males, genital involvement encompassed lesions on the pubis, shaft, foreskin, glans, scrotum, and perineum [7]. For females, it included lesions on the mons pubis, labia majora, labia minora, anterior commissure, interlabial groove, and perineum [7]. Patients with lesions in the inguinal folds and intergluteal cleft but without involvement in the aforementioned anatomical sites were excluded from the study [7].
All patients received bimekizumab in accordance with the Summary of Product Characteristics [29]. They were followed up until week 16, receiving two subcutaneous injections of 160 mg each at weeks 0, 4, 8, 12, 16 [29]. No concurrent topical or systemic agents were administered in conjunction with bimekizumab treatment.
Ethical Considerations
This study adhered to established clinical standards and did not require approval from the institutional review board. All patients provided written consent for the retrospective collection of their anonymous data. The research was conducted in compliance with the Helsinki Declaration of 1964 and its subsequent amendments.
Assessment
In accordance with our institution standard protocol, assessments of effectiveness and safety were conducted at baseline, week 4, and week 16. During each dermatological examination, the following parameters were evaluated:
Static Physician Global Assessment of Genitalia ( sPGA-G): this clinician-reported outcome measure, developed specifically for grading the severity of genital psoriasis, assesses erythema, plaque elevation, and scaling on a 6-point scale (0: clear; 5: very severe) [6].
Psoriasis Area and Severity Index (PASI) score: including PASI75, PASI90, and PASI100 (percentages of patients who achieved a percentage reduction of 75%, 90%, and 100% from baseline, respectively).
Proportion of patients achieving an absolute PASI of 2 or less at each visit.
Dermatology-Life-Quality-Index (DLQI).
Percentage of patients with DLQI≥10 (indicating a severe impact on quality of life).
Data Analysis
Data analysis was conducted utilizing descriptive statistics. Continuous variables were presented as the mean and standard deviation (SD), while categorical variables were represented as the absolute number and percentage. To assess differences between baseline and follow-up visits, the Wilcoxon matched-pair rank test was employed. A P value less than 0.05 was considered statistically significant. Subgroup analyses were performed to assess the impact of previous exposure to other biologics and the presence of obesity as a comorbidity on the effectiveness of bimekizumab. All analyses were carried out using GraphPad Prism software v8.0.
Results
Patient Population
A total of 65 patients were enrolled in this study, with 46 of them being males (70.8%). The average age of the patients was 50.4±14.2 years. Detailed demographic characteristics and clinical features of all patients at baseline are presented in Table 1. The mean body mass index (BMI) among the patients was 26.7±5.6 kg/m2. Fourteen patients were obese (21.5%), with a BMI ≥30. Additionally, 6 patients (9.2%) had a concomitant diagnosis of psoriatic arthritis. Approximately 3/4 of the patients (74.6%) had at least one cardio-metabolic comorbidity, which included conditions such as obesity, arterial hypertension, cardiovascular disease, type II diabetes mellitus, and hyperlipidemia. Interestingly, 19 patients (29.2%) had experienced a previous SARS-CoV-2 infection. The patients had a mean history of psoriasis of 13.9±11.3 years. At baseline, the mean PASI was 18.3±9.0, indicating the severity of psoriasis. DLQI at baseline had an average score of 17.1±8.8, highlighting a substantial impact on the quality of life for these patients. Fifty-one patients reported a severe impairment of their quality of life, with a DLQI score of ≥10 (81%). Twenty-five patients had previously failed at least one biological therapy (38.5%), while 38 patients were bio-naive (61.5%) (Table 1). A significant proportion of patients, specifically 63 out of 65 (96.9%), successfully completed the 16-week treatment course. The remaining patients were lost to follow-up.
Table 1.
Demographics and General Clinical Characteristics of Patients With Genital Psoriasis at Baseline.
| Characteristic | All Patients (N = 65) |
|---|---|
| Age (years) | 50.4 (14.2%) |
| Male sex | 46 (70.8%) |
| BMI (kg/m2) | 26.7 (5.6%) |
| Comorbidities | |
| • Psoriatic Arthritis | 6 (9.2%) |
| • Obesity | 14 (21.5%) |
| • Type 2 diabetes | 5 (7.7%) |
| • Hyperlipidemia | 10 (15.4%) |
| • Hypertension | 15 (23.1%) |
| • Cardiovascular diseases | 3 (4.6%) |
| Previous SARS-CoV-2 infection | 19 (29.2%) |
| Mean age at baseline (years) | 48.2 (14.9) |
| Mean duration of psoriasis (years) | 13.9 (11.3) |
| Psoriasis on special locations | |
| • Genital | 65 (100%) |
| • Scalp | 48 (73.8%) |
| • Palmo-plantar | 19 (29.2%) |
| • Nails | 25 (38.5%) |
| Mean PASI at baselinea | 18.3 (9.0%) |
| Mean DLQI at baseline a | 17.1 (8.8%) |
| Previous exposure to biologics | 25 (38.5%) |
| Adalimumab | 12 (18.5%) |
| Brodalumab | 1 (1.5%) |
| Ixekizumab | 2 (3.1%) |
| Risankizumab | 2 (3.1%) |
| Secukinumab | 9 (13.8%) |
| Tildrakizumab | 1 (1.5%) |
| Ustekinumab | 3 (4.6%) |
BMI = body mass index; DLQI = Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index.
PASI and DLQI scores were available for 63 patients at baseline. Continuous variables were reported as mean (standard deviation), while categorical variables were expressed as absolute number (percentage).
Effectiveness Assessment
At baseline, more than half of the patients had a moderate-to-severe genital involvement, defined as a s-PGA-g of 3 or more (Figure 1 and Table 2). Among the 63 patients evaluated for sPGA-G at the 4-week mark, 48 patients achieved a sPGA-G score of clear (76.2%), while 11 patients achieved a score of almost clear (17.5%). Impressively, at the 16-week visit, 98.4% of the assessed patients had achieved a sPGA-G score of clear, demonstrating the remarkable efficacy of bimekizumab in clearing genital psoriasis (Figure 1, Table 2).
Figure 1.
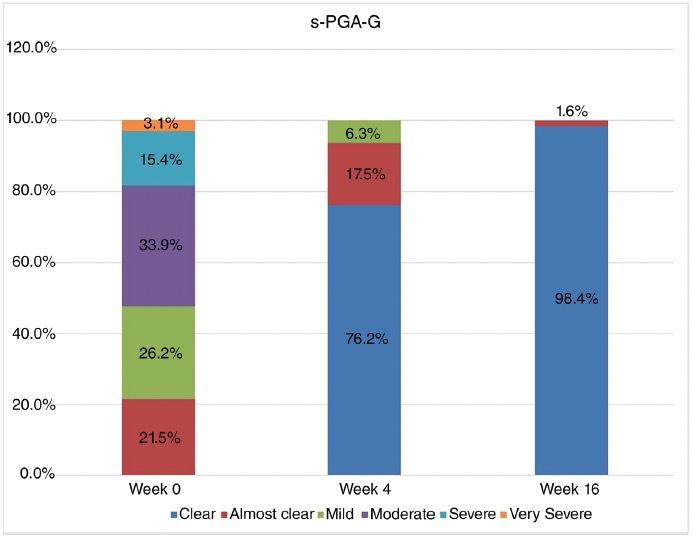
Static genital PGA score (Static Physician Global Assessment of Genitalia, sPGA-G) distribution over time. Data were expressed as absolute number (percentage).
Table 2.
Static Genital PGA score (sPGA-G) distribution over time.
| sPGA-G | |||
|---|---|---|---|
| Baseline (N = 65) | 4-Week Visit (N = 63) | 16-Week Visit (N = 63) | |
| Clear | - | 48 (76.2%) | 62 (98.4%) |
| Almost clear | 14 (21.5%) | 11 (17.5%) | 1 (1.6%) |
| Mild | 17 (26.2%) | 2 (6.3%) | - |
| Moderate | 22 (33.9%) | - | - |
| Severe | 10 (15.4%) | - | - |
| Very severe | 2 (3.1%) | - | - |
sPGA-G = Static Physician Global Assessment of Genitalia.
PASI Improvement
At baseline, PASI was recorded for 63 patients, and 61 of them completed 16 weeks of follow-up. In our study, there was a notable increase in the percentage of patients achieving PASI75, PASI90, and PASI100 between the 4-week and 16-week marks of bimekizumab treatment (Figure 2). Specifically, PASI75 was achieved by 43 patients after 4 weeks (70.5%) and by 57 patients after 16 weeks (93.4%) ( Figure 2). Additionally, PASI90 increased from 29 patients at 4 weeks (47.5%) to 48 patients at 16 weeks (78.7%). The percentage of patients achieving PASI100 increased from 25 at 4 weeks (41%) to 42 after 16 weeks of treatment (68.9%) (Figure 2).
Figure 2.
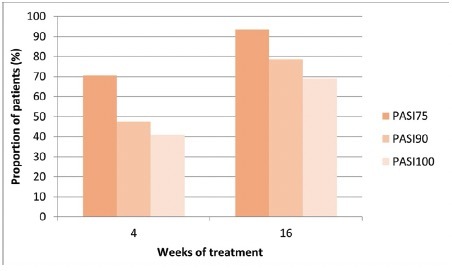
Percentage of patients achieving Psoriasis Area and Severity Index (PASI) 75, PASI90 and PASI 100 over time. Baseline, N = 63; week 4, N = 61; week 16, N = 61;; PASI75, at least a 75% improvement from baseline in PASI; PASI90, at least a 90% improvement from baseline in PASI. PASI100, 100% improvement from baseline in PASI. PASI score was available for 63 patients at baseline and for 61 patients at weeks 4 and 16.
The mean PASI decreased significantly from 18.3±9.0 at baseline to 4.3±13 after 4 weeks of treatment (P < 0.001) and was further reduced to 1.1±3.5 after 16 weeks of therapy (P < 0.001) (Table 3). At baseline, only 1 patient (1.6%) had a PASI score < 2, while 28 patients (46.7%) and 47 patients (78.3%) had a PASI score < 2 after 4 weeks and 16 weeks of treatment, respectively (Table 3).
Table 3.
Mean PASI and DLQI, Percentage of Patients with PASI≤2, and Percentage With DLQI≥10 Over Time.
| Time Point | Mean PASI | PASI≤2 |
|---|---|---|
| Baseline | 18.3 (9.0) | - |
| 4 weeks | 4.3 (13.0) | 28 (46.7%) |
| 16 weeks | 1.1 (3.5) | 47 (78.3%) |
| Time Point | Mean DLQI | DLQI ≥ 10 |
| Baseline | 17.1 (8.8) | 51 (81.0%) |
| 4 weeks | 2.9 (4.0) | 5 (8.3%) |
| 16 weeks | 0.5 (1.4) | 0 |
PASI and DLQI scores were available for 63 patients at baseline and for 61 patients at weeks 4 and 16.
DLQI = Dermatology Life Quality Index; PASI: Psoriasis Area and Severity Index.
DLQI Improvement
At baseline, DLQI scores were available for 63 patients and 61 of them had a 4- and 16-week follow-up. The rapid improvement in PASI scores was paralleled by a decrease in the mean DLQI throughout the study period (Table 3). The DLQI, which was 17.1±8.8 at baseline, significantly improved to 2.9±4 at 4 weeks (P < 0.001, paired sample t-test) and further dropped to 0.5±1.4 at 16 weeks (P < 0.001, paired sample t-test). The number of patients reporting severe impairment of quality of life (DLQI ≥10) decreased from 51 patients (81%) at baseline to 5 patients (8.3%) after 4 weeks of treatment. None of the patients had a DLQI≥10 after 16 weeks of treatment (Table 3).
Given the impressive response of genital psoriasis to bimekizumab in almost all of our patient, in our study we found no significant impact of prior biologics exposure on the improvement of sPGA-G or on the likelihood of achieving PASI75, PASI90, or PASI100 during the study (Figure 3). Similarly, the presence of obesity as a comorbidity did not appear to have a statistically significant effect on the bimekizumab-induced improvement of sPGA-G values. Obesity did not significantly influence the likelihood of achieving PASI75, PASI90, or PASI100 during the study (Figure 3). These findings suggest that bimekizumab is effective in improving genital psoriasis and associated quality of life, regardless of prior biologic exposure or the presence of obesity as a comorbidity.
Figure 3.
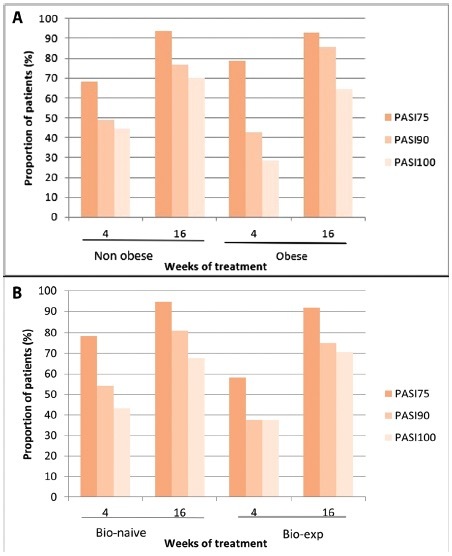
(A) Patients achieving Psoriasis Area and Severity Index (PASI) 75, PASI90 and PASI100 during treatment in relation to previous exposure to biologic therapy. (B) Percentage of patients achieving PASI 75, PASI90 and PASI 100 over time in relation to the presence of obesity as a comorbidity. PASI75, at least a 75% improvement from baseline in PASI; PASI90, at least a 90% improvement from baseline in PASI. PASI100, 100% improvement from baseline in PASI. PASI score was available for 63 patients at baseline and for 61 patients at weeks 4 and 16.
Conclusions
To date, no specific guidelines are available regarding the treatment of genital psoriasis with biological drugs. However, a few real-world experiences have been recently published on the role of anti-IL-23 and anti-IL17A drugs [16,30]. Real-world evidence on the effectiveness of bimekizumab in this subset of patients are extremely limited.
When examining our study population at baseline, we observed similarities in their characteristics when compared to the participants in phase-3 clinical trials evaluating bimekizumab in psoriatic patients [20–23]. One notable exception was the lower mean PASI at baseline in our study. This discrepancy can be attributed to the stringent inclusion criteria typically employed in clinical trials. Of particular interest, our patients exhibited a higher mean DLQI compared to those involved in the aforementioned trials, underscoring the substantial impact of psoriasis on our patients quality of life [20–23].
The data presented in our study highlight the remarkable effectiveness of bimekizumab in the treatment of genital psoriasis. We observed rapid and significant improvements in the severity of genital psoriasis, as measured by the sPGA-G score, with 98.4% of patients achieving a clear sPGA-G score after just 16 weeks of treatment.
Compared with ixekizumab, the only biological treatment specifically approved for genital psoriasis treatment, we observed better clinical responses at week 16 [31]. As a matter of fact, the study on ixekizumab by Guenther et al showed that 75% of patients achieved a clear or almost clear sPGA-G score after 52 weeks of treatment, with 60% achieving complete clearance [10]. Similarly, Sotirou et al achieved a sPGA-G score of almost clear or clear in 68.8% and 93.8% of patients treated with ixekizumab at weeks 16 and 24, respectively [16]. These findings suggest that bimekizumab may lead to a more rapid and effective improvement in genital psoriasis compared to ixekizumab, but head-to-head comparison studies are needed to confirm this observation. Our findings could also be partially explained with a moderate severity of genital psoriasis in our cohort, as one third of the patients had a s-PGA-G of 3, as shown in Table 2.
In comparison to the results from randomized clinical trials with bimekizumab, our study demonstrated comparable or slightly superior rates of improvement in key psoriasis severity measures, including PASI75, PASI90, and PASI100, at both the 4-week and 16-week time points [23].
Specifically, at the 4-week mark, our study showed higher rates of patients achieving PASI100 response compared to those reported in clinical trials [20–23]. By the 16-week time point, the rates of improvement in PASI scores in our study were very similar to those reported in the clinical trials. In terms of how bimekizumab treatment affected the well-being of patients, it is worth noting that after 16 weeks, all individuals reported that their psoriasis was no longer having a negative impact on their quality of life.
In contrast to earlier research, which suggested that treatment responses were better in biologically naive (patients who had not previously received biologic treatments) compared to biologically experienced individuals, our study did not find significant differences in how patients responded to bimekizumab treatment, regardless of their prior exposure to biologics [32–35].
Previous research has shown a direct correlation between BMI and psoriasis prevalence and severity, along with evidence that psoriasis symptoms improve with weight reduction and increased physical activity [36–41].
It is worth noting that many anti-TNFα biologic treatments for psoriasis have shown better outcomes in individuals with normal or slightly higher body weight compared to those who are obese [39]. Similarly, IL-17 inhibitors like secukinumab, ixekizumab, and brodalumab, while generally highly effective regardless of body weight, tend to yield a more favorable response in patients with normal body weight compared to those who are overweight or obese [39–42]. Our findings did not align with these associations, as it did not reveal significant differences in treatment response to bimekizumab between obese and non-obese patients.
The absence of a statistically significant difference in the response to bimekizumab between non-obese and obese patients in our study could be attributed to the relatively small sample size. Additionally, it is worth considering that the superior efficacy of bimekizumab, which inhibits both IL17A and IL17F, in treating psoriatic patients compared to secukinumab, which only inhibits IL-17A, might have lessened the impact of obesity [23]. However, this hypothesis contrasts with the fact that brodalumab, which blocks the IL-1 receptor A and inhibits the biological activity of IL-17A, IL-17F, IL-17E, and IL-17C, has shown greater effectiveness in non-obese psoriatic patients in various studies [12,34,43].
The study does have several limitations that should be acknowledged. First, the study enrolled a relatively small number of patients, underscoring the need for further research to assess the long-term optimal management of genital psoriasis patients in real-life settings. Additionally, the study did not include an active comparator, which could have been ixekizumab, to provide a more comprehensive comparison. Furthermore, it is worth noting that 73% of the study population consisted of men, even though genital psoriasis is somewhat more common in male compared to female psoriasis patients, as reported in previous studies [44]. Moreover, the study assessed treatment response only up to the 16-week mark and only a 18.5% of patients had a severe or very severe genital psoriasis. Investigating the long-term impact of bimekizumab on genital symptoms and sexual activity is crucial, as the effects of treatment on sexual activity within a relationship may take longer to become evident in some cases. The involvement of difficult-to-treat areas can severely impact the patients quality of life and, in this context, both anti-IL-23 and anti-IL-17 drugs have shown promising data [45,46]. The role of bimekizumab in this setting has not been explored yet, thus our study provides initial real-world data, even though they are limited by a small sample size and a relatively short observation period.
In this study, a 16-week course of bimekizumab treatment demonstrated highly favorable outcomes for patients with genital psoriasis. Notably, 98.4% of patients achieved a clear sPGA-G score within 16 weeks of treatment, and there were consistent improvements in PASI scores over the 4 to 16-week treatment period. Furthermore, the study revealed a significant reduction in the mean DLQI score, indicating an improvement in patients quality of life. Noteworthy is the low number of patients reporting severe impairment in quality of life (DLQI ≥10) after only four weeks of treatment, with none experiencing this level of impairment by the end of the 16-week period.
Contrary to some existing literature, our study did not observe differences on the effectiveness of bimekizumab between bio-naïve and bio-experienced subgroups or between non-obese and obese patient groups. It is important to acknowledge the limitations of our study, primarily the relatively small cohort size, which may have influenced these findings.
In summary, our preliminary findings are promising and suggest that bimekizumab holds great potential as a treatment option for genital psoriasis. However, further research with larger sample sizes and longer-term follow-up is essential to validate these results conclusively.
Acknowledgements
Editorial assistance was provided by Luca Giacomelli, PhD, Valeria Benedusi, PhD, Aashni Shah and Valentina Attanasio (Polistudium SRL, Milan, Italy). This assistance was supported by internal funds.
Footnotes
Funding: None.
Competing Interests: D. Orsini has been a speaker and/or consultant for Abbvie, LeoPharma, UCB, Bristol-Meyer-Squibb and Boehringer-Ingelheim. P. Malagoli has been a speaker for AbbVie, Lilly, Novartis, Janssen-Cilag, Celgene, Leopharma, and Almirall. A. Balato has received honoraria for participation in advisory boards, meetings, or as speaker for AbbVie, Celgene, Janssen-Cilag, Eli Lilly, Novartis Pharma, Pfizer, Sanofi-Genzyme, and UCB Pharma. L. Bianchi has received honoraria as a speaker or consultant for AbbVie, Janssen, Almirall Eli-Lilly, Leopharma, Novartis, Sanofi, Pfizer and UCB Pharma. G. Caldarola reports consulting fees or honorarium and payment for lectures from Lilly and Novartis. C. De Simone reports consulting fees or honorarium from Abbvie, Amgen, Novartis, Celgene, Sanofi, UCB Pharma, Janssen and Lilly and payment for lectures from Abbvie, Lilly, Novartis, UCB Pharma, and Celgene. M. Esposito has served as speaker and/or consultant for AbbVie, Almirall, Biogen, Celgene, Eli Lilly, Janssen, Leo Pharma, Novartis, Sanofi Genzyme, UCB. M. C. Fargnoli has served on advisory boards, received honoraria for lectures and research grants from AMGEN, Almirall, AbbVie, BMS, Galderma, Kyowa Kirin, Leo Pharma, Pierre Fabre, UCB, Lilly, Pfizer, Janssen, MSD, Novartis, Sanofi-Regeneron, Sun Pharma. P. Gisondi has been a consultant and/or speaker for Abbvie, Almirall, Amgen, Janssen, Leo-Pharma, Eli-Lilly, Novartis, Pierre Febre, Sandoz, Sanofi and UCB. M. Burlando has acted as a speaker and consultant for AbbVie, Janssen, Amgen, Novartis, Eli Lilly, UCB Pharma. C. G. Carrera has served as a board participant or speaker for Abbvie, Lilly, Janssen, Novartis, Celgene, Almirall, and Leopharma. P. Dapavo has been a speaker for Novartis, Abbvie, Sanofi, UCB, Janssen, Lilly, and LeoPharma. F. M. Gaiani acted as a speaker or consultant for Novartis, Abbvie, Eli Lilly, Celgene, LeoPharma, and Almirall. L. Gargiulo has been a consultant for Almirall A. Giunta received grants or is an investigator for Biogen and Lilly; and is a consultant/advisory board/speaker for AbbVie, Almirall, Celgene, Janssen, Leo Pharma, Eli Lilly, Merck Sharpe Dohme, Novartis, Pfizer, Sandoz and UCB. F. Loconsole served on advisory boards and/or received honoraria for lectures from Abbvie, Janssen-Cilag, Novartis, Lilly, Sanofi. A. V. Marzano reports consultancy/advisory boards disease-relevant honoraria from AbbVie, Boehringer-Ingelheim, Novartis, Pfizer, Sanofi and UCB. M. Megna acted as a speaker or consultant for Abbvie, Eli Lilly, Janssen, Leo-Pharma, UCB and Novartis. A. Narcisi has served on advisory boards, received honoraria for lectures and research grants from Almirall, Abbvie, Leo Pharma, Celgene, Eli Lilly, Janssen, Novartis, Sanofi-Genzyme, Amgen and Boehringer Ingelheim. A. Offidani acted as a speaker and consultant for Abbvie, Eli Lilly, Novartis, Celgene, Sanofi, Galderma, Leo Pharma, Pierre Fabre. M. Venturini served as a speaker or advisory board member for Abbvie, Almirall, Amgen, Eli-LILLY, Galderma, Leo Pharma, Novartis, Pierre Fabre and UCB Pharma. A. Costanzo has served as an advisory board member, consultant and has received fees and speaker’s honoraria or has participated in clinical trials for Abbvie, Almirall, Biogen, LEO Pharma, Lilly, Janssen, Novartis, Pfizer, Sanofi Genzyme, and UCB-Pharma. No other disclosures were reported.
Authorship: All authors have contributed significantly to this publication.
Ethics Statement: Institutional review board approval was exempted as the study protocol did not deviate from standard clinical practice. All included patients had provided written consent for retrospective study of data collected during routine clinical practice (demographics, clinical scores). The study was performed in accordance with the Helsinki Declaration of 1964 and its later amendments. Data collection and handling complied with applicable laws, regulations, and guidance regarding patient protection, including patient privacy.
References
- 1.Meeuwis KAP, Potts Bleakman A, van de Kerkhof PCM, et al. Prevalence of genital psoriasis in patients with psoriasis. J Dermatolog Treat. 2018;29(8):754–760. doi: 10.1080/09546634.2018.1453125. [DOI] [PubMed] [Google Scholar]
- 2.Staubach P, Plavic-Radeka N, Peveling-Oberhag A, et al. High prevalence and little awareness in patients with chronic inflammatory skin diseases and genital involvement. J Dtsch Dermatol Ges. 2021;19(10):1443–1448. doi: 10.1111/ddg.14437. [DOI] [PubMed] [Google Scholar]
- 3.Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180(3):647–656. doi: 10.1111/bjd.17147. [DOI] [PubMed] [Google Scholar]
- 4.Yang EJ, Beck KM, Sanchez IM, Koo J, Liao W. The impact of genital psoriasis on quality of life: a systematic review. Psoriasis (Auckl) 2018;8:41–47. doi: 10.2147/PTT.S169389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelly A, Ryan C. Genital Psoriasis: Impact on Quality of Life and Treatment Options. Am J Clin Dermatol. 2019;20(5):639–646. doi: 10.1007/s40257-019-00447-5. [DOI] [PubMed] [Google Scholar]
- 6.Ryan C, Guenther L, Foley P, et al. Ixekizumab provides persistent improvements in health-related quality of life and the sexual impact associated with moderate-to-severe genital psoriasis in adult patients during a 52-week, randomised, placebo-controlled, phase 3 clinical trial. J Eur Acad Dermatol Venereol. 2022;36(4):e277–e279. doi: 10.1111/jdv.17836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calianno G, Esposito M, Fidanza R, Palmieri M, Fargnoli MC. Ixekizumab improves disease severity, clinical symptoms and quality of life in patients with genital psoriasis: A 24-week real-life experience. Dermatol Ther. 2021;34(4):e14993. doi: 10.1111/dth.14993. [DOI] [PubMed] [Google Scholar]
- 8.Hong JJ, Mosca ML, Hadeler EK, Brownstone ND, Bhutani T, Liao WJ. Genital and Inverse/Intertriginous Psoriasis: An Updated Review of Therapies and Recommendations for Practical Management. Dermatol Ther (Heidelb) 2021;11(3):833–844. doi: 10.1007/s13555-021-00536-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beck KM, Yang EJ, Sanchez IM, Liao W. Treatment of Genital Psoriasis: A Systematic Review. Dermatol Ther (Heidelb) 2018;8(4):509–525. doi: 10.1007/s13555-018-0257-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guenther L, Potts Bleakman A, Weisman J, et al. Ixekizumab Results in Persistent Clinical Improvement in Moderate-to-Severe Genital Psoriasis During a 52 Week, Randomized, Placebo-Controlled, Phase 3 Clinical Trial. Acta Derm Venereol. 2020;100(1):adv00006. doi: 10.2340/00015555-3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armstrong AW, Read C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA. 2020;323(19):1945–1960. doi: 10.1001/jama.2020.4006. [DOI] [PubMed] [Google Scholar]
- 12.Gargiulo L, Ibba L, Malagoli P, et al. Brodalumab for the treatment of plaque psoriasis in a real-life setting: a 3 years multicenter retrospective study-IL PSO (Italian landscape psoriasis) Front Med (Lausanne) 2023;10:1196966. doi: 10.3389/fmed.2023.1196966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yosipovitch G, Foley P, Ryan C, et al. Ixekizumab Improved Patient-Reported Genital Psoriasis Symptoms and Impact of Symptoms on Sexual Activity vs Placebo in a Randomized, Double-Blind Study. J Sex Med. 2018;15(11):1645–1652. doi: 10.1016/j.jsxm.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Merola JF, Ghislain PD, Dauendorffer JN, et al. Ixekizumab improves secondary lesional signs, pain and sexual health in patients with moderate-to-severe genital psoriasis. J Eur Acad Dermatol Venereol. 2020;34(6):1257–1262. doi: 10.1111/jdv.16181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yeh CP, Huang YW, Tsai TF. Comparison of the relative efficacy of different biologics in different body areas in patients with moderate to severe psoriasis receiving biologics and tofacitinib in phase 3 randomized controlled trials: a 15-year single-center experience. Expert Rev Clin Pharmacol. 2022;15(7):887–895. doi: 10.1080/17512433.2022.2103538. [DOI] [PubMed] [Google Scholar]
- 16.Sotiriou E, Bakirtzi K, Papadimitriou I, et al. A head-to-head comparison of risankizumab and ixekizumab for genital psoriasis: a real-life, 24-week, prospective study. J Eur Acad Dermatol Venereol. 2022;36(5):e359–e361. doi: 10.1111/jdv.17880. [DOI] [PubMed] [Google Scholar]
- 17.Ryan C, Menter A, Guenther L, et al. Efficacy and safety of ixekizumab in a randomized, double-blinded, placebo-controlled phase IIIb study of patients with moderate-to-severe genital psoriasis. Br J Dermatol. 2018;179(4):844–852. doi: 10.1111/bjd.16736. [DOI] [PubMed] [Google Scholar]
- 18.Soung J, Jennifer CC, Gooderham M. 33054 Improvement in Touch Avoidance in Patients with Genital Psoriasis Treated with Ixekizumab: 52-Week Results of a Phase 3 Clinical Trial in Patients with Moderate-to-Severe Genital Psoriasis (IXORA-Q) Journal of the American Academy of Dermatology. 2022;87(3):AB69. doi: 10.1016/j.jaad.2022.06.309. [DOI] [Google Scholar]
- 19.Kolbinger F, Loesche C, Valentin MA, et al. β-Defensin 2 is a responsive biomarker of IL-17A-driven skin pathology in patients with psoriasis. J Allergy Clin Immunol. 2017;139(3):923–932.e8. doi: 10.1016/j.jaci.2016.06.038. [DOI] [PubMed] [Google Scholar]
- 20.Warren RB, Blauvelt A, Bagel J, et al. Bimekizumab versus Adalimumab in Plaque Psoriasis. N Engl J Med. 2021;385(2):130–141. doi: 10.1056/NEJMoa2102388. [DOI] [PubMed] [Google Scholar]
- 21.Gordon KB, Foley P, Krueger JG, et al. Bimekizumab efficacy and safety in moderate to severe plaque psoriasis (BE READY): a multicentre, double-blind, placebo-controlled, randomised withdrawal phase 3 trial. Lancet. 2021;397(10273):475–486. doi: 10.1016/S0140-6736(21)00126-4. [DOI] [PubMed] [Google Scholar]
- 22.Reich K, Papp KA, Blauvelt A, et al. Bimekizumab versus ustekinumab for the treatment of moderate to severe plaque psoriasis (BE VIVID): efficacy and safety from a 52-week, multicentre, double-blind, active comparator and placebo controlled phase 3 trial. Lancet. 2021;397(10273):487–498. doi: 10.1016/S0140-6736(21)00125-2. [DOI] [PubMed] [Google Scholar]
- 23.Reich K, Warren RB, Lebwohl M, et al. Bimekizumab versus Secukinumab in Plaque Psoriasis. N Engl J Med. 2021;385(2):142–152. doi: 10.1056/NEJMoa2102383. [DOI] [PubMed] [Google Scholar]
- 24.Megna M, Battista T, Potestio L, et al. A case of erythrodermic psoriasis rapidly and successfully treated with Bimekizumab. J Cosmet Dermatol. 2023;22(3):1146–1148. doi: 10.1111/jocd.15543. [DOI] [PubMed] [Google Scholar]
- 25.Valenti M, Gargiulo L, Ibba L, Pavia G, Narcisi A, Costanzo A. Sub-erythrodermic psoriasis successfully treated with bimekizumab: A case report. Dermatol Ther. 2022;35(12):e15952. doi: 10.1111/dth.15952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gargiulo L, Narcisi A, Ibba L, et al. Effectiveness and safety of bimekizumab for the treatment of plaque psoriasis: a reallife multicenter study-IL PSO (Italian landscape psoriasis) Front Med (Lausanne) 2023;10:1243843. doi: 10.3389/fmed.2023.1243843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Piaserico S, Riedl E, Pavlovsky L, et al. Comparative effectiveness of biologics for patients with moderate-to-severe psoriasis and special area involvement: week 12 results from the observational Psoriasis Study of Health Outcomes (PSoHO) Front Med ( Lausanne) 2023;10:1185523. doi: 10.3389/fmed.2023.1185523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gisondi P, Fargnoli MC, Amerio P, et al. Italian adaptation of EuroGuiDerm guideline on the systemic treatment of chronic plaque psoriasis. Ital J Dermatol Venerol. 2022;157(Suppl 1 to No 1):1–78. doi: 10.23736/S2784-8671.21.07132-2. [DOI] [PubMed] [Google Scholar]
- 29.European Medicines Agency. Bimzelx (bimekizumab): summary of product characteristics. 2023. [Accessed on September 6, 2023]. Available fromt: https://www.ema.europa.eu/en/documents/product-information/bimzelxepar-product-information_it.pdf.
- 30.Orsini D, Gargiulo L, Ibba L, et al. Effectiveness of risankizumab in plaque psoriasis with involvement of difficult-to-treat areas: a real-world experience from two referral centers. J Dermatolog Treat. 2023;34(1):2220849. doi: 10.1080/09546634.2023.2220849. [DOI] [PubMed] [Google Scholar]
- 31.European Medicines Agency. Taltz (ixekizumab): summary of product characteristics. 2023. [Accessed on September 20, 2023]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/taltz.
- 32.Gargiulo L, Ibba L, Malagoli P, et al. A risankizumab super responder profile identified by long-term real-life observation-IL PSO (ITALIAN LANDSCAPE PSORIASIS) J Eur Acad Dermatol Venereol. 2024;38(1):e113–e116. doi: 10.1111/jdv.19464. [DOI] [PubMed] [Google Scholar]
- 33.Mastorino L, Castelli F, Stroppiana E, et al. Risankizumab shows faster response in bio naïve than in bio-experienced psoriatic patients. J Eur Acad Dermatol Venereol. 2022;36(10):e838–e841. doi: 10.1111/jdv.18314. [DOI] [PubMed] [Google Scholar]
- 34.Mastorino L, Cariti C, Susca S, et al. Brodalumab efficacy in bionaïve psoriasis patients: real-life experience of 202 subjects up to 48 weeks. J Dermatolog Treat. 2022;33(8):3211–3213. doi: 10.1080/09546634.2022.2125265. [DOI] [PubMed] [Google Scholar]
- 35.Gargiulo L, Ibba L, Pavia G, et al. Real-Life Effectiveness and Safety of Risankizumab in 131 Patients Affected by Moderate-to-Severe Plaque Psoriasis: A 52-Week Retrospective Study. Dermatol Ther (Heidelb) 2022;12(10):2309–2324. doi: 10.1007/s13555-022-00795-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7(7):CD011972. doi: 10.1002/14651858.CD011972.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ogawa K, Stuart PE, Tsoi LC, et al. A Transethnic Mendelian Randomization Study Identifies Causality of Obesity on Risk of Psoriasis. J Invest Dermatol. 2019;139(6):1397–1400. doi: 10.1016/j.jid.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jensen P, Skov L. Psoriasis and Obesity. Dermatology. 2016;232(6):633–639. doi: 10.1159/000455840. [DOI] [PubMed] [Google Scholar]
- 39.Kaushik SB, Lebwohl MG. Psoriasis: Which therapy for which patient: Psoriasis comorbidities and preferred systemic agents. J Am Acad Dermatol. 2019;80(1):27–40. doi: 10.1016/j.jaad.2018.06.057. [DOI] [PubMed] [Google Scholar]
- 40.Polo TCF, Corrente JE, Miot LDB, Papini SJ, Miot HA. Dietary patterns of patients with psoriasis at a public healthcare institution in Brazil. An Bras Dermatol. 2020;95(4):452–458. doi: 10.1016/j.abd.2020.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Paroutoglou K, Papadavid E, Christodoulatos GS, Dalamaga M. Deciphering the Association Between Psoriasis and Obesity: Current Evidence and Treatment Considerations. Curr Obes Rep. 2020;9(3):165–178. doi: 10.1007/s13679-020-00380-3. [DOI] [PubMed] [Google Scholar]
- 42.Zafiriou E, Daponte AI, Siokas V, Tsigalou C, Dardiotis E, Bogdanos DP. Depression and Obesity in Patients With Psoriasis and Psoriatic Arthritis: Is IL-17-Mediated Immune Dysregulation the Connecting Link? Front Immunol. 2021;12:699848. doi: 10.3389/fimmu.2021.699848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Blair HA. Brodalumab: A Review in Moderate to Severe Plaque Psoriasis. Drugs. 2018;78(4):495–504. doi: 10.1007/s40265-018-0888-4. [DOI] [PubMed] [Google Scholar]
- 44.Meeuwis KA, de Hullu JA, Massuger LF, van de Kerkhof PC, van Rossum MM. Genital psoriasis: A systematic literature review on this hidden skin disease. Acta Derm Venereol. 2011;91(1):5–11. doi: 10.2340/00015555-0988. [DOI] [PubMed] [Google Scholar]
- 45.Cortese A, Gargiulo L, Ibba L, et al. Anti-interleukin-17 and anti-interleukin-23 biologic drugs for genital psoriasis: a single-center retrospective comparative study. Dermatol Reports. 2023;15(3):9692. doi: 10.4081/dr.2023.9692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trovato E, Cortonesi G, Orsini C, et al. Anti-IL23for nail psoriasis in real life: Results of efficacy and safety during a 52-week period. Dermatol Ther. 2022;35(7):e15506. doi: 10.1111/dth.15506. [DOI] [PubMed] [Google Scholar]


