Abstract.
Significance
People with Parkinson’s disease (PD) experience changes in fine motor skills, which is viewed as one of the hallmark signs of this disease. Due to its non-invasive nature and portability, functional near-infrared spectroscopy (fNIRS) is a promising tool for assessing changes related to fine motor skills.
Aim
We aim to compare activation patterns in the primary motor cortex using fNIRS, comparing volunteers with PD and sex- and age-matched control participants during a fine motor task and walking. Moreover, inter and intrahemispheric functional connectivity (FC) was investigated during the resting state.
Approach
We used fNIRS to measure the hemodynamic changes in the primary motor cortex elicited by a finger-tapping task in 20 PD patients and 20 controls matched for age, sex, education, and body mass index. In addition, a two-minute walking task was carried out. Resting-state FC was also assessed.
Results
Patients with PD showed delayed hypoactivation in the motor cortex during the fine motor task with the dominant hand and delayed hyperactivation with the non-dominant hand. The findings also revealed significant correlations among various measures of hemodynamic activity in the motor cortex using fNIRS and different cognitive and clinical variables. There were no significant differences between patients with PD and controls during the walking task. However, there were significant differences in interhemispheric connectivity between PD patients and control participants, with a statistically significant decrease in PD patients compared with control participants.
Conclusions
Decreased interhemispheric FC and delayed activity in the primary motor cortex elicited by a fine motor task may one day serve as one of the many potential neuroimaging biomarkers for diagnosing PD.
Keywords: Parkinson’s disease, functional near-infrared spectroscopy, cortical activity, resting state, functional connectivity
1. Introduction
Parkinson’s disease (PD) is a progressive neurological condition that affects movement. The condition is due to the loss of neurons in the substantia nigra of the brain, which is the area responsible for the production of dopamine. This neurotransmitter is essential for movement. The loss of neurons causes a reduction in dopamine production, leading to the characteristic symptoms of the condition: tremors, rigidity, bradykinesia, and postural instability.1 As of today, there is no cure for PD, but treatment that can provide significant relief from symptoms is available, and neuroimaging techniques can be used to monitor the progress of the disease and predict its severity. Therefore, the diagnosis of PD is based on the presence of these symptoms, together with a detailed history of the patient’s condition.2 The Unified Parkinson’s Disease Rating Scale (UPDRS) is widely used for diagnosing PD.3 The criteria are based on tremor, rigidity, bradykinesia, and postural instability.
The condition has been well studied, and there is a good understanding of the underlying causes. There is, however, a need for new diagnostic techniques that are objective and provide a measure of disease severity. New neuroimaging techniques are needed for the diagnosis and monitoring of PD progression due to the complexity and variability of the disease. Current imaging techniques may not fully capture the early stages or the full spectrum of PD symptoms.4 One such technique is functional near-infrared spectroscopy (fNIRS), which is a portable, non-invasive optical neuroimaging technique that measures changes in brain activity by estimating oxygenated and deoxygenated hemoglobin near the brain’s surface, thus enabling the investigation of cortical activity, which can be altered in PD. At the same time, participants move freely.5 Unlike traditional imaging methods such as functional magnetic resonance imaging (fMRI), fNIRS allows participants to move and speak relatively easily, making it well suited to exploring the full complement of neuroimaging research questions and serving as a viable alternative to fMRI due to its unique advantages such as portability, resiliency to motion artifacts, and tolerance to metallic implants; these make it more suitable for patients with PD who may have difficulty remaining still or have deep brain devices not compatible with fMRI.5,6 In addition, fNIRS is safe, portable, lightweight, cost-effective, and less sensitive to motion artifacts and environmental noise than other imaging techniques.7,8
fNIRS can potentially study movement disorders such as PD as it can overcome movement restrictions and enable experiments in more natural conditions.9 Focusing specifically on fine motor skills, fNIRS has been used to discriminate between different motor responses and levels of motor complexity, making it a relevant tool for monitoring changes related to fine motor skills.10 In addition, fNIRS is a promising approach to studying the presumed contribution of dysfunction within the pre-frontal cortex (PFC) to difficulties in PD patients.11 Furthermore, fNIRS has been used to assess fine motor tasks in PD patients during stepping tasks12 and under deep brain stimulation.13 It has been shown that fNIRS can detect changes in cortical activation patterns during fine motor tasks in PD patients. For instance, Bonilauri et al.14 found that early-stage PD patients showed higher activation in motor and occipital areas compared with those with moderate PD, who had increased activation in frontal areas, suggesting a shift in brain activation patterns with disease progression. Simpson and Mak15 suggested that transcranial direct current stimulation alters motor cortex oxygenation in both PD patients and healthy controls but does not modulate its task-related activity.
When assessing the cognitive status of PD patients, research has shown fine motor skills to be impaired. Furthermore, this impaired cognitive status may exacerbate such difficulties in PD patients.16 Using fNIRS to measure cortical blood flow changes related to fine motor skills, researchers can assess relevant motor symptoms in PD patients and determine whether patients have mild cognitive impairment or not.17 Overall, fNIRS has the potential to study fine motor tasks in PD patients and to monitor changes related to the development of the disease.
Furthermore, fNIRS can assess the brain’s resting-state functional connectivity (RSFC). RSFC measures the temporal correlation among spatially remote brain regions that exhibit synchronous low-frequency fluctuations in blood oxygenation level-dependent signals.18 RSFC has been used to compare differences in functional connectivity (FC) between patients with PD and age-matched controls using fMRI.19 Comparing RSFC between these two groups can provide insights into the pathophysiology of PD and help identify potential biomarkers for early diagnosis and treatment.19
In the present study, fNIRS was used to examine the hemodynamic response to fine motor skills in the motor cortex of PD patients and control participants. First, correlations between the hemodynamic response function (HRF) parameters and clinical markers were analyzed. Thereafter, motor cortex activity during a walking task was measured, and the RSFC in these patients was assessed.
2. Methods
2.1. Ethics Statement
The study protocol was designed according to the Declaration of Helsinki and reviewed and approved by the Institutional Review Board (registration number 77-21). The study was conducted at the Neurology Department of the Central Hospital “Dr. Ignacio Morones Prieto” in Mexico. The recruiting period was from October 2021 to October 2022. Before testing, all participants provided signed informed consent.
2.2. Participants
Twenty patients were enrolled in our clinic and were diagnosed according to the criteria of the United Kingdom PD Society Brain Bank.20 All PD patients were on anti-Parkinson drugs. Daily Levodopa equivalent doses were determined from total daily doses for participants using anti-Parkinson medicines using a conversion formula.21 In the present analysis, we included patients with a tremor-dominant () or akinetic-rigid subtype ().22 A neurologist (FJRR) used the Hoehn and Yahr (HY) scale23 and the Movement Disorder Society-Sponsored Revision of the Unified Parkinson Disease Rating Scale (MDS-UPDRS, part III) to determine the severity of the disease.3 Freezing of gait severity was assessed with the freezing of gait questionnaire (FOGQ).24 All evaluations detailed in this study were conducted while the subjects were in their “off” medication state ( after the last anti-parkinsonian medication dose was taken). The MDS-UPDRS part III scores were additionally validated by an independent rater.
The control group was composed of 20 age- and sex-matched healthy participants without movement disorders. All participants underwent the Spanish version of the Montreal Cognitive Assessment (MoCA) for global cognitive function assessment.25 Severe cognitive impairment at baseline was defined as a score on the MoCA examination, and individuals with this score or below were excluded from this study.
2.3. fNIRS Data Acquisition
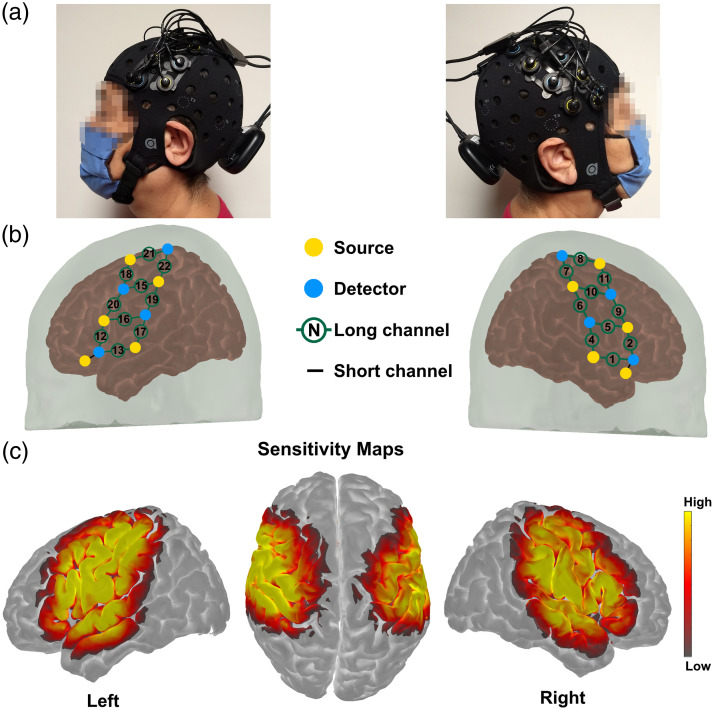
The portable fNIRS system Brite MKII (Artinis Medical Systems BV, the Netherlands) was employed for measuring cortical brain activity in the present study. This system employs 10 dual-wavelength light-emitting diodes (LEDs) centered at 757 and 843 nm, with a sampling frequency of 25 Hz, which is sufficiently high enough to avoid aliasing effects of blood pressure, respiratory, and cardiac frequencies. Participants in the study were outfitted with a black neoprene head cap, with sizes varying between 54 and 60 cm, based on their head circumference, as depicted in Fig. 1(a). The cap was placed on the head of the participant, and the fNIRS optodes were securely held throughout data collection. There were eight detectors and 10 near-infrared sources; the optodes were placed 3 cm apart for the 20 long channels and 1.5 cm apart for the two short channels. The sources and detectors were positioned in the cap holders before the cap was worn by the participant and then manually adjusted to achieve an efficient optode-scalp coupling, as determined in real time by the acquisition software (Oxysoft, Artinis Medical Systems BV, the Netherlands). A three-dimensional digitizer (Patriot, Polhemus Inc., Colchester, Vermont, United States) was used to record the position. The 20 long channels [10 per hemisphere, as shown in Fig. 1(b)] were utilized to measure changes in hemoglobin, which serves as a proxy measure of brain activity, across bilateral motor regions of the brain [Fig. 1(c)], and the two short channels were used to minimize the effects of superficial hemodynamics.26,27
Fig. 1.
(a) Photographs of optode array placement on one participant’s head. (b) Registered probe geometry: yellow dots indicate the 10 sources, and cyan dots specify the eight detectors. Black numbers in green circles indicate long separation channels, and the black lines denote SSCs. (c) Sensitivity profile displayed in a log10 scale.
2.4. Experimental Protocol 1: Fine Motor Task
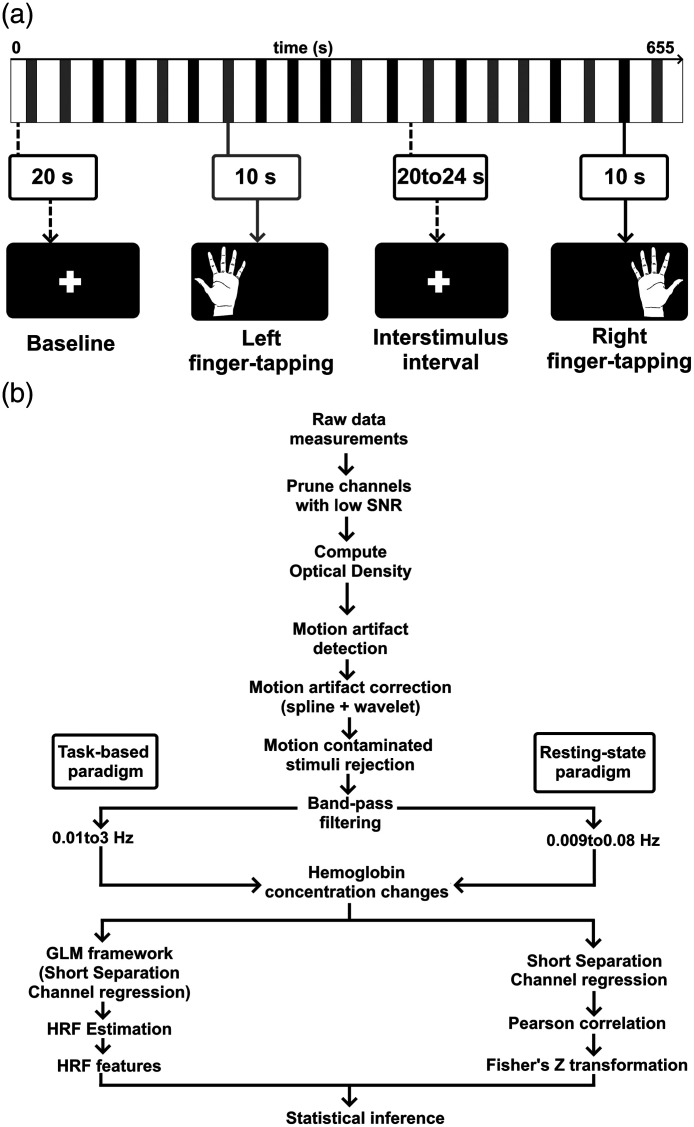
A finger-tapping exercise activated the primary motor cortex (M1), and its task-related hemodynamic activity was measured. Next, subjects were told to tap their thumbs consecutively with the other fingers of the same hand (i.e., thumb and index finger tapping, thumb and middle finger tapping, etc.). The action was performed in brief bursts of 10 s, interspersed with rest periods ranging from 20 to 24 s. This variable interstimulus period was intended to prevent task events from becoming phase-locked to physiological hemodynamic oscillations28 and to reduce anticipatory effects.29 In addition, video cues were used to instruct movement initiation and offset. The motor task lasted around 11 min and was divided into 20 blocks (10 with the left hand and 10 with the right hand, randomly allocated), as depicted in Fig. 2(a).
Fig. 2.
(a) Visualization of the experimental protocol, which includes two types of finger-tapping tasks: left and right. The entire experiment took 11 min. (b) Flow chart of the processing steps used in this study.
2.5. Experimental Protocol 2: A 2-min Walk
The participants initially conducted a 2-min walking exercise with a baseline standing period of 20 s. The walking response was analyzed as a single trial from to 25 s, but 2 min were allocated to see if the patients presented gait freeze. During the over-ground walking condition, participants walked back and forth along a 6 m straight path with 180-deg turns at either end. A research team member walked beside the participants to ensure their safety and stood by them when they turned.
2.6. Experimental Protocol 3: RSFC
Participants were instructed to remain as motionless as possible in a seating position for 6 min in a dimly lighted space. Participants were asked to close their eyes, remain relaxed, and refrain from concentrating on anything specific. A research team member monitored participant conduct during the trial to ensure they did not doze off. In addition, after the trial, participants were asked if they had fallen asleep at any moment. None of the participants reported that they had fallen asleep.
2.7. fNIRS Data Processing and Analysis
2.7.1. Fine motor task
To estimate the HRF elicited by the left and right finger-tapping tasks, the following procedure was implemented.
Data were processed and analyzed in MATLAB R2017b (The MathWorks, Natick, Massachusetts, United States) using Homer330 and custom-made scripts, as illustrated in Fig. 2(b). First, the raw time series went through a quality control process in which readings with very high or meager optical density (OD) changes, low signal-to-noise ratio (SNR), or very long source-detector separation were excluded from further analysis, using the hmrR_PruneChannels function (ranging from 1e−7 to 1e7, SNRthresh = 2, SDrange = 0.45). Then, the raw data were transformed into units of change in OD (). Motion artifacts were identified on a channel-by-channel basis using the hmrR_MotionArtifactByChannel function. Briefly, if the signal amplitude was larger than 0.5 (AMPthresh) or the standard deviation augmented by a factor of 15 (SDthresh) over a time window of 1 s (tMotion), then a segment of the time series of 1s length (tMask) was identified as motion artifact. The data in the motion-contaminated segments were interpolated by a cubic spline using the hmrR_MotionCorrectSpline function with a smoothing parameter of to avoid corrupting the clean segments adjacent to motion artifacts,31,32 and motion artifacts were corrected using the hmrR_MotionCorrectWavelet with an interquartile range of 1.5.33 To ensure the exclusion of any trials with residual motion artifacts, we conducted motion artifact rejection within a to 10 s window subsequent to our motion-artifact correction efforts, specifically to eliminate trials that continued to exhibit motion-related characteristics using the hmrR_StimRejection function. Finally, the OD data were bandpass filtered between 0.01 and 3 Hz to minimize the effect of slow drifts34 The time-series were converted to oxy-(HbO) and deoxygenated (HbR) hemoglobin employing the modified Beer–Lambert law, implemented in the hmrR_OD2Conc function with a partial pathlength factor of 1 for each wavelength, thus yielding units of -mm.35 The individual HRFs were estimated via a general linear model (GLM; hmrR_GLM). The analyzed range was from 2 s before stimulus onset to 25 s post-stimulation. The GLM was solved by ordinary least squares.36 A consecutive series of 1-s broad Gaussian functions interspaced 1 s was used as the basis function. Channels spaced by less than 1.5 cm were considered short separation, and the short channel with the most significant correlation was used to regress the superficial hemodynamics from the estimated HRF.37,38 A polynomial correction of order three was implemented.
2.7.2. Two-min walk
Changes in oxygenated hemoglobin (HbO) from baseline standing to walking were used as a proxy for cortical activation.39 The same procedure described in the fine motor task paradigm was implemented to process the fNIRS signals. Although individual trials in experiments might present limitations, the application of the GLM approach in fNIRS single trial analysis has demonstrated robustness, even in one-shot experiments.40
2.7.3. Resting-state
After motion artifact detection and correction, as described in the paragraphs above, the remaining clean segments were then bandpass filtered into the FC band (0.009 to 0.08 Hz) using the hmrR_BandPassFilt function41,42 and converted to hemoglobin concentrations.
2.8. Functional Connectivity
FC analysis was performed only on the time points identified as free of motion artifacts; discarding the motion-artifact segments has been shown to preserve interhemispheric FC.32 A procedure to remove spontaneous fNIRS fluctuations common to all channels was implemented using short separation channel (SSC) signal regression. First, the SSC signal was computed as the average time course of all short channels ( in our case): . Then, the SSC signal was regressed from the measured fNIRS time-series using a Tikhonov regularized estimator:
| (1) |
where the regularization parameter was chosen as .41 The residual time-series was then computed as follows:
| (2) |
generates channel-based connectivity matrices, computing the Pearson correlation between all channels. To obtain an index of intrahemispheric connectivity, channel-to-channel correlation coefficients in the left and right hemispheres, respectively, were averaged separately for each participant.43 An index of interhemispheric connectivity was calculated by averaging all channels and their contralateral homologs.
2.9. Statistical Analysis
A paired sample -test was used to compare the mean change in hemoglobin during the pre-task baseline period and the task period to assess channel activation.44 Comparisons between PD patients and controls were made through a non-parametric Wilcoxon–Mann–Whitney test for the task-based paradigms because part of the data was not normally distributed, according to a Shapiro–Wilk test.45 Five different metrics extracted from the hemodynamic response were compared between controls and PD patients. Furthermore, relationships between fNIRS data from channels demonstrating statistically significant group differences and clinical variables (Table 1) were examined with Spearman’s correlation coefficients, and corrections for false positives were carried out using a false-discovery rate (FDR) adjustment.46,47 To further investigate if the side of onset mediates the correlations between clinical variables and fNIRS features, all data from PD patients were stratified according to the side of onset (11 left and 9 right), and then, a partial correlation analysis was conducted to assess the relationship between HRF features and clinical variables while controlling for age and years of education.
Table 1.
Demographic and clinical features of PD patients and controls. Unless otherwise indicated, the mean values are given, with standard deviations in parenthesis.
| PD group | Control group | -Value | |
|---|---|---|---|
| Age (years) | 69.9 (10.1) | 65.6 (9.9) | 0.23 |
| Proportion of men (%) | 60 | 45 | 0.52 |
| Education (years) | 9.3 (4.1) | 12.1 (5.2) | 0.06 |
| Reported handedness (right/left) | 20/0 | 20/0 | 1.00 |
| MoCA | 21.6 (3.8) | 26.2 (2.7) | <0.001 |
| Unified PD rating scale III | 37.4 (18.6) | N/A | N/A |
| Disease duration (years) | 8.2 (3.8) | N/A | N/A |
| Freezing of gait questionnaire (range) | 2.7 (0 to 15) | N/A | N/A |
| Levodopa equivalent daily dosage (mg) | 707.5 (394.8) | N/A | N/A |
| HY scale (range) | 2.9 (2 to 4) | N/A | N/A |
| Side of symptom onset (right/left) | 9/11 | N/A | N/A |
For the intra and interhemispheric FC, Pearson’s coefficients were transformed to a Fisher -score using before computing the Wilcoxon–Mann–Whitney tests.48
In all experimental paradigms, corrections for multiple comparisons within-subject and between-group were performed using the FDR adjustment.46,47 Categorical variables were compared using the Chi-square test.
3. Results and Discussion
3.1. Clinical Evaluation
Table 1 summarizes the clinical and demographic characteristics of the studied groups. No significant differences were found in age, proportion of male participants, years of education, or body mass index. However, the cognitive dysfunction of PD patients was significantly higher than that of control participants (), as shown by previous studies.49–51 Because cognitive impairment is common in patients with PD and can occur at any stage of the disease,52 those with mild cognitive impairment were included in this study.
3.2. Cortical Activation during Fine Motor Movement
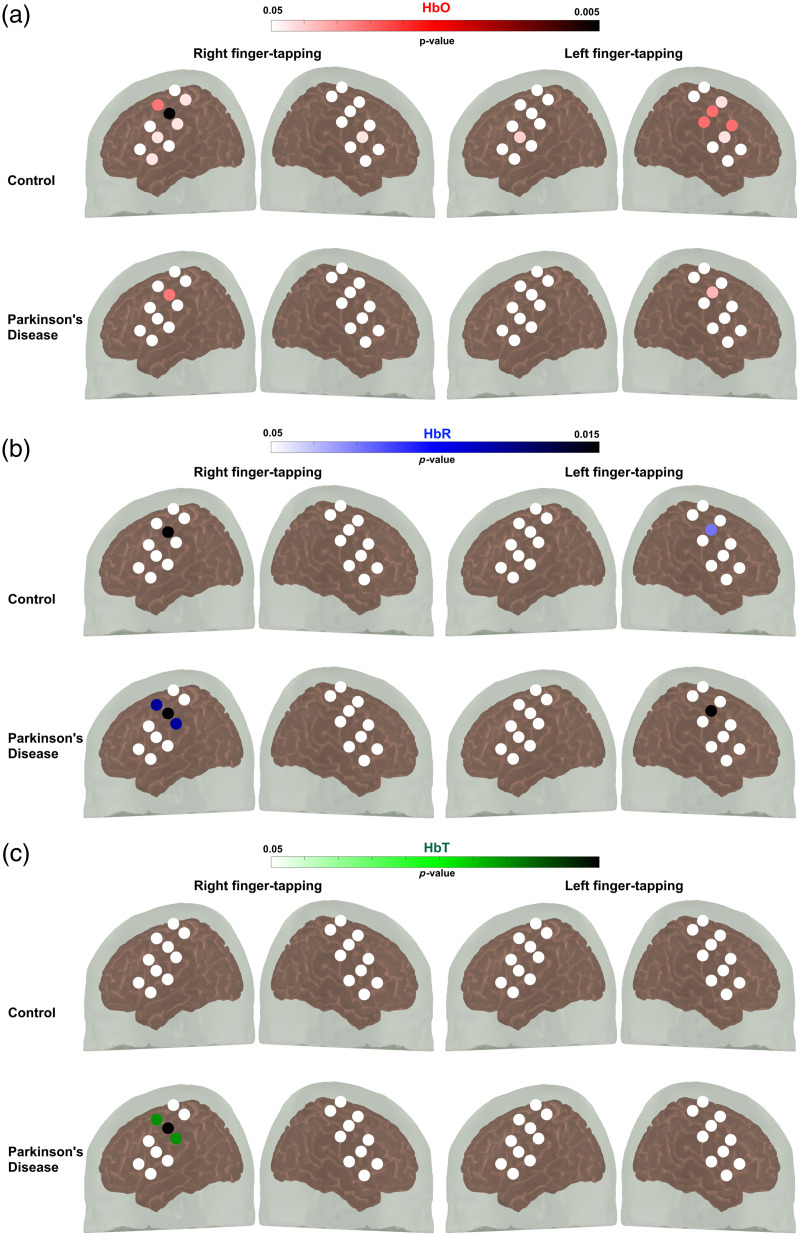
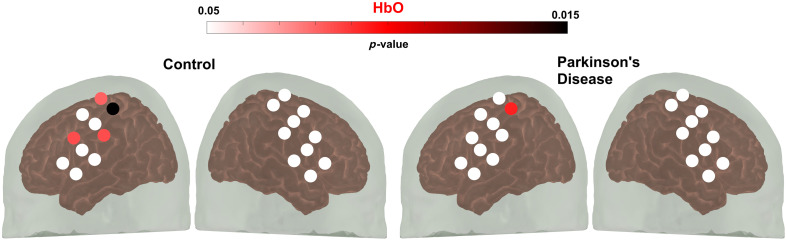
Channels that showed significant hemodynamic activation in the motor cortex evoked by finger tapping of both the dominant (right) and non-dominant hand (left) are shown in Fig. 3.
Fig. 3.
Paired sample -test was used to compare the mean change in hemoglobin during the pre-task baseline period and the task period to assess channel activation. The color gradient indicates the -value during the finger-tapping task. Channels in white demonstrate no statistically significant variations in hemoglobin levels between the pre-task baseline and task periods. (a) Oxyhemoglobin (HbO), (b) deoxyhemoglobin (HbR), and (c) total hemoglobin (HbT).
Five different features of the HRF were assessed for all hemoglobin types: peak value, time to peak (also known as peak latency), area under the HRF curve (AUC), mean value, and slope from stimulus onset to peak because studies have revealed that the peak latency and form, which vary across and within persons for each hemoglobin signal, task, and brain area, may be significant variables to research.53,54 For example, a larger time-to-peak in the HRF means it takes longer for the HbO concentration to peak following neuronal activation.
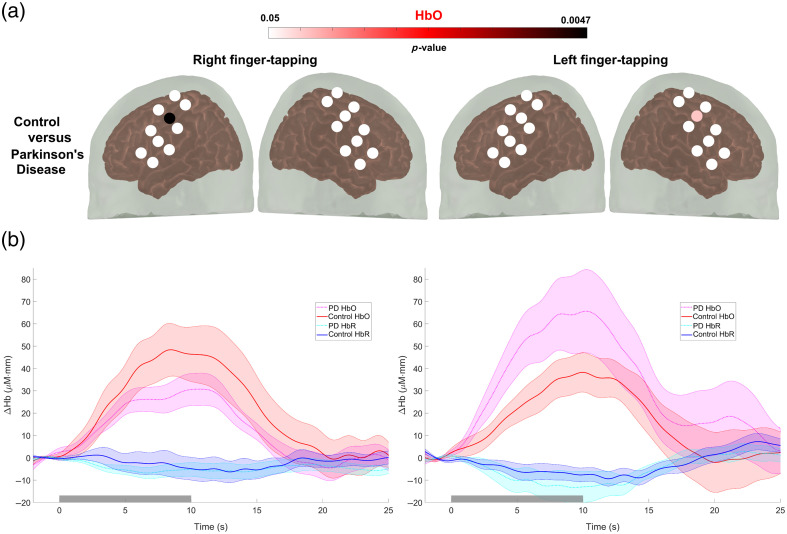
Channel-by-channel comparisons revealed significant peak latency differences between the PD and control groups in channel 15 for right finger tapping and channel 10 for left finger tapping, as shown in Fig. 4(a) (see Table S1 in the Supplementary Material for correlation values corrected for false positives). The results depicted in Fig. 4(b) indicate that patients with PD had delayed hypoactivation in the motor cortex during the fine motor task with the dominant hand and delayed hyperactivation with the non-dominant hand, as measured by near-infrared spectroscopy. This delayed activation may explain the difficulty of patients with PD to perform bimanual movements, as revealed by Wu et al.55 using fMRI. Furthermore, our findings are supported by previous studies that have found hypoactivation in the contralateral primary motor cortex elicited with a simple finger-tapping task in patients with a similar HY stage.56
Fig. 4.
Group comparisons. (a) A Wilcoxon–Mann–Whitney test with the FDR correction for false positives was used to detect group differences in mean oxyhemoglobin (HbO) changes during the finger-tapping task. The color gradient indicates a -value of group differences. White channels indicate no statistically significant differences between the two groups. No significant differences were found in either HbR or HbT. (b) Average waveforms at channel 15 (right finger tapping) and channel 10 (left finger tapping). The gray rectangle denotes stimulus onset and duration.
3.3. Correlation Analysis during Fine Motor Movement
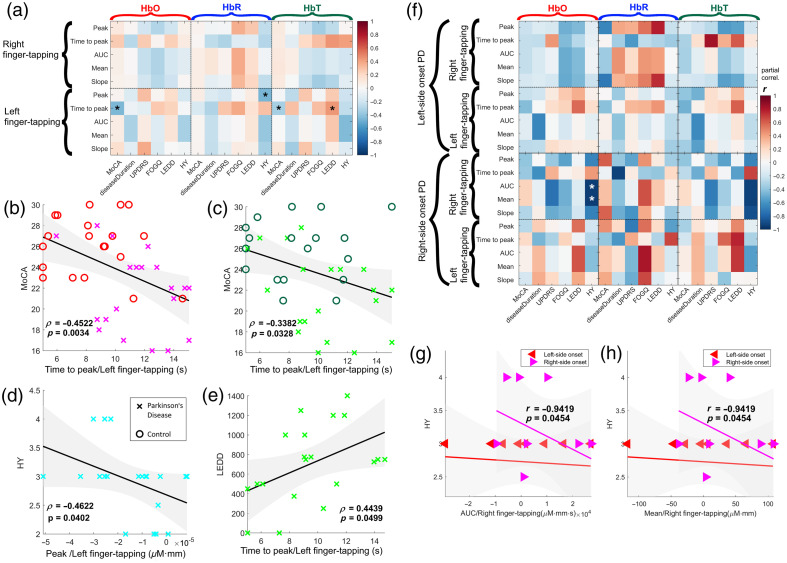
Our findings suggest significant correlations among various measures of hemodynamic activity in the motor cortex using fNIRS and different cognitive and clinical variables. After considering only channels with a significant difference between groups and correcting correlations for false positives, only the activity on channel 10, corresponding to left finger tapping, showed statistically significant correlations with clinical variables. A breakdown of each of the correlations is shown in Fig. 5 (see Table 2 for correlation values corrected for false positives).
Fig. 5.
Correlation analysis. (a) Correlation matrix between clinical variables and fNIRS-derived metrics. The asterisk sign (*) denotes a significant relationship (). (b) Correlation between MoCA score and time-to-peak during left finger tapping (HbO). (c) Correlation between MoCA scores and time-to-peak during right finger tapping (HbO). (d) Correlation between HY scale and peak amplitude of left finger tapping (HbR). (e) Correlation between l-dopa equivalent daily dose and time-to-peak during left finger tapping and (f) correlation between MoCA scores and time-to-peak during left finger tapping. Shaded areas denote 95% confidence intervals.
Table 2.
Correlation values between HRF features and clinical variables. Significant values after the FDR correction are displayed in bold.
| Spearman’s correlation | HbO |
HbR |
HbT |
||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MoCA | disease duration | UPDRS-3 | FOGQ | LEDD | HY | MoCA | disease duration | UPDRS-3 | FOGQ | LEDD | HY | MoCA | disease duration | UPDRS-3 | FOGQ | LEDD | HY | ||
| Right | Peak | 0.11 | 0.01 | -0.19 | -0.30 | -0.20 | 0.05 | 0.04 | -0.03 | -0.02 | 0.36 | 0.27 | -0.25 | 0.09 | -0.01 | -0.22 | -0.34 | -0.26 | 0.02 |
| Time to Peak | 0.26 | -0.28 | 0.26 | 0.03 | 0.24 | 0.09 | 0.01 | -0.25 | -0.31 | -0.13 | -0.12 | -0.29 | 0.08 | -0.11 | 0.21 | 0.30 | 0.39 | 0.41 | |
| AUC | 0.06 | 0.01 | -0.24 | -0.31 | -0.18 | -0.12 | 0.07 | 0.12 | 0.04 | 0.37 | 0.17 | -0.04 | 0.11 | 0.07 | -0.19 | -0.22 | -0.14 | -0.20 | |
| Mean | 0.06 | 0.01 | -0.24 | -0.31 | -0.18 | -0.12 | 0.07 | 0.12 | 0.04 | 0.37 | 0.17 | -0.04 | 0.11 | 0.07 | -0.19 | -0.22 | -0.14 | -0.20 | |
| Slope | 0.05 | 0.03 | -0.19 | -0.28 | -0.21 | 0.01 | 0.07 | -0.09 | -0.06 | 0.33 | 0.21 | -0.25 | 0.10 | -0.01 | 0.24 | -0.35 | -0.30 | -0.11 | |
| Left | Peak | 0.03 | -0.14 | 0.30 | 0.02 | 0.18 | -0.18 | 0.08 | -0.14 | -0.20 | -0.03 | 0.08 | -0.46 | -0.02 | -0.26 | 0.27 | -0.03 | 0.26 | -0.21 |
| Time to Peak | -0.45 | -0.07 | -0.10 | 0.26 | 0.22 | -0.02 | -0.16 | -0.30 | 0.28 | 0.38 | 0.14 | 0.30 | -0.34 | 0.29 | -0.11 | 0.30 | 0.44 | -0.25 | |
| AUC | 0.07 | -0.32 | 0.09 | -0.13 | 0.14 | -0.32 | 0.02 | -0.23 | -0.04 | 0.01 | 0.12 | -0.38 | 0.00 | -0.22 | 0.02 | -0.04 | 0.27 | -0.43 | |
| Mean | 0.07 | -0.32 | 0.09 | -0.13 | 0.14 | -0.32 | 0.02 | -0.23 | -0.04 | 0.01 | 0.12 | -0.38 | 0.00 | -0.22 | 0.02 | -0.04 | 0.27 | -0.43 | |
| Slope | 0.17 | -0.11 | -0.11 | -0.15 | 0.00 | -0.03 | 0.03 | -0.24 | -0.10 | 0.09 | 0.06 | -0.33 | 0.12 | -0.35 | 0.28 | -0.18 | 0.07 | -0.13 | |
The correlation between time to peak during left finger tapping of both HbO and HbT and MoCA is moderate (, ) and significant (, , respectively); this stronger negative relationship between the time it takes for HbT and HbO to peak during a motor task with the non-dominant hand and cognitive performance possibly indicates that cognitive processes require optimal oxygen and blood supply to the brain57 and impairments of cerebral oxygenation present in PD patients have an effect on cognitive performance.
The association between peak HRF during left finger tapping of HbR and HY scale is moderate () and significant (), indicating a negative relationship between the peak of HbR in the right hemisphere and the severity of PD symptoms. Previous works supported this finding; for instance, Junqué et al.58 pointed out that asymmetries have been reported in PD, with left-hemisphere functions relatively more preserved than right-hemisphere ones; this suggests that the right hemisphere may play an important role in PD symptoms. In addition, although not explicitly addressing PD, Goldstein et al.59 suggested that normal aging has a functionally asymmetric effect on the right hemisphere, which could potentially contribute to PD symptoms. However, further research is needed to confirm this potential negative relationship between the amplitude of deoxyhemoglobin in the right hemisphere and the severity of PD symptoms.
The correlation between time to peak during left finger tapping of HbT and levodopa equivalent daily dose is significantly moderate (, ), suggesting a significant positive relationship between the time it takes for total hemoglobin to peak in the right hemisphere and medicament dosage. This may be explained by the effect of levodopa absorption and delivery to the brain.60 No association was seen in disease duration, UPDRS score, or FOGQ.
The correlation between AUC during right finger tapping and the HY scale was found to be statistically significant: , , indicating a strong negative association between the two variables, as shown in Figs. 5(f)–5(g). The correlation coefficient of −0.9419 suggests that, after accounting for age and education, an increase in the HY scale is associated with a decrease in the AUC of the HRF in patients with right-side onset but not in patients with left-side onset. The same results were obtained with the mean value of the HRF during right finger tapping and the HY scale [Fig. 5(h)]. This supports a neural inefficiency hypothesis in which patients with more disability show a decrease in the activity evoked by finger tapping with the dominant hand. This result may be explained by the limited sample size and the fact that all patients with an HY of 4 are patients with right-side onset. Our results contrast with the findings of Zhu et al.,61 in which left-dominant symptom patients showed more severe gait impairments as well as more severe sleep-related dysfunction.
3.4. Motor Cortex Activation during Walking
There was a significant increase in activity in the primary motor cortex for the simple walking task compared with baseline in both the control group (four channels, , ) and the PD group (one channel, , ), as depicted in Fig. 6. All active channels were found in the left hemisphere. However, there were no significant differences between patients with PD and controls; this could be explained due to two findings. First, normal walking does not significantly increase blood oxygenation in the PFC,62 and although it is expected in the motor cortex, it was not observed in our data. We hypothesize this may be due to technical limitations of the fNIRS probe used, such as suboptimal optode separation. Second, many studies reported that increased cortical activity during walking has confounding effects due to motion artifacts.63 Further research is required to determine its causes.
Fig. 6.
Paired sample -test was used to compare the mean change in hemoglobin during the pre-task baseline period and the walking task period to assess channel activation. The color gradient indicates the -value during the walking task. Channels in white demonstrate no statistically significant variations in hemoglobin levels between the pre-task baseline and task periods.
3.5. FC Analysis during Walking
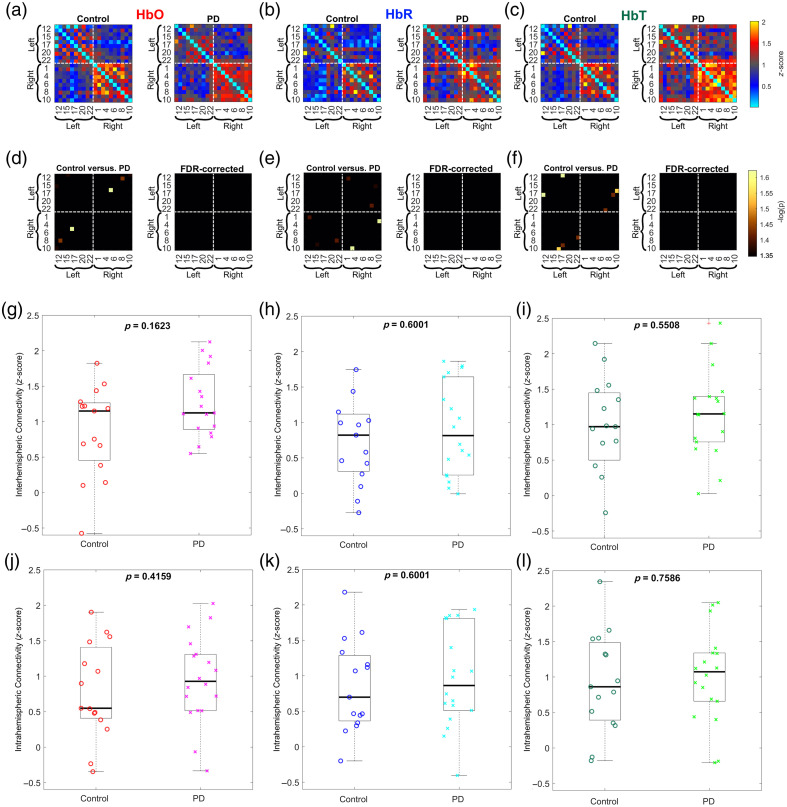
Several factors might explain the lack of significant differences in FC observed in the study comparing patients with PD and the control group during a walking task (Fig. 7). The inherent variability in task execution and the heterogeneity of the condition could result in increased variability in observed FC patterns, potentially masking any subtle differences.
Fig. 7.
FC during walking: (a)–(c) color-coded average FC matrices (Fisher’s -score) between channels for both groups calculated for HbO, HbR, and HbT, respectively. Warm colors denote positive correlations and cold colors denote negative correlations. (d)–(f) Statistical comparison between controls and PD patients. Only statistically significant correlations are shown (). After the FDR correction, no significant differences were found. (g)–(i) Group comparison of interhemispheric connectivity computed for HbO, HbR, and HbT, respectively. (j)–(l) Group comparison of intrahemispheric connectivity computed for HbO, HbR, and HbT, respectively.
3.6. RSFC Analysis
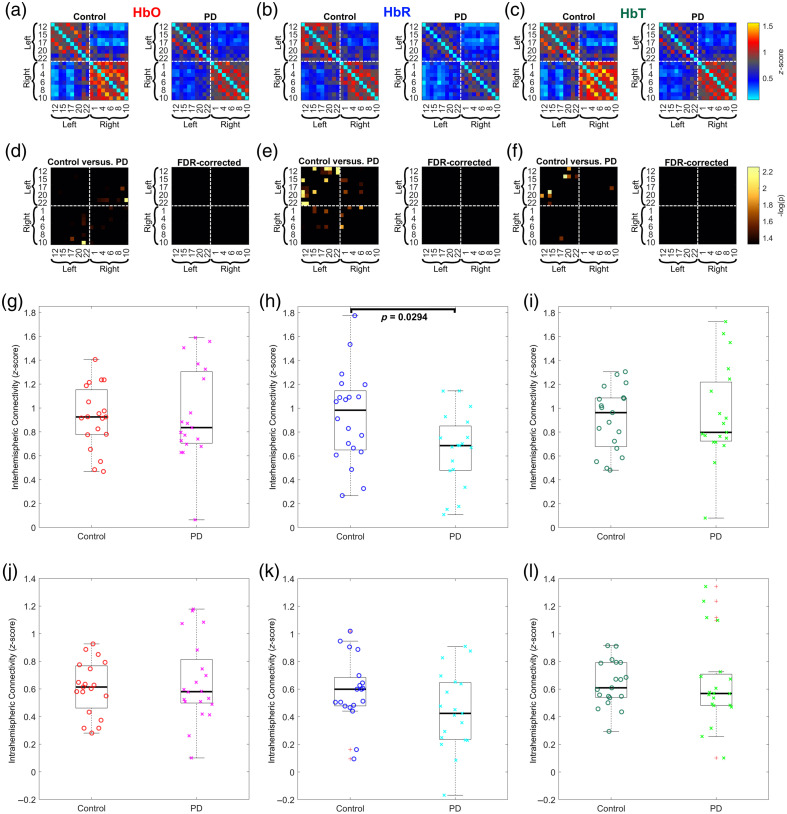
We hypothesized that there might be differences in RSFC between PD patients and control participants. The -score transformed correlation matrices are shown in Figs. 8(a)–8(c). Our analysis revealed significant differences in several channels, particularly in the HbR contrast, as depicted in Figs. 8(d)–8(f). In addition, a general trend of decreased positive connections in the PD group was observed. However, these results did not survive the FDR correction. This trend of decreased connectivity in PD patients confirms observations made in previous studies.64
Fig. 8.
FC: (a)–(c) Color-coded average FC matrices (Fisher’s -score) between channels for both groups calculated for HbO, HbR, and HbT, respectively. Warm colors denote positive correlations, and cold colors denote negative correlations. (d)–(f) Statistical comparison between controls and PD patients. Only statistically significant correlations are shown (). After the FDR correction, no significant differences were found. (g)–(i) Group comparison of interhemispheric connectivity computed for HbO, HbR, and HbT, respectively. A statistically significant decrease in interhemispheric connectivity in PD patients compared with controls was found in HbR measurements. (j)–(l) Group comparison of intrahemispheric connectivity computed for HbO, HbR, and HbT, respectively.
Our analysis revealed significant differences between the two groups regarding the strength of interhemispheric connectivity, as shown in Figs. 8(g)–8(i). More specifically, a statistically significant decrease in interhemispheric connectivity in PD patients compared with controls was found in HbR measurements [see Fig. 8(h)]. The existing literature studying brain networks has associated a decreased interhemispheric FC with symptoms of PD. Gan et al.65,66 found that PD patients with impulse control disorders had abnormal interhemispheric RSFC, and those patients with levodopa-induced dyskinesia had altered interhemispheric connectivity. The study found that PD patients with freezing of gait had decreased interhemispheric connectivity in certain brain regions compared with PD patients without freezing of gait and healthy controls.67 These findings imply the possibility of using decreased interhemispheric FC as one of many potential neuroimaging biomarkers for diagnosing PD, albeit it is shared by other neurodegenerative conditions such as Alzheimer’s disease.68
Our intrahemispheric connectivity analysis revealed trends between the groups, but these results did not pass the FDR threshold. Specifically, control participants showed stronger positive connections within a hemisphere than PD patients (see Figs. 8(j)–8(l)].
4. Conclusion
During fine motor movement, patients with PD had delayed hypoactivation in the motor cortex with the dominant hand and delayed hyperactivation with the non-dominant hand. This delayed activation may explain the difficulty that patients with PD have performing fine motor movements and may be interpreted in line with the neural inefficiency hypothesis, as the delayed and abnormal activation patterns indicate inefficient neural processing during motor tasks.
A significantly higher cognitive dysfunction in PD patients could further complicate these findings. The cognitive dysfunction noted in PD patients compared with control participants could exacerbate the inefficiencies in neural processing, impacting both motor and cognitive functions.
The significant correlations between measures of hemodynamic activity in the right motor cortex during left finger tapping and cognitive or clinical variables underline the intertwined nature of motor and cognitive impairments in PD, suggesting that any compensatory mechanisms may be limited by concurrent cognitive decline.
Although there were significantly active channels in the left motor cortex for the walking task in both the control and PD groups, there were no significant differences between patients with PD and controls. Our findings suggest that interhemispheric FC is crucial for motor function and that motor impairments are reflected as disruptions in this connectivity. Therefore, both activation in the primary motor cortex elicited by a fine motor task and interhemispheric FC may be proposed as neuroimaging biomarkers for assessing PD. However, caution should be exercised in its interpretation because decreased interhemispheric connectivity is also present in other neurodegenerative diseases, such as Alzheimer’s disease.
The study faced several limitations that may impact the generalizability and depth of the findings. First, the technical constraints of the fNIRS probe, such as suboptimal optode separation, could limit the accuracy of cortical activation measurements, particularly during complex tasks such as walking. Second, the omission of turn registration in the walking task might have overlooked significant aspects of motor function in PD. Finally, the relatively limited sample size encourages further research with enhanced methodologies and larger cohorts to validate these findings comprehensively.
Further research is needed to confirm these results and explore its potential in studying PD.
Supplementary Material
Acknowledgments
This work was supported in part (E. G.) by the “IxM CONAHCYT” program, project 528, and “Frontier Science” project 20884. We would like to thank Katia Moreno and Arturo González for their assistance in some of the acquisitions and Michelle Goodrick for editing and reviewing the English language version of this paper. We sincerely appreciate the time and effort from the editor and reviewers who provided valuable comments and insights.
Biographies
Edgar Guevara is a CONAHCYT research fellow at UASLP-CIACYT. He received his PhD in biomedical engineering from École Polytechnique de Montréal. He is a National System of Researchers level 2 member and has received numerous competitive grants and scholarships. His research interests include non-invasive medical diagnosis using optical imaging, functional connectivity, spectroscopy, and biomedical signal processing. He is a member of SPIE and the Society for Functional Near-infrared Spectroscopy (SfNIRS).
Francisco Javier Rivas-Ruvalcaba is a movement disorder neurologist in Guadalajara, México. He is a member of the Movement Disorder Society (MDS) and the Mexican Society for the Study of Movement Disorders (SOMMA). He completed the neurology course at the San Luis Potosi Autonomous University (UASLP) and then made a fellowship in movement disorders at the Hospital Civil de Guadalajara Fray Antonio Alcalde.
Eleazar Samuel Kolosovas-Machuca received his electronic engineering degree, his MS, and this PhD in applied sciences from the Autonomous University of San Luis Potosí (UASLP), Mexico, in 2006, 2009, and 2014, respectively. Since 2017, he has been a full professor at the UASLP. He is involved in researching infrared thermography and biosensors for medical applications. He is the author or co-author of more than 40 scientific articles.
Miguel Ramírez-Elías is a full professor at the School of Sciences of the Universidad Autónoma de San Luis Potosí (México). He received his PhD in applied sciences from the Universidad Autónoma de San Luis Potosí. His research is focused on biophotonics and data science for medical applications. He has been the project leader for several research projects and a reviewer for international grant organizations. He is a member of the National System of Researchers and SPIE.
Ramón Díaz de León Zapata obtained his degree in electronics engineering, his MSc in computer science from the Technological Institute of SLP where he is a professor-researcher, and his doctorate in applied sciences from the Autonomous University of SLP. He has been recognized as a National Researcher level 1 by the National Council of Humanities Science and Technology (CONAHCyT, Mexico), His recent research topics include electronic instrumentation and computer simulation.
Jose Luis Ramirez-GarciaLuna is a clinician -scientist aiming to revolutionize healthcare by implementing innovative research strategies, with over 10 years of experience as an emergency doctor and wound care expert. He has proven leadership and collaboration in multicultural and multidisciplinary research teams in Canada, the United States, Latin America, and Europe. He has experience in bridging the gap between fundamental and clinical research, as well as among research and innovation. He also has ample experience in the communication of research results to scientific and lay audiences, in addition to the media. He has proven learning agility by conducting cutting-edge projects in multiple fields, including surgery, tissue engineering, biomaterials, immunology, and artificial intelligence.
Ildefonso Rodríguez-Leyva, MD, PhD, FAAN, FAES, FANA, is a full professor of neurology at the School of Medicine of the UASLP. He is a member of the National System of Researchers of Mexico, a former president of the Council of Neurology of Mexico, AMCEMIG (Mexican Association for the Study of Headache), and an editor-in-chief of the Revista Mexicana de Neurociencia. His research areas include multiple fields of Neurology, with multiple publications. He is a fellow of the AAN, AES, and ANA and an active member of multiple neurological societies.
Contributor Information
Edgar Guevara, Email: edgar.guevara@uaslp.mx.
Francisco Javier Rivas-Ruvalcaba, Email: franrivasr07@gmail.com.
Eleazar Samuel Kolosovas-Machuca, Email: samuel.kolosovas@uaslp.mx.
Miguel Ramírez-Elías, Email: miguel.fciencias@gmail.com.
Ramón Díaz de León Zapata, Email: ramon.dd@slp.tecnm.mx.
Jose Luis Ramirez-GarciaLuna, Email: ramirezgl@gmail.com.
Ildefonso Rodríguez-Leyva, Email: ildefonso.rodriguez@uaslp.mx.
Disclosures
The authors declare no financial or commercial conflicts of interest.
Code and Data Availability
The data supporting this study’s findings are available in Zenodo at https://zenodo.org/record/7966830, Ref. 69.
References
- 1.Kumar D. K., “Parkinson’s disease and its symptoms,” in Techniques for Assessment of Parkinsonism for Diagnosis and Rehabilitation, Arjunan S. P., Kumar D. K., Eds., pp. 25–30, Springer, Singapore: (2022). [Google Scholar]
- 2.Armstrong M. J., Okun M. S., “Diagnosis and treatment of Parkinson disease: a review,” JAMA 323(6), 548–560 (2020). 10.1001/jama.2019.22360 [DOI] [PubMed] [Google Scholar]
- 3.Goetz C. G., et al. , “Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): process, format, and clinimetric testing plan,” Mov. Disord. Off. J. Mov. Disord. Soc. 22(1), 41–47 (2007). 10.1002/mds.21198 [DOI] [PubMed] [Google Scholar]
- 4.Mitchell T., et al. , “Emerging neuroimaging biomarkers across disease stage in Parkinson disease: a review,” JAMA Neurol. 78(10), 1262–1272 (2021). 10.1001/jamaneurol.2021.1312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ayaz H., et al. , “Optical imaging and spectroscopy for the study of the human brain: status report,” Neurophotonics 9(S2), S24001 (2022). 10.1117/1.NPh.9.S2.S24001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Devezas M. Â. M., “Shedding light on neuroscience: two decades of functional near-infrared spectroscopy applications and advances from a bibliometric perspective,” J. Neuroimaging 31(4), 641–655 (2021). 10.1111/jon.12877 [DOI] [PubMed] [Google Scholar]
- 7.Gao Y., et al. , “Deep learning-based motion artifact removal in functional near-infrared spectroscopy,” Neurophotonics 9(4), 041406 (2022). 10.1117/1.NPh.9.4.041406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li R., et al. , “Current opinions on the present and future use of functional near-infrared spectroscopy in psychiatry,” Neurophotonics 10(1), 013505 (2023). 10.1117/1.NPh.10.1.013505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Faria D. D., et al. , “Task-related brain activity and functional connectivity in upper limb dystonia: a functional magnetic resonance imaging (fMRI) and functional near-infrared spectroscopy (fNIRS) study,” Neurophotonics 7(4), 045004 (2020). 10.1117/1.NPh.7.4.045004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hill A. P., Bohil C. J., “Applications of optical neuroimaging in usability research,” Ergon. Des. 24(2), 4–9 (2016). 10.1177/1064804616629309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nieuwhof F., et al. , “Measuring prefrontal cortical activity during dual task walking in patients with Parkinson’s disease: feasibility of using a new portable fNIRS device,” Pilot Feasibility Stud. 2(1), 59 (2016). 10.1186/s40814-016-0099-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pelicioni P. H. S., et al. , “People with Parkinson’s disease exhibit reduced cognitive and motor cortical activity when undertaking complex stepping tasks requiring inhibitory control,” Neurorehabil. Neural Repair 34(12), 1088–1098 (2020). 10.1177/1545968320969943 [DOI] [PubMed] [Google Scholar]
- 13.Morishita T., et al. , “Changes in motor-related cortical activity following deep brain stimulation for Parkinson’s disease detected by functional near infrared spectroscopy: a pilot study,” Front. Hum. Neurosci. 10, 629 (2016). 10.3389/fnhum.2016.00629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonilauri A., et al. , “Whole-head functional near-infrared spectroscopy as an ecological monitoring tool for assessing cortical activity in Parkinson’s disease patients at different stages,” Int. J. Mol. Sci. 23(23), 14897 (2022). 10.3390/ijms232314897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson M. W., Mak M., “Modulating cortical hemodynamic activity in Parkinson’s disease using focal transcranial direct current stimulation: a pilot functional near-infrared spectroscopy study,” Brain Topogr. 36(6), 926–935 (2023). 10.1007/s10548-023-01002-6 [DOI] [PubMed] [Google Scholar]
- 16.Dahdal P., et al. , “Fine motor function skills in patients with Parkinson disease with and without mild cognitive impairment,” Dement. Geriatr. Cogn. Disord. 42(3–4), 127–134 (2016). 10.1159/000448751 [DOI] [PubMed] [Google Scholar]
- 17.Otte K., et al. , “Instrumental assessment of stepping in place captures clinically relevant motor symptoms of Parkinson’s disease,” Sensors 20(19), 5465 (2020). 10.3390/s20195465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Devignes Q., et al. , “Resting-state functional connectivity in frontostriatal and posterior cortical subtypes in Parkinson’s disease-mild cognitive impairment,” Mov. Disord. Off. J. Mov. Disord. Soc. 37(3), 502–512 (2022). 10.1002/mds.28888 [DOI] [PubMed] [Google Scholar]
- 19.Veréb D., et al. , “Modulation of cortical resting state functional connectivity during a visuospatial attention task in Parkinson’s disease,” Front. Neurol. 13, 927481 (2022). 10.3389/fneur.2022.927481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes A. J., et al. , “Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases,” J. Neurol. Neurosurg. Psychiatry 55(3), 181–184 (1992). 10.1136/jnnp.55.3.181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tomlinson C. L., et al. , “Systematic review of levodopa dose equivalency reporting in Parkinson’s disease,” Mov. Disord. Off. J. Mov. Disord. Soc. 25(15), 2649–2653 (2010). 10.1002/mds.23429 [DOI] [PubMed] [Google Scholar]
- 22.Schiess M., et al. , “Parkinson’s disease subtypes: clinical classification and ventricular cerebrospinal fluid analysis,” Parkinsonism Relat. Disord. 6(2), 69–76 (2000). 10.1016/S1353-8020(99)00051-6 [DOI] [PubMed] [Google Scholar]
- 23.Hoehn M., Yahr M., “Parkinsonism: onset, progression, and mortality,” Neurology 17(5), 442–427 (1967). 10.1212/WNL.17.5.427 [DOI] [PubMed] [Google Scholar]
- 24.Taghizadeh G., et al. , “Clinimetrics of the freezing of gait questionnaire for Parkinson disease during the ‘off’ state,” Basic Clin. Neurosci. 12(1), 69–78 (2021). 10.32598/bcn.12.6.882.11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nasreddine Z. S., et al. , “The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment,” J. Am. Geriatr. Soc. 53(4), 695–699 (2005). 10.1111/j.1532-5415.2005.53221.x [DOI] [PubMed] [Google Scholar]
- 26.Brigadoi S., Cooper R. J., “How short is short? Optimum source–detector distance for short-separation channels in functional near-infrared spectroscopy,” Neurophotonics 2(2), 025005 (2015). 10.1117/1.NPh.2.2.025005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tachtsidis I., Scholkmann F., “False positives and false negatives in functional near-infrared spectroscopy: issues, challenges, and the way forward,” Neurophotonics 3(3), 031405 (2016). 10.1117/1.NPh.3.3.031405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yeung M. K., “An optical window into brain function in children and adolescents: a systematic review of functional near-infrared spectroscopy studies,” NeuroImage 227, 117672 (2021). 10.1016/j.neuroimage.2020.117672 [DOI] [PubMed] [Google Scholar]
- 29.Lloyd-Fox S., Blasi A., Elwell C. E., “Illuminating the developing brain: the past, present and future of functional near infrared spectroscopy,” Neurosci. Biobehav. Rev. 34(3), 269–284 (2010). 10.1016/j.neubiorev.2009.07.008 [DOI] [PubMed] [Google Scholar]
- 30.Huppert T. J., et al. , “HomER: a review of time-series analysis methods for near-infrared spectroscopy of the brain,” Appl. Opt. 48(10), D280–D298 (2009). 10.1364/AO.48.00D280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scholkmann F., et al. , “How to detect and reduce movement artifacts in near-infrared imaging using moving standard deviation and spline interpolation,” Physiol. Meas. 31(5), 649–662 (2010). 10.1088/0967-3334/31/5/004 [DOI] [PubMed] [Google Scholar]
- 32.Selb J. J., et al. , “Effect of motion artifacts and their correction on near-infrared spectroscopy oscillation data: a study in healthy subjects and stroke patients,” J. Biomed. Opt. 20(5), 056011 (2015). 10.1117/1.JBO.20.5.056011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Novi S. L., et al. , “Functional near-infrared spectroscopy for speech protocols: characterization of motion artifacts and guidelines for improving data analysis,” Neurophotonics 7(1), 015001 (2020). 10.1117/1.NPh.7.1.015001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Di Rosa E., et al. , “Reward motivation and neurostimulation interact to improve working memory performance in healthy older adults: a simultaneous tDCS-fNIRS study,” NeuroImage 202, 116062 (2019). 10.1016/j.neuroimage.2019.116062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Delpy D. T., et al. , “Estimation of optical pathlength through tissue from direct time of flight measurement,” Phys. Med. Biol. 33(12), 1433–1442 (1988). 10.1088/0031-9155/33/12/008 [DOI] [PubMed] [Google Scholar]
- 36.Ye J. C., et al. , “NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy,” NeuroImage 44(2), 428–447 (2009). 10.1016/j.neuroimage.2008.08.036 [DOI] [PubMed] [Google Scholar]
- 37.Saager R. B., Berger A. J., “Direct characterization and removal of interfering absorption trends in two-layer turbid media,” J. Opt. Soc. Am. A Opt. Image Sci. Vis. 22(9), 1874–1882 (2005). 10.1364/JOSAA.22.001874 [DOI] [PubMed] [Google Scholar]
- 38.Gagnon L., et al. , “Short separation channel location impacts the performance of short channel regression in NIRS,” NeuroImage 59(3), 2518–2528 (2012). 10.1016/j.neuroimage.2011.08.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stuart S., et al. , “Pre-frontal cortical activity during walking and turning is reliable and differentiates across young, older adults and people with Parkinson’s disease,” Front. Neurol. 10, 536 (2019). 10.3389/fneur.2019.00536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.von Lühmann A., et al. , “Using the general linear model to improve performance in fNIRS single trial analysis and classification: a perspective,” Front. Hum. Neurosci. 14, 30 (2020). 10.3389/fnhum.2020.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mesquita R. C., Franceschini M. A., Boas D. A., “Resting state functional connectivity of the whole head with near-infrared spectroscopy,” Biomed. Opt. Express 1(1), 324–336 (2010). 10.1364/BOE.1.000324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang T., et al. , “Altered complexity in resting-state fNIRS signal in autism: a multiscale entropy approach,” Physiol. Meas. 42(8), 085004 (2021). 10.1088/1361-6579/ac184d [DOI] [PubMed] [Google Scholar]
- 43.Bruyn N. D., et al. , “Functional network connectivity is altered in patients with upper limb somatosensory impairments in the acute phase post stroke: a cross-sectional study,” PLoS ONE 13(10), e0205693 (2018). 10.1371/journal.pone.0205693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Husain S. F., et al. , “Cortical haemodynamic response measured by functional near infrared spectroscopy during a verbal fluency task in patients with major depression and borderline personality disorder,” eBioMedicine 51, 102586 (2020). 10.1016/j.ebiom.2019.11.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Razali N. M., Wah Y. B., “Power comparisons of Shapiro-Wilk, Kolmogorov-Smirnov, Lilliefors and Anderson-darling tests,” J. Stat. Model. Anal. 2(1), 21–33 (2011). [Google Scholar]
- 46.Benjamini Y., Hochberg Y., “Controlling the false discovery rate: a practical and powerful approach to multiple testing,” J. R. Stat. Soc. Ser. B Methodol. 57(1), 289–300 (1995). 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 47.Hayasaka S., “Functional connectivity networks with and without global signal correction,” Front. Hum. Neurosci. 7, 880 (2013). 10.3389/fnhum.2013.00880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abdalmalak A., et al. , “Effects of systemic physiology on mapping resting-state networks using functional near-infrared spectroscopy,” Front. Neurosci. 16, 803297 (2022). 10.3389/fnins.2022.803297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bacia T., “Problems with electrical signs of epileptic focal discharges recorded from the scalp,” Acta Neurol. Scand. 89(S152), 9–16 (1994). 10.1111/j.1600-0404.1994.tb05177.x [DOI] [PubMed] [Google Scholar]
- 50.Firbank M. J., et al. , “Longitudinal change in 99mTcHMPAO cerebral perfusion SPECT in Parkinson’s disease over one year,” J. Neurol. Neurosurg. Psychiatry 76(10), 1448–1451 (2005). 10.1136/jnnp.2004.058685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Menéndez-González M., et al. , “Frontotemporal lobe degeneration as origin of scans without evidence of dopaminergic deficit,” Front. Neurol. 9, 335 (2018). 10.3389/fneur.2018.00335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aarsland D., et al. , “Parkinson disease-associated cognitive impairment,” Nat. Rev. Dis. Primer 7(1), 1–21 (2021). 10.1038/s41572-021-00280-3 [DOI] [PubMed] [Google Scholar]
- 53.Hocke L. M., et al. , “Automated processing of fNIRS data—a visual guide to the pitfalls and consequences,” Algorithms 11(5), 67 (2018). 10.3390/a11050067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Uga M., et al. , “Optimizing the general linear model for functional near-infrared spectroscopy: an adaptive hemodynamic response function approach,” Neurophotonics 1(1), 015004 (2014). 10.1117/1.NPh.1.1.015004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wu T., et al. , “Neural correlates of bimanual anti-phase and in-phase movements in Parkinson’s disease,” Brain 133(8), 2394–2409 (2010). 10.1093/brain/awq151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Buhmann C., et al. , “Pharmacologically modulated fMRI—cortical responsiveness to levodopa in drug‐naive hemiparkinsonian patients,” Brain 126(2), 451–461 (2003). 10.1093/brain/awg033 [DOI] [PubMed] [Google Scholar]
- 57.Herold F., et al. , “Functional near-infrared spectroscopy in movement science: a systematic review on cortical activity in postural and walking tasks,” Neurophotonics 4(4), 041403 (2017). 10.1117/1.NPh.4.4.041403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Junqué C., Roig C., Litvan I., “Unilateral left cerebral deterioration documented by CT, MRI, and neuropsychological studies: a possible case of pick’s disease,” Dev. Neuropsychol. 4(4), 295–302 (1988). 10.1080/87565648809540413 [DOI] [Google Scholar]
- 59.Goldstein G., Shelly C., “Does the right hemisphere age more rapidly than the left?” J. Clin. Neuropsychol. 3(1), 65–78 (1981). 10.1080/01688638108403114 [DOI] [PubMed] [Google Scholar]
- 60.Stocchi F., et al. , “Clinical efficacy of a single afternoon dose of effervescent levodopa-carbidopa preparation (CHF 1512) in fluctuating Parkinson disease,” Clin. Neuropharmacol. 30(1), 18 (2007). 10.1097/01.WNF.0000236762.77913.C6 [DOI] [PubMed] [Google Scholar]
- 61.Zhu S., et al. , “The association between clinical characteristics and motor symptom laterality in patients with Parkinson’s disease,” Front. Neurol. 12, 663232 (2021). 10.3389/fneur.2021.663232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mirelman A., et al. , “Increased frontal brain activation during walking while dual tasking: an fNIRS study in healthy young adults,” J. NeuroEng. Rehabil. 11(1), 85 (2014). 10.1186/1743-0003-11-85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vitorio R., et al. , “fNIRS response during walking—artefact or cortical activity? A systematic review,” Neurosci. Biobehav. Rev. 83, 160–172 (2017). 10.1016/j.neubiorev.2017.10.002 [DOI] [PubMed] [Google Scholar]
- 64.Kajiyama Y., et al. , “Decreased frontotemporal connectivity in patients with Parkinson’s disease experiencing face pareidolia,” NPJ Park. Dis. 7(1), 1–9 (2021). 10.1038/s41531-021-00237-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gan C., et al. , “Abnormal interhemispheric resting state functional connectivity in Parkinson’s disease patients with impulse control disorders,” NPJ Parkinsons Dis. 7(1), 1–7 (2021). 10.1038/s41531-021-00205-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gan C., et al. , “Altered interhemispheric synchrony in Parkinson’s disease patients with levodopa-induced dyskinesias,” NPJ Parkinsons Dis. 6(1), 1–7 (2020). 10.1038/s41531-020-0116-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jin C., et al. , “Integrating structural and functional interhemispheric brain connectivity of gait freezing in Parkinson’s disease,” Front. Neurol. 12, 609866 (2021). 10.3389/fneur.2021.609866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li K.-C., et al. , “Distinct patterns of interhemispheric connectivity in patients with early- and late-onset Alzheimer’s disease,” Front. Aging Neurosci. 10, 261 (2018). 10.3389/fnagi.2018.00261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Guevara E., et al. , “Functional near-infrared spectroscopy reveals delayed hemodynamic changes in the primary motor cortex during fine motor tasks and decreased interhemispheric connectivity in Parkinson’s disease patients,” Zenodo (2023). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data supporting this study’s findings are available in Zenodo at https://zenodo.org/record/7966830, Ref. 69.