Abstract
Introduction
The introduction of Janus Kinase inhibitors (JAKi) seems to revolutionize the field of alopecia areata (AA) therapeutics. However, real-world data are still missing.
Objectives
To provide evidence about effectiveness and safety of tofacitinib and baricitinib in AA in real-world settings and describe baseline disease characteristics and patients profiles that are considered good candidates for JAKi in the daily practice. Furthermore, we intended to investigate potential correlations between baseline characteristics and treatment outcomes.
Methods
We retrospectively reviewed the databases of two tertiary Hospitals in Greece, to identify individuals of any age currently being treated with systemic JAKi for severe AA.
Results
We identified 42 individuals, including 3 adolescents. In our cohort, 52.3% (22/42) were under tofacitinib and 47.6% (20/42) under baricitinib treatment. Efficacy analysis was performed on the subgroup of 30 patients that had completed at least a 3-month follow-up on treatment. In the latter group, mean time on treatment was 10 months. Mean Severity of Alopecia Tool and mean Dermatology Life Quality Index scores decreased from 84.46% and 12.86 at baseline, to 43.26% and 6.63, respectively. Complete response (CR) was recorded in 4 (13.33%), partial in 12 (40%) and no response in 14 patients (46.66%), correspondingly. Seventeen out of 42 (40.5%) individuals in total, reported at least 1 adverse event. No patient required hospitalization. Among 15 patients (35.7%) who got COVID-19, one suffered from serious infection. The 3 adolescents achieved CR with no significant adverse events.
Conclusions
Real-world data suggest efficacy and safety of JAKi in severe forms of AA. Tolerability is optimal in younger individuals.
Keywords: alopecia areata, janus kinase inhibitors, tofacitinib, baricitinib, real-world
Introduction
Alopecia areata (AA) is a non-scarring alopecia that greatly affects patients quality of life. Disease severity depends on the extend of the body area involvement, ranging from mild severity in localized forms (plaque type AA) to severe generalized clinical types of AA, such as alopecia totalis (AT) or alopecia universalis (AU). Topical and intralesional steroids are considered first-line therapeutic modalities for localized plaque type AA, while systemic use of steroids and other immunomodulating agents are preserved for generalized forms of the disease. Despite their beneficial effect on AA, conventional treatments have been linked to important side-effects and high rates of recurrence after treatment cessation that limit their use.
After many years of scant or no development in the area of AA therapeutics, the introduction of Janus Kinase inhibitors (JAKi) seems to revolutionize the field, providing promising treatment outcomes in severe forms of the disease [1]. The mode of action of JAKi implicates intracellular interruption of the JAK-STAT pathway. Tofacitinib is a potent, selective inhibitor of the JAK family (JAK 1/3), approved for the treatment of psoriatic arthritis, rheumatoid arthritis, ankylosing spondylitis, ulcerative colitis in adults and juvenile idiopathic arthritis in patients ≥ 2 years of age. Tofacitinib, though not officially indicated, was the first drug of the group of JAKi used in AA. Recently, FDA approved baricitinib for the treatment of severe AA. Baricitinib exerts its effects by inhibiting JAK1 and JAK2 enzymes.
Data emerging from real-world settings in regards with the use of JAKi in AA are limited [2–5].
Objectives
In the current study, we aimed to provide evidence about effectiveness and safety of the use of tofacitinib and baricitinib for AA in real-world settings and describe baseline disease characteristics and patients profiles that are considered good candidates for JAKi in the daily practice. Furthermore, we intended to investigate potential correlations between baseline characteristics and treatment outcomes.
Methods
Towards the aims of the study, we retrospectively reviewed the databases of two tertiary Hospitals in Greece for the last five years, to identify individuals of any age currently being treated with systemic JAKi for severe AA. The standard of care for AA in both centers conforms to the published guidelines and depends on the clinical type, disease severity, age of the patient and response to previous treatments. The study received Institutional Ethical Committee approval.
Upon their first visit, all patients provide written informed consent for collecting their data, and history and physical and dermatoscopic examination is carried out. Disease severity is determined by the use of the Severity of Alopecia Tool (SALT) that estimates the extent of alopecia in percentages. ΔSALT score was used as a variable in order to assess the percentage change observed in SALT score at baseline and at the most recent follow up visit for each patient. Treatment response was classified as no response (NR) with <30% of regrowth, partial response (PR) with 30%–90% of regrowth and complete response (CR) with >90% of regrowth. Patients were also asked to fill in a Dermatology Life Quality Index (DLQI) questionnaire in Greek. Clinical and dermatoscopic photos were collected and saved in the databases. Efficacy outcomes were obtained from individuals that had completed at least a 3-month follow-up period on treatment, whilst safety outcomes emerged from the whole studied group. According to the regulations in Greece, before prescribing JAKi to our patients for any indication, we have to ask for approval by the National Medical Organization. Spearman correlation and Kruskal-Wallis tests were used to compare the variables using IBM SPSS Statistics version 29.0. Statistical analyses were performed using IBM SPSS Statistics version 29.0.
Results
Demographics and Baseline Clinical Characteristics
We identified 42 individuals (25 females and 17 males), including 3 adolescents. The mean age was 39.5 (SD: 14.4, range: 13–60 years). All participants suffered from severe AA (mean baseline SALT score 80.21% [SD 24.09%]). Patients sociodemographic and clinical characteristics are summarized in Table 1.
Table 1.
Epidemiologic and clinical characteristics of the patients included in the study
| Variables | All patients (N = 42) | |
|---|---|---|
| Socio-demographic features | ||
| Age, years, mean (SD) | 39.52 (14.48) | |
| Time since first onset, years mean (SD) | 8.28 (10.73) | |
| Sex Male, % (N) | 40.47% (17/42) | |
| Sex Female, % (N) | 59.52% (25/42) | |
| Time since last episode of AA, years, mean (SD) | 3.32 (4.16) | |
| Previous treatments, % (N) | ||
| topical steroids | 76% (32/42) | |
| intralesional injections | 45.2% (19/42) | |
| topical calcineurin inhibitors | 14.2% (6/42) | |
| DPCP | 14.2% (6/42) | |
| Anthralin | 9.5% (4/42) | |
| systemic cortisone | 71% (30/42) | |
| cyclosporine | 52% (22/42) | |
| JAKi | 4.7% (2/42) | |
| PRP | 9.5% (4/42) | |
| hydroxychloroquine | 4.7% (2/42) | |
| Comorbidities, % (N) | ||
| thyroid disease | 38% (16/42) | |
| myasthenia gravis | 4.7% (2/42) | |
| diabetes mellitus | 4.7% (2/42) | |
| vitiligo | 2% (1/42) | |
| hypertension | 4.7% (2/42) | |
| asthma | 2% (1/42) | |
| rheumatoid arthritis | 2% (1/42) | |
| Severity of the disease | ||
| Basal SALT score mean (SD) | 80.21 (24.09) | |
| Current SALT score mean (SD) | 43.27 (39.44) | |
| Treatment characteristics | ||
| Baricitinib | Tofacitinib | |
| Mean treatment time (months) | 2.7 | 11.7 |
| Maintenance dose (mg/day) | 4mg/day | 10mg/day (17/22) |
| 20mg/day (5/22) | ||
Jaki=Janus kinase inhibitor; PRP=Platelet Rich Plasma; SALT = Severity of Alopecia Tool; SD=Standard deviation;
The mean time since AA first onset and last AA episode were 8.28 and 3.32 years, correspondingly. Mean baseline SALT score was 80.21% (SD: 24.09%) and the mean baseline DLQI score was 12.59 (SD: 3.96). Statistically significant difference was recorded in mean baseline DLQI score between men (11.15) and women (14.17, P = 0.04), whilst no statistically significant difference was observed in the corresponding mean SALT scores (mean baseline SALT for men was 78.38% and 89.11% for women, P = 0.161). The latter observation indicates that AA has a greater impact on females as compared to males.
The most frequent clinical type of alopecia areata in this cohort was universalis (40.4%), followed by plaque type AA covering 25–75% of the scalp (16.6%) and totalis seen in 14.2%. Family history of AA was reported by 26.1% (11/42) of the patients, while 52.3% (22/42) could not recall other first- or second-degree relatives suffering from AA. From the recorded comorbidities, thyroid disease was the first in the list, found in 38% (16/42) of the participants.
The most common previously used treatments were topical steroids (32/42), systemic steroids (30/42), cyclosporine (22/42), and intralesional steroids (19/42). Adverse events to previous treatment included hypertension (~41%), hypertrichosis (~35%), Cushing syndrome (~35%) and topical atrophy (~17%).
In our cohort, 52.3% (22/42) of the patients were under tofacitinib and 47.6% (20/42) under baricitinib and the mean time on current treatment was 7.4 months (SD: 6.46, range: 1–30 months).
Table 1 summarizes patients epidemiological and clinical characteristics.
Clinical Outcomes
Mean time on treatment of all participants was 7.4 months (SD 6.46, 1–30), whilst mean time to first signs of hair regrowth was 2.66 months. All patients on baricitinib received the recommended dose of 4 mg per day. In this group one patient switched from tofacitinib due to treatment failure (started with 10mg/day for 6 months and 20 mg/day for 3 months with no response and continued afterwards with 4 mg/day baricitinib). The maintenance dose for all the 20 patients receiving baricitinib was 4 mg/day.
In the tofacitinib group, 21 patients started with 10 mg/day, except from one patient who started with a daily dose of 5mg, due to history of metabolic syndrome. This individual had developed Cushing syndrome due to the previous long-term use of systemic steroids. After 11 months of tofacitinib, he switched to baricitinib, due to lack of efficacy. Five patients from the tofacitinib group received a maintenance dose of 20 mg/day, whilst 17 of them received a maintenance dose of 10 mg/day. For all patients under tofacitinib the maximum dose was 20 mg/day for 1–6 months, depending on treatment tolerance. The maintenance dose was determined by the response to treatment and the patient’s tolerance to the drug.
All the 3 adolescents that were treated with tofacitinib, experienced >90% of hair regrowth.
Efficacy Analysis and Correlations
Efficacy analysis was performed on the subgroup of 30 patients that had completed at least a 3-month follow-up on treatment. The rest twelve patients that had not completed the first 3-month follow-up visit were excluded, due to lack of efficacy data at this time point.
In the 30 individuals analyzed, the mean time on treatment was 10 months. Their mean SALT and mean DLQI scores decreased from 84.46% and 12.86 at baseline, to 43.26% and 6.63, respectively. CR was recorded in 4 patients (13.33%), PR in 12 patients (40%) and no response in 14 patients (46.66%) patients. Figure 1 illustrates clinical examples of the three groups. There was statistically significant difference in the treatment duration among the 3 response subgroups (p= 0.002). The majority of non-responders (64.2%) belonged to the group of AU.
Figure 1.
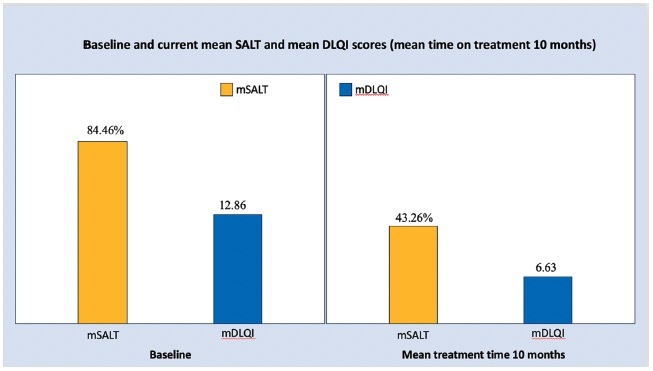
Efficacy analysis of 30 individuals that had completed at least 3 months on treatment with Janus Kinase inhibitors revealed a significant reduction in mean Severity of Alopecia Tool (SALT) and Dermatology Life Quality Index (DLQI) scores from baseline to the last follow up visit (mean treatment time 10 months).
Figure 2.
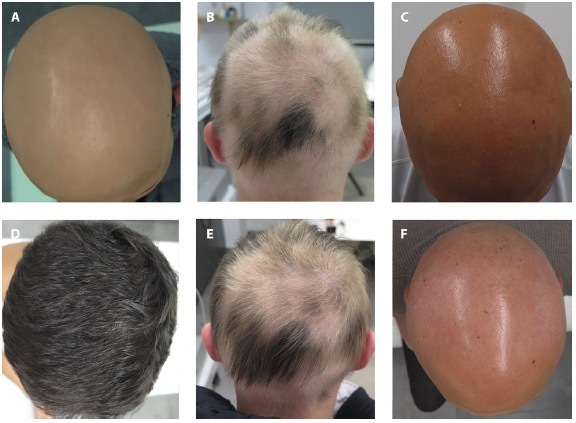
(A,D) Clinical picture of a patient at baseline (A) who achieved complete response after 9 months of treatment (D). (B,E) Clinical picture of a patient at baseline partial (B) who achieved partial response after 6 months of treatment (C). (C,F) Clinical picture of a patient at baseline partial (C) who failed to respond after 9 months of treatment (F).
No statistically significant differences were observed between sex and ΔSALT score and thus effectiveness does not appear to be related to sex (P = 0.323, two-sided test). We divided the patients into three age groups (< 25 years, 25–50 years and > 50 years) and we found no statistical correlation between ΔSALT score and age (p=0.693). ΔSALT score did not significantly differ among the 7 clinical types of alopecia areata (P = 0.207, the significance level is 0.05) and was not affected by time since the last episode of AA (rs: 0.373, P > 0.05). All performed correlations are presented in Table 2.
Table 2.
Correlations and non-parametric tests
| Correlations | P-value |
|---|---|
| ΔSALT score and patient age | rs: 0.596, p>0.05 |
| ΔSALT score and patient age (among the 3 age groups) | P = 0.693 |
| ΔSALT score and clinical types of AA | P = 0.207 |
| ΔSALT score and time since last episode of AA | rs: 0.373, p>0.05 |
| Clinical response and treatment duration | P = 0.002 |
AA = AA: alopecia areata; SALT = Severity of Alopecia Tool.
Safety Outcomes
Twenty five out of 42 (59.5%) individuals experienced no adverse events (AE), whilst 17/42 (40.5%) reported at least 1 AE, during JAKi treatment. Thirteen patients (30.9%) presented with one AE, 7.1% (3/42) presented with two and 2.3% (1/42) presented with three AE. No patient required hospitalization for JAKi-related AE. Fifteen patients (35.7%) got COVID-19 infection during treatment. Among them, two out of 42 (4.7%) individuals were totally asymptomatic, 8/42 (19%) presented with mild infection (fever up to 37.5°C, myalgia, weakness and cough) and 4/42 (9.5%) presented with moderate symptom (fever up to 38.5°C for 2 days, weakness and sore throat). One individual (2.3%) suffered from serious COVID-19 disease (fever up to 40°C for 3 days, headache, cough, weakness). It is of particular importance to report that the 3 adolescent patients did not experience any kind of AE or laboratory abnormalities, except of persistent common warts in one of them. Table 3 summarizes the AEs observed in our cohort.
Table 3.
The most common adverse events in patients with alopecia areata treated with baricitinib and tofacitinib in our cohort
| Type of adverse event | Percentage (%) |
|---|---|
|
| |
| Elevated liver enzymes and lipid elevation | 4.7 |
|
| |
| Lipid elevation | 9.5 |
|
| |
| COVID-19 infection | 35.7 |
| • mild symptoms | 19.0 |
| • moderate symptoms | 9.5 |
| • serious symptoms | 2.3 |
| • asymptomatic | 4.7 |
|
| |
| Herpes zoster and herpes simplex | 2.3 |
|
| |
| None | 59.5 |
Conclusions
Severe AA is a challenging-to-treat immune mediated disorder, characterized by treatment resistance and high recurrence rate. Until recently, there were no safe and effective treatments for AA that could offer consistent and long-lasting results. Topical, intralesional and systemic treatment with corticosteroids, contact immunotherapy, and systemic immunomodulating drugs as cyclosporine, methotrexate and azathioprine have been administered with variable efficacy outcomes, although the abovementioned agents are used as off label treatments in cases of AA [6].
In the last years, several studies on tofacitinib, baricitinib and other JAK inhibitors have demonstrated encouraging outcomes [7]. Tofacitinib and baricitinib showed to be effective and well-tolerated also in our cases of AA, achieving almost 50% reduction in the mean SALT score. Furthermore, 16/30 patients responded to treatment, and 13.33% achieved an improvement in the SALT score of >90%. Similar results were recorded by Kenedy Crispin et al in an open-label study, evaluating the use of oral tofacitinib and reporting 64% of patients responding to the therapy and 32% of them achieving a SALT score reduction >50%. Accordingly, Liu et al report >50% improvement in SALT score in 42% of their patients [8]. Similar, were the results from the BRAVE-AA1 and BRAVE-AA2 clinical trials where 19.7% and 34% of patients achieving SALT≤20 in the 2 and 4 mg baricitinib arms, respectively [9,10]. A recent meta-analysis of JAKi in the treatment of AA, reported that from 289 patients, there were 45.7% good responders, and 21.4% partial responders. Interestingly, the researchers found no difference in the response rates between tofacitinib, baricitinib, and ruxolitinib [11]. Our treatment response rates comply with that from other trials and we confirm the efficacy of these new drugs in treating AA at a real world setting. Except of tofacitinib and baricitinib, efficacy of upatacitinib in AA has been evaluated in a study including patients concomitantly suffering from both, atopic dermatitis and AA. The investigators recorded a significant reduction of the mean SALT score from baseline (95.1 ±9 .6) to the 4th week of treatment (77.6 ± 28.2, P = 0.0087), highlighting that the group of JAKi opens a new horizon in the treatment of AA [12].
In our studied population we included 3 adolescents and no statistical correlation was identified regarding the efficacy outcome and the age of the patient. In this specific age group, no differences were identified regarding the adverse events recordings. Similarly low is the adverse event rate from analogous studies [13–15].
Our observations also pose a few questions regarding treatment handling during infections [16,17]. From the meta-analysis report, the most common adverse events observed are upper respiratory (18.2%), urinary tract infections (2.2%) and total infections (24.6%) [11]. The observation period of our study included the COVID-19 pandemic period and maybe this is the reason for the high incidence of COVID-19 infection rates recorded. Whether treatment with JAKi should be discontinued, as advised in cases of other chronic inflammatory diseases, is under discussion.
Considering COVID-19 disease, our results indicate that JAKi have a satisfactory safety profile, as long as patients are compliant and discontinue treatment during infection [18]. Although there are reports proposing JAKi as a potential treatment for COVID-19, we recommend JAKi discontinuation, especially in symptomatic patients.
Overall, complication rates and adverse events during treatment with JAKi are low, and so far, no hospitalization for JAK inhibitor-related adverse events is reported in the literature.
Addressing safety and efficacy of SARS-CoV-2 vaccination in patients receiving immunotherapeutics, Gresham et al outlines that there is a possibility of decreased immune response and vaccine immunogenicity in patients on systemic immunotherapies, particularly in patients receiving azathioprine, cyclosporine, methotrexate, or JAKis [19]. Additionally, Seror et al conducted a study in order to investigate the immune response to COVID-19 vaccination in patients treated with JAKis. 113 patients with rheumatoid arthritis or psoriatic arthritis receiving baricitinib (56/113, 50%), tofacitinib (30/113, 27%) or upadacitinib (27/113, 24%) were included and their immune response to COVID-19 vaccination was evaluated. The overall response rate to the vaccination in patients treated with JAKis remained high. Non-responders were mostly older patients, and patients receiving upadacitinib. Serological assessment 2 weeks after vaccination should be recommended for patients aged 65 years and older or treated with upadacitinib to secure a high safety profile [20].
In conclusion, our results demonstrate that tofacitinib and baricitinib are effective and safe therapeutic modalities for AA in real-world settings. Currently, only baricitinib is officially indicated for AA.
Limitation of our study is the short follow up on treatment and the small number of participants.
Footnotes
Funding: None.
Competing Interests: None.
Authorship: All authors have contributed significantly to this publication.
References
- 1.Triyangkulsri K, Suchonwanit P. Role of janus kinase inhibitors in the treatment of alopecia areata. Drug Des Devel Ther. 2018;12:2323–2335. doi: 10.2147/DDDT.S172638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Husein-ElAhmed H, Abdulla N, Al-Obaidli A, Ali-Alam M, Steinhoff M. Real-world experience and long-term evaluation of tofacitinib in refractory alopecia areata: A prospective, open-label, single-center study in Asian Arab population. Dermatol Ther. 2022;35(12):e15871. doi: 10.1111/dth.15871. [DOI] [PubMed] [Google Scholar]
- 3.Zhan J, Cao J, Chen F, Jin Y, Huang C. Real-data on the use of baricitinib in adolescents with severe alopecia areata. J Eur Acad Dermatol Venereol. 2023 Apr 17; doi: 10.1111/jdv.19121. [DOI] [PubMed] [Google Scholar]
- 4.Hernandez-Montoya C, Ruiz-Villaverde R. The Role of Tofacitinib in the Management of Alopecia Totalis. Sultan Qaboos Univ Med J. 2019;19(1):e77–e78. doi: 10.18295/squmj.2019.19.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanchez-Diaz M, Diaz-Calvillo P, Rodriguez-Pozo JA, Tercedor-Sánchez J, Cantudo-Cuenca MR, Molina-Leyva A, Arias-Santiago S. Tofacitinib for Treatment of Alopecia Areata: Real-world Evidence and Factors Associated with Therapeutic Response. Acta Derm Venereol. 2022;102:adv00736. doi: 10.2340/actadv.v102.2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meah N, Wall D, York K, et al. The Alopecia Areata Consensus of Experts (ACE) study: Results of an international expert opinion on treatments for alopecia areata. J Am Acad Dermatol. 2020;83(1):123–130. doi: 10.1016/j.jaad.2020.03.004. [DOI] [PubMed] [Google Scholar]
- 7.Kennedy Crispin M, Ko JMC, et al. Safety and efficacy of the JAK inhibitor tofacitinib citrate in patients with alopecia areata. JCI Insight. 2016;1(15):e89776. doi: 10.1172/jci.insight.89776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Liu LY, Craiglow BG, Dai F, King BA. Tofacitinib for the treatment of severe alopecia areata and variants: A study of 90 patients. J Am Acad Dermatol. 2017;76(1):22–28. doi: 10.1016/j.jaad.2016.09.007. [DOI] [PubMed] [Google Scholar]
- 9.King B, Ko J, Forman S, et al. Efficacy and safety of the oral Janus kinase inhibitor baricitinib in the treatment of adults with alopecia areata: Phase 2 results from a randomized controlled study. J Am Acad Dermatol. 2021;85(4):847–853. doi: 10.1016/j.jaad.2021.05.050. [DOI] [PubMed] [Google Scholar]
- 10.King B, Ohyama M, Kwon OZ, et al. Two Phase 3 Trials of Baricitinib for Alopecia Areata. N Engl J Med. 2022;386(18):1687–1699. doi: 10.1056/NEJMoa2110343. [DOI] [PubMed] [Google Scholar]
- 11.Phan K, Sebaratnam DF. JAK inhibitors for alopecia areata: a systematic review and meta-analysis. J Eur Acad Dermatol Venereol. 2019;33(5):850–856. doi: 10.1111/jdv.15489. [DOI] [PubMed] [Google Scholar]
- 12.Chiricozzi A, Balato A, Fabbrocini G, et al. Beneficial effects of upadacitinib on alopecia areata associated with atopic dermatitis: A multicenter retrospective study. J Am Acad Dermatol. 2023;89(6):1251–1253. doi: 10.1016/j.jaad.2023.05.001. [DOI] [PubMed] [Google Scholar]
- 13.Craiglow BG, Liu LY, King BA. Tofacitinib for the treatment of alopecia areata and variants in adolescents. J Am Acad Dermatol. 2017;76(1):29–32. doi: 10.1016/j.jaad.2016.09.006. [DOI] [PubMed] [Google Scholar]
- 14.Castelo-Soccio L. Experience with oral tofacitinib in 8 adolescent patients with alopecia universalis. J Am Acad Dermatol. 2017;76(4):754–755. doi: 10.1016/j.jaad.2016.11.038. [DOI] [PubMed] [Google Scholar]
- 15.Moussa A, Eisman S, Kazmi A, et al. Treatment of moderate-to-severe alopecia areata in adolescents with baricitinib: A retrospective review of 29 patients. J Am Acad Dermatol. 2023;88(5):1194–1196. doi: 10.1016/j.jaad.2022.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Ghazawi FM, Lim M, Dutz JP, Kirchhof MG. Infection risk of dermatologic therapeutics during the COVID-19 pandemic: an evidence-based recalibration. Int J Dermatol. 2020;59(9):1043–1056. doi: 10.1111/ijd.15028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ZaZahedi Niaki O, Anadkat MJ, Chen ST, et al. Navigating immunosuppression in a pandemic: A guide for the dermatologist from the COVID Task Force of the Medical Dermatology Society and Society of Dermatology Hospitalists. J Am Acad Dermatol. 2020;83(4):1150–1159. doi: 10.1016/j.jaad.2020.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guimarães PO, Quirk D, Furtado RH, et al. Tofacitinib in Patients Hospitalized with Covid-19 Pneumonia. N Engl J Med. 2021;385(5):406–415. doi: 10.1056/NEJMoa2101643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gresham LM, Marzario B, Dutz J, Kirchhof MG. An evidence-based guide to SARS-CoV-2 vaccination of patients on immunotherapies in dermatology. J Am Acad Dermatol. 2021;84(6):1652–1666. doi: 10.1016/j.jaad.2021.01.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seror R, Camus M, Salmon JH, et al. Do JAK inhibitors affect immune response to COVID-19 vaccination? Data from the MAJIK-SFR Registry. Lancet Rheumatol. 2022;4(1):e8–e11. doi: 10.1016/S2665-9913(21)00314-3. [DOI] [PMC free article] [PubMed] [Google Scholar]


