Abstract
Three promoters have been identified as having potentially important regulatory roles in governing expression of the fla/che operon and of sigD, a gene that lies near the 3′ end of the operon. Two of these promoters, fla/che PA and PD-3, lie upstream of the >26-kb fla/che operon. The third promoter, PsigD, lies within the operon, immediately upstream of sigD. fla/che PA, transcribed by EςA, lies ≥24 kb upstream of sigD and appears to be largely responsible for sigD expression. PD-3, transcribed by EςD, has been proposed to participate in an autoregulatory positive feedback loop. PsigD, a minor ςA-dependent promoter, has been implicated as essential for normal expression of the fla/che operon. We tested the proposed functions of these promoters in experiments that utilized strains that bear chromosomal deletions of fla/che PA, PD-3, or PsigD. Our analysis of these strains indicates that fla/che PA is absolutely essential for motility, that PD-3 does not function in positive feedback regulation of sigD expression, and that PsigD is not essential for normal fla/che expression. Further, our results suggest that an additional promoter(s) contributes to sigD expression.
Motility and chemotaxis functions in Bacillus subtilis are encoded within the fla/che operon. This large (>26-kb) operon includes both structural and regulatory components required for motility (6, 19, 32). The proximal region of the operon includes genes that encode the hook and basal body (HBB) complex, a structure that is required for tethering the flagellar filament to the cell. The distal-most region of the fla/che operon encodes the flagellum-specific sigma factor, ςD (19). ςD activity is required for transcription of the genes encoding flagellin (hag) and for the motA and motB genes, which encode the motor proteins that drive flagellar rotation (21, 22). In addition, ςD is also partially responsible for expression of the anti-sigma factor, FlgM (14, 18). FlgM antagonizes ςD activity in vivo (3, 8) and binds directly to ςD in vitro (2). FlgM may inhibit ςD activity either by binding to ςD protein in a manner that precludes it from forming a stable complex with core RNA polymerase or by binding to the ςD-holoenzyme and interfering with its function (4). In Escherichia coli and Salmonella enterica serovar Typhimurium, it has been demonstrated that FlgM is exported through the HBB to allow activation of the ςD homolog (11, 15). It is believed that FlgM activity is also controlled by export through the HBB in B. subtilis (5, 21, 24). Expression of the fla/che operon thus controls motility in a complex manner. First, HBB components are expressed concurrently with ςD. Subsequent assembly of the HBB structure allows export of FlgM. This activates ςD to promote transcription of ςD-dependent motility genes.
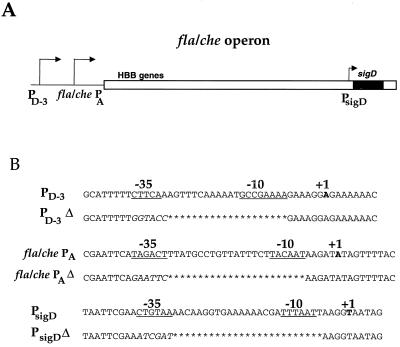
Recent studies have implicated three promoters in the expression of the fla/che operon and the sigD gene (1, 6) (see schematic, Fig. 1A). One of these promoters, PsigD, is located within the fla/che operon, immediately upstream of the sigD gene. Transcription from PsigD is dependent upon ςA, the major, vegetative sigma factor in B. subtilis. Previous studies indicated that PsigD contributed only weakly to overall expression of the sigD gene (1). However, genetic data suggested that this slight level of expression might be required to control temporal regulation of the entire fla/che operon (1). This requirement would presumably be indirect, since the location of PsigD precludes it from directly promoting transcription of the fla/che operon (see schematic in Fig. 1A).
FIG. 1.
(A) Schematic of the fla/che operon. The intergenic region upstream of the operon is indicated by the black line. The white box indicates fla/che operon sequences; the sigD gene, at the distal end of the operon, is indicated by a black bar. The location of the HBB genes, in the proximal portion of the operon, is indicated. The relative positions of each promoter are indicated by arrows. The identity of the promoter is indicated, in boldface, below the arrows. fla/che PA and PsigD are both ςA-dependent promoters. PD-3 is a ςD-dependent promoter. It has been suggested (6) that initial expression of the fla/che operon involves transcription from fla/che PA. Such expression would allow HBB assembly and export of FlgM, yielding active ςD. Thus, EςD could then initiate transcription from PD-3 to upregulate sigD expression, contributing to peak levels of ςD activity observed at the end of exponential growth. PsigD has been implicated as having a regulatory role in fla/che expression (1). (B) Sequences of the three promoters are indicated. The −35 and −10 regions are underlined. The +1 position is indicated in boldface. For the promoter deletions, sequences which have been deleted are indicated by asterisks. Sequences that have been replaced by restriction sites are indicated in italics.
Two additional promoters, fla/che PA and PD-3, have been identified. These promoters lie upstream of the entire fla/che operon (Fig. 1A). Deletion of fla/che PA, a ςA-dependent promoter eliminates motility (6). Moreover, the fla/che PAΔ strain exhibits a dramatic reduction in ςD protein levels. Additionally, ςD-dependent gene expression is abolished (6). These phenotypes apparently occur, in part, because the loss of fla/che PA-dependent transcription of the operon gives rise to impaired export of FlgM. When flgM is deleted concurrently with the fla/che PA, expression of a ςD-dependent reporter fusion is restored (6). PD-3, the other promoter upstream of the operon, is thought to be involved in this restoration of ςD-dependent motility gene expression (6). PD-3, located approximately 130 bp upstream of fla/che PA, is a ςD-dependent promoter. Its activity is induced, by as much as 10-fold, in the absence of FlgM (6). Based on these data, it has been suggested that inactivation of FlgM triggers an autoregulatory positive feedback loop at PD-3. Specifically, activation of transcription from PD-3 has been proposed to increase sigD gene expression, resulting in an accumulation of ςD protein, which gives rise to increased expression from ςD-dependent promoters (including PD-3).
We sought to clarify the roles of each of the three promoters in regulating expression of the fla/che operon and the sigD gene. Our approach was to construct strains that carry chromosomal deletions of fla/che PA, PD-3, and PsigD, either singly or in combination. Moreover, we generated strains that carry insertional disruptions of flgM in addition to the promoter deletion(s). Our results indicate that the fla/che PA is the major promoter responsible for fla/che expression and that fla/che PA is essential for motility. In our experiments PD-3 did not exhibit autoregulatory positive feedback control over sigD gene expression. Moreover, PD-3-mediated transcriptional activation of the fla/che operon is insufficient to promote motility. Further, we found that PsigD is not essential for normal expression of the fla/che operon or for ςD-dependent motility functions. Finally, our results suggest that there is an additional promoter(s) that mediates sigD expression.
MATERIALS AND METHODS
Construction of strains.
Strains of B. subtilis are listed in Table 1. Strains LMB214 and LMB216 have been described previously (6). However, because the original strains were lost, they were reconstructed for this study. The procedure for reconstructing the strains was identical to the one used in the original construction (6) and is essentially the same as the procedure described below for the construction of LMB226 and LMB228. For LMB214 and LMB216, however, the integrational plasmid pWE4-int, which contains the fla/che PA deletion and a wild-type version of PD-3, was utilized. PCR primers OWE7 and OWE8B were utilized, as described below, to identify fla/che PA deletion strains.
TABLE 1.
B. subtilis strains used in this study
| Strain | Genotype | Source or derivation (reference)a |
|---|---|---|
| LMB1 | trpC2 | E. Ferrari, I168b |
| LMB10 | trpC2 sigD::pLM5 (Cmr) | M. J. Chamberlin, CB100b (6) |
| LMB214 | trpC2 fla/che PAΔ | Transform [LMB1: pWE4-int, Cmr]c (6; this study) |
| LMB216 | trpC2 fla/che PAΔ flgM::mini-Tn10 (Spr) | Transform [LMB214: LMB213 (6), Spr] (6; this study) |
| LMB226 | trpC2 fla/che PAΔ PD-3Δ | Transform [LMB1: pSS7-1, Cmr]c (this study) |
| LMB228 | trpC2 fla/che PAΔ PD-3Δ flgM::mini-Tn10 (Spr) | Transform [LMB226: LMB213 (6), Spr] (this study) |
| LMB232 | trpC2 PsigDΔ | Transform [LMB1: pJW14, Cmr]c (this study) |
| LMB233 | trpC2 fla/che PAΔ PD-3Δ PsigDΔ | Transform [LMB226: pJW14, Cmr]c (this study) |
| LMB234 | trpC2 fla/che PAΔ PD-3Δ PsigDΔ flgM::mini-Tn10 (Spr) | Transform [LMB228: pJW14, Cmr]c (this study) |
| LMB241 | trpC2 sigD::pLM5(Cmr) flgM::mini-Tn10 (Spr) | Transform [LMB10: LMB213 (6)] (this study) |
| LMB243 | trpC2 PsigDΔ SPβc2Δ2φ[hag-cat-lacZ], Neor | Transduce [LMB232: HB4187 (J. Helmann), Neor]d (this study) |
| LMB244 | trpC2 SPβc2Δ2φ[hag-cat-lacZ], Neor | Transduce [LMB1: HB4187 (J. Helmann), Neor]d (this study) |
| LMB247 | trpC2 sigD::pLM5(Cmr) SPβc2Δ2φ[hag-cat-lacZ], Neor | Transduce [LMB10: HB4187 (J. Helmann), Neor]d (this study) |
Transformation of [recipient strain: with chromosomal DNA from this strain, selecting for this resistance].
Previous name of strain.
This strain was subsequently cured of the chloramphenicol resistance marker.
Transduction of [recipient strain: with transducing lysate from this strain, selecting for this resistance].
Strains bearing deletions of both fla/che PA and PD-3 (LMB226 and LMB228) were constructed by integration and subsequent curing of the plasmid pSS7-1. Beginning with plasmid pWE4 (6), which contained the fla/che PA deletion, oligonucleotide-mediated site-directed mutagenesis was employed to delete PD-3, using primer OWE5B (5′-GTATAATTTAATAAATTTTGCATTTTTGGTACCGAAAGGAGAAAAACAGAATTCTGC-3′). This results in replacement of PD-3 with a KpnI site (see Fig. 1B). The resulting double-deletion sequences were subcloned as a PstI fragment into pJM102 (26) to yield pSS7-1. The procedures utilized were essentially identical to those described elsewhere (6). pSS7-1 was digested and concatemerized and then transformed into LMB1 according to standard procedures (23); finally, it was plated onto Luria-Bertani medium (LB) plus 7 μg of chloramphenicol per ml to select for transformants. Double-crossover integrants were identified by PCR. For detection of the fla/che PA deletion, primers OWE7 (5′-GTGAGGACATTTTTTTACACTG-3′) and OWE8B (5′-CCCTCAATATCCTTGTCGAG-3′) were used. The deletion yields a product of 75 bp, while the wild-type product is approximately 100 bp. The PD-3 deletion was detected with primers OSS4 (5′-GCAGAATTTCTGTTTTTTCTCC-3′) and OSS5 (5′-CCTGGGTTGAAAGTCTTTCTATG-3′). The deletion and wild-type products also differ by approximately 25 bp. The plasmid sequences were “cured” from the strain by growing them without selection for several passages. Chloramphenicol-sensitive candidates were identified by replica patching onto selective and nonselective plates. Chloramphenicol-sensitive isolates were screened by PCR to identify cured deletion strains. To generate LMB228 (flgM::mini-Tn10, Spcr) LMB226 was transformed with chromosomal DNA (100 ng) from strain LMB213 (6) according to standard protocols (23). Spcr candidates were rescreened by PCR, with the OWE7-OWE8B and OSS4-OSS5 primer pairs, to ensure that they retained the promoter deletions. Strains were also microscopically examined for ςD-dependent autolysin activity to confirm the presence of the flgM mutation.
PsigDΔ strains were generated by integration and subsequent curing of plasmid pJW14. The SphI-EcoRI fragment of pLM112 (9) that contains PsigD was subcloned into pGEM7zf+ (Promega) and then subjected to oligonucleotide-mediated site-directed mutagenesis using primer OJW7 (5′-CCCGACGTCGCATGCTGCATATTCGAATCGATTAAGGTATTAGGGGGATACATGC-3′). This results in the replacement of PsigD with a ClaI restriction site (Fig. 1B). This deletion fragment was subcloned, replacing the wild-type version of the SphI-EcoRI portion of pLM112 to yield the plasmid pJW9. pJW9 includes an approximately 900-bp fragment from the SalI site upstream of the sigD gene to the EcoRI site within sigD; the vector sequences are derived from the integrational plasmid pJM102. Because this plasmid could not be efficiently cured out of integrant strains, an additional 500-bp HindIII-SalI fragment from pJH6-2 (9) was subcloned into pJW9, resulting in plasmid pJW14. pJW14 was integrated as a double crossover into LMB1, LMB226, and LMB228 as described above. Double crossovers were identified by PCR using primers 5′sigdel (5′-GCATGCTGCATATTCG-3′) and OJWS3′ (5′-CAGCGCGTCCAAAGCA-3′). The deletion yields a product of 73 bp, whereas the wild-type product is 98 bp. Plasmid sequences were cured as described above. For all deletion strains, DNA surrounding the deletion was amplified by PCR and sequenced to ensure that no extraneous mutations had occurred.
For construction of hag-lacZ reporter strains, a lysate from B. subtilis HB4187 (SPβc2Δ2φ[hag-cat-lacZ], Neor) was generated. HB4187 was grown overnight on an LB-neomycin plate; a colony was then inoculated into Difco Antibiotic Medium No. 3 (i.e., Penassay broth) and grown to light turbidity at 37°C with aeration. To generate SPβ phage lysate, the culture was transferred to 50°C and incubated for 90 min with aeration. Cellular debris was removed by centrifugation at 8,000 rpm in an SS34 rotor for 10 min. After transfer to a fresh tube, a drop of chloroform was added to the lysate, which was stored at 4°C. Strains LMB243 and LMB244 were generated by transducing strains LMB232 and LMB1, respectively, with this lysate. LMB1 and LMB232 were grown to mid-log phase in Difco Antibiotic Medium No. 3 (Penassay broth [PAB]) at 37°C with aeration. After addition of an equal volume of lysate, this incubation was continued for an additional 20 min. Cells were collected by centrifugation and then washed with 5 ml of 1× SC (0.15 M NaCl, 0.01 M sodium citrate; pH 7.0). Cells were then plated onto LB-neomycin to select for transductants that carried the hag-lacZ reporter.
RNA isolation and primer extension.
Cells were grown in complex sporulation medium (2× SG) at 37°C with aeration. At T0 and T0.5, 25 to 50 ml of culture was transferred to Falcon conical centrifuge tubes, pelleted, and snap frozen in dry ice-ethanol. Cells were lysed by 3-min incubation in disruption buffer (30 mM Tris, pH 8; 50 mM EDTA; 100 mM NaCl; 1 mg of lysozyme per ml). This was followed by incubation with 50 U of RQ1 DNase (Promega) and 0.5 mg of proteinase K per ml. Samples were extracted with phenol-chloroform-isoamyl alcohol (25:24:1) until the interface cleared and then precipitated with ethanol and 0.3 M sodium acetate. RNA samples were examined by formaldehyde agarose gel electrophoresis, performed according to standard procedures (28), to ensure that they were intact.
Primer extensions were conducted essentially as described previously (23). For detection of fla/che PA and PD-3 transcripts, primer OWE3 (5′-AATATCCGCTCGTCTCAAGGCAT-3′) was used, yielding extension products of 123 and 259 bases, respectively. For detection of PsigD transcripts, primer OJSPE2 (5′-GCACTGATTTCGGCAGTCCGACAG-3′) was used, yielding an extension product of 164 bases. For control rpsB reactions, primer RPSBE (5′-GTGACCGAAGTGAACAGG-3′) was used, also yielding an extension product of 164 bases. The following modifications were made to the protocol: unincorporated label was removed from primers using a NICK-Column (Pharmacia); prior to the annealing reaction, 0.6 pmol of labeled primer was ethanol precipitated with 50 μg of sample RNA and then centrifuged for ≥15 min in a microcentrifuge at 4°C; and air-dried pellets were resuspended in 8 μl of 1 mM vanadyl-ribonucleoside complex (VRC) for annealing. Primer extension products were resolved alongside sequencing reactions of pWE1 (6) for fla/che PA and PD-3 and of pLM112 (9) for PsigD.
Autoradiography was carried out a −80°C with intensifying screens for 5 to 15 days. Exposed X-ray films were scanned with a UMAX scanner into Adobe Photoshop 4.0.
Western blots.
Cells were grown in 2× SG to t0.5. Then, 10 to 20 ml of culture was collected by centrifugation and washed in ice-cold STE (150 mM NaCl, 10 mM Tris-Cl, 100 mM EDTA). Cells were lysed by sonication as described previously (18), and cellular debris was removed from the protein extracts by centrifugation. For detection of ςD, 50 μg of protein was resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) on a 12% gel and then transferred to nitrocellulose. The filter was stained with Ponceau-S (Sigma) to ensure equal loading and transfer of protein samples across all lanes. The filter was blocked with TBST (10 mM Tris, pH 7.5; 150 mM NaCl; 0.1% Triton X-100) plus 5% powdered skim milk for 2 h at room temperature, incubated with 1:1,000 anti-ςD polyclonal antibody 2855 (9) for 2 h at room temperature, washed four times for 10 min each time in TBST, incubated with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Sigma) diluted 1:2,000 in TBST for 1 h, and washed four times for 10 min each time in TBST. ςD protein was visualized using BCIP (5-bromo-4-chloro-3-indolylphosphate)–nitroblue tetrazolium substrate (Sigma) in distilled, deionized water.
For detection of flagellin, the procedure was as described above except that 10 μg of protein was resolved on a 10% PAGE mini-gel. The anti-flagellin polyclonal antibody (25) was used at a 1:2,000 dilution. In addition, an anti-ςA antibody (9) was used simultaneously with the flagellin antibody at a 1:2,000 dilution. The result obtained with these two antibodies used together was as expected from pilot experiments in which the flagellin and ςA antibodies were used individually (i.e., there was no extraneous cross-reactivity observed when the antibodies were used simultaneously).
Swarm assay.
A swarm assay was conducted according to the method of Fein and Rogers (7). Cells were patched from a fresh overnight LB plate to a swarm assay plate (Difco Antibiotic Medium No. 3 plus 0.4% Bacto-agar) and incubated in a humidified chamber at 37°C overnight.
β-Galactosidase assay.
To assay expression from the hag-lacZ transcriptional fusion, cells were grown in 2× SG medium at 37°C with aeration. Samples were removed every 18 min and stored on ice. β-Galactosidase assays were performed essentially as described previously (6). Assays were performed in duplicate or triplicate samples, and values were averaged.
RESULTS
fla/che expression initiated from PD-3 is insufficient to promote motility and does not appear to function in positive-feedback regulation of sigD expression.
Previous work demonstrated that ςD-dependent gene expression and motility were abolished in a fla/che PAΔ mutant strain (6). Concurrent deletion of fla/che PA and flgM resulted in restoration of the subset of ςD-dependent activities that were examined (6), but the question of whether disruption of flgM also restores motility in a fla/che PAΔ strain background was not addressed. However, the proposed model (6) predicts that motility would be exhibited by the fla/che PAΔ flgM mutant strain; basal levels of ςD would be activated in the absence of FlgM activity, resulting in expression of the fla/che operon from PD-3. Consequently, the HBB genes would be expressed concurrently with the ςD-dependent motility genes, allowing assembly of functional flagella.
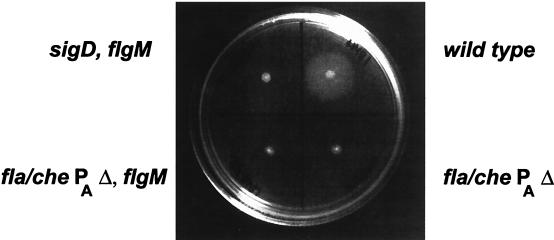
To test this prediction, we examined the fla/che PAΔ, flgM::mini-Tn10 strain (LMB216) for motility. Figure 2 shows the results of a swarm plate assay. In this assay, cells were inoculated onto semisolid agar plates (7); cells that were motile swarm outward from the point of inoculation, making a halo of growth, whereas nonmotile cells were restricted to the point of inoculation. The fla/che PAΔ flgM::mini-Tn10 strain (LMB216) fails to swarm, indicating that it is not motile. Examination of live cells by light microscopy confirmed that LMB216 is nonmotile (data not shown).
FIG. 2.
Swarm assay of LMB216. Cells of the indicated genotype were inoculated onto PAB–0.4% agar plates and grown at 37°C overnight. LMB1, the wild-type strain, has swarmed from the point of inoculation, which indicates that this strain is motile. LMB241 (sigD::pLM5 flgM::mini-Tn10), the negative control strain, exhibits growth from the position of inoculation, but does not swarm. LMB214 (fla/che PAΔ) and LMB216 (fla/che PAΔ flgM::mini-Tn10) also do not swarm, indicating that they are nonmotile. This indicates that in LMB216, activation of ςD by the removal of FlgM does not restore motility.
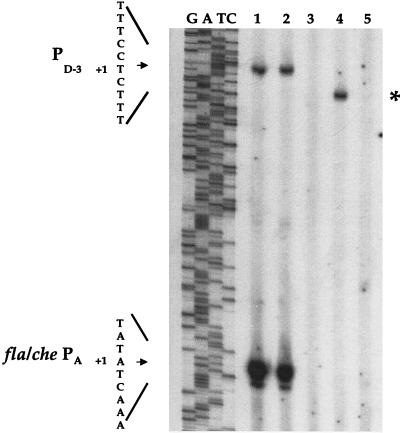
In order to gain insight into this surprising result, we examined RNA from the LMB216 strain for evidence of transcription from PD-3. Figure 3 (lane 4, asterisk) shows the presence of a primer extension product that corresponds to the PD-3-specific transcript. This PD-3-specific product is shorter than the PD-3-specific product of the wild-type strain (lane 1); the shortened length of the PD-3-specific product in LMB216 results from the deletion of fla/che PA sequences, which lie downstream of PD-3 (Fig. 1). The level of PD-3 product appears to be similar for LMB216 and the wild-type strain. However, overall fla/che transcription is greatly reduced in LMB216 compared to the wild type since LMB216 lacks the fla/che PA-specific product, which comprises the vast majority of fla/che primer extension products in the wild-type sample (Fig. 3, lane 1). The primer extension results indicate that PD-3-mediated transcription of the fla/che operon is induced in LMB216. However, this expression of fla/che genes is reduced relative to the wild type.
FIG. 3.
Primer extension analysis of fla/che PA and PD-3. A 50 μg portion of total RNA was isolated at T0 and subjected to primer extension analysis using primer OWE-3. In parallel, OWE-3 was utilized in a dideoxy sequencing reaction on plasmid pWE-1, which includes the fla/che promoter region and flgB. Lanes of the sequencing reaction are indicated as G, A, T, and C. Lane 1, LMB1 (wild type); lane 2, LMB232 (PsigDΔ); lane 3, LMB214 (fla/che PAΔ); lane 4, LMB216 (fla/che PAΔ flgM::mini-Tn10); lane 5, LMB234 (fla/che PAΔ PD-3Δ PsigDΔ flgM::mini-Tn10). The +1 position for PD-3 and fla/che PA is indicated to the left. The sequence indicated corresponds to the template strand (i.e., is complementary to the sequence indicated in Fig. 1B). The asterisk indicates the position of the truncated PD-3-specific primer extension product in LMB216 that results from internal deletion of sequences corresponding to the fla/che PA. The result in lane 1 indicates that expression from fla/che PA greatly exceeds expression from PD-3 in the wild-type strain at the end of logarithmic growth. This is the time of maximal ςD activity (25), suggesting that activation of PD-3 is not serving a positive autoregulatory role in the induction of ςD activity. Lane 3 shows that in the fla/che PAΔ strain, primer extension products corresponding to both the fla/che PA and the PD-3 transcripts are absent, indicating that expression from fla/che PA is required for ςD-dependent transcription at PD-3. Lane 4 shows that disruption of flgM, in a fla/che PA background, results in activation of PD-3. However, Fig. 1 demonstrates that this expression of the fla/che operon from PD-3 is insufficient to promote motility. Lane 2 shows that a PsigDΔ strain resembles the wild type with regard to expression of the fla/che operon. Lane 5 shows the absence of fla/che PA- and PD-3-specific primer extension products in a fla/che PAΔ PD-3Δ PsigDΔ flgM::mini-Tn10 strain (LMB234). In control primer extension reactions utilizing a primer specific for the ribosomal rpsB gene, the LMB216 (lane 3), LMB234 (lane 5), and wild-type (lane 1) samples yielded comparable results (data not shown), indicating that the lack of signal observed in lanes 3 and 5 is a direct result of the promoter deletions.
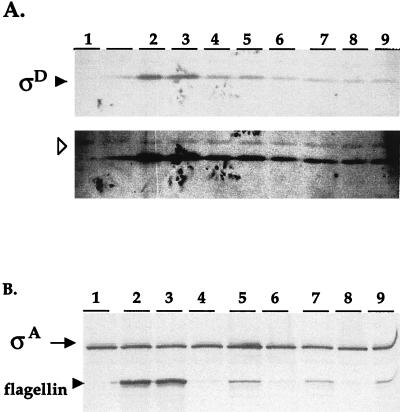
Figure 4A (lane 5) indicates that ςD is present in the fla/che PAΔ flgM::mini-Tn10 strain (LMB216). However, ςD expression is reduced in LMB216 relative to ςD expression in the wild-type strain (Fig. 4A, lane 1). Finally, LMB216 appears to express ςD-dependent motility genes. Figure 4B (lane 5) indicates that flagellin protein is expressed in LMB216, albeit at reduced levels compared to the wild type (lane 1). Altogether, our analysis of LMB216 indicates that the fla/che operon is expressed in the fla/che PAΔ flgM::mini-Tn10 mutant background at reduced levels relative to wild-type expression. ςD and ςD-dependent motility genes are also expressed at reduced levels, and the strain is nonmotile. This indicates that fla/che expression mediated by PD-3 is insufficient to support motility.
FIG. 4.
(A) Western blot analysis of ςD. (Upper panel) A total of 50 μg of protein, isolated from cultures at T0.5, was fractionated by SDS-PAGE and probed with antibodies raised against ςD. The blot was stained with Ponceau-S, prior to antibody incubation, to ensure equivalent loading across lanes. Lane 1, LMB10 (sigD::pLM5); lane 2, LMB1 (wild type); lane 3, LMB232 (PsigDΔ); lane 4, LMB214 (fla/che PAΔ); lane 5, LMB216 (fla/che PAΔ flgM::mini-Tn10); lane 6, LMB226 (fla/che PAΔ PD-3Δ); lane 7, LMB228 (fla/che PAΔ PD-3Δ flgM::mini-Tn10); lane 8, LMB233 (fla/che PAΔ PD-3Δ PsigDΔ); lane 9, LMB234 (fla/che PAΔ PD-3Δ PsigDΔ flgM::mini-Tn10). (Lower panel) Overexposing the same blot allows visualization of a cross-reacting band (arrowhead), which indicates uniform loading of samples. The lane adjacent to lane 1 also contains protein extract from the sigD null strain; spillover from lane 2 is evident in this lane. Comparison of lanes 1 and 3 shows that deletion of fla/che PA results in a reduction in the ςD protein level. Comparison of lanes 3 and 4 indicates that disruption of flgM, in a fla/che PAΔ background, does not result in increased ςD levels, even though transcription from PD-3 is activated in this strain background. Lanes 8 and 9 show that ςD is detected in strains that lack fla/che PA, PD-3, and PsigD, indicating that an additional promoter(s) contributes to sigD gene expression. A comparison of lanes 8 and 9 with lanes 6 and 7 reveals that ςD detected in strains that lack all three known promoters is comparable to ςD detected in isogenic strains that lack fla/che PA and PD-3 but retain PsigD; this indicates that PsigD activity does not contribute significantly to the ςD that is observed. Lane 2 indicates that ςD is expressed at apparently wild-type levels in a strain that lacks PsigD but retains the other promoters. (B) Western blot analysis of flagellin protein. A total of 10 μg of protein, isolated from cultures at T0.5, was fractionated by SDS-PAGE and probed with antibodies specific for flagellin and for ςA as a loading control. Lane 1, LMB10 (sigD::pLM5, null mutant); lane 2, LMB1 (wild type); lane 3, LMB232 (PsigDΔ); lane 4, LMB214 (fla/che PAΔ); lane 5, LMB216 (fla/che PAΔ flgM::mini-Tn10); lane 6, LMB226 (fla/che PAΔ PD-3Δ); lane 7, LMB228 (fla/che PAΔ PD-3Δ flgM::mini-Tn10); lane 8, LMB233 (fla/che PAΔ PD-3Δ PsigDΔ); lane 9, LMB234 (fla/che PAΔ PD-3Δ PsigDΔ flgM::mini-Tn10). In a fla/che PAΔ strain, flagellin expression is diminished (lane 3). However, disruption of flgM in the fla/che PAΔ strain background results in observable flagellin expression (lane 4) because ςD-dependent gene expression is activated. This expression of flagellin remains reduced relative to wild-type expression (compare lanes 4 and 1). Lane 2 shows that a PsigDΔ strain exhibits essentially wild-type expression of flagellin protein, indicating that PsigD is not required for normal expression of this motility gene.
In addition, our data enable us to make inferences about the role of PD-3 in the autoregulatory control of sigD expression. The primer extension data indicate that transcription from PD-3 is essentially abolished in LMB214, the fla/che PAΔ strain (Fig. 3, lane 3). The fla/che PAΔ flgM strain (LMB216) exhibits a severalfold induction of PD-3 transcript relative to LMB214 (Fig. 3, lane 4). However, there is no commensurate increase in ςD levels in LMB216 relative to LMB214 (Fig. 4A, compare lanes 4 and 5). This indicates that the PD-3 transcript does not contribute significantly to ςD protein levels, suggesting that PD-3 is not part of an autoregulatory feedback mechanism for inducing sigD expression.
Simultaneous deletion of fla/che PA, PD-3, and PsigD suggests that other promoters contribute to expression of sigD.
The fla/che PAΔ strain (LMB214) exhibits expression of ςD in the absence of transcription from fla/che PA and PD-3 (Fig. 3, lane 3; Fig. 4A, lane 3). We sought to determine whether PsigD could be responsible for this ςD expression. We constructed strain LMB233, which bears chromosomal deletions of fla/che PA, PD-3, and PsigD. In addition, we constructed LMB234, which is isogenic to LMB233 but which also bears a disruption of the flgM gene. We confirmed that LMB233 and LMB234 lack the PsigD-specific product primer extension product that is evident for the wild-type strain (Fig. 5). Additionally, Fig. 3 (lane 5) shows that primer extension products specific to the fla/che PA and PD-3 transcripts are absent in LMB234, a finding consistent with the chromosomal deletion of those promoters in this strain; a control primer extension utilizing a primer for the ribosomal rpsB gene yielded comparable results for LMB234 and the wild-type strain, indicating that the lack of primer extension products for fla/che PA, PD-3, and PsigD results directly from deletion of these promoters in LMB234.
FIG. 5.
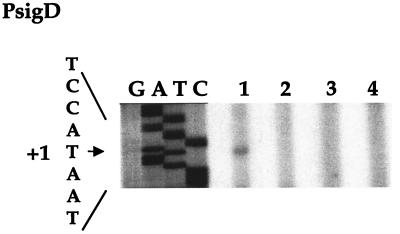
Primer extension analysis of PsigD. A total of 50 μg of total RNA was isolated at T0 and subjected to primer extension analysis using primer OSPE2. In parallel, OSPE2 was utilized in a dideoxy sequencing reaction on plasmid pLM112, which contains the PsigD promoter region and the 5′ half of the sigD gene. Lanes of the sequencing reaction are indicated as G, A, T, and C. Lane 1, LMB1 (wild type); lane 2, LMB232 (PsigDΔ); lane 3, LMB233 (fla/che PAΔ PD-3Δ PsigDΔ); lane 4, LMB234 (fla/che PAΔ PD-3Δ PsigDΔ flgM::mini-Tn10). The +1 position for PsigD is indicated to the left. The sequence indicated corresponds to the template strand (i.e., it is complementary to the sequence indicated in Fig. 1). Lane 1 shows the primer extension product corresponding to the weakly expressed transcript that initiates at PsigD. Lanes 2 to 4 show that this product is absent in PsigDΔ strains.
Surprisingly, we were able to detect ςD in protein extracts of LMB233 and LMB234 (Fig. 4, lanes 8 and 9). This indicates that an additional promoter(s) must contribute to the expression of sigD. Moreover, Fig. 4A indicates that strains LMB233 and LMB234 exhibit ςD levels comparable to those exhibited by strains LMB226 and LMB228. These strains are isogenic to LMB233 and LMB234, respectively, but retain a wild-type copy of PsigD. Two inferences can be drawn from the comparison of these strains. First, PsigD does not contribute significantly to the pool of ςD protein that is expressed in the absence of fla/che PA and PD-3 activity. Second, an additional promoter(s) appears to account for the bulk of the ςD expression in the fla/che PAΔ PD-3Δ strain background.
PsigD is not essential for ςD expression or for motility functions.
It has been suggested that PsigD is required for normal expression of the fla/che operon (1). We explored this possibility by constructing strain LMB232, which carries a chromosomal deletion of PsigD. We confirmed that the primer extension product specific to PsigD is absent in this strain (Fig. 5, lane 4). Primer extension products specific to the fla/che PA and PD-3 transcripts, however, are detected at levels comparable to those found in the wild-type strain (Fig. 4, lane 2). This suggests that expression of the fla/che operon is normal in the absence of PsigD.
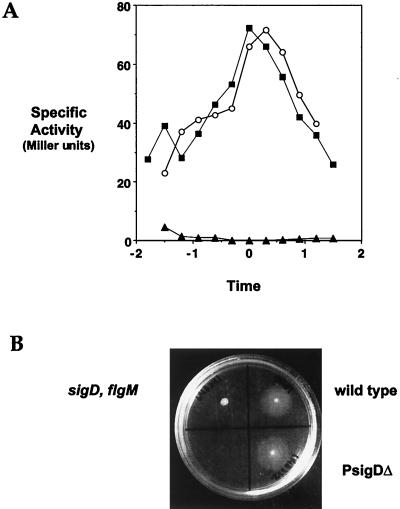
We next examined ςD protein levels and ςD activity. On a Western blot, LMB232 exhibits ςD protein at a level roughly comparable to that found in the wild-type strain (Fig. 3B, lane 3). ςD activity was assessed in two ways. First, we examined flagellin levels in protein lysates isolated from LMB232 at T0.5 and found that flagellin expression is comparable to expression in the wild-type strain (Fig. 3A, lanes 2 and 3). Second, to assess ςD activity throughout growth, we examined the expression of a hag-cat-lacZ transcriptional fusion in strain LMB243. This strain bears the chromosomal deletion of PsigD, as well as a reporter construct consisting of the strong, ςD-dependent promoter of the flagellin (hag) gene driving expression of β-galactosidase. Figure 6A shows that the profile of hag-lacZ-dependent β-galactosidase activity in the LMB243 (PsigDΔ) background is similar to the activity profile exhibited by a wild-type strain bearing the same reporter fusion. This indicates that ςD activity, throughout growth, is normal in the PsigDΔ mutant. In addition, the PsigDΔ strain is motile (Fig. 6B). Altogether, our data indicate that PsigD is not required for fla/che expression, ςD expression, ςD-dependent motility gene expression, or motility.
FIG. 6.
(A) hag-lacZ expression in the PsigDΔ strain. β-Galactosidase activity was monitored in strains bearing hag-lacZ reporter constructs throughout growth from approximately an optical density at 600 nm of 0.1. The x axis represents the time in culture, where 0 h represents the end of logarithmic growth. Symbols: ○, LMB244 (wild type); ■, LMB243 (PsigDΔ); ▴, LMB247 (sigD::pLM5). The PsigDΔ and wild-type strains exhibit comparable patterns of hag-lacZ expression throughout growth. This suggests that PsigD is not required for regulation of ςD expression or activity. (B) Swarm assay of LMB232 (PsigDΔ). Cells of the indicated genotype were inoculated onto PAB–0.4% agar plates and grown at 37°C overnight. LMB232 swarms, like the wild-type strain, indicating that PsigD is not required for motility. LMB241 (sigD::pLM5 flgM::mini-Tn10), the nonmotile negative control strain, does not swarm.
DISCUSSION
Our results allow us to draw several inferences about the relative roles of fla/che PA, PD-3, and PsigD in regulating expression of the fla/che genes and in controlling motility. A number of the salient features of our findings are summarized in Fig. 7.
FIG. 7.
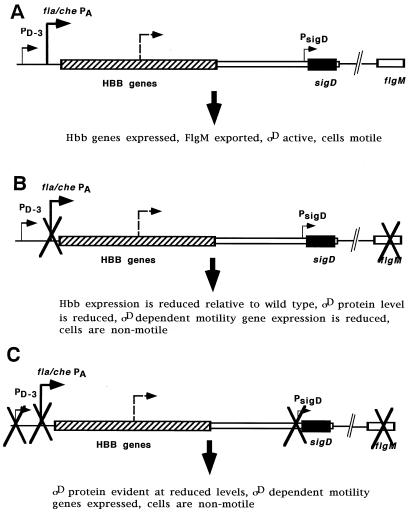
Schematic representation of the fla/che operon and the promoters (arrows) that function in fla/che gene expression. This size of the arrow is proportional to the relative contribution of the designated promoter in gene expression. The dotted arrow represents the previously undescribed promoter(s), which has not yet been localized within the operon. (A) In a wild-type cell, fla/che PA makes the predominant contribution to fla/che expression. PD-3 and PsigD play minor roles. PD-3 is activated because fla/che PA-dependent expression of the HBB genes allow efficient export of FlgM, with consequent activation of ςD. (B) In the absence of fla/che PA activity, sigD is expressed, albeit at reduced levels, from the undefined promoter (dotted line) and PsigD. The resulting ςD is inactive unless flgM is also disrupted. flgM disruption allows activation of ςD, with consequent activation of PD-3 transcription, and ςD-dependent gene expression. ςD-dependent gene expression is reduced relative to wild-type levels, and cells are nonmotile (see Discussion). (C) ςD protein is observed, at reduced levels, in strains that bear deletions of all three known promoters (fla/che PA, PD-3, and PsigD), indicating that another promoter(s) contributes to sigD expression.
fla/che PA appears to be essential for fla/che expression and for motility. Moreover, since expression of ςD and ςD-dependent motility genes is reduced in fla/che PA deletion strains, fla/che PA activity appears to be required for normal expression of these genes, as well. In wild-type cells, the primer extension data indicate that the majority of fla/che transcripts originate from fla/che PA; PD-3 transcripts comprise only a minor fraction of the fla/che transcripts. Moreover, analysis of the fla/che PAΔ mutant indicates that fla/che PA activity is required for expression of the PD-3-specific transcript, as well as for the fla/che PA-specific transcript. ςD protein is evident in a mutant strain that lacks the fla/che PA, but this ςD is incapable of activating transcription from PD-3. This inactivity of ςD is probably a consequence of the lack of HBB gene expression in the absence of fla/che PA activity, which precludes the inactivation of FlgM. PD-3-mediated expression of fla/che genes can be achieved in a fla/che PAΔ strain only if the flgM gene is also disrupted. In this context, in which fla/che gene expression is mediated entirely through PD-3, the overall level of fla/che expression is substantially lower than is observed when fla/che PA is active. Primer extension and Western blot data indicate that the expression of HBB (i.e., flgB) transcript and ςD protein are both reduced relative to the wild type in the fla/che PAΔ flgM double mutant strain. Moreover, this strain background exhibits a reduction of ςD-dependent motility gene expression; Western blot data indicates that flagellin protein is diminished relative to wild-type levels of expression. Finally, the strain that lacks fla/che PA and flgM is nonmotile.
A possible explanation for this lack of motility is that expression of motility genes is reduced below some critical threshold level. As stated above, fla/che expression, which is solely dependent upon PD-3 in this context, is greatly reduced relative to the wild type. Moreover, ςD protein is also reduced relative to the wild type. In addition, this reduction in ςD protein is accompanied by a reduced level of flagellin expression; since it has previously been shown that ςD-dependent motility genes are coordinately regulated in response to ςD activity levels (3), we presume that the expression of other ςD-dependent motility genes is also reduced. Thus, in the fla/che PAΔ flgM::mini-Tn10 strain, the HBB components, sigD, and the ςD-dependent motility genes are all simultaneously expressed at significantly reduced levels. The process of flagellar formation is thought to be highly conserved among B. subtilis and the enteric bacteria, i.e., Salmonella spp. and E. coli. In these enteric bacteria, analysis of stoichiometric ratios of HBB components has revealed that as many as 26 subunits of some components are required in each HBB structure, whereas only 5 or fewer subunits of other components are required (13). Moreover, the assembly process has been found to occur in a stepwise manner and is stalled at particular junctures in mutant backgrounds where structural components are lacking (13, 29, 30). Taking this into account, we think it is possible that the simultaneous and dramatic reduction in expression of the fla/che operon, sigD, and ςD-dependent motility genes could give rise to a situation where one or more of the flagellar components is limiting for assembly. This may underlie the lack of motility that we observe in the strain that lacks fla/che PA and FlgM activities.
An alternative hypothesis to explain this lack of motility is rooted in observations of fla/che expression in the mutant strains that we have examined. In the strain that lacks fla/che PA activity, PD-3 is apparently not transcribed, and ςD protein is reduced relative to the wild type. In the strain that lacks both fla/che PA and FlgM activities, PD-3 transcription is significantly induced, but ςD protein levels remain unchanged. There are two possible explanations for this apparent inability of the PD-3 transcript to contribute to sigD gene expression. The first is that, by comparison with the other relevant promoters, PD-3 makes such a slight contribution to sigD expression that its effect is not sufficient to be detected by Western blot. The second hypothesis is that the transcript that originates at PD-3 terminates upstream of the sigD gene. This hypothesis could explain the observed lack of motility if the PD-3 transcript terminated prior to completing transcription of all of the HBB component genes. In this case, the lack of one or more HBB gene products would stall assembly of the flagella. Such a hypothesis would require that the PD-3 transcript terminate earlier in the operon than the fla/che PA transcript in order to account for the observation that fla/che PA is essential for motility. We have examined the fla/che operon sequence for clues as to a mechanism by which such a PD-3-specific termination event might occur. The PD-3 transcript does not appear to contain an open reading that might be subject to attenuation. Cursory examination of the 133-bp region between PD-3 and fla/che PA (L.M.-M., unpublished observation) suggested that it might contain a site(s) for mediating rho-dependent termination (12). However, we have generated a strain isogenic to LMB216 that bears a disruption in rho (27) and find that motility is not restored (unpublished data). This suggests that rho-dependent termination of the PD-3 transcript in the proximal region of the operon is not the basis for the lack of motility in LMB216. A second possibility is that the transcript that initiates from fla/che PA is specifically subject to some antitermination mechanism. Our cursory analysis of the fla/che operon sequence has not revealed any obvious intrinsic terminators. Moreover, we have not identified any potential target sites for known antitermination mechanisms.
Our results indicate the central role of the fla/che PA in regulating expression of the fla/che operon and of the sigD gene. However, our results indicate that the regulatory roles of PD-3 and PsigD are quite minor. First, as discussed previously, primer extension data indicates that PD-3 makes only a small contribution to fla/che gene expression in wild-type cells. Moreover, the RNA samples utilized for our primer extension experiments were isolated at the time points when ςD expression and activity are maximal. This suggests that PD-3 activation is not the primary factor responsible for the induction of sigD expression and ςD activity. Other data are also consistent with this notion that PD-3 activation does not have a role in the positive autoregulation of sigD expression. Specifically, as described above, induction of transcription from PD-3, in a fla/che PAΔ strain background, does not result in a commensurate increase in ςD levels. Together, these data suggest that PD-3 does not contribute significantly to sigD expression, either in the presence or in the absence of fla/che PA activity.
Our results also indicate that PsigD is not involved in the regulation of fla/che expression. Whereas others (1) have concluded that deletion of PsigD results in delayed activation, as well as reduced levels, of fla/che expression, our results indicate that fla/che expression and ςD activation are normal in PsigDΔ mutant strains. One factor that could account for the difference between these studies is the nature of the deletion strains that were employed. The earlier study utilized chromosomal insertions of plasmids carrying variants of a sigD-lacZ reporter (with or without PsigD); our study employed a deletion of PsigD in its normal chromosomal context. Aside from being expendable for fla/che regulation, our data indicate that PsigD activity is not required for the ςD expression that is observed in fla/che PAΔ strains. Further, ςD levels are comparable in isogenic strains that either carry deletions of fla/che PA and PD-3 or that carry deletions of all three promoters. This indicates that PsigD activity makes very little contribution to the pool of ςD that is observed in strains lacking fla/che PA and PD-3 activity. Altogether, our results indicate that PsigD is not required for motility and that PsigD contributes negligibly to overall ςD levels in cells grown in rich medium under our culture conditions. Recently, it has been suggested that the primary role of PsigD in vivo may be to allow expression of ςD-dependent functions unrelated to motility (e.g., autolysin genes) under conditions in which it is undesirable to induce motility gene expression (31).
Finally, our results indicate that an additional, previously undescribed promoter(s) contributes to sigD expression, since we detect ςD protein and activity in strains where the known promoters have all been deleted. We postulate that this promoter(s) exhibits ςA-dependent activity, since it does not appear to be regulated by FlgM. It seems likely that this putative ςA-dependent promoter(s) lies upstream of PsigD, since the −10 region of PsigD is 30 bases upstream of the initiating codon and no other promoter consensus sequences are found within this region. We have conducted a sequence pattern search of the fla/che operon for sequence elements closely related to the EςA promoter consensus. We have identified at least two potential candidate promoters in intergenic regions internal to the operon that could contribute to expression of sigD; however, it should be noted that PsigD is not within an intergenic region. It will be of interest to test the two aforementioned candidates, as well as other sequences in the 26-kb operon that bear close relationship to the EςA promoter consensus, to assess whether they exhibit promoter activity in vitro and in vivo. The functional promoter(s) can then be tested to determine whether it is required for the sigD expression that we have observed in fla/che PAΔ strains. Finally, it would be of interest to determine the relative contribution of this promoter(s) to overall expression of sigD and to define the conditions under which the promoter(s) is required in vivo.
ACKNOWLEDGMENTS
We thank Carol Gross for support and for helpful suggestions on the manuscript. We thank Sonia Santa Anna-Arriola for construction of LMB226 and LMB228 and Boni Cruz for assisting with the strain constructions. We are grateful to John Helmann for reagents.
This work was supported by an NSF-CAREER grant MCB-900932 to L.M.-M. J.W. was supported by a supplement to the CAREER grant to L.M.-M. and by NIH-RIMI (Research Infrastructure in Minority Institutions) supplemental award (5 P20 RR11805). During the later stages of this work, J.W. was funded by a Minority Post-doctoral Supplement Award to the NIH R01 grant GM32678 to Carol Gross.
REFERENCES
- 1.Allmansberger R. Temporal regulation of sigD from Bacillus subtilis depends on a minor promoter in front of the gene. J Bacteriol. 1997;179:6531–6535. doi: 10.1128/jb.179.20.6531-6535.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertero M G, Gonzales B, Tarricone C, Ceciliani F, Galizzi A. Overproduction and characterization of the Bacillus subtilis anti-sigma factor FlgM. J Biol Chem. 1999;17:12103–12107. doi: 10.1074/jbc.274.17.12103. [DOI] [PubMed] [Google Scholar]
- 3.Caramori T, Barilla D, Nessi C, Sacchi L, Galizzi A. Role of FlgM in ςD-dependent gene expression in Bacillus subtilis. J Bacteriol. 1996;178:3113–3118. doi: 10.1128/jb.178.11.3113-3118.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chadsey M S, Karlinsey J E, Hughes K T. The flagellar anti-ς factor FlgM actively dissociates Salmonella typhimurium ς28 polymerase holoenzyme. Genes Dev. 1998;12:3123–3136. doi: 10.1101/gad.12.19.3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y F, Helmann J D. Restoration of motility to an Escherichia coli fli A flagellar mutant by a Bacillus subtilis sigma factor. Proc Natl Acad Sci USA. 1992;89:5123–5127. doi: 10.1073/pnas.89.11.5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Estacio W, Santa Anna-Arriola S, Adedipe M, Márquez-Magaña L M. Dual promoters are responsible for transcription initiation of the fla/che operon in Bacillus subtilis. J Bacteriol. 1998;180:3548–3555. doi: 10.1128/jb.180.14.3548-3555.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fein J E, Rogers H J. Autolytic enzyme-deficient mutants of Bacillus subtilis 168. J Bacteriol. 1976;127:1427–1442. doi: 10.1128/jb.127.3.1427-1442.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fredrick K, Helmann J D. FlgM is a primary regulator of ςD activity, and its absence restores motility to a sinR mutant. J Bacteriol. 1996;178:7010–7013. doi: 10.1128/jb.178.23.7010-7013.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmann J D, Márquez L M, Chamberlin M J. Cloning, sequencing and disruption of the Bacillus subtilis ς28 gene. J Bacteriol. 1988;170:1568–1574. doi: 10.1128/jb.170.4.1568-1574.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helmann J D. Alternative sigma factors and the regulation of flagellar gene expression. Mol Microbiol. 1991;5:2875–2882. doi: 10.1111/j.1365-2958.1991.tb01847.x. [DOI] [PubMed] [Google Scholar]
- 11.Hughes K T, Gillen K L, Semon M J, Karlinsey J E. Sensing structural intermediates in bacterial flagellar assembly by export of a negative regulator. Science. 1993;262:1277–1280. doi: 10.1126/science.8235660. [DOI] [PubMed] [Google Scholar]
- 12.Ingham C J, Dennis J, Furneaux P A. Autogenous regulation of transcription termination factor Rho and the requirement for Nus factors in Bacillus subtilis. Mol Microbiol. 1999;31:651–63. doi: 10.1046/j.1365-2958.1999.01205.x. [DOI] [PubMed] [Google Scholar]
- 13.Jones C J, Macnab R M. Flagellar assembly in Salmonella typhimurium: analysis with temperature-sensitive mutants. J Bacteriol. 1990;172:1327–1339. doi: 10.1128/jb.172.3.1327-1339.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones C J, Macnab R M, Okino H, Aizawa S-I. Stoichiometric analysis of the flagellar hook-(basal-body) complex of Salmonella typhimurium. J Mol Biol. 1990;212:377–387. doi: 10.1016/0022-2836(90)90132-6. [DOI] [PubMed] [Google Scholar]
- 15.Kutsukake K. Excretion of the anti-sigma factor through a flagellar substructure couples flagellar gene expression with flagellar assembly in Salmonella typhimurium. Mol Gen Genet. 1994;243:605–612. doi: 10.1007/BF00279569. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Zuber P. A molecular switch controlling competence and motility: competence regulatory factors ComS, MecA, and ComK control ςD-dependent gene expression in Bacillus subtilis. J Bacteriol. 1998;180:4243–4251. doi: 10.1128/jb.180.16.4243-4251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Macnab R M. Flagella and motility. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 123–145. [Google Scholar]
- 18.Márquez L, Helmann J D, Ferrari E, Parker H M, Ordal G W, Chamberlin M J. Studies of ςD-dependent functions in Bacillus subtilis. J Bacteriol. 1990;172:3435–3443. doi: 10.1128/jb.172.6.3435-3443.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Márquez-Magaña L, Chamberlin M J. Characterization of the sigD transcription unit of Bacillus subtilis. J Bacteriol. 1994;176:2427–2434. doi: 10.1128/jb.176.8.2427-2434.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mirel D B, Lauer P, Chamberlin M J. Identification of flagellar synthesis regulatory and structural genes in a ςD-dependent operon of Bacillus subtilis. J Bacteriol. 1994;176:4492–4500. doi: 10.1128/jb.176.15.4492-4500.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mirel D B, Lustre V M, Chamberlin M J. An operon of Bacillus subtilis motility genes transcribed by the sigma D form of RNA polymerase. J Bacteriol. 1992;174:4197–204. doi: 10.1128/jb.174.13.4197-4204.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mirel D B, Chamberlin M J. The Bacillus subtilis flagellin gene (hag) is transcribed by the ς28 form of RNA polymerase. J Bacteriol. 1989;174:3095–3101. doi: 10.1128/jb.171.6.3095-3101.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Moran C P., Jr . Measuring gene expression in Bacillus. In: Harwood C R, Cutting S M, editors. Molecular biological methods for Bacillus. Chichester, England: John Wiley & Sons, Ltd.; 1990. pp. 262–282. [Google Scholar]
- 24.Ordal G W, Parker H M, Kirby J K. Complementation and characterization of a chemotaxis mutant in Bacillus subtilis. J Bacteriol. 1985;164:802–810. doi: 10.1128/jb.164.2.802-810.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ordal G W, Márquez-Magaña L, Chamberlin M J. Motility and chemotaxis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biology, biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 765–784. [Google Scholar]
- 26.Perego M. Integrational vectors for genetic manipulation in Bacillus subtilis. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biology, biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 615–624. [Google Scholar]
- 27.Quirk P G, Dunkley E A, Jr, Lee P, Krulwich T A. Identification of a putative Bacillus subtilis rho gene. J Bacteriol. 1993;175:647–654. doi: 10.1128/jb.175.3.647-654.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Suzuki T, Iino T, Horiguchi T, Yamaguchi S. Incomplete flagellar structures in nonflagellate mutants of Salmonella typhimurium. J Bacteriol. 1978;133:904–915. doi: 10.1128/jb.133.2.904-915.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki T, Komeda Y. Incomplete flagellar structures in Escherichia coli mutants. J Bacteriol. 1981;145:1036–1041. doi: 10.1128/jb.145.2.1036-1041.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang D H, von Kalckreuth J, Allmansberger R. Synthesis of the sigmaD protein is not sufficient to trigger expression of motility functions in Bacillus subtilis. J Bacteriol. 1999;181:2942–2946. doi: 10.1128/jb.181.9.2942-2946.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zuberi A R, Ying C W, Weinrich M R, Ordal G W. Transcriptional organization of a cloned chemotaxis locus of Bacillus subtilis. J Bacteriol. 1990;172:1870–1876. doi: 10.1128/jb.172.4.1870-1876.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]