Summary
Background
Primary and secondary myelofibrosis (PMF and SMF) are malignant diseases of hematopoietic stem cell characterized by the neoplastic myeloproliferation and a strong inflammatory milieu. The prognostic nutritional index (PNI) integrates information on albumin and absolute lymphocyte count (ALC) and reflects the inflammatory, nutritional and immune status of a patient. The clinical and prognostic significance of albumin, ALC and PNI in patients with myelofibrosis has not been previously investigated.
Methods
We retrospectively analyzed a cohort of 83 myelofibrosis patients treated in our institution from 2006 to 2017. Albumin, ALC and PNI were assessed in addition to other disease specific markers.
Results
The PMF and SMF patients had significantly lower ALC and PNI but similar albumin compared to controls. Lower albumin was significantly associated with older age and parameters reflecting more aggressive disease biology (e.g. anemia, lower platelet levels, higher lactate dehydrogenase (LDH), circulatory blasts, transfusion dependency, blast phase disease), inflammation (higher C reactive protein (CRP), constitutional symptoms) and higher degree of bone marrow fibrosis. Lower ALC was significantly associated with lower white blood cells (WBC) and lower circulatory blasts. Low PNI was associated with lower albumin, lower ALC, anemia, lower WBCs, lower serum iron and lower transferrin saturation. There was no difference in albumin, ALC and PNI regarding the driver mutations. In multivariate analysis adjusted for age and gender, low albumin (hazard ratio [HR] = 4.61, P = 0.001), low ALC (HR = 3.54, P = 0.004) and Dynamic International Prognostic Scoring System (DIPSS) (HR = 2.45, P = 0.001) were able to predict inferior survival independently of each other. Accordingly, low PNI (HR = 4.32, P< 0.001) predicted poor survival independently of DIPSS (HR = 3.31, P< 0.001).
Conclusion
Assessing albumin, ALC and PNI might improve prognostication in patients with myelofibrosis and could assist in recognition of patients under increased risk of death.
Keywords: Philadelphia chromosome negative myeloproliferative neoplasm, Primary myelofibrosis, Secondary myelofibrosis, Survival, Nutrition
Introduction
Philadelphia chromosome negative (Ph-) myeloproliferative neoplasms (MPN) [1] are clonal disorders of the hematopoietic stem cell. Most patients carry a mutation in either of Janus-kinase-2 (JAK2), calreticulin (CALR) or myeloproliferative leukemia virus oncogene (MPL) genes [2] resulting in a constitutive activation of JAK/signal transducer and activator of transcription (STAT) signalling pathway and a highly inflammatory milieu typical for these diseases [3]. Primary myelofibrosis (PMF) exhibits the most aggressive biological behavior among the Ph-MPNs and bears the highest risk of transformation to acute leukemia and death [4, 5]. It is characterized by megakaryocytic proliferation and atypia, progressive bone marrow fibrosis, leukoerythroblastic blood smear and development of hepatosplenomegaly due to induction of extramedullary hematopoiesis. Patients often suffer from debilitating constitutional symptoms and develop varying numbers and degree of myeloid lineage cytopenias. Patients with two other Ph-MPNs, polycythemia rubra vera (PRV) and essential thrombocythemia (ET), can also develop bone marrow fibrosis and PMF-related features during the disease course [6], when these conditions are termed secondary myelofibrosis (SMF).
The PMF and SMF patients have similar clinical presentation and experience a similar clinical course. The risk of death in MF patients can be determined using the International Prognostic Scoring System (IPSS) [7] at the time of diagnosis and the Dynamic International Prognostic Scoring System (DIPSS) [8] during the course of the disease. Both prognostic systems assign scores for the patient’s age, white blood cell (WBC) count, hemoglobin level, presence of circulatory blasts and the presence of constitutional symptoms.
Serum albumin concentration and absolute lymphocyte count (ALC) are traditional measures of nutritional status that are also influenced by other nonnutritional factors, such as inflammation, stress and specific illness [9–15]. The prognostic nutritional index (PNI), as proposed by Onodera et al. [16], integrates information on these two parameters and is calculated as PNI = serum albumin (g/l) + [5 × ALC (× 109/l)]. This simple indirect measure of immunocompetence, inflammatory and nutritional status has been shown to be predictive of overall survival and perioperative complications in various malignancies [17–22].
A subset of myelofibrosis patients present with a significant weight loss and cachexia which represents an important clinical problem [23]. Similarly, the majority of myelofibrosis patients present with lymphocytopenia and a disturbance in lymphocyte subsets [24]; however, potential prognostic implications of low serum albumin, low ALC and PNI have not been previously studied in patients with MF.
In this study, we aimed to investigate clinical associations of serum albumin concentration, ALC and PNI in patients with MF and assess potential prognostic significance of these parameters.
Patients, materials and methods
Patients
A total of 83 patients with MF who were treated in University Hospital Dubrava in the period from 2006 to 2017 were retrospectively analyzed. All patients fulfilled the World Health Organization (WHO) 2016 criteria for the diagnosis of PMF [1] and the International Working Group for Myelofibrosis Research and Treatment (IWG-MRT) criteria for the diagnosis of SMF [6]. A total of 69 (83.1%) patients were evaluated at the time of establishing the diagnosis and 14 (16.9%) patients were evaluated at the time of referral to our institution. All patients provided a written informed consent for the molecular analyses. The study was approved by the Institutional Review Board. All procedures were in accordance with the ethical standards of the responsible committee on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
The degree of bone marrow fibrosis was determined according to the current European consensus [25]. Serum albumin concentration and ALC were recorded in addition to other demographic, hemato-logical and clinical parameters, e.g. age, gender, WBC count, circulatory blasts, hemoglobin level, mean corpuscular volume (MCV), red cell distribution width (RDW), platelet count, C reactive protein (CRP) level, lactate dehydrogenase (LDH) level, serum iron level, total iron binding capacity (TIBC), transferrin saturation, ferritin level, presence of constitutional symptoms, blast phase disease, transfusion dependency, JAK2, CALR and MPL mutational status. Disease was staged according to the DIPSS, spleen and liver size were assessed by palpation. The PNI was calculated as serum albumin (g/l) + [5 × ALC (× 109/l)]. Additionally, albumin, ALC and PNI of PMF and SMF patients were compared to 30 age and gender matched healthy controls.
Molecular analyses
The deoxyribonucleic acid (DNA) was isolated from full blood by QIAamp DNA Blood Mini Kit (Qiagen, Hliden, Germany, ID 51104), JAK2 V617F was assessed by allele-specific PCR as described previously [26], CALR1 and MPL exon 10 mutations were screened by high-resolution melting dye assays [27, 28] and any sample sequence that deviated from normal was Sanger sequenced.
Statistical analyses
The normality of data distribution was tested using the Kolmogorov-Smirnov test. Numerical variables are presented as either median with interquartile range (IQR), or as arithmetic mean ± standard deviation (SD) depending on the normality of distribution. Categorical variables are presented as proportions. The Mann Whitney U-test, T-test, Kruskal-Wallis test, the χ2-test and the Spearman rank correlation were utilized where appropriate. The Jonckheere-Terpstra test for trend was used to test trends of increase in numerical values over DIPSS risk categories. Survival analyses [29] were performed using the methods of Kaplan and Meier, the Cox-Mantel version of the log-rank test [30] and the Cox regression analysis. Receiver operating characteristic (ROC) curve analysis using survival status as a classification variable was performed in order to determine optimal cut-off values for survival analyses. P values <0.05 were considered significant. Associations of different prognostic factors with survival were screened for via a custom-made MS Excel workbook [31]. Analyses were performed using MedCalc Statistical Software version 17.6 (MedCalc Software BVBA, Ostend, Belgium).
Results
Overview of myelofibrosis patients
In our study, we analyzed a total of 83 patients with MF, 63 (75.3%) of whom were diagnosed with PMF and 20 (24.7%) with SMF. Mean patient age was 65.6 ± 10.4 years, with 51/83 (61.4%) patients of male gender. Patient characteristics are listed in Table 1.
Table 1.
Patient characteristics
| Number of patients | 83 |
| Diagnosis | PMF 63/83 (75.9%) SMF 20/83 (24.1%) |
| Age (years) | 65.6 ± 10.4 |
| Gender | Male 51/83 (61.4%) Female 32/83 (38.6%) |
| Bone marrow fibrosis | Grade 0–I 33/83 (39.8%) Grade II–III 50/83 (60.2%) |
| JAK2 mutations | 52/80 (65%) |
| CALR mutations | 8/63 (12.7%) |
| MPL mutations | 2/63 (3.2%) |
| Constitutional symptoms | 30/82 (36.6%) |
| Massive splenomegaly | 19/80 (23.8%) |
| Blast phase disease | 8/83 (9.6%) |
| WBC (× 109/l) | 11 IQR (6.9–17.4) |
| >1% circulatory blasts | 30/82 (36.6%) |
| Hemoglobin level (g/l) | 113.6 ± 25.9 |
| Platelets (× 109/l) | 336 IQR (178–574) |
| RDW (%) | 19.6 IQR (18.1–21.1) |
| LDH (U/l) | 539 IQR (326–766.5) |
| CRP (mg/l) | 5.3 IQR (2–15.1) |
| Albumin (g/l) | 44 IQR (40–46) |
| ALC (× 109/l) | 1.5 IQR (1–1.9) |
| PNI | 50.5 IQR (47–55.5) |
PMF primary myelofibrosis, SMF secondary myelofibrosis, JAK2 Janus kinase 2, CALR calreticulin, MPL myeloproliferative leukemia virus oncogene, WBC white blood cells, IQR interquartile range, RDW red cell distribution width, LDH lactate dehydrogenase, CRP C reactive protein, ALC absolute lymphocyte count, PNI prognostic nutritional index
Median follow up of our cohort of patients was 51 months. Median overall survival was 67 months and it did not differ between PMF and SMF patients.
Serum albumin concentration
The median serum albumin concentration was 44g/l, IQR (40–46) and it did not significantly differ between PMF, SMF and controls (P = 0.865) as shown in Fig. 1a. Only a small subset of patients, 7/83 (8.4%), presented with hypoalbuminemia, defined as a serum albumin level lower than 35g/l.
Fig. 1.
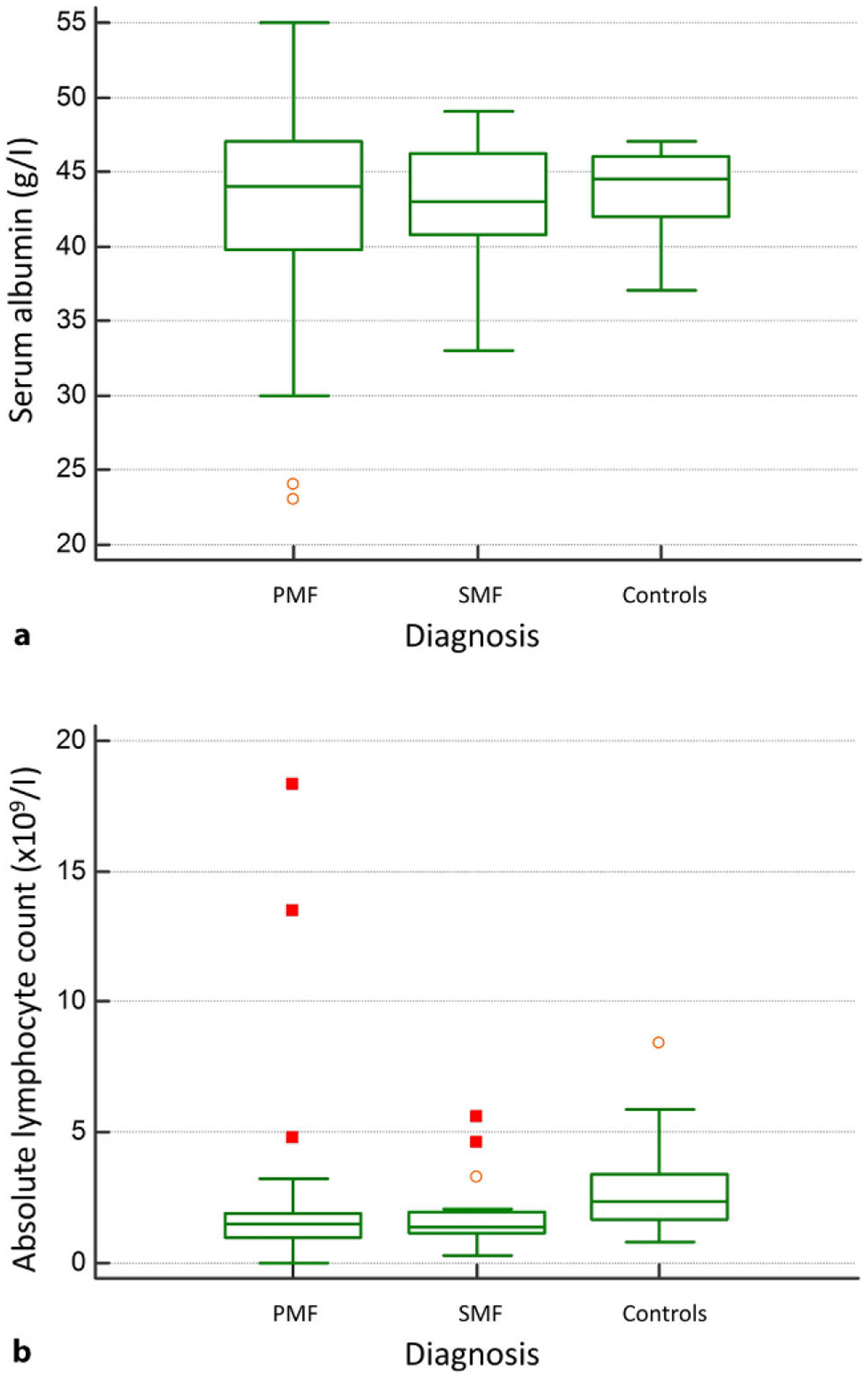
a Serum albumin concentration and b absolute lymphocyte count (ALC) in patients with primary myelofibrosis (PMF), secondary myelofibrosis (SMF) and healthy controls
Higher serum albumin concentration was significantly associated with younger age (Rho = −0.25, P = 0.026), higher hemoglobin level (Rho = 0.41, P< 0.001), lower percentage of circulatory blasts (Rho = −0.33, P = 0.002), higher platelet count (Rho = 0.26, P = 0.021), lower LDH level (Rho = −0.39, P< 0.001), lower RDW (Rho = −0.37, P = 0.001), lower CRP level (Rho = −0.5, P< 0.001), higher serum iron level (Rho = 0.38, P = 0.003), higher TIBC (Rho = 0.28, P = 0.029), absence of constitutional symptoms (median 42g/l vs. 44.5g/l for patients with and without constitutional symptoms, P = 0.001), absence of blast phase disease (median 35g/l vs. 44g/l for patients with and without leukemic transformation of myelofibrosis, P< 0.001), transfusion independency (median 40g/l vs. 44g/l for transfusion dependent and transfusion independent patients, P = 0.019) and lower degree of bone marrow fibrosis (Rho = −0.25, P = 0.035) as depicted in Fig. 2a. There was a clear and significant trend of decrease in serum albumin values over DIPSS risk categories (P< 0.001) as shown in Fig. 2b. There was no statistically significant correlation between serum albumin concentration and gender, WBC count, ALC, spleen and liver size, MCV, transferrin saturation, ferritin level, JAK2, CALR or MPL mutational status.
Fig. 2.
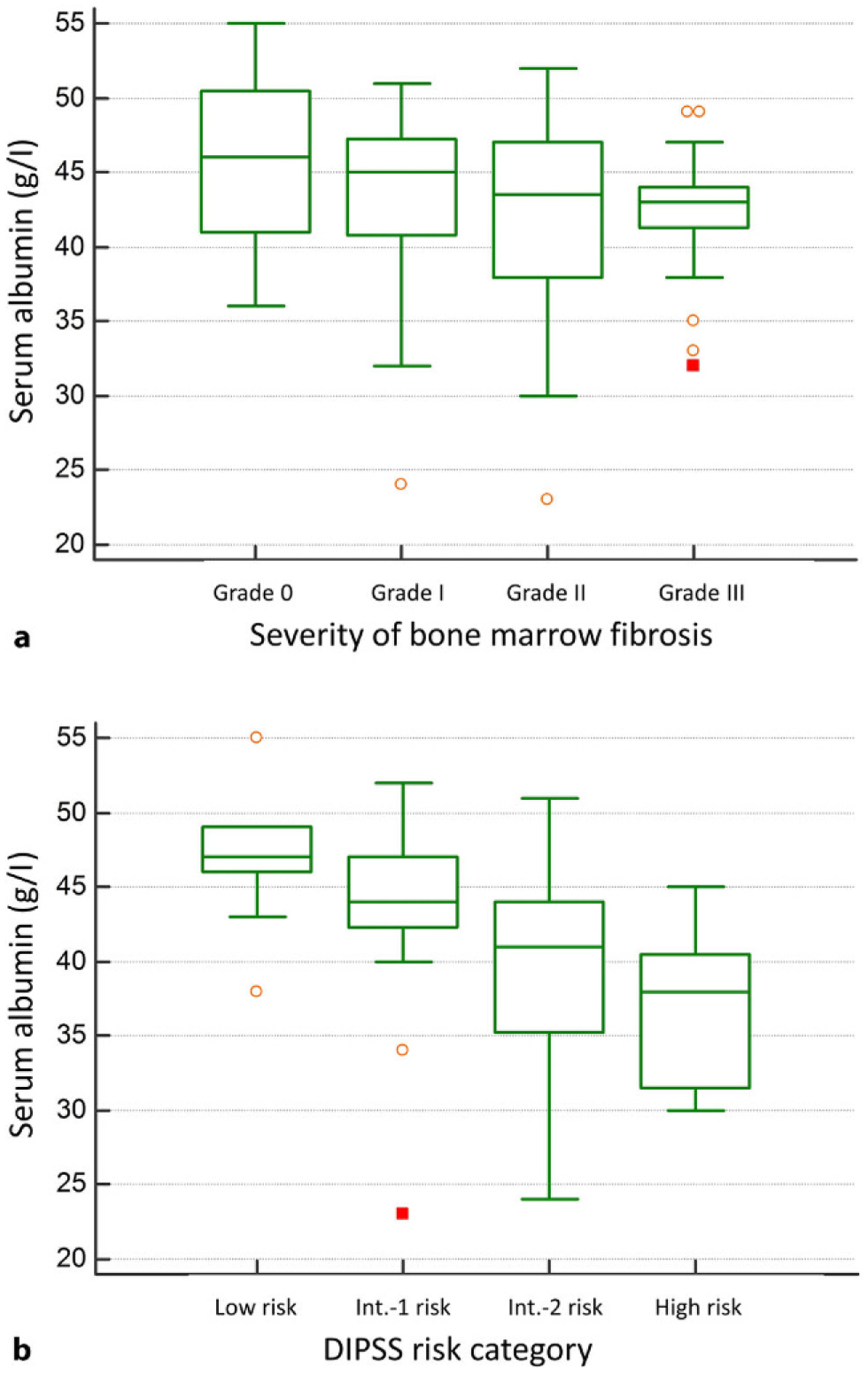
a Serum albumin concentration regarding the degree of bone marrow fibrosis and b Serum albumin concentration regarding the Dynamic International Prognostic Scoring System (DIPSS) risk categories. Int. intermediate
Optimal serum albumin cut-off value for survival analyses was determined using the ROC curve analysis and patients were divided into high albumin (>42g/l) and low albumin (≤42g/l) groups. Low albumin was univariately associated with shorter overall survival (HR = 6.49, P< 0.001) as shown in Fig. 3a. This association remained significant in the Cox regression model adjusted for age, gender and DIPSS (low albumin HR = 3.84, P< 0.001; DIPSS HR = 2.51, P< 0.001; age and gender not significant).
Fig. 3.
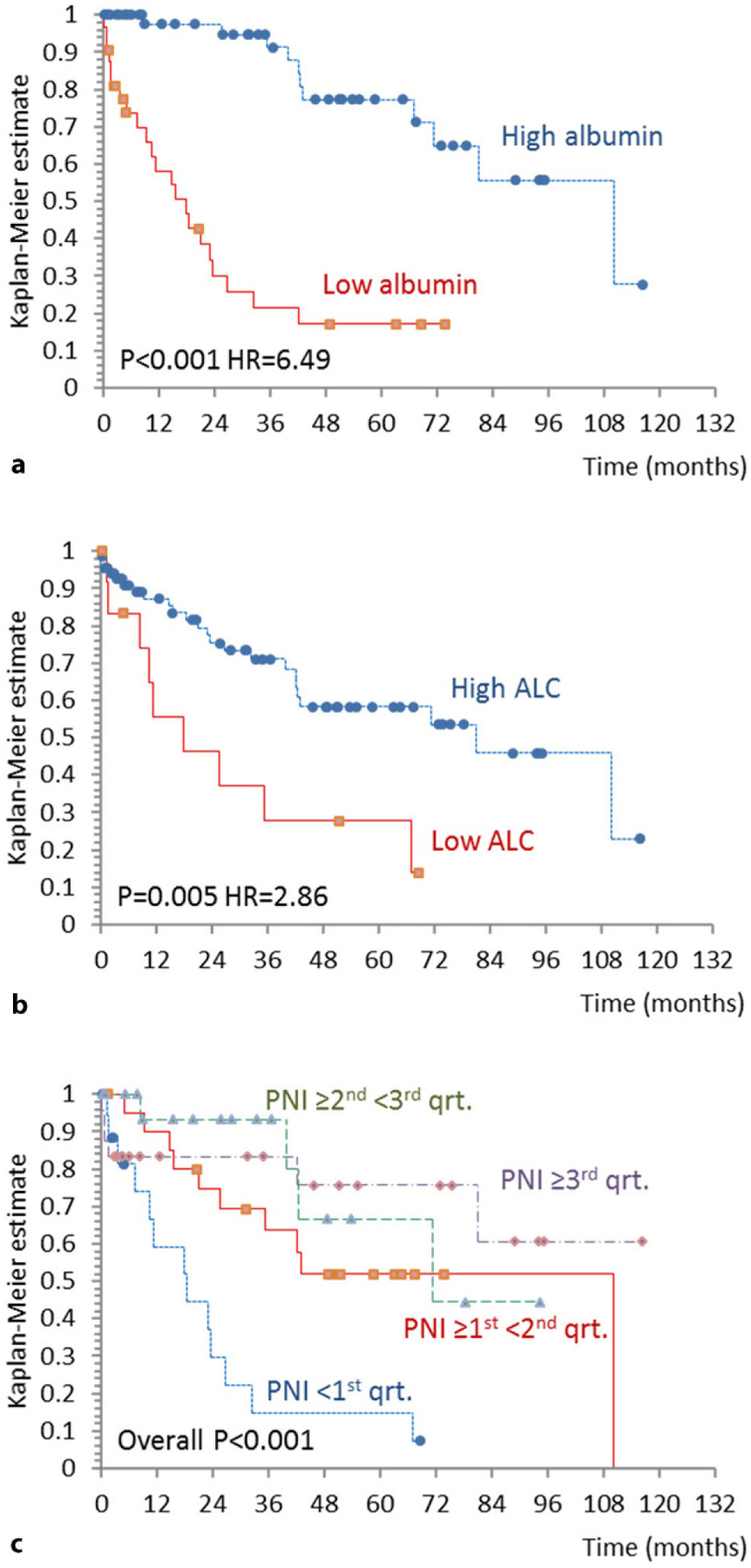
Overall survival (OS) of patients with myelofibrosis stratified by a serum albumin concentration, b absolute lymphocyte count (ALC) and c prognostic nutritional index (PNI). HR hazard ratio, qrt. quartile
Absolute lymphocyte count
Median ALC was 1.5 × 109/l, IQR (1–1.9) and it differed significantly between patients and controls (P< 0.001). Although there was no significant difference between PMF and SMF patients with respect to ALC, both subgroups had significantly lower values in comparison to controls (P< 0.05 for both comparisons) as depicted in Fig. 1b. A subset of 16/83 (19.3%) patients presented with ALC values lower than 1 × 109/l.
Higher ALC was significantly associated with higher WBC count (Rho = 0.48, P< 0.001) and higher percentage of circulatory blasts (Rho = 0.28, P = 0.010). We did not observe statistically significant correlations with other tested parameters (age, gender, hemoglobin level, platelet count, LDH level, CRP level, albumin, MCV, RDW, parameters of iron metabolism, spleen or liver size, constitutional symptoms, transfusion dependency, blast phase disease, degree of bone marrow fibrosis, JAK2, CALR or MPL mutational status). In addition, ALC did not correlate with DIPSS.
Optimal ALC cut-off value for survival analyses was determined by the ROC curve analysis and patients were divided into high ALC (>0.86 × 109/l) and low ALC (≤0.86 × 109/l) groups. Low ALC group presented with significantly shorter overall survival (HR = 2.86, P = 0.005) as shown in Fig. 3b. Low ALC remained significantly associated with poor survival in the multivariate Cox regression model adjusted for age, gender and DIPSS (low ALC HR = 3.65, P = 0.002; DIPSS HR = 3.66, P< 0.001; age and gender not significant). We could also demonstrate that low ALC, low albumin and DIPSS can predict inferior overall survival independently of each other in the model including low ALC, low albumin and DIPSS, age and male gender as specified in Table 2.
Table 2.
Age and gender adjusted Cox regression analysis model demonstrating independent prognostic properties of low albumin, low absolute lymphocyte count (ALC), and Dynamic International Prognostic Scoring System (DIPSS) in patients with myelofibrosis
| Low albumin | 4.61 | [1.96–10.85] | 0.001* |
| Low ALC | 3.54 | [1.5–8.37] | 0.004* |
| DIPSS | 2.45 | [1.42–4.22] | 0.001* |
| Age (years) | 1.02 | [0.98–1.07] | 0.338 |
| Male gender | 0.79 | [0.36–1.71] | 0.548 |
HR hazard ratio, C.I. confidence interval, ALC absolute lymphocyte count, DIPSS Dynamic International Prognostic Scoring System
Statistically significant result (P< 0.05)
Prognostic nutritional index (PNI)
We further investigated how PNI, which incorporates information on serum albumin concentration and ALC, affects survival of patients with MF. Median PNI was 50.5, IQR (47–55.5) and it did not statistically significantly differ between PMF and SMF patients but was lower in both groups of patients when compared to healthy controls (P< 0.05 for both comparisons). Patients were divided into subgroups based on the PNI score quartiles and we could demonstrate that there is a significant difference in overall survival between patient groups (P< 0.001) as shown in Fig. 3c. Patients with PNI lower than the first quartile (PNI< 47) had significantly inferior overall survival in comparison to all the other subgroups (P< 0.008 for all comparisons, considering the Bonferroni correction for multiple comparisons). Patients in other three subgroups based on PNI quartiles had similar disease course and did not significantly differ in overall survival. Having PNI< 47 remained statistically significantly associated with shorter overall survival in the Cox regression model adjusted for age, gender and DIPSS (PNI< 47 HR = 4.32, P< 0.001; DIPSS HR = 3.31, P< 0.001; age and gender not significant).
Patients with the PNI lower than the first quartile expectedly had lower serum albumin concentration (median 38.5g/l vs. 45g/L, P< 0.001) and lower ALC (median 0.93 × 109/l vs. 1.5 × 109/l, P< 0.001), but also had lower hemoglobin levels (100.5 ± 16.7 vs. 117.1 ± 26.8g/l, P = 0.018), lower WBC count (median 6.9 vs. 11.4 × 109/l, P = 0.044), lower serum iron level (median 5.3 vs. 13.2 μmol/l, P = 0.006) and lower transferrin saturation (median 8% vs. 27%, P = 0.020) compared to those with the higher PNI, but did not significantly differ in other disease-specific parameters.
Discussion
To the best of our knowledge, this study is first to investigate clinical correlations and prognostic significance of serum albumin, ALC and PNI in patients with MF and to report their DIPSS-independent prognostic properties.
Although being traditionally considered as measures of nutritional status [9–12], serum albumin concentration and ALC are strongly affected by a variety of other factors, most notably inflammation [13, 32]. As we have shown in our current study, lower serum albumin level was associated with parameters reflecting more aggressive disease biology (higher LDH level, higher percentage of circulatory blasts, blast phase disease, transfusion dependency), higher inflammatory milieu (higher CRP level, presence of constitutional symptoms) and also with parameters that could reflect nutritional status of PMF/SMF patients (lower serum iron level, anemia). Lower serum albumin was associated with an increasing degree of bone marrow fibrosis suggesting that these two phenomena might be regulated by a similar cytokine profile. Associations of low serum albumin with a large number of aforementioned negative prognostic parameters were reflected in inferior overall survival in our cohort of patients. Treatment of MF patients with the JAK1/2 inhibitor ruxolitinib typically results in a reduction of inflammatory cytokines, improvement of constitutional symptoms and a reduction in spleen size, but also in an improvement in body weight and serum albumin concentration [33]. Survival advantage seen in a subset of ruxolitinib-treated patients might be at least in part mediated through the improvement in nutritional status; however, role of albumin as prognostic marker in this setting has not yet been evaluated.
Our finding of a decreased ALC in MF patients in comparison to healthy controls is in line with the previous observation of lymphocytopenia as a characteristic feature of the disease [24]. Remarkably, ALC did not show statistically significant associations with most parameters of disease severity, except positive correlations with WBC count and circulatory blasts, which are considered to be negative prognostic markers [7]. In contrast, higher ALC in our cohort of patients seems to bear positive prognostic implications, and its effect on improved survival could be mediated by decreased susceptibility to infections as observed in other clinical situations [34]. A recent large retrospective study that investigated factors associated with infections in patients with MF unfortunately did not investigate lymphocyte count as a possible factor [35], leaving this issue unresolved. In addition, neither serum albumin, nor ALC were associated with mutations in JAK2, CALR and MPL genes suggesting that their changes do not reflect driver mutation-specific phenomena.
Our most striking observation was that low serum albumin, low ALC and DIPSS all seem to provide additional prognostic information and could predict inferior overall survival independently of each other (Table 2). This suggests that all three parameters reflect different pathophysiological processes and assessing all three of them might improve prognostication of patients with MF. Due to their independent associations with survival in these patients, it is reasonable to combine information on serum albumin and ALC into a composite index abbreviated PNI as previously proposed by Onodera et al. [16]. The PNI is considered to reflect inflammatory, nutritional and immune status of an oncological patient and was shown to be of prognostic value in a variety of malignant neoplastic diseases [17–22]. As we have observed in our cohort of MF patients, a quarter of patients presenting with the lowest PNI values (<47) had lower WBC count, lower hemoglobin level, lower serum iron level and lower transferrin saturation in addition to lower serum albumin concentration and lower ALC which all might have been affected by the nutritional status of a patient. A quarter of patients presenting with the lowest PNI values experienced unfavorable disease course with the significantly shorter overall survival in comparison to other three subcohorts (Fig. 3c). Interestingly, all patients with PNI≥ 47 had similar prognosis and their survival was not significantly affected by the assignment to the particular PNI subgroup. Therefore, only reaching below a critical PNI threshold seems to be negatively affecting survival through a process that might be independent of disease-specific mechanisms measured by the currently established DIPSS prognostic system.
The main limitations of our work are single institution experience, retrospective study design, small number of patients and heterogeneous patient population. Retrospective study design prevents us from assessing anthropometric measures of nutritional status, such as body mass index, waist circumference or triceps skin fold, as well as correlating them to parameters of interest. In addition, study was conducted over an 11-year period during which patients were exposed to different disease-oriented and other supportive therapies that might modulate effects of measured parameters. A subset of patients that were treated upfront with ruxolitinib (at the time of diagnosis) was relatively small and most of intermediate 2 and high-risk patients received the drug later during the course of the disease (when it became available), and not synchronous with the study baseline. Short follow-up, small number of events and consequent statistical power limitations prevent us from properly investigating whether studied phenomena persist in a subcohort of ruxolitinib treated patients. Nevertheless, our study identifies serum albumin, ALC and PNI as useful and easy to obtain parameters that bear valuable clinical information in patients with MF. Hence, future studies investigating these parameters in larger prospective cohorts of patients are warranted, especially from the point of view of new and emerging therapies with cachexia modulatory properties.
In conclusion, serum albumin, ALC and DIPSS predict survival independently of each other. The PNI integrates information on serum albumin and ALC and similarly provides DIPSS-independent prognostic information. Assessing these parameters could improve prognostication of patients with myelofibrosis and help in recognition of patients under an increased risk of death, therefore enabling timely supportive interventions.
Footnotes
Conflict of interest M. Lucijanic, I. Veletic, D. Rahelic, V. Pejsa, D. Cicic, M. Skelin, A. Livun, K.M. Tupek, T. Stoos-Veic, T. Lucijanic, A. Maglicic, and R. Kusec declare that they have no competing interests.
Ethical standards All procedures performed in studies involving human participants were in accordance with the ethical standards of the Institutional Review Board and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all subjects in whom molecular studies were performed.
Contributor Information
Marko Lucijanic, Hematology Department, University Hospital Dubrava, Av. Gojka Suska 6, 10000 Zagreb, Croatia.
Ivo Veletic, Department of Leukemia, The University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Dario Rahelic, Endocrinology, Diabetes and Metabolic Disorders Department, University Hospital Dubrava, Zagreb, Croatia; School of Medicine, University of Zagreb, Zagreb, Croatia.
Vlatko Pejsa, Hematology Department, University Hospital Dubrava, Av. Gojka Suska 6, 10000 Zagreb, Croatia; School of Medicine, University of Zagreb, Zagreb, Croatia.
David Cicic, Hematology Department, University Hospital Dubrava, Av. Gojka Suska 6, 10000 Zagreb, Croatia.
Marko Skelin, Pharmacy Department, General Hospital Sibenik, Sibenik, Croatia.
Ana Livun, Divison of Molecular Diagnosis and Genetics, Clinical Department of Laboratory Diagnostics, University Hospital Dubrava, Zagreb, Croatia.
Katarina Marija Tupek, Divison of Molecular Diagnosis and Genetics, Clinical Department of Laboratory Diagnostics, University Hospital Dubrava, Zagreb, Croatia.
Tajana Stoos-Veic, Department of Clinical Cytology and Cytometry, University, Hospital Dubrava, Zagreb, Croatia; Faculty of Medicine, University of Osijek, Osijek, Croatia.
Tomo Lucijanic, Endocrinology, Diabetes and Metabolic Disorders Department, University Hospital Dubrava, Zagreb, Croatia.
Ana Maglicic, Health Care Center Vojnic, Vojnic, Croatia.
Rajko Kusec, Hematology Department, University Hospital Dubrava, Av. Gojka Suska 6, 10000 Zagreb, Croatia; School of Medicine, University of Zagreb, Zagreb, Croatia; Divison of Molecular Diagnosis and Genetics, Clinical Department of Laboratory Diagnostics, University Hospital Dubrava, Zagreb, Croatia.
References
- 1.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and a cute leukemia. Blood. 2016;127(20):2391–405. [DOI] [PubMed] [Google Scholar]
- 2.Sliwa T, Beham-Schmid C, Burgstaller S, et al. Austrian recommendations for the management of primary myelofibrosis, post-polycythemia vera myelofibrosis and post-essential thrombocythemia myelofibrosis: an expert statement. Wien Klin Wochenschr. 2017;129(9–10):293–302. [DOI] [PubMed] [Google Scholar]
- 3.Hasselbalch HC. Perspectives on chronic inflammation in essential thrombocythemia, polycythemia vera, and myelofibrosis: is chronic inflammation a trigger and driver of clonal evolution and development of accelerated atherosclerosis and second cancer? Blood. 2012;119(14):3219–25. [DOI] [PubMed] [Google Scholar]
- 4.Hultcrantz M, Kristinsson SY, Andersson TM, et al. Patterns of survival among patients with myeloproliferative neoplasms diagnosed in Sweden from 1973 to 2008: a population-based study. JClinOncol. 2012;30(24):2995–3001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yogarajah M, Tefferi A. Leukemic transformation in myeloproliferative neoplasms: a literature review on risk, characteristics, and outcome. Mayo Clin Proc. 2017;92(7):1118–28. [DOI] [PubMed] [Google Scholar]
- 6.Barosi G, Mesa RA, Thiele J, et al. Proposed criteria for the diagnosis of post-polycythemia vera and post-essential thrombocythemia myelofibrosis: a consensus statement from the International Working Group for Myelofibrosis Research and Treatment. Leukemia. 2008;22(2):437–8. [DOI] [PubMed] [Google Scholar]
- 7.Cervantes F, Dupriez B, Pereira A, et al. New prognostic scoring system for primary myelofibrosis based on a study of the International Working Group for Myelofibrosis Research and Treatment. Blood. 2009;113(13):2895–901. [DOI] [PubMed] [Google Scholar]
- 8.Passamonti F, Cervantes F, Vannucchi AM, et al. A dynamic prognostic model to predict survival in primary myelofibrosis: a study by the IWG-MRT (International Working Group for Myeloproliferative Neoplasms Research and Treatment). Blood. 2010;115(9):1703–8. [DOI] [PubMed] [Google Scholar]
- 9.Tayek JA. Albumin synthesis and nutritional assessment. Nutr Clin Pract. 1988;3(6):219–21. [DOI] [PubMed] [Google Scholar]
- 10.Fock RA, Blatt SL, Beutler B, et al. Study of lymphocyte subpopulations in bone marrow in a model of protein-energy malnutrition. Nutrition. 2010;26(10):1021–8. [DOI] [PubMed] [Google Scholar]
- 11.Rocha NP, Fortes RC. Total lymphocyte count and serum albumin as predictors of nutritional risk in surgical patients. Arq Bras Cir Dig. 2015;28(3):193–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saito H, Nomura K, Hotta M, Takano K. Malnutrition induces dissociated changes in lymphocyte count and subset proportion in patients with anorexia nervosa. Int J Eat Disord. 2007;40(6):575–9. [DOI] [PubMed] [Google Scholar]
- 13.Bharadwaj S, Ginoya S, Tandon P, et al. Malnutrition: laboratory markers vs nutritional assessment. Gastroenterol Rep. 2016;4(4):272–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Z, Pereira SL, Luo M, Matheson EM. Evaluation of blood Biomarkers associated with risk of malnutrition in older adults: a systematic review and meta-analysis. Nutrients. 2017;9(8):E829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Blackburn GL, Bistrian BR, Maini BS, Schlamm HT, Smith MF. Nutritional and metabolic assessment of the hospitalized patient. JPEN J Parenter Enteral Nutr. 1977;1(1):11–22. [DOI] [PubMed] [Google Scholar]
- 16.Onodera T, Goseki N, Kosaki G. Prognostic nutritional index in gastrointestinal surgery of malnourished cancer patients. Nihon Geka Gakkai Zasshi. 1984;85(9):1001–5. [PubMed] [Google Scholar]
- 17.Perisa V, Zibar L, Knezovic A, Perisa I, Sincic-Petricevic J, Aurer I. Prognostic nutritional index as a predictor of prognosis in patients with diffuse large B cell lymphoma. Wien Klin Wochenschr. 2017;129(11–12):411–9. [DOI] [PubMed] [Google Scholar]
- 18.Sun K, Chen S, Xu J, Li G, He Y. The prognostic significance of the prognostic nutritional index in cancer: a systematic review and meta-analysis. J Cancer Res Clin Oncol. 2014;140(9):1537–49. [DOI] [PubMed] [Google Scholar]
- 19.Okada S, Shimada J, Kato D, Tsunezuka H, Teramukai S, Inoue M. Clinical significance of prognostic nutritional index after surgical treatment in lung cancer. Ann Thorac Surg. 2017;104(1):296–302. [DOI] [PubMed] [Google Scholar]
- 20.Yang Y, Gao P, Chen X, et al. Prognostic significance of preoperative prognostic nutritional index in colorectal cancer: results from a retrospective cohort study and ameta-analysis. Oncotarget. 2016;7(36):58543–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee JY, Kim HI, Kim YN, et al. Clinical significance of the prognostic nutritional index for predicting short- and long-term surgical outcomes after gastrectomy: a retrospective analysis of 7781 gastric cancer patients. Medicine (Baltimore). 2016;95(18):e3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SH, Chung MJ, Kim B, et al. The significance of the prognostic nutritional index for all stages of pancreatic cancer. Nutr Cancer. 2017;69(3):512–9. [DOI] [PubMed] [Google Scholar]
- 23.Scherber RM, Mesa RA. Relevance of weight loss, splenomegaly, and hypocholesterolemia in the treatment of myeloproliferative neoplasms—implications for a JAK2 inhibitor era. Oncol Hematol Rev. 2011;7(1):61–3. [Google Scholar]
- 24.Cervantes F, Hernandez-Boluda JC, Villamor N, Serra A, Montserrat E. Assessment of peripheral blood lymphocyte subsets in idiopathic myelofibrosis. Eur J Haematol. 2000;65(2):104–8. [DOI] [PubMed] [Google Scholar]
- 25.Thiele J, Kvasnicka HM, Facchetti F, Franco V, van der Walt J, Orazi A. European consensus on grading bone marrow fibrosis and assessment of cellularity. Haematologica. 2005;90(8):1128–32. [PubMed] [Google Scholar]
- 26.Baxter EJ, Scott LM, Campbell PJ, et al. Acquired mutation of the tyrosine kinase JAK2 in human myeloproliferative disorders. Lancet. 2005;365(9464):1054–61. [DOI] [PubMed] [Google Scholar]
- 27.Bilbao-Sieyro C, Santana G, Moreno M, et al. High resolution melting analysis: a rapid and accurate method to detect CALR mutations. PLoS ONE. 2014;9(7):e103511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pardanani A, Guglielmelli P, Lasho TL, et al. Primary myelofibrosis with or without mutant MPL: comparison of survival and clinical features involving 603 patients. Leukemia. 2011;25(12):1834–9. [DOI] [PubMed] [Google Scholar]
- 29.Lucijanic M, Petrovecki M. Analysis of censored data. Biochem Med. 2012;22(2):151–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lucijanic M, Skelin M, Lucijanic T. Survival analysis, more than meets the eye. Biochem Med. 2017;27(1):14–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lucijanic M Survival analysis in clinical practice: analyze your own data using an Excel workbook. Croat Med J. 2016;57(1):77–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nunez J, Minana G, Bodi V, et al. Low lymphocyte count and cardiovascular diseases. Curr Med Chem. 2011;18(21):3226–33. [DOI] [PubMed] [Google Scholar]
- 33.Mesa RA, Verstovsek S, Gupta V, et al. Effects of ruxolitinib treatment on metabolic and nutritional parameters in patients with myelofibrosis from COMFORT-I. Clin Lymphoma Myeloma Leuk. 2015;15(4):214–221.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gluck T, Kiefmann B, Grohmann M, Falk W, Straub RH, Scholmerich J. Immune status and risk for infection in patients receiving chronic immunosuppressive therapy. J Rheumatol. 2005;32(8):1473–80. [PubMed] [Google Scholar]
- 35.Polverelli N, Breccia M, Benevolo G, et al. Risk factors for infections in myelofibrosis: role of disease status and treatment. A multicenter study of 507 patients. Am J Hematol. 2017;92(1):37–41. [DOI] [PubMed] [Google Scholar]


