Abstract
In recent years, new targeted therapies have been developed to treat patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) breast cancer. Some of these therapies have not just become the new therapy standard but also led to significantly longer overall survival rates. The cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) have become the therapeutic standard for first-line therapy. Around 70 – 80% of patients are treated with a CDK4/6i. In recent years, a number of biomarkers associated with progression, clonal selection or evolution have been reported for CDK4/6i and their endocrine combination partners. Understanding the mechanisms behind treatment efficacy and resistance is important. A better understanding could contribute to planning the most effective therapeutic sequences and utilizing basic molecular information to overcome endocrine resistance. One study with large numbers of patients which aims to elucidate these mechanisms is the Comprehensive Analysis of sPatial, TempORal and molecular patterns of ribociclib efficacy and resistance in advanced Breast Cancer patients (CAPTOR BC) trial. This overview summarizes the latest clinical research on resistance to endocrine therapies, focusing on CDK4/6 inhibitors and discussing current study concepts.
Keywords: breast cancer, therapy, CDK4/6
Introduction
In 2009, a preclinical trial first noted that cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i) could play a special role in the treatment of breast cancer patients with hormone receptor-positive (HR+)/human epidermal growth factor receptor 2-negative (HER2−) disease 1 . Since then, the three CDK4/6is ribociclib, palbociclib and abemaciclib have become part of the standard first-line treatment provided to HR+/HER2− patients with advanced disease 2 , 3 , 4 . Two studies have looked at the use of CDK4/6i in the adjuvant setting and reported a benefit with regards to invasive recurrence-free survival 5 , 6 . Abemaciclib has already been approved for use in the adjuvant setting while ribociclib is still awaiting approval.
The widespread use of these substances in the treatment algorithms of patients with breast cancer clearly shows that understanding the modes of action of and resistance mechanisms to CDK4/6i is an important precondition for developing therapies for this group of patients.
In this context, both retrospective analyses and prospective studies are investigating whether and how patient groups can be identified who would particularly benefit from or be disadvantaged by therapy with CDK4/6i.
Some concepts have focused on the efficacy of the endocrine combination partner by determining mutations in the estrogen receptor gene (ESR1) . Others are studying the molecular changes which occur during CDK4/6i therapy or investigating the overall effect of molecular or immunological patterns on their efficacy. A number of study programs have been set up in recent years which specifically focus on identifying these mechanisms.
This overview presents the current status of clinical and translational research. Current national and international study programs investigating the modes of action of CDK4/6i are also described. In Germany, for example, attention has focused on the CAPTOR BC trial, which is extensively collecting biomaterials to identify markers for both efficacy and resistance.
CDK4/6 Inhibitors
High preclinical activity in HR+/HER2− cell lines
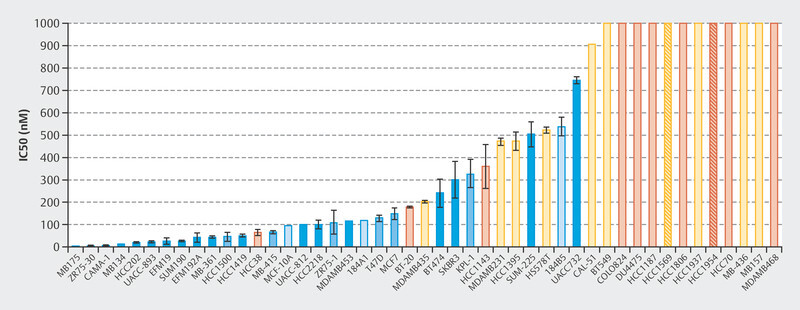
The efficacy of treatment with palbociclib was initially investigated in a number of cell lines 1 . A total of 47 cell lines were studied to identify the different molecular subtypes of breast cancer and the variability between patients. Fig. 1 shows that particularly with HR+, luminal cell lines responded well to treatment with palbociclib 1 . This study and other preclinical studies were followed by early clinical trials with palbociclib, in particular the PALOMA-1/TRIO-18 trial which led to the approval of palbociclib for the treatment of metastatic breast cancer 7 . During the initial preclinical studies with cell lines 1 , extensive tests were already being carried out to determine which biomarkers correlated with response in addition to hormone receptor positivity. Microchip technology was used for genome-wide evaluation to determine which genes correlated preclinically with palbociclib efficacy. Around 450 genes were identified where expression varied between cell lines which responded to palbociclib and cell lines that did not 1 . In sensitive cell lines, the expression of genes which coded for the retinoblastoma protein (Rb) and cyclin D increased but the expression of CDKN2A decreased 1 . These early studies already showed that the mode of action of CDK4/6i therapy depends on molecular markers. Following this and other studies, large randomized studies of advanced breast cancer were carried out which led to the approval of CDK4/6i for this indication.
Fig. 1.
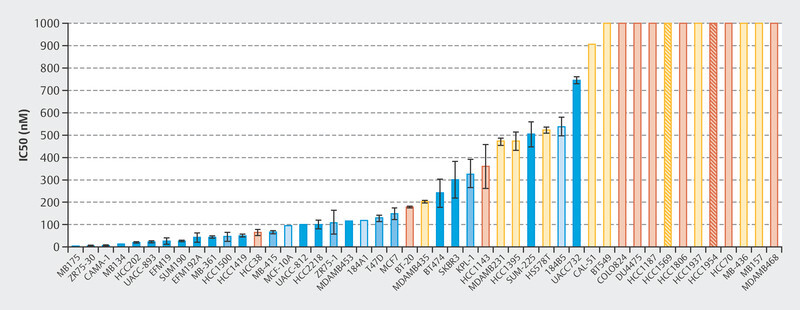
Concentrations of palbociclib required to inhibit 50% of cell growth 1 .
Introduction of CDK4/6 Inhibitors into Clinical Practice
Studies to establish the use of CDK4/6 inhibitors
Studies with the three CDK4/6is palbociclib, ribociclib and abemaciclib were carried out in different clinical scenarios and with different endocrine combination partners. The findings of the studies which investigated the use of CDK4/6i as first line-therapy can be grouped together. These trials mainly included patients in whom the probability of endocrine resistance was low. The PALOMA-2 (palbociclib), MONALEESA-2 (ribociclib) and MONARCH-3 (abemaciclib) trials therefore chose an aromatase inhibitor as the combination partner. But there are also a number of studies which included patients with a high probability of endocrine resistance. These were usually patients who were receiving more advanced therapy lines or with rapid progression under an aromatase inhibitor. Fulvestrant was selected as the combination partner in the PALOMA-3 (palbociclib) and MONARCH-2 (abemaciclib) trials. Two other studies investigated additional questions. The MONALEESA-3 trial which included patients receiving both early and advanced therapy lines also provides results for ribociclib + fulvestrant as a first-line therapy. The MONALEESA-7 trial provides results for ribociclib + an aromatase inhibitor in exclusively premenopausal patients.
All studies showed comparable effects with regards to progression-free survival. Median progression-free survival (PFS) almost doubled in all of the studies. With regards to overall survival, however, the studies showed clinically relevant differences. The two palbociclib studies did not achieve a statistically significant prolongation of overall survival, whereas the remaining studies showed a statistically significant reduction in the relative mortality risk of around 25%. Although the recent interim analysis of the MONARCH-3 trial seems to suggest an overall survival benefit, the final analysis has not yet been published. Table 1 provides an overview of the most important randomized phase III trials with CDK4/6i.
Table 1 Overview of randomized phase III trials with palbociclib, abemaciclib and ribociclib for the treatment of patients with advanced breast cancer.
| N | Therapy | Last patient in | PFS HR (95% CI) |
Median PFS CDK4/6i | Placebo* |
OS HR (95% CI) |
Median OS CDK4/6i | Placebo* |
Percentage with de novo metastasis | Percentage of patients with DFI < 12 months | References | |
|---|---|---|---|---|---|---|---|---|---|---|
| * = interim analysis, ** = not reported, DFI = disease-free interval, HR = hazard ratio, NA = not applicable, OS = overall survival, PFS = progression-free survival | ||||||||||
| MONALEESA-2 | 668 | ribociclib ± letrozole | 03/2015 | 0.56 (0.43 – 0.72) | 25.3 | 16.0 | 0.76 (0.63 – 0.93) | 53.9 | 51.2 | 34% | 18% | 41 , 42 |
| MONARCH-3 | 493 | abemaciclib ± NSAI | 11/2015 | 0.54 (0.41 – 0.72) | 28.2 | 14.8 | 0.76 (0.58 – 0.97) | 67.1 | 54.1 | 40% | ** | 43 , 44 |
| PALOMA-2 | 666 | palbociclib ± letrozole | 07/2014 | 0.58 (0.46 – 0.72) | 24.8 | 14.5 | 0.96 (0.78 – 1.18) | 63.9 | 51.4 | 37% | 22% | 45 , 46 |
| MONALEESA-7 | 672 | ribociclib ± ET | 08/2016 | 0.55 (0.44 – 0.69) | 23.8 | 13.0 | 0.71 (0.54 – 0.95) | 58.7 | 48.0 | 19% | 4.3% | 47 , 48 , 49 |
| MONALEESA-3 | 726 | ribociclib ± fulvestrant | 06/2016 | 0.59 (0.48 – 0.73) | 20.5 | 12.8 | 0.72 (0.57 – 0.92) | 53.7 | 41.5 | 40% | 5.4% | 50 , 51 , 52 , 53 |
| MONARCH-2 | 669 | abemaciclib ± fulvestrant | 12/2015 | 0.55 (0.45 – 0.68) | 16.9 | 9.3 | 0.76 (0.61 – 0.95) | 46.7 | 37.3 | NA | NA | 54 , 55 |
| PALOMA-3 | 521 | palbociclib ± fulvestrant | 08/2014 | 0.46 (0.36 – 0.59) | 9.5 | 4.6 | 0.81 (0.64 – 1.03) | 34.8 | 28.0 | NA | NA | 8 , 56 , 57 |
| DAWNA-1 | 361 | dalpiciclib* ± fulvestrant | 09/2020* | 0.42 (0.31 – 0.58) | 15.7 | 7.2 | not yet reported | not yet reported | NA | NA | 58 |
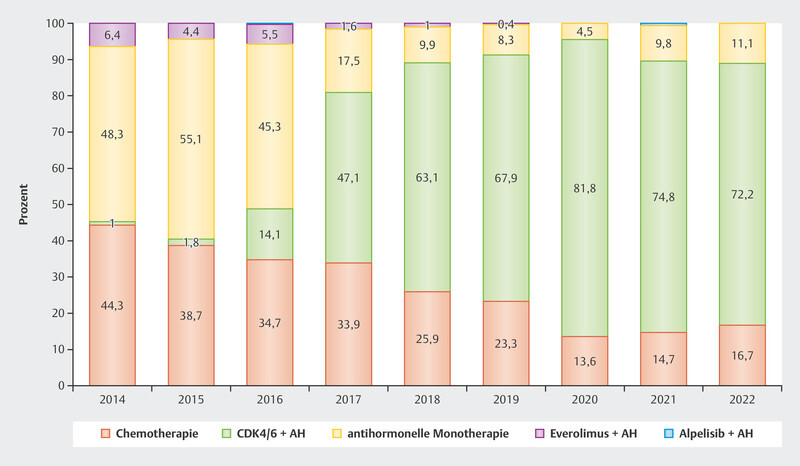
The data were so convincing that since the approval of CDK4/6i in 2016 and 2017 in Germany around 70 – 80% of patients with advanced HR+/HER2− breast cancer receive first-line therapy with CDK4/6i ( Fig. 2 ). Understanding the resistance and efficacy mechanisms is particularly important in this context to establish effective therapy sequences for these patients.
Fig. 2.
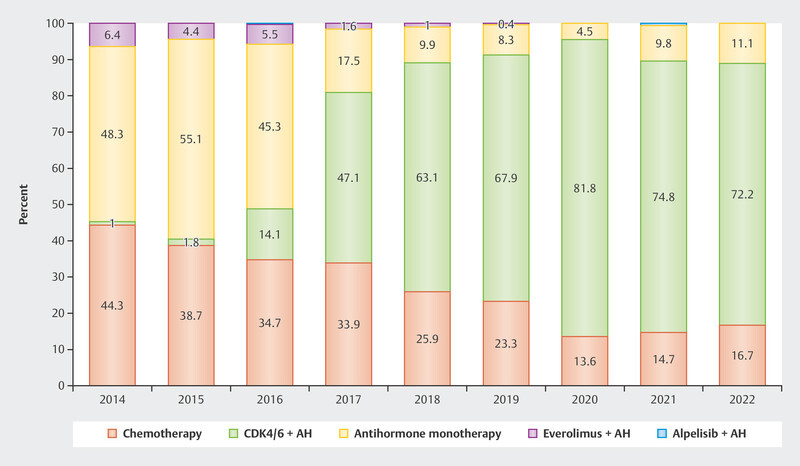
Use of different therapeutic options as first-line therapy in patients with advanced HR+/HER2− breast cancer 2 .
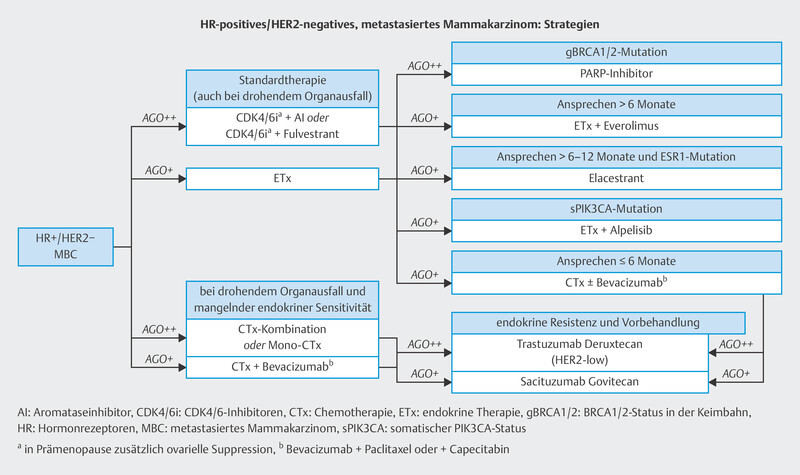
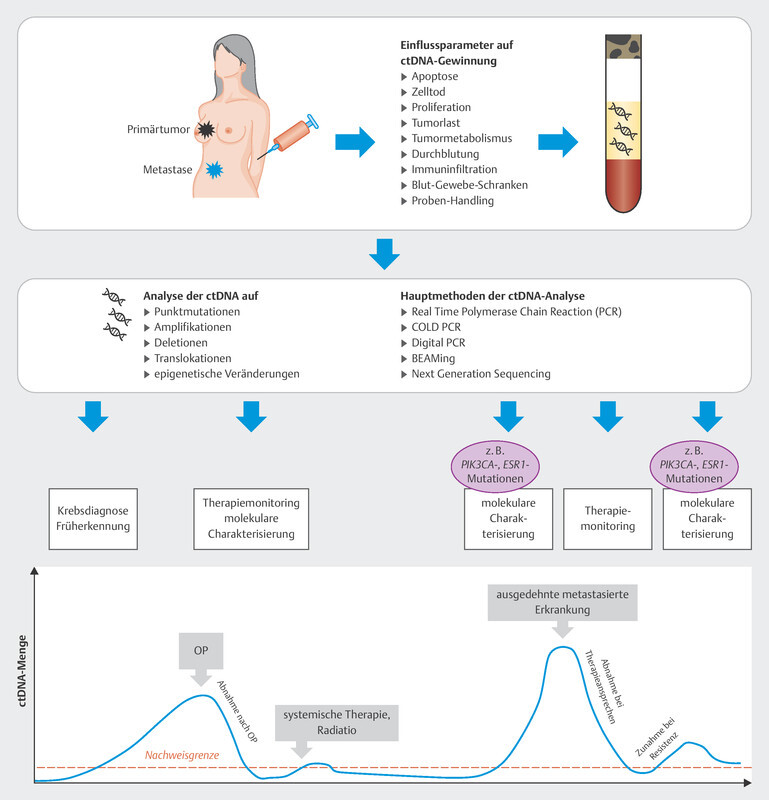
In the therapy recommendations issued by the Breast Commission of the AGO ( Fig. 3 ), three sequential therapies which are initiated after failure of CDK4/6i therapy are now based on predictive molecular markers. Poly (ADP-ribose) polymerase inhibitors (PARPi) are indicated in patients with confirmed BRCA1/2 germline mutation; the phosphatidylinositol 3-kinase inhibitor (PI3Ki) alpelisib is prescribed to patients with PIK3CA mutation, and the oral selective estrogen receptor degrader (SERD) elacestrant has been approved in the USA for patients with confirmed mutation in the estrogen receptor gene ESR1 . Elacestrant has not yet been approved for use in Europe (as at 09/2023), although the EMA has already issued a positive opinion for elacestrant. The identification of mutations using plasma-based circulating tumor DNA (ctDNA) is expected to become increasingly important in this context. Both PIK3CA mutations and ESR1 mutations can be determined using ctDNA. The scope of ctDNA analysis in clinical care and research will increase even further in the coming years. Fig. 4 shows the process, influencing factors and possible clinical applications.
Fig. 3.
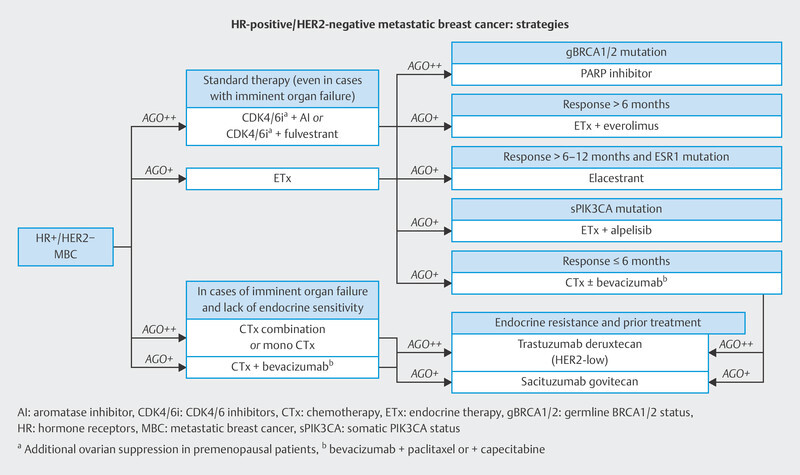
Treatment algorithm of the Breast Commission of the AGO outlining sequential therapies for patients with HR+/HER2− breast cancer in an advanced therapy setting.
Fig. 4.
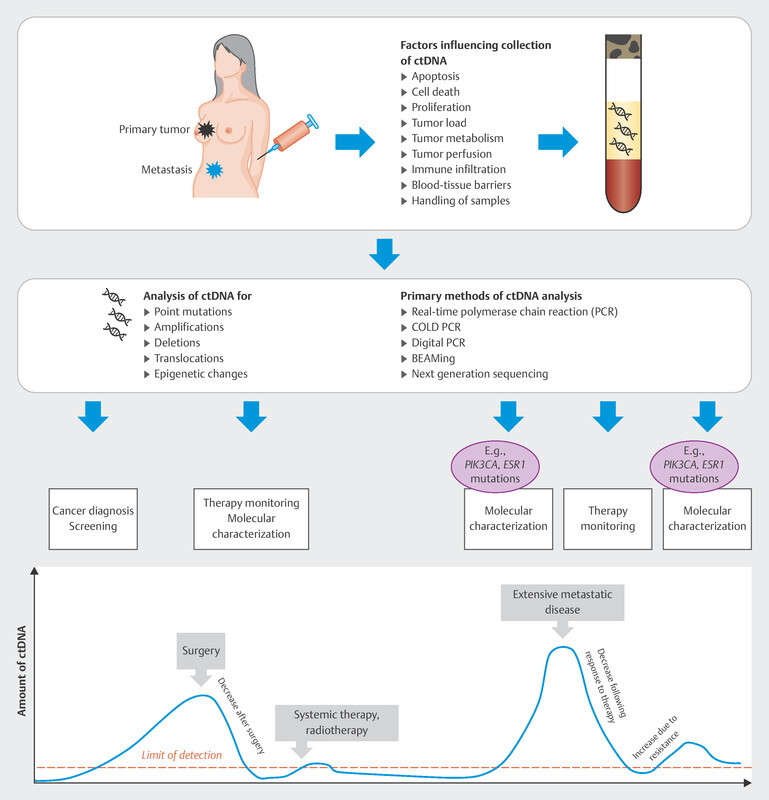
Possible course and clinical application of ctDNA analysis.
Biomarker Investigations in Large Randomized Studies
Initial findings on clonal evolution in the PALOMA-3 trial
Most large randomized studies now also include extensive translational research programs. These programs have collected important information which can help to predict treatment efficacy and resistance based on the use of biomarkers.
The PALOMA-3 trial was one of the first studies which extensively investigated the clonal evolution of disease under CDK4/6i therapy 8 , 9 . Using ctDNA obtained from blood samples, investigations were carried out to determine which mutations are most commonly found at the end of therapy in potentially relevant genes compared to the start of therapy. The most common mutations were in the two genes ESR1 and PIK3CA . Mutations in RB1 occurred more frequently in the palbociclib arm of the study 9 . Mutations in PIK3CA (relating to the PI3Ki alpelisib) and probably, in the near future, also mutations in ESR1 (relating to the SERD elacestrant) are actionable mutations. The PALOMA-3 trial shows the importance of mutation analysis carried out immediately before the start of therapy as it provides the basis for treating patients in accordance with their identified mutation status.
PIK3CA and ESR1 mutations in the MONARCH-2 trial
In the MONARCH-2 trial, patients with endocrine resistance were treated with abemaciclib and fulvestrant or fulvestrant alone. Samples of ctDNA taken just before starting therapy were investigated for mutations in PIK3CA and ESR1 10 . A mutation in PIK3CA was found in 44% of patients and an ESR1 mutation was confirmed in 59% of patients. In this trial, however, these biomarkers had no impact on patientsʼ prognosis 10 .
Biomarker examinations in the MONALEESA study program
In the MONALEESA trials 2, 3 and 7, extensive pooled analyses of gene expression and other genomic analyses were carried out in a large group of patients. More than 1150 patients were categorized into their respective intrinsic subtypes using gene expression analysis with PAM50. Even though these breast cancers are often described as luminal-like, this clinical assessment is only an estimation. In addition to luminal-A (46.8%) and luminal-B tumors (24.0%), the pooled MONALEESA-2, 3 and 7 analyses also found tumors with HER2-enriched (12.6%), normal-like (14.1%) and basal-like (2.4%) intrinsic subtypes. What was interesting was that the therapeutic effect of ribociclib was found to differ across the different subgroups. With a hazard ratio (HR) of 0.40 (95% CI: 0.26 – 0.62), the effect appeared to be greatest in the group with HER2-enriched tumors. This is probably due to the pronounced endocrine resistance in this group, which became evident when results were compared to outcomes with monotherapy. More than one third of patients experienced primary progression under endocrine monotherapy. The addition of ribociclib probably overcame resistance in a majority of these patients. Similarly, a majority of patients with basal-like tumors demonstrated early progression under endocrine monotherapy which was resolved by the addition of ribociclib (HR = 1.14; 95% CI: 0.46 – 2.83) 11 .
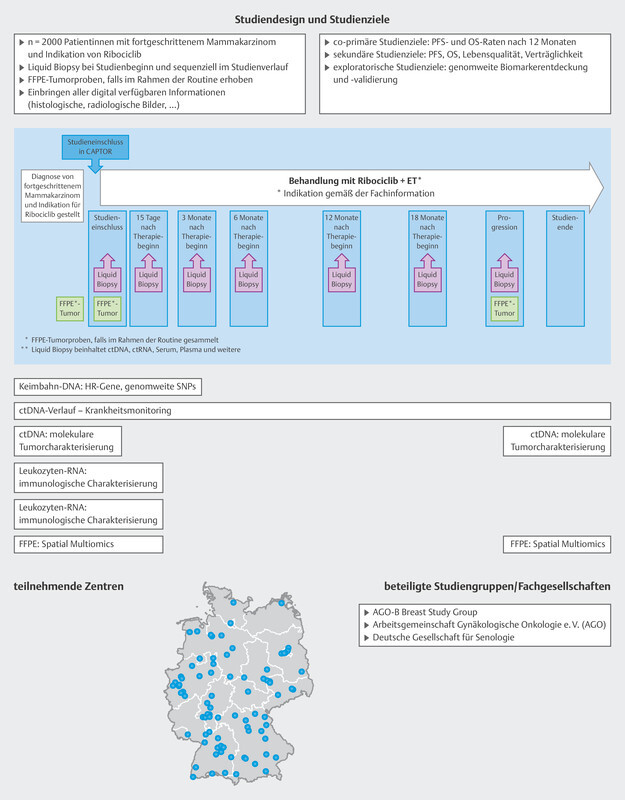
Extensive analysis of tumor mutations and gene amplifications was also carried out in a pooled patient group (n = 1703) from the MONALEESA-2, 3 and 7 trials 12 . At the start of treatment with ribociclib and endocrine therapy, ctDNA was analyzed with regards to mutations and amplifications of around 550 genes using next generation sequencing. The most common finding was an alteration in PIK3CA (33%), which in most cases was a mutation. The most commonly amplified genes were FDF3, FDF4 and FGF19 (8 – 9%). Some genes were identified if a mutation under endocrine monotherapy was associated with a very short median PFS which was then significantly improved by additonally administering ribociclib. These genes were FRS2, MDM2, PRKCA, AKT1, BRCA1/2 and ERBB2 . Conversely, no improvement in median PFS following the addition of ribociclib was found for mutations in CHD4, CDKN2A/B/C and ATM . These analyses have given rise to a number of hypotheses, but they show that gene mutations and amplifications play a role in the context of endocrine resistance which has not yet been scientifically investigated. It is therefore clear that a clinically simple method (blood sampling and ctDNA analysis) can help to determine predictive markers which will then be used to direct further therapy ( Fig. 4 ). This is also being offered to patients with progression in the CAPTOR BC trial in the context of a scientific subproject ( Fig. 8 ).
Fig. 8.
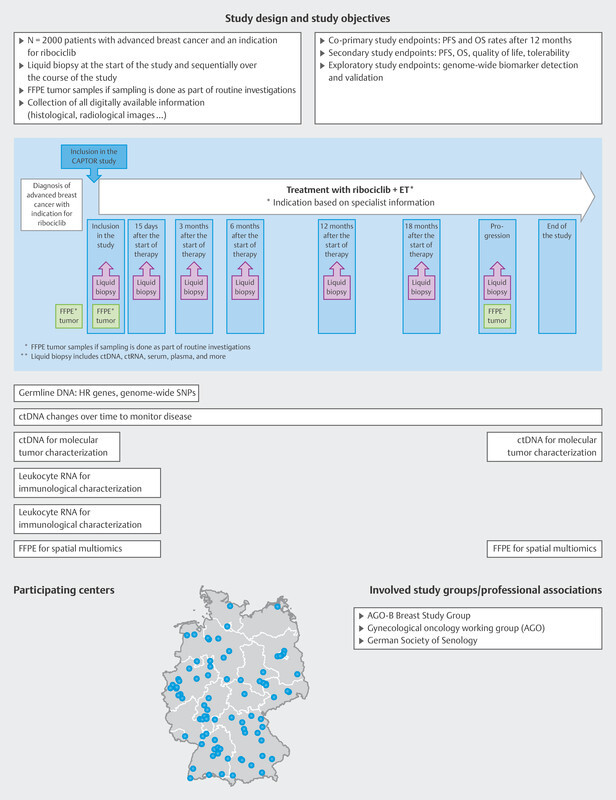
Study design of the CAPTOR BC trial and planned molecular analyses. ET = endocrine therapy, FFPE = formalin-fixed, paraffin-embedded tissues, HR = homologous recombination, OS = overall survival, PFS = progression-free survival, SNP = single-nucleotide polymorphism
Therapy Monitoring with ctDNA Analysis
Monitoring of CDK4/6i therapy with ribociclib – the BioItaLEE trial
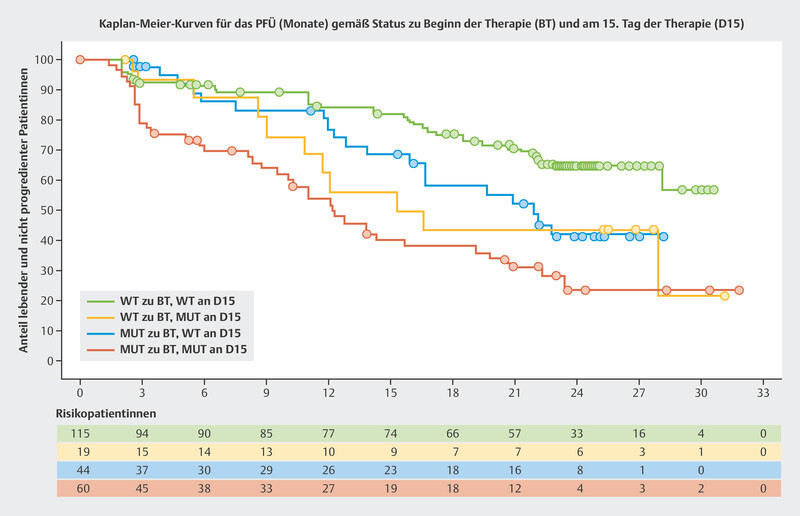
The BioItaLEE trial is a potentially pioneering study into the monitoring of ribociclib plus endocrine therapy using ctDNA. In this study, the amount of tumor-specific ctDNA was determined prior to the start of therapy and 15 days after starting therapy and correlated with the median PFS 13 . Patients were divided into four groups based on the amount of ctDNA. After a median follow-up time of 26.9 months, clear prognostic patterns with regards to median PFS were identified:
no ctDNA at the start of therapy → no ctDNA after 15 days: median PFS not achieved
no ctDNA prior to starting therapy → ctDNA newly present after 15 days: median PFS 15.9 months
ctDNA present before the start of therapy → decrease in ctDNA after 15 days: median PFS 21.9 months
ctDNA present before the start of therapy → ctDNA present after 15 days: median PFS 12.3 months
The Kaplan-Meier curves are shown in Fig. 5 . Modern tumor-specific biomarkers obtained through ctDNA analysis could allow the success of therapy to be estimated after just a short treatment period.
Fig. 5.
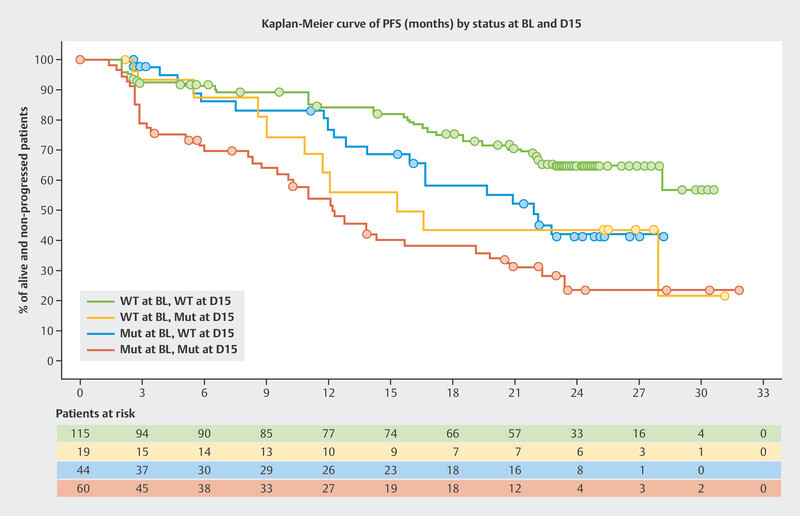
Progression-free survival (PFS) in the BioItaLEE trial depending on changes in ctDNA between the start of therapy (BL) and 15 days after the start of therapy (D15) 13 . Mut = mutated, WT = wildtype.
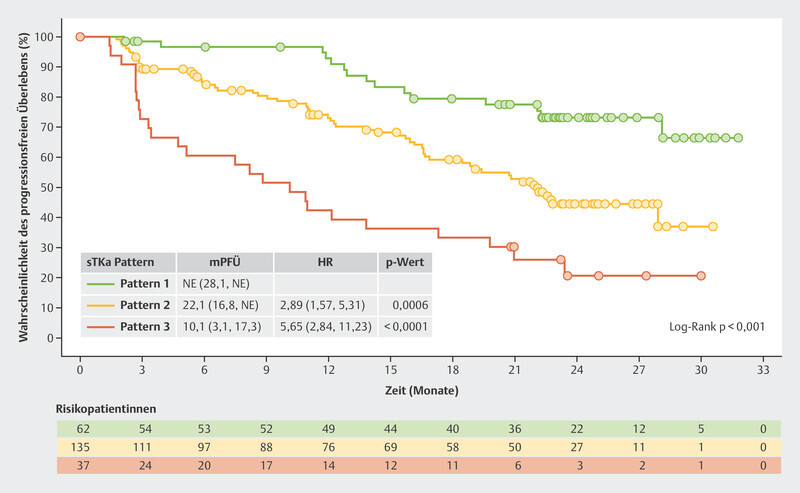
The BioItaLEE trial also analyzed other biomarkers such as serum thymidine kinase (sTK), a proliferation marker which correlates with the effect of CDK4/6i 14 . Of particular interest were patients where sTK was found to be suppressed under the limit of detection (LOD) after both 15 days of therapy and 28 days of therapy ( Fig. 6 , Pattern 1). This group had the best prognosis. With a median follow-up time of 26.9 months, this group did not achieve the median PFS. Patients in whom suppression of sTK under the LOD was initially achieved after 15 days of therapy but who then showed an increase again after 28 days of therapy ( Fig. 6 , Pattern 2) had a median PFS of 22.1 months. Patients who showed no decrease in sTK under the LOD after 15 days of therapy ( Fig. 6 , Pattern 3) had the poorest median PFS with just 10.1 months 15 .
Fig. 6.
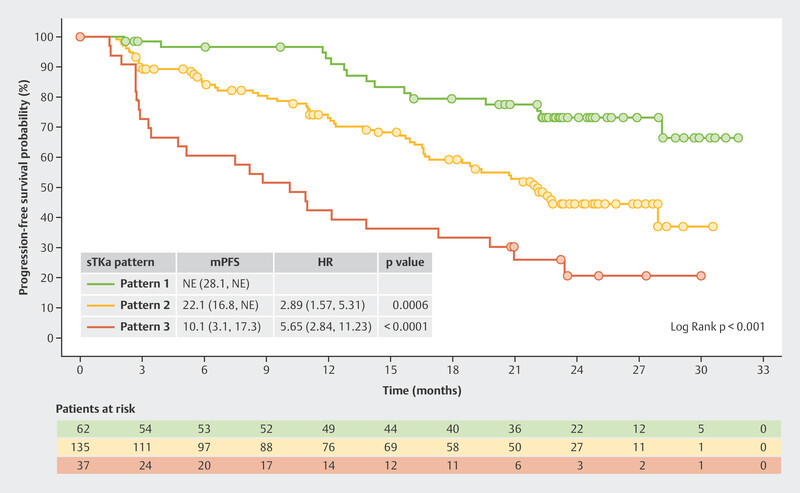
Progression-free survival in the BioItaLEE trial depending on the changes in serum thymidine kinase activity under therapy with ribociclib 15 . HR = hazard ratio, mPFS = median progression-free survival, sTKa = serum thymidine kinase activity
Homologous Recombination and Endocrine Resistence
New molecular patterns associated with endocrine resistence
Data from the MSK-IMPACT cohort on prognosis under CDK4/6i therapy has already been presented at the 2021 San Antonio Breast Cancer Symposium (SABCS). BRCA2 germline mutation was found to have an unfavorable prognostic effect in patients under CDK4/6i therapy. Compared to patients with BRCA2 wildtype, patients with BRCA2 mutation had a higher risk of progression (HR = 2.32; 95% CI: 1.38 – 3.91) 16 .
Other analyses in this cohort investigated the effect of complex mutation patterns on prognosis under CDK4/6i therapy 17 . The differentiation of tumors according to complex mutation patterns (signatures or profiles) is an attempt to determine different prognostic categories based on these mutation patterns. During the pathogenesis of tumors, different irritants and circumstances lead to characteristic mutation profiles. 18 . Such mutation profiles can be developed for different types of mutations (single base pair, doublet base pair, insertion-deletion mutations). The analysis of the MSK-IMPACT cohort focused on single base pair mutations (SBS) 17 for which a recently published work described 96 different signatures 19 ; these signatures are also available from the Catalogue of Somatic Mutations in Cancer (COSMIC) 20 .
Some of these SBS signatures are found more often in breast cancer and can be divided into the following etiological groups: clock-like signatures, apolipoprotein B mRNA editing enzyme, catalytic polypeptide (APOBEC) signature, homologous recombination deficiency (HRD) signature, smoking-related and mismatch repair-related signatures.
The presented clinical data refer to the change in the mutation profile of the primary tumor when it metastasizes and also to the impact of the mutation profile on the prognosis of patients treated with CDK4/6i as their first-line therapy. The two mutation profiles which were found to have increased most during the progression of disease from early-stage HR+/HER2− breast cancer to metastatic disease were the APOBEC and HRD signatures 17 . Significant differences were found with regard to the prognostic importance of the mutation profiles under first-line therapy with a CDK4/6i. Patients with few mutations had a median PFS of 17.8 months, patients with an APOBEC signature had a median PFS of 12.3 months and patients with an HRD signature had a median PFS of only 7.6 months 17 .
To what extent this knowledge can be used to determine subsequent therapies or therapy sequences must be elucidated in future studies. Currently, treatment with a CDK4/6i is and remains the standard first-line therapy for patients with advanced HR+/HER2− breast cancer. However, given the short median PFS of patients with an HRD signature, the question arises whether these patients should not rather be treated with PARPi. The HRD group consisted of only 10.5% of patients treated with CDK4/6i in the MSK-IMPACT cohort, representing only a small number of the patients in the study, meaning that further studies or real-world data are needed for a conclusive assessment.
ESR1 Mutations and Endocrine Combination Partners
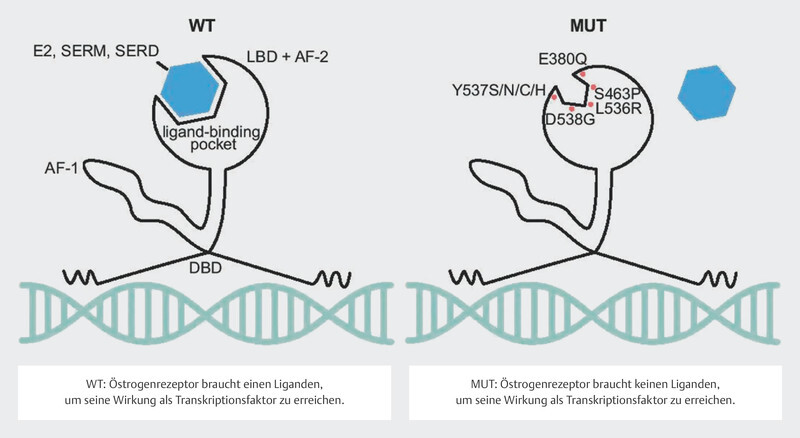
Around 30 – 40% of tumors in patients with advanced endocrine-resistant breast cancer were found to have a mutation in the estrogen receptor gene ESR1 21 , 22 , 23 . These mutations can be determined with a relatively high sensitivity using serum ctDNA 24 , 25 . It should be easy to determine these mutations over the clinical course of disease. The mutations in the gene segments of ESR1 coding for the ligand-binding domain were correlated with a poorer clinical outcome 24 , 25 , 26 , presumably because these mutations lead to changes in protein structure in the sense of a constitutively active form of the estrogen receptor 27 , 28 ( Fig. 7 ). Their frequency and the clonal selection of ESR1 mutations under endocrine therapy have focused interest in endocrine resistance on this mutation.
Fig. 7.
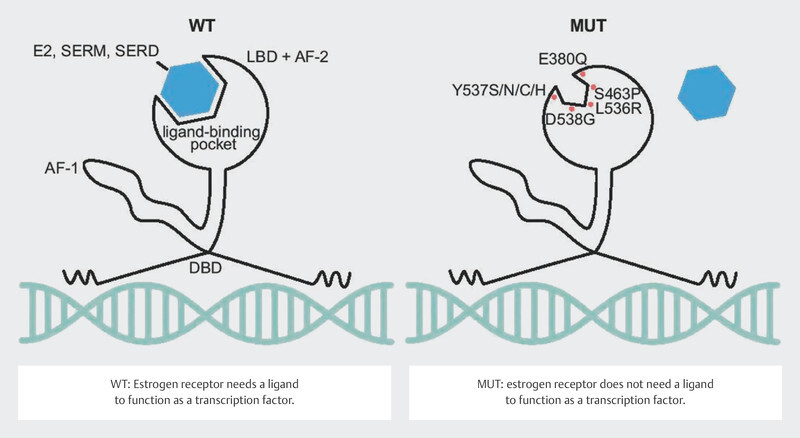
Effect of ESR1 mutation on the function of the estrogen receptor (Creative Commons Licence 4.0, 59 ). LBD: ligand-binding domain, AF-2: activating factor-2 domain, DBD: DNA-binding domain, AF-1: activating factor 1 domain, E2: estradiol, SERM: selective estrogen receptor modulator, SERD: selective estrogen receptor degrader, WT: wildtype, MUT: mutated (Source: Brett JO, Spring LM, Bardia A et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res 2021; 23: 85. doi:10.1186/s13058-021-01462-3.) © 2021. The Author(s). Licensed under a Creative Commons Attribution 4.0 International License ( http://creativecommons.org/licenses/by/4.0/ ). Changes: Addition of explanations for WT and MUT in the image.
ESR1 mutations and the oral SERD elacestrant
The SERD fulvestrant which is administered intramuscularly has been approved to treat patients with advanced breast cancer for more than 20 years. With the development of oral SERDs, research activities into this class of substances have increased significantly. In the USA, the oral SERD elacestrant has already been approved to treat patients with hormone therapy-resistant advanced breast cancer if ESR1 mutations are confirmed in the patientʼs ctDNA. In the EMERALD approval study 29 , patients with ESR1 mutation who received elacestrant had a longer median PFS than patients who received standard endocrine therapy (HR 0.55; 95% CI: 0.39 – 0.77). Elacestrant did not improve median PFS in patients without ESR1 mutation (HR 0.86; 95% CI: 0.63 – 1.19) 29 .
ESR1 mutations and fulvestrant as first-line therapy
In addition to the approval studies, studies have also focused on biomarkers. The PADA-1 trial is particularly important in this context. In this study, ctDNA was used to determine whether patients who were receiving first-line therapy with an aromatase inhibitor and palbociclib had an ESR1 mutation. If the presence of ESR1 mutation in serum was confirmed and the patient showed no clinical progression, patients were randomized into groups: in one group the endocrine combination partner of the aromatase inhibitor was switched to fulvestrant while the other group continued to receive treatment with an aromatase inhibitor and palbociclib. 172 patients were randomized. The median PFS under continuation of the aromatase inhibitor therapy was 5.7 months; the median PFS if therapy was switched to fulvestrant was 11.9 months (HR 0.61; 95% CI: 0.43 – 0.86) 30 .
The CAPTOR Study Program
CAPTOR BC study design
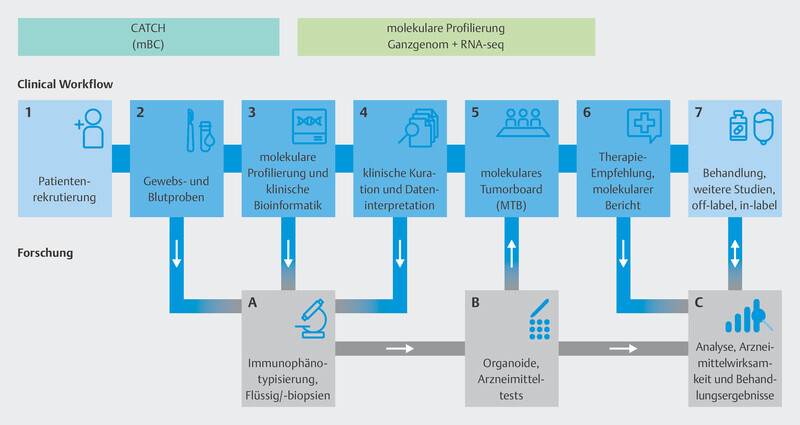
The CAPTOR BC study is a phase IV trial which investigates resistance and the mechanisms of action for combination therapy with ribociclib and endocrine therapy. The study is also investigating several new research concepts to find new genes and pathways which could identify a priori patients for whom ribociclib would be effective as well as those for whom ribociclib will not be effective. This could provide additional information on which therapy sequences will be most appropriate. The CAPTOR BC trial also records all subsequent therapies initiated after the end of therapy with ribociclib.
As the study is hoping to include large numbers of patients, the aim has been to simplify the practical implementation of the study while simultaneously achieving the extensive study goals ( Fig. 8 ).
Patients are included if routine clinical examination finds that ribociclib is indicated as part of their first-line therapy. Patients will be followed up until death, for a maximum period of 4 years, or until the end of the overall study (November 2027). Blood samples are taken on inclusion in the study and each patient is requested to provide the study with paraffinized tumor samples. Up to 7 more blood samples are taken over the course of the study ( Fig. 8 ). The patientʼs quality of life is also recorded using patient-reported outcome tools and a paper-based questionnaire or app.
This means that extensive information and biomaterials can be obtained with a relatively simple study design. Some of the scientific objectives of the CAPTOR BC trial are described in more detail below.
Detection of agnostic biomarkers in the CAPTOR BC trial
Despite numerous studies which have already investigated the mechanisms of action of CDK4/6i-based therapies, many methods have not yet been exhausted. Up to now, the use of agnostic approaches was largely ruled out, purely because of the rather small case numbers in the studies carried out to date. Modern methods of analysis are being used to carry out genome-wide identifications of gene expression, genetic mutations and gene copy alterations in a short space of time. If the case numbers are sufficiently high, new genes and signalling pathways which were not previously taken into consideration will be identified. A good example of this was the discovery of more than 300 gene loci which explain more than 40% of familial breast cancer risks 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 . The first big association studies were able to discover and validate the breadth of the genetic basis of the risk of breast cancer. Other examples are the immunomodulatory genes identified using a similar approach in the context of the SUCCESS trial which correlate with neutropenia and prognosis 39 . The large number of patients (n = 2000) in the CAPTOR BC study paves the way to carry out several such genome-wide analyses.
ctDNA in scientific and healthcare research in the CAPTOR BC trial
Just how varied the options for ctDNA analysis are has been described. A number of scientific and healthcare applications are conceivable, both in the context of therapy monitoring and for the molecular characterization of diseases. Serial blood sampling is being carried out as part of the CAPTOR BC trial, and the samples are prepared and processed in accordance with the latest state of knowledge of ctDNA analysis. One of the scientific objectives is to learn as much as possible about the course of disease progression under CDK4/6i therapy. The basis for this will be the analysis of ctDNA obtained at the time of progression. As part of the CAPTOR BC study, patients who have received treatment with a CDK4/6i and for whom marker-guided therapy is now indicated will be offered a ctDNA analysis under study conditions at the time of progression. Research is currently focused on investigating whether there are other molecular genomic markers in addition to the genes PIK3CA, ESR1 and BRCA1/2 that could help to identify appropriate subsequent therapies. One of the programs in this field of study is the multicenter program “Comprehensive Assessment of clinical feaTures and biomarkers to identify patients with advanced or metastatic breast Cancer for marker-driven trials in Humans” (CATCH) of the National Center for Tumor Diseases (NCT).
New Therapeutic Approaches in Clinical Studies
The CATCH study program
The speed of drug development processes has increased significantly in recent years. As the understanding of how the human genome functions and the importance of relevant mechanisms on the development of breast cancer has increased, more targeted drugs have been approved, i.e., drugs which address a target partly determined by molecular testing. Examples include CDK4/6i (palbociclib, ribociclib, abemaciclib), the PIK3CAi alpelisib, PARPi (olaparib, talazoparib), immune checkpoint inhibitors (ICIs) (atezolizumab, pembrolizumab), antibody-drug conjugates (ADCs) (sacituzumab-govitecan, trastuzumab-deruxtecan), the AKT inhibitor capivasertib, the oral SERD elacestrant and others.
As drug developments have occurred in parallel, the current therapy standard is usually not reflected in study populations. One example of this is alpelisib and the SOLAR-1 trial. This study included almost no patients who had received pretreatment with CDK4/6i, even though treatment with CDK4/6i is now the standard first-line therapy. This makes it clear how important it is to carry out therapies and, specifically, sequential therapies in molecularly informed trials.
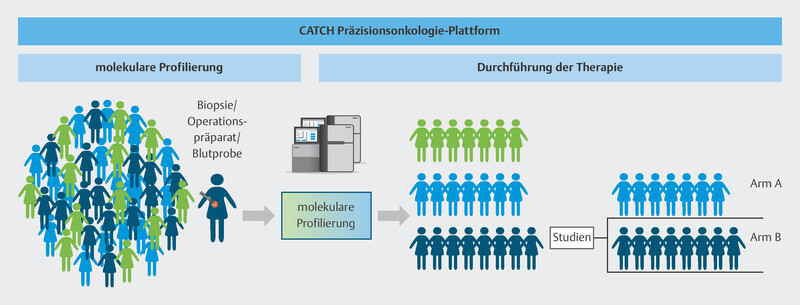
The multicenter CATCH program is a platform which aims to provide a basis to test the efficacy of individualized cancer drugs (precision oncology), specificially drugs for metastatic breast cancer, and to provide comprehensive access to controlled evidence-based precision oncology structures.
To do this, the individual genetic makeup of therapy-resistant metastasis in breast cancer patients is analyzed using modern molecular biological procedures to identify molecular points of attack ( Fig. 9 ). Based on specific molecular changes, patients will be offered targeted therapies in further clinical studies, either in the context of approval studies or as off-label therapies ( Fig. 10 ). These drugs should delay disease progression while maintaining patientsʼ quality of life. The pilot phase of the trial showed that it was possible to delay disease progression for longer in around one third of patients than is currently possible with standard therapies, and that a positive impact on disease progression could be expected even for patients who had received many prior therapies 40 . The information obtained in this study will be used over the longer term in subsequent studies to develop innovative targeted effective substances and modern diagnostic processes which will be transferred to standard care. The CATCH program is independent of the CAPTOR BC trial, but for patients, both study concepts may be closely connected. After disease progress is identified in the CAPTOR BC trial and the relevant molecular characterization is carried out, studies such as CATCH will be used to offer new and innovative therapies to patients based on their molecular characteristics.
Fig. 9.
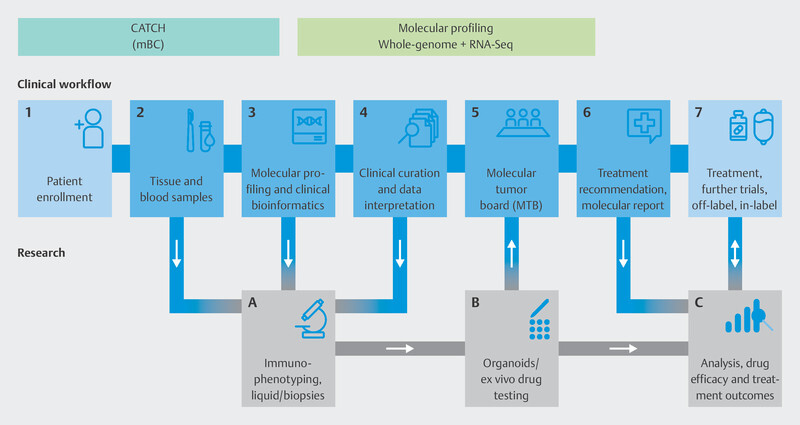
Technical procedure used for molecular characterization in the CATCH program.
Fig. 10.
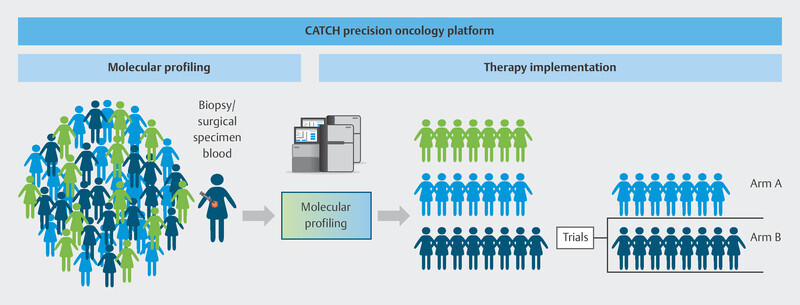
Therapy allocation based on molecular characteristics in the CATCH program.
Outlook
The large number and the quality of analyses of molecular biomarkers show ever more clearly that molecular characterization of breast cancer offers benefits to patients. The use of some markers is already standard when determining the indications for approved therapies. Large study programs such as the CAPTOR BC and CATCH studies, which include high numbers of patients, provide the basis for the creation of future treatment concepts which will expand this body of knowledge for the benefit of patients.
Acknowledgements
This work was partly the result of funding from onkowissen.de and Novartis. Neither of the companies had any share in the compilation of or recommendations in the manuscript. The authors are solely responsible for the contents of the manuscript.
Danksagung
Diese Arbeit entstand teilweise in Folge von Förderungen der Firmen onkowissen.de und Novartis. Keine der Firmen hatte einen Anteil an der Erstellung und den Empfehlungen des Manuskriptes. Für den Inhalt des Manuskriptes sind alleine die Autoren verantwortlich.
Footnotes
Conflict of Interest/Interessenkonflikt B. A. received honoria and travel grants from AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Onkowissen, Daiichi Sankyo and Pfizer. C. B. is an employee of Novartis Deutschland GmbH. E. B. received honoraria from Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, B Braun and onkowissen.de for clinical research management and/or medical education activities. S. Y. B. has received honoraria from Roche, Novartis, Pfizer, MSD, Teva, AstraZeneca. N. D. has received honoraria from MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen, Gilead, MCI Healthcare. P. A. F. received honoraria from Novartis, Pfizer, Roche, Amgen, Celgene, onkowissen.de, Daiichi Sankyo, AstraZeneca, Merck Sharp & Dohme, Eisai, Puma and Teva. His institution conducts research with funding from Novartis and BioNtech. T. E. has received honoraria from AstraZeneca, Eli Lilly, Daiichi Sankyo, Gilead, GSK, MSD, Novartis, Pfizer, Roche, Stemline. T. N. F. has participated on advisory boards for Amgen, Daiichi Sankyo, Novartis, Pfizer, and Roche and has received honoraria for lectures from Amgen, Celgene, Daiichi Sankyo, Roche, Novartis and Pfizer. A. D. H. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal and Pfizer. C. C. H. has received honoraria from Pfizer, Novartis, Roche, AstraZeneca, Eisai and Daiichi Sankyo. H. H. received speaker honoraria from Novartis. H. N. received honoraria for lectures and/or consulting from Amgen, AstraZeneca, Daiichi Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz, Seagen. W. J. has received research grants and/or honoraria from Sanofi Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene and Johnson & Johnson. H.-C. K. has received honoraria from Pfizer, Novartis, Seagen, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lilly, SurgVision, Onkowissen, Gilead, Daiichi Sankyo and MSD, travel support from Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro and owns stock of Theraclion SA and Phaon Scientific GmbH. D. L. received honoraria from Amgen, AstraZeneca, Eli Lilly, High5md, Gilead, GSK, Loreal, MSD, Novartis, Onkowissen, Pfizer, Seagen, Teva. M. P. L. has participated on advisory boards for AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi Sankyo, PharmaMar, Roche, SamanTree, Sysmex and Hexal and has received honoraria for lectures from MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Grünenthal, pfm, Gilead, AstraZeneca, and Eisai. V. M. received speaker honoraria from Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 Oncology, Medscape, Gilead. Consultancy honoraria from Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead. Institutional research support from Novartis, Roche, Seagen, Genentech. Travel grants: Roche, Pfizer, Daiichi Sankyo. B. R. reports research grants from Roche, Menarini, Lilly, Inivata. Honoraria from AZ, Roche. C. R. is an employee of Novartis Deutschland GmbH. A. S. received research grants from Celgene, Roche, honoraria from Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, Clinsol, Connectmedica, Gilead, GSK, I-MED, Lilly, MCI Deutschland, Metaplan, MSD, Nanostring, Novartis, Onkowissen.de, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, Streamedup, Teva, Tesaro, Thieme and travel support from Celgene, Pfizer, Roche. K. S. received travel support from Gilead and Lilly. F.-A. T. received speaker and consultancy honoraria from AstraZeneca, Genomic Health, Gilead, GSK, Hexal, MSD, Novartis, Onkowissen, Pfizer, Roche, Tesaro. H. T. received honoraria from Novartis, Roche, Celgene, Teva, Pfizer, AstraZeneca and travel support from Roche, Celgene, and Pfizer. M. T. has participated on advisory boards for AstraZeneca, Celgene, Clovis, Daiichi Sankyo, Eisai, Gilead Science, Grünenthal, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen, and Roche and has received honoraria for lectures from Amgen, Aurikamed, Celgene, Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor and AstraZeneca and has received trial funding from Exact Sciences and Endomag Manuscript support was provided by Amgen, ClearCut, pfm medical, Roche, Servier, Vifor. M. U. all honoraria went to the institution/employer: Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi Aventis, Novartis, Pierre Fabre, Seagen, Gilead. P. W. has received honoraria from Roche, Novartis, Amgen, AstraZeneca, Pfizer, MSD, Clovis, Tesaro, Celgene, Teva, Eisai, Daiichi Sankyo, Seagen and Eli Lilly. The other authors (L. L. V, M. R., S. H., V. T., I. J.-B., D. A., M. B., M. S., J. R., I. N.) have no conflict of interest to declare for this specific work./ B. A. erhielt Honorare und Reisekosten von AstraZeneca, Gilead, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Onkowissen, Daiichi Sankyo und Pfizer. C. B. ist Angestellte der Firma Novartis Deutschland GmbH. E. B. erhielt Honorare von Gilead, Ipsen, Sanofi, Sandoz, SunPharma, AstraZeneca, Novartis, Hexal, BMS, Lilly, Pfizer, Roche, MSD, B Braun und onkowissen.de für klinisches Forschungsmanagement und/oder Aktivitäten der medizinischen Fortbildung. S. Y. B. erhielt Honorare von Roche, Novartis, Pfizer, MSD, Teva, AstraZeneca. N. D. erhielt Honorare von MSD, Roche, AstraZeneca, Teva, Pfizer, Novartis, Seagen, Gilead, MCI Healthcare. P. A. F. erhielt Honorare von Novartis, Pfizer, Roche, Amgen, Celgene, onkowissen.de, Daiichi Sankyo, AstraZeneca, Merck Sharp & Dohme, Eisai, Puma und Teva. Seine Institution betreibt Forschung mit finanzieller Unterstützung von Novartis und BioNtech. T. E. erhielt Honorare von AstraZeneca, Eli Lilly, Daiichi Sankyo, Gilead, GSK, MSD, Novartis, Pfizer, Roche, Stemline. T. N. F. hat in Beiräten mitgewirkt für Amgen, Daiichi Sankyo, Novartis, Pfizer und Roche und hat Vortragshonorare erhalten von Amgen, Celgene, Daiichi Sankyo, Roche, Novartis und Pfizer. A. D. H. erhielt Sprecher- und Beraterhonorare von AstraZeneca, Genomic Health, Roche, Novartis, Celgene, Lilly, MSD, Eisai, Teva, Tesaro, Daiichi Sankyo, Hexal und Pfizer. C. C. H. erhielt Honorare von Pfizer, Novartis, Roche, AstraZeneca, Eisai und Daiichi Sankyo. H. H. erhielt Sprecherhonorare von Novartis. H. N. erhielt Vortragshonorare und/oder Honorare für Beratertätigkeiten vn Amgen, AstraZeneca, Daiichi Sankyo, Exact Sciences, Gilead, Lilly, MSD, Mylan, Novartis, Pierre Fabre, Pfizer, Roche, Sandoz, Seagen. W. J. erhielt Forschungsstipendien und/oder Honorare von Sanofi Aventis, Daiichi Sankyo, Novartis, Roche, Pfizer, Lilly, AstraZeneca, Chugai, GSK, Eisai, Cellgene und Johnson & Johnson. H.-C. K. erhielt Honorare von Pfizer, Novartis, Seagen, Roche, Genomic Health/Exact Sciences, Amgen, AstraZeneca, Riemser, Carl Zeiss Meditec, Teva, Theraclion, Janssen-Cilag, GSK, LIV Pharma, Lilly, SurgVision, Onkowissen, Gilead, Daiichi Sankyo and MSD, Reisekostenzuschüsse von Carl Zeiss Meditec, LIV Pharma, Novartis, Amgen, Pfizer, Daiichi Sankyo, Tesaro und besitzt Aktien von Theraclion SA und Phaon Scientific GmbH. D. L. erhielt Honorare von Amgen, AstraZeneca, Eli Lilly, High5md, Gilead, GSK, Loreal, MSD, Novartis, Onkowissen, Pfizer, Seagen, Teva. M. P. L. hat in Beiräten mitgewirkt für AstraZeneca, Lilly, MSD, Novartis, Pfizer, Eisai, Gilead, Exact Sciences, Pierre Fabre, Grünenthal, Daiichi Sankyo, PharmaMar, Roche, SamanTree, Sysmex und Hexal und erhielt Vortragshonorare von MSD, Lilly, Roche, Novartis, Pfizer, Exact Sciences, Daiichi Sankyo, Grünenthal, pfm, Gilead, AstraZeneca und Eisai. V. M. erhielt Sprecherhonorare von Amgen, AstraZeneca, Daiichi Sankyo, Eisai, GSK, Pfizer, MSD, Medac, Novartis, Roche, Teva, Seagen, Onkowissen, high5 oncology, Medscape, Gilead. Beraterhonorare von Hexal, Roche, Pierre Fabre, Amgen, ClinSol, Novartis, MSD, Daiichi Sankyo, Eisai, Lilly, Sanofi, Seagen, Gilead, institutionelle Forschungsförderung von Novartis, Roche, Seagen, Genentech. Reisekostenzuschüsse von Roche, Pfizer, Daiichi Sankyo. B. R. erhielt Forschungsstipendien von Roche, Menarini, Lilly, Inivata, Honorare von AZ, Roche. C. R. ist Angestellter der Firma Novartis Deutschland GmbH. A. S. erhielt Forschungsstipendien von Celgene, Roche, Honorare von Amgen, AstraZeneca, Aurikamed, Bayer, Celgene, Clinsol, Connectmedica, Gilead, GSK, I-MED, Lilly, MCI Deutschland, Metaplan, MSD, Nanostring, Novartis, Onkowissen.de, Promedicis, Pfizer, Pierre Fabre, Roche, Seagen, Streamedup, Teva, Tesaro, Thieme und Reisekostenzuschüsse von Celgene, Pfizer, Roche. K. S. erhielt Reisekostenzuschüsse von Gilead und Lilly. F.-A. T. erhielt Sprecher- und Beraterhonorare von AstraZeneca, Genomic Health, Gilead, GSK, Hexal, MSD, Novartis, Onkowissen, Pfizer, Roche, Tesaro. H. T. erhielt Honorare von Novartis, Roche, Celgene, Teva, Pfizer, AstraZeneca und Reisekostenzuschüsse von Roche, Celgene und Pfizer. M. T. hat in Beiräten mitgewirkt für AstraZeneca, Celgene, Clovis, Daiichi Sankyo, Eisai, Gilead Science, Grünenthal, GSK, Lilly, MSD, Novartis, Organon, Pfizer, Pierre Fabre, Seagen und Roche, erhielt Vortragshonorare von Amgen, Aurikamed, Celgene, Clovis, Daiichi Sankyo, Eisai, GSK, Lilly, MSD, Roche, Novartis, Organon, Pfizer, Seagen, Exact Sciences, Viatris, Vifor und AstraZeneca und erhielt Studienfinanzierung von Exact Sciences und Endomag; Unterstützung für das Manuskript kam von Amgen, ClearCut, pfm medical, Roche, Servier, Vifor. M. U. Alle Honorare wurden an die Institution/den Arbeitergeber abgeführt: Abbvie, Amgen, AstraZeneca, Daiichi Sankyo, Eisai, Lilly, MSD, Myriad Genetics, Pfizer, Roche, Sanofi Aventis, Novartis, Pierre Fabre, Seagen, Gilead. P. W. erhielt Honorare von Roche, Novartis, Amgen, AstraZeneca, Pfizer, MSD, Clovis, Tesaro, Celgene, Teva, Eisai, Daiichi Sankyo, Seagen und Eli Lilly. Die anderen Autoren und Autorinnen (L. L. V, M. R., S. H., V. T., I. J.-B., D. A., M. B., M. S., J. R., I. N.) haben keine Interessenkonflikte zu melden.
References/Literatur
- 1.Finn R S, Dering J, Conklin D et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11:R77. doi: 10.1186/bcr2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engler T, Fasching P A, Luftner D et al. Implementation of CDK4/6 Inhibitors and its Influence on the Treatment Landscape of Advanced Breast Cancer Patients – Data from the Real-World Registry PRAEGNANT. Geburtshilfe Frauenheilkd. 2022;82:1055–1067. doi: 10.1055/a-1880-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneeweiss A, Ettl J, Luftner D et al. Initial experience with CDK4/6 inhibitor-based therapies compared to antihormone monotherapies in routine clinical use in patients with hormone receptor positive, HER2 negative breast cancer – Data from the PRAEGNANT research network for the first 2 years of drug availability in Germany. Breast. 2020;54:88–95. doi: 10.1016/j.breast.2020.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thill M, Kolberg-Liedtke C, Albert U S et al. AGO Recommendations for the Diagnosis and Treatment of Patients with Locally Advanced and Metastatic Breast Cancer: Update 2023. Breast Care (Basel) 2023;18:306–315. doi: 10.1159/000531579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harbeck N, Rastogi P, Martin M et al. Adjuvant abemaciclib combined with endocrine therapy for high-risk early breast cancer: updated efficacy and Ki-67 analysis from the monarchE study. Ann Oncol. 2021;32:1571–1581. doi: 10.1016/j.annonc.2021.09.015. [DOI] [PubMed] [Google Scholar]
- 6.Slamon D, Stroyakovskiy D, Yardley D et al. Phase III NATALEE trial of ribociclib + endocrine therapy as adjuvant treatment in patients with HR+/HER2− early breast cancer. ASCO Annual Meeting. 2023;2023:LBA500. [Google Scholar]
- 7.Finn R S, Crown J P, Lang I et al. The cyclin-dependent kinase 4/6 inhibitor palbociclib in combination with letrozole versus letrozole alone as first-line treatment of oestrogen receptor-positive, HER2-negative, advanced breast cancer (PALOMA-1/TRIO-18): a randomised phase 2 study. Lancet Oncol. 2015;16:25–35. doi: 10.1016/S1470-2045(14)71159-3. [DOI] [PubMed] [Google Scholar]
- 8.Turner N C, Ro J, Andre F et al. Palbociclib in Hormone-Receptor-Positive Advanced Breast Cancer. N Engl J Med. 2015;373:209–219. doi: 10.1056/NEJMoa1505270. [DOI] [PubMed] [Google Scholar]
- 9.OʼLeary B, Cutts R J, Liu Y et al. The Genetic Landscape and Clonal Evolution of Breast Cancer Resistance to Palbociclib plus Fulvestrant in the PALOMA-3 Trial. Cancer Discov. 2018;8:1390–1403. doi: 10.1158/2159-8290.CD-18-0264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tolaney S M, Toi M, Neven P et al. Clinical Significance of PIK3CA and ESR1 Mutations in Circulating Tumor DNA: Analysis from the MONARCH 2 Study of Abemaciclib plus Fulvestrant. Clinical Cancer Research. 2022;28:1500–1506. doi: 10.1158/1078-0432.Ccr-21-3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prat A, Chaudhury A, Solovieff N et al. Correlative Biomarker Analysis of Intrinsic Subtypes and Efficacy Across the MONALEESA Phase III Studies. J Clin Oncol. 2021;39:1458–1467. doi: 10.1200/JCO.20.02977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andre F, Su F, Solovieff N et al. Pooled ctDNA analysis of the MONALEESA (ML) phase III advanced breast cancer (ABC) trials. J Clin Oncol. 2020;38:1009. doi: 10.1200/JCO.2020.38.15_suppl.1009. [DOI] [PubMed] [Google Scholar]
- 13.Bianchini G, Malorni L, Arpino G et al. Circulating tumor DNA (ctDNA) dynamics in patients with hormone receptor positive (HR+)/HER2 negative (HER2-) advanced breast cancer (aBC) treated in first line with ribociclib (R) and letrozole (L) in the BioItaLEE trial. San Antonio Breast Cancer Symposium. 2021;2021:GS3-07. [Google Scholar]
- 14.Bagegni N, Thomas S, Liu N et al. Serum thymidine kinase 1 activity as a pharmacodynamic marker of cyclin-dependent kinase 4/6 inhibition in patients with early-stage breast cancer receiving neoadjuvant palbociclib. Breast Cancer Res. 2017;19:123. doi: 10.1186/s13058-017-0913-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malorni L, Bianchini G, Caputo R et al. Serum thymidine kinase activity in patients with HR-positive/HER2-negative advanced breast cancer treated with ribociclib plus letrozole: Results from the prospective BioItaLEE trial. Eur J Cancer. 2023;186:1–11. doi: 10.1016/j.ejca.2023.03.001. [DOI] [PubMed] [Google Scholar]
- 16.Safonov A, Bandlamudi C, Tallón de Lara P et al. Comprehensive genomic profiling of patients with breast cancer identifies germline-somatic interactions mediating therapy resistanc. San Antonio Breast Cancer Symposium. 2021;2021:GS4-08. [Google Scholar]
- 17.Marra A, Gazzo A, Gupta A et al. Mutational signature analysis reveals patterns of genomic instability linked to resistance to endocrine therapy (ET) ± CDK 4/6 inhibition (CDK4/6i) in estrogen receptor-positive/HER2-negative (ER+/HER2-) metastatic breast cancer (MBC) Ann Oncol. 2022;33 7:S88–S121. doi: 10.1016/annonc/annonc1089. [DOI] [Google Scholar]
- 18.Alexandrov L B, Stratton M R. Mutational signatures: the patterns of somatic mutations hidden in cancer genomes. Curr Opin Genet Dev. 2014;24:52–60. doi: 10.1016/j.gde.2013.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alexandrov L B, Kim J, Haradhvala N J et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578:94–101. doi: 10.1038/s41586-020-1943-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.COSMIC Mutational Signatures (v3.3 – June 2022)- Single Base Substitution (SBS) Signatures 2022. Online (Stand: 12.11.2022):https://cancer.sanger.ac.uk/signatures/sbs/
- 21.Toy W, Weir H, Razavi P et al. Activating ESR1 Mutations Differentially Affect the Efficacy of ER Antagonists. Cancer Discov. 2017;7:277–287. doi: 10.1158/2159-8290.CD-15-1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Robinson D R, Wu Y M, Vats P et al. Activating ESR1 mutations in hormone-resistant metastatic breast cancer. Nat Genet. 2013;45:1446–1451. doi: 10.1038/ng.2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spoerke J M, Gendreau S, Walter K et al. Heterogeneity and clinical significance of ESR1 mutations in ER-positive metastatic breast cancer patients receiving fulvestrant. Nat Commun. 2016;7:11579. doi: 10.1038/ncomms11579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang P, Bahreini A, Gyanchandani R et al. Sensitive Detection of Mono- and Polyclonal ESR1 Mutations in Primary Tumors, Metastatic Lesions, and Cell-Free DNA of Breast Cancer Patients. Clin Cancer Res. 2016;22:1130–1137. doi: 10.1158/1078-0432.CCR-15-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schiavon G, Hrebien S, Garcia-Murillas I et al. Analysis of ESR1 mutation in circulating tumor DNA demonstrates evolution during therapy for metastatic breast cancer. Sci Transl Med. 2015;7:313ra182. doi: 10.1126/scitranslmed.aac7551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang K, Hong R, Xu F et al. Clinical value of circulating ESR1 mutations for patients with metastatic breast cancer: a meta-analysis. Cancer Manag Res. 2018;10:2573–2580. doi: 10.2147/CMAR.S173193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fanning S W, Mayne C G, Dharmarajan V et al. Estrogen receptor alpha somatic mutations Y537S and D538 G confer breast cancer endocrine resistance by stabilizing the activating function-2 binding conformation. Elife. 2016;5:e12792. doi: 10.7554/eLife.12792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fanning S W, Jeselsohn R, Dharmarajan V et al. The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells. Elife. 2018;7:e37161. doi: 10.7554/eLife.37161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bidard F C, Kaklamani V G, Neven P et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results From the Randomized Phase III EMERALD Trial. J Clin Oncol. 2022;40:3246–3256. doi: 10.1200/JCO.22.00338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bidard F C, Hardy-Bessard A C, Dalenc F et al. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): a randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022;23:1367–1377. doi: 10.1016/S1470-2045(22)00555-1. [DOI] [PubMed] [Google Scholar]
- 31.Breast Cancer Association Consortium . Dorling L, Carvalho S, Allen J et al. Breast Cancer Risk Genes – Association Analysis in More than 113,000 Women. N Engl J Med. 2021;384:428–439. doi: 10.1056/NEJMoa1913948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fachal L, Aschard H, Beesley J et al. Fine-mapping of 150 breast cancer risk regions identifies 191 likely target genes. Nat Genet. 2020;52:56–73. doi: 10.1038/s41588-019-0537-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Garcia-Closas M, Couch F J, Lindstrom Set al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci Nat Genet 201345392–398.398e1–398e2 10.1038/ng.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghoussaini M, Fletcher O, Michailidou K et al. Genome-wide association analysis identifies three new breast cancer susceptibility loci. Nat Genet. 2012;44:312–318. doi: 10.1038/ng.1049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Michailidou K, Beesley J, Lindstrom S et al. Genome-wide association analysis of more than 120,000 individuals identifies 15 new susceptibility loci for breast cancer. Nat Genet. 2015;47:373–380. doi: 10.1038/ng.3242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michailidou K, Hall P, Gonzalez-Neira Aet al. Large-scale genotyping identifies 41 new loci associated with breast cancer risk Nat Genet 201345353–361.361e1–361e2 10.1038/ng.2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milne R L, Kuchenbaecker K B, Michailidou K et al. Identification of ten variants associated with risk of estrogen-receptor-negative breast cancer. Nat Genet. 2017;49:1767–1778. doi: 10.1038/ng.3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michailidou K, Lindstrom S, Dennis J et al. Association analysis identifies 65 new breast cancer risk loci. Nature. 2017;551:92–94. doi: 10.1038/nature24284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fasching P A, Liu D, Scully S et al. Identification of Two Genetic Loci Associated with Leukopenia after Chemotherapy in Breast Cancer Patients. Clin Cancer Res. 2022;28:3342–3355. doi: 10.1158/1078-0432.CCR-20-4774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hlevnjak M, Schulze M, Elgaafary S et al. CATCH: A Prospective Precision Oncology Trial in Metastatic Breast Cancer. JCO Precis Oncol. 2021;5:PO.20.00248. doi: 10.1200/PO.20.00248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hortobagyi G N, Stemmer S M, Burris H A et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N Engl J Med. 2022;386:942–950. doi: 10.1056/NEJMoa2114663. [DOI] [PubMed] [Google Scholar]
- 42.Hortobagyi G N, Stemmer S M, Burris H A et al. Ribociclib as First-Line Therapy for HR-Positive, Advanced Breast Cancer. N Engl J Med. 2016;375:1738–1748. doi: 10.1056/NEJMoa1609709. [DOI] [PubMed] [Google Scholar]
- 43.European Medicines Agency Verzenios Summary of product characteristics (SmPC) 2022. Online (Stand: 26.03.2022):https://www.ema.europa.eu/en/documents/product-information/verzenios-epa-product-information_en.pdf
- 44.Goetz M P, Toi M, Campone M et al. MONARCH 3: Abemaciclib As Initial Therapy for Advanced Breast Cancer. J Clin Oncol. 2017;35:3638–3646. doi: 10.1200/JCO.2017.75.6155. [DOI] [PubMed] [Google Scholar]
- 45.Finn R S, Martin M, Rugo H S et al. Palbociclib and Letrozole in Advanced Breast Cancer. N Engl J Med. 2016;375:1925–1936. doi: 10.1056/NEJMoa1607303. [DOI] [PubMed] [Google Scholar]
- 46.Finn R S, Rugo H S, Dieras V C et al. Overall survival (OS) with first-line palbociclib plus letrozole (PAL+LET) versus placebo plus letrozole (PBO+LET) in women with estrogen receptor–positive/human epidermal growth factor receptor 2–negative advanced breast cancer (ER+/HER2− ABC): Analyses from PALOMA-2. J Clin Oncol. 2022;40:LBA1003. doi: 10.1200/JCO.2022.40.17_suppl.LBA1003. [DOI] [Google Scholar]
- 47.Im S A, Lu Y S, Bardia A et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N Engl J Med. 2019;381:307–316. doi: 10.1056/NEJMoa1903765. [DOI] [PubMed] [Google Scholar]
- 48.Tripathy D, Im S-A, Colleoni M et al. Abstract PD2-04: Updated overall survival (OS) results from the phase III MONALEESA-7 trial of pre- or perimenopausal patients with hormone receptor positive/human epidermal growth factor receptor 2 negative (HR+/HER2−) advanced breast cancer (ABC) treated with endocrine therapy (ET) ± ribociclib. Cancer Res. 2021;81:PD2-04. doi: 10.1158/1538-7445.Sabcs20-pd2-04. [DOI] [Google Scholar]
- 49.Tripathy D, Im S A, Colleoni M et al. Ribociclib plus endocrine therapy for premenopausal women with hormone-receptor-positive, advanced breast cancer (MONALEESA-7): a randomised phase 3 trial. Lancet Oncol. 2018;19:904–915. doi: 10.1016/S1470-2045(18)30292-4. [DOI] [PubMed] [Google Scholar]
- 50.Fasching P A, Esteva F J, Pivot X et al. Patient-reported outcomes (PROs) in advanced breast cancer (ABC) treated with ribociclib plus fulvestrant: Results from MONALEESA-3. Ann Oncol. 2018 doi: 10.1093/annonc/mdy272.282. [DOI] [Google Scholar]
- 51.Slamon D J, Neven P, Chia S et al. Overall Survival Results from the Phase 3 MONALEESA-3 Study of Fulvestrant ± Ribociclib in Postmenopausal Patients With HR+/HER2− Advanced Breast Cancer. Ann Oncol. 2019;30 5:v851–v934. doi: 10.1093/annonc/mdz394. [DOI] [Google Scholar]
- 52.Slamon D J, Neven P, Chia S et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2020;382:514–524. doi: 10.1056/NEJMoa1911149. [DOI] [PubMed] [Google Scholar]
- 53.Slamon D J, Neven P, Chia S et al. Phase III Randomized Study of Ribociclib and Fulvestrant in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: MONALEESA-3. J Clin Oncol. 2018;36:2465–2472. doi: 10.1200/JCO.2018.78.9909. [DOI] [PubMed] [Google Scholar]
- 54.Sledge G W, jr., Toi M, Neven P et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020;6:116–124. doi: 10.1001/jamaoncol.2019.4782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sledge G W, jr., Toi M, Neven P et al. MONARCH 2: Abemaciclib in Combination With Fulvestrant in Women With HR+/HER2- Advanced Breast Cancer Who Had Progressed While Receiving Endocrine Therapy. J Clin Oncol. 2017;35:2875–2884. doi: 10.1200/JCO.2017.73.7585. [DOI] [PubMed] [Google Scholar]
- 56.Cristofanilli M, Rugo H S, Im S-A et al. Overall survival (OS) with palbociclib (PAL) + fulvestrant (FUL) in women with hormone receptor–positive (HR+), human epidermal growth factor receptor 2–negative (HER2–) advanced breast cancer (ABC): Updated analyses from PALOMA-3. J Clin Oncol. 2021;39:1000. doi: 10.1200/JCO.2021.39.15_suppl.1000. [DOI] [Google Scholar]
- 57.Turner N C, Slamon D J, Ro J et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N Engl J Med. 2018;379:1926–1936. doi: 10.1056/NEJMoa1810527. [DOI] [PubMed] [Google Scholar]
- 58.Xu B, Zhang Q, Zhang P et al. Dalpiciclib or placebo plus fulvestrant in hormone receptor-positive and HER2-negative advanced breast cancer: a randomized, phase 3 trial. Nat Med. 2021;27:1904–1909. doi: 10.1038/s41591-021-01562-9. [DOI] [PubMed] [Google Scholar]
- 59.Brett J O, Spring L M, Bardia A et al. ESR1 mutation as an emerging clinical biomarker in metastatic hormone receptor-positive breast cancer. Breast Cancer Res. 2021;23:85. doi: 10.1186/s13058-021-01462-3. [DOI] [PMC free article] [PubMed] [Google Scholar]