Abstract
Common histidine-to-aspartate (His-to-Asp) phosphorelay signaling systems involve three types of signaling components: a sensor His kinase, a response regulator, and a histidine-containing phosphotransfer (HPt) protein. In the fission yeast Schizosaccharomyces pombe, two response regulators, Mcs4 and Prr1, have been identified recently, and it was shown that they are involved in the signal transduction implicated in stress responses. Furthermore, Mcs4 appears to be involved in mitotic cell-cycle control. However, neither the HPt phosphotransmitter nor His kinase has been characterized in S. pombe. In this study, we identified a gene encoding an HPt phosphotransmitter, named Spy1 (S. pombe YPD1-like protein). The spy1+ gene showed an ability to complement a mutational lesion of the Saccharomyces cerevisiae YPD1 gene, which is involved in an osmosensing signal transduction. The result from yeast two-hybrid analysis indicated that Spy1 interacts with Mcs4. To gain insight into the function of Spy1, a series of genetic analyses were conducted. The results provided evidence that Spy1, together with Mcs4, plays a role in regulation of the G2/M cell cycle progression. Spy1-deficient cells appear to be precocious in the entry to M phase. In the proposed model, Spy1 modulates Mcs4 in a negative manner, presumably through a direct His-to-Asp phosphorelay, operating upstream of the Sty1 mitogen-activated protein kinase cascade.
Common prokaryotic signal transduction mechanisms are generally referred to as “histidine-to-aspartate (His-to-Asp) phosphorelay systems” (or “two-component regulatory systems”). Such a His-to-Asp phosphorelay involves two or more of the common signal transducers, a sensor exhibiting histidine (His) kinase activity, a response regulator containing a phosphoaccepting receiver, and a histidine-containing phosphotransmitter (HPt) (4, 5,12, 23, 29, 39). To date, numerous instances of His-to-Asp phosphorelay systems, involved in a wide variety of adaptive responses to environmental stimuli, have been reported for many bacterial species. The His-to-Asp phosphorelay system was once thought to be restricted to prokaryotes. However, many instances have recently been reported for diverse eukaryotic species, including yeasts (17, 18), fungi (3), slime molds (7, 32, 41), and even higher plants (6, 13, 15, 40). Thus, the His-to-Asp phosphorelay is a paradigm of intracellular signal transduction through protein phosphorylation in both prokaryotes and eukaryotes.
In eukaryotes, the best-characterized His-to-Asp phosphorelay is the osmoresponsive signal transduction in the budding yeast Saccharomyces cerevisiae (20, 43). Together, the three components Sln1 (sensor His kinase), Ypd1 (HPt phosphotransmitter), and Ssk1 (response regulator) are involved in the His-to-Asp phosphorelay signaling pathway. A striking fact is that the yeast His-to-Asp phosphorelay pathway is directly linked to a eukaryotic mitogen-activated protein kinase (MAPK) signaling cascade (termed the HOG1 [high-osmolarity glycerol response]) cascade. The fission yeast Schizosaccharomyces pombe is an alternative model microorganism with which to gain an insight into how a bacterial type of signal transduction mechanism is integrated into a eukaryotic signal transduction cascade. Nonetheless, clarification of such a His-to-Asp phosphorelay system in S. pombe is at a very early stage.
In S. pombe, so far, two response regulators, named Prr1 and Mcs4, have been uncovered and characterized (8, 28, 33, 37). The Prr1 response regulator has a typical phosphoaccepting receiver domain, preceded by a mammalian heat shock factor-like DNA-binding domain. It was demonstrated that Prr1 is responsible for transcriptional regulation of some genes (e.g., trr1+ and ctt1+), which are induced by oxidative stress (28). The Mcs4 response regulator appears to be the counterpart (or homologue) of the S. cerevisiae Ssk1 response regulator, as judged by the fact that their amino acid sequences are very similar to each other and that Mcs4 functions immediately upstream of an S. pombe stress-activated MAPK cascade (8, 33, 37). This particular MAPK cascade has recently been characterized extensively, and it includes MAPK Sty1 (also known as Spc1 and Phh1) (16, 21, 35), MAPKK Wis1 (34), and MAPKKK Wak1 (also known as Wik1) (33, 37). The Sty1 MAPK cascade is considered to be analogous to the S. cerevisiae HOG1 MAPK cascade. In contrast to the HOG1 MAPK cascade, however, the Sty1 MAPK cascade is activated by multiple environmental stresses, including osmotic stress, oxidative stress, heat shock, and UV light (9, 10, 21, 33, 35, 36, 38). More interestingly, it is known that the Sty1 MAPK cascade links stress signaling with control of sexual differentiation in S. pombe (16, 36). Furthermore, the Sty1 MAPK cascade was suggested to somehow integrate stress sensing into control of mitosis (35). Thus, the Sty1 MAPK cascade appears to be crucial for linking stress sensing with two processes fundamental to all eukaryotes, namely, control of both mitosis and meiosis. In this context, it should be noted that the mcs4+ gene was originally identified as a mutation which is capable of suppressing the lethal phenotype (the so-called “mitotic catastrophe”) caused by the cdc2-3w and wee1-50 double mutations (24). These and other previous results supported the idea that, together with the Sty1 MAPK cascade, a His-to-Asp phosphorelay pathway involving Mcs4 plays a role in a presumed stress-responsive control of mitosis. However, such a putative His-to-Asp phosphorelay in S. pombe is entirely elusive, because neither His kinase nor the HPt phosphotransmitter has been characterized. In this study, an HPt phosphotransmitter of S. pombe was identified and characterized. This newly uncovered HPt phosphotransmitter, named Spy1, was suggested to play a role, together with Mcs4, in control of the timing of the mitotic initiation (or the G2/M transition).
MATERIALS AND METHODS
Strains, plasmids, and media.
The S. pombe strains and plasmids used in this study are listed in Table 1. These strains were grown either in YPD medium (1% yeast extract [Difco], 2% Polypeptone [Wako], 2% glucose) containing 10 μg of adenine per ml or in SD medium (0.67% yeast nitrogen base without amino acids [Difco], 2% glucose) supplemented with the necessary growth requirements in standard amounts. EMM minimal medium and MEA medium were also used (25).
TABLE 1.
Yeast strains and plasmids relevant to this study
| Strain of plasmid | Genotype | Source |
|---|---|---|
| Strains | ||
| S. pombe | ||
| JY333 | h− leu1-32 ade6-M216 | M. Yamamoto |
| JY741 | h− leu1-32 ade6-M216 ura4-D18 | M. Yamamoto |
| JY746 | h+ leu1-32 ade6-M210 ura4-D18 | M. Yamamoto |
| Gp14 | h− leu1-32 ade6-M216 ura4-D18 mcs4::ura4+ | G. Cottarel (8) |
| M132 | h− leu1-32 cdc25-22 | H. Okayama |
| KI001 | h− leu1-32 ade6-M216 ura4-D18 spy1::ura4+ | This study |
| KI002 | h− leu1-32 ade6-M216 ura4-D18 spy1::ura4+ mcs4::ura4+ | This study |
| KI003 | h− leu1-32 ura4-D18 spy1::ura4+ cdc25-22 | This study |
| KI004 | h− leu1-32 ade6-M216 ura4-D18 spy1::ura4+ sty1::ura4+ | This study |
| JM1160 | h+ leu1-32 ade6-M216 ura4-D18 sty1::ura4+ | J. B. A. Millar |
| S. cerevisiae CUY1 | MATα ura3-52 leu2-3 his3-200 YPD1::LEU2/pGB22 [pGB22;pRS413(HIS3) GAL1p-PTP2] | T. Suzuki (40) |
| Plasmids | ||
| pREP1 | nmt1 promoter, LEU2 marker | K. Maundrell |
| pKA013 | spy1+ gene under control of nmt1 promoter | This study |
| pKA018 | spy1HQ gene under control of nmt1 promoter | This study |
| pCUY326 | ADH1 promoter, URA3 marker | C. Ueguchi |
| pKA014 | spy1+ gene under control of ADH1 promoter | This study |
| pKA019 | spy1HQ gene under control of ADH1 promoter | This study |
Purification and phosphorylation of Spy1 and Mcs4.
The spy1+ or mcs4+ coding sequence was placed under the T7 promoter on pET22b(+), which is an Escherichia coli expression vector (Novagen, Madison, Wis.). The appropriately constructed plasmid was transferred into E. coli BL21(DE3). The cells were grown in Luria-Bertani medium in the presence of IPTG (isopropyl-β-d-thiogalactopyranoside). A cleared-cell lysate was obtained by use of an Aminco French pressure cell. This sample was applied to an Ni column with the rapid affinity purification pET His-Tag system supplied by Novagen. Other methods were those recommended by the supplier. The purified Spy1 protein (8 μg) was incubated with urea-treated E. coli cytoplasmic membrane (20 μg) at 37°C in the presence of 0.05 mM [γ-32P]ATP (10,000 cpm pmol−1), 200 mM KCl, and 5 mM MgCl2. After incubation, the samples were subjected to sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE), followed by autoradiography. For phosphate transfer experiment, radioactively phosphorylated Spy1 protein was purified by means of gel filtration as described previously (1, 14). Other details were the same as those described previously (40).
Northern hybridization analysis.
Northern hybridization analysis was carried out as described previously (2). Exponentially growing cells in YPD medium were collected and resuspended in fresh YPD medium containing 0.9 M KCl or 1 mM H2O2. A total RNA fraction was prepared from the cells at each time. After denaturation with formamide-formaldehyde, RNA (5 μg) was analyzed on a 1.4% agarose gel containing formaldehyde, followed by alkali blotting onto Hybond-N+ membrane (Amersham International). Hybridization was carried out with 32P-labelled probe, which specifically encompassed the gpd1+, ctt1+, or leu1+ coding sequence, at 65°C for 2 h in Rapid-hyb buffer, as recommended by the supplier (Amersham International).
Plasmid construction.
For construction of pKA013 (named pREP1-Spy1 in Fig. 7), in which the spy1+ gene is controlled under the nmt1 promoter, the coding sequence of spy1+ was PCR amplified with the primers (5′-TTCTAACATATGAGTGTATATCGTGATAACATG and 5′-GGGGATCCAAAGGCTAGGTACTTTGAC). After digestion with NdeI and BamHI, the fragment was cloned in the same site of pREP1 (19). For construct pKA018, which is identical to pKA013 except that the His-221 of Spy1 was replaced with Gln, site-directed mutagenesis was carried out with the oligonucleotide 5′-pGATCCTTTAAGGAATTGCCCCAACGAGGAAAGC. For S. cerevisiae complementation analysis, two plasmids (2 μm origin, URA3 marker) were constructed, and named pKA014 and pKA019, respectively. pKA014 carries the S. pombe spy1+ gene, which was placed under the S. cerevisiae ADH1 promoter (named pSpy1 in Fig. 2), whereas pKA019 carries the His-221-to-Gln mutation in the spy1 gene (named pSpy1HQ in Fig. 2). For two-hybrid analysis, three plasmids, pKA027, pKA015, and pKA030, were constructed. To construct pKA027, the mcs4+ gene was PCR amplified with primers 5′-ATGAATTCCATATGCGCATTTGGTTTAAAAAAG and 5′-GCTAGTCGACTCGACCGCGAAAACGGC. After digestion with EcoRI and BamHI, the fragment encoding mcs4+ was cloned in the same site of pGBT9. pKA015 was constructed as follows. An NdeI-BamHI fragment carrying the spy1+ gene was isolated from pKA013, treated with T4 DNA polymerase, and then cloned into an SmaI site of pGAD424. pKA030 was identical to pKA015, except that the His-221 of Spy1 was replaced with Gln.
FIG. 7.
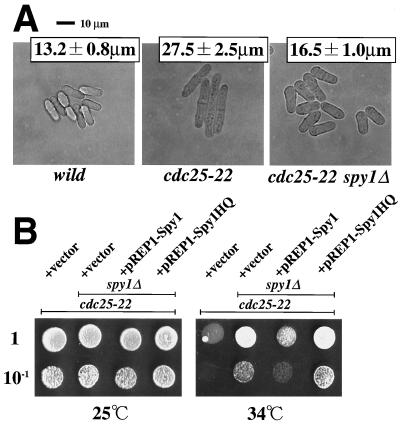
spy1Δ mutation can function as a suppressor for cdc25-22 mutation. (A) The indicated cell types growing exponentially in EMM medium at 30°C were photographed. The average cell size of septated cells was determined from 20 individuals (± standard deviation). The cdc25-22 mutant and cdc25-22 spy1Δ double mutant which carry each of the plasmids indicated were spotted onto EMM plates at the proper dilution. Cells were grown at 25 or 34°C for 3 days and photographed.
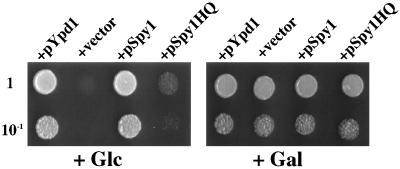
FIG. 2.
The spy1+ gene is able to complement the mutational lesion of the YPD1 gene of S. cerevisiae. The ypd1Δ mutant carrying a plasmid harboring a composite PTP2 gene (PGAL1-PTP2) cannot grow on SC medium supplemented by glucose, while it can grow on SC medium supplemented with galactose (see the text) (+vector). Plasmid pKA014 carrying the recombinant spy1+ gene (designated as pSpy1) was transferred into the ypd1Δ mutant, and then the viability of the transformed cells (about 2 × 102 and 2 × 103) was examined by spotting on galactose-synthetic complete (SC) medium (Gal) or glucose-SC medium (Glc). The cells were incubated for 3 days at 30°C. The same analyses were carried out for the plasmids carrying the mutant spy1 gene (designated as pSpy1HQ) as well as the budding yeast YPD1 gene (designated as pYpd1).
Two-hybrid analysis.
The kit used for two-hybrid analysis (MATCHMAKER; Clontech) was obtained through Toyobo Co. The kit included the vectors pGBT9, providing the GAL4 DNA-binding domain (TRP1 marker), and pGAD424, providing the GAL4 activation domain (LEU2 marker). Analysis was carried out according to the manual by using strain HF7c as a host.
Gene disruption.
For spy1 disruption, 4,684 bp of an SphI-BamHI fragment carrying the spy1+ gene was amplified by PCR with the appropriate primers and cloned on pUC18 to construct plasmid pHAI 207 (Fig. 1A). Then the MunI and XhoI region in the spy1 open reading frame (ORF) was replaced with a ura4+ cassette and used for linear transformation after VspI digestion (Fig. 1A). Stable Ura4+ transformants were selected for a diploid strain (JY741 × JY746), and then the spy1::ura4+ construct on the chromosome was confirmed by Southern hybridization. After sporulation, Ura+ haploid segregants were analyzed.
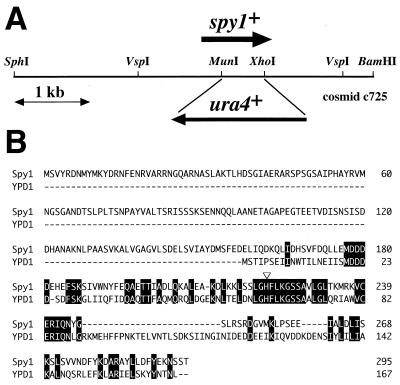
FIG. 1.
The spy1+ gene encodes an HPt phosphotransmitter. (A) Schematic representation of the S. pombe chromosomal region encompassing the spy1+ gene. This region is carried in the c725 cosmid, whose entire nucleotide sequence has been determined (GenBank accession no. AL034352). In this study, an spy1Δ strain was constructed by inserting the ura4+ marker, as shown schematically. (B) The deduced amino acid sequence of Spy1 was aligned with that of the budding yeast Ypd1 protein. The open triangle indicates the presumed phosphorylation site (histidine). The amino acids identical in Spy1 and Ypd1 are highlighted.
RESULTS
Identification of the spy1+ gene that encodes an HPt phosphotransmitter.
An extensive inspection of the current genome sequence database for S. pombe revealed the occurrence of a gene encoding a protein highly homologous to the S. cerevisiae Ypd1 protein. As shown in Fig. 1, this gene was found as an ORF in cosmid c725 from chromosome II (gene name, SPBC725.02; GenBank accession no. AL034352). This putative gene, named spy1+ (S. pombe YPD1 homologue), specifies an uninterrupted ORF encoding a protein of 295 amino acids. Its C-terminal region, consisting of about 140 amino acids, has 39% identity to that of Ypd1 (Fig. 1B). In particular, their amino acid sequences around the presumed phosphorylation sites (His-221 in Spy1) are particularly conserved (Fig. 1B). Spy1 has an extension at its N terminus (to the amino acid position of 157), which is absent in Ypd1.
To examine whether or not Spy1 can function as an HPt phosphotransmitter in a His-to-Asp phosphorelay, a complementation experiment employing a ypd1Δ mutant of S. cerevisiae was carried out (Fig. 2). As previously known, disruption of the YPD1 gene results in lethality, presumably due to an excessive phosphorylation (or activation) of the HOG1 MAPK (30). Such a hyperphosphorylation event in the ypd1Δ cells can be eliminated by overproduction of the Ptp2 protein tyrosine phosphatase. Therefore, the ypd1Δ cells carrying a plasmid harboring a galactose-inducible PTP2 gene (designated as Pgal-PTP2) can grow on SC medium in which the glucose was replaced with galactose, while they cannot grow on standard SC medium (Fig. 2; +vector) (30). When the spy1+ gene of S. pombe was introduced into such ypd1Δ cells, the transformed cells were capable of growing on the SC medium, as in the case of the cells carrying the plasmid-borne YPD1 gene (Fig. 2; +pSpy1 and +pYpd1, respectively). A mutant spy1 gene encoding the Spy1 protein having an amino acid substitution (His-221 to Gln) at the presumed phosphorylation site showed no ability to do so (Fig. 2; +pSpy1HQ). These results demonstrated that Spy1 is capable of functioning as an HPt phosphotransmitter.
Spy1 interacts with Mcs4.
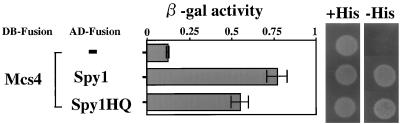
It is known that Ypd1 functions as an intermediate of phosphorelay towards the downstream target, Ssk1, in S. cerevisiae (30). By analogy, it was assumed for S. pombe that Spy1 most likely functions together with Mcs4, which appears to be the homologue of Ssk1. To examine this, yeast two-hybrid analyses were adopted by using Mcs4 and Spy1 as bait and prey, respectively (Fig. 3). A positive result was obtained, as judged by both the β-galactosidase and histidine-auxotrophy assays, suggesting that Spy1 and Mcs4 interact with each other. Interestingly, the mutant Spy1HQ protein also showed such an interaction. To analyze whether Spy1 specifically interacts with Mcs4, another S. pombe response regulator, Prr1, was also used as bait in the two-hybrid analysis. However, Prr1 fused to the DNA-binding domain of Gal4 showed evident activity by itself. Accordingly, thus far, we have not been able to address this.
FIG. 3.
Yeast two-hybrid analysis. Two-hybrid analysis was carried out with the combination indicated. The results are shown as β-galactosidase activity and histidine auxotrophy (+His or −His). In this experiment, it should be noted that neither AD-Spy1 nor AD-Spy1HQ alone showed significant β-galactosidase activity (0.13 and 0.18 U, respectively) (data not shown). DB-Fusion and AD-Fusion indicate proteins which are fused with the GAL4 DNA-binding domain and GAL4 activation domain, respectively.
Spy1 undergoes phosphorylation and transfers its phosphate to Mcs4.
It is crucial to ask the question of whether or not Spy1 is capable of undergoing phosphorylation at the putative phosphoaccepting histidine site (His-221). This could be assessed by employing the E. coli cytoplasmic membrane that contains the overproduced ArcB hybrid sensor His kinase (40). When some heterologous HPt phosphotransmitters were incubated with the E. coli cytoplasmic membrane in the presence of ATP, they can acquire a phosphoryl group at a certain histidine residue, as previously demonstrated for the higher plant (Arabidopsis) HPt phosphotransmitters (40). This artificial in vitro phosphorylation system was used to show that Spy1 has the ability to undergo phosphorylation. The Spy1 protein was purified, together with its mutant derivative, which has the His-to-Gln substitution at the position of 221 (Fig. 4A). These purified proteins were incubated with the E. coli cytoplasmic membrane in the presence of [γ-32P]ATP under the in vitro conditions established previously (40). The results showed that the wild-type Spy1 protein is capable of undergoing phosphorylation in a manner catalyzed by the E. coli cytoplasmic membrane, while the mutant protein is not (Fig. 4B).
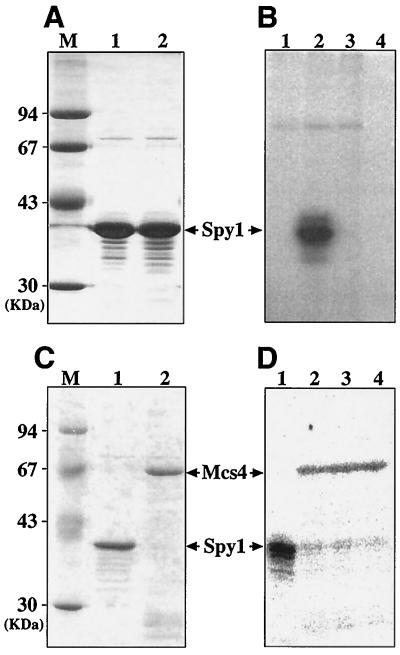
FIG. 4.
In vitro phosphorylation of Spy1 and Mcs4. (A) Both histidine-tagged Spy1 (lane 1) and Spy1HQ (lane 2) proteins were purified as described in Materials and Methods. They were analyzed by SDS-PAGE followed by staining with Coomassie brilliant blue (16 μg each). Molecular mass markers are shown in lane M. (B) The purified Spy1 protein was incubated with the E. coli membrane in the presence of [γ-32P]ATP for 30 min, as also described in Materials and Methods. The samples were analyzed by SDS-PAGE followed by autoradiography (lane 1, membrane alone; lane 2, membrane plus Spy1; lane 3, membrane plus Spy1HQ; and lane 4, Spy1 alone). (C) The histidine-tagged Mcs4 (lane 2) was purified and analyzed by SDS-PAGE, followed by staining with Coomassie brilliant blue. Spy1 protein used for the phosphate transfer experiment was also indicated (lane 1). (D) Autoradiogram showing phosphotransfer between phospho-Spy1 and Mcs4. 32P-labelled phospho-Spy1 was purified as described previously (lane 1) (1, 14) and then was incubated with the purified Mcs4 protein at 16°C. Aliquots were removed 0.5 min (lane 2), 1 min (lane 3), and 5 min (lane 4) later and analyzed by SDS-PAGE.
Next, the phosphorylated Spy1 was purified and incubated with purified Mcs4 protein. As shown in Fig. 4D, the phosphoryl group on Spy1 was apparently transferred to Mcs4. From these biochemical data, it was suggested that the Spy1 protein has the ability to acquire a phosphoryl group at the histidine site at position 221 and transfer its phosphate to Mcs4.
Function of Spy1 in stress response.
The results presented above are compatible with the idea that Spy1 and Mcs4 together constitute a His-Asp phosphorelay pathway in S. pombe. To address this genetically, we attempted to construct an spy1 deletion mutant by creating an spy1::ura4+ allele on the chromosome (Fig. 1A); the VspI DNA segment encompassing the spy1::ura4+ construct was integrated into a diploid strain via homologous recombination. Tetrad dissection of asci from heterozygous diploids gave rise to four viable spores on germination that showed a 2:2 segregation of uracil auxotrophs to uracil prototrophs (data not shown). This indicated that the spy1+ gene is not essential for growth under standard growth conditions. This is in a sharp contrast to the fact that the ypd1Δ mutant is lethal in S. cerevisiae (30). The natures of Spy1-deficient cells were then characterized in comparison with those of Mcs4-deficient cells.
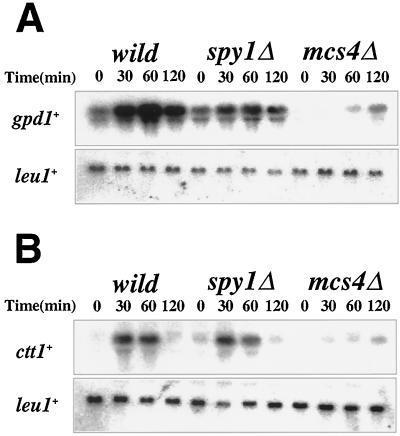
It is known that the gpd1+ gene encoding glycerol-3-phosphate dehydrogenase is induced by osmotic stress (e.g., 0.9 M KCl in medium) in a manner dependent on Mcs4 as well as the Sty1 MAPK cascade (2, 33, 36, 42). The ctt1+ gene encoding catalase is also under the same regulatory circuit—in this case, in response to oxidative stress (e.g., H2O2 treatment) (33). It was reported that Mcs4-deficient cells are osmosensitive for growth, although not as severely as Sty1-deficient cells (33, 37). Based on these results, the expression of gpd1+ and ctt1+ in Spy1-deficient cells was examined by Northern hybridization, after the cells had been subjected to either osmotic or oxidative stresses (Fig. 5). In Mcs4-deficient cells, the osmoinducible expression of gpd1+ and the H2O2-inducible expression of ctt1+ were markedly impaired, as previously reported (33). However, the regulatory profiles of gpd1+ and ctt1+ were not significantly altered in the spy1Δ background, compared with those in the wild-type background. Furthermore, Spy1-deficient cells grew well on SD agar plates containing either 1 M KCl or 2 mM H2O2 (data not shown). Therefore, no evidence was obtained that implicated Spy1 in the stress-responsive signal transduction pathway, as far as the osmotic and oxidative stress responses were concerned.
FIG. 5.
Spy1-deficient strain shows normal stress responses. (A) Northern hybridization showing osmotic induction of gpd1+ mRNA in the spy1Δ mutant. RNA was prepared before and after the addition of 0.9 M KCl at the indicated time and hybridized with gpd1+ probe. The same filter was also hybridized with the leu1+ probe for the control of the loading amount. (B) Northern hybridization showing oxidative induction of ctt1+ mRNA in the spy1Δ mutant. RNA was prepared before and after the addition of 1 mM H2O2 at the indicated time and hybridized with ctt1+ probe. The same filter was also hybridized with leu1+ probe for control of the loading amount.
Function of Spy1 in control of the mitotic cell cycle.
Mcs4 appears to be involved, not only in the stress-responsive signaling, but also in a signaling circuitry of mitotic control, as demonstrated previously (8, 33, 37). The main engine of the mitotic G2/M transition in S. pombe consists of the Cdc13 (cyclin B)/Cdc2 kinase, the Wee1/Mik1 tyrosine kinases, and the Cdc25 phosphatase (11). As documented previously, certain S. pombe mutants (e.g., cdc25-22) that are delayed in the timing of the mitotic G2/M transition divide at a cell length longer than the wild-type cells, whereas other mutants (e.g., wee1-50) resulting in an advancement of the G2/M transition divide at a shorter cell length (11, 22, 31). Based on such hallmarks of mutational lesions of the mitotic control, it was previously reported that Mcs4-deficient cells are delayed in the timing of the G2/M transition, thereby exhibiting an elongated cell size, as confirmed in Fig. 6C (8, 33, 37). Spy1-deficient cells were then assessed in terms of this particular aspect.
FIG. 6.
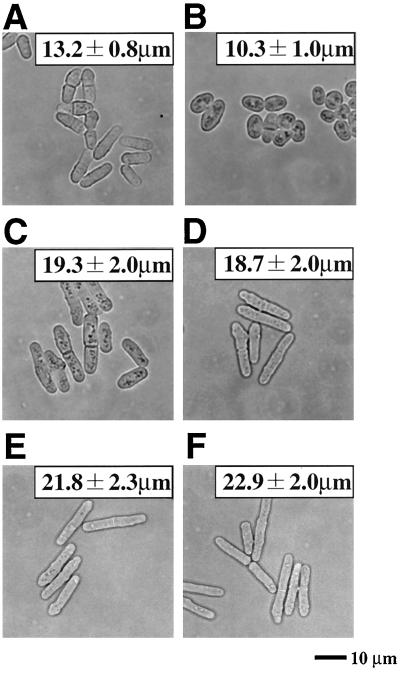
Initiation of mitosis is accelerated in the spy1Δ mutant. (A) Wild type. (B) spy1Δ. (C) mcs4Δ. (D) mcs4Δ spy1Δ. (E) sty1Δ. (F) sty1Δ spy1Δ. Each of indicated cell types growing exponentially in EMM medium at 30°C was photographed. The average cell size of septated cells was determined from 20 individuals (± standard deviation).
To assess the timing of cell division, Spy1-deficient cells were grown in a given medium, and then, at the exponential growth phase, they were observed under a phase-contrast microscope. The average cell length at division (i.e., length of septated cell) was statistically measured (Fig. 6B). As mentioned above, Mcs4-deficient cells were elongated (Fig. 6C; 19.3 ± 2.0 μm) and were significantly longer than wild-type cells (Fig. 6A; 13.2 ± 0.8 μm). This is quite consistent with the previous result, which suggested that the mcs4Δ mutant is delayed in the timing of the G2/M transition (8, 33, 37). In sharp contrast, it was found that Spy1-deficient cells had an ovoid morphology, which was significantly shorter (Fig. 6B; 10.3 ± 1.0 μm) than the wild type. Such a short ovoid morphology is indicative of precocious entry into the M phase. It should be noted that this morphological change of the spy1Δ mutant was suppressed by introducing the plasmid-borne spy1+ gene, but not by the mutant spy1-HQ gene (data not shown).
To conduct critical epistatic analyses, we constructed an mcs4Δ and spy1Δ double mutant, and also constructed an sty1Δ and spy1Δ double mutant. As mentioned above, Mcs4 was considered to function upstream of the Sty1 MAPK. The sty1Δ single mutant showing an elongated cell size is also impaired in control of the G2/M transition (Fig. 6E), as well documented previously (21, 35). Here the mcs4Δ and spy1Δ double mutant showed a cell size (18.7 ± 2.0 μm) very similar to that of the mcs4Δ single mutant (Fig. 6C and D). The sty1Δ and spy1Δ double mutant also showed an elongated cell size (22.9 ± 2.0 μm) very similar to that of the sty1Δ single mutant (Fig. 6E and F). These results of epistatic analyses strongly suggest that Spy1 functions upstream of Mcs4 and Sty1 in a presumed linear signaling pathway.
Altogether from the results shown in Fig. 6, one can reasonably propose the following scenario. Spy1, together with Mcs4, is involved in a signaling pathway for mitotic control of the cell cycle in S. pombe. Mcs4 is a positive regulator for progression of the G2/M transition, whereas Spy1 functions upstream of Mcs4 as a negative regulator for Mcs4 through the presumed His-to-Asp phosphorelay, which operates upstream of the Sty1 MAPK cascade.
A link between Spy1 and the mechanism underlying control of the G2/M transition.
If the spy1+ gene is indeed involved in a signal transduction pathway that somehow regulates the mitotic cell cycle (particularly, the G2/M transition), one can expect that the spy1Δ mutation should display a genetic interaction with the well-documented main controller of the G2/M transition [i.e., the Cdc13 (cyclin B)/Cdc2 kinase, the Wee1/Mik1 tyrosine kinases, and the Cdc25 phosphatase]. To address this issue, we employed the well-known cdc25-22 mutant and constructed its double mutant with spy1Δ. They were characterized in terms of the cell length at division, as explained above (Fig. 7). The temperature-sensitive cdc25-22 mutant showed an elongated cell size even at 30°C (Fig. 7A). (The size was about twice that of the wild type). This mitotic lesion in cdc25-22 was clearly affected by introduction of the spy1Δ mutation, as judged by the fact that the cdc25-22 and spy1Δ double mutant showed a shorter cell size. Furthermore, when the temperature sensitivity for growth of cdc25-22 on the SD agar plate at 34°C was examined, it was found that the spy1Δ mutation served as an extragenic suppressor for growth, at least partially (Fig. 7B). This notion was further supported by the results of appropriate control experiments, in which the spy1+ gene was reintroduced into the double mutant. The results also revealed the functional importance of the His-221 phosphorylation site of Spy1. However, the temperature-sensitive phenotype of cdc25-22 was not suppressed at 37°C by introduction of the spy1Δ mutation (data not shown). In any event, the observed genetic interactions between the cdc25 and spy1 mutations is compatible with the idea that Spy1 plays a role in the signaling pathway of the mitotic control per se.
DISCUSSION
The idea that there is a link between a His-to-Asp phosphorelay system and cell cycle control in S. pombe first came from an intriguing finding obtained by three groups (8, 33, 37). They collectively showed that the mcs4+ gene, which was originally identified as a suppressor of the mitotic catastrophe phenotype of a cdc2-3w wee1-50 double mutant (24), encodes a response regulator, named Mcs4, which acts upstream of the Wak1 (or Wik1)-Wis1-Sty1 (or Spc1) stress-activated MAPK cascade. Based on these findings, it was considered that a His-to-Asp phosphorelay involving the Mcs4 response regulator is part of a sensor system for multiple environmental signals that modulates the timing of entry into mitosis by regulating the Wak1 (Wik1)-Wis1-Sty1 (Spc1) MAPK cascade. Nevertheless, clarification of the presumed His-to-Asp phosphorelay system is at a very early stage. Altogether the results in this study showed that the Spy1 HPt phosphotransmitter functions upstream of the Mcs4 response regulator and most likely regulates Mcs4 function in a negative manner through a direct His-to-Asp phosphorelay. Consequently, Spy1-deficient cells enter mitosis precociously, thereby resulting in a short and ovoid cell morphology. It may also be worth mentioning that such a phenotype of spy1Δ is exaggerated when grown on a minimal medium, but is less evident on a rich medium (data not shown).
The fission yeast cell cycle is controlled at two major points: in G1 at entry into S phase (initiation of DNA replication) and in G2 at the initiation of mitosis (G2/M transition). Genetic and physiological studies have revealed that the timing of both transitions requires attainment of a critical cell size (27). The Sty1 MAPK gene was identified as a gene that affects cell size at division. Sty1-deficient cells show an elongated cell morphology, due to the delay of mitosis (21, 35). Sty1 appears to influence, directly or indirectly, the activity of the Cdc2-Cdc13 (cyclin B) cell cycle control machinery by an as yet unknown mechanism that is most likely independent of both the Wee1 tyrosine kinase and Cdc25 protein phosphatase (35). It was previously suggested that the Mcs4 response regulator controls the timing of mitotic initiation by running this Sty1-dependent mechanism in a positive manner (33, 37). Here we showed that Spy1 regulates such a function of Mcs4 in a negative manner. Our genetic results are fully consistent with these views, because the spy1 mutation showing a precocious entry into mitosis is not evident in both the mcs4Δ and sty1Δ backgrounds, but it clearly affects the phenotype of the cdc25-22 mutant. Mcs4 was previously suggested to control the timing of mitosis also through an additional Sty1-independent pathway (33). In this context, our result is indicative that Spy1 functions through a Sty1-dependent pathway. In any case, our results in this study further highlight an important role for the Spy1-Mcs4 phosphorelay system in coordinated cell cycle progression in response to environmental stimuli. Nevertheless, the underlying molecular mechanism through which the presumed Spy1-to-Mcs4 phosphorelay mediates an effect upon the cell cycle control machinery is not clear and appears to be complex, as discussed further below.
It should also be mentioned briefly that Mcs4 is important not only for mitotic control, as mentioned above, but also for osmotic and oxidative stress responses that are dependent on the Sty1 MAPK and the Atf1 and Pap1 bZIP transcriptional factors, as indeed confirmed in this study (Fig. 5) (20, 33, 37). Our results from Spy1-deficient cells did not support the view that Spy1 is also implicated, through Mcs4, in such transcriptional regulation of the gpd1+ and ctt1+ genes in response to osmotic and oxidative stresses. In any case, the fact that we could not detect any sign of the presumed up-regulation of gpd1+ and ctt1+ genes in the spy1Δ mutant may suggest the occurrence of another spy1+-like HPt phosphotransmitter in S. pombe. Clarification of this interesting issue must also await further studies. It should also be mentioned that Nguyen et al. recently characterized the function of the S. pombe mpr1+ gene, which is identical to spy1+, with special emphasis on oxidative stress response (26). They showed that, in their mpr1Δ cells, an elevated level of Sty1 phosphorylation did not increase further in response to oxidative stress, whereas the Sty1 phosphorylation in the wild-type cells was markedly induced. It was also shown that Mpr1 binds to Mcs4 in response to oxidative stress. Based on these findings, they proposed the model that oxidative stress stimuli are transmitted by a His-to-Asp (Mpr1/Spy1 to Mcs4) phosphorelay to the Sty1 MAPK cascade. However, they showed that the expression of ctt1+ mRNA can be induced normally in mpr1Δ cells, as we also have demonstrated (Fig. 5). From the physiological viewpoint, we would like to argue that Spy1 (or Mpr1) is not involved crucially, if at all, in the oxidative stress response, as far as the induction of the ctt1+ gene is concerned. Thus, the physiological importance of the spy1+-dependent modulation of the Sty1 phosphorylation in response to oxidative stress should be addressed carefully.
The molecular mechanism by which the presumed His-to-Asp phosphorelay involving Spy1 and Mcs4 operates in S. pombe is not yet clear. In S. cerevisiae, a multistep His-to-Asp phosphorelay that consists of Sln1, Ypd1, and Ssk1 directly regulates the Ssk2/Ssk22 MAPKKKs in osmosensing (20, 43). The currently proposed model is that Sln1 His kinase phosphorylates Ypd1, which in turn negatively regulates Ssk1 through a phosphotransfer. The nonphosphorylated form of Ssk1 functions as a positive regulator of Ssk2/Ssk22. If a homologous phosphorelay operates in S. pombe, then, an Sln1-like His kinase is expected to exist. An inspection of the fission yeast databases revealed the presence of (at least) three proteins encoded by typical His kinase genes: sensor 1 with 2,344 amino acids, GenBank accession no. AL031543 (SPCC74.06); sensor 2 with 2,310 amino acids, GenBank accession no. Z98978 (SPAC27E2.09); and sensor 3 with 1,639 amino acids, GenBank accession no. AL157734 (SPAC1834). Each of these predicted proteins, like Sln1, has a typical His kinase domain followed by a receiver domain. Two of them most likely correspond to each of those described previously and named Mak1 and Mak2 (20, 33). In any case, this fact suggests that the situation with regard to the His-to-Asp phosphorelay in the fission yeast is more complex than that in the budding yeast. This is consistent with the fact that the budding yeast HOG1 MAPK cascade appears to be activated only by osmotic stress, while the fission yeast Sty1 MAPK cascade is implicated in a wide range of stress responses (20). In any event, clarification of a link between these multiple His kinases and the Spy1-Mcs4 components is entirely elusive, and experiments along these lines are under way in our laboratory.
ACKNOWLEDGMENTS
We thank G. Cottarel for the mcs4Δ strain and plasmid; H. Saito, T. Maeda, and C. Ueguchi for the S. cerevisiae strain and plasmid; and J. B. A. Millar, H. Okayama, K. Tanaka, M. Yamamoto, and Y. Watanabe for S. pombe strains and the cDNA library.
This study was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, and Culture of Japan.
REFERENCES
- 1.Aiba H, Mizuno T, Mizushima S. Transfer of phosphoryl group between two regulatory proteins involved in osmoregulatory expression of ompF and ompC genes in Escherichia coli. J Biol Chem. 1989;264:8563–8567. [PubMed] [Google Scholar]
- 2.Aiba H, Yamada H, Ohmiya R, Mizuno T. The osmo-inducible gpd1+ gene is a target of the signaling pathway involving Wis1 MAP-kinase kinase in fission yeast. FEBS Lett. 1995;376:199–201. doi: 10.1016/0014-5793(95)01277-4. [DOI] [PubMed] [Google Scholar]
- 3.Alex L A, Borkovich K A, Simon M I. Hyphal development in Neurospora crassa: involvement of a two-component histidine kinase. Proc Natl Acad Sci USA. 1996;93:3416–3421. doi: 10.1073/pnas.93.8.3416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Appleby J L, Parkinson J S, Bourret R B. Signal transduction via the multi-step phosphorelay: not necessarily a road less traveled. Cell. 1996;86:845–848. doi: 10.1016/s0092-8674(00)80158-0. [DOI] [PubMed] [Google Scholar]
- 5.Bourret R B, Borvich K A, Simon M I. Signal transduction pathways involving protein phosphorylation in prokaryotes. Annu Rev Biochem. 1991;60:401–441. doi: 10.1146/annurev.bi.60.070191.002153. [DOI] [PubMed] [Google Scholar]
- 6.Chang C, Kwok S F, Bleeker A B, Meyerowitz E M. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;274:539–544. doi: 10.1126/science.8211181. [DOI] [PubMed] [Google Scholar]
- 7.Chang W T, Thomason P A, Gross J D, Newell P C. Evidence that the RdeA protein is a component of a multistep phosphorelay modulating rate of development in Dictyostelium. EMBO J. 1998;17:2809–2816. doi: 10.1093/emboj/17.10.2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cottarel G. Mcs4, a two-component system response regulator homologue, regulates the Schizosaccharomyces pombe cell cycle control. Genetics. 1997;147:1043–1051. doi: 10.1093/genetics/147.3.1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Degols G, Russell P. Discrete roles of the Spc1 kinase and the Atf1 transcription factor in the UV response of Schizosaccharomyces pombe. Mol Cell Biol. 1997;17:3356–3363. doi: 10.1128/mcb.17.6.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Degols G, Shiozaki K, Russell P. Activation and regulation of the Spc1 stress-activated protein kinase in Schizosaccharomyces pombe. Mol Cell Biol. 1996;16:2870–2877. doi: 10.1128/mcb.16.6.2870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunphy W G. The decision to enter mitosis. Trends Cell Biol. 1994;4:202–207. doi: 10.1016/0962-8924(94)90142-2. [DOI] [PubMed] [Google Scholar]
- 12.Hoch J A, Silhavy T J. Two-component signal transduction. Washington, D.C.: American Society for Microbiology; 1995. [Google Scholar]
- 13.Imamura A, Hanaki N, Umeda H, Nakamura A, Suzuki T, Ueguchi C, Mizuno T. Response regulators implicated in His-to-Asp phosphotransfer signaling in Arabidopsis. Proc Natl Acad Sci USA. 1998;95:2691–2696. doi: 10.1073/pnas.95.5.2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ishige K, Nagasawa S, Tokishita S, Mizuno T. A novel device of bacterial signal transducers. EMBO J. 1994;13:5195–5202. doi: 10.1002/j.1460-2075.1994.tb06850.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kakimoto T. CKI1, a histidine kinase homolog implicated in cytokinin signal transduction. Science. 1996;274:982–985. doi: 10.1126/science.274.5289.982. [DOI] [PubMed] [Google Scholar]
- 16.Kato T, Okazaki H, Murakami S, Stetter P, Fantes P, Okayama H. Stress signal, mediated by a Hog1-like MAP kinase, controls sexual development in fission yeast. FEBS Lett. 1996;378:207–212. doi: 10.1016/0014-5793(95)01442-x. [DOI] [PubMed] [Google Scholar]
- 17.Maeda T, Takekawa M, Saito H. Activation of yeast PBS2 MAPKK by MAPKKKs or by binding of an SH3-containing osmosensor. Science. 1995;269:554–558. doi: 10.1126/science.7624781. [DOI] [PubMed] [Google Scholar]
- 18.Maeda T, Wurgler-Murphy S M, Saito H. A two-component system that regulates an osmosensing MAP kinase cascade in yeast. Nature. 1994;369:242–245. doi: 10.1038/369242a0. [DOI] [PubMed] [Google Scholar]
- 19.Maundrell K. Thiamine-repressible expression vectors pREP and pRIP for fission yeast. Gene. 1993;123:127–130. doi: 10.1016/0378-1119(93)90551-d. [DOI] [PubMed] [Google Scholar]
- 20.Millar J B A. Stress-activated MAP kinase (mitogen-activated protein kinase) pathways of budding and fission yeasts. Biochem Soc Symp. 1999;64:49–62. [PubMed] [Google Scholar]
- 21.Millar J B A, Buck V, Wilkinson M G. Pyp1 and Pyp2 PTPases dephosphorylate an osmosensing MAP kinase controlling cell size at division in fission yeast. Genes Dev. 1995;9:2117–2130. doi: 10.1101/gad.9.17.2117. [DOI] [PubMed] [Google Scholar]
- 22.Millar J B A, Russell P. The cdc25 M-phase inducer: an unconventional protein phosphatase. Cell. 1992;68:407–410. doi: 10.1016/0092-8674(92)90177-e. [DOI] [PubMed] [Google Scholar]
- 23.Mizuno T. His-Asp phosphotransfer signal transduction. J Biochem (Tokyo) 1998;123:555–563. doi: 10.1093/oxfordjournals.jbchem.a021972. [DOI] [PubMed] [Google Scholar]
- 24.Molz L, Booher R, Young P, Beach D. cdc2 and the regulation of mitosis: six interacting mcs genes. Genetics. 1989;122:773–782. doi: 10.1093/genetics/122.4.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moreno S, Klar A, Nurse P. Molecular genetic analysis of fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1991;194:795–823. doi: 10.1016/0076-6879(91)94059-l. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen A N, Lee A, Place W, Shiozaki K. Multistep phosphorelay proteins transmit oxidative stress signals to the fission yeast stress-activated protein kinase. Mol Biol Cell. 2000;11:1169–1181. doi: 10.1091/mbc.11.4.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nurse P. Genetic control of cell size at cell division in yeast. Nature. 1975;256:547–551. doi: 10.1038/256547a0. [DOI] [PubMed] [Google Scholar]
- 28.Ohmiya R, Kato C, Yamada H, Aiba H, Mizuno T. A fission yeast gene (prr1+) that encodes a response regulator implicated in oxidative stress response. J Biochem (Tokyo) 1999;125:1061–1066. doi: 10.1093/oxfordjournals.jbchem.a022387. [DOI] [PubMed] [Google Scholar]
- 29.Parkinson J S, Kofoid E C. Communication modules in bacterial signalling proteins. Annu Rev Genet. 1992;26:71–112. doi: 10.1146/annurev.ge.26.120192.000443. [DOI] [PubMed] [Google Scholar]
- 30.Posas F, Wurgler-Murphy S M, Maeda T, Witten E A, Thai T C, Saito H. Yeast HOG1 MAP kinase cascade is regulated by a multistep phosphorelay mechanism in the SLN1-YPD1-SSK1 two-component osmosensor. Cell. 1996;86:865–875. doi: 10.1016/s0092-8674(00)80162-2. [DOI] [PubMed] [Google Scholar]
- 31.Russell P, Nurse P. Negative regulation of mitosis by wee1+, a gene encoding a protein kinase homolog. Cell. 1987;49:559–567. doi: 10.1016/0092-8674(87)90458-2. [DOI] [PubMed] [Google Scholar]
- 32.Schuster S C, Noegel A A, Oehmen F, Gerisher G, Simon M I. The hybrid histidine kinase DokA is part of the osmotic response system of Dictyostelium. EMBO J. 1996;15:3880–3889. [PMC free article] [PubMed] [Google Scholar]
- 33.Shieh J C, Wilkinson M G, Buck V, Morgan B A, Makino K, Millar J B A. The Mcs4 response regulator coordinately controls the stress activated Wak1-Wis1-Sty1 MAP kinase pathway and fission yeast cell cycle. Genes Dev. 1997;11:1008–1022. doi: 10.1101/gad.11.8.1008. [DOI] [PubMed] [Google Scholar]
- 34.Shiozaki K, Russell P. Counteractive roles of protein phosphatase 2C (PP2C) and a MAP kinase kinase homologue in the osmoregulation of fission yeast. EMBO J. 1995;14:492–502. doi: 10.1002/j.1460-2075.1995.tb07025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shiozaki K, Russell P. Cell-cycle control linked to extracellular environment by MAP kinase pathway in fission yeast. Nature. 1995;378:739–743. doi: 10.1038/378739a0. [DOI] [PubMed] [Google Scholar]
- 36.Shiozaki K, Russell P. Conjugation, meiosis, and the osmotic stress response are regulated by Spc1 kinase through Atf1 transcription factor in fission yeast. Genes Dev. 1996;10:2276–2288. doi: 10.1101/gad.10.18.2276. [DOI] [PubMed] [Google Scholar]
- 37.Shiozaki K, Shiozaki M, Russell P. Mcs4 mitotic catastrophe suppressor regulates the fission yeast cell cycle through the Wik1-Wis1-Spc1 kinase cascade. Mol Biol Cell. 1997;8:409–419. doi: 10.1091/mbc.8.3.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shiozaki K, Shiozaki M, Russell P. Heat stress activates fission yeast Spc1/Sty1 MAPK by a MEKK-independent mechanism. Mol Biol Cell. 1998;9:1339–1349. doi: 10.1091/mbc.9.6.1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stock J B, Ninfa A J, Stock A M. Protein phosphorylation and regulation of adaptive responses in bacteria. Microbiol Rev. 1989;53:450–490. doi: 10.1128/mr.53.4.450-490.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzuki T, Imamura A, Ueguchi C, Mizuno T. Histidine-containing phosphotransfer (HPt) signal transducers implicated in His-to-Asp phosphorelay in Arabidopsis. Plant Cell Physiol. 1998;39:1258–1268. doi: 10.1093/oxfordjournals.pcp.a029329. [DOI] [PubMed] [Google Scholar]
- 41.Wang N, Shaulsky G, Escalante R, Loomis W F. A two-component histidine kinase gene that functions in Dictyostelium development. EMBO J. 1996;15:3890–3898. [PMC free article] [PubMed] [Google Scholar]
- 42.Wilkinson M G, Samuels M, Takeda T, Toone W M, Shieh J, Toda T, Millar J B A, Jones N. The Atf1 transcription factor is a target for the Sty1 stress-activated MAP kinase pathway in fission yeast. Genes Dev. 1996;10:2289–2301. doi: 10.1101/gad.10.18.2289. [DOI] [PubMed] [Google Scholar]
- 43.Wurgler-Murphy S M, Saito H. Two-component signal transducers and MAPK cascades. Trends Biol Sci. 1997;22:172–176. doi: 10.1016/s0968-0004(97)01036-0. [DOI] [PubMed] [Google Scholar]