Abstract
PARTNER is a prospective, phase II–III, randomized controlled clinical trial that recruited patients with triple-negative breast cancer1,2, who were germline BRCA1 and BRCA2 wild type3. Here we report the results of the trial. Patients (n = 559) were randomized on a 1:1 basis to receive neoadjuvant carboplatin–paclitaxel with or without 150 mg olaparib twice daily, on days 3 to 14, of each of four cycles (gap schedule olaparib, research arm) followed by three cycles of anthracycline-based chemotherapy before surgery. The primary end point was pathologic complete response (pCR)4, and secondary end points included event-free survival (EFS) and overall survival (OS)5. pCR was achieved in 51% of patients in the research arm and 52% in the control arm (P = 0.753). Estimated EFS at 36 months in the research and control arms was 80% and 79% (log-rank P > 0.9), respectively; OS was 90% and 87.2% (log-rank P = 0.8), respectively. In patients with pCR, estimated EFS at 36 months was 90%, and in those with non-pCR it was 70% (log-rank P < 0.001), and OS was 96% and 83% (log-rank P < 0.001), respectively. Neoadjuvant olaparib did not improve pCR rates, EFS or OS when added to carboplatin–paclitaxel and anthracycline-based chemotherapy in patients with triple-negative breast cancer who were germline BRCA1 and BRCA2 wild type. ClinicalTrials.gov ID: NCT03150576.
Subject terms: Breast cancer, Chemotherapy, Targeted therapies
A study details the results of the PARTNER trial, a prospective, randomized controlled trial of the use of neoadjuvant olaparib with carboplatin–paclitaxel chemotherapy in patients with triple-negative breast cancer who were germline BRCA1 and BRCA2 wild type.
Main
Olaparib, the first poly(ADP-ribose) polymerase (PARP) inhibitor to be developed, is effective in treating women with breast cancer who have germline pathogenic variants in BRCA1 and/or BRCA2 (gBRCAm), both in the metastatic6,7 and the adjuvant setting8. The PARTNER trial tested olaparib in the neoadjuvant setting in two cohorts. One cohort consisted of the patients with gBRCAm who had early breast cancer and the report of that cohort is in progress (J.E.A. et al., manuscript in preparation). The other cohort (reported here) consisted of patients with triple-negative breast cancer (TNBC) who were germline BRCA1 and BRCA2 wild type (gBRCAwt)). In addition, all tumours had a basal-like phenotype as defined by immunohistochemistry (Methods). The standard of care for many years for TNBC had been anthracycline- and taxane-based chemotherapy9. However, there was emerging evidence from neoadjuvant trials (now published) that carboplatin is a useful addition to this standard treatment10,11. In our trial, olaparib was given 48 h after carboplatin–paclitaxel (gap schedule) for four cycles in the neoadjuvant setting and was followed by anthracycline-based chemotherapy before surgery.
TNBCs in patients who are gBRCAwt frequently exhibit homologous recombination deficiency, and widespread genomic instability1 similar to that seen in breast cancers in patients with gBRCAm. Defects in homologous recombination repair can occur through numerous mechanisms including the loss of BRCA1 and BRCA2 function within the breast cancer, thus resulting in a gBRCAm-like phenotype12, which could potentially be treated with PARP inhibitors. ‘Genomic scars’, typically found in gBRCAm-related tumours, have also been identified in tumours that are gBRCAwt13. Typical rearrangement signatures with high numbers of tandem duplications have been linked to a subgroup of TNBC(gBRCAwt) with a homologous recombination deficiency profile14. This suggests that TNBC(gBRCAwt) includes a targetable molecular group outside the gBRCA population that could also benefit from PARP inhibition. In homologous-recombination-deficient cells, PARP inhibition results in synthetic lethality by preventing repair of single-strand breaks, which leads to problems downstream with double-strand repair15,16. PARP inhibitors therefore could work in synergy with DNA-damaging agents such as platinums, which cause both single- and double-strand breaks. In high-grade serous ovarian cancer, widespread adoption of PARP inhibitors in gBRCAwt tumours has already occurred and has been supported by evidence of their activity in those cancers that demonstrate homologous recombination deficiency17,18.
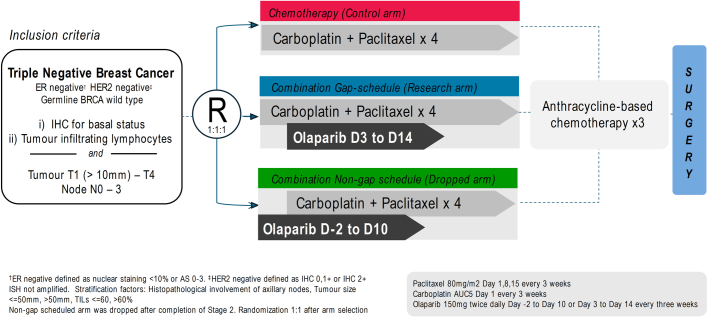
The PARTNER trial used olaparib in the experimental group as an addition to carboplatin–paclitaxel followed by anthracycline-based chemotherapy. The first stage of the trial examined the safety of the combination and the second stage investigated optimal scheduling of olaparib in combination with platinum chemotherapy, which has never been established. The combination had previously been tested in patients with relapsed ovarian cancer19, but the dose and schedule used required reduced doses of carboplatin, and the combination resulted in response rates similar to, rather than superior to, those for carboplatin–paclitaxel alone. Therefore, after these results, olaparib has been scheduled after completion of chemotherapy, when full and continuous doses are used8. The third stage of the PARTNER trial investigated the efficacy of the same chemotherapy and olaparib in the 48-h gap schedule research arm (Extended Data Fig. 1). This paper details the results of the PARTNER trial, a prospective, randomized controlled trial in the TNBC(gBRCAwt) cohort.
Extended Data Fig. 1. Trial Flow Chart: PARTNER Trial for the TNBC Cohort.
Trial Flow Chart: PARTNER Trial for the TNBC Cohort.
Patients and treatment
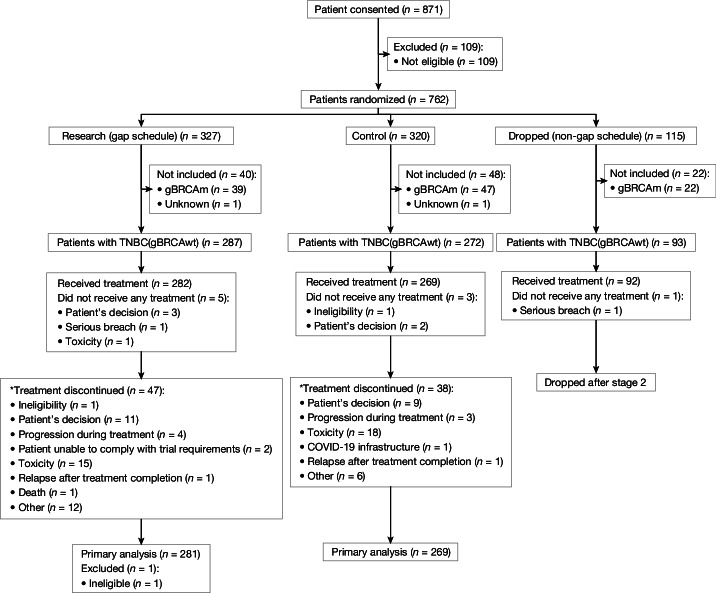
From September 2016 to December 2021, 559 patients with TNBC(gBRCAwt) (287 research arm (gap schedule); 272 control arm) were randomized at 29 UK centres (CONSORT diagram, Fig. 1). Recruitment was extended by around 6 months to decrease the risk of losing participants due to the COVID-19 pandemic, which had considerably slowed recruitment during 2020.
Fig. 1. Trial CONSORT diagram.
*The first main reason for treatment discontinuation was reported.
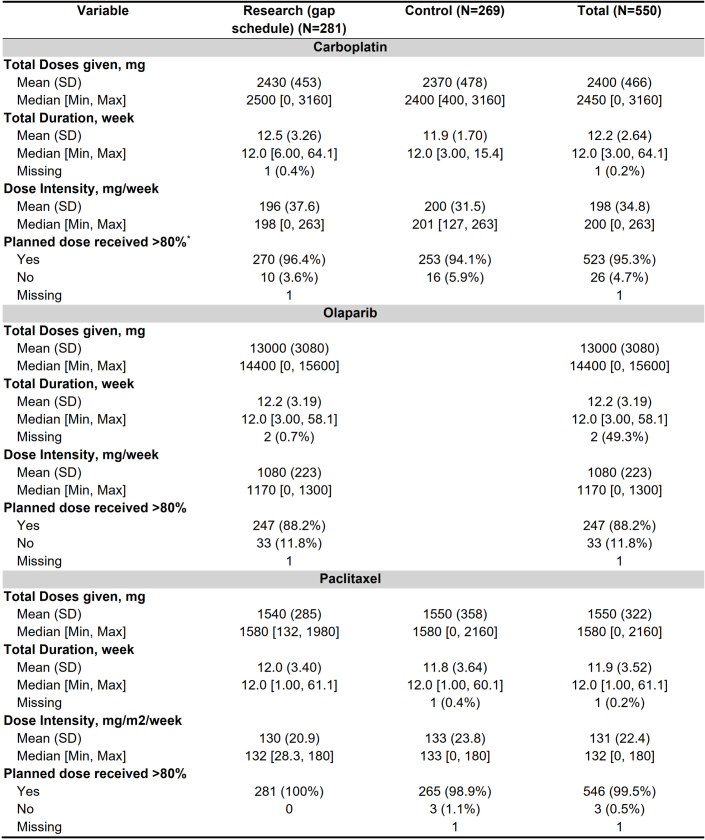
The data cutoff for analysis was 30 November 2023 with a median follow-up of 38 months. Five patients (3 opt-out, 1 breach, 1 toxicity) in the research arm (gap schedule) and three patients (1 ineligible, 2 opt-out) in the control arm did not receive any treatment after randomization. In the research arm (gap schedule), one patient was found to be ineligible after receiving treatment. The resulting modified intention-to-treat population consisted of 550 patients. The demographics and pretreatment disease characteristics were well balanced between the two arms (Table 1). The median patient age was 49 (range 23 to 71) years; 95.1% of patients had a tumour diameter of less than 50 mm; 22.5% had a tumour-infiltrating lymphocyte (TIL) score ≥ 60%; 94.8% had an Eastern Cooperative Oncology Group performance status of 0; and 36.5% had prior oophorectomy or were post-menopausal. In the research arm (gap schedule), 88.2% received at least 80% of the planned olaparib with a median dose intensity of 1,170 mg per week (Extended Data Table 1). There were no differences between the research (gap schedule) and control arms for patients receiving at least 80% of the planned carboplatin (95%) and paclitaxel doses (99%) (Extended Data Table 1). Surgery was carried out after the treatment was completed in 98.2% of the patients (276 research (gap schedule); 264 control). In patients who had surgery, 73.9% had breast-conserving wide local excision and 61.5% had sentinel node biopsy. A total of 447 (81.3%) patients received local radiotherapy according to centre protocols, after completion of trial treatment and surgery (Table 1).
Table 1.
Baseline characteristics and surgery carried out for the patients with at least one dose of treatment
| Variable | Research (gap schedule) (n = 281) | Control (n = 269) | Total (n = 550) |
|---|---|---|---|
| Median age (range)a | 49.6 (23.9, 70.9) | 48.4 (23.2, 71.0) | 49.1 (23.2, 71.0) |
| Ethnicity, n (%) | |||
| White | 228 (81.1%) | 217 (80.7%) | 445 (80.9%) |
| Mixed | 2 (0.7%) | 2 (0.7%) | 4 (0.7%) |
| Asian or Asian British | 4 (1.4%) | 7 (2.6%) | 11 (2.0%) |
| Black or Black British | 9 (3.2%) | 4 (1.5%) | 13 (2.4%) |
| Unknown | 38 (13.5%) | 39 (14.5%) | 77 (14.0%) |
| Tumour size, n (%) | |||
| ≤50 mm | 265 (94.3%) | 258 (95.9%) | 523 (95.1%) |
| >50 mm | 16 (5.7%) | 11 (4.1%) | 27 (4.9%) |
| Axillary lymph node involvement at diagnosis by biopsy and/or imaging, n (%) | |||
| No | 188 (66.9%) | 188 (69.9%) | 376 (68.4%) |
| Yes | 93 (33.1%) | 81 (30.1%) | 174 (31.6%) |
| TIL score, n (%) | |||
| <60% | 215 (76.5%) | 211 (78.4%) | 426 (77.5%) |
| ≥60% | 66 (23.5%) | 58 (21.6%) | 124 (22.5%) |
| Eastern Cooperative Oncology Group performance status, n (%) | |||
| 0 | 260 (92.5%) | 258 (95.9%) | 518 (94.2%) |
| 1 | 21 (7.5%) | 11 (4.1%) | 32 (5.8%) |
| HER2 immunohistochemisty status, n (%) | |||
| 0 | 233 (82.9%) | 220 (81.8%) | 453 (82.4%) |
| 1 | 28 (10.0%) | 24 (8.9%) | 52 (9.5%) |
| 2 | 20 (7.1%) | 25 (9.3%) | 45 (8.2%) |
| Post-menopausal (oophorectomy before diagnosis or natural menopause) | |||
| Yes | 95 (35.4%) | 96 (37.5%) | 191 (36.5%) |
| No | 173 (64.6%) | 160 (62.5%) | 333 (63.5%) |
| Surgery after neoadjuvant treatmentb, n (%) | 276 (98.2%) | 264 (98.1%) | 540 (98.2%) |
| Breast surgery for the protocol-treated breast cancerb, n (%) | |||
| Breast-conserving wide local excision | 200 (72.5%) | 199 (75.4%) | 399 (73.9%) |
| Mastectomy | 79 (28.6%) | 67 (25.4%) | 146 (26.5%) |
| Reconstruction | 24 (8.7%) | 16 (6.1%) | 40 (7.4%) |
| Axillary surgery for the protocol-treated breast cancerc, n (%) | |||
| Sentinel node biopsy | 166 (60.1%) | 166 (62.9%) | 332 (61.5%) |
| Axillary clearance | 88 (31.9%) | 78 (29.5%) | 166 (30.7%) |
| Axillary sampling | 42 (15.2%) | 43 (16.3%) | 85 (15.7%) |
| Radiotherapy (local) after completion of protocol treatment | 231 (82.2%) | 216 (80.3%) | 447 (81.3%) |
aMaximum age of 71 was due to rounding.
bA total of ten patients (five in each group) had missing surgery information. The denominators are the number of patients in each group.
cEach patient may have several surgeries; denominators are the number of patients who had a surgery.
Extended Data Table 1.
Summary of treatment received during the first regimen by treatment arm
*Planned dose for carboplatin was based on derived dose using the Calvert formula.
Efficacy
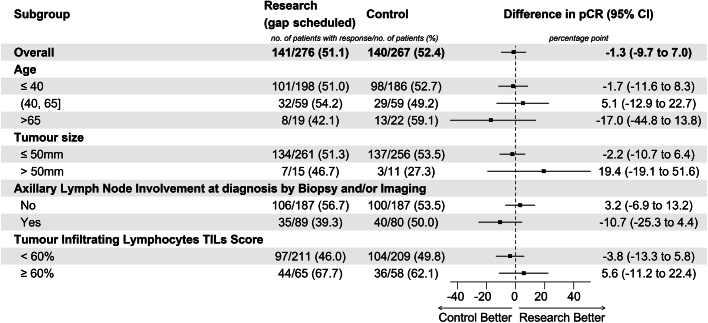
A total of 543 patients had pathological response data at surgery available, of which 141/276 (51.1%) in the research arm (gap schedule) and 140/267 (52.4%) in the control arm had a pCR with a difference of −1.3% (95% confidence interval (CI) −9.7% to 7.0%, P value = 0.753; Fig. 2a). The result of no significant difference was consistent in all pre-specified subgroups and after imputing missing data over a range of assumptions (Extended Data Table 2 and Extended Data Fig. 2). The proportion of patients with pCR was higher for those with tumours with TILs ≥ 60% (65%) compared to those with TILs < 60% (47.9%) with a difference of 17.2% (95% CI 7.2% to 26.5%, P value < 0.001; Fig. 2b). There were no significant differences in pCR rate in each TIL group between the research (gap schedule) and control arms (Extended Data Fig. 2).
Fig. 2. pCR rate by treatment arm and TIL group.
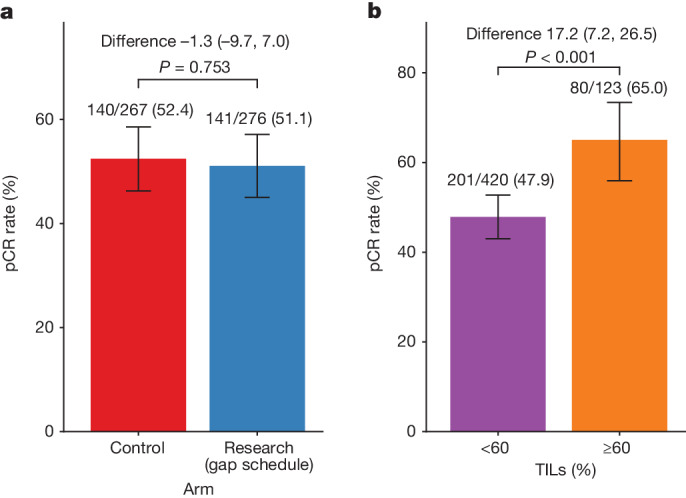
a,b, pCR rate by treatment arm (a) and TIL group (≥60% versus <60%) (b). Data were analysed from a total of 276 patients in the research (gap schedule) and 267 control arms. Error bars, 95% CI of the proportion based on exact method. The statistical test was based on the two-tailed chi-squared test.
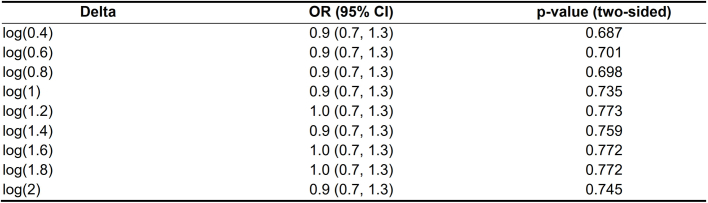
Extended Data Table 2.
Logistic regression analysis of primary endpoint imputing missing data with delta-adjusted pattern mixture model
The imputation was based on delta-adjusted pattern mixture model adjusting for stratification factors with a range of delta values. The pattern mixture model of a binary endpoint can be expressed in the following form:
Where represents the difference in the log-odds of between non-missing and missing, are observed covariates, which is stratification factors here, and is a missing indicator of . While implies data missing at random, implies better outcome in missing patients and otherwise. All the missing pCR was imputed with the same . For example, , and indicates patients with missing pCR would have an odds of 0.5, 1 and 1.5 times achieving a pCR compared to non-missing patients.
Extended Data Fig. 2. Forest plot of the primary end-point in subgroups.
The data are the numbers and proportions of patients with pathological complete response (pCR). Data were analysed from a total of 276 in research (gap schedule) and 267 control arms. Estimates are the difference in the pCR and 95% CI based on the score method between research arm and control arm.
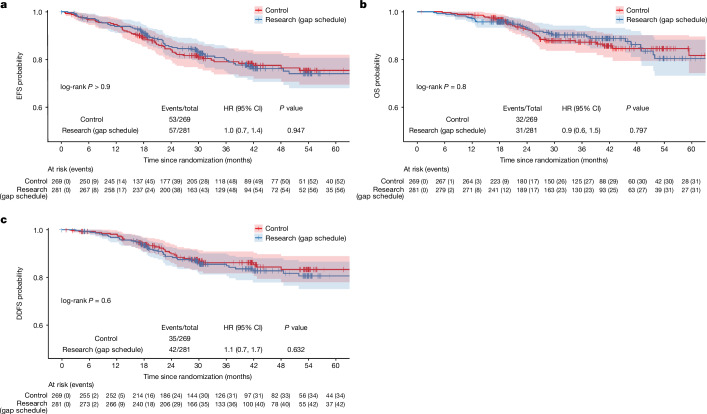
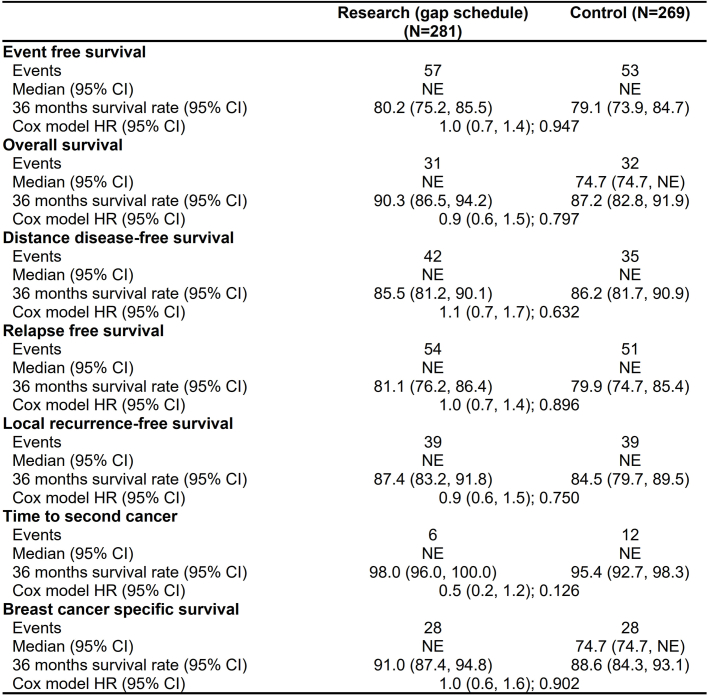
A total of 110 patients (57 research (gap schedule); 53 control) had an event or died with a hazard ratio (HR) of 0.9 (95% CI 0.6 to 1.4, P = 0.781; Fig. 3a). A detailed breakdown of the event types is shown in Extended Data Table 3. The estimated EFS rate at 36 months was 80.2% (95% CI 75.2 to 85.5) in the research arm (gap schedule) and 79.1% (95% CI 73.9 to 84.7) in the control arm, with a median EFS not reached in either (Extended Data Table 4).
Fig. 3. Kaplan–Meier curves of key survival end points.
a–c, EFS (a), OS (b) and DDFS (c) in patients in the modified intention-to-treat group by treatment arm.
Extended Data Table 3.
Summary of events observed during the trial follow-up
Note: each patient may have multiple events during the follow-up.
Relapse: patient completely responded to trial treatment but relapsed after trial treatment completed.
Progression on trial treatment: patient’s disease has progressed during trial treatment.
Progression after completion of trial treatment: patient’s disease did not completely respond to trial treatment and progressed after trial treatment finished.
First second primary: the first occurrence of a new primary cancer diagnosed after randomisation.
Extended Data Table 4.
Summary of time-to-event outcomes
NE: not estimated.
A total of 63 patients (31 research (gap schedule); 32 control) died, with an HR of 0.9 (95% CI 0.6 to 1.5, P = 0.8; Fig. 3b). The estimated OS rate at 36 months was 90.3% (95% CI 86.5 to 94.2) in the research arm (gap schedule) with a median OS not reached, and 87.2% (95% CI 82.8 to 91.9) in the control arm with a median OS of 74.7 months (Extended Data Table 4).
A total of 77 patients (42 research (gap schedule); 35 control) had a distant relapse or died with an HR of 1.1 (95% CI 0.7 to 1.7, P = 0.632; Fig. 3c). The estimated distant disease-free survival (DDFS) rate at 36 months was 85.5% (95% CI 81.2 to 90.1) in the research arm (gap schedule) and 86.2% (95% CI 81.7 to 90.9) in the control arm, with a median DDFS not reached in either (Extended Data Table 4).
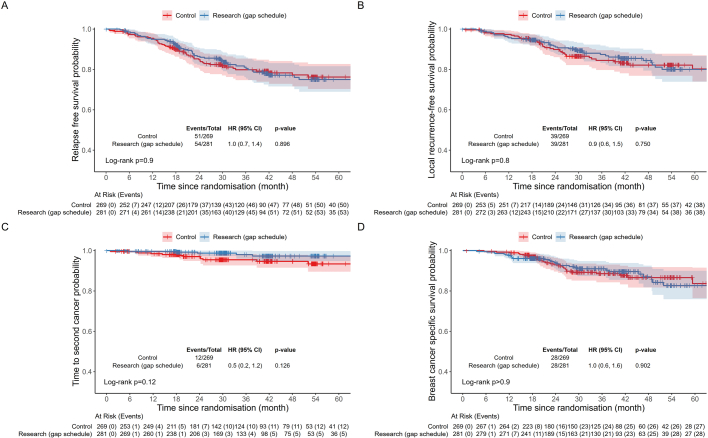
Similarly, no difference was observed in relapse-free survival (HR = 1.0, 95% CI 0.7 to 1.4, P = 0.896), local recurrence-free survival (HR = 0.9, 95% CI 0.6 to 1.5, P = 0.750), time to second cancer (HR = 0.5, 95% CI 0.2 to 1.2, P = 0.126) or breast cancer-specific survival (HR = 1.0, 95% CI 0.6 to 1.6, P = 0.902; Extended Data Fig. 3a–d). Neither arm reached a median on these time-to-event outcomes, except the control arm for OS (74.7 months) and breast cancer-specific survival (74.7 months; Extended Data Table 4).
Extended Data Fig. 3. Additional Kaplan-Meier curves of survival endpoints (part 1).
Relapse free survival (A), local recurrence free survival (B), time-to-second cancer (C) and breast cancer specific survival (D) by treatment arm (control and research arm).
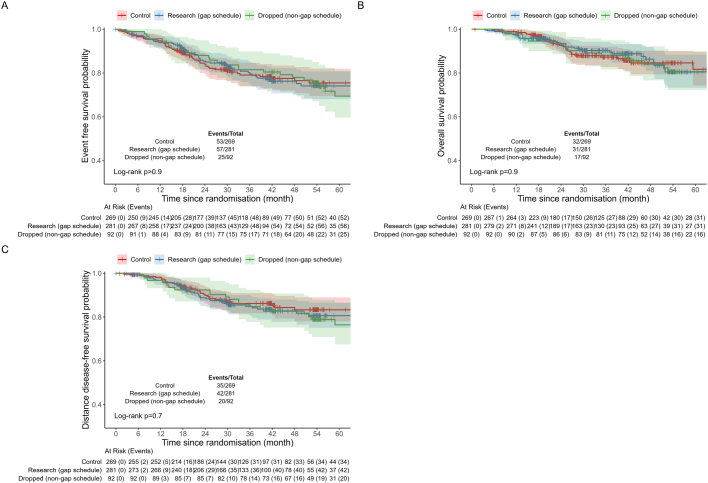
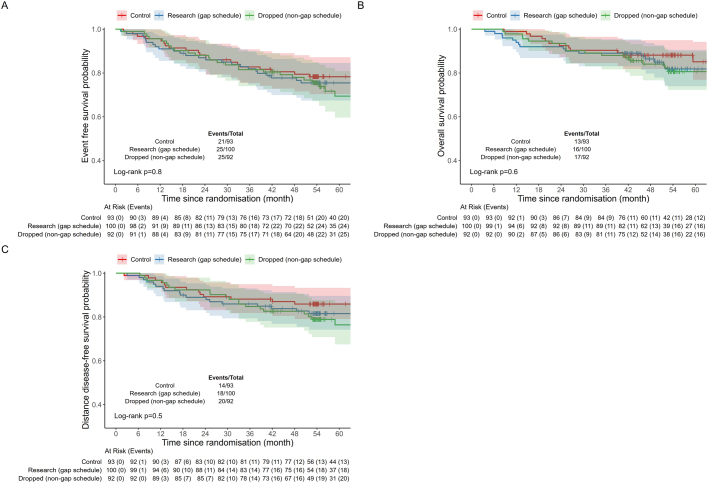
An exploratory analysis was carried out including the 92 patients randomized into the dropped arm (non-gap schedule), compared with patients at stage 3, control and research (gap schedule) arms (Extended Data Fig. 4), and with patients at stage 2, control and research (gap schedule) arms (Extended Data Fig. 5). There were no significant differences for estimated EFS, OS or DDFS in any comparisons.
Extended Data Fig. 4. Additional Kaplan-Meier curves of survival endpoints (part 2).
Relapse free survival (A), overall survival (B), and distant disease-free survival (C) by treatment arm (control, research arm, and dropped arm) in all patients.
Extended Data Fig. 5. Additional Kaplan-Meier curves of survival endpoints (part 3).
Relapse free survival (A), overall survival (B), and distant disease-free survival (C) by treatment arm [control, research arm (gap-scheduled), and dropped arm (non-gap-scheduled)) in patients who were assessed at stage II.
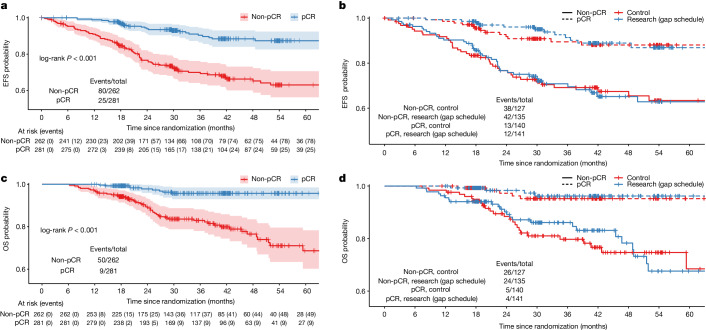
Kaplan–Meier curves of EFS and OS by pathological response and treatment group are presented in Fig. 4. The estimated 36-month EFS rate was 90.4% (95% CI 86.4 to 94.5) in the patients with a pCR as compared with 70% (95% CI 64.2 to 76.2) in those with a non-pCR (HR = 0.3, 95% CI 0.2 to 0.4; P < 0.001). Similarly, more deaths were observed in patients with a non-pCR (P < 0.001). The estimated 36-month OS rate was 95.7% (95% CI 93.0 to 98.5) in the patients with a pCR as compared with 83% (95% CI 78 to 88.2) in those with a non-pCR (HR = 0.2, 95% CI 0.1 to 0.3; P < 0.001). Fewer events and deaths were observed in patients with a pCR compared to those with a non-pCR, regardless of the treatment received (Fig. 4b,d).
Fig. 4. Kaplan–Meier curves of survival by pathological response and treatment arm.
a,b, EFS by pathological response (a) and by pathological response and treatment arm (b). c,d, OS by pathological response (c) and by pathological response and treatment arm (d) in patients in the modified intention-to-treat group.
Safety and toxicity
A total of 551 (282 research (gap schedule); 269 control) patients were evaluated for safety. A total of 47 (16.6%) patients in the research arm (gap schedule) and 38 (14.1%) in the control arm stopped treatment early with toxicity being the most common reason (21 research (gap schedule); 22 control group). Of patients who discontinued trial treatment early, eight in the research (gap schedule) arm and three in the control arm had further neoadjuvant treatment outside the protocol.
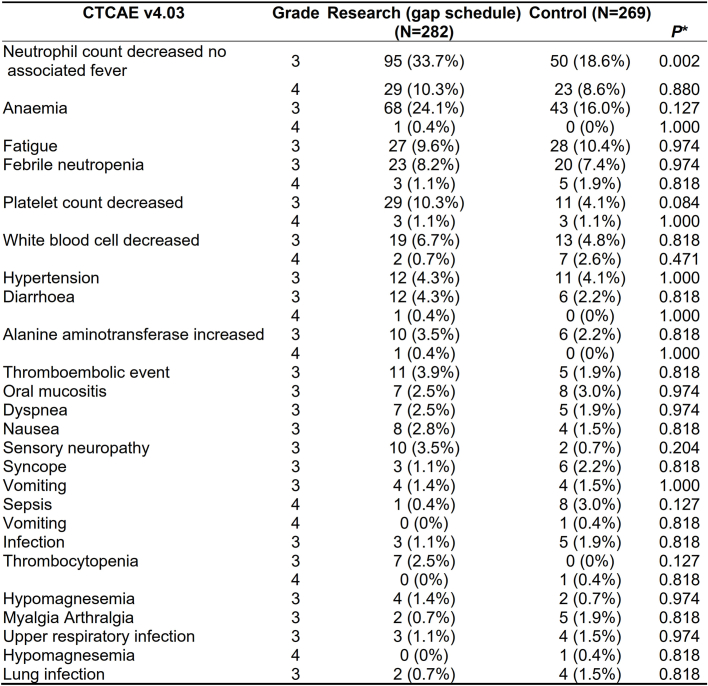
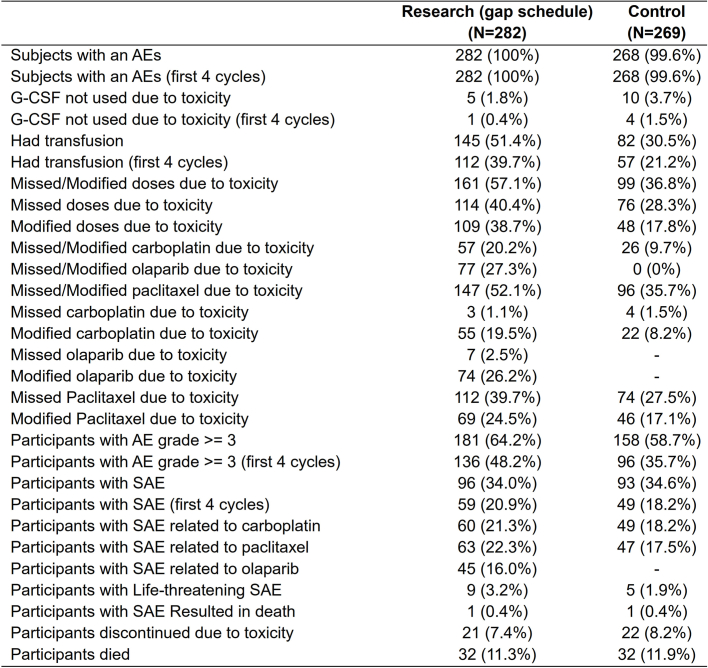
Patients in the research arm experienced slightly more adverse events (AEs) of grade ≥3 than those in the control arm (64.2% versus 58.7%; Table 2). The number of serious AEs (SAEs) related to carboplatin was slightly higher in the research arm (60 (21.3%)) than in the control arm (49 (18.2%)) and the number of SAEs related to paclitaxel was also slightly higher in the research arm (63 (22.3%)) than in the control arm (47 (17.5%)). A total of 45 (16.0%) patients in the research arm had an SAE related to olaparib. During the whole treatment period, the number of patients who had a transfusion was higher in the research arm (145 (51.4%)) than in the control arm (82 (30.5%)). A summary of the worst AE grade ≥3 experienced per patient in at least 1% of patients is shown in Extended Data Table 5, and the only AE that is significantly worse in the research arm compared with the control arm is neutropenia without associated fever (research arm, 95 (33.7%); control arm, 50 (18.6%); P = 0.002). More patients in the research arm than patients in the control arm had a missed or modified dose of carboplatin (20.2% versus 9.7%, respectively) or a missed or modified dose of paclitaxel (52.1% versus 35.7%, respectively) due to toxicity (see Extended Data Table 6 for full details).
Table 2.
Summary of AEs in the study period
| Research (gap schedule) (n = 282) | Control (n = 269) | |
|---|---|---|
| Any AEs | 282 (100%) | 268 (99.6%) |
| AE grade ≥3 | 181 (64.2%) | 158 (58.7%) |
| Any SAE | 96 (34.0%) | 93 (34.6%) |
| SAE related to carboplatin | 60 (21.3%) | 49 (18.2%) |
| SAE related to paclitaxel | 63 (22.3%) | 47 (17.5%) |
| SAE related to olaparib | 45 (16.0%) | − |
| Missed doses due to toxicity | 114 (40.4%) | 76 (28.3%) |
| Modified doses due to toxicity | 109 (38.7%) | 48 (17.8%) |
| Treatment discontinued because of toxicity | 21 (7.4%) | 22 (8.2%) |
| Red cell transfusion required during chemotherapy | 145 (51.4%) | 82 (30.5%) |
Extended Data Table 5.
Summary of AE grade ≥ 3 experienced per patient by term and grade in at least 1% of non-BRCA patients
*P-values (two-sided) after controlled the false discovery rate from tests on each toxicity and toxicity grades with fisher’s exact method.
Extended Data Table 6.
Detailed Safety and Toxicity Summary
Quality of life
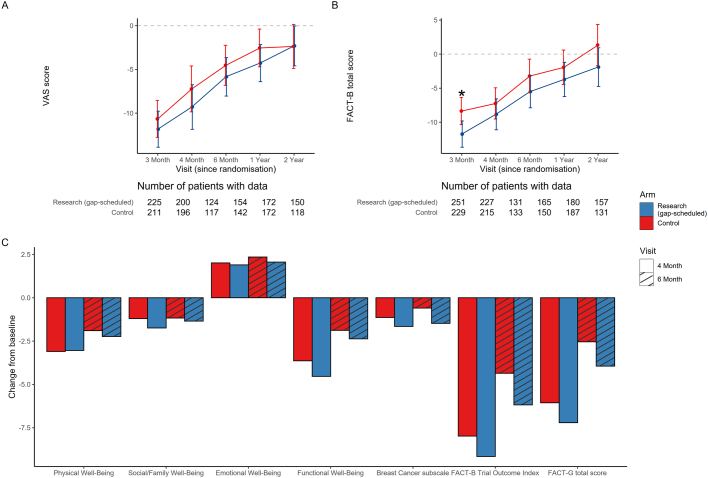
A total of 522 patients (268 research (gap schedule); 254 control) consented to the quality of life sub-study. Please refer to Methods, Study procedures for explanation of the health-related quality of life (HRQOL) measures. The mean EQ-5D-5L (EuroQol 5 Dimension 5 Level) visual analogue scale score and Functional Assessment of Cancer Therapy—Breast (FACT-B) total score were consistently slightly higher (slightly better HRQOL) for the control arm compared with the research arm (gap schedule) throughout the study period (Extended Data Fig. 6a,b). However, the only data showing a significantly worse HRQOL for patients in the research (gap schedule) arm compared with those in the control arm was at the 3-month time point for the FACT-B total score (Extended Data Fig. 6c). A decrease was observed in both the control and research (gap schedule) arms at 4 and 6 months in physical well-being, social and family well-being, functional well-being, breast cancer subscale, FACT-B trial outcome index and FACT-B total score from the baseline group, with slightly larger decreases in the research arm (gap schedule), which were non-significant.
Extended Data Fig. 6. EQ-5D-5L visual analogue scale (VAS) scores and FACT-B total scores.
Least-squares mean change from baseline of EQ-5D-5L visual analogue scale (VAS) score (A), FACT-B total score (B) and FACT-B subscales with trial outcome index and FACT-B total score at 4 months and 6 months. Asterisk indicates a statistical difference between the treatment arms of p < 0.05 based on two-tailed t-test with Tukey’s adjustment for multiple comparisons. Error bars, 95% CI of the estimated change score.
Discussion
This neoadjuvant trial tested low-dose, intermittent olaparib, 150 mg twice daily by mouth for 12 days, starting on day 3 until day 14, every 3 weeks for four cycles, concurrently with four cycles of carboplatin–paclitaxel, in patients with TNBC(gBRCAwt). This treatment was followed by three cycles of anthracycline-based chemotherapy. There was no improvement in either estimated EFS and OS at 36 months or pCR rate at surgery from the addition of olaparib. Our original hypothesis was that low-dose olaparib would work in synergy with platinum-based chemotherapy to enhance lethal damage to cancer cells and increase the rate of pCR. This hypothesis has been disproved in the TNBC(gBRCAwt) cohort. However, results for the gBRCAm cohort from the PARTNER trial (J.E.A. et al., manuscript in preparation) showed significantly improved EFS and OS for patients in the research arm (gap schedule) compared with those in the control arm and even more so compared with those in the dropped arm (non-gap schedule). This confirms that olaparib was given at the optimal dose and schedule in combination with carboplatin–paclitaxel in the TNBC(gBRCAwt) cohort, and the lack of activity demonstrated in this group is not due to suboptimal dose or schedule. Pre-planned translational work has commenced on available samples in this cohort to establish whether there are smaller subgroups that can be identified with biomarkers of response to the gap-schedule olaparib.
There remains an important unanswered question on the utility of olaparib in early breast cancer, which the results from the PARTNER trial TNBC(gBRCAwt) cohort help to inform. It is not known whether patients with early TNBC(gBRCAwt) would benefit from adjuvant olaparib. To date, in this group there have been randomized controlled trials directly comparing adjuvant olaparib with the standard of care in patients with residual disease following neoadjuvant chemotherapy. However, as the PARTNER trial showed no hint of activity in the TNBC(gBRCAwt) cohort at this dose and schedule, and when this is compared with the major benefit of the same treatment in patients with gBRCAm breast cancer (J.E.A. et al., manuscript in preparation), it seems unlikely that there would be any effect for adjuvant olaparib treatment in the whole TNBC(gBRCAwt) group.
A strength of the PARTNER trial is the detailed, upfront characterization of patients’ breast cancers. In addition, all patients were prospectively tested for gBRCA pathogenic variants and therefore in our first reporting here, we can confirm that all patients were gBRCAwt. In addition, all recruited patients had basal-like TNBC on the basis of immunohistochemistry assessments, and the patients in the non-basal group were excluded. Non-basal TNBCs, although triple negative, share few of the biological and genomic features of basal-like TNBC, and respond less well to most systemic treatments20–22. Therefore, their inclusion in randomized controlled trials of TNBC add non-informative data to the analyses that could affect results. Nevertheless, the designation of basal-like TNBC(gBRCAwt) by immunohistochemistry, although readily available in most centres, is not perfect and this group will undoubtedly include some tumours that are non-basal by molecular testing. Another strength is that the PARTNER trial is one of the few studies that can provide detailed safety data for the combination of chemotherapy and PARP inhibitor as a treatment for early-stage breast cancer. The combination was generally well tolerated and no significant differences in treatment discontinuation due to toxicity were observed between groups. Of note however, more patients required a blood transfusion for treatment-induced anaemia with olaparib (51.4% versus 30.5%). High rates of symptomatic anaemia have also been described with the use of talazoparib monotherapy (39.3%) in the neoadjuvant setting22. Our data are therefore consistent with the known toxicity profile of PARP inhibitors, enhanced by the additional effect of platinum on the bone marrow. Despite this, the delivery of chemotherapy and olaparib was generally more than 90% and rates of pCR were high, comparable with the best from previously reported studies10. Notably, the GeparOLA trial reported overall low rates of grade 3–4 anaemia from olaparib (2.9%) and carboplatin (18.9%) in combination with paclitaxel23. This is probably explained by weekly dosing of carboplatin as well as the reduced dose of continuous olaparib.
Although the COVID-19 pandemic occurred during the trial, causing a pause in recruitment, the members of the teams at our recruiting centres and in the central Cambridge team ensured that enrolment into the TNBC(gBRCAwt) cohort was completed with minimum delay. Another strength was the inclusion of carboplatin in both the control and the research groups, which drove changes in the standard of care in the UK’s National Health Service with recruiting centres adopting carboplatin-based chemotherapy early. In addition, now that the trial is completed it can inform adjuvant and neoadjuvant treatments for TNBC in 2024. Last, a major strength of the study is the strong and comprehensive translational research component (not reported here), with tumour tissue collection from all patients, and fresh tissue collection from patients treated in selected centres. In addition, patients from selected centres have had longitudinal circulating tumour DNA samples collected, and a cohort of patient-derived tumour xenografts have been developed in the Cambridge Centre. The translational research that is ongoing will be published later and should add to the knowledge base for TNBC(gBRCAwt) and accelerate the application of precision medicine and personalized treatment in this group of patients.
There were no major limitations in the study from a methodological or practical point of view. This study alongside the results from the gBRCAm cohort highlights the importance of the availability of rapid assessment of gBRCA pathogenic variants. The early knowledge of these biomarkers or their absence is now critical to ensure that patients with TNBC(gBRCAwt) and gBRCAm receive the most appropriate neoadjuvant regimens. Testing patients for germline pathogenic variants in BRCA1, BRCA2 and also PALB2 (ref. 24) in a clinically relevant time frame is essential to equip clinicians with the necessary information to apply a precision medicine approach.
The landscape of treatment for TNBC has changed considerably since planning for this study started in 2012. The CREATE-X trial demonstrated a significant benefit in patients with TNBC from 6 months of adjuvant capecitabine given after neoadjuvant chemotherapy that left residual disease25. The GEICAM–CIBOMA trial also addressed this question but showed no improvement with capecitabine in patients in the TNBC group as a whole, although it did report benefit in patients in the non-basal TNBC group26. Therefore, there is some evidence to change the standard of care to use adjuvant capecitabine for non-basal TNBC with residual disease after neoadjuvant chemotherapy.
The positive outcomes of KEYNOTE 522 (refs. 27,28) have resulted in the immune checkpoint inhibitor pembrolizumab becoming licensed for use as neoadjuvant and adjuvant therapy in TNBC by the US Food and Drug Administration29, the European Medicines Evaluation Agency30 and the National Institute for Health and Care Excellence31. KEYNOTE 522 has yet to report biomarker results defining the basal-like TNBC and gBRCAm cohorts, which would help to guide the standard of care for patients in these groups. However, pembrolizumab is now standard of care in early TNBC(gBRCAwt) in the neoadjuvant and adjuvant settings. Results from the NeoTRIP trial that tested neoadjuvant atezolizumab with chemotherapy32 showed emerging signals of benefit from immunotherapy in patients with TNBC tumours with evidence of immune activation and remodelling in the tumour microenvironment33. Other published evidence confirms the prognostic importance of DNA-damage immune response signatures and stromal TILs34. Our analysis also confirmed that a high level of TILs (≥60%) correlated positively with increasing response rates to neoadjuvant treatments as has been shown by other groups35, although olaparib did not seem to be more active in tumours with higher TIL counts.
Conclusions
In conclusion, the PARTNER trial is a neoadjuvant study that has tested the addition of olaparib with a gap schedule to platinum-based chemotherapy in patients with basal-like TNBC who are known to be gBRCAwt. The trial did not show a benefit from the addition of olaparib in the dose and schedule used, either in rates of pCR or estimated EFS and OS. This is in marked contrast to the significant positive effect of the same dose and schedule in patients with gBRCAm breast cancer (J.E.A., manuscript in preparation). Further translational analysis will provide insights into the biology of TNBC(gBRCAwt) and may identify predictive biomarkers at baseline or after treatment to improve outcomes for patients with neoadjuvant olaparib.
Methods
Patient and tumour characteristics
Patients aged between 16 and 70 years with histologically confirmed stage T1–4, N0–3 (tumour or axillary lymph node diameter ≥10 mm) invasive breast cancer, confirmed ER-negative and HER2-negative, and Eastern Cooperative Oncology Group performance status 0–1 were eligible. Other key inclusion criteria were patient fitness to receive the trial chemotherapy regimen and availability of slides and paraffin-embedded tissue blocks from the pretreatment biopsy. Patients were excluded if they had T0 tumour in the absence of axillary node ≥10 mm, apparent distant metastases, prior history of invasive breast cancer in the past 5 years or any previous chemotherapy or targeted agent used for the treatment of cancer in the past 5 years. The PARTNER trial protocol (NCT03150576) was approved by the North West – Haydock Research Ethics Committee (ref: 15/NW/0926) and the trial was carried out in accordance with the Declaration of Helsinki and the European Clinical Trials Directives 2001/20/EC. All patients provided an initial written informed consent that covered pathological review of the local slides and biopsy tissue at the Cambridge Centre, with additional biomarker review carried out centrally (EGFR, CK5, CK6 and AR) to confirm basal-like TNBC. If the biomarkers confirmed this, the patients proceeded to full consent for the main study at the local centre. Following trial entry, all patients were tested for pathogenic variants of germline BRCA1 and BRCA2 (gBRCAm). Those with gBRCAwt were included in this cohort, whereas those with gBRCAm were included in another cohort. The trial was sponsored by Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, and financed by a project grant from AstraZeneca, who also supplied olaparib. Cancer Research UK endorsed the trial and financed the sample collections for the translational studies that will be reported separately. The funders had no role in the study data collection, analysis, interpretation or writing of this report.
Treatment
The trial was open label and was carried out in three stages. Stage 1 assessed the safety of combining olaparib with carboplatin and paclitaxel, and stage 2 compared two different schedules of olaparib and carboplatin–paclitaxel to ‘pick-the-winner’. In stages 1 and 2, eligible patients were randomly assigned using a minimization method in a 1:1:1 ratio, with a web-based central randomization system. Patients in the control group received chemotherapy alone: carboplatin at area under the curve 5 (AUC5) intravenously on day 1 with paclitaxel 80 mg m−2 intravenously on days 1, 8 and 15 every 3 weeks for four cycles. During stages 1 and 2, there were two randomized research arms in which intermittent dosing of olaparib at 150 mg twice a day by mouth (p.o) for 12 days was added to carboplatin–paclitaxel for each of four cycles. The schedule in the first research arm was olaparib at 150 mg twice a day on days −2 to day +10 of each of four cycles of carboplatin with paclitaxel and this was designated as the non-gap schedule. The schedule in the second research arm was olaparib at 150 mg twice a day by mouth from day +3 to day +14 and this was designated as the gap schedule. Patients were treated on a 3-weekly basis for four cycles in the control or research arms and then all patients had three cycles of standard local anthracycline-based chemotherapy without olaparib before surgery. The research arm (gap schedule) was subsequently selected and taken forwards to stage 3 and the non-gap schedule arm was the ‘dropped arm’. This decision was based on the recommendation of the independent data safety monitoring committee (IDSMC), which based guidance on pre-specified criteria of safety, convenience and compliance, and efficacy.
In stage 3, patients were randomly assigned with a 1:1 ratio to either the control or research arm (gap schedule olaparib). Patients who had been randomized to the control arm and the gap schedule research arm in stages 1 and 2 were also included in this analysis. Stratification factors at randomization included tumour size (≤50 mm versus >50 mm), axillary lymph node involvement at diagnosis by biopsy and/or imaging (yes versus no) and TILs (<60% versus ≥60%). G-CSF was given as per local practice. A flow chart of the trial treatment is shown in Extended Data Fig. 1.
Study procedures
Patients were clinically assessed before the beginning of every cycle until the end of treatment or disease progression. Breast surgery was carried out after 21 weeks of chemotherapy with or without olaparib and was followed by radiotherapy as per local standard protocols. After surgery, patients were followed 6-monthly for 2 years and then annually for up to 10 years. Local histopathology reports from primary surgery were centrally reviewed independently by two readers (clinicians and pathologists) blinded to the treatment allocation, for each report (E.P., H.M.E., L.M.D. and A.F.), and if there were any differences in any of the response criteria (Extended Data Fig. 7), consensus for each patient was reached after discussion. AEs were reported for each cycle of protocol treatment using NCI CTCAE version 4.03. Participation in the quality of life (QOL) sub-study was optional and this metric was assessed using two measures. The first was the EQ-5D-5L measure, which is a self-report survey that measures QOL across 5 domains: mobility, self-care, usual activities, pain/discomfort and anxiety/depression. The second was the FACT-B, which is a 37-item instrument designed to measure five domains of HRQOL in patients with breast cancer. Both questionnaires were completed by patients before randomization, following completion of four cycles, completion of seven cycles, surgery and radiotherapy, and annually for 2 years from completion of surgery.
Extended Data Fig. 7. Central pathology review Case Report Form.
Central pathology review Case Report Form.
Statistical analysis
In this three-stage phase II–III trial, stage 1 assessed the safety of the addition of olaparib to 3-weekly carboplatin with weekly paclitaxel chemotherapy. Stage 2 selected the ‘winner’ from two research arms, and stage 3 assessed pCR at surgery after neoadjuvant treatment in all patients. The primary end point was comparison of pCR rates between the research and control groups. Details of the statistical analysis plan for each stage are provided in the Supplementary Information. Secondary survival end points were all calculated from the date of randomization to the date of first event and included: EFS (local or distant recurrence, diagnosis of a second cancer or death from any cause); relapse-free survival (local or distant recurrence or death from any cause, excluding patients who relapsed before surgery); breast cancer-specific survival (death from breast cancer or death after breast cancer relapse); DDFS (distant recurrence or death from any cause); local recurrence-free survival (local recurrence or death from any cause); OS (death from any cause); time to second cancer (diagnosis of a second cancer). Other secondary end points were: residual cancer burden; pCR in breast alone; radiological response; safety and quality of life.
In this TNBC(gBRCAwt) cohort, a total of 454 patients were needed to test with 90% power and 5% significance level the null hypothesis of no difference in pCR rate between the two groups, versus the alternative of 50% in the control group and 65% in the research group. Allowing for a non-compliance of 5%, it was planned to recruit a total of 478 patients TNBC(gBRCAwt) between the control and the selected research group.
The main analysis was conducted on the basis of the modified intention-to-treat principle, which included all randomized, eligible patients excluding only those who did not start treatment. The safety analyses included patients who had at least one dose of trial treatment. The differences between binary outcomes were compared using the chi-squared test and the CI of the differences was based on the score method36. Kaplan–Meier plots were generated for time-to-event outcomes and groups were compared using the log-rank test. HRs were estimated using the Cox regression model. The subscales of the EQ-5D-5L and FACT-B questionnaires were derived according to standard-scoring manuals. Analyses of changes from the baseline over time and differences between the two groups for subscales were carried out with repeated measures analysis of covariance, adjusting for the baseline level, time, treatment and interaction of time and treatment. Although it was assumed that the data were ‘missing at random’, a sensitivity analysis for data ‘missing not at random’ was carried out for the primary end point. All statistical analyses were carried out in R (v4.1.0) and all P values are based on two-tailed tests.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Portfolio reporting summaries, source data, extended data, supplementary information, acknowledgements, peer review information; details of author contributions and competing interests; and statements of data and code availability are available at 10.1038/s41586-024-07384-2.
Supplementary information
Summary from protocol; and PARTNER trial consortium members.
Acknowledgements
We thank the patients, and the families and friends who supported them, for participating in this trial; our ethics committee, our independent data and safety monitoring committee and the trial management group for their advisory roles; the PARTNER trial consortium members, past and present (Supplementary Information); and I. Cizaite for preparing and proofreading the manuscript. This trial was sponsored by Cambridge University Hospitals NHS Foundation Trust and the University of Cambridge, and financed by a project grant from AstraZeneca, who also supplied olaparib. Cancer Research UK provided peer review and endorsement for the study and financed the sample collections for the translational studies, which will be reported separately. We also acknowledge the National Institute for Health and Care Research Cambridge Biomedical Research Centre and the Cancer Research UK Cambridge Centre for their financial support for staff and infrastructure costs. The funders had no role in data collection or analysis. Once the trial group had interpreted the data, the results were then shared with the AstraZeneca scientists. In addition, we thank the Cancer Molecular Diagnostics Laboratory and The Precision Breast Cancer Institute Team for their support for sample collection; Cambridge Tissue Bank (NIHR203312) for sample assessment and diagnostics; Cambridge Clinical Trials Centre – Cancer Theme for their core staff support; the clinical trials support staff at all participating sites; and Addenbrookes Charitable Trust for financing the post of the chief investigator (2015–2018). We acknowledge Cancer Research UK (CRUKE/14/048) and AstraZeneca (1994-A093777).
Extended data figures and tables
Author contributions
J.E.A.: chief investigator, overall oversight, design, protocol development, management, data interpretation and manuscript writing; site principal investigator for co-ordinating central site. K.P.: clinical fellow contributing to manuscript writing, data interpretation, trial safety management and protocol development. L.G.: senior trials co-ordinator dealing with day-to-day management of the trial and all the trial team. A.D.: main statistician dealing with data analysis. N.D.: senior statistician overseeing data analysis. C.H.: senior data manager overseeing data collection team. L.M.D.: clinical fellow, contributing to trial safety management and pathology report assessment. R.L.: clinical fellow, contributing to trial safety management and protocol development. A.F.: clinical fellow, contributing to trial safety management and pathology report assessment. A.N.R.: clinical trials practitioner and contributed to case report development. J.R.W.: senior clinical trials practitioner and contributed to case report development and sample management. A. Chabbra: senior pharmacist contributed to trial development. W.Q.: original lead statistician. A.-L.V.: original senior trials co-ordinator. R.M.H.: lead programmer for development of case report form and data collection proformas. Site principal investigators (recruitment and consent of patients): S.C., T.H., D.T., R.V., M.P., S.A., D.G., S.R., A.B., J.P.B., S.S., E.S., L.C.S., M.D., C.A.P., M.M., M.J.C., M.B.M., A. Chakrabarti, R.R.R. and N.C.L. Site principal investigators with trial management group responsibilities: J.C.N., K.M., A.C.A. (co-chair of trial management group), E.R.C. (co-chair of trial management group). P.C.S.: trial pathologist. E.M.: trial development and support. M.T.: medical genetics lead. E.P.: senior trial pathologist. H.M.E.: design, management, data interpretation and manuscript writing.
Peer review
Peer review information
Nature thanks Aditya Bardia, Maria Eleni Hatzipanagiotou and the other, anonymous, reviewer(s) for their contribution to the peer review of this work. Peer reviewer reports are available.
Data availability
Data collected in the PARTNER study will be made available to researchers whose full proposal for their use of the data has been approved by the PARTNER trial management group and whose research includes a clear and comprehensive research plan with statistical considerations adequately completed. The data required for the approved, specified purposes and the trial protocol will be provided after completion of a data sharing agreement. Data sharing agreements will be set up by the trial steering and management groups and will include clear instructions on publication, reporting and usage policy. A minimum dataset of anonymized data will be made available after full publication of the trial and related work. Requests for data should be addressed to ja344@cam.ac.uk.
Competing interests
The authors declare competing financial interests in the form of research grants and honoraria for lectures. The funders of the research grants and honoraria had no role in the study design, data collection, analysis, interpretation or writing of the manuscript. J.E.A. reports honoraria, conference attendance travel support and a grant from AstraZeneca; and honoraria from Esai and Pfizer for lectures. M.M. reports shares in AstraZeneca. M.B.M. reports advisory board membership of Roche, Pfizer, MSD, Daiichi Sankyo, Gilead, AstraZeneca, Novartis, the Menarini Group, Genomic Health (Precision Medicine) and Seagen; speaker honoraria from Roche, BMS, Seagen, Pfizer, Daiichi Sankyo, AstraZeneca, Lilly, MSD, Genomic Health (Precision Medicine), Eisai and Novartis; and meeting expenses from Roche, Eli Lilly, Novartis and MSD. R.R.R. reports honoraria from Daiichi Sankyo, AstraZeneca, Novartis and Pfizer; membership of advisory boards for Daiichi Sankyo, Eli Lilly, Pfizer and AstraZeneca; and travel and conference attendance from BMS, Pfizer and Roche. P.C.S. reports that their partner is employed by AstraZeneca. N.C.L. reports shares in AstraZeneca. A.C.A. reports research funding paid to their institution from AstraZeneca; conference fees and travel expenses from Roche and Novartis; conference fees from MSD; membership of Roche and AstraZeneca advisory boards; and a grant for an educational project from Gilead. E.R.C. reports honoraria from AstraZeneca, Eli Lilly, Novartis, Pfizer and Roche; membership of advisory boards for AstraZeneca, Eli Lilly, Pfizer, Menarini Stemline UK and Novartis; consultancy for Pfizer; conference fees, travel and accommodation from Roche and Novartis; an educational grant from Daiichi Sankyo; and research funding and support from SECA and AstraZeneca. E.P. reports honoraria from Roche, Novartis and AstraZeneca. The remaining authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
is available for this paper at 10.1038/s41586-024-07384-2.
Supplementary information
The online version contains supplementary material available at 10.1038/s41586-024-07384-2.
References
- 1.Koboldt, D. C. et al. Comprehensive molecular portraits of human breast tumours. Nature490, 61–70 (2012). [DOI] [PMC free article] [PubMed]
- 2.Perou CM. Molecular stratification of triple-negative breast cancers. Oncologist. 2011;16:61–70. doi: 10.1634/theoncologist.2011-S1-61. [DOI] [PubMed] [Google Scholar]
- 3.Schettini F, et al. Clinical, radiometabolic and immunologic effects of olaparib in locally advanced triple negative breast cancer: the OLTRE window of opportunity trial. Front. Oncol. 2021;11:686776. doi: 10.3389/fonc.2021.686776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortazar P, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384:164–172. doi: 10.1016/S0140-6736(13)62422-8. [DOI] [PubMed] [Google Scholar]
- 5.Litton JK, et al. Standardized definitions for efficacy end points in neoadjuvant breast cancer clinical trials: NeoSTEEP. J. Clin. Oncol. 2023;41:4433–4442. doi: 10.1200/JCO.23.00435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tutt A, et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and advanced breast cancer: a proof-of-concept trial. Lancet. 2010;376:235–244. doi: 10.1016/S0140-6736(10)60892-6. [DOI] [PubMed] [Google Scholar]
- 7.Robson M, et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N. Engl. J. Med. 2017;377:523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 8.Tutt ANJ, et al. Adjuvant olaparib for patients with BRCA1- or BRCA2-mutated breast cancer. New Engl. J. Med. 2021;384:2394–2405. doi: 10.1056/NEJMoa2105215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Early Breast Cancer Trialists' Collaborative Group (EBCTCG). Comparisons between different polychemotherapy regimens for early breast cancer: meta-analyses of long-term outcome among 100 000 women in 123 randomised trials. Lancet. 2012;379:432–444. doi: 10.1016/S0140-6736(11)61625-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Loibl S, et al. Addition of the PARP inhibitor veliparib plus carboplatin or carboplatin alone to standard neoadjuvant chemotherapy in triple-negative breast cancer (BrighTNess): a randomised, phase 3 trial. Lancet Oncol. 2018;19:497–509. doi: 10.1016/S1470-2045(18)30111-6. [DOI] [PubMed] [Google Scholar]
- 11.Mason, S. R. et al. Platinum-based chemotherapy for early triple-negative breast cancer. Cochrane Database Syst. Rev.https://doi.org//10.1002/14651858.CD014805.pub2 (2023). [DOI] [PMC free article] [PubMed]
- 12.Staaf J, et al. Whole-genome sequencing of triple-negative breast cancers in a population-based clinical study. Nat. Med. 2019;25:1526–1533. doi: 10.1038/s41591-019-0582-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vollebergh MA, et al. Genomic patterns resembling BRCA1- and BRCA2-mutated breast cancers predict benefit of intensified carboplatin-based chemotherapy. Breast Cancer Res. 2014;16:R47. doi: 10.1186/bcr3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nik-Zainal S, et al. Landscape of somatic mutations in 560 breast cancer whole-genome sequences. Nature. 2016;534:47–54. doi: 10.1038/nature17676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bryant HE, et al. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature. 2005;434:913–917. doi: 10.1038/nature03443. [DOI] [PubMed] [Google Scholar]
- 16.Farmer H, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434:917–921. doi: 10.1038/nature03445. [DOI] [PubMed] [Google Scholar]
- 17.Arora S, et al. FDA approval summary: olaparib monotherapy or in combination with bevacizumab for the maintenance treatment of patients with advanced ovarian cancer. Oncologist. 2021;26:e164–e172. doi: 10.1002/onco.13551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olaparib for Maintenance Treatment of Relapsed, Platinum-Sensitive Ovarian, Fallopian Tube or Peritoneal Cancer after 2 or More Courses of Platinum-Based Chemotherapy (NICE, 2023); https://www.nice.org.uk/guidance/ta908/resources/olaparib-for-maintenance-treatment-of-relapsed-platinumsensitive-ovarian-fallopian-tube-or-peritoneal-cancer-after-2-or-more-courses-of-platinumbased-chemotherapy-pdf-82615433389765.
- 19.Oza AM, et al. Olaparib combined with chemotherapy for recurrent platinum-sensitive ovarian cancer: a randomised phase 2 trial. Lancet Oncol. 2015;16:87–97. doi: 10.1016/S1470-2045(14)71135-0. [DOI] [PubMed] [Google Scholar]
- 20.Lehmann BD, et al. Refinement of triple-negative breast cancer molecular subtypes: implications for neoadjuvant chemotherapy selection. PLoS ONE. 2016;11:e0157368. doi: 10.1371/journal.pone.0157368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Masuda H, et al. Differential response to neoadjuvant chemotherapy among 7 triple-negative breast cancer molecular subtypes. Clin. Cancer Res. 2013;19:5533–5540. doi: 10.1158/1078-0432.CCR-13-0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Litton JK, et al. Neoadjuvant talazoparib in patients with germline BRCA1/2 mutation-positive, early-stage triple-negative breast cancer: results of a phase II study. Oncologist. 2023;28:845–855. doi: 10.1093/oncolo/oyad139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fasching PA, et al. Neoadjuvant paclitaxel/olaparib in comparison to paclitaxel/carboplatinum in patients with HER2-negative breast cancer and homologous recombination deficiency (GeparOLA study) Ann. Oncol. 2021;32:49–57. doi: 10.1016/j.annonc.2020.10.471. [DOI] [PubMed] [Google Scholar]
- 24.Tung NM, et al. TBCRC 048: phase II study of olaparib for metastatic breast cancer and mutations in homologous recombination-related genes. J. Clin. Oncol. 2020;38:4274–4282. doi: 10.1200/JCO.20.02151. [DOI] [PubMed] [Google Scholar]
- 25.Masuda N, et al. Adjuvant capecitabine for breast cancer after preoperative chemotherapy. N. Engl. J. Med. 2017;376:2147–2159. doi: 10.1056/NEJMoa1612645. [DOI] [PubMed] [Google Scholar]
- 26.Lluch A, et al. Phase III trial of adjuvant capecitabine after standard neo-/adjuvant chemotherapy in patients with early triple-negative breast cancer (GEICAM/2003-11_CIBOMA/2004-01) J. Clin. Oncol. 2020;38:203–213. doi: 10.1200/JCO.19.00904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schmid P, et al. Event-free survival with pembrolizumab in early triple-negative breast cancer. New Engl. J. Med. 2022;386:556–567. doi: 10.1056/NEJMoa2112651. [DOI] [PubMed] [Google Scholar]
- 28.Schmid P, et al. Pembrolizumab for early triple-negative breast cancer. New Engl. J. Med. 2020;382:810–821. doi: 10.1056/NEJMoa1910549. [DOI] [PubMed] [Google Scholar]
- 29.Shah M, et al. FDA approval summary: pembrolizumab for neoadjuvant and adjuvant treatment of patients with high-risk early-stage triple-negative breast cancer. Clin. Cancer Res. 2022;28:5249–5253. doi: 10.1158/1078-0432.CCR-22-1110. [DOI] [PubMed] [Google Scholar]
- 30.European Commission approves KEYTRUDA® (pembrolizumab) plus chemotherapy as neoadjuvant treatment, then continued as adjuvant monotherapy after surgery for locally advanced or early-stage triple-negative breast cancer at high risk of recurrence. Merckhttps://www.merck.com/news/european-commission-approves-keytruda-pembrolizumab-plus-chemotherapy-as-neoadjuvant-treatment-then-continued-as-adjuvant-monotherapy-after-surgery-for-locally-advanced-or-early-stage-triple/ (2022).
- 31.Pembrolizumab for Neoadjuvant and Adjuvant Treatment of Triple-Negative Early or Locally Advanced Breast Cancer (NICE, 2022); https://www.nice.org.uk/guidance/ta851.
- 32.Gianni L, et al. Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple-negative, early high-risk and locally advanced breast cancer: NeoTRIP Michelangelo randomized study. Ann. Oncol. 2022;33:534–543. doi: 10.1016/j.annonc.2022.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Wang XQ, et al. Spatial predictors of immunotherapy response in triple-negative breast cancer. Nature. 2023;621:868–876. doi: 10.1038/s41586-023-06498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharma P, et al. Validation of the DNA damage immune response signature in patients with triple-negative breast cancer from the SWOG 9313c trial. J. Clin. Oncol. 2019;37:3484–3492. doi: 10.1200/JCO.19.00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li S, et al. Predictive and prognostic values of tumor infiltrating lymphocytes in breast cancers treated with neoadjuvant chemotherapy: a meta-analysis. Breast. 2022;66:97–109. doi: 10.1016/j.breast.2022.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Miettinen O, Nurminen M. Comparative analysis of two rates. Stat. Med. 1985;4:213–226. doi: 10.1002/sim.4780040211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary from protocol; and PARTNER trial consortium members.
Data Availability Statement
Data collected in the PARTNER study will be made available to researchers whose full proposal for their use of the data has been approved by the PARTNER trial management group and whose research includes a clear and comprehensive research plan with statistical considerations adequately completed. The data required for the approved, specified purposes and the trial protocol will be provided after completion of a data sharing agreement. Data sharing agreements will be set up by the trial steering and management groups and will include clear instructions on publication, reporting and usage policy. A minimum dataset of anonymized data will be made available after full publication of the trial and related work. Requests for data should be addressed to ja344@cam.ac.uk.