Abstract
Objectives
The cerebral vessels may be affected in primary systemic vasculitis (PSV), but little is known about cerebrovascular events (CVEs) in this population. This study aimed to determine the frequency of CVEs at the time of diagnosis of PSV, to identify factors associated with CVEs in PSV, and to explore features and outcomes of stroke in patients with PSV.
Methods
Data from adults newly diagnosed with PSV within the Diagnostic and Classification Criteria in VASculitis (DCVAS) study were analysed. Demographics, risk factors for vascular disease, and clinical features were compared between patients with PSV with and without CVE. Stroke subtypes and cumulative incidence of recurrent CVE during a prospective 6-month follow-up were also assessed.
Results
The analysis included 4828 PSV patients, and a CVE was reported in 169 (3.50%, 95% CI 3.00–4.06): 102 (2.13% 95% CI 1.73–2.56) with stroke and 81 (1.68% 95% CI 1.33–2.08) with transient ischemic attack (TIA). The frequency of CVE was highest in Behçet’s disease (9.5%, 95% CI 5.79–14.37), polyarteritis nodosa (6.2%, 95% CI 3.25–10.61), and Takayasu’s arteritis (6.0%, 95% CI 4.30–8.19), and lowest in microscopic polyangiitis (2.2%, 95% CI 1.09–3.86), granulomatosis with polyangiitis (2.0%, 95% CI 1.20–3.01), cryoglobulinaemic vasculitis (1.9%, 95% CI 0.05–9.89), and IgA-vasculitis (Henoch-Schönlein) (0.4%, 95% CI 0.01–2.05). PSV patients had a 11.9% cumulative incidence of recurrent CVE during a 6-month follow-up period.
Conclusion
CVEs affect a significant proportion of patients at time of PSV diagnosis, and the frequency varies widely among different vasculitis, being higher in Behçet’s. Overall, CVE in PSV is not explained by traditional vascular risk factors and has a high risk of CVE recurrence.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12251-1.
Keywords: Stroke, Primary systemic vasculitis, Transient ischaemic attack, Cerebrovascular event
Introduction
Primary systemic vasculitis (PSV) comprises a heterogeneous group of inflammatory diseases affecting large, medium, and/or small vessels in different organs, including extra- and intra-cranial involvement. Overall, vasculitis is a rare cause of stroke, but its frequency in patients with stroke varies globally [1, 2]. The epidemiologic data may be dependent on the local experience and evaluation strategies used for diagnosing rare causes of stroke, such as PSV, even in young people where the proportion of stroke due to vasculitis is thought to be higher [3, 4]. Moreover, people with PSV seem to have a greater risk of cerebrovascular disease when compared to the general population, particularly those with Takayasu’s arteritis (TAK) [5], giant cell arteritis (GCA) [6, 7], anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis [8–10], or Behçet’s disease [11]. This increased risk may relate to direct involvement of vasculitis in the central nervous system (CNS) [12, 13], extracranial organ involvement (e.g. heart) [14], accelerated rates of atherosclerosis [15], or potential side effect of treatments for PSV. Stroke early in disease course is perhaps more likely directly related to vasculitis than to atherosclerosis, other vascular or cardiac conditions, or treatment complications, which may tend to accrue and be more significant later in PSV disease course.
However, little is known about the frequency and features of stroke at PSV onset. The frequency of stroke in PSV has been studied in relatively small and heterogeneous cohorts, most commonly retrospectively assessed over the whole disease course [10, 16–25]). Ischaemic stroke has been described as the most common subtype of stroke in these patients [16, 22], but there are limited available data on the frequency of haemorrhagic stroke and cerebral venous thrombosis (CVT) in PSV [26–30], except for CVT in Behçet’s disease [31].
This study aimed to determine the frequency and subtypes of stroke and transient ischaemic attack (TIA) at the time of diagnosis of PSV and to identify features associated with occurrence of cerebrovascular events (CVEs) in PSV. In addition, recurrence of stroke/TIA and stroke-related disability were explored in patients with PSV and compared to patients without PSV.
Methods
Study design and patients
This investigation was a multinational observational sub-study of the Diagnostic and Classification Criteria in VASculitis (DCVAS) study, which has been described in detail elsewhere [32]. Consecutive adult patients where PSV was a potential diagnosis for current illness were recruited from academic and community practices, between January 2011 and December 2017. All cases were enrolled within 2 years of diagnosis, except for patients with TAK and PAN who were enrolled up to 5 years after diagnosis to increase recruitment chances. Patients were prospectively followed for 6 months after enrolment date.
From the DCVAS database, data were extracted on patients with a diagnosis of PSV confirmed by the submitting physician at 6-months, as per the DCVAS study protocol [32] including large vessel-vessel (GCA, TAK), medium-vessel (polyarteritis nodosa (PAN)), small-vessel [eosinophilic granulomatosis with polyangiitis (EGPA), granulomatosis with polyangiitis (GPA), microscopic angiitis (MPA)], Behçet’s disease, and other forms of vasculitis. Data were also extracted on patients with a diagnosis of other autoimmune/systemic illnesses with a similar presentation to PSV (“PSV mimics”), which was used as a comparator group. Patients with single-organ vasculitis, including primary angiitis of the central nervous system, or diagnosed with two forms of vasculitis were excluded. Data from all PSV cases were analysed cross-sectionally.
All DCVAS sites were invited and agreed a priori to participate in the pre-specified Stroke sub-study (listed in Online Resource 1). The Stroke sub-study included patients experiencing any CVE from symptom onset until a vasculitis or a vasculitis mimic diagnosis and consisted in retrospective collection of data at enrolment date, and prospective data collection during the 6-month follow-up period in the main study. Based on the literature, assuming a combined 7% frequency of CVE in PSV [6–11, 16, 17, 20–23], a minimum sample of 140 stroke patients was calculated.
Primary, secondary, and exploratory objectives
The primary aim of this study was to determine the frequency of CVE in patients with PSV (question 1). Secondary aims were to analyse factors associated with CVE occurrence in PSV (question 2); and to assess the different characteristics of stroke subtypes, disability, and 6-month recurrence rate in PSV patients (question 3). Exploratory aims included comparing patients with CVE and PSV with “PSV mimics” (question 4). Data from all PSV cases included in the DCVAS were analysed cross-sectionally to answer questions 1 and 2. Cross-sectional and prospective data from the Stroke sub-study were used to answer questions 3 and 4.
Data collection and definitions
Organ involvement, including CVE, laboratory and imaging findings, present from first symptom onset to diagnosis of PSV/PSV mimics, were recorded in the DCVAS study [33]. Dates of organ involvement manifestations, including CVE occurrence dates, were not available. The stroke sub-study case report form (CRF) included additional data elements on CVE (stroke and TIA) during the above-mentioned time periods, including diagnosis and aetiological investigations (Online Resource 5) made by each centre investigator, as per local standard of care. Clinical definitions were used for ischaemic stroke, TIA, haemorrhagic stroke [intracerebral haemorrhage and subarachnoid haemorrhage (SAH)], and cerebral venous thrombosis and supported by imaging data [34, 35]. Aetiology of ischaemic CVE was defined according to ‘Trial of ORG 10172 in acute stroke treatment’ (TOAST) classification categories [36]. Causes for intracerebral haemorrhage were noted separately (small-vessel disease, vascular malformation, amyloid angiopathy, haematological disorders, cryptogenic). Asymptomatic ischaemic or haemorrhagic cerebral lesions were not considered to be CVE. The arterial territory involved in ischaemic events was classified combining the clinical presentation [37] and confirmed by the reported imaging. Stroke data documented in the CRFs were reviewed case by case by two neurologists (RG, MS). The diagnosis, aetiology and location of strokes were agreed upon by consensus after full review of all data. The original brain imaging scans were not available for review.
Comorbidities prior to the symptom onset of the current illness (PSV or mimics), including history of CVE (‘Previous CVE’), and the Vasculitis Damage Index (VDI) [38] at 6 months were captured. In the stroke sub-study, cumulative incidence of recurrent CVE during the 6-month period of follow-up was determined. Stroke disability at 6-month follow-up was assessed with the modified Rankin scale (mRankin) [39].
Statistical analysis
Descriptive analyses were conducted on the frequency and characteristics of CVEs in PSV (all) and each individual forms of PSV. Continuous variables were expressed as means (standard deviations), or medians (interquartile range). Categorical variables were expressed as absolute numbers and percentages.
Patients with PSV and CVE were compared to patients with PSV without CVE regarding features potentially associated with the occurrence of CVE (comorbidities, organ involvement, presence of new onset headache or peripheral neuropathy). Univariate analysis included Pearson chi-square/Fisher exact test, t Student test, or Mann–Whitney test, as appropriate. Age, disease duration, ethnicity, sex and variables that were significant in the univariate analysis were included in a binary logistic regression model used to identify factors associated with CVE for the whole PSV group. Results are summarized as odds ratios (OR) and 95% confidence intervals (CI). Finally, descriptive statistics on stroke were calculated for PSV mimics and patients in the Stroke-Biobank, as done for patients with PSV. Two-sided p-values were considered statistically significant when < 0.05. Statistical analyses were performed using SPSS Statistic Editor version 28.
Standard protocol approvals, registrations, and patient consents
The study was approved by the Berkshire Research Ethics Committee (10/H505/19). The DCVAS study is listed in the ClinicalTrials.gov database (NCT01066208). All sites obtained any additional ethical and institutional approvals required for their jurisdiction. The DCVAS study is in accordance with the 1964 Declaration of Helsinki and ethical approval was obtained by national and local ethics committees in accordance with national legislation. All patients signed an informed consent form.
Data availability
Data from the DCVAS study used for this study analyses are available from the DCVAS Steering Committee on reasonable request.
Results
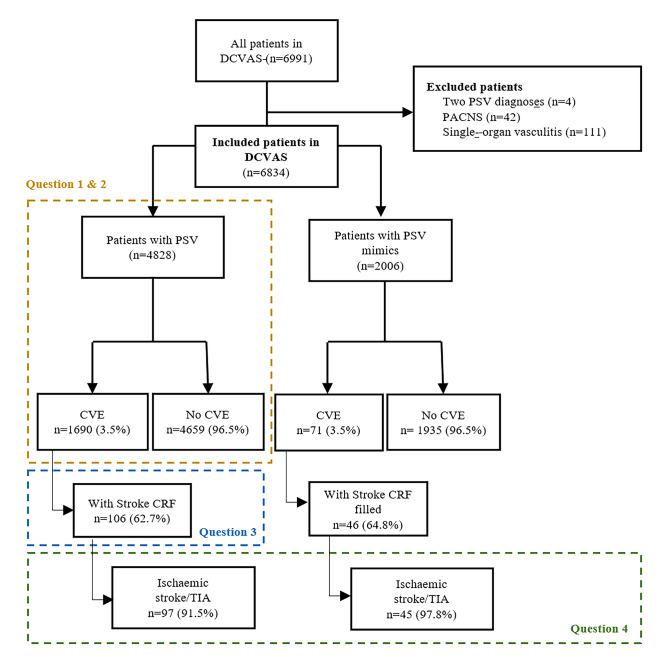
From the initial 6991 patients with data submitted into the DCVAS study, 6834 patients were included (4828 with PSV and 2006 with PSV mimics) (Fig. 1). Patients with PSV had a median age of 61.0 (IQR 30.0) years, 59.4% were female, and the median disease duration was 10 (IQR 10.0) months (5.9% with no exact disease duration available) (Table 1).
Fig. 1.
Flowchart of patients included in the study. IMM Instituto de Medicina Molecular, DCVAS—Diagnostic & Classification Criteria in Vasculitis study; PSV primary systemic vasculitis; PACNS primary angiitis of the central nervous system; CVE cerebrovascular event; CRF case report form; TIA transient ischaemic attack
Table 1.
Comparison of patients with primary systemic vasculitis with and without cerebrovascular events regarding baseline characteristics, systemic organ involvement and cardiovascular risk factors
| With CVE (n = 169) |
Without CVE (n = 4659) | p-Value | |
|---|---|---|---|
| Median age (IQR) | 59.0 (35.0) | 61.0 (30.0) | 0.37 |
| Median disease duration (IQR) (months) | 11.0 (11.0) | 10.0 (10.0) | 0.03 |
| Female sex (%) | 98 (58.0) | 2770 (59.5) | 0.70 |
| Ethnicity | |||
| White (%) | 112 (66.3) | 3357 (72.1) | 0.10 |
| Black/African (%) | 5 (3.0) | 61 (1.3) | 0.08 |
| Asian (%) | 32 (18.9) | 822 (17.6) | 0.67 |
| Middle East (%) | 23 (13.6) | 357 (7.7) | 0.005 |
| Latin (%) | 1 (0.6) | 97 (2.1) | 0.134 |
| Systemic involvement | |||
| Skin (%) | 54 (32.0) | 1464 (31.4) | 0.88 |
| Cardiovascular (%) | 56 (33.1) | 1108 (23.8) | 0.005 |
| Musculoskeletal (%) | 83 (49.1) | 2451 (52.6) | 0.37 |
| Chest/pulmonary (%) | 59 (34.9) | 1861 (39.9) | 0.19 |
| Gastrointestinal (%) | 51 (30.2) | 1076 (23.1) | 0.03 |
| Eye (%) | 78 (46.2) | 1309 (28.1) | < 0.001 |
| Gynaecologic/urological (%) | 27 (16.0) | 525 (11.3) | 0.06 |
| Ear, nose, and throat (%) | 54 (32.0) | 2010 (43.1) | 0.04 |
| Median total VDI (IQR) | 83 (49.1) | 2451 (52.6) | 0.37 |
| Other neurological symptoms | |||
| New headache (%) | 57 (33.7) | 1152 (24.7) | 0.008 |
| Neuropathy (%) | 14 (8.3) | 357 (7.7) | 0.776 |
| Cardiovascular risk factors | |||
| Hypertension (%) | 57 (33.7) | 1370 (29.4) | 0.23 |
| Diabetes (%) | 20 (11.8) | 391 (8.4) | 0.112 |
| Dyslipidaemia (%) | 27 (16.0) | 553 (11.9) | 0.11 |
| Ever smoker (%) | 66 (40.0) | 1739 (38.8) | 0.76 |
| Coronary heart disease (%) | 5 (3.0) | 250 (5.4) | 0.11 |
| Heart failure (%) | 1 (0.6) | 76 (1.6) | 0.24 |
| Peripheral vascular disease (%) | 3 (1.8) | 86 (1.8) | 0.62 |
| Previous CVECVE (%) | 15 (8.9) | 86 (1.8) | < 0.001 |
| Malignancy (%) | 17 (10.1) | 273 (5.9) | 0.02 |
CVE cerebrovascular event; previous CVE previous history of CVE; PSV primary systemic vasculitis; IQR intra-quartile range; VDI vasculitis damage index
CVE frequencies differed between PSVs
A total of 183 CVEs (any type) occurred in 169 (3.50%, 95% CI 3.00–4.06) patients diagnosed with PSV. Specifically, 102 (2.13% 95% CI 1.73–2.56) were stroke and 81 (1.68% 95% CI 1.33, 2.08) were TIA (Table 2). The frequency of CVE was highest in Behçet’s disease (9.5%, 95% CI 5.79–14.37), PAN (6.2%, 95% CI 3.25–10.61), and TAK (6.0%, 95% CI 4.30–8.19), and lowest in GCA (3.6%, 95% CI 2.59–4.77), EGPA (2.9%, 95% CI 1.45–5.09), MPA (2.2%, 95% CI 1.09–3.86), GPA (2.0%, 95% CI 1.20–3.01), cryoglobulinaemic vasculitis (1.9%, 95% CI 0.05–9.89) and IgA vasculitis (Henoch-Schönlein, (0.4%, 95% CI 0.01–2.05).
Table 2.
Frequency of cerebrovascular events after onset of primary systemic vasculitis
| Any CVE N (%) |
TIA N (%) |
Stroke N (%) |
Total N (%) |
|
|---|---|---|---|---|
| All primary systemic vasculitis | 169 (3.5) | 81 (1.7) | 102 (2.2) | 4828 (100.0) |
| Large-vessel vasculitis | ||||
| Takayasu’s arteritis | 38 (6.0) | 19 (3.0) | 22 (3.5) | 630 (13.0) |
| Giant cell arteritis | 43 (3.6) | 18 (1.5) | 28 (2.3) | 1206 (25.0) |
| Isolated aortitis | 1 (2.9) | 1 (2.9) | 0 (0) | 35 (0.7) |
| Other large-vessel vasculitis | 4 (4.7) | 3 (3.5) | 2 (2.4) | 85 (1.8) |
| Medium-vessel vasculitis | ||||
| Polyarteritis nodosa | 12 (6.2) | 4 (2.1) | 8 (4.1) | 193 (4.0) |
| Small-vessel vasculitis | ||||
| Granulomatosis with polyangiitis | 20 (2.0) | 10 (1.0) | 13 (1.3) | 1021 (21.1) |
| Eosinophilic granulomatosis with polyangiitis | 11 (2.9) | 3 (0.8) | 8 (2.1) | 382 (7.9) |
| Microscopic polyangiitis | 11 (2.2) | 6 (1.2) | 8 (1.6) | 505 (10.5) |
| Other small-vessel vasculitis | 5 (2.6) | 3 (1.6) | 2 (1.1) | 189 (3.9) |
| IgA vasculitis (Henoch-Schönlein) | 1 (0.4) | 1 (0.4) | 0 (0) | 270 (5.6) |
| Cryoglobulinaemic vasculitis | 1 (1.9) | 1 (1.9) | 1 (1.9) | 54 (1.1) |
| Other | ||||
| Behçet’s disease | 19 (9.5) | 12 (6.0) | 7 (3.5) | 201 (4.2) |
| Other with no specific vessel size | 3 (5.3) | 0 (0) | 3 (5.3) | 57 (1.2) |
CVE cerebrovascular event; TIA transient ischemic attack
Vasculitis and non-vasculitis related factors influence the occurrence of CVE
Univariate analyses comparing patients with PSV with and without CVE regarding baseline characteristics, systemic organ involvement, comorbidities/cardiovascular risk factors are summarized in Table 1. In the multivariate analysis (Online Resource 2), patients with Previous CVE (OR 4.09, 95% CI 2.11–7.92, p < 0.001) and malignancy (OR 2.51, 95% CI 1.29–3.92, p = 0.004) were more likely to experience a CVE. Eye (OR 2.37, 95% CI 1.67–3.37, p < 0.001), cardiovascular (OR 1.56, 95% CI 1.08–2.25, p = 0.02), and gastrointestinal (OR 1.54, 95% CI 1.05–2.28, p = 0.03) involvement of vasculitis were each associated with a higher risk of CVE, while PSV with ear-nose-throat involvement (OR 0.59, 95% CI 0.41–0.86, p = 0.006) were less likely to experience a CVE. Presence of a new headache (OR 1.65, 95% CI 1.12–2.44, p = 0.01) was also more common in PSV with CVE. Traditional cardiovascular risk factors (arterial hypertension, diabetes, dyslipidaemia) were not associated with increased risk of CVE.
Data on inflammatory parameters were available in 69.8% of patients with PSV, and no differences were found between patients with or without CVE in median maximum values of serum C reactive protein (CRP) (36.5 [IQR 107.0] vs 46.6 [IQR 94.8 mg/L, p = 0.38) or erythrocyte sedimentation rate (52.5 [IQR 47.0] vs 59.0 [IQR 56.0] mm/hr, p = 0.30).
Description of demographic characteristics of each PSV is displayed in Online Resource 3. The occurrence of CVE was associated with the presence of new-onset headache (36.8% vs 16.5%, p = 0.04) and eye involvement (78.9% vs 53.8%, p = 0.03) in the Behçet’s subgroup, while in the PAN subgroup, only gastrointestinal involvement was associated with the occurrence of CVE (75.0% vs 43.6%, p = 0.03). Skin (21.1% vs 6.9%, p = 0.006) and eye (44.7% vs 13.9%, p < 0.001) involvement was more common and median VDI scores were higher (3.0 [IQR 4.0] vs 1.0 [IQR 2.0], p < 0.001) in patients with TAK with CVE compared to those without CVE. Hypertension was more common in patients with GCA and CVE (62.8% vs 45.7%, p = 0.03), CVE did not associate with any other organ involvement in GCA. Previous CVE (14.0% vs 3.3%, p = 0.004) was more common in TAK (7.9% vs 0.8%, p = 0.009) and GCA (14.0% vs 3.3%, p = 0.004) patients with CVE, compared to patients without.
In the patients with small-vessel PSV, the occurrence of CVE was associated with older median age (66.0 [IQR 8.0] vs 59.0 [23.0], p = 0.04), higher median CRP (115.0 [IQR 137.0] vs 55.0 [IQR 113.28] mg/L, p = 0.02), higher median VDI (3.0 [IQR 3.0] vs 2.0 [IQR 2.0], p < 0.001), hypertension (45.2% vs 26.1%, p = 0.005), Previous CVE (11.9% vs 1.7%, p < 0.001), and malignancy (14.3% vs 5.4%, p = 0.03). In the subgroup of patients with ANCA-positive EGPA, GPA, and MPA (n = 1561), similar results were observed except for malignancy, which was no longer significant (11.8% vs 5.6% p = 0.13).
In PSV most CVEs are ischemic and have a significant recurrence rate at 6-months
The Stroke sub-study CRF was filled out in 106 of 169 (63%) cases of PSV with reported CVE in the DCVAS database. Brain imaging reports were available in 96.2% of CRFs. Compared to cases with CVE without available data (n = 63), cases of CVE with available data in the Stroke CRF (n = 106) were older (median age 64.0 [IQR 33.0] vs 51.0 [IQR 35.0] years, p = 0.05), more frequently White (73.6% vs 54.0%, p = 0.009), less likely Asian (12.3% vs 30.2%, p = 0.004), and more frequently ever-smokers (47.1% vs 27.9%, p = 0.02). Diagnoses were also more frequently GCA (31.8% vs 14.3%, p = 0.008) and EGPA (9.3% vs 1.6%, p = 0.041) and less frequently TA (15.9% vs 33.3%, p = 0.008).
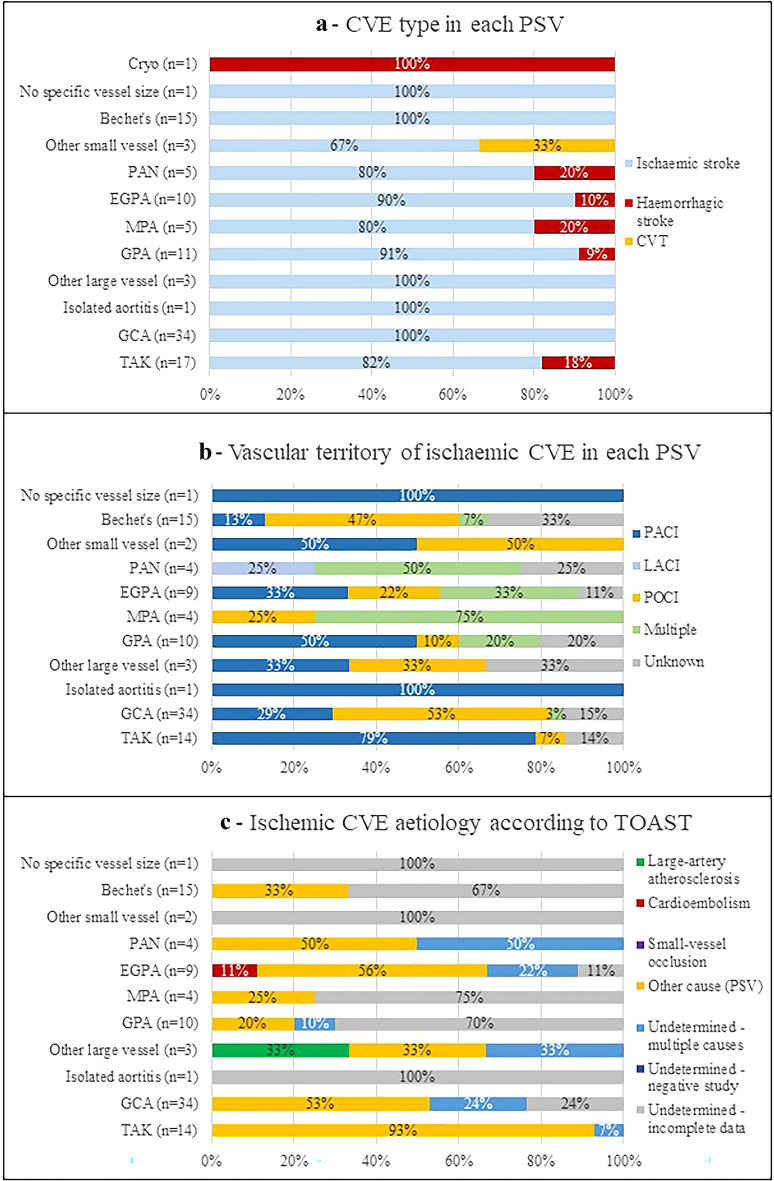
Most CVEs were ischaemic (n = 97; 91.5%), of which 49 (50.5%) TIA, and eight (7.5%) were haemorrhagic (parenchymal haematomas: 3 in TAK, 1 in EGPA, 1 in MPA; subarachnoid haemorrhage: 1 in GPA, 1 in cryoglobulinaemia; and 1 parenchymal haematoma with simultaneous subarachnoid haemorrhage in PAN). One (0.9%) cerebral venous thrombosis (CVT) was reported in a patient with ‘other small vessel PSV’ diagnosis (Fig. 2a).
Fig. 2.
Characteristics of cerebral vascular events in each form of primary systemic vasculitis. a Type of cerebral vasculitis events. Ischaemic CVE was more common than haemorrhagic stroke in most PSVs. b Vascular territory of involvement in ischaemic cerebral vasculitis events. c Aetiology of ischaemic cerebral vasculitis events. CVE was attributed to vasculitis and classified in ‘Other determined cause’ in several of the PSVs. In EGPA, two CVE were attributed to cardioembolism, one associated with vasculitis-related cardiac ulceration, and one associated to a non-PSV related cardiomyopathy. The cause of CVE was not possible to ascertain in 32% of all PSVs. CVE cerebral vasculitis events; Cryo cryoglobulinaemic vasculitis; PAN polyarteritis nodosa; EGPA eosinophilic granulomatosis with polyangiitis; MPA microscopic polyangiitis; GPA granulomatosis with polyangiitis; GCA giant cell arteritis; TAK Takayasu’s arteritis; CVT cerebral venous thrombosis; TACI total anterior circular infarction; PACI partial anterior circular infarction; LACI lacunar infarction; POCI posterior circulation infarction; PSV primary systemic vasculitis
Regarding ischaemic CVEs, anterior circulation ischaemia was present in 35 (36.1%) patients (particularly those with TAK), posterior circulation in 32 (32.7%) (especially in GCA), and multiple territories in 12 (12.2%) (distinctly in MPA, PAN and EGPA) (Fig. 2b). About half of ischaemic strokes (n = 47; 48.5%) were classified as ‘other determined aetiology’, all of which were attributed to PSV (Fig. 2c). Additionally, PSV was also one of the aetiologies in 11 of 15 patients with stroke attributed to ‘two or more causes’. Other causes of CVE identified simultaneously with PSV included cardioembolism (n = 5), large-vessel atherosclerosis (n = 2), small-vessel occlusion (n = 2), and infection (herpes encephalitis) (n = 1).
The cause of haemorrhagic CVE was at least partially attributed to PSV in three cases and, in four cases, to non-inflammatory cerebral small-vessel disease. No vascular malformations were described in patients with SAH. Three cases of asymptomatic cerebral aneurysms were reported: 2 in patients with related ischaemic stroke (GCA and Behçet’s), and 1 in a patient with TAK and hypertensive parenchymal haemorrhage (PH).
Information on treatment of ischaemic CVE was available in 92 cases: 63 (68.5%) cases were given aspirin and 8 (8.7%) two antiplatelet drugs. Eleven cases (12.0%) were given heparin and 10 (11.0%) oral anticoagulation. Regarding acute treatment of ischaemic stroke, only one case was treated with recombinant tissue plasminogen activator (rtPA) and one case with large vessel PSV was submitted to endovascular procedure (stenting).
In the Stroke sub-study, follow-up data regarding CVE recurrence data were available for 101 patients, and 12 (11.9%) had a recurrent CVE at 6-months follow-up: 6 (5.9%) stroke and 6 (5.9%) TIA. Regarding stroke disability, as measured by the mRankin score, data were available for 94 patients: 11 (11.7%) were dependent (score higher than 3), and five patients had died (mRankin 6) one due to 6-month CVE, 3 due to infection, and one of an unknown cause.
Most clinical features of ischaemic CVE were comparable between patients with and without PSV
An analysis was conducted to explore whether features of CVE in the PSV DCVAS cohort (n = 97) differed from those present in patients without PSV from the DCVAS ‘PSV mimics’ cohort (n = 45) (Fig. 1). ‘PSV mimics’ included a heterogenous group of disorders, from other neurological and rheumatological conditions to infectious diseases (Online Resource 4).
Cardiovascular risk factors, stroke locations, disability and treatment were not different between groups. ‘PSV mimics’ patients were younger (median 48.0 [IQR 21.0] vs 61.0 [IQR 34.0] years, p = 0.02), less affected by malignancy (2.2% vs 13.7%, p = 0.03), and had lower frequencies of chest (15.6% vs 35.8%, p = 0.01), ear–nose–throat (13.3% vs 33.7%, p = 0.008), and gynaecological/urological (4.4% vs 17.9%, p = 0.02) involvement than those with PSV. Patients with PSV mimics had a higher frequency of CVE due to cardioembolism than patients with PSV (8.9% vs 1.0%, p = 0.04). Six-month CVE cumulative incidence was also similar (PSV mimics 6.8% vs PSV 11.9% p = 0.27), as well as disability (dependent PSV mimics 5.4% vs dependent PSV 11.7%, p = 0.23).
Discussion
This study, based on data from the DCVAS cohort, has shown that CVE occurs in about 3.5% of patients with PSV at time of diagnosis. Rates of CVE varied among the different types of PSVs. Being an uncommon presentation, CVE may be challenging to diagnose and manage in patients with PSV. This study identified features that may help clinicians recognize CVE risk in PSV and showed that CVE in these patients is associated with significant disability and short-term recurrence.
Frequency of CVE varied according to PSV type. The highest frequencies were seen in Behçet’s and PAN, where previous limited data was available [40]. Interestingly, in Behçet’s disease, arterial ischaemic stroke was more common than CVT, a well-recognized neurological manifestation of this PSV [31], highlighting that these patients are also at risk of arterial ischaemic stroke as suggested before [41]. Regarding large vessel vasculitis, frequency of CVEs (3.6%) in GCA is similar to previous studies looking at stroke at the time of diagnosis [42, 43]. However, the TAK cohort had a lower frequency (6.0%) of CVE than what was previously reported [44], which could be explained by demographic differences, as the majority of the cases of TAK included in the DCVAS study were Asian, where lower rates of stroke have been described (1.2–1.3%) [44]. In the small-vessel vasculitis cohort CVE was less common, despite an expected higher risk of cardiovascular disease in this population [22, 24]. Cerebral small-vessel vasculitis may be more challenging to diagnose than the territorial infarcts associated with the large-vessel vasculitis, especially if detailed imaging (i.e. brain MRI) is not available. In the DCVAS EGPA cohort, CVE frequency (2.9%) was not much different compared to GCA, highlighting that the variability of CVE frequency across the different PSV is not explained by vessel type involvement alone, and should be recognized in all types of vasculitis.
The different types of PSV appear to have different predisposing factors for CVE occurrence. Overall and in patients with TAK, PAN, or Behçet’s disease, traditional vascular risk factors, such as smoking, arterial hypertension, diabetes, and dyslipidaemia, did not associate with the presence of CVE. On the other hand, a higher burden of vasculitis-related damage did associate with higher CVE risk, suggesting that CVE may be directly related to the vasculitic process. Additionally, in the small-vessel vasculitis group, although arterial hypertension associated with CVE, so did CRP, a marker of inflammation shown to be associated with cerebral small-vessel disease in MPO-ANCA-associated vasculitis at onset [9], again linking inflammation to cerebrovascular disease in these patients. Malignancy associated with a higher risk of CVE in our PSV patients and this association was not explained by the presence of concomitant vascular comorbidities, and the mechanisms of this association deserve to be further explored, especially in small-vessel vasculitis not associated with ANCA. We have confirmed and association between eye symptoms and CVE in the whole PSV cohort and this was likely driven by TAK and Behçet’s, highlighting the importance of ophthalmological assessment in patients with CVE and possible vasculitis [45].
Regarding the clinical features of stroke, ischaemic events were more frequent than haemorrhagic events, as expected. Large-vessel vasculitis associated with ischaemic territorial infarcts: in GCA most commonly affecting the posterior circulations and in TAK the anterior circulation, as previously described [42, 46, 47]. Recognizing these specific patterns of cerebrovascular involvement may be of help in differentiating GCA from TAK [47]. Stroke in small-vessel vasculitis presented with more heterogeneous features, frequently involving multiple vascular territories. During case review, aetiology was also more difficult to ascertain in these cases when compared to large-vessel vasculitis, because there was no obvious stenosis/occlusion or vascular wall enhancement as clear markers of a vasculitis-related mechanism of stroke. Again, these features may explain why it might be harder to recognize and classify stroke in these patients.
Finally, although direct vascular involvement is usually the easy go-to aetiology when assessing a PSV patient with stroke, our results also highlight that myocarditis/myocardiopathy needs to be considered as potential causes of ischemic stroke/TIA in PSV.
In exploratory comparisons, except for more widespread organ involvement, patients with PSV are not much different from the heterogenous group of the patients with mimics of PSV. Both PSV and PSV mimics have been reported to be more common in young patients with stroke than in the older population [4, 48].
The current study measured a 12% 6-month cumulative incidence of recurrent CVE, despite most patients being on at least one antiaggregant. Although this rate was not significantly different compared to PSV mimics (7%), this may be due to low sample size. We report a higher rate of recurrent events in PSV at 6 months than the rate reported at 12 months (3–6%) in a large study including patients of all ages [49] and of that reported at 5-years (9.4%) in young adults [50].
This study has several limitations. To seize the opportunity of studying a large group of patients with PSV, the Stroke substudy design was added to the main DCVAS study, to collect minimal additional information, and some design limitations could not be overcome (i.e. retrospective nature, time of scheduled visits). Because it was a large world-wide multicentre study, it was not possible to guarantee a standardized evaluation of CVE among all centres (i.e. previous medication; NIHSS not always available). However, this likely reflects real-world clinical practice where stroke work up is not uniform across centres [4]. To overcome this bias, all data on DCVAS study and Stroke CRF were reviewed by two neurologists to confirm the aetiologies of the CVEs. However, the investigators could not review the original imaging, and concomitant possible silent strokes or microvascular leukoencephalopathy could not be thoroughly assessed. Regarding the exploratory comparison with patients with CVE without vasculitis, the ‘PSV mimics’ group included a highly heterogenous group of diseases, which may halter direct comparisons. However, this is the main group of differential diagnosis of stroke associated with raised inflammatory markers or systemic involvement, and reflects clinical practice.
Despite its limitations, this study provides a comprehensive and unique insight into CVE in PSV. Its multicentric nature allowed the inclusion of a large number of patients with PSV and the estimation of frequencies of CVE. It also focuses on occurrence of CVE from symptom onset until diagnosis, compared to most previous studies that included patients only after diagnosis of PSV. This is important because stroke may be one of the first manifestations of PSV and understanding its clinical features may contribute to an early diagnosis of PSV. Moreover, CVEs at early stages of PSV are less likely to reflect side effects of long-term treatments such as glucocorticoid-related diabetes mellitus or infection. Though CVEs seem to be more frequent in PSV patients than in the general population, mainly occurring within the first years of PSV diagnosis [10, 51], lifelong stroke risk in PSV is not yet well-studied in prospective studies.
In conclusion, this study describes the prevalence and type of CVEs among patients with PSV. CVEs affect a minority of patients with PSV, but the frequency varies widely among different vasculitis. CVE in PSV is not explained by traditional vascular risk factors and has a moderate risk of short-term recurrence. These results have important implications to help identify patients with PSV who present with CVE and provide insight into the pathophysiology of CVEs in PSV.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Katherine Gibbons for data extraction from DCVAS database.
Funding
The Diagnostic & Classification Criteria in Vasculitis (DCVAS) study, which included the development of this classification criteria, was funded by grants from the American College of Rheumatology (ACR), the European Alliance of Associations for Rheumatology (EULAR), the Vasculitis Foundation, and the University of Pennsylvania Vasculitis Center.
Declarations
Conflicts of interest
R Geraldes reports no disclosures relevant to the manuscript. M Santos reports no disclosures relevant to the manuscript. C Ponte reports no disclosures relevant to the manuscript. A Craven reports no disclosures relevant to the manuscript. L Barra reports no disclosures relevant to the manuscript. JC Robson reports no disclosures relevant to the manuscript. N Hammam reports no disclosures relevant to the manuscript. J Springer no disclosures relevant to the manuscript. J Henes reports no disclosures relevant to the manuscript. A Hocevar reports no disclosures relevant to the manuscript. J Putaala reports no disclosures relevant to the manuscript. E Santos reports no disclosures relevant to the manuscript. L Rajasekhar reports no disclosures relevant to the manuscript. T Daikeler reports no disclosures relevant to the manuscript. O Karadag reports no disclosures relevant to the manuscript. A Costa reports no disclosures relevant to the manuscript. N Khalidi reports no disclosures relevant to the manuscript. C Pagnoux reports no disclosures relevant to the manuscript. P Canhão reports no disclosures relevant to the manuscript Teresa. P. Melo reports no disclosures relevant to the manuscript A C. Fonseca reports no disclosures relevant to the manuscript. J M. Ferro reports no disclosures relevant to the manuscript. J E. Fonseca reports no disclosures relevant to the manuscript. R Suppiah reports no disclosures relevant to the manuscript. R A. Watts reports no disclosures relevant to the manuscript. P Grayson reports no disclosures relevant to the manuscript. P A. Merkel reports no disclosures relevant to the manuscript. R A. Luqmani reports no disclosures relevant to the manuscript. R Watts disclosures: Editor in Chief Oxford Medical Case Reports—since 2012; Data monitoring committees; Chair DMEC for the BIOVASS trial funded by NIHR; Chair trial steering committee Combivas trial funded MRC and GSK; Royalties Oxford Textbook of Rheumatology Editor 4th edition published 2014.
Ethics
All human and animal studies have been approved by the appropriate ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. It should also be stated clearly in the text that all persons gave their informed consent prior to their inclusion in the study.
References
- 1.Boot E, Ekker MS, Putaala J, et al. Ischaemic stroke in young adults: a global perspective. J Neurol Neurosurg Psychiatry. 2020;91:411–417. doi: 10.1136/jnnp-2019-322424. [DOI] [PubMed] [Google Scholar]
- 2.Jacob MA, Ekker MS, Allach Y, et al. Global differences in risk factors, etiology, and outcome of ischemic stroke in young adults—a worldwide meta-analysis. Neurology. 2022;98:e573–e588. doi: 10.1212/wnl.0000000000013195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferro JM. Vasculitis of the central nervous system. Rev Prat. 1998;245:766–776. doi: 10.1007/s004150050285. [DOI] [PubMed] [Google Scholar]
- 4.Ferro JM, Massaro AR, Mas J-L. Aetiological diagnosis of ischaemic stroke in young adults. Lancet Neurol. 2010;9:1085–1096. doi: 10.1016/S1474-4422(10)70251-9. [DOI] [PubMed] [Google Scholar]
- 5.Goel R, Chandan JS, Thayakaran R, et al. Cardiovascular and renal morbidity in Takayasu arteritis: a population-based retrospective cohort study from the United Kingdom. Arthritis Rheumatol (Hoboken, NJ) 2021;73:504–511. doi: 10.1002/ART.41529. [DOI] [PubMed] [Google Scholar]
- 6.Robson JC, Kiran A, Maskell J, et al. Which patients with giant cell arteritis will develop cardiovascular or cerebrovascular disease? A clinical practice research datalink study. J Rheumatol. 2016;43:1085–1092. doi: 10.3899/jrheum.151024. [DOI] [PubMed] [Google Scholar]
- 7.Ungprasert P, Wijarnpreecha K, Koster MJ, et al. Cerebrovascular accident in patients with giant cell arteritis: a systematic review and meta-analysis of cohort studies. Semin Arthritis Rheum. 2016;46:361–366. doi: 10.1016/j.semarthrit.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 8.Houben E, Penne EL, Voskuyl AE, et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology (Oxford) 2018;57:555–562. doi: 10.1093/rheumatology/kex338. [DOI] [PubMed] [Google Scholar]
- 9.Tani H, Nagai K, Hosokawa T, et al. Occurrence of cerebral small vessel disease at diagnosis of MPO-ANCA-associated vasculitis. J Neurol. 2019;266:1708–1715. doi: 10.1007/s00415-019-09318-9. [DOI] [PubMed] [Google Scholar]
- 10.Berti A, Matteson EL, Crowson CS, et al. Risk of cardiovascular disease and venous thromboembolism among patients with incident ANCA-associated vasculitis: a 20-year population-based cohort study. Mayo Clin Proc. 2018;93:597–606. doi: 10.1016/j.mayocp.2018.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu C-Y, Yu H-S, Chai C-Y, et al. Increased ischemic stroke risk in patients with Behçet’s disease: a nationwide population-based cohort study. PLoS ONE. 2019;14:e0218652. doi: 10.1371/journal.pone.0218652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bond KM, Nasr D, Lehman V, et al. Intracranial and extracranial neurovascular manifestations of Takayasu arteritis. AJNR Am J Neuroradiol. 2017;38:766–772. doi: 10.3174/ajnr.A5095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Larivière D, Sacre K, Klein I, et al. Extra- and intracranial cerebral vasculitis in giant cell arteritis: an observational study. Medicine (Baltimore) 2014;93:e265. doi: 10.1097/MD.0000000000000265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Misra DP, Shenoy SN. Cardiac involvement in primary systemic vasculitis and potential drug therapies to reduce cardiovascular risk. Rheumatol Int. 2017;37:151–167. doi: 10.1007/S00296-016-3435-1. [DOI] [PubMed] [Google Scholar]
- 15.Clifford AH, Cohen Tervaert JW. Cardiovascular events and the role of accelerated atherosclerosis in systemic vasculitis. Atherosclerosis. 2021;325:8–15. doi: 10.1016/j.atherosclerosis.2021.03.032. [DOI] [PubMed] [Google Scholar]
- 16.Duarte MM, Geraldes R, Sousa R, et al. Stroke and transient ischemic attack in Takayasu’s arteritis: a systematic review and meta-analysis. J Stroke Cerebrovasc Dis. 2016;25:781–791. doi: 10.1016/J.JSTROKECEREBROVASDIS.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Ahn SS, Han M, Park Y-B, et al. Incidence, prevalence and risk of stroke in patients with Takayasu arteritis: a nationwide population-based study in South Korea. Stroke Vasc Neurol. 2021;7(2):149–157. doi: 10.1136/svn-2020-000809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ray JG, Mamdani MM, Geerts WH. Giant cell arteritis and cardiovascular disease in older adults. Heart. 2005;91:324–328. doi: 10.1136/HRT.2004.037481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pariente A, Guédon A, Alamowitch S, et al. Ischemic stroke in giant-cell arteritis: French retrospective study. J Autoimmun. 2019;99:48–51. doi: 10.1016/J.JAUT.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 20.Fauci AS, Haynes BF, Katz P, Wolff SM. Wegener’s granulomatosis: prospective clinical and therapeutic experience with 85 patients for 21 years. Ann Intern Med. 1983;98:76. doi: 10.7326/0003-4819-98-1-76. [DOI] [PubMed] [Google Scholar]
- 21.Sehgal M, Swanson JW, DeRemee RA, et al. Neurologic manifestations of Churg-Strauss syndrome. Mayo Clin Proc. 1995;70:337–341. doi: 10.4065/70.4.337. [DOI] [PubMed] [Google Scholar]
- 22.Ahn SS, Han M, Yoo J, et al. Risk of stroke in systemic necrotizing vasculitis: a nationwide study using the national claims database. Front Immunol. 2021;12:629902. doi: 10.3389/fimmu.2021.629902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aviña-Zubieta JA, Mai A, Amiri N, et al. Risk of myocardial infarction and stroke in patients with granulomatosis with polyangiitis (Wegener’s): a population-based study. Arthritis Rheumatol (Hoboken, NJ) 2016;68:2752–2759. doi: 10.1002/art.39762. [DOI] [PubMed] [Google Scholar]
- 24.Suppiah R, Judge A, Batra R, et al. A model to predict cardiovascular events in patients with newly diagnosed Wegener’s granulomatosis and microscopic polyangiitis. Arthritis Care Res (Hoboken) 2011;63:588–596. doi: 10.1002/acr.20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monti S, Craven A, Klersy C, et al. Association between age at disease onset of anti-neutrophil cytoplasmic antibody-associated vasculitis and clinical presentation and short-term outcomes. Rheumatology. 2021;60:617–628. doi: 10.1093/RHEUMATOLOGY/KEAA215. [DOI] [PubMed] [Google Scholar]
- 26.Yamada Y, Ando S, Umeda Y, et al. A case of multiple cerebral hemorrhage caused by sudden increase of eosinophil in a patient with eosinophilic granulomatosis with polyangiitis. Rinsho Shinkeigaku. 2018;58:565–569. doi: 10.5692/clinicalneurol.cn-001188. [DOI] [PubMed] [Google Scholar]
- 27.Oomura M, Yamawaki T, Naritomi H, et al. Polyarteritis nodosa in association with subarachnoid hemorrhage. Intern Med. 2006;45:655–658. doi: 10.2169/internalmedicine.45.1632. [DOI] [PubMed] [Google Scholar]
- 28.Isoda K, Nuri K, Shoda T, et al. Microscopic polyangiitis complicated with cerebral infarction and hemorrhage: a case report and review of literature. Japanese J Clin Immunol. 2010;33:111–115. doi: 10.2177/jsci.33.111. [DOI] [PubMed] [Google Scholar]
- 29.Hyun S-J, Hwang S-N, Nam T-K, et al. Takayasu’s arteritis complicated with subarachnoid hemorrhage and hematomyelia-case report. Neurol Med Chir (Tokyo) 2011;51:119–122. doi: 10.2176/nmc.51.119. [DOI] [PubMed] [Google Scholar]
- 30.de Nogueira RC, de Oliveira EF, Conforto AB, et al. Takayasu’s arteritis and cerebral venous thrombosis: comorbidity or coincidence? Arq Neuropsiquiatr. 2012;70:741–742. doi: 10.1590/s0004-282x2012000900017. [DOI] [PubMed] [Google Scholar]
- 31.De Sousa DA, Mestre T, Ferro JM. Cerebral venous thrombosis in Behçet’s disease: a systematic review. J Neurol. 2011;258:719–727. doi: 10.1007/S00415-010-5885-9. [DOI] [PubMed] [Google Scholar]
- 32.Craven A, Robson J, Ponte C, et al. ACR/EULAR-endorsed study to develop diagnostic and classification criteria for vasculitis (DCVAS) Clin Exp Nephrol. 2013;17:619–621. doi: 10.1007/s10157-013-0854-0. [DOI] [PubMed] [Google Scholar]
- 33.Bischof A, Jaeger VK, Hadden RDM, et al. Peripheral neuropathy in antineutrophil cytoplasmic antibody-associated vasculitides: Insights from the DCVAS study. Neurol Neuroimmunol Neuroinflamm. 2019 doi: 10.1212/NXI.0000000000000615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aho K, Harmsen P, Hatano S, et al. Cerebrovascular disease in the community: results of a WHO collaborative study. Bull World Health Organ. 1980;58:113–130. [PMC free article] [PubMed] [Google Scholar]
- 35.Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madden KP, Karanjia PN, Adams HP, et al. Accuracy of initial stroke subtype diagnosis in the TOAST study. Neurology. 1995;45:1975–1979. doi: 10.1212/WNL.45.11.1975. [DOI] [PubMed] [Google Scholar]
- 37.Bamford J, Sandercock P, Dennis M, et al. Classification and natural history of clinically identifiable subtypes of cerebral infarction. Lancet. 1991;337:1521–1526. doi: 10.1016/0140-6736(91)93206-O. [DOI] [PubMed] [Google Scholar]
- 38.Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the vasculitis damage index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40:371–380. doi: 10.1002/art.1780400222. [DOI] [PubMed] [Google Scholar]
- 39.Banks JL, Marotta CA. Outcomes validity and reliability of the modified Rankin scale: implications for stroke clinical trials—a literature review and synthesis. Stroke. 2007;38:1091–1096. doi: 10.1161/01.STR.0000258355.23810.c6. [DOI] [PubMed] [Google Scholar]
- 40.Berlit P, Krämer M. Consensus Group for the C. Cerebral involvement in systemic vasculitides: extracts from the guideline of the German neurological society. Neurol Res Pract. 2019 doi: 10.1186/s42466-019-0016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chuang K, Chang H. Risk of ischemic heart diseases and stroke in Behçet disease: a systematic review and meta-analysis. Eur J Clin Invest. 2022 doi: 10.1111/eci.13778. [DOI] [PubMed] [Google Scholar]
- 42.Gonzalez-Gay MA, Vazquez-Rodriguez TR, Gomez-Acebo I, et al. Strokes at time of disease diagnosis in a series of 287 patients with biopsy-proven giant cell arteritis. Medicine (Baltimore) 2009;88:227–235. doi: 10.1097/MD.0b013e3181af4518. [DOI] [PubMed] [Google Scholar]
- 43.Berger CT, Wolbers M, Meyer P, et al. High incidence of severe ischaemic complications in patients with giant cell arteritis irrespective of platelet count and size, and platelet inhibition. Rheumatology (Oxford) 2009;48:258–261. doi: 10.1093/rheumatology/ken480. [DOI] [PubMed] [Google Scholar]
- 44.Kim H, Barra L. Ischemic complications in Takayasu’s arteritis: a meta-analysis. Semin Arthritis Rheum. 2018;47:900–906. doi: 10.1016/j.semarthrit.2017.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Kong F, Huang X, Su L, et al. Risk factors for cerebral infarction in Takayasu arteritis: a single-centre case–control study. Rheumatology (Oxford) 2021;61:281–290. doi: 10.1093/rheumatology/keab308. [DOI] [PubMed] [Google Scholar]
- 46.Couture P, Chazal T, Rosso C, et al. Cerebrovascular events in Takayasu arteritis: a multicenter case-controlled study. J Neurol. 2018;265:757–763. doi: 10.1007/s00415-018-8744-8. [DOI] [PubMed] [Google Scholar]
- 47.Gribbons KB, Ponte C, Carette S, et al. Patterns of arterial disease in Takayasu arteritis and giant cell arteritis. Arthritis Care Res (Hoboken) 2020;72:1615–1624. doi: 10.1002/acr.24055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Putaala J. Ischemic stroke in young adults. Contin Lifelong Learn Neurol. 2020;26:386–414. doi: 10.1212/CON.0000000000000833. [DOI] [PubMed] [Google Scholar]
- 49.Flach C, Muruet W, Wolfe CDA, Bhalla A, Douiri A. Risk and secondary prevention of stroke. Stroke. 2020;51:2435–2444. doi: 10.1161/STROKEAHA.120.028992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Putaala J, Haapaniemi E, Metso AJ, et al. Recurrent ischemic events in young adults after first-ever ischemic stroke. Ann Neurol. 2010;68:661–671. doi: 10.1002/ANA.22091. [DOI] [PubMed] [Google Scholar]
- 51.De BH, Aouba A. An updated review of cardiovascular events in giant cell arteritis. J Clin Med. 2022;11:1005. doi: 10.3390/jcm11041005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the DCVAS study used for this study analyses are available from the DCVAS Steering Committee on reasonable request.