Abstract
Introduction
The prevalence of major and mild cognitive impairment (CI) in type-2 diabetes older patients is 15–25% and 30–60%, respectively, thus affecting quality of life and health outcomes. There is, therefore, the need of head-to-head studies aiming at identifying the optimal treatment for individuals with type-2 diabetes at increased risk of mild and major CI. This study focuses on the risk of developing mild and major CI in Danish patients treated with dipeptidyl peptidase-4 inhibitors (DPP-4i) and glucagon-like peptide-1 analogues (GLP-1a) using administrative and healthcare registers.
Methods
An active comparator design with a 3-year follow-up period was used. The main outcome was the hospital admission with a diagnosis of mild CI or major CI. Multivariate Cox Regression analysis was performed using the high-dimensional propensity score to obtain adjusted Hazard Ratio (HR) estimates. Inverse probability of treatment weighting (IPTW) and marginal structured model were used to calculate risk differences while accounting for the variations of confounders throughout the follow-up period.
Results
Our results show a significant higher risk of major CI between DPP-4i and GLP-1a in unadjusted [HR (95% CI) = 3.13 (2.45–4.00), p < 0.001] and adjusted analyses [HR (95% CI) = 1.58 (1.22–2.06), p = 0.001]. No statistically significant differences were observed for mild CI. IPTW resulted stable throughout the follow-up period. Marginal structure modeling (β (95% CI) = 0.022 (0.020–0.024), p < 0.001) resulted in a higher risk of major CI for DPP-4i when compared to GLP-1a.
Discussion
DPP-4i was associated with an increased risk of developing major CI when compared to GLP-1a among older individuals with type-2 diabetes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00415-024-12300-9.
Keywords: Glucagon-like peptide-1 analogues, Dipeptidyl peptidase-4 inhibitors, Major cognitive impairment, Mild cognitive impairment, Older individuals, Register-based study
Introduction
As the prevalence of type-2 diabetes continue to rise, there is a growing interest in understanding its impact on major and mild cognitive impairment (CI), especially among older individuals. CI poses a significant burden on older patients diagnosed with type-2 diabetes, substantially impacting their quality of life and overall health outcomes [1, 2]. Research suggests a complex interplay between diabetes-related factors, such as hyperglycaemia, insulin resistance, and chronic inflammation, contributing to cognitive decline. These impairments often manifest as deficits in memory, attention, processing speed, and executive function. Moreover, the presence of vascular complications exacerbates cognitive decline in diabetic patients. The multifaceted nature of this relationship underscores the urgent need for targeted interventions and comprehensive management strategies to alleviate the cognitive burden associated with type-2 diabetes, especially among older individuals, thereby enhancing the holistic well-being of affected individuals [1, 2].
Studies have shown that the prevalence of major CI in type-2 diabetes patients is approximately 15–25%, while the prevalence of mild CI is approximately 30–60%. This is not surprising considering that type-2 diabetes is a risk factor for both major and mild CI [2].
Recent literature emphasizes the necessity to optimize the pharmacological treatment in type-2 diabetes to effectively mitigate the risk of major and mild CI, particularly among individuals at an elevated risk of these clinical conditions. Specifically, there is a recognized need for head-to-head studies aiming to identify the optimal treatment for individuals with type-2 diabetes at an increased risk of major and mild CI [3].
Incretin-based treatments, which are widely used glucose-lowering drugs, have recently shown promise in improving cognitive functions among individuals with type-2 diabetes, positioning themselves as potential therapeutic agents for major CI [4, 5]. Notably, a recent meta-analysis of cohort studies revealed that dipeptidyl peptidase-4 inhibitors (DPP-4i) can significantly improve cognitive functions in patients with type-2 diabetes compared to other classes of glucose-lowering agents (i.e., sulfonylurea and metformin) [4]. In addition, glucagon-like peptide-1 analogues (GLP-1a) have garnered increased attention as pharmacological treatments capable of improving cognitive functions, supported by positive results from preclinical studies and some observational research [6, 7].
Furthermore, to the best of our knowledge, no head-to-head studies investigating the risks of major and mild CI have been conducted between DPP-4i and GLP-1a using real-world data.
This register-based study utilized the Danish Administrative and Healthcare Registers to compare the risk of a new diagnosis of major CI or mild CI among individuals aged 65 or older exposed to DPP-4i versus those exposed to GLP-1a.
Methods
Study design and data source
This registry-based cohort study utilized data from six Danish Administrative and Healthcare Registers, including the Danish Civil Registration System [8], the Danish National Patient Register [9], The Danish Register of Causes of Death [10], the Danish National Prescription Registry [11], the Population Education Registry [12], and the nationwide Register of Laboratory Results for Research [13]. Each Danish citizen, at birth or immigration, is assigned a personal civil registration (CPR) number, which serves as a unique identifier in national administrative databases. The CPR number enables access to comprehensive, virtually lifelong information on demographic characteristics, cause of death, claimed prescriptions at community pharmacies, hospital discharges, and laboratory measurements.
Study population
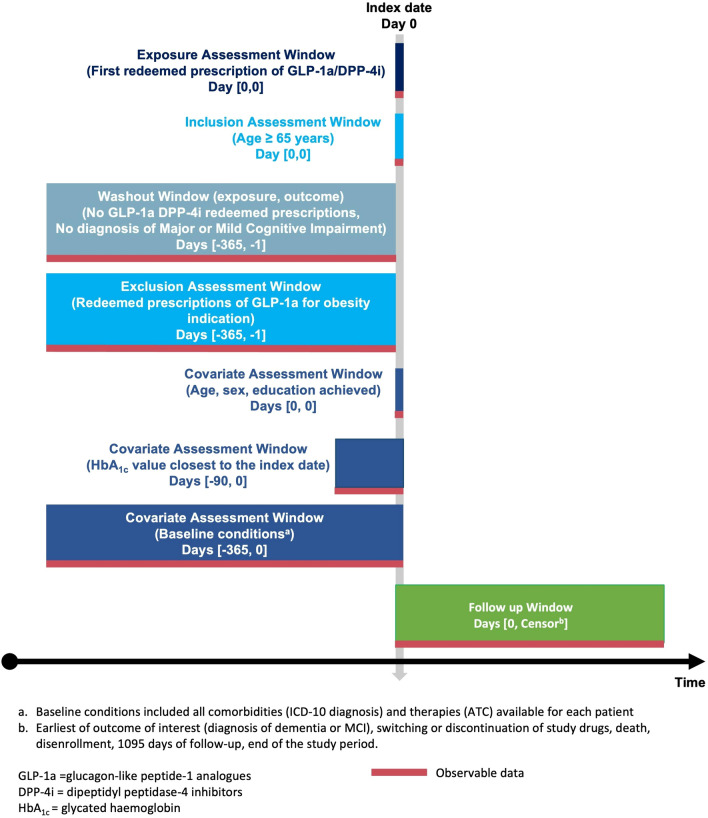
The source population encompassed all Danish citizens aged 65 years and older with type-2 diabetes between 2007 and 2018 [11]. From this source population, our study population was selected, consisting exclusively of individuals with at least one redeemed prescription for GLP-1a [Anatomical Therapeutic Chemical (ATC) system: A10BJ] or DPP-4i [ATC code: A10BH] for type-2 diabetes. Specifically, GLP-1a included exenatide (A10BJ01) and liraglutide (A10BJ02), while DPP-4i included sitagliptin (A10BH01), vildagliptin (A10BH02), saxagliptin (A10BH03), alogliptin (A10BH04), and linagliptin (A10BH05). The study population was tracked through Danish registries from the date of the first redeemed prescription for the drugs of interest, known as the index date (Fig. 1).
Fig. 1.
Study design diagram
The study population was divided into two cohorts: individuals treated with DPP-4i and those treated with GLP-1a.
Follow-up
Individuals in the two cohorts were followed for 3 years from the index date until the occurrence of the outcome or were censored in case of emigration, the end of follow-up, death, or the end of data coverage. Censoring also applied to individuals who switched from one treatment to the other. The period 2007–2018 for registered prescriptions was chosen to ensure a minimum of 3 years of follow-up for all included subjects. The decision for a 3-year follow-up period was based on a previous Danish study, which reported a median follow-up time of 3.5 years before the occurrence of a major CI diagnosis in Randomized Controlled Trials [14]. However, in registers, a distinction between those with or without major CI treated with GLP-1a was observed already after 2–3 years of treatment [14].
Outcomes
The main outcomes were hospital admission with a primary diagnosis of mild CI (ICD-10: G31.84, and F06.7) and hospital admission with a diagnosis of major CI, defined as a diagnosis of dementia in Alzheimer’s disease (ICD-10: F00), vascular dementia (ICD-10: F01), dementia in other diseases classified elsewhere (ICD-10: F02), unspecified dementia (ICD-10: F03), Alzheimer’s disease (ICD-10: G30), other degenerative diseases of the nervous system, not elsewhere classified (ICD-10: G31.0, G31.1, G31.83). Primary diagnoses were obtained from the Danish National Patient Register [9] and the mentioned ICD-10 codes were carefully checked used as guideline the Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition, Text Revision (DSM-5-TR) for major and mild neurocognitive disorders [15] and the recommended codes by Allan et al. [16]. The validity of major CI diagnoses in the Danish National Patient Register has been previously assessed and it was found to have positive predictive values more than 80% [17, 18].
Statistical analysis
A descriptive analysis was conducted for all the variables assessed at the index date, including age, sex, HbA1c, educational level, and comorbidities. HbA1c measurements were retrieved using the Nomenclature for Properties and Units code 27,300 from the nationwide Register of Laboratory Results for Research [13]. Educational level and comorbidities were retrieved from the Population Education Registry [12] and the Danish National Patient Register [9], respectively. The methods and results were reported following the HARmonized Protocol Template to Enhance Reproducibility (HARPER) guidelines [19] (Supplementary Table S1). Continuous variables were presented as mean and SD, while categorical variables were expressed as frequencies and percentages. Crude Cox Regression survival analysis was conducted to assess the Hazard Ratio (HR) with the corresponding 95% confidence interval (CI) for major and mild CI in the DPP-4i group compared to the GLP-1a group (intention-to-treat analysis).
Multivariate Cox regression analysis was performed using the high-dimensional propensity score (HDPS) to obtain adjusted HR estimates. The HDPS was calculated using all available information regarding prescription drugs and clinical discharges for each patient, following the methodology described by Shakibfar et al. [20–22]. The HDPS has demonstrated superiority over traditional adjustment approaches in pharmacoepidemiologic studies in a Nordic setting [20]. The positivity assumption of the HDPS was checked using a Love plot.
In all analyses, a p value <0.05 was considered statistically significant. Data management was performed using SAS, version 9.4 (SAS Institute Inc., Cary, NC, USA), and analyses were conducted using R, version 3.5.0.
Sensitivity analysis
To ensure statistically significant results in adjusted Cox regression, three sensitivity analyses were conducted:
The first sensitivity analysis aimed to address potential variations in the presence of confounders over the follow-up. The HDPS was recalculated annually during the follow-up, as outlined in paragraph 2.5. Boxplots illustrating the distribution of the HDPS over the follow-up years were employed to depict any changes. Subsequently, the inverse probability of treatment weighting (IPTW) [23] was computed and used in marginal structure models [24]. The computation of outcomes was determined as the risk difference for the outcomes.
The second sensitivity analysis was performed to account for misclassification bias of the outcomes. Specifically, we wanted to check if potential misclassifications in the registers regarding a diagnosis of major CI could affect our results. Correction of HR estimates and 95% CI for bias due to outcome misclassification was carried out according to the Brenner and Gefeller’s methodology [25]. The analysis was performed considering non-differential misclassification and a sensitivity of 71%, which has been previously been observed in validation studies of these diagnoses in the Danish National Patient Register [25].
The third sensitivity analysis involved including treatment discontinuation (measured with adherence) as an additional censoring criterion. Treatment discontinuation with DPP-4i and GLP-1a was assessed using adherence. Adherence was calculated through the medication possession ratio (MPR) [26]. The duration of each individual medical prescription was calculated by considering the defined daily doses (DDD) provided by the WHO ATC in 2023, the amount of DDD redeemed at each dispensing, and the recommended dosage based on therapeutic indications in Denmark provided by Medicin.dk [27]. A grace period of 90 days was permitted, and the grace period was also considered at the end of the treatment episode, following the approach described by Pazzagli et al. [28–30].
Ethics
In Denmark, every patient record/information is pseudonymized before analysis, eliminating the need for informed consent or ethical approval in registry-based studies. The University of Copenhagen, where the analysis occurred, and Statistics Denmark (project number 707278) hold utilization data approval from the Regional Capital Area Data Protection Agency.
Results
Baseline characteristics of the study population
The study encompassed a population of 36,115 individuals aged over 65 with type-2 diabetes [mean (±SD) = 69.7(±7.6) years] who had initiated treatment with either GLP-1a (n = 11,745; 32.5%) or DPP-4i (n = 24,370; 67.5%). Liraglutide was the most prescribed GLP-1a (n = 10,718; 91.3%), while sitagliptin held the highest prescription rate among DPP-4i (n = 16,961; 69.6%). Individuals treated with DPP-4i were older compared to those receiving GLP-1a (71 vs. 67 years), with a similar percentage of males in the GLP-1a group as in the DPP-4i group. Highly prevalent comorbidities included hypercholesterolemia (77.7%) and hypertension (72.7%) in the overall population. Hypercholesterolemia, hypertension, major depression, ischemic heart disease, obesity, infection, and COPD were more frequently observed in patients treated with GLP-1a, while cerebrovascular disease and schizophrenia were more prevalent in those treated with DPP-4i. Mean (±SD) HbA1c values were higher in individuals treated with GLP-1a compared to DPP-4i [67.9 (±16.5) mmol/mol vs. 62.1 (±16.1) mmol/mol]. Additional demographic and clinical characteristics of the patients included in the study are detailed in Table 1, and the analysed covariates are presented in Supplementary Figure S1.
Table 1.
Descriptive analysis of GLP-1a and DPP-4i users
| GLP-1a (n = 11,745) |
DPP-4i (n = 24,370) |
Total (n = 36,115) | |
|---|---|---|---|
| Mean age (± SD) | 67.2 (± 5.8) | 71.0 (± 8.1) | 69.7 (± 7.6) |
| HbA1c (mmol/mol)—mean (± SD) | 67.9 (± 16.5) | 62.1 (± 16.1) | 63.9 (± 16.3) |
| HbA1c—latest measurement prior to the index date—median (days) | 19 | 27 | 21 |
| Sex, n (%) | |||
| Males | 6898 (58.7) | 13,945 (57.2) | 20,843 (57.7) |
| Females | 4847 (41.3) | 10,425 (42.8) | 15,272 (42.3) |
| Drugs (ATC code), n (%) | |||
| Exenatide (A10BJ01) | 1027 (8.7) | 1027 (2.8) | |
| Liraglutide (A10BJ02) | 10,718 (91.3) | 10,718 (29.7) | |
| Sitagliptin (A10BH01) | 16,961 (69.6) | 16,961 (47.0) | |
| Vildagliptin (A10BH02) | 2915 (12.0) | 2915 (8.1) | |
| Saxagliptin (A10BH03) | 1536 (6.3) | 1536 (4.3) | |
| Alogliptin (A10BH04) | 228 (0.9) | 228 (0.6) | |
| Linagliptin (A10BH05) | 2730 (11.2) | 2730 (7.6) | |
| Educational level | |||
| No education | 47 (0.4) | 91 (0.4) | 138 (0.4) |
| Compulsory school and 10th grade | 4,733 (41.4) | 10,717 (45.7) | 15,450 (44.3) |
| Vocational education and training and adult education | 257 (2.2) | 576 (2.5) | 833 (2.4) |
| Upper secondary certificate (gymnasium) | 282 (2.5) | 658 (2.8) | 940 (2.7) |
| Academic profession degrees | 4187 (36.6) | 8004 (34.1) | 12,191 (34.9) |
| Bachelor and diploma degree | 1075 (14.9) | 2926(12.5) | 4631(13.3) |
| Candidatus and master’s degree | 159 (1.4) | 332 (1.4) | 491 (1.4) |
| PhD | 19 (0.2) | 30 (0.1) | 49 (0.1) |
| Other | 54 (0.5) | 126 (0.5) | 180 (0.5) |
| Comorbidities n (%) | |||
| Hypertension | 8982 (76.5) | 17,276 (70.9) | 26,258 (72.7) |
| Traumatic brain injury | 354 (3.0) | 753 (3.1) | 1107 (3.1) |
| Schizophrenia | 24 (0.2) | 81 (0.3) | 105 (0.3) |
| Major depression | 1826 (15.5) | 3478 (14.3) | 5304 (14.7) |
| Bipolar disorders | 28 (0.2) | 51 (0.2) | 79 (0.2) |
| Ischemic heart disease | 3530 (30.1) | 6518 (26.7) | 10,048 (27.8) |
| Cerebrovascular disease | 1274 (10.8) | 3281 (13.5) | 4555 (12.6) |
| Obesity | 3555 (30.3) | 6614 (14.8) | 7169 (19.9) |
| Hypercholesterolemia | 9624 (81.9) | 18,447 (75.7) | 28,071 (77.7) |
| Infection | 5854 (49.8) | 11,707 (48.0) | 17,561 (48.6) |
| COPD | 1980 (16.9) | 3850 (15.8) | 5830 (16.1) |
| Inflammatory disease | 1559 (13.3) | 3224 (13.2) | 4783 (13.2) |
| Alcohol use disorder | 377 (3.2) | 857 (3.5) | 1234(3.4) |
ATC Anatomical Therapeutic Chemical system code, COPD chronic obstructive pulmonary disease, DPP-4i dipeptidyl peptidase-4 inhibitors, GLP-1a glucagon-like peptide-1 analogues
Major and mild cognitive impairment
A total of 525 patients (1.5%) were registered with a diagnosis of major CI, while 79 patients (0.2%) were diagnosed with mild CI. Cox regression analysis revealed a significant difference for major CI between users of DPP-4i and GLP-1a in both crude [HR (95% CI) = 3.13 (2.45–4.00), p < 0.001] and adjusted analyses [HR (95% CI) = 1.58 (1.22–2.06), p = 0.001]. However, no statistically significant differences were observed for mild CI between GLP-1a and DPP-4i users in both crude [HR (95% CI) = 1.62 (0.97–2.71), p = 0.07] and adjusted analyses [HR (95% CI) = 1.32 (0.75–2.33), p = 0.34] (Table 2).
Table 2.
Univariate and multivariate cox regression for major and mild cognitive impairment
| Outcome | Drug classes | N. events (%) | Crude HR (95% CI) | Adjusted HR (95% CI) |
|---|---|---|---|---|
| Major cognitive impairment | GLP-1a (Ref. group) | 81 (0.7) | – | – |
| DPP-4i | 444 (1.8) | 3.13 (2.45–4.00) | 1.58 (1.22–2.06) | |
| Mild cognitive impairment | GLP-1a (Ref. group) | 25 (0.2) | – | – |
| DPP-4i | 54 (0.2) | 1.62 (0.97–2.71) | 1.32 (0.75–2.33) |
HR hazard ratio, CI confidence interval, DPP-4i dipeptidyl peptidase-4 inhibitors, GLP-1a glucagon-like peptide-1 analogues
Sensitivity analysis
In the first sensitivity analysis, IPTW was calculated, and its stability was maintained throughout the follow-up period (Fig. 2). Marginal structure modeling revealed a positive association for DPP-4i (β (95% CI) = 0.022 (0.020–0.024), p < 0.001) with major CI, indicating a higher risk for major CI in the cohort exposed to DPP-4i compared to those exposed to GLP-1a.
Fig. 2.
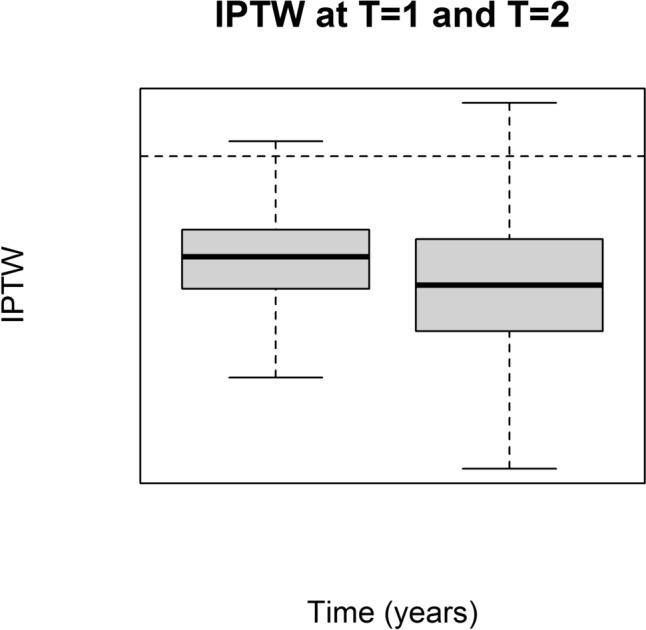
Inverse probability of treatment weighting (IPTW) after 1 and 2 years of follow-up
In the second sensitivity analysis, we observed no discernible impact of misclassification of the study outcome on the results of the main analysis (Supplementary Figure S2).
In the third sensitivity analysis, adherence rates for both drugs were high with MPR (95% CI) at 102 (98–114)% for DPP-4i and 82 (73–106)% for GLP-1a, respectively. Even when treatment discontinuation was considered as an additional censoring criterion, Cox Regression analysis for major CI remained significant both before [HR (95% CI) = 8.4 (5.73–12.30), p < 0.001] and after adjustment [HR (95% CI) = 4.03 (2.71–5.98), p = 0.002], highlighting a major risk associated with DPP-4i compared to GLP-1a.
Discussion
To the best of our knowledge, this is the first head-to-head study evaluating the impact of DPP-4i and GLP-1a on neurocognitive outcomes (i.e., major and mild CI) in older patients with type-2 diabetes, using real-world data. Our results show a statistically significant difference in major CI risk between DPP-4i and GLP-1a, both before and after adjustment, with notably higher risk associated with DPP-4i. Similar findings have been reported in existing scientific literature.
It should highlight that previous studies conducted in Denmark have observed a reduced risk of major CI among GLP-1a users when compared to non-users. Specifically, a nested case–control study encompassing all antidiabetic drugs in the Danish population showed a lower OR for major CI among individuals exposed to GLP-1a in comparison to those not exposed to pharmacological treatment for type-2 diabetes (OR: 0.58; 95% CI: 0.50–0.67) [31]. In another case–control study, each Danish patient exposed to GLP-1a exhibited the lowest risk of major CI compared to all other antidiabetic drugs combined (no head-to-head comparisons) [HR (95% CI) = 0.89 (0.86–0.93)] [14].
While we did not conduct mechanistic pharmacological studies, we speculated and hypothesized, drawing on evidence from pharmacological research, that the pharmacological action of DPP4-i and their inability to cross the blood–brain barrier offer a plausible explanation for differences in the risk of developing major and mild CI when compared to GLP-1a [6, 31, 32]. Specifically, GLP-1a can cross the blood–brain barrier, exhibiting pro-cognitive effects by improving neurogenesis, inflammation, oxidative stress, and cerebral glucose metabolism [31, 33, 34]. On the other hand, the DPP-4i linagliptin does not cross the blood–brain barrier and exhibits neuroprotective effects primarily attributed to peripheral functions through the inhibition of GLP-1 degradation [32]. Linagliptin has been proved to be a good substrate for P-glycoprotein, a factor that may significantly contribute to the drug’s efflux from the rat’s brain, despite its extensive distribution to other organs. The study also suggested that physiological alterations in Alzheimer’s disease could potentially result in reduced blood–brain barrier resistance, allowing for better penetration of the drug into the central nervous system [35, 36]. Another recent study found that omarigliptin, a novel once-weekly DPP-4i, can cross the blood–brain barrier due to its lipophilic properties [36]. The relationship between DPP-4i and cognitive function is complex and not fully understood. It may involve interactions with various factors such as age, disease duration, the presence of diabetic complications, and individual genetics. It is noteworthy that this protective effect of DPP-4i might be more pronounced in younger patients and those without diabetic complications [37]. In addition, we observed that individuals who had a redeemed prescription for GLP-1a had mean higher baseline HbA1c values measured closest to the index date, potentially underestimating the risk of major CI for DPP4-i, rather than overestimating it. This aligns with the findings of a previous study that showed an increased risk of major CI in type-2 diabetes patients with rising HbA1c (a proxy for poor glycaemic control) compared to those with lower levels [38].
IPTW consistently indicated a positive association between DPP-4i and major CI, reinforcing our primary findings. The robustness of our results was further supported by high adherence levels in both drug classes, as evidenced by MPR. Notably, patients with a redeemed prescription of DPP-4i exhibited better adherence, suggesting that differences in outcomes were not attributed to variations in medication compliance. Furthermore, even after including treatment discontinuation as an additional censoring criterion during the follow-up, Cox regression analysis continued to reveal a significant and substantial risk of major CI associated with DPP-4i exposure.
Strengths and limitations
The study’s strengths lie in its population-based approach and the extensive analysis of a large number of subjects encompassing the entire Danish population [39]. Furthermore, the use of rigorous statistical methods effectively minimized residual confounding [23, 24]. However, the study has its limitations. The number of patients experiencing the outcomes was relatively low, possibly due to potential misclassification of major and mild CI, especially for non-severe cases that did not require hospitalization. In cases where the disease is mild and managed in outpatient settings by healthcare professionals, such diagnoses may not be recorded in the National Patient Register. In addition, the available data only included diagnoses based on clinical admissions for major and mild CI, often established in outpatient clinics. It is essential to acknowledge that while the validation study conducted by Phung et al. [17] demonstrated higher validity in diagnosing major CI, it may not fully capture the complexities inherent in real-world diagnostic scenarios and their associated validity.
Conclusion
The use of DPP-4i was associated with a higher risk of developing major CI in comparison to GLP-1a users among older individuals with type-2 diabetes during the initial 3 years of treatment in Denmark from 2007 to 2018. Future research is necessary to validate these results and delve into the underlying mechanisms. In addition, it should more thoroughly evaluate the clinical relevance of these findings for older patients with type-2 diabetes.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
VB, MAB, and MS conceived and designed the study. VB, MAB, and MS designed and supervised the research and analyzed the data. VB wrote the original draft and MAB, CC, and MS reviewed and edited with inputs from all the authors. VB, MAB, CC, ES, EC, and MS participated in the interpretation of data, revised, and approved the final article as submitted.
Funding
Open access funding provided by Copenhagen University.
Data availability
Data are stored on secure servers on Statistics Denmark and cannot be shared according to Statistics Denmark regulations. Access to Statistics Denmark servers and the associated data can be granted by Statistics Denmark upon adequate permissions.
Declarations
Conflicts of interest
All the authors declare that there is no conflict of interest.
References
- 1.Aderinto N, Olatunji G, Abdulbasit M, et al. The impact of diabetes in cognitive impairment: a review of current evidence and prospects for future investigations. Medicine. 2023;102:e35557. doi: 10.1097/MD.0000000000035557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srikanth V, Sinclair AJ, Hill-Briggs F, et al. Type 2 diabetes and cognitive dysfunction—towards effective management of both comorbidities. Lancet Diabetes Endocrinol. 2020;8:535–545. doi: 10.1016/S2213-8587(20)30118-2. [DOI] [PubMed] [Google Scholar]
- 3.Wu C-Y, Shapiro L, Ouk M, et al. Glucose-lowering drugs, cognition, and dementia: the clinical evidence. Neurosci Biobehav Rev. 2022;137:104654. doi: 10.1016/j.neubiorev.2022.104654. [DOI] [PubMed] [Google Scholar]
- 4.Chai S, Liu F, Yu S, et al. Cognitive protection of incretin-based therapies in patients with type 2 diabetes mellitus: a systematic review and meta-analysis based on clinical studies. J Diabetes Investig. 2023;14:864–873. doi: 10.1111/jdi.14015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nowell J, Blunt E, Edison P. Incretin and insulin signaling as novel therapeutic targets for Alzheimer’s and Parkinson’s disease. Mol Psychiatry. 2023;28:217–229. doi: 10.1038/s41380-022-01792-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tang H, Shao H, Shaaban CE, et al. Newer glucose-lowering drugs and risk of dementia: a systematic review and meta-analysis of observational studies. J Am Geriatr Soc. 2023;71:2096–2106. doi: 10.1111/JGS.18306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mantik KEK, Kim S, Gu B, et al. Repositioning of anti-diabetic drugs against dementia: insight from molecular perspectives to clinical trials. Int J Mol Sci. 2023;24:11450. doi: 10.3390/ijms241411450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pedersen CB. The Danish civil registration system. Scand J Public Health. 2011;39:22–25. doi: 10.1177/1403494810387965. [DOI] [PubMed] [Google Scholar]
- 9.Schmidt M, Schmidt SAJ, Sandegaard JL, et al. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449. doi: 10.2147/CLEP.S91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Helweg-Larsen K. The Danish register of causes of death. Scand J Public Health. 2011;39:26–29. doi: 10.1177/1403494811399958. [DOI] [PubMed] [Google Scholar]
- 11.Wallach Kildemoes H, Toft Sørensen H, Hallas J. The Danish national prescription registry. Scand J Public Health. 2011;39:38–41. doi: 10.1177/1403494810394717. [DOI] [PubMed] [Google Scholar]
- 12.Jensen VM, Rasmussen AW. Danish education registers. Scand J Public Health. 2011;39:91–94. doi: 10.1177/1403494810394715. [DOI] [PubMed] [Google Scholar]
- 13.Arendt JFH, Hansen AT, Ladefoged SA, et al. Existing data sources in clinical epidemiology: laboratory information system databases in Denmark. Clin Epidemiol. 2020;12:469–475. doi: 10.2147/CLEP.S245060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nørgaard CH, Friedrich S, Hansen CT, et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimers Dement (N Y) 2022;8:e12268. doi: 10.1002/trc2.12268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. Washington, DC: American Psychiatric Association; 2022. [Google Scholar]
- 16.Allan LM, Wheatley A, Smith A et al (2019) Read codes for general practitioner dementia quality outcomes framework register. https://www.ncbi.nlm.nih.gov/books/NBK549015/. Accessed 26 Feb 2024
- 17.Phung TKT, Andersen BB, Høgh P, et al. Validity of dementia diagnoses in the Danish hospital registers. Dement Geriatr Cogn Disord. 2007;24:220–228. doi: 10.1159/000107084. [DOI] [PubMed] [Google Scholar]
- 18.Thygesen SK, Christiansen CF, Christensen S, et al. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish national registry of patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang SV, Pottegård A, Crown W, et al. HARmonized Protocol Template to Enhance Reproducibility of hypothesis evaluating real-world evidence studies on treatment effects: a good practices report of a joint ISPE/ISPOR task force. Pharmacoepidemiol Drug Saf. 2023;32:44–55. doi: 10.1002/pds.5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallas J, Pottegård A. Performance of the high-dimensional propensity score in a Nordic healthcare model. Basic Clin Pharmacol Toxicol. 2017;120:312–317. doi: 10.1111/bcpt.12716. [DOI] [PubMed] [Google Scholar]
- 21.Rassen JA, Blin P, Kloss S, et al. High-dimensional propensity scores for empirical covariate selection in secondary database studies: planning, implementation, and reporting. Pharmacoepidemiol Drug Saf. 2023;32:93–106. doi: 10.1002/pds.5566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shakibfar S, Andersen M, Sessa M. AI-based disease risk score for community-acquired pneumonia hospitalization. iScience. 2023;26:107027. doi: 10.1016/j.isci.2023.107027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chesnaye NC, Stel VS, Tripepi G, et al. An introduction to inverse probability of treatment weighting in observational research. Clin Kidney J. 2022;15:14–20. doi: 10.1093/ckj/sfab158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168:656–664. doi: 10.1093/aje/kwn164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brenner H, Gefeller O. Use of the positive predictive value to correct for disease misclassification in epidemiologic studies. Am J Epidemiol. 1993;138:1007–1015. doi: 10.1093/oxfordjournals.aje.a116805. [DOI] [PubMed] [Google Scholar]
- 26.Kim D, Cha J. Association between medical complications according to continuity of care and medication adherence in patients with hypertension in Korea: a national population-based cohort study. BMJ Open. 2023;13:e073404. doi: 10.1136/bmjopen-2023-073404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.pro.medicin.dk—information om medicin. https://pro.medicin.dk/. Accessed 26 Feb 2024
- 28.Pazzagli L, Andersen M, Sessa M. Pharmacological and epidemiological considerations while constructing treatment episodes using observational data: a simulation study. Pharmacoepidemiol Drug Saf. 2022;31:55–60. doi: 10.1002/pds.5366. [DOI] [PubMed] [Google Scholar]
- 29.Pazzagli L, Liang D, Andersen M, et al. Rationale and performances of a data-driven method for computing the duration of pharmacological prescriptions using secondary data sources. Sci Rep. 2022;12:6245. doi: 10.1038/s41598-022-10144-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meaidi M, Støvring H, Rostgaard K, et al. Pharmacoepidemiological methods for computing the duration of pharmacological prescriptions using secondary data sources. Eur J Clin Pharmacol. 2021;77:1805–1814. doi: 10.1007/s00228-021-03188-9. [DOI] [PubMed] [Google Scholar]
- 31.Wium-Andersen IK, Osler M, Jørgensen MB, et al. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: a nested case–control study. Eur J Endocrinol. 2019;181:499–507. doi: 10.1530/EJE-19-0259. [DOI] [PubMed] [Google Scholar]
- 32.Mousa S, Ayoub B. Repositioning of dipeptidyl peptidase-4 inhibitors and glucagon like peptide-1 agonists as potential neuroprotective agents. Neural Regen Res. 2019;14:745. doi: 10.4103/1673-5374.249217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Athauda D, Foltynie T. The glucagon-like peptide 1 (GLP) receptor as a therapeutic target in Parkinson’s disease: mechanisms of action. Drug Discov Today. 2016;21:802–818. doi: 10.1016/j.drudis.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 34.Hunter K, Hölscher C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012;13:1–6. doi: 10.1186/1471-2202-13-33/FIGURES/2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Srinivas NR. Linagliptin-role in the reversal of Aβ-mediated impairment of insulin signaling and reduced neurotoxicity in AD pathogenesis: some considerations. CNS Neurosci Ther. 2015;21:962–963. doi: 10.1111/cns.12475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ayoub BM, Mowaka S, Safar MM, et al. Repositioning of Omarigliptin as a once-weekly intranasal anti-parkinsonian agent. Sci Rep. 2018;8:8959. doi: 10.1038/s41598-018-27395-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen K-C, Chung C-H, Lu C-H, et al. Association between the use of dipeptidyl peptidase 4 inhibitors and the risk of dementia among patients with type 2 diabetes in Taiwan. J Clin Med. 2020;9:660. doi: 10.3390/jcm9030660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cho S, Ok Kim C, Cha B, et al. The effects of long-term cumulative HbA1c exposure on the development and onset time of dementia in the patients with type 2 diabetes mellitus: hospitalbased retrospective study (2005–2021) Diabetes Res Clin Pract. 2023;201:110721. doi: 10.1016/j.diabres.2023.110721. [DOI] [PubMed] [Google Scholar]
- 39.Wang W, Battini V, Carnovale C, et al. A novel approach for pharmacological substantiation of safety signals using plasma concentrations of medication and administrative/healthcare databases: a case study using Danish registries for an FDA warning on lamotrigine. Pharmacol Res. 2023;193:106811. doi: 10.1016/j.phrs.2023.106811. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are stored on secure servers on Statistics Denmark and cannot be shared according to Statistics Denmark regulations. Access to Statistics Denmark servers and the associated data can be granted by Statistics Denmark upon adequate permissions.