Abstract
Introduction
This study aimed to analyze the association between baseline National Institutes of Health Stroke Scale (NIHSS) scores and clinical outcomes in patients with large core infarctions undergoing endovascular treatment (EVT), a relationship that remains unclear.
Methods
Data were obtained from the MAGIC study, a prospective multicenter cohort study focusing on patients with acute large core ischemic stroke. This analysis evaluated the impact of NIHSS scores on EVT outcomes in patients with large core infarctions. Primary outcome metrics included favorable outcomes (modified Rankin Scale [mRS] of 0–3 at 90 days), while secondary outcomes encompassed shifts in mRS scores, functional independence (mRS score of 0–2), mRS score of 0–4, and successful recanalization rates. Adverse events considered were symptomatic intracranial hemorrhage (sICH) and mortality.
Results
A total of 490 patients were enrolled in this study. Higher baseline NIHSS scores were inversely correlated with favorable outcomes (adjusted odds ratio [OR] in model 3, 0.848 [0.797–0.903], P < 0.001), particularly in patients with NIHSS scores above 20 (adjusted OR in model 3, 0.518 [0.306–0.878] vs. 0.290 [0.161–0.523]). Regarding adverse events, higher baseline NIHSS scores significantly correlated with increased 90-day mortality rates (adjusted OR in model 3, 1.129 [1.072–1.189], P < 0.001). This correlation became insignificant when baseline NIHSS scores exceeded 22. Additionally, baseline NIHSS scores partially mediated the association between age (indirect effect = − 0.0005, 19.39% mediated) and sex (indirect effect = 0.0457, 25.08% mediated) with the primary outcome.
Conclusions
The findings indicate that higher baseline NIHSS scores correlate with poorer outcomes and increased mortality, particularly when scores exceed 20. Moreover, age and sex indirectly influence favorable outcomes through their association with baseline NIHSS scores.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40120-024-00588-8.
Keywords: Endovascular therapy, National Institutes of Health Stroke Scale score, Large core infarctions
Key Summary Points
| Why this study was conducted? |
| A few randomized clinical trials have shown that patients with large cerebral infarction had better functional outcomes with EVT than with medical care. |
| We hypothesize that there is a correlation between NIHSS score and prognosis of patients with large core infarction. |
| What was learned from this study? |
| Patients with a higher baseline NIHSS score are less likely to have a favorable outcome, especially in those with NIHSS scores greater than 20. |
| Age and sex indirectly affect favorable outcomes through their association with baseline NIHSS score. |
Introduction
Endovascular therapy (EVT) has been shown to significantly improve prognosis and reduce mortality rates compared to standard drug therapy in cases of anterior circulation large vessel occlusion with small core infarctions and large ischemic penumbra [1–4]. However, the effectiveness of EVT in patients with large core infarctions remains controversial. Recent evidence from five randomized clinical trials indicates that patients with large cerebral infarction achieve better functional outcomes with EVT than with medical care alone [5–9]. Despite these favorable outcomes in the EVT group, less than 30% of patients achieved a 90-day modified Rankin Scale (mRS) score of 0–2, and the 90-day mortality rate remained above 18%, even with a high rate of successful revascularization [5–9]. These findings suggest that factors such as ischemic core volume, collateral circulation, and severity of neurological impairment may influence the outcomes of large core infarctions following EVT.
The National Institutes of Health Stroke Scale (NIHSS) is a widely used 15-item impairment scale for assessing stroke severity, recommended by the National Stroke Foundation guideline as a valid tool for emergency department evaluations [10]. The literature suggests that the NIHSS score is independently associated with the functional prognosis of patients with acute ischemic stroke receiving EVT [11]. Furthermore, a combination of NIHSS consciousness scores and ASPECTS has been identified as a favorable predictor of functional independence [12]. However, the relationship between NIHSS scores and clinical outcomes in patients with large core infarctions undergoing EVT remains unclear. This study aimed to analyze this association, focusing on baseline NIHSS scores and their impact on the clinical outcomes of patients with large core infarctions undergoing EVT.
Methods
Study Design and Patient Populations
The data for this study was obtained from the MAGIC registry, a program focusing on patients with acute large core ischemic strokes undergoing EVT in real-world clinical settings. MAGIC involved 38 stroke centers across 12 provinces in China, each performing a minimum of 15 thrombectomy procedures annually from December 2021 to February 2023. There are 3 centers with an annual thrombectomy volume of more than 300 cases, 9 centers with more than 200 cases, 16 centers with more than 100 cases, and 10 centers with 50 to 100 cases. Moreover, most of the centers participating in the study are national advanced stroke centers. In addition, our country has a large population base. In our study, a total of 98 patients were included from the Xin Qiao Hospital, and affiliated Qujing Hospital of Kunming Medical University included 80 patients. The study adhered to the Declaration of Helsinki, received ethics committee approval from all participating centers, and patient consent was duly obtained.
The eligibility criteria for patient inclusion were (1) age 18 years or older; (2) acute ischemic stroke due to large vessel occlusion in the anterior circulation, specifically in the internal carotid artery or M1/M2 segments of the middle cerebral artery; (3) a pre-stroke mRS score of 0 to 2 (the scale ranges from 0 to 6, with higher scores indicating greater disability and a score of 6 indicating death); (4) an Alberta Stroke Program Early CT Score (ASPECTS) of 0 to 5 based on non-contrast CT scans performed within 24 h of stroke onset (defined as the time the patient was last known to be well).
Patients were excluded if they had (1) a pre-stroke mRS score greater than 2; (2) insufficient follow-up information regarding 90-day outcomes; (3) serious or terminal illness.
Of the 745 patients registered with large infarctions, 255 in the standard medical treatment (SMT) group were excluded, resulting in the inclusion of 490 patients in the EVT group upon admission (eFig. 1).
Data Collection
The study collected a comprehensive range of data, including demographic information, vascular risk factors (such as hypertension, hyperlipidemia, diabetes mellitus, and smoking), NIHSS scores at admission, ASPECTS, time metrics, and clinical outcomes. Symptomatic intracerebral hemorrhage (sICH) was classified according to the Heidelberg bleeding classification [13]. Neuroimaging data, crucial for the study, were analyzed by experienced neurologists of an independent core laboratory who were blinded to both clinical outcomes and imaging data. In instances of disagreement regarding patient data, a final evaluation was reached through a consensus among two experienced vascular neurologists and one neuroradiologist. Study participants were separated on the basis of baseline NIHSS tertile: a low NIHSS group (NIHSS scores ranging from 10 to 14), a medium NIHSS group (NIHSS scores ranging from 15 to 19), and a high NIHSS group (NIHSS scores of ≥ 20).
Outcome Measurement
The primary outcome of the study was defined as achieving a mRS score between 0 and 3 at 90 days post-treatment, indicating a favorable outcome. The mRS is a categorical scale used for assessing disability in patients with stroke, ranging from 0 (no symptoms) to 6 (death). Assessments were conducted by neurologists trained in mRS evaluation and blinded to treatment group allocations. Follow-up was scheduled for 90 ± 7 days after treatment, with options for in-person hospital visits or remote assessments via telephone or video call for those unable to attend in person. Secondary outcomes were diverse and included shifts in mRS scores across its full range, achievement of functional independence (mRS score of 0 to 2), attainment of an mRS score between 0 to 4, and the rate of successful recanalization. Successful recanalization was quantified using the modified Thrombolysis in Cerebral Infarction (mTICI) score of 2b to 3, which ranges from 0 (no reperfusion) to 3 (complete recanalization), based on angiographic evidence [14].
Adverse events monitored in the study included sICH, classified according to the Heidelberg bleeding classification, and mortality within 90 days post-treatment. These measures provided comprehensive insights into the outcomes and potential risks associated with the treatment.
Statistical Analysis
In the statistical analysis of the study, categorical and binary variables were compared using the chi-square (χ2) test or Fisher’s exact test as appropriate. For continuous variables that did not follow a normal distribution, the Mann–Whitney U test and the Kruskal–Wallis test were used.
Baseline characteristics and outcomes were presented in the study as follows: continuous and ordinal variables were reported as interquartile ranges (IQR), while categorical variables were expressed as percentages. For the analysis of binary outcomes and shifts in the mRS scores, multivariable binary logistic regression was employed. The results from these logistic regression analyses were presented as odds ratios (OR) and beta coefficients with 95% confidence intervals (CI). A methodical approach to variable selection was applied in the selection of covariates [15].
To control for confounding factors that might affect outcomes, three regression models were developed. Model 1 included age, medical history (including hypertension, hyperlipidemia, diabetes, and atrial fibrillation), baseline ASPECTS, occlusion site, and onset-to-puncture time. Model 2 added several clinically relevant variables to model 1, including etiologic classifications of ischemic stroke, American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral score (ASITN/SIR), intravenous thrombolysis, systolic and diastolic blood pressure, and smoking. Model 3 further included sex, onset-to-imaging, and onset-to-recanalization time.
The study employed restricted cubic splines analysis with four knots to explore the relationship between baseline NIHSS scores and adverse events. The nonlinearity of this association was assessed using the Wald-χ2 test. Additionally, curves demonstrating the association between baseline NIHSS scores and the percentage of 90-day mortality were constructed. For the key statistical analyses, R software version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria), SPSS statistical software version 27 (IBM Corp., Armonk, NY, USA), and GraphPad Prism version 8 (GraphPad Software, La Jolla, CA, USA) were used. All tests were two-sided and considered statistically significant at a P value < 0.05.
Results
Patient Characteristics
The study included 490 patients from the registry who were eligible for EVT treatment. Of these, 42.7% were female. The median age of the patients was 69 years (interquartile range [IQR] 59–78 years). Key time metrics included a median imaging time of 292.50 min (IQR 157.75–459.00 min), puncture time of 362.00 min (IQR 240.00–547.00 min), and recanalization time of 449.50 min (IQR 326.00–657.25 min). The median baseline NIHSS score was 17 (IQR 14.00–20.00). Successful reperfusion was achieved in 423 patients, accounting for 86.3% of the cohort. The methods of EVT varied, with 251 patients (51.2%) undergoing aspiration, 109 (22.2%) receiving SWIM (Solitaire stent With Intracranial support catheter for Mechanical thrombectomy), 87 (17.8%) undergoing stent thrombectomy, 25 (5.1%) receiving balloon treatment, 5 (1.0%) treated with intra-arterial tirofiban, 3 (0.6%) undergoing mechanical fragmentation, 3 (0.6%) receiving stenting, and 3 (0.6%) missing data on the method of EVT. Additionally, 2 patients (0.4%) underwent intra-arterial thrombolysis, 1 patient (0.2%) experienced spontaneous reperfusion, and 1 patient (0.2%) underwent both mechanical fragmentation and intra-arterial thrombolysis.
Vascular risk factors varied among groups defined by baseline NIHSS scores. Patients in the low and medium NIHSS groups had higher rates of smoking and hyperlipidemia, while those in the low and high NIHSS groups more frequently had hypertension and diabetes. The low NIHSS group exhibited a lower incidence of atrial fibrillation compared to the medium and high NIHSS groups. In terms of stroke etiology, large-artery atherosclerosis-related strokes were more common in the low and medium NIHSS groups, whereas cardioembolic strokes predominated in the high NIHSS group. Detailed patient characteristics are presented in Table 1.
Table 1.
Baseline patient characteristics by categorical baseline NIHSS
| Characteristic | All (n = 490) | Low NIHSS group (n = 152) | Medium NIHSS group (n = 174) | High NIHSS group (n = 164) | χ2/H | P value |
|---|---|---|---|---|---|---|
| Age, median (IQR), years | 69 (59–78) | 67.00 (57.25–76.00) | 67 (57–77) | 73 (64–79) | H = 11.574 | 0.003 |
| ≥ 65 | 250/490 (51.0) | 70/152 (46.1) | 79/174 (45.4) | 101/164 (61.6) | χ2 = 6.970 | 0.031 |
| Sex, frequency, (%) | ||||||
| Female | 209/490 (42.7) | 59/152 (38.8) | 66/174 (37.9) | 84/164 (51.2) | χ2 = 7.421 | 0.024 |
| Male | 281/490 (57.3) | 93/152 (61.2) | 108/174 (62.1) | 80/164 (48.8) | ||
| SBP, median (IQR), mmHg | 146.00 (128.00–164.00) | 145.00 (128.00–160.50) | 144.00 (125.00–160.00) | 149.00 (130.00–170.00) | H = 6.246 | 0.044 |
| DBP, median (IQR), mmHg | 86.00 (75.00–96.00) | 86.00 (77.00–93.00) | 85.00 (73.00–97.25) | 88.00 (78.00–100.00) | H = 4.734 | 0.094 |
| Baseline ASPECTS, median (IQR) | 4 (2–5) | 4 (3–4) | 4 (2–5) | 3 (2–5) | H = 14.518 | < 0.001 |
| Medical history, frequency (%) | ||||||
| Smoking, current or past | 151/490 (30.8) | 51/152 (33.6) | 55/174 (31.6) | 45/164 (27.4) | χ2 = 1.463 | 0.481 |
| Hypertension | 297/490 (60.6) | 98/152 (64.5) | 95/174 (54,6) | 104/64 (63.4) | χ2 = 4.125 | 0.127 |
| Hyperlipidemia | 106/490 (21.6) | 43/152 (28.3) | 37/174 (21.3) | 26/164 (15.9) | χ2 = 7.218 | 0.027 |
| Diabetes | 73/490 (14.9) | 22/152 (14.5) | 24/174 (13.8) | 27/164 (16.5) | χ2 = 0.506 | 0.776 |
| Atrial fibrillation | 221/490 (45.1) | 62/152 (40.8) | 72/174 (41.4) | 87/164 (53.0) | χ2 = 6.298 | 0.043 |
| Occlusion site, frequency (%) | ||||||
| ICA | 206/490 (42.0) | 51/152 (33.6) | 71/174 (40.8) | 84/164 (51.2) | χ2 = 10.581 | 0.032 |
| M1 | 233/490 (47.2) | 84/152 (55.3) | 85/174 (48.9) | 64/164 (39.0) | ||
| M2 | 51/490 (10.4) | 17/152 (11.2) | 18/174 (10.3) | 16/164 (9.8) | ||
| Toast, frequency (%) | ||||||
| Large artery atherosclerosis | 146/490 (29.8) | 54/152 (35.5) | 54/174 (31.0) | 38/164 (23.2) | χ2 = 14.684 | 0.023 |
| Cardioembolism | 277/490 (56.5) | 71/152 (46.7) | 97/174 (55.7) | 109/164 (66.5) | ||
| Other causes | 20/490 (4.1) | 8/152 (5.3) | 9/174 (5.2) | 3/164 (1.8) | ||
| Unknown | 47/490 (9.6) | 19/152 (12.5) | 14/174 (8.0) | 14/164 (8.5) | ||
| Intravenous thrombolysis (IVT), frequency (%) | 122/490 (24.9) | 35/152 (23.0) | 51/174 (29.3) | 36/164 (22.0) | χ2 = 2.858 | 0.240 |
| ASITN/SIR collateral grade | ||||||
| 0–1 | 239/490 (48.8) | 57/152 (37.5) | 75/174 (43.1) | 107/164 (65.2) | χ2 = 31.401 | < 0.001 |
| 2 | 169/490 (34.5) | 64/152 (42.1) | 66/174 (37.9) | 39/164 (23.8) | ||
| 3–4 | 82/490 (16.7) | 31/152 (20.4) | 33/174 (19.0) | 18/164 (11.0) | ||
| Procedure variables | ||||||
| Onset to imaging, min, median (IQR) | 292.50 (157.75–459.00) | 320.50 (173.75–533.75) | 326.50 (161.00–486.25) | 226.00 (142.25–399.75) | H = 8.073 | 0.018 |
| Onset to puncture, min, median (IQR) | 362 (240–547) | 380.00 (258.50–625.00) | 393.00 (248.00–553.50) | 324.00 (225.00–490.00) | H = 8.850 | 0.012 |
| Onset to recanalization, min, median (IQR) | 449.50 (326.00–657.25) | 472.00 (342.50–750.00) | 468.00 (347.00–692.25) | 420.00 (300.00–580.00) | H = 8.982 | 0.011 |
| First choice of EVTa | ||||||
| Stent thrombectomy | 87/487 (17.8) | 36/151 (23.7) | 26/172 (14.9) | 25/164 (15.2) | χ2 = 20.587 | 0.422 |
| Balloon | 25/487 (5.1) | 8/151 (5.3) | 11/172 (6.3) | 6/164 (3.7) | ||
| Stenting | 3/487 (0.6) | 1/151 (0.7) | 1/172 (0.6) | 1/164 (0.6) | ||
| Intra-arterial thrombolysis | 2/487 (0.4) | 2/151 (1.3) | 0 | 0 | ||
| Intra-arterial tirofiban | 5/487 (1.0) | 2/151 (1.3) | 1/172 (0.6) | 2/164 (1.2) | ||
| Mechanical fragmentation | 3/487 (0.6) | 2/151 (1.3) | 0 | 1/164 (0.6) | ||
| Aspiration | 251/487 (51.2) | 71/151 (46.7) | 93/172 (53.4) | 87/164 (53) | ||
| Swim | 109/487 (22.2) | 29/151 (19.1) | 40/172 (23.0) | 40/164 (24.4) | ||
| Spontaneous reperfusion | 1/487 (0.2) | 0 | 0 | 1/164 (0.6) | ||
| Mechanical fragmentation and intra-arterial thrombolysis | 1/487 (0.2) | 0 | 0 | 1/164 (0.6) | ||
IQR interquartile range, SBP systolic pressure, DBP diastolic pressure, ICA internal carotid artery, Toast Trial of Org 10172 in Acute Stroke Treatment, M1 M1 of middle cerebral artery, M2 M2 of middle cerebral artery, NIHSS National Institutes of Health Stroke Scale score, ASITN/SIR American Society of Interventional and Therapeutic Neuroradiology/Society of Interventional Radiology collateral score, ASPECTS Alberta Stroke Program Early Computed Tomography Score, EVT endovascular therapy, SWIM Solitarie stent With Intracranial support catheter for Mechanical thrombectomy
aThe first choice of EVT has missing in three patients
Baseline NIHSS as a Continuous Variable and Outcomes
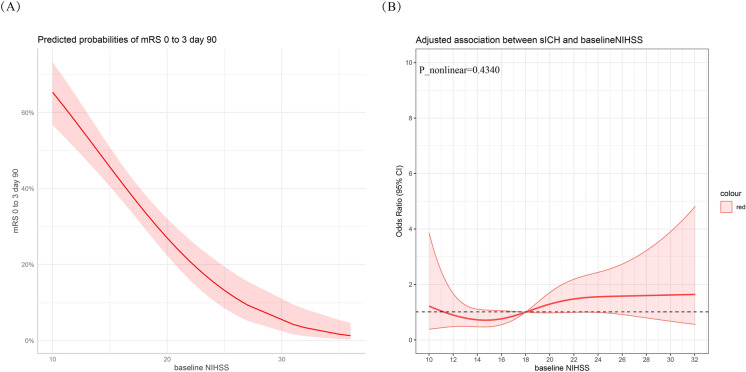
In the univariable logistic regression analysis, a significant association was observed between the baseline NIHSS score and a reduced likelihood of achieving the primary outcome (unadjusted OR 0.847 [0.807–0.888], P < 0.001, Fig. 2a). This association remained significant after adjusting for confounders in models 1, 2, and 3 (Table 2). Specifically, each one-point increase in the NIHSS score was associated with a 13.3% increase in the odds of a shift in the mRS score range (adjusted OR in model 3, 1.133 [1.084–1.180], P < 0.001) and a 12.2% decrease in the odds of achieving functional independence (mRS 0–2, adjusted OR in model 3, 0.878 [0.821–0.939], P < 0.001). While the baseline NIHSS score showed a 3.6% decrease in the odds of successful recanalization (adjusted OR in model 3, 0.964 [0.907–1.024], P = 0.230) per one-point increase, this relationship was not statistically significant.
Fig. 2.
Relationship between baseline NIHSS with primary outcome and symptomatic intracranial hemorrhage (sICH). a Relationship between baseline NIHSS with primary outcome. b Relationship between baseline NIHSS with sICH in a restricted cubic spline model
Table 2.
Association of continuous baseline NIHSS with outcomes
| Characteristic | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | |
| Primary outcome | ||||||||
| mRS0–3 | 0.85 (0.81–0.89)a | < 0.001 | 0.86 (0.81–0.91)a | < 0.001 | 0.86 (0.81–0.91)a | < 0.001 | 0.85 (0.80–0.90)a | < 0.001 |
| Secondary outcome | ||||||||
| mRS at 90 days | 1.17 (1.12–1.21)b | < 0.001 | 1.14 (1.09–1.18)b | < 0.001 | 1.13 (1.08–1.18)b | < 0.001 | 1.13 (1.09–1.18)b | < 0.001 |
| mRS0–2 | 0.88 (0.83–0.93)a | < 0.001 | 0.88 (0.83–0.94)a | < 0.001 | 0.88 (0.83–0.94)a | < 0.001 | 0.88 (0.82–0.94)a | < 0.001 |
| mRS0–4 | 0.83 (0.80–0.87)a | < 0.001 | 0.85 (0.81–0.90)a | < 0.001 | 0.85 (0.80–0.90)a | < 0.001 | 0.85 (0.80–0.90)a | < 0.001 |
| Successful recanalization | 0.11 (0.92–1.01)a | 0.106 | 0.96 (0.91–1.01)a | 0.139 | 0.96 (0.91–1.02)a | 0.19 | 0.96 (0.91–1.02)a | 0.23 |
| Adverse events | ||||||||
| sICH | 1.04 (0.99–1.09)a | 0.08 | 1.03 (0.98–1.09)a | 0.29 | 1.02 (0.97–1.09)a | 0.43 | 1.02 (0.97–1.09)a | 0.43 |
| AICH | 1.03 (0.99–1.07) | 0.14 | 1.02 (0.98–1.06)a | 0.43 | 1.02 (0.97–1.06)a | 0.47 | 1.02 (0.98–1.06)a | 0.45 |
| Mortality at 90 days | 1.17 (1.12–1.22)a | < 0.001 | 1.14 (1.09–1.19)a | < 0.001 | 1.13 (1.08–1.19)a | < 0.001 | 1.13 (1.07–1.19)a | < 0.001 |
Model 1 was adjusted for age, medical history including hypertension, hyperlipidemia, diabetes, atrial fibrillation, baseline ASPECTS, occlusion site, onset to puncture
Model 2 was adjusted for confounders in model 1 plus toast, ASITN/SIR, intravenous thrombolysis, SBP, DBP, smoking
Model 3 was adjusted for confounders in model 2 plus sex, onset to imaging, onset to recanalization
mRS modified Rankin Scale score, NIHSS National Institutes of Health Stroke Scale score, mTICI modified Thrombolysis in Cerebral Infarction Score, sICH symptomatic intracranial hemorrhage, AICH any intracranial hemorrhage, SBP systolic pressure, DBP diastolic pressure, Toast Trial of Org 10172 in Acute Stroke Treatment
aThe odds ratios were estimated from a binary logistic regression model
bThe odds ratios were estimated from an ordinal regression model
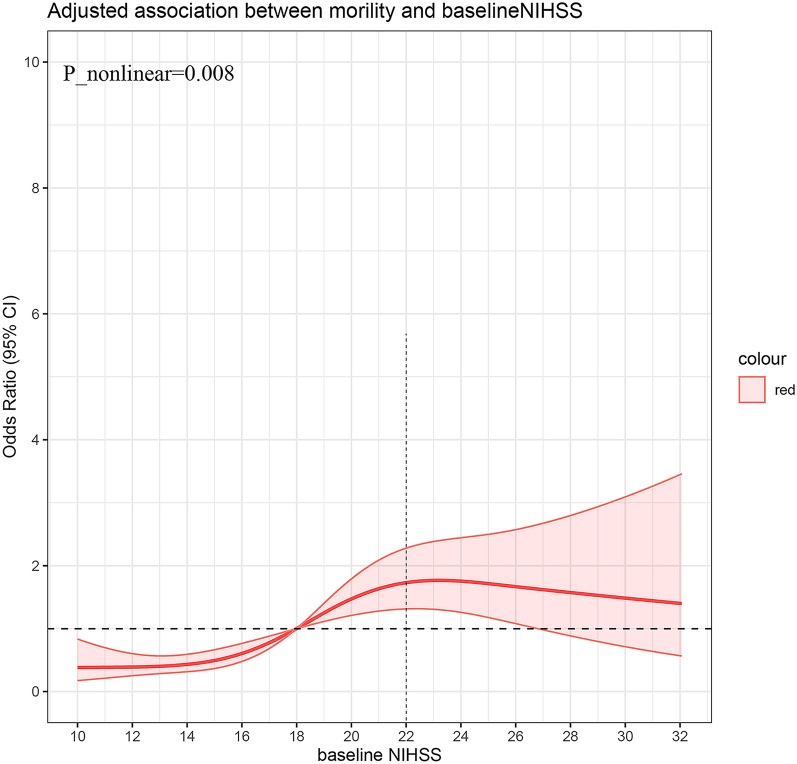
The association between adverse events and NIHSS score is depicted in Figs. 1 and 2b. For NIHSS scores below 22, there was an increasing trend in mortality rate with increasing NIHSS scores. However, the curve plateaued after reaching an NIHSS score of 22 (Fig. 1). Conversely, no non-linear relationship was observed between sICH and baseline NIHSS score (P nonlinearity = 0.4340, Fig. 2b).
Fig. 1.
Association of mortality at 90 days and National Institutes of Health Stroke score (NIHSS)
Baseline NIHSS as a Categorical Variable and Outcomes
Table 3 presents the outcomes of the study population. Among the 490 patients included in the analysis, 181 (36.9%) achieved favorable outcomes (mRS score of 0–3), 205 (41.8%) died, and 65 (13.3%) experienced sICH. The participants were trichotomized according to their baseline NIHSS score: 154 (31.4%) in the low NIHSS score (10–14) group, 174 (35.5%) in the medium NIHSS score group (15–20), and 162 (33.1%) in the high NIHSS score (≥ 20) group. The likelihood of achieving favorable outcomes was notably higher in the low NIHSS score group (53.3%), comparatively lower in the medium NIHSS group (38.5%), and lowest in the high NIHSS score group (20.1%). Rates of successful recanalization were 90.8% for the low NIHSS group, 87.9% for the medium NIHSS group, and 80.5% for the high NIHSS group.
Table 3.
Association of categorical baseline NIHSS with outcome
| Characteristic | Frequencies | χ2 | P value | Unadjusted | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Unadjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | Adjusted OR (95% CI) | P value | ||||
| Primary outcome | |||||||||||
| mRS0–3 | 37.54 | < 0.001 | |||||||||
| NIHSS 10–14 | 81/152 (53.3) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 67/127 (38.5) | 0.55 (0.35–0.85)a | 0.008 | 0.53 (0.32–0.90)a | 0.04 | 0.53 (0.32–0.90)a | 0.02 | 0.52 (0.31–0.88)a | 0.02 | ||
| NIHSS ≥ 20 | 33/164 (20.1) | 0.22 (0.13–0.36)a | < 0.001 | 0.29 (0.16–0.52)a | < 0.001 | 0.29 (0.16–0.52)a | < 0.001 | 0.29 (0.16–0.52)a | < 0.001 | ||
| Secondary outcome | |||||||||||
| mRS at 90 days | 61.76 | < 0.001 | |||||||||
| NIHSS 10–14 | Reference | NA | Reference | NA | Reference | NA | Reference | NA | |||
| NIHSS 15–19 | 0.60 (0.41–0.88)b | 0.009 | 0.63 (0.41–0.95)b | 0.07 | 0.63 (0.41–0.95)b | 0.03 | 0.62 (0.40–0.94)b | 0.02 | |||
| NIHSS ≥ 20 | 0.23 (0.15–0.35)b | < 0.001 | 0.34 (0.21–0.54)b | < 0.001 | 0.34 (0.21–0.54)b | < 0.001 | 0.33 (0.21–0.53)b | < 0.001 | |||
| mRS0–2 | 20.41 | < 0.001 | |||||||||
| NIHSS 10–14 | 44/152 (28.9) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 45/174 (25.9) | 0.86 (0.53–1.40)a | 0.53 | 0.88 (0.51–1.54)a | 0.89 | 0.88 (0.51–1.54)a | 0.66 | 0.89 (0.51–1.56)a | 0.692 | ||
| NIHSS ≥ 20 | 16/164 (9.8) | 0.27 (0.14–0.50)a | < 0.001 | 0.32 (0.16–0.64)a | < 0.001 | 0.32 (0.16–0.64)a | 0.001 | 0.32 (0.16–0.64)a | 0.001 | ||
| mRS0–4 | 54.25 | < 0.001 | |||||||||
| NIHSS 10–14 | 107/152 (70.4) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 93/174 (53.4) | 0.48 (0.31–0.76)a | 0.002 | 0.43 (0.25–0.75)a | 0.01 | 0.43 (0.25–0.75)a | 0.003 | 0.43 (0.25–0.74)a | 0.002 | ||
| NIHSS ≥ 20 | 48/164 (29.3) | 0.17 (0.11–0.28)a | < 0.001 | 0.23 (0.13–0.41)a | < 0.001 | 0.23 (0.13–0.41)a | < 0.001 | 0.23 (0.13–0.41)a | < 0.001 | ||
| Successful recanalization | 7.68 | 0.02 | |||||||||
| NIHSS 10–14 | 138/152 (90.8) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 153/174 (87.9) | 0.74 (0.36–1.51)a | 0.41 | 0.72 (0.33–1.56)a | 0.54 | 0.72 (0.33–1.56)a | 0.40 | 0.71 (0.33–1.55)a | 0.39 | ||
| NIHSS ≥ 20 | 132/164 (80.5) | 0.42 (0.21–0.82)a | 0.01 | 0.41 (0.19–0.90)a | 0.02 | 0.41 (0.19–0.90)a | 0.03 | 0.41 (0.19–0.90)a | 0.03 | ||
| Safety outcome | |||||||||||
| sICH | 9.76 | 0.008 | |||||||||
| NIHSS 10–14 | 19/152 (12.5) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 14/174 (8.0) | 0.61 (0.30–1.27)a | 0.19 | 0.62 (0.27–1.42)a | 0.10 | 0.62 (0.27–1.42)a | 0.26 | 0.63 (0.27–1.43)a | 0.27 | ||
| NIHSS ≥ 20 | 32/164 (19.5) | 1.70 (0.92–3.14)a | 0.09 | 1.36 (0.64–2.89)a | 0.35 | 1.36 (0.64–2.89)a | 0.42 | 1.36 (0.64–2.90)a | 0.42 | ||
| AICH | 3.58 | 0.17 | |||||||||
| NIHSS 10–14 | 55/152 (36.2) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 56/174 (32.2) | 0.84 (0.53–1.32)a | 0.45 | 0.80 (0.49–1.32)a | 0.34 | 0.80 (0.49–1.32)a | 0.38 | 0.81 (0.50–1.33)a | 0.41 | ||
| NIHSS ≥ 20 | 69/164 (42.1) | 1.28 (0.81–2.02)a | 0.28 | 1.10 (0.66–1.83)a | 0.60 | 1.10 (0.66–1.83)a | 0.71 | 1.10 (0.66–1.84)a | 0.70 | ||
| Mortality at 90 days | 43.91 | < 0.001 | |||||||||
| NIHSS 10–14 | 36/152 (23.7) | Reference | NA | Reference | NA | Reference | NA | Reference | NA | ||
| NIHSS 15–19 | 70/174 (40.2) | 2.17 (1.34–3.51)a | 0.002 | 2.16 (1.24–3.79)a | 0.02 | 2.16 (1.24–3.79)a | 0.007 | 2.19 (1.25–3.84)a | 0.006 | ||
| NIHSS ≥ 20 | 99/164 (60.4) | 4.91 (3.01–7.99)a | < 0.001 | 3.28 (1.86–5.80)a | < 0.001 | 3.28 (1.86–5.81)a | < 0.001 | 3.29 (1.86–5.80)a | < 0.001 | ||
Model 1 was adjusted for age, medical history including hypertension, hyperlipidemia, diabetes, atrial fibrillation, baseline ASPECTS, occlusion site, onset to puncture
Model 2 was adjusted for confounders in model 1 plus stroke causative mechanism, ASITN/SIR, intravenous thrombolysis, SBP, DBP, smoking
Model 3 was adjusted for confounders in model 2 plus sex, onset to imaging, onset to recanalization
mRS modified Rankin Scale score, NIHSS National Institutes of Health Stroke Scale score, mTICI modified Thrombolysis in Cerebral Infarction Score, sICH symptomatic intracranial hemorrhage, AICH any intracranial hemorrhage, SBP systolic pressure, DBP diastolic pressure, Toast Trial of Org 10172 in Acute Stroke Treatment
aThe odds ratios were estimated from a binary logistic regression model
bThe odds ratios were estimated from an ordinal regression model
In the adjusted analysis, the probability of primary outcomes (favorable outcomes) was significantly lower in both the medium and high NIHSS groups compared to the low NIHSS group, with adjusted ORs in model 3 of 0.518 (0.306–0.878) and 0.290 (0.161–0.523), respectively. Additionally, lower NIHSS scores were associated with better outcomes when defined as functional independence (mRS 0–2), with adjusted ORs in model 3 of 0.894 (0.511–1.560) for the medium group and 0.317 (CI 0.156–0.640) for the high group, and decreased odds of shifts in scores over the entire range of mRS (adjusted OR in model 3, 0.615 [0.403–0.938] for the medium group and 0.333 [0.209–0.531] for the high group). The 90-day mortality rate was significantly higher in the high NIHSS score group (60.4%), followed by the medium NIHSS group (40.2%), and lowest in the low NIHSS group (23.7%, P < 0.001). The incidence of sICH was highest in the high NIHSS score group (19.5%), followed by the low NIHSS group (12.5%), and lowest in the medium NIHSS group (8.0%, P = 0.008). The distribution of mRS scores at 90 days and rates of successful recanalization are depicted in eFig. 2.
Subgroup Analysis
Subgroup analyses showed that the association of baseline NIHSS score and favorable outcome have large effects in male patients, those with age less than 75, patients with baseline ASPECTS 3 to 5, large-artery atherosclerosis, middle cerebral artery M1 segment stenosis, those without traditional vascular risks factors such as hypertension, hyperlipidemia, diabetes mellitus, and smoking (eFig. 3).
Mediation Analysis
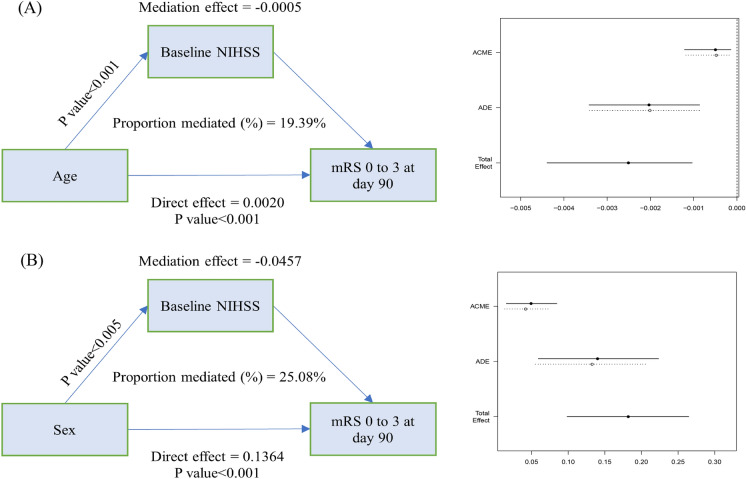
In the multivariable linear regression analysis, several predictors of baseline NIHSS scores were identified, including age, sex, hyperlipidemia, diastolic blood pressure, ASITN/SIR, and ASPECTS (eTable 1). To further investigate the relationship between baseline NIHSS scores and the likelihood of a favorable outcome, mediation analysis was conducted. This analysis revealed that the effects of age and sex on favorable outcomes were partially mediated by the baseline NIHSS score. As illustrated in Fig. 3, there were significant regression coefficients between age and sex with favorable outcomes and between baseline NIHSS score and favorable outcomes. Specifically, Fig. 3a demonstrated that the baseline NIHSS score significantly mediated the association between age and favorable outcomes (indirect effect = − 0.0005, 19.39% mediated). Similarly, Fig. 3b showed that the baseline NIHSS score significantly mediated the association between sex and favorable outcomes (indirect effect = − 0.0457, 25.08% mediated). These findings suggest that baseline NIHSS score plays a crucial role in the relationship between demographic factors (age and sex) and clinical outcomes in patients with large core infarctions undergoing EVT.
Fig. 3.
Mediation analysis. a The effect of age on primary outcome can partially mediated via baseline NIHSS score. b The effect of sex on primary outcome can partially mediated via baseline NIHSS score. Unstandardized indirect effects were computed for each of 1000 bootstrapped samples, and the 95% confidence interval was computed by determining the indirect effects at the 2.5th and 97.5th percentiles. ACME average causal mediation effects (indirect effect), ADE average direct effects
Discussion
This study suggests that a NIHSS score correlates with poorer outcomes and increased mortality. Specifically, an NIHSS score above 20 significantly reduces the likelihood of a favorable clinical outcome. Furthermore, factors such as age and sex appear to indirectly influence the probability of a favorable outcome, mediated by their association with the baseline NIHSS score. Although only a small proportion (≤ 10%) of acute ischemic strokes evolve into large infarctions [16–18], these cases are associated with significant morbidity and mortality [19]. Over 50% of large core infarctions are prone to developing malignant cerebral edema, often leading to rapid neurological decline within the first 2–3 days post-onset [20, 21]. Recent studies have focused on the effectiveness of EVT in treating large core infarctions, with concerns centered around the potential for non-beneficial recanalization and risks of reperfusion injuries such as hemorrhage [22]. However, recent randomized controlled trials have shown that EVT significantly improves clinical outcomes and reduces mortality in patients with large core infarctions compared to standard medical therapy [5–8]. Consistent with these findings, 36.9% of patients in this study achieved a favorable outcome (mRS score of 0–3) following EVT. However, this is still lower than the effectiveness in patients with traditional small core infarctions and large vessel occlusions in the ischemic penumbra [23], suggesting that there were a few differences between the two groups and the ischemic core volume, collateral circulation degree, and severity of neurological impairment and so on might affect the outcomes of large core infarctions after EVT.
The NIHSS is a critical tool for assessing stroke severity and guiding treatment decisions. It provides a comprehensive evaluation of stroke severity, which is crucial for determining the risk of clinical deterioration and selecting appropriate treatment strategies, especially before undertaking EVT. Existing research reports a correlation between NIHSS scores and clinical symptoms, as well as recanalization rates following EVT [24–26]. In this study, a higher baseline NIHSS score was associated with a reduced likelihood of achieving a favorable outcome, particularly in patients with NIHSS scores > 20. Regarding adverse events, an increased mortality risk was observed in patients with large infarctions. Interestingly, this correlation plateaued when the NIHSS score exceeded 22, indicating a lack of significance beyond this threshold. These findings suggest that the baseline NIHSS score is a valuable prognostic tool for clinicians, helping predict the effectiveness of EVT and associated mortality risks in patients with large core infarctions. Therefore, the NIHSS score can assist healthcare professionals in making informed decisions regarding EVT candidacy and effectively communicating prognoses to patients and their families.
The study revealed a significant correlation between older age and poorer neurological outcomes in patients with stroke, suggesting that advancing age is associated with higher baseline NIHSS scores [27]. Age has been recognized as an independent risk factor for ischemic stroke, with stroke prevalence demonstrably increasing with age, as reported by the American Heart Association [10]. Research conducted by Ospel et al. has highlighted that the impact of a single-point increase in the NIHSS score is approximately equivalent to a 3-year increase in patient age in terms of its effect on the likelihood of a favorable outcome post-stroke [28]. Furthermore, older patients often show more pronounced cerebral small vessel disease, which can worsen stroke outcomes and consequently increase NIHSS scores [29]. Additionally, aging may alter the body’s response to injury and its capacity for repair, potentially leading to more severe stroke manifestations and higher NIHSS scores [29]. These insights underscore the critical interplay between patient age, baseline NIHSS score, and the likelihood of favorable outcomes in patients with ischemic stroke, emphasizing the need for age-specific considerations in stroke management and prognosis.
Numerous studies have established a link between gender and prognosis in acute ischemic stroke, with evidence suggesting that gender differences may influence stroke outcomes [27, 30, 31]. An Italian study observed that older women who suffer from a stroke tend to experience worse functional outcomes, as measured by the NIHSS score, compared to their male counterparts [32]. Consistent with these findings, our research also indicated that women generally presented with higher NIHSS scores indicating more severe neurological damage. The literature has highlighted that women may be at an increased risk due to certain factors and often experience poorer post-stroke outcomes than men [33]. For instance, hypertension is more prevalent among elderly women than in men, possibly as a result of the regulatory effects of estrogen receptors on the sympathetic nervous system. Notably, the incidence of hypertension is lower in premenopausal women compared to men [34]. Additionally, obesity, more prevalent in women, not only serves as an independent risk factor for stroke but also contributes to hypertension [35]. Furthermore, our data revealed that the rate of favorable outcomes (mRS score 0–3) was significantly lower in women (26.8%) compared to men (44.5%) (P < 0.001). These findings highlight the critical role of gender in stroke outcomes, with both age and sex partially accounting for differences in baseline NIHSS scores and, consequently, stroke prognosis.
The key findings of this study hold significant clinical implications, particularly for surgical decision-making and patient communication. Surgeons require a reliable tool to evaluate surgical indications and effectively convey the prognosis to patients and their families. This research proposes using the patient’s NIHSS score at admission as a basis for preliminary assessment, serving as a foundation for discussions between healthcare providers, patients, and their families. In cases where the admission NIHSS score is 20 points or higher, it is crucial to thoroughly inform the patient’s family before surgery about the low probability of a favorable prognosis. This information is vital to assist them in making informed decisions regarding the surgical intervention.
This study has several limitations. Firstly, it uses retrospectively analyzed data from a prospective collection, which may affect the broader applicability of the findings. Additionally, the study mainly involves a Chinese patient cohort characterized by a high prevalence of intracranial artery stenosis. This demographic specificity might limit the relevance of the results to other populations. Furthermore, as a result of the potential for type I errors resulting from multiple comparisons, the conclusions drawn from secondary outcome analyses should be considered preliminary and warrant further investigation. Another limitation is the absence of perfusion imaging in the evaluation, which is particularly significant for patients with severe neurological deficits, as it helps in identifying specific radiological profiles correlating with clinical severity. However, the study employs the ASPECTS for assessing the extent of ischemic damage in cerebral infarction, leveraging the advantages of cranial computed tomography’s simplicity, speed, and widespread availability.
Conclusions
This study indicates that a higher baseline NIHSS score correlates with poorer outcomes and increased mortality. Specifically, a baseline NIHSS score above 20 significantly reduces the likelihood of a favorable clinical outcome. Furthermore, it reveals that age and sex indirectly influence the probability of a favorable outcome by affecting the baseline NIHSS score.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Medical Writing and Editorial Assistance
MJE editor (www.mjeditor.com) provided English editing services during the preparation of this manuscript, and The Incubation Project of Weihai Municipal Hospital affiliated with Shandong University (FH-2021-XY01) is funding this support.
Author Contributions
All authors made significant contributions to the completion of this article and have approved the final manuscript. The specific contributions are as follows: Pengfei Wang and Changwei Guo take responsibility for the conception, design and revising draft critically for important intellectual content and final approval of the version to be published; Lingyu Zhang and Jinfu Ma were involved in the design of the study, analysis and interpretation of data, drafting the article, and contributed equally to this paper. Mengmeng Wang and Lin Zhang worked on drafting the article and the analysis and interpretation of data. Wenzhe Sun and Honghong Ji worked on the conception and design and revise the article. Chengsong Yue and Jiacheng Huang worked on acquisition and analysis of data. Wenji Zi and Fengli Li worked on acquisition and analysis of data. The corresponding author attests that all listed authors meet the authorship criteria. All authors read and approved the final manuscript.
Funding
The Incubation Project of Weihai Municipal Hospital affiliated with Shandong University (FH-2021-XY01) is funding the journal’s fee.
Declarations
Conflict of Interest
Lingyu Zhang, Jinfu Ma, Mengmeng Wang, Lin Zhang, Wenzhe Sun, Honghong Ji, Chengsong Yue, Jiacheng Huang, Wenjie Zi, Fengli Li, Changwei Guo, Pengfei Wang have nothing to disclose.
Ethical Approval
The study was approved by the ethics committee of the Xinqiao Hospital (Second Affiliated Hospital), Army Medical University, (ChiCTR2100051664), and the local ethics committee of each site. Informed consent was acquired from each patient and/or their legal surrogates according to the Declaration of Helsinki. Once the written consent was obtained, the investigators could access the corresponding secondary information related to the initial morbidity with permission.
Footnotes
Lingyu Zhang and Jinfu Ma contributed equally.
Contributor Information
Changwei Guo, Email: changw_guo@163.com.
Pengfei Wang, Email: wpf5287598@163.com.
References
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med. 2015;372(1):11–20. doi: 10.1056/NEJMoa1411587. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372(11):1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 3.Nogueira RG, Jadhav AP, Haussen DC, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med. 2018;378(1):11–21. doi: 10.1056/NEJMoa1706442. [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378(8):708–718. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bendszus M, Fiehler J, Subtil F, et al. Endovascular thrombectomy for acute ischaemic stroke with established large infarct: multicentre, open-label, randomised trial. Lancet. 2023;402(10414):1753–1763. doi: 10.1016/S0140-6736(23)02032-9. [DOI] [PubMed] [Google Scholar]
- 6.Huo X, Ma G, Tong X, et al. Trial of endovascular therapy for acute ischemic stroke with large infarct. N Engl J Med. 2023;388(14):1272–1283. doi: 10.1056/NEJMoa2213379. [DOI] [PubMed] [Google Scholar]
- 7.Sarraj A, Hassan AE, Abraham MG, et al. Trial of endovascular thrombectomy for large ischemic strokes. N Engl J Med. 2023;388(14):1259–1271. doi: 10.1056/NEJMoa2214403. [DOI] [PubMed] [Google Scholar]
- 8.Yoshimura S, Sakai N, Yamagami H, et al. Endovascular therapy for acute stroke with a large ischemic region. N Engl J Med. 2022;386(14):1303–1313. doi: 10.1056/NEJMoa2118191. [DOI] [PubMed] [Google Scholar]
- 9.Costalat V, Lapergue B, Albucher JF, et al. Evaluation of acute mechanical revascularization in large stroke (ASPECTS ≤ 5) and large vessel occlusion within 7 h of last-seen-well: the laste multicenter, randomized, clinical trial protocol. Int J Stroke. 2023;19(1):114–119. doi: 10.1177/17474930231191033. [DOI] [PubMed] [Google Scholar]
- 10.Kwah LK, Diong J. National Institutes of Health Stroke Scale (NIHSS) J Physiother. 2014;60(1):61. doi: 10.1016/j.jphys.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 11.Linfante I, Starosciak AK, Walker GR, et al. Predictors of poor outcome despite recanalization: a multiple regression analysis of the NASA registry. J Neurointerv Surg. 2016;8(3):224–229. doi: 10.1136/neurintsurg-2014-011525. [DOI] [PubMed] [Google Scholar]
- 12.Cheng Z, Geng X, Rajah GB, et al. NIHSS consciousness score combined with ASPECTS is a favorable predictor of functional outcome post endovascular recanalization in stroke patients. Aging Dis. 2021;12(2):415–424. doi: 10.14336/AD.2020.0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Kummer R, Broderick JP, Campbell BC, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015;46(10):2981–2986. doi: 10.1161/STROKEAHA.115.010049. [DOI] [PubMed] [Google Scholar]
- 14.Liu X, Dai Q, Ye R, et al. Endovascular treatment versus standard medical treatment for vertebrobasilar artery occlusion (BEST): an open-label, randomised controlled trial. Lancet Neurol. 2020;19(2):115–122. doi: 10.1016/S1474-4422(19)30395-3. [DOI] [PubMed] [Google Scholar]
- 15.Bursac Z, Gauss CH, Williams DK, et al. Purposeful selection of variables in logistic regression. Source Code Biol Med. 2008;3:17. doi: 10.1186/1751-0473-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao Z, Chang X, Zhou H, et al. A cohort study of decompressive craniectomy for malignant middle cerebral artery infarction: a real-world experience in clinical practice. Medicine (Baltimore) 2015;94(25):e1039. doi: 10.1097/MD.0000000000001039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahme R, Curry R, Kleindorfer D, et al. How often are patients with ischemic stroke eligible for decompressive hemicraniectomy? Stroke. 2012;43(2):550–552. doi: 10.1161/STROKEAHA.111.635185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zha AM, Sari M, Torbey MT. Recommendations for management of large hemispheric infarction. Curr Opin Crit Care. 2015;21(2):91–98. doi: 10.1097/MCC.0000000000000184. [DOI] [PubMed] [Google Scholar]
- 19.Liebeskind DS, Jüttler E, Shapovalov Y, et al. Cerebral edema associated with large hemispheric infarction. Stroke. 2019;50(9):2619–2625. doi: 10.1161/STROKEAHA.118.024766. [DOI] [PubMed] [Google Scholar]
- 20.Qureshi AI, Suarez JI, Yahia AM, et al. Timing of neurologic deterioration in massive middle cerebral artery infarction: a multicenter review. Crit Care Med. 2003;31(1):272–277. doi: 10.1097/00003246-200301000-00043. [DOI] [PubMed] [Google Scholar]
- 21.Wartenberg KE. Malignant middle cerebral artery infarction. Curr Opin Crit Care. 2012;18(2):152–163. doi: 10.1097/MCC.0b013e32835075c5. [DOI] [PubMed] [Google Scholar]
- 22.Leslie-Mazwi TM, Altschul D, Simonsen CZ. Thrombectomy for patients with large infarct core in practice: where should the pendulum swing? Stroke. 2021;52(10):3118–3120. doi: 10.1161/STROKEAHA.121.034754. [DOI] [PubMed] [Google Scholar]
- 23.Jadhav AP, Desai SM, Jovin TG. Indications for mechanical thrombectomy for acute ischemic stroke: current guidelines and beyond. Neurology. 2021;97(20 Suppl 2):S126–S136. doi: 10.1212/WNL.0000000000012801. [DOI] [PubMed] [Google Scholar]
- 24.Aoki J, Suzuki K, Kanamaru T, et al. Association between initial NIHSS score and recanalization rate after endovascular thrombectomy. J Neurol Sci. 2019;403:127–132. doi: 10.1016/j.jns.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 25.Boxerman JL, Jayaraman MV, Mehan WA, et al. Clinical stroke penumbra: use of National Institutes of Health Stroke Scale as a surrogate for CT perfusion in patient triage for intra-arterial middle cerebral artery stroke therapy. AJNR Am J Neuroradiol. 2012;33(10):1893–1900. doi: 10.3174/ajnr.A3102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanacker P, Heldner MR, Amiguet M, et al. Prediction of large vessel occlusions in acute stroke: National Institute of Health Stroke Scale is hard to beat. Crit Care Med. 2016;44(6):e336–e343. doi: 10.1097/CCM.0000000000001630. [DOI] [PubMed] [Google Scholar]
- 27.Roy-O'Reilly M, McCullough LD. Age and sex are critical factors in ischemic stroke pathology. Endocrinology. 2018;159(8):3120–3131. doi: 10.1210/en.2018-00465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ospel JM, Brown S, Kappelhof M, et al. Comparing the prognostic impact of age and baseline National Institutes of Health Stroke Scale in acute stroke due to large vessel occlusion. Stroke. 2021;52(9):2839–2845. doi: 10.1161/STROKEAHA.120.032364. [DOI] [PubMed] [Google Scholar]
- 29.Li T, Huang Y, Cai W, et al. Age-related cerebral small vessel disease and inflammaging. Cell Death Dis. 2020;11(10):932. doi: 10.1038/s41419-020-03137-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodríguez-Castro E, Rodríguez-Yáñez M, Arias S, et al. Influence of sex on stroke prognosis: a demographic, clinical, and molecular analysis. Front Neurol. 2019;10:388. doi: 10.3389/fneur.2019.00388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Purroy F, Vicente-Pascual M, Arque G, et al. Sex-related differences in clinical features, neuroimaging, and long-term prognosis after transient ischemic attack. Stroke. 2021;52(2):424–433. doi: 10.1161/STROKEAHA.120.032814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Santalucia P, Pezzella FR, Sessa M, et al. Sex differences in clinical presentation, severity and outcome of stroke: results from a hospital-based registry. Eur J Intern Med. 2013;24(2):167–171. doi: 10.1016/j.ejim.2012.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Psaltopoulou T, Sergentanis TN, Panagiotakos DB, et al. Mediterranean diet, stroke, cognitive impairment, and depression: a meta-analysis. Ann Neurol. 2013;74(4):580–591. doi: 10.1002/ana.23944. [DOI] [PubMed] [Google Scholar]
- 34.Cherian L. Women and ischemic stroke. Neurol Clin. 2023;41(2):265–281. doi: 10.1016/j.ncl.2022.10.001. [DOI] [PubMed] [Google Scholar]
- 35.Ghazi L, Annabathula RV, Bello NA, et al. Hypertension across a woman's life cycle. Curr Hypertens Rep. 2022;24(12):723–733. doi: 10.1007/s11906-022-01230-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.