Abstract
Coronary computed tomography angiography (CCTA) has emerged as a pivotal tool for diagnosing and risk-stratifying patients with suspected coronary artery disease (CAD). Recent advancements in image analysis and artificial intelligence (AI) techniques have enabled the comprehensive quantitative analysis of coronary atherosclerosis. Fully quantitative assessments of coronary stenosis and lumen attenuation have improved the accuracy of assessing stenosis severity and predicting hemodynamically significant lesions. In addition to stenosis evaluation, quantitative plaque analysis plays a crucial role in predicting and monitoring CAD progression. Studies have demonstrated that the quantitative assessment of plaque subtypes based on CT attenuation provides a nuanced understanding of plaque characteristics and their association with cardiovascular events. Quantitative analysis of serial CCTA scans offers a unique perspective on the impact of medical therapies on plaque modification. However, challenges such as time-intensive analyses and variability in software platforms still need to be addressed for broader clinical implementation. The paradigm of CCTA has shifted towards comprehensive quantitative plaque analysis facilitated by technological advancements. As these methods continue to evolve, their integration into routine clinical practice has the potential to enhance risk assessment and guide individualized patient management. This article reviews the evolving landscape of quantitative plaque analysis in CCTA and explores its applications and limitations.
Keywords: Coronary computed tomography angiography, Artificial intelligence, Quantitative plaque analysis, Coronary artery atherosclerosis
INTRODUCTION
Cardiovascular disease (CVD) is the leading cause of morbidity and mortality in developed countries. Coronary computed tomography angiography (CCTA) is a rapidly evolving diagnostic imaging modality. Multiple clinical studies have demonstrated its efficacy for diagnosing and stratifying patients with suspected coronary artery disease (CAD). Recent guidelines from American and European societies have endorsed CCTA as an initial testing modality for assessing symptomatic CAD [1,2].
One of CCTA’s key strengths is its ability to characterize coronary atherosclerotic plaques. Owing to its three-dimensional (3D), noninvasive nature, CCTA enables the comprehensive assessment of coronary plaques throughout the entire coronary tree. Conventional clinical assessment using CCTA involves visual estimation and qualitative evaluations of stenosis and plaque type. However, recent advancements in image analysis and artificial intelligence (AI) techniques have enabled the comprehensive quantitative analysis of plaque composition, volume, and degree of stenosis. This quantitative plaque assessment can significantly improve the diagnosis of CAD and the prediction of subsequent cardiac events. Furthermore, serial evaluation of quantitative plaque characteristics facilitates evaluating treatment response to drugs that favorably modulate coronary plaques and enables effective monitoring of CAD progression.
This review provides a comprehensive overview of prior studies, future applications, and potential limitations of quantitatively assessing coronary artery plaques using CCTA.
Application of Quantitative Plaque Analysis in Stenosis Evaluation
Numerous clinical studies have consistently reported the high diagnostic accuracy of CCTA, particularly in excluding obstructive CAD among symptomatic patients with a low-to-intermediate pretest probability of CAD. However, traditional visual assessment often overestimates the degree of stenosis compared to invasive reference standards [3]. Moreover, relying solely on the anatomical evaluation of stenosis severity has demonstrated limited diagnostic accuracy in identifying hemodynamically significant stenoses [4]. Consequently, recent research has focused on addressing the limitations of CCTA for stenosis evaluation.
Quantitative Assessment of Stenosis Severity
Conventional qualitative analysis of coronary atherosclerotic lesions is based on different categories of diameter stenosis: none (no visible stenosis), minimal (1%–24% estimated stenosis of the coronary luminal diameter), mild (25%–49%), moderate (50%–69%), severe (70%–99%), or occluded (100%) [5]. Recent advances in CT workstations and specialized plaque analysis (PA) software have facilitated semi-automated or fully automated quantification of coronary artery stenosis. For example, Boogers et al. [6] demonstrated that automated quantification of stenosis severity on CCTA exhibited a good correlation with quantitative coronary angiography (QCA) and improved diagnostic accuracy compared to visual assessment alone. Furthermore, machine learning and deep learning enable a fully automated stenosis evaluation [7]. In a study by Hong et al. [8] employing a deep learning approach featuring the M-net CNN architecture, a fully quantitative assessment of area stenosis and diameter stenosis demonstrated an outstanding correlation (r = 0.984 for minimal luminal area and r = 0.957 for diameter stenosis) with expert readers with a rapid processing time of < 32 seconds. In another recent study, Lin et al. [9] developed a fully automated deep learning-based diameter and area stenosis evaluation method that employed invasive coronary angiography and intravascular ultrasound (IVUS) as the reference standard. The agreement for the minimal luminal area between the deep learning algorithm and IVUS evaluation was strong, with an interclass correlation coefficient of 0.904 (Fig. 1). In addition, Griffin et al. [10] demonstrated the effectiveness of AI-based software that enables the rapid and accurate identification and exclusion of high-grade stenosis, with good agreement with QCA. Recently, AI-based coronary stenosis quantification software exhibited a high discriminatory ability for anatomic stenosis across vessel segments, including area under the receiver operating characteristic curve (AUC) values of 0.92 and 0.93 at 50% and 70% thresholds [11]. Adopting a fully automated CT stenosis evaluation can enhance the utility of CCTA, enabling faster, more reproducible, and more accurate clinical reporting.
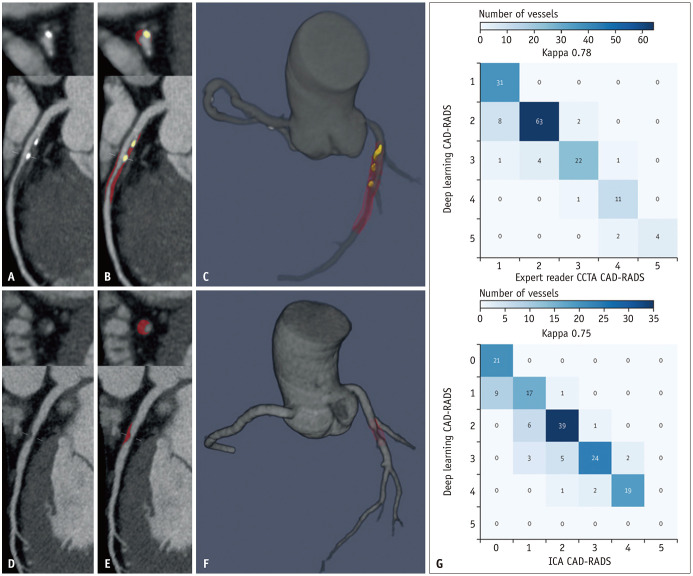
Fig. 1. Deep-learning coronary artery plaque analysis. A-F: Case examples of deep-learning plaque segmentation in the proximal to mid LAD (A-C). Case examples of deep-learning plaque segmentation in the mid-LAD (D-F). Curved multiplanar reformation CCTA images (A, D). Deep-learning segmentation of calcified plaque (yellow) and noncalcified plaque (red) (B, E). Three-dimensional rendered view of the coronary tree (C, F). G: Per-vessel CAD-RADS categorization by deep learning versus expert readers and ICA. CAD-RADS categorical agreement between deep learning and experts and between deep learning and ICA was strong (unweighted Cohen’s κ coefficient = 0.78 and κ = 0.75, respectively), and there was 99% (between deep learning and experts) and 97% (between deep learning and ICA) agreement within one CAD-RADS category. Adapted from Lin et al. Lancet Digit Health 2022;4:e256-e265, with permission of Elsevier [9]. LAD = left anterior descending, CCTA = coronary computed tomography angiography, CAD-RADS = Coronary Artery Disease Reporting and Data System, ICA = invasive coronary angiography.
Quantitative Assessment of Lumen Density to Detect Hemodynamically Significant Lesion: TAG and CDD
Previous invasive studies have revealed significant disparities between angiographically and functionally significant lesions, as assessed by the fractional flow reserve (FFR) [4]. A similar gap exists between CCTA and invasive FFR. Recent developments in quantitative analysis have demonstrated the potential of CCTA to evaluate the functional significance of lesions, most notably using CT-FFR, especially in patients with intermediate stenosis, which necessitates further assessment of functional significance [12,13]. The use of CT-FFR in intermediate lesions on CCTA has a Class IIa recommendation in the American College of Cardiology/American Heart Association guidelines for patients with chest pain syndromes and a strong recommendation in the guidelines of the European Society of Cardiology [1,2]. Although CT-FFR is a widely used tool for assessing the hemodynamic significance of lesions, it has limitations. CT-FFR entails additional costs and nitroglycerin administration and requires high-quality contrast-enhanced CT images free of artifacts or noise. Furthermore, a recent multicenter observational study showed that CT-FFR has a low positive predictive value (PPV) and is associated with a higher cost than conventional stress-imaging approaches [14]. Only one vendor, HeartFlow, currently provides CT-FFR based on computational fluid dynamics. Recently, other approaches assessing the functional significance of lesions using machine learning and deep learning applied to coronary plaques have shown a high correlation with invasive FFR [15,16]. An alternative tool, CT myocardial perfusion, can also offer a functional assessment of CAD [17]. However, significant limitations constrain its widespread use in daily clinical practice, including artifacts from CT imaging such as beam hardening, misregistration, image noise, motion artifacts, and requiring a second scan using pharmacological stress, with the disadvantages of extra radiation and altered scheduling times. Alternatively, several other quantitative analysis methods, such as the transluminal attenuation gradient (TAG) and contrast density drop (CDD), have been proposed to assess coronary stenosis’s functional significance by analyzing lumen density changes.
TAG is the linear regression coefficient between luminal attenuation and axial distance throughout a specific vessel, with higher TAG values associated with higher stenosis severity [18,19,20]. In an initial study, Choi et al. [19] investigated the value of TAG in 370 major coronary arteries, measuring 7263 intervals of 5 mm length. In correlation with CCTA and invasive coronary angiography, there was a consistent and significant decrease in TAG levels in vessels with a higher degree of stenosis. Furthermore, TAG has shown an incremental value over CCTA alone in detecting functionally significant coronary artery stenosis [21]. However, a decline in intraluminal attenuation was noted, along with a reduction in vessel diameter [22]. Prior studies have shown that TAG and transluminal diameter gradient do not offer additional diagnostic value compared to CCTA alone for detecting significant ischemia [23]. Further studies are needed to standardize and validate the TAG evaluation using a larger number of patients.
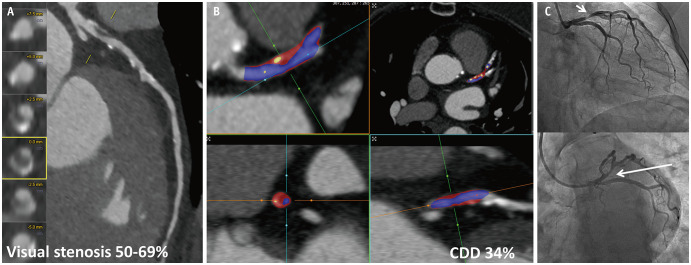
CDD, another quantitative method for assessing changes in luminal contrast density over a coronary lesion, is the maximum percentage difference in contrast densities relative to the proximal reference cross-section, with a higher CDD indicating hemodynamically significant lesions (Fig. 2) [24,25,26]. Dey et al. [24] compared the coronary plaque burden using CCTA in patients with acute coronary syndrome (ACS) and those with stable CAD. Their findings showed that higher CDD values reliably distinguished patients with ACS from those with stable CAD, along with plaque parameters such as noncalcified plaque (NCP), total plaque burden, and stenosis [24]. Diaz-Zamudio et al. [25] examined whether automated quantitative measurements of plaque features from CCTA could predict the presence of ischemia using myocardial perfusion imaging at various stenosis severity levels. In that study, CDD was strongly associated with ischemia in vessels with > 70% stenosis. Hell et al. [26] explored whether TAG and CDD could serve as indicators of the hemodynamic significance of coronary artery stenoses by comparing the values of invasively measured FFR. They demonstrated that the diagnostic accuracy (specificity, 75%; sensitivity, 33%; PPV, 35%; negative predictive value, 73%) of CDD was superior to TAG for identifying hemodynamically significant lesions. Furthermore, in a study using machine learning techniques to develop a model for predicting hemodynamically significant ischemia, the combination of CDD and plaque assessment showed a diagnostic performance comparable to that of CT-FFR in identifying invasive FFR-defined ischemia [27]. Despite the potential applications of CDD, certain limitations should be noted. Validation of the clinical application of the CDD is limited, and only one quantitative program can provide the required data. Additionally, imaging artifacts, such as beam hardening or metallic artifacts, and the timing of contrast acquisition can affect the assessment of changes in luminal density throughout the coronary arteries.
Fig. 2. Images of a 73-year-old female who presented with typical chest pain. A: Multiplanar reformat of CCTA demonstrating a borderline (50%–69%) stenotic lesion in the proximal LAD. B: Quantitative analysis of CDD using the Autoplaque software. The start and end points were selected manually, and the calculation yielded a CDD of 34. C: Invasive coronary angiography showing 75% stenosis of the proximal LAD (arrows). CCTA = coronary computed tomography angiography, LAD = left anterior descending, CDD = contrast density drops.
Application of Quantitative Analysis for Plaque Burden Assessment
An essential advantage of CCTA is its ability to assess total plaque burden. Traditionally, plaque burden has been estimated using quantitative analysis of calcified plaques in coronary artery calcification (CAC) scanning, the Agatston score, or semi-quantitative visual evaluation of plaque extent in several coronary segments. However, recent studies have demonstrated that the quantitative assessment of coronary plaques in CCTA enables a more comprehensive and robust analysis of plaques within individual segments and across the entire coronary artery tree.
Conventional Semi-Quantitative Approach: CAC and Semi-Quantitative CCTA Scores
CAC scoring is considered an effective method for the early detection of CAD, especially in asymptomatic primary prevention populations, compared to conventional clinical risk scores such as the Framingham 10-year risk score [28,29]. The CAC plaque burden was quantified using the method described by Agatston et al. [30] and categorized as none (CAC = 0), mild (1–100), moderate (101–300), severe (301–1000), or extensive (> 1000) [5]. Although previous studies have shown that CAC is a strong and independent predictor of future adverse cardiovascular events and has incremental prognostic value in predicting CVD events [31,32,33], it does not account for NCP. Purely calcified plaques are stable and unlikely to cause ACS events. NCP, mainly low-density NCP, are the most rupture-prone plaques. Furthermore, the utility of serial CAC assessment is limited, given the tendency of preventive medications such as statins to potentially elevate CAC scores while simultaneously decreasing the risk of CVD.
In CCTA imaging, semi-quantitative scoring systems, such as the Segment Involvement Score (SIS), Segment Stenosis Score (SSS), and modified Duke CAD index, have been conventionally employed to assess the coronary plaque burden. The SIS provides a simple measure of the overall coronary plaque burden by assigning a score of 1 to each coronary artery segment with detectable atherosclerotic plaques, irrespective of plaque severity [34]. In contrast, the SSS and modified Duke index incorporate both the severity and extent of coronary artery plaques [35]. Although these semi-quantitative scores offer CAD plaque assessment, they only provide an approximation of the CAD burden.
Quantitative Plaque Analysis Software
Recent advancements have introduced specialized software that enables quantitative evaluation of plaques at both the lesion and patient levels. These tools measure plaque composition, volume, coronary stenosis, and positive remodeling. Various quantitative CT software options are now available, employing diverse approaches encompassing automated or semi-automated techniques for detecting the lumen border. Several FDA-approved quantitative PA software options are available, including QAngio, SUREPlaque, Autoplaque, vascuCAP, Cleerly, and automated AI-PA from HeartFlow (Table 1) [15,36,37,38,39,40,41,42,43].
Table 1. FDA-cleared quantitative plaque analysis softwares.
| Software | Vendor | FDA approval | Key features | Plaque types analyzed | Key validation studies |
|---|---|---|---|---|---|
| QAngio | Medis Medical Imaging Systems, Leiden, the Netherlands | 510k 2006 | Stenosis, plaque volume, vessel volume, remodeling index, plaque types | Necrotic core, fibrofatty, fibrous, dense calcium | Boogers et al. [36], de Graaf et al. [37] |
| SUREplaque | Canon Medical Systems, Otawara, Japan | 510k 2004 | Stenosis, plaque volume, vessel volume, plaque types | Low density non calcified, non-calcified, calcified | Fujimoto et al. [38], Voros et al. [39] |
| Cleerly | Cleerly Healthcare, New York, NY, USA | 510k 2019 | Stenosis, plaque volume, vessel volume, remodeling index, plaque types | Low density non calcified, non-calcified, calcified | Choi et al. [40] |
| vascuCAP | Elucid Bioimaging, Wenham, MA, USA | 510k 2017 | Stenosis, plaque volume, vessel volume, remodeling index, plaque types | Lipid rich necrotic core, matrix, calcified plaque | Sheahan et al. [41] |
| Autoplaque | Cedars-Sinai Medical Center, Los Angeles, CA, USA | 510k 2012 | Stenosis, plaque volume, composition, and burden, vessel volume, remodeling index, contrast density drop, plaque types | Non-calcified, calcified, low density non calcified, necrotic core, fibrous fatty, fibrous, dense calcium | Dey et al. [15], Dey et al. [42] |
| HeartFlow Plaque Analysis | HeartFlow, Mountain View, CA, USA | 510k 2022 | Plaque volume, vessel volume, plaque types | Low CT attenuation plaque, non-calcified, calcified | Tzimas et al. [43] |
The semi-automated plaque assessment process across various platforms involves several key steps. Initially, automated algorithms were used to extract the centerline of the coronary artery. Automated methods detect the boundaries of the lumen and outer vessel wall using mathematical or rule-based approaches. The lumen of a coronary artery is typically segmented based on cross-sectional CCTA images, transforming the vessel’s entire length into a single volume. Subsequently, the reader manually adjusted the detected boundaries in multiplanar reconstructed or cross-sectional views, with the extent of adjustment dependent on the quality of the CCTA images. Plaque was identified as all voxels between the lumen and vessel wall boundaries, and the software automatically measured the plaque.
There is no standardized nomenclature for describing plaque volumes or components, leading to varied terminology across software vendors. Plaque size is typically measured volumetrically in cubic millimeters (mm3), and analysis can be conducted at the per-lesion, per-coronary segment, per-vessel, or per-patient level. The plaque area on a 2D CCTA cross-section can also be determined as the plaque area (mm2). Similar to IVUS methodology, the percentage of the overall vessel volume occupied by plaque on CCTA can be calculated as the “percent atheroma volume,” “plaque burden volume ratio,” or simply “plaque burden.” To account for differences in patient sex and body size, plaque volume was normalized to vessel volume, reducing variability and providing a more optimal method of reporting the coronary atherosclerotic plaque burden [44]. In 2D cross-sectional PA, plaque area is indexed to vessel area (mm2) to calculate the “cross-sectional plaque burden,” typically at the site of maximal stenosis. Additional parameters automatically calculated by PA software include plaque length, plaque thickness, remodeling index, and the ratio of the maximal vessel dimension within a lesion to that at a proximal “normal” reference point.
Software vendors exhibit high heterogeneity in the terminology and thresholds used to define plaque components. Calcified plaque, or “dense calcium”, is generally defined by a density ≥ 350 Hounsfield unit (HU). NCP, referred to as “fibrotic” or “medium density” plaque, is often further categorized into fibrous and fibro-fatty components. Low-density NCP, typically < 30 HU, may be labeled as “necrotic core,” “lipid-rich,” “lipid-rich necrotic core,” or simply “low attenuation plaque” [24,45]. While default HU thresholds are set for most software platforms, users can adjust them. Some vendors use adaptive scan-specific thresholds that are automatically adjusted based on lumen attenuation, considering their influence on the absolute HU of plaque components.
Software-based plaque measurement accuracy and reproducibility depend on image quality and reader experience. Puchner et al. [46] found that iterative reconstruction algorithms enhanced CCTA-derived cross-sectional plaque burden, correlating more strongly with IVUS than traditional methods. Stolzmann et al. [47] revealed excellent inter-reader reproducibility and a high correlation with IVUS for plaque burden using CCTA, regardless of the reconstruction algorithm used in an ex vivo study. Various software vendors have shown robust intraobserver and interobserver agreements for plaque volumes. However, there is a lack of data on inter-platform reproducibility.
The QAngio software (Medis Medical Imaging Systems, Leiden, the Netherlands) was validated against IVUS, demonstrating a strong correlation between lumen area stenosis and plaque burden. Moreover, the quantification of plaque subtypes, including fibrofatty, fibrous, and calcified volumes, exhibited excellent correlation with those assessed by IVUS. The well-established (Progression of AtheRosclerotic PlAque DetermIned by Computed Tomographic Angiography Imaging) PARADIGM registry examined serial changes in plaque components using Medis QAngio for at least 2 years between baseline and follow-up scans for over 2000 patients. The findings of multiple substudies revealed important factors modulating plaque progression, including baseline plaque burden and statin therapy [48,49].
SUREplaque (Canon Medical Systems, Otawara, Japan) was also validated against IVUS in a prospective study focusing on the accuracy of 3D quantitative PA using CCTA compared to IVUS with radiofrequency backscatter analysis. Although there was a wide limit of agreement, the overall mean differences in the total plaque assessment were minor [39]. Furthermore, low-density NCP correlated with the necrotic core and fibrofatty tissue on IVUS. Compared to the invasive coronary angiography findings of the culprit lesion, CCTA features of plaque disruption in patients with unstable angina demonstrated good sensitivity (53%–81%) and specificity (82%–95%) [50].
Cleerly Healthcare (New York, USA) offers AI-based, fully automated CCTA analysis with manual adjustment, if necessary. The software has been validated primarily through studies comparing it with expert plaque quantification or QCA analyses. Initial validation studies reported excellent diagnostic performance for detecting > 70% and > 50% stenosis compared to the consensus of three level-3 expert CCTA readers [40]. In a sub-study of the PARADIGM registry, the software proved effective in evaluating a large patient population for detecting small changes in plaque burden and reducing measurement variability compared with the initially used software [51].
vascuCAP (Elucid Bioimaging, Wenham, MA, USA) is another plaque quantification software initially validated against carotid CT imaging histopathological findings. This study demonstrated a strong correlation between calcification and lipid-rich necrotic core [41]. The software was also applied in the Effect of Vascepa on Improving Coronary Atherosclerosis in People with High Triglycerides Taking Statin Therapy (EVAPORATE) randomized trial, which assessed changes in plaque morphology in a trial evaluating the efficacy of icosapent ethyl in patients with hypertriglyceridemia. Evaluation at three time points, baseline, 9 months, and 18 months of follow-up, showed potential in assessing plaque changes early in follow-up at 9 months [52]. Furthermore, this study suggests that assessing more detailed changes in plaque characteristics, such as maximal wall thickness and increases in cap thickness, might be feasible.
Autoplaque (Cedars-Sinai Medical Center, Los Angeles, CA, USA) is widely used and was initially validated and compared with IVUS, showing an excellent correlation of quantified NCPs between the software and IVUS. The software, utilized in large multicenter clinical trials, such as the Scottish Computed Tomography of the HEART (SCOT-HEART) and Rapid Assessment of Potential Ischemic Heart Disease with computerized tomography coronary angiography (RAPID CTCA), demonstrated its capability to improve the identification of patients at high risk for adverse CVD events. Furthermore, quantitative plaque burden assessment improved the assessment of lesion-specific ischemia and predicted lesions requiring revascularization by implementing machine-learning techniques [15,53]. Most recently, the application of a rapid AI tool enabled fully automated plaque quantification, showing good-to-excellent agreement between automated plaque and expert reader measurements of the total plaque volume and diameter stenosis [9].
HeartFlow (Mountain View, CA, USA) offers an automated AI-PA that was validated against expert reader plaque quantification using Autoplaque software. Pearson’s correlation coefficient demonstrated a highly significant correlation between AI-PA and CT readers when the overall total atherosclerotic plaques were assessed [43]. The tool’s accuracy was also compared with that of IVUS, demonstrating that the total plaque volume, vessel, lumen, and plaque subtype volumes derived from the AI-PA tool were highly correlated with those derived from IVUS in per-lesion analysis.
Clinical Application of Quantitative Plaque Assessment
Risk Prediction
Total Plaque Burden
The total plaque burden derived from CCTA has demonstrated a predictive value for subsequent cardiac events [54,55]. In a prospective study analyzing the results of the PARADIGM registry involving 1345 patients, the additional value of semiautomated quantitative total plaque volume over qualitative CCTA evaluation methods improved the prediction of rapid plaque progression and adverse clinical outcomes [56]. More recently, Lin et al. [9] demonstrated that a deep learning-based plaque quantification system could predict the risk of myocardial infarction.
Plaque Subtypes
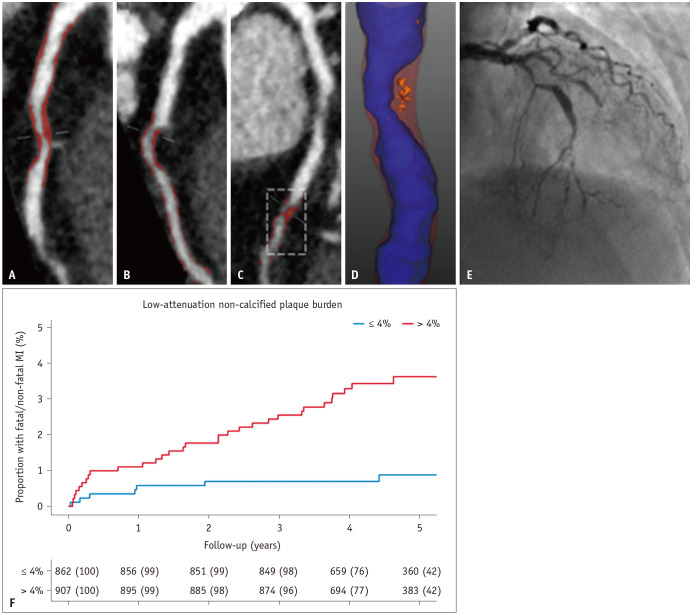
A growing body of evidence suggests that the specific plaque phenotypes are more strongly associated with the risk of plaque rupture and increased cardiovascular events. In quantitative PA using CCTA imaging, the plaque subtype was differentiated based on HU. The CT findings of low-HU attenuation plaques signified the presence of high intraplaque lipid content. Quantification of LAP holds promise as a marker of high-risk plaques and a prognostic indicator. Previous studies have shown that increased low-density NCP increases the risk of plaque rupture and myocardial infarction. For example, Chang et al. [54] performed quantitative PAs in 234 patients with ACS and 234 matched controls in the Incident Coronary Syndromes Identified by Computed Tomography (ICONIC) sub-study of the Coronary CT Angiography Evaluation for Evaluation of Clinical Outcomes: An International Multicenter Registry (CONFIRM) registry. They found that the total, calcified, and fibrous plaque volumes did not differ significantly between patients with ACS and controls. In contrast, the fibrofatty plaque and necrotic core volumes were substantially higher in patients with ACS than in controls. In addition, in the SCOT-HEART trial, LAP burden was the strongest predictor of fatal or nonfatal myocardial infarction beyond the cardiovascular risk score, CAC score, or obstructive coronary artery stenoses [55]. They found that patients with an LAP burden > 4% had a nearly five times higher risk of myocardial infarction (Fig. 3). Similar observations were reported for RAPID-CTCA. In patients with suspected ACS, LAP burden is a significant predictor of 1-year death or recurrent myocardial infarction [57]. Patients with an LAP burden above the median had an approximately 8-fold increased risk of adverse CVD outcomes, outperforming conventional stenosis-based approaches.
Fig. 3. Plaque characteristics. A: Proximal LAD. B: First diagonal. C: Mid LAD. D: Mid-LAD plaque with blue lumen, red noncalcified plaque, and orange LAP. E: Invasive coronary angiography. F: Cumulative incidence of MI in patients with and without a LAP burden greater than 4%. Adapted from Williams et al. Circulation 2020;141:1452-1462, with permission of Wolters Kluwer Health [55]. LAD = left anterior descending, LAP = low attenuation plaque, MI = myocardial infarction.
In contrast to low-density plaques, high-density calcium is considered a stabilized plaque phenotype associated with low CVD risk. Early studies using noncontrast CAC scoring CT scans have shown that calcium density is inversely related to coronary heart disease and CVD risk at any CAC volume level [58]. This density assessment can also be applied to quantitative CCTA based on the HU threshold. In a substudy of ICONIC, van Rosendael et al. [59] showed that patients who experienced subsequent ACS events had not only a high burden of low-density NCP but also a significantly low burden of high-density calcium, defined as > 1000 HU, compared with those without ACS events. Moreover, statin treatment is associated with an increased volume of high-density calcified plaques, suggesting that increased calcium densification may be related to plaque healing and a reduced risk of plaque rupture [48].
Plaque Distribution
In addition to the evaluation of plaque morphology and burden, CCTA permits the accurate determination of plaque distribution and vessel curvature. The quantitative assessment of these geometric characteristics has improved the risk prediction of future CVD events. In a serial CCTA study of 1478 patients, proximally located lesions tend to have more significant lipid-density plaque components and progress rapidly [60]. Another study investigated the incremental prognostic values of quantitative adverse geometric characteristic assessments, including ostial to plaque distance, vessel tortuosity, and lesion at bifurcation, for future ACS in a sub-study of the ICONIC study [61]. This study found that CCTA-derived adverse geometric characteristics were significantly associated with the risk of future ACS-causing culprit lesions and conventional CCTA assessments, including diameter stenosis, adverse plaque characteristics, and quantitative plaque characteristics.
Plaque Radiomics
Radiomics is a method for extracting imaging features (radiomic features) from medical images using data characterization algorithms. This serves as a potential quantitative approach to enhance the precise phenotyping of diseases. Several studies have shown that applying radiomics to CCTA can improve the identification of vulnerable plaque characteristics. Kolossváry et al. [62] compared radiomics-based ML models with visual and histogram-based assessments of ex vivo CCTA, using histological examination as a reference standard for detecting advanced atherosclerotic lesions. This study showed that the radiomics-based ML model improved the discrimination of plaques from advanced atherosclerotic lesions, which are associated with a higher risk of future myocardial infarction. In addition, Lin et al. [63] found distinct radiomic features in culprit lesions in acute myocardial infarction compared to nonculprit lesions in the same patients and with lesions in stable CAD patients. Recent studies have also suggested that the CCTA-derived radiomic signature of coronary plaques enables better identification of rapid plaque progression and improved prediction of future adverse cardiac events compared to conventional morphological plaque parameters [64,65]. Integrating radiomic analysis with AI-based plaque assessment can enhance the detection of patients at an elevated risk of future cardiovascular events, potentially warranting more aggressive preventive interventions. Nevertheless, the clinical application of radiomics in CCTA PA is in its early phases, and additional studies are required to validate its efficacy. The development of standardized radiomics approaches is essential to ensure consistency and reliability across research settings and clinical practices.
Monitoring Medical Therapy with Serial CCTA Scans
An essential advantage of the quantitative analysis of plaque composition is that plaque changes over time can be assessed as objective indicators through serial CCTA. Table 2 summarizes previous studies that explored the association between conventional clinical risk factors and changes in quantitative plaque characteristics using serial CCTA analysis [66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85]. Previous studies on patients who underwent serial CCTA examinations have reported associations between clinical factors, laboratory values, and changes in quantitative plaque characteristics. For example, the presence of conventional risk factors such as diabetes or high low-density lipoprotein (LDL) cholesterol levels is associated with accelerated plaque progression [66,67,69,71,72,73,74]. Patients at high risk of atherosclerotic cardiovascular disease (ASCVD) with an increased ASCVD risk score demonstrated more rapid plaque progression, including calcified plaques, fibrofatty plaques, and LAP, and exhibited more newly developed adverse plaques [77]. Plaque changes exhibited sex-related distinctions, indicating more favorable alterations in women. Women demonstrate slower NCP progression and faster calcified plaque progression than men [75,76]. Otaki et al. [68] also showed that a reduction in LDL-cholesterol level was associated with a reduction in all components of the NCP, including LAP.
Table 2. Studies exploring the association between clinical risk factors and serial plaque changes.
| Study | Patients (n) | Population | Study type | Intervention (groups) | Software | Plaque measures | Follow up* | Results | |
|---|---|---|---|---|---|---|---|---|---|
| LDL-C | |||||||||
| Shin et al., 2017 [66] | 147 | Participants who underwent serial CCTA | Prospective observational | F/u LDL-C < 70 mg/dL: 37 | QAngio | PV, dense calcium | 3.2 years | Patients with LDL-C below 70 mg/dL displayed a significant attenuation in PP | |
| F/u LDL-C ≥ 70 mg/dL: 110 | |||||||||
| Svanteson et al., 2019 [67] | 68 | Patients with IJD and carotid artery plaque(s) underwent CCTA before and after statin treatment | Prospective observational | RA 45 | Plaque Analysis, Comprehensive Cardiac, Philips Healthcare | TP, CP, MP | 4.7 years | Patients who had obtained the LDL-C treatment target at follow-up, experienced reduced progression of both CAC and TP volume | |
| AS 15 | |||||||||
| Psoriatic arthritis 8 | |||||||||
| Otaki et al., 2019 [68] | 154 | Patients with serial CCTA | Retrospective observational | LDL-C decrease 85 | Autoplaque | TP, NCP, LAP | 4 years | There was interval reduction in TP, LAP, MLAP, and MAP volumes in patients with LDL-C decrease | |
| No LDL-C decrease 69 | |||||||||
| Shi et al., 2022 [69] | 208 | DM patients | Retrospective observational | LDL controlled 75 | cvi42, Circle Cardiovascular Imaging | TP, CP, NCP, LD-NCP | Unavailable | Increase in CACs was independently associated with the annual change of NCPV and LD-NCPV in LDL-C uncontrolled patient | |
| LDL uncontrolled 133 | |||||||||
| Sun et al., 2022 [71] | 240 | Patients over 60 years old | Retrospective observational | Intensive lipid lowering 66 | QAngio | TP, CP, NCP, Fibrous, FF, lipid rich plaque | 2 years | Intensive lipid-lowering group demonstrated a higher progression in calcified PV, CACS, and PCPV, and a significantly greater attenuation in FF and lipid-rich PV | |
| Lipid lowering 110 | |||||||||
| Control 64 | |||||||||
| Hirai et al., 2023 [70] | 81 | ACS patients | Retrospective observational | LDL-C < 70 48 | Vitrea, Canon | TP, CP, LAP, Fibrous | 1 year | CP volume was significantly increased in the LDL-C < 70 group | |
| LDL-C ≥ 70 33 | |||||||||
| Percent change in LAP volume in the LDL < 70 group was significantly lower than in the LDL-C ≥ 70 group | |||||||||
| Diabetes | |||||||||
| Nakanishi et al., 2016 [73] | 142 | Patients who were clinically referred for serial CCTA | Propensity-matched study | DM 71 | QAngio | TP, NCP, CP, Fibrous, FF, LAP | 3.4 years | Diabetic patients showed a 2-fold greater progression in normalized TPV than non-diabetes patients. DM was associated with normalized TP and NCP progression | |
| Non-DM 71 | |||||||||
| Kim et al., 2018 [72] | 1602 | PARADIGM | Prospective observational | No DM 326 | QAngio | PV, CP NCP, LAP, FF, NC | 3.8 years | Percent changes in overall PV and NC volume were significantly greater in those with DM | |
| Propensity score matching | DM 326 | ||||||||
| Won et al., 2019 [74] | 1296 | PARADIGM | Prospective observational | According to glycemic status: normal, pre-DM, and DM | QAngio | TP | 3.2 years | Adjusted OR for PP was higher in DM than non-DM | |
| Sex difference | |||||||||
| Lee et al., 2020 [76] | 1255 | Suspected CAD | Prospective observational | Women 543 | QAngio | TP, CP, NCP, LAP | 2 years | Women was associated with greater calcified PV progression but slower noncalcified PV progression than in men | |
| Men 712 | |||||||||
| El Mahdiui et al., 2021 [75] | 211 | Patients underwent CCTA | Prospective observational | Men 146 | QAngio | TP, CP, NCP, Fibrous, FF, NC | 6.2 years | Women under 55 years demonstrated significantly greater reduction in fibrous and non-calcified PAV over time compared to age-matched men | |
| Women 65 | |||||||||
| Other risk factors | |||||||||
| Han et al., 2020 [77] | 1005 | PARADIGM without known CAD | Prospective observational | ASCVD risk | QAngio | TP, CP, NCP, LAP | 3.3 years | Annualized progression rate of PAV for TP, CP, and NCP was associated with increasing ASCVD risk score | |
| Low 463 | |||||||||
| Intermediated 373 | |||||||||
| High 169 | |||||||||
| Weber et al., 2020 [78] | 350 | Patients underwent serial CCTA | Retrospective observational | - | QAngio | TP, CP, NCP, LAP | 3.6 years | Men and typical angina were identified as risk factors for fast TPV progression, while HDL-C had a protective effect | |
| Won et al., 2020 [80] | 1143 | PARADIGM with available data on TyG index and diabetic status | Prospective observational | TyG index | QAngio | TP, CP, Fibrous, FF | 3.2 years | Risk of PP and rapid PP was increased in highest TyG index compared to that in lowest TyG index | |
| Lowest 382 | |||||||||
| Middle 388 | |||||||||
| Highest 373 | |||||||||
| Won et al., 2022 [79] | 830 | PARADIGM | Prospective observational | Baseline hemoglobin | QAngio | TP | 3.2 years | Hemoglobin change was independently associated with a decrease in annualized total PVC | |
| Won et al., 2022 [81] | 95 | PARADIGM | Prospective observational | Normal SBP 40 | QAngio | TP, CP, Fibrous, FF, NC | 3.5 years | SBP maintain ≥ 118.5 mm Hg and baseline total PV independently influenced coronary PP | |
| Elevated SBP 55 | |||||||||
| Ben Zekry et al., 2022 [84] | 1234 | PARADIGM | Prospective observational | East-Asian 955 | QAngio | TP, CP, Fibrous, FF | 8.5 years | East Asians with PP had more clinical risk factors and higher plaque burden at baseline | |
| Caucasian 279 | |||||||||
| Li et al., 2020 [82] | 396 | T2DM patients | Prospective Observational | Non-progression 253 | - | TP | 2.3 years | Long-term glycemic variability is associated with accelerated PP | |
| Progression 143 | |||||||||
| Wang et al., 2021 [83] | 116 | Serial CCTA without prior CAD history | Retrospective, observational | PP (-) 84 | Syngo.via VB10B | TP | 30.8 months | Elevated baseline Lp(a) level was an independent risk factor for PP | |
| Kaiser et al., 2022 [85] | 191 | Sable CAD, serial CCTA at baseline and 1 year | A substudy of randomized controlled trial | Lp(a) ≥ 70 43 | Autoplaque | TP, CP, NCP, FF, low density plaque | 1 year | Lp(a) is associated with accelerated progression of coronary LAP | |
| Lp(a) < 70 148 | |||||||||
*The mean or median values.
CCTA = coronary computed tomography angiography, F/u = follow up, LDL-C = low density lipoprotein cholesterol, PV = plaque volume, PP = plaque progression, IJD = inflammatory joint diseases, RA = rheumatoid arthritis, AS = ankylosing spondylitis, TP = total plaque, CP = calcified plaque, MP = mixed plaque, CAC = coronary artery calcium, NCP = non-CP, LAP = low attenuation plaque, MLAP = medium LAP, MAP = medium attenuation plaque, DM = diabetes mellitus, LD-NCPV = low-density noncalcified PV, FF = fibro-fatty, ACS = acute coronary syndrome, NC = necrotic core, CAD = coronary artery disease, PAV = percentage atheroma volume, ASCVD = atherosclerotic cardiovascular disease, HDL = high density lipoprotein, TyG = triglyceride glucose index, PVC = plaque volume change, SBP = systolic blood pressure, Lp(a) = lipoprotein(a)
Furthermore, a recent study showed that higher lipoprotein(a) levels are associated with accelerated progression of coronary LAP [83,85]. Beyond the established risk factors, research has explored the link between plaque progression and variables such as triglyceride levels, hemoglobin changes, and blood pressure control maintenance [79,80,81]. Consistent with the established link between CVD risk factors and plaque modification, changes in these risk factors can induce favorable changes in plaque characteristics, potentially mitigating the risk of future CVD events.
Table 3 summarizes prior research using serial CCTA quantitative analysis to assess changes in coronary artery plaques in response to therapies [48,49,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108]. Studies have consistently shown that statin use is associated with reduced or slower progression of the overall coronary plaque volume and reduced high-risk plaque features, while accelerating the progression of calcified plaque volume in patients with suspected CAD [49,86,87,88,91,93], acute myocardial infarction [90] and human immunodeficiency virus [89,92,94]. These findings suggest that the benefits of statins and lowering LDL cholesterol levels in CVD risk reduction can be evaluated by serial monitoring of quantitative CT PA, namely, favorable modification of plaque subtypes (Fig. 4).
Table 3. Studies assessing quantitative plaque changes on serial CCTA in response to therapies.
| Study | Patients (n) | Population | Study type | Intervention (groups) | Software | Plaque measures | Follow up* | Results | |
|---|---|---|---|---|---|---|---|---|---|
| Statin | |||||||||
| Hoffmann et al., 2010 [86] | 63 | Serial CCTA studies | Retrospective observational | Statin* | Vitrea | TP, NCP, MP, CP | 25 months | Statins significantly slowed the growth of NCP but did not significantly affect the growth rate of MP or CP | |
| Inoue et al., 2010 [87] | 32 | Suspected CAD, no baseline statin | Prospective observational | Statin 24 | SUREPlaque | LAP, intermediate, calcified based on HU | 12 months | Statin treatment results in significant reduction of TP and LAP volumes | |
| No statin 8 | |||||||||
| Zeb et al., 2013 [88] | 100 | No history of CAD and serial CCTA at an interscan interval of 1 year | Retrospective observational | Statin 60 | Vitrea | TP, NCP, MP, CP, LAP | 406 days | Mean plaque volume difference between statin and non-statin users was statistically significant for both LAP and NCP volumes | |
| No statin 40 | |||||||||
| Lo et al., 2015 [89] | 40 | HIV-infected patients on stable ART, and LDL-C between 70–130 mg/dL | Prospective randomized | Atorvastatin 19 | Aquarius iNtuition, Terarecon | TP, NCP | 1 year | Atorvastatin reduced NCP volume relative to placebo | |
| No statin 21 | |||||||||
| Auscher et al., 2015 [90] | 96 | Acute MI patients | Prospective randomized | Intensive statin 48 | QAngio | TP, NC, FF, Fibrous, CP | 1 year | Plaque composition changed over 1 year with an increase in total dense calcium volume in the intensive care group and a decreased in the usual care group | |
| Standard statin 48 | |||||||||
| Li et al., 2016 [91] | 206 | Suspected CAD | Prospective observational | Intensive 55 | CardIQ Xpress 2.0 | LAP, TP, PPV | 18 months | LAP volume, TP volume, and PPV showed significant regression among intensive-statin compared with no-statin group | |
| Moderate 85 | |||||||||
| No statin 66 | |||||||||
| Nou et al., 2016 [92] | 40 | HIV-infected patients on stable ART with subclinical coronary atherosclerosis and LDL-C less than 130 mg/dL | Prospective randomized | Atorvastatin 19 | - | TP, NCP, CP | 12 months | Change in oxLDL significantly correlated with changes in NCP volume, TP volume | |
| Placebo 21 | |||||||||
| Lee et al., 2018 [49] | 1255 | serial CCTA at an interscan interval of ≥ 2 years | Prospective observational | Statin naïve 474 | QAngio | PV, CP NCP, LAP, FF, Fibrous | 3.4 years | Lesions in statin-taking patients displayed a slower rate of overall PAV progression but more rapid progression of calcified PAV | |
| Statin taking 781 | |||||||||
| Smit et al., 2020 [93] | 202 | Suspected CAD | Prospective observational | Statin (+) 161 | QAngio | TP, CP, NCP | 6.4 years | Statin use showed an independent association with annual progression of CP. Statin use was borderline significantly associated with a reduced progression of NCP | |
| Statin (-) 41 | |||||||||
| Foldyna et al., 2020 [94] | 40 | HIV-infected patients | Prospective randomized | Atorvastatin 19 | Aquarius iNtuition, TeraRecon | TP, CP, FF, Fibrous | 12 months | Statins suppressed progression of fibrotic plaque, with a trend towards reducing fatty plaque and no significant effect on CP | |
| Placebo 21 | |||||||||
| van Rosendael et al., 2021 [48] | 857 | PARADIGM | Prospective observational | Statin (+) 548 | QAngio | LAP, FF, Fibrous, low-density calcium, high density calcium | 3.4 years | Statin therapy was associated with volume decreases in LAP and FF plaque and greater progression of high-density CP and 1K plaque | |
| Statin (-) 309 | |||||||||
| Other lipid lowering treatment | |||||||||
| Alfaddagh et al., 2017 [96] | 285 | Stable CAD on statins | Prospective randomized | Omega-3 ethyl ester 143 | SUREPlaque | TP, CP, NCP, FF, Fibrous | 30 months | No difference was observed in NCP volume, between the 2 treatment groups | |
| Control 142 | |||||||||
| Budoff et al., 2020 [98] | 80 | Patients with stenoses with ≥ 20% persistently elevated TG levels | Prospective randomized | IPE 31 | QAngio | TP, NCP, LAP, FF, CP | 18 months | IPE demonstrated significant regression of LAP | |
| Placebo 37 | |||||||||
| Motoyama et al., 2022 [95] | 210 | ACS patients | Retrospective observational | No EPA/DHA 69 | QAngio | TP, CP, NCP, LAP, Fibrous, FF | 24 months | Addition of high-dose EPA to statin therapy was associated with a lower rate of plaque progression | |
| Low dose EPA + DHA 51 | |||||||||
| High dose EPA + DHA 20 | |||||||||
| High dose EPA alone 70 | |||||||||
| Baumann et al., 2022 [97] | 23 | Patients underwent CCTA | Prospective observational | PCSK 9 inhibitor | Syngo VE36A | TP, CP, NCP | 1 year | TPV, CPV, NCPV, lumen volume, and functional plaque parameters did not change significantly | |
| Pérez et al., 2023 [99] | 104 | Familial hypercholesterolemia without ASCVD | Phase IV clinical trial | Alirocumab, PCSK9 inhibitor | QAngio | TP, CP, Fibrous, FF, NC | 78 weeks | Alirocumab + high-intensity statin induced increased calcified, fibrous plaque, and decreased FF, necrotic plaque | |
| Biology therapy in psoriasis patients | |||||||||
| Elnabawi et al., 2019 [100] | 290 | Severe psoriasis | Prospective observational | TNF-a, IL 12/23, IL 17 inhibitor vs. placebo | QAngio | TP, NCP, CP, LAP | 1 year | Biology therapy is associated with decreased NCP, FF, necrotic burden | |
| Choi et al., 2020 [101] | 209 | Biologic naïve psoriasis patients | Prospective observational | Mild to moderate psoriasis 212 | vascuCAP | LRNC | 1 year | Biologic therapy had a reduction in LRNC | |
| Severe psoriasis 77 | |||||||||
| Other medication | |||||||||
| Budoff et al., 2017 [102] | 138 | Symptomatic hypogonadism | Prospective randomized | Testosterone treatment 73 | QAngio | TP, NCP, LAP, FF, CP | 1 year | Treatment with testosterone gel for 1 year compared with placebo was associated with a significantly greater increase in NCP volume | |
| Placebo 65 | |||||||||
| Lee et al., 2017 [103] | 40 | DM patients | Prospective randomized | Sarpogrelate + aspirin: 20 | Brilliance Workspace V4.5; Philips Healthcare | TP, NCP, CP | 6 months | Sarpogrelate treatment may decrease coronary artery plaque volume, particularly the NCP, in DM patients | |
| Aspirin: 20 | |||||||||
| Matsumoto et al., 2017 [104] | 54 | Recent ACS patients | Prospective randomized | One of 3 VIA 2291 doses (25 mg, 50 mg, 100 mg) or placebo | SUREPlaque, | LAP, FF, Fibrous, dense calcium | 6 months | VIA-2291 resulted in slowed PP compared with placebo across different plaque subtypes in patients with recent ACS | |
| Vaidya et al., 2018 [105] | 80 | Recent ACS (< 1 month) | Prospective observational | Colchicine + OMT 40 | GE Advantage workstation v4.5 | CP, NCP, LAP, TAV | 12.6 months | Colchicine therapy significantly reduced LAPV | |
| OMT alone 40 | |||||||||
| Shaikh et al., 2020 [106] | 66 | DM patients | Prospective randomized | Aged garlic extract 37 | QAngio | TP, NCP, CP, LAP | 1 year | Aged garlic extract group exhibited a statistically significant regression in normalized LAP | |
| Placebo 29 | |||||||||
| Aldana-Bitar et al., 2023 [107] | 74 | Patients with nonvalvular atrial fibrillation using apixaban or rivaroxaban | Prospective randomized | Apixaban 29 | AW 4.6 GE Healthcare | TP, CP, NCP | 12 months | Significantly lower CP progression in the apixaban group | |
| Rivaroxaban 45 | |||||||||
| Heinsen et al., 2023 [108] | 204 | Asymptomatic DM patients | Prospective observational | Liraglutide (+) 55 | QAngio | TP, CP, Fibrous, FF, NC | 1 year | A greater increase in fibrous plaque volume was seen in the Lira+ vs. the Lira- group | |
| Liraglutide (-) 149 | |||||||||
*The mean or median values.
CCTA = coronary computed tomography angiography, TP = total plaque, NCP = noncalcified plaque, MP = mixed plaque, CP = calcified plaque, CAD = coronary artery disease, LAP = low attenuation plaque, HU = Hounsfield unit, HIV = human immunodeficiency virus, ART = antiretroviral therapy, LDL-C = low density lipoprotein cholesterol, MI = myocardial infarction, NC = necrotic core, FF = fibro-fatty, PPV = percent plaque volume, oxLDL = oxidized LDL, PAV = percentage atheroma volume, IPE = icosapent ethyl, ACS = acute coronary syndrome, EPA = epicosapentaenoic acid, DHA = docosahexaenoic acid, ASCVD = atherosclerotic cardiovascular disease, LRNC = lipid rich necrotic core, DM = diabetes mellitus, OMT = optimal medical therapy
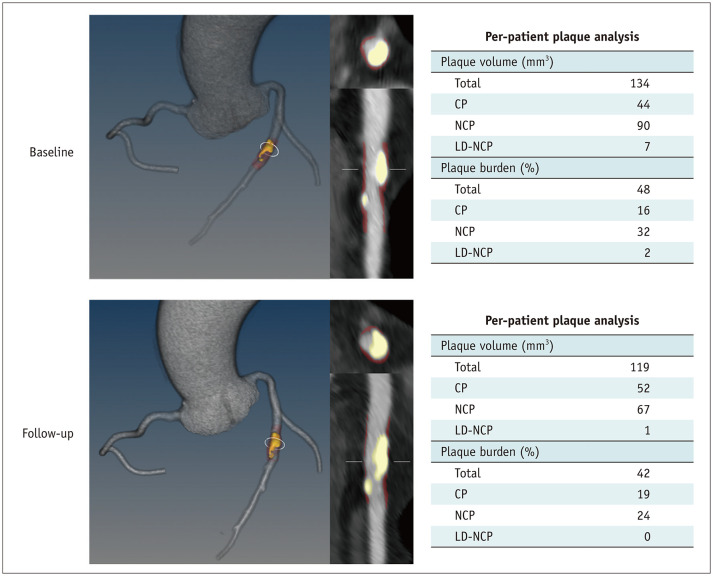
Fig. 4. 3D rendered view of the coronary tree and quantitative plaque volume from a 64-year-old woman who was treated with high-intensity statins. The interscan interval is 2.3 years. CP increased (yellow overlay in 3D and 2D images), and noncalcified and LD-NCPs decreased (red overlay). Changes in plaque volume and burden are presented in tables. D = dimensional, CP = calcified plaque, LD-NCP = low-density NCP, NCP = noncalcified plaque.
Studies have also shown that serial CCTA can monitor plaque changes in patients receiving other lipid-lowering therapies [95,96,97,98,99]. As noted above, the EVAPORATE study revealed that icosapent ethyl was associated with a significant regression of LAP volume compared to placebo over 18 months [98]. More recently, the Effect of Alirocumab on Atherosclerotic Plaque Volume, Architecture and Composition (ARCHITECT) study demonstrated that treatment with the PCSK9 inhibitor alirocumab and a high-intensity statin for 78 weeks in patients with familial hypercholesterolemia induced significant plaque regression of the coronary artery and plaque stabilization with an increase in calcified and fibrous plaques, accompanied by a reduction in fibrofatty and necrotic plaques [99].
Several studies have examined the effects of medication on plaque modification. The ongoing WARRIOR CCTA (NCT05035056) sub-study evaluating plaque changes by serial CCTA, in which symptomatic women with nonobstructive CAD are randomized to usual care or intensive medical therapy (statins, angiotensin-converting enzyme inhibitors, aspirin), may shed further light on the effects of renin-angiotensin-aldosterone system inhibitors on the atherosclerotic process in addition to ACS, stroke, and cardiac mortality. In another study, the impact of evolocumab on coronary artery plaque volume and composition by CCTA and microcalcification by 18F-sodium fluoride (18F-NaF) PET (EVOLVE study, NCT03689946) was studied to determine the effects of evolocumab on changes in coronary plaque volume, as measured by serial CCTA and microcalcification activity using serial 18F-NaF PET. Future studies will provide critical mechanistic insights into plaque characteristics that may inform clinical trials of novel lipid-lowering agents or other preventive strategies for reducing the risk of CVD.
Studies have also shown changes in coronary artery plaques when treating conditions unrelated to cholesterol treatment. Budoff et al. [102] investigated the effects of testosterone treatment on coronary plaques in older men with low testosterone levels in a double-blinded, placebo-controlled trial. They found that 1 year of testosterone gel treatment was associated with an increased volume of noncalcified coronary artery plaques without changes in the CAC score, as measured by serial CCTA scans. Elnabawi et al. [100] evaluated the changes in coronary artery plaques in psoriasis patients treated with biologic therapies, such as anti-tumor necrosis factor, anti-interleukin (IL) 12/23, and anti-IL 17. They observed a favorable modification, primarily a reduction in the NCP burden, without significant changes in calcified plaques. The study noted diminished inflammatory phenotypes, including fibrofatty plaques and necrotic cores, and biomarkers in patients with psoriasis receiving biological treatment. Furthermore, studies have explored the influence of drugs such as sarpogrelate [103], colchicine [105], aged garlic extract [106], and liraglutide [108] on modifications in plaque composition. These findings suggest that serial CCTA can be expanded to evaluate overall CVD risk assessment in patients with various conditions that may facilitate CVD progression and are at high risk for CVD.
Limitations and Barriers to Implementation
One challenge in implementing quantitative analysis in clinical practice is the time required for the analysis. Most methods are semi-automated and need human interaction to refine the detected vessel contours. Significant time investment is necessary when handling cases with high plaque burden and poor image quality. Consequently, much of this software has been primarily applied in research because its speed and labor-intensive nature are significant barriers to its clinical deployment. Recently, several AI-based CCTA PA software programs, which rapidly perform with minimal subjective adjustment, have been approved by the FDA for clinical use. Accelerating the speed of analysis and improving access to these software tools are essential factors in promoting their broader adoption in clinical practice.
Furthermore, despite validation using invasive imaging or expert manual measurements, each software platform may yield very different results for plaque volumes. Head-to-head comparisons of the latest technologies have yet to be conducted.
The quality of CT images, and consequently, the accuracy of quantitative plaque measurements, can be influenced by various factors, including CCTA and clinical parameters such as imaging protocol, contrast timing, scan parameters, reconstruction technique, temporal and spatial resolution, heart rhythm variability, and patient-specific factors [109,110,111]. Addressing these limitations requires the establishment of standardized imaging protocols, guidelines, and quality assurance measures to ensure the consistency and comparability of results in clinical practice and research involving CCTA. In addition, validating quantitative analysis software across multiple CT vendors and diverse patient cohorts, including populations with varying clinical and imaging characteristics, is essential to address validity concerns and enhance the reliability of the findings.
The principal limitation of the practical clinical use of various quantitative plaque measurements is that physicians do not yet know how to use these data to guide patient management. Some studies have attempted to establish a reference threshold for quantitative plaque volume based on CCTA. HeartFlow AI-PA recently established age- and sex-based nomograms for plaque volumes derived from a large cohort of 11808 patients who underwent clinically indicated CCTA [43]. A staging system was proposed for absolute total plaque volume and percentage atheroma volume based on lesions’ anatomical and functional significance on invasive QCA and FFR [112]. Although plaque measurements have been shown to add predictive information regarding cardiac events, there currently needs to be a consensus on applying these findings to individual patient management. One straightforward application is the assessment of therapy effectiveness through consecutive measurements from serial CCTA studies. Nonetheless, as AI methods continue to improve the various software used for quantitative plaque measurement, these assessments will soon be widely used in the practical care of patients with coronary atherosclerosis.
CONCLUSION
The paradigm of CCTA image analysis has moved beyond the visual assessment of coronary artery stenosis to include the characteristics and quantitative analysis of coronary plaques. CCTA-derived plaque volume and composition measurements can now be efficiently performed using semi-automated software, demonstrating strong correlations with IVUS results. Quantitative analysis of coronary plaques improves subsequent cardiac event prediction and enables a more precise assessment of temporal plaque changes on serial imaging. Moreover, applying AI techniques such as deep learning will facilitate the complete automation of coronary plaque and stenosis quantification. Furthermore, there is the potential to identify new “high-risk” plaque phenotypes through ongoing software and AI advancements. Integrating quantitative PA with factors such as stenosis severity and high-risk plaque characteristics may contribute to a more comprehensive cardiovascular risk assessment in patients undergoing CCTA. However, for these analyses to be incorporated into clinical practice, conducting studies demonstrating how changes in plaque properties lead to improved outcomes is essential.
Footnotes
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
- Conceptualization: Donghee Han.
- Data curation: Su Nam Lee, Donghee Han.
- Formal analysis: Su Nam Lee, Donghee Han.
- Funding acquisition: Donghee Han, Damini Dey, Daniel S. Berman.
- Investigation: Su Nam Lee, Donghee Han.
- Methodology: Su Nam Lee, Andrew Lin.
- Project administration: Su Nam Lee, Donghee Han.
- Resources: Su Nam Lee, Andrew Lin, Donghee Han.
- Software: Andrew Lin, Damini Dey, Donghee Han.
- Supervision: Damini Dey, Daniel S. Berman.
- Validation: Andrew Lin, Damini Dey, Donghee Han.
- Visualization: Andrew Lin, Damini Dey, Donghee Han.
- Writing—original draft: Su Nam Lee, Donghee Han.
- Writing—review & editing: all authors.
Funding Statement: The work was supported in part by a grant from the Miriam and Sheldon G. Adelson Medical Research Foundation. Outside of the current work, Dr. Dey is funded by the National Institute of Health/National Heart, Lung, and Blood Institute grants (1R01HL148787-01A1 and 1R01HL151266) and the Winnick Family Foundation as well as a grant from the Miriam and Sheldon G. Adelson Medical Research Foundation.
References
- 1.Knuuti J, Wijns W, Saraste A, Capodanno D, Barbato E, Funck-Brentano C, et al. 2019 ESC guidelines for the diagnosis and management of chronic coronary syndromes. Eur Heart J. 2020;41:407–477. doi: 10.1093/eurheartj/ehz425. [DOI] [PubMed] [Google Scholar]
- 2.Gulati M, Levy PD, Mukherjee D, Amsterdam E, Bhatt DL, Birtcher KK, et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: a report of the American College of Cardiology/American Heart Association joint committee on clinical practice guidelines. Circulation. 2021;144:e368–e454. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 3.Arbab-Zadeh A, Hoe J. Quantification of coronary arterial stenoses by multidetector CT angiography in comparison with conventional angiography methods, caveats, and implications. JACC Cardiovasc Imaging. 2011;4:191–202. doi: 10.1016/j.jcmg.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 4.Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, et al. Angiographic versus functional severity of coronary artery stenoses in the FAME study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. doi: 10.1016/j.jacc.2009.11.096. [DOI] [PubMed] [Google Scholar]
- 5.Cury RC, Leipsic J, Abbara S, Achenbach S, Berman D, Bittencourt M, et al. CAD-RADSTM 2.0 - 2022 coronary artery disease-reporting and data system: an expert consensus document of the Society of Cardiovascular Computed Tomography (SCCT), the American College of Cardiology (ACC), the American College of Radiology (ACR), and the North America Society of Cardiovascular Imaging (NASCI) J Cardiovasc Comput Tomogr. 2022;16:536–557. doi: 10.1016/j.jcct.2022.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Boogers MJ, Schuijf JD, Kitslaar PH, van Werkhoven JM, de Graaf FR, Boersma E, et al. Automated quantification of stenosis severity on 64-slice CT: a comparison with quantitative coronary angiography. JACC Cardiovasc Imaging. 2010;3:699–709. doi: 10.1016/j.jcmg.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 7.Yang DH. Application of artificial intelligence to cardiovascular computed tomography. Korean J Radiol. 2021;22:1597–1608. doi: 10.3348/kjr.2020.1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hong Y, Commandeur F, Cadet S, Goeller M, Doris MK, Chen X, et al. Deep learning-based stenosis quantification from coronary CT angiography. Proc SPIE Int Soc Opt Eng. 2019;10949:109492I. doi: 10.1117/12.2512168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin A, Manral N, McElhinney P, Killekar A, Matsumoto H, Kwiecinski J, et al. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: an international multicentre study. Lancet Digit Health. 2022;4:e256–e265. doi: 10.1016/S2589-7500(22)00022-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffin WF, Choi AD, Riess JS, Marques H, Chang HJ, Choi JH, et al. AI evaluation of stenosis on coronary CTA, comparison with quantitative coronary angiography and fractional flow reserve: a CREDENCE trial substudy. JACC Cardiovasc Imaging. 2023;16:193–205. doi: 10.1016/j.jcmg.2021.10.020. [DOI] [PubMed] [Google Scholar]
- 11.Dundas J, Leipsic JA, Sellers S, Blanke P, Miranda P, Ng N, et al. Artificial intelligence-based coronary stenosis quantification at coronary CT angiography versus quantitative coronary angiography. Radiol Cardiothorac Imaging. 2023;5:e230124. doi: 10.1148/ryct.230124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Madsen KT, Nørgaard BL, Øvrehus KA, Jensen JM, Parner E, Grove EL, et al. Prognostic value of coronary CT angiography-derived fractional flow reserve on 3-year outcomes in patients with stable angina. Radiology. 2023;308:e230524. doi: 10.1148/radiol.230524. [DOI] [PubMed] [Google Scholar]
- 13.Patel MR, Nørgaard BL, Fairbairn TA, Nieman K, Akasaka T, Berman DS, et al. 1-year impact on medical practice and clinical outcomes of FFRCT: the ADVANCE registry. JACC Cardiovasc Imaging. 2020;13(1 Pt 1):97–105. doi: 10.1016/j.jcmg.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 14.Mittal TK, Hothi SS, Venugopal V, Taleyratne J, O’Brien D, Adnan K, et al. The use and efficacy of FFR-CT: real-world multicenter audit of clinical data with cost analysis. JACC Cardiovasc Imaging. 2023;16:1056–1065. doi: 10.1016/j.jcmg.2023.02.005. [DOI] [PubMed] [Google Scholar]
- 15.Dey D, Gaur S, Ovrehus KA, Slomka PJ, Betancur J, Goeller M, et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: a multicentre study. Eur Radiol. 2018;28:2655–2664. doi: 10.1007/s00330-017-5223-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nous FMA, Budde RPJ, Lubbers MM, Yamasaki Y, Kardys I, Bruning TA, et al. Impact of machine-learning CT-derived fractional flow reserve for the diagnosis and management of coronary artery disease in the randomized CRESCENT trials. Eur Radiol. 2020;30:3692–3701. doi: 10.1007/s00330-020-06778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mushtaq S, Conte E, Pontone G, Baggiano A, Annoni A, Formenti A, et al. State-of-the-art-myocardial perfusion stress testing: static CT perfusion. J Cardiovasc Comput Tomogr. 2020;14:294–302. doi: 10.1016/j.jcct.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 18.Choi JH, Koo BK, Yoon YE, Min JK, Song YB, Hahn JY, et al. Diagnostic performance of intracoronary gradient-based methods by coronary computed tomography angiography for the evaluation of physiologically significant coronary artery stenoses: a validation study with fractional flow reserve. Eur Heart J Cardiovasc Imaging. 2012;13:1001–1007. doi: 10.1093/ehjci/jes130. [DOI] [PubMed] [Google Scholar]
- 19.Choi JH, Min JK, Labounty TM, Lin FY, Mendoza DD, Shin DH, et al. Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. JACC Cardiovasc Imaging. 2011;4:1149–1157. doi: 10.1016/j.jcmg.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Yoon YE, Choi JH, Kim JH, Park KW, Doh JH, Kim YJ, et al. Noninvasive diagnosis of ischemia-causing coronary stenosis using CT angiography: diagnostic value of transluminal attenuation gradient and fractional flow reserve computed from coronary CT angiography compared to invasively measured fractional flow reserve. JACC Cardiovasc Imaging. 2012;5:1088–1096. doi: 10.1016/j.jcmg.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Wong DT, Ko BS, Cameron JD, Nerlekar N, Leung MC, Malaiapan Y, et al. Transluminal attenuation gradient in coronary computed tomography angiography is a novel noninvasive approach to the identification of functionally significant coronary artery stenosis: a comparison with fractional flow reserve. J Am Coll Cardiol. 2013;61:1271–1279. doi: 10.1016/j.jacc.2012.12.029. [DOI] [PubMed] [Google Scholar]
- 22.Park EA, Lee W, Park SJ, Kim YK, Hwang HY. Influence of coronary artery diameter on intracoronary transluminal attenuation gradient during CT angiography. JACC Cardiovasc Imaging. 2016;9:1074–1083. doi: 10.1016/j.jcmg.2015.10.028. [DOI] [PubMed] [Google Scholar]
- 23.Bom MJ, Driessen RS, Stuijfzand WJ, Raijmakers PG, Van Kuijk CC, Lammertsma AA, et al. Diagnostic value of transluminal attenuation gradient for the presence of ischemia as defined by fractional flow reserve and quantitative positron emission tomography. JACC Cardiovasc Imaging. 2019;12:323–333. doi: 10.1016/j.jcmg.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 24.Dey D, Achenbach S, Schuhbaeck A, Pflederer T, Nakazato R, Slomka PJ, et al. Comparison of quantitative atherosclerotic plaque burden from coronary CT angiography in patients with first acute coronary syndrome and stable coronary artery disease. J Cardiovasc Comput Tomogr. 2014;8:368–374. doi: 10.1016/j.jcct.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Zamudio M, Fuchs TA, Slomka P, Otaki Y, Arsanjani R, Gransar H, et al. Quantitative plaque features from coronary computed tomography angiography to identify regional ischemia by myocardial perfusion imaging. Eur Heart J Cardiovasc Imaging. 2017;18:499–507. doi: 10.1093/ehjci/jew274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hell MM, Dey D, Marwan M, Achenbach S, Schmid J, Schuhbaeck A. Non-invasive prediction of hemodynamically significant coronary artery stenoses by contrast density difference in coronary CT angiography. Eur J Radiol. 2015;84:1502–1508. doi: 10.1016/j.ejrad.2015.04.024. [DOI] [PubMed] [Google Scholar]
- 27.Lin A, van Diemen PA, Motwani M, McElhinney P, Otaki Y, Han D, et al. Machine learning from quantitative coronary computed tomography angiography predicts fractional flow reserve-defined ischemia and impaired myocardial blood flow. Circ Cardiovasc Imaging. 2022;15:e014369. doi: 10.1161/CIRCIMAGING.122.014369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hecht HS. Coronary artery calcium: utilization for primary prevention of CHD. Curr Cardiol Rep. 2011;13:465–474. doi: 10.1007/s11886-011-0217-y. [DOI] [PubMed] [Google Scholar]
- 29.Kim SY, Suh YJ, Kim NY, Lee S, Nam K, Kim J, et al. A modified length-based grading method for assessing coronary artery calcium severity on non-electrocardiogram-gated chest computed tomography: a multiple-observer study. Korean J Radiol. 2023;24:284–293. doi: 10.3348/kjr.2022.0826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M, Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. doi: 10.1016/0735-1097(90)90282-t. [DOI] [PubMed] [Google Scholar]
- 31.Budoff MJ, Shaw LJ, Liu ST, Weinstein SR, Mosler TP, Tseng PH, et al. Long-term prognosis associated with coronary calcification: observations from a registry of 25,253 patients. J Am Coll Cardiol. 2007;49:1860–1870. doi: 10.1016/j.jacc.2006.10.079. [DOI] [PubMed] [Google Scholar]
- 32.Detrano R, Guerci AD, Carr JJ, Bild DE, Burke G, Folsom AR, et al. Coronary calcium as a predictor of coronary events in four racial or ethnic groups. N Engl J Med. 2008;358:1336–1345. doi: 10.1056/NEJMoa072100. [DOI] [PubMed] [Google Scholar]
- 33.Lee JG, Kim H, Kang H, Koo HJ, Kang JW, Kim YH, et al. Fully automatic coronary calcium score software empowered by artificial intelligence technology: validation study using three CT cohorts. Korean J Radiol. 2021;22:1764–1776. doi: 10.3348/kjr.2021.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ayoub C, Erthal F, Abdelsalam MA, Murad MH, Wang Z, Erwin PJ, et al. Prognostic value of segment involvement score compared to other measures of coronary atherosclerosis by computed tomography: a systematic review and meta-analysis. J Cardiovasc Comput Tomogr. 2017;11:258–267. doi: 10.1016/j.jcct.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 35.Min JK, Shaw LJ, Devereux RB, Okin PM, Weinsaft JW, Russo DJ, et al. Prognostic value of multidetector coronary computed tomographic angiography for prediction of all-cause mortality. J Am Coll Cardiol. 2007;50:1161–1170. doi: 10.1016/j.jacc.2007.03.067. [DOI] [PubMed] [Google Scholar]
- 36.Boogers MJ, Broersen A, van Velzen JE, de Graaf FR, El-Naggar HM, Kitslaar PH, et al. Automated quantification of coronary plaque with computed tomography: comparison with intravascular ultrasound using a dedicated registration algorithm for fusion-based quantification. Eur Heart J. 2012;33:1007–1016. doi: 10.1093/eurheartj/ehr465. [DOI] [PubMed] [Google Scholar]
- 37.de Graaf MA, Broersen A, Kitslaar PH, Roos CJ, Dijkstra J, Lelieveldt BP, et al. Automatic quantification and characterization of coronary atherosclerosis with computed tomography coronary angiography: cross-correlation with intravascular ultrasound virtual histology. Int J Cardiovasc Imaging. 2013;29:1177–1190. doi: 10.1007/s10554-013-0194-x. [DOI] [PubMed] [Google Scholar]
- 38.Fujimoto S, Kondo T, Kodama T, Fujisawa Y, Groarke J, Kumamaru KK, et al. A novel method for non-invasive plaque morphology analysis by coronary computed tomography angiography. Int J Cardiovasc Imaging. 2014;30:1373–1382. doi: 10.1007/s10554-014-0461-5. [DOI] [PubMed] [Google Scholar]
- 39.Voros S, Rinehart S, Qian Z, Vazquez G, Anderson H, Murrieta L, et al. Prospective validation of standardized, 3-dimensional, quantitative coronary computed tomographic plaque measurements using radiofrequency backscatter intravascular ultrasound as reference standard in intermediate coronary arterial lesions: results from the ATLANTA (assessment of tissue characteristics, lesion morphology, and hemodynamics by angiography with fractional flow reserve, intravascular ultrasound and virtual histology, and noninvasive computed tomography in atherosclerotic plaques) I study. JACC Cardiovasc Interv. 2011;4:198–208. doi: 10.1016/j.jcin.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 40.Choi AD, Marques H, Kumar V, Griffin WF, Rahban H, Karlsberg RP, et al. CT evaluation by artificial intelligence for atherosclerosis, stenosis and vascular morphology (CLARIFY): a multi-center, international study. J Cardiovasc Comput Tomogr. 2021;15:470–476. doi: 10.1016/j.jcct.2021.05.004. [DOI] [PubMed] [Google Scholar]
- 41.Sheahan M, Ma X, Paik D, Obuchowski NA, St Pierre S, Newman WP, 3rd, et al. Atherosclerotic plaque tissue: noninvasive quantitative assessment of characteristics with software-aided measurements from conventional CT angiography. Radiology. 2018;286:622–631. doi: 10.1148/radiol.2017170127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dey D, Schepis T, Marwan M, Slomka PJ, Berman DS, Achenbach S. Automated three-dimensional quantification of noncalcified coronary plaque from coronary CT angiography: comparison with intravascular US. Radiology. 2010;257:516–522. doi: 10.1148/radiol.10100681. [DOI] [PubMed] [Google Scholar]
- 43.Tzimas G, Gulsin GS, Everett RJ, Akodad M, Meier D, Sewnarain K, et al. Age- and sex-specific nomographic CT quantitative plaque data from a large international cohort. JACC Cardiovasc Imaging. 2024;17:165–175. doi: 10.1016/j.jcmg.2023.05.011. [DOI] [PubMed] [Google Scholar]
- 44.Park HB, Lee BK, Shin S, Heo R, Arsanjani R, Kitslaar PH, et al. Clinical feasibility of 3D automated coronary atherosclerotic plaque quantification algorithm on coronary computed tomography angiography: comparison with intravascular ultrasound. Eur Radiol. 2015;25:3073–3083. doi: 10.1007/s00330-015-3698-z. [DOI] [PubMed] [Google Scholar]
- 45.Matsumoto H, Watanabe S, Kyo E, Tsuji T, Ando Y, Otaki Y, et al. Standardized volumetric plaque quantification and characterization from coronary CT angiography: a head-to-head comparison with invasive intravascular ultrasound. Eur Radiol. 2019;29:6129–6139. doi: 10.1007/s00330-019-06219-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Puchner SB, Ferencik M, Maehara A, Stolzmann P, Ma S, Do S, et al. Iterative image reconstruction improves the accuracy of automated plaque burden assessment in coronary CT angiography: a comparison with intravascular ultrasound. AJR Am J Roentgenol. 2017;208:777–784. doi: 10.2214/AJR.16.17187. [DOI] [PubMed] [Google Scholar]
- 47.Stolzmann P, Schlett CL, Maurovich-Horvat P, Maehara A, Ma S, Scheffel H, et al. Variability and accuracy of coronary CT angiography including use of iterative reconstruction algorithms for plaque burden assessment as compared with intravascular ultrasound-an ex vivo study. Eur Radiol. 2012;22:2067–2075. doi: 10.1007/s00330-012-2464-8. [DOI] [PubMed] [Google Scholar]
- 48.van Rosendael AR, van den Hoogen IJ, Gianni U, Ma X, Tantawy SW, Bax AM, et al. Association of statin treatment with progression of coronary atherosclerotic plaque composition. JAMA Cardiol. 2021;6:1257–1266. doi: 10.1001/jamacardio.2021.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lee SE, Chang HJ, Sung JM, Park HB, Heo R, Rizvi A, et al. Effects of statins on coronary atherosclerotic plaques: the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1475–1484. doi: 10.1016/j.jcmg.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 50.Madder RD, Chinnaiyan KM, Marandici AM, Goldstein JA. Features of disrupted plaques by coronary computed tomographic angiography: correlates with invasively proven complex lesions. Circ Cardiovasc Imaging. 2011;4:105–113. doi: 10.1161/CIRCIMAGING.110.957282. [DOI] [PubMed] [Google Scholar]
- 51.Cardoso R, Choi AD, Shiyovich A, Besser SA, Min JK, Earls J, et al. How early can atherosclerosis be detected by coronary CT angiography? Insights from quantitative CT analysis of serial scans in the PARADIGM trial. J Cardiovasc Comput Tomogr. 2023;17:407–412. doi: 10.1016/j.jcct.2023.08.012. [DOI] [PubMed] [Google Scholar]
- 52.Buckler AJ, Doros G, Kinninger A, Lakshmanan S, Le VT, Libby P, et al. Quantitative imaging biomarkers of coronary plaque morphology: insights from EVAPORATE. Front Cardiovasc Med. 2023;10:1204071. doi: 10.3389/fcvm.2023.1204071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kwan AC, McElhinney PA, Tamarappoo BK, Cadet S, Hurtado C, Miller RJH, et al. Prediction of revascularization by coronary CT angiography using a machine learning ischemia risk score. Eur Radiol. 2021;31:1227–1235. doi: 10.1007/s00330-020-07142-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang HJ, Lin FY, Lee SE, Andreini D, Bax J, Cademartiri F, et al. Coronary atherosclerotic precursors of acute coronary syndromes. J Am Coll Cardiol. 2018;71:2511–2522. doi: 10.1016/j.jacc.2018.02.079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Williams MC, Kwiecinski J, Doris M, McElhinney P, D’Souza MS, Cadet S, et al. Low-attenuation noncalcified plaque on coronary computed tomography angiography predicts myocardial infarction: results from the multicenter SCOT-HEART trial (Scottish computed tomography of the heart) Circulation. 2020;141:1452–1462. doi: 10.1161/CIRCULATIONAHA.119.044720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee SE, Sung JM, Rizvi A, Lin FY, Kumar A, Hadamitzky M, et al. Quantification of coronary atherosclerosis in the assessment of coronary artery disease. Circ Cardiovasc Imaging. 2018;11:e007562. doi: 10.1161/CIRCIMAGING.117.007562. [DOI] [PubMed] [Google Scholar]
- 57.Meah MN, Tzolos E, Wang KL, Bularga A, Dweck MR, Curzen N, et al. Plaque burden and 1-year outcomes in acute chest pain: results from the multicenter RAPID-CTCA trial. JACC Cardiovasc Imaging. 2022;15:1916–1925. doi: 10.1016/j.jcmg.2022.04.024. [DOI] [PubMed] [Google Scholar]
- 58.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, et al. Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA. 2014;311:271–278. doi: 10.1001/jama.2013.282535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.van Rosendael AR, Narula J, Lin FY, van den Hoogen IJ, Gianni U, Al Hussein Alawamlh O, et al. Association of high-density calcified 1K plaque with risk of acute coronary syndrome. JAMA Cardiol. 2020;5:282–290. doi: 10.1001/jamacardio.2019.5315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bax AM, Yoon YE, Gianni U, Ma X, Lu Y, Lee BC, et al. Plaque character and progression according to the location of coronary atherosclerotic plaque. Am J Cardiol. 2021;158:15–22. doi: 10.1016/j.amjcard.2021.07.040. [DOI] [PubMed] [Google Scholar]
- 61.Han D, Lin A, Kuronuma K, Tzolos E, Kwan AC, Klein E, et al. Association of plaque location and vessel geometry determined by coronary computed tomographic angiography with future acute coronary syndrome-causing culprit lesions. JAMA Cardiol. 2022;7:309–319. doi: 10.1001/jamacardio.2021.5705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kolossváry M, Karády J, Kikuchi Y, Ivanov A, Schlett CL, Lu MT, et al. Radiomics versus visual and histogram-based assessment to identify atheromatous lesions at coronary CT angiography: an ex vivo study. Radiology. 2019;293:89–96. doi: 10.1148/radiol.2019190407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lin A, Kolossváry M, Cadet S, McElhinney P, Goeller M, Han D, et al. Radiomics-based precision phenotyping identifies unstable coronary plaques from computed tomography angiography. JACC Cardiovasc Imaging. 2022;15:859–871. doi: 10.1016/j.jcmg.2021.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chen Q, Xie G, Tang CX, Yang L, Xu P, Gao X, et al. Development and validation of CCTA-based radiomics signature for predicting coronary plaques with rapid progression. Circ Cardiovasc Imaging. 2023;16:e015340. doi: 10.1161/CIRCIMAGING.123.015340. [DOI] [PubMed] [Google Scholar]
- 65.Chen Q, Pan T, Wang YN, Schoepf UJ, Bidwell SL, Qiao H, et al. A coronary CT angiography radiomics model to identify vulnerable plaque and predict cardiovascular events. Radiology. 2023;307:e221693. doi: 10.1148/radiol.221693. [DOI] [PubMed] [Google Scholar]
- 66.Shin S, Park HB, Chang HJ, Arsanjani R, Min JK, Kim YJ, et al. Impact of intensive LDL cholesterol lowering on coronary artery atherosclerosis progression: a serial CT angiography study. JACC Cardiovasc Imaging. 2017;10:437–446. doi: 10.1016/j.jcmg.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 67.Svanteson M, Rollefstad S, Kløw NE, Hisdal J, Ikdahl E, Sexton J, et al. Effects of long-term statin-treatment on coronary atherosclerosis in patients with inflammatory joint diseases. PLoS One. 2019;14:e0226479. doi: 10.1371/journal.pone.0226479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Otaki Y, Tamarappoo B, Cadet SJ, Doris M, Arnson Y, Huynh PT, et al. Decrease in LDL-C is associated with decrease in all components of noncalcified plaque on coronary CTA. Atherosclerosis. 2019;285:128–134. doi: 10.1016/j.atherosclerosis.2019.04.201. [DOI] [PubMed] [Google Scholar]
- 69.Shi R, Gao Y, Shen LL, Shi K, Wang J, Jiang L, et al. The effect of LDL-C status on the association between increased coronary artery calcium score and compositional plaque volume progression in statins-treated diabetic patients: evaluated using serial coronary CTAs. Cardiovasc Diabetol. 2022;21:121. doi: 10.1186/s12933-022-01556-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hirai K, Kawasaki T, Soejima T, Kajiyama K, Fukami Y, Asada S, et al. Impact of intensive low-density lipoprotein cholesterol-lowering therapy on coronary artery plaques in acute coronary syndrome. Am J Cardiol. 2023;204:84–91. doi: 10.1016/j.amjcard.2023.07.049. [DOI] [PubMed] [Google Scholar]
- 71.Sun T, Wang Y, Wang X, Hu W, Li A, Li S, et al. Effect of long-term intensive cholesterol control on the plaque progression in elderly based on CTA cohort study. Eur Radiol. 2022;32:4374–4383. doi: 10.1007/s00330-022-08594-w. [DOI] [PubMed] [Google Scholar]
- 72.Kim U, Leipsic JA, Sellers SL, Shao M, Blanke P, Hadamitzky M, et al. Natural history of diabetic coronary atherosclerosis by quantitative measurement of serial coronary computed tomographic angiography: results of the PARADIGM study. JACC Cardiovasc Imaging. 2018;11:1461–1471. doi: 10.1016/j.jcmg.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 73.Nakanishi R, Ceponiene I, Osawa K, Luo Y, Kanisawa M, Megowan N, et al. Plaque progression assessed by a novel semi-automated quantitative plaque software on coronary computed tomography angiography between diabetes and non-diabetes patients: a propensity-score matching study. Atherosclerosis. 2016;255:73–79. doi: 10.1016/j.atherosclerosis.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 74.Won KB, Lee SE, Lee BK, Park HB, Heo R, Rizvi A, et al. Longitudinal assessment of coronary plaque volume change related to glycemic status using serial coronary computed tomography angiography: a PARADIGM (progression of atherosclerotic plaque determined by computed tomographic angiography imaging) substudy. J Cardiovasc Comput Tomogr. 2019;13:142–147. doi: 10.1016/j.jcct.2018.12.002. [DOI] [PubMed] [Google Scholar]
- 75.El Mahdiui M, Smit JM, van Rosendael AR, Neglia D, Knuuti J, Saraste A, et al. Sex differences in coronary plaque changes assessed by serial computed tomography angiography. Int J Cardiovasc Imaging. 2021;37:2311–2321. doi: 10.1007/s10554-021-02204-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee SE, Sung JM, Andreini D, Al-Mallah MH, Budoff MJ, Cademartiri F, et al. Sex differences in compositional plaque volume progression in patients with coronary artery disease. JACC Cardiovasc Imaging. 2020;13:2386–2396. doi: 10.1016/j.jcmg.2020.06.034. [DOI] [PubMed] [Google Scholar]
- 77.Han D, Berman DS, Miller RJH, Andreini D, Budoff MJ, Cademartiri F, et al. Association of cardiovascular disease risk factor burden with progression of coronary atherosclerosis assessed by serial coronary computed tomographic angiography. JAMA Netw Open. 2020;3:e2011444. doi: 10.1001/jamanetworkopen.2020.11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Weber C, Deseive S, Brim G, Stocker TJ, Broersen A, Kitslaar P, et al. Coronary plaque volume and predictors for fast plaque progression assessed by serial coronary CT angiography-A single-center observational study. Eur J Radiol. 2020;123:108805. doi: 10.1016/j.ejrad.2019.108805. [DOI] [PubMed] [Google Scholar]
- 79.Won KB, Lee BK, Heo R, Park HB, Lin FY, Hadamitzky M, et al. Longitudinal quantitative assessment of coronary atherosclerotic plaque burden related to serum hemoglobin levels. JACC Asia. 2022;2:311–319. doi: 10.1016/j.jacasi.2021.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Won KB, Lee BK, Park HB, Heo R, Lee SE, Rizvi A, et al. Quantitative assessment of coronary plaque volume change related to triglyceride glucose index: the progression of atherosclerotic plaque determined by computed tomographic angiography imaging (PARADIGM) registry. Cardiovasc Diabetol. 2020;19:113. doi: 10.1186/s12933-020-01081-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Won KB, Park HB, Heo R, Lee BK, Lin FY, Hadamitzky M, et al. Longitudinal quantitative assessment of coronary atherosclerosis related to normal systolic blood pressure maintenance in the absence of established cardiovascular disease. Clin Cardiol. 2022;45:873–881. doi: 10.1002/clc.23870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Li S, Tang X, Luo Y, Wu B, Huang Z, Li Z, et al. Impact of long-term glucose variability on coronary atherosclerosis progression in patients with type 2 diabetes: a 2.3 year follow-up study. Cardiovasc Diabetol. 2020;19:146. doi: 10.1186/s12933-020-01126-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang X, Shan DK, Dou GH, Ding YP, Jing J, Che HB, et al. Lipoprotein(a) is associated with coronary atheroma progression: analysis from a serial coronary computed tomography angiography study. J Geriatr Cardiol. 2021;18:996–1007. doi: 10.11909/j.issn.1671-5411.2021.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ben Zekry S, Sreedharan S, Han D, Sellers S, Ahmadi AA, Blanke P, et al. Comparison of coronary atherosclerotic plaque progression in East Asians and Caucasians by serial coronary computed tomographic angiography: a PARADIGM substudy. J Cardiovasc Comput Tomogr. 2022;16:222–229. doi: 10.1016/j.jcct.2021.09.012. [DOI] [PubMed] [Google Scholar]
- 85.Kaiser Y, Daghem M, Tzolos E, Meah MN, Doris MK, Moss AJ, et al. Association of lipoprotein(a) with atherosclerotic plaque progression. J Am Coll Cardiol. 2022;79:223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hoffmann H, Frieler K, Schlattmann P, Hamm B, Dewey M. Influence of statin treatment on coronary atherosclerosis visualised using multidetector computed tomography. Eur Radiol. 2010;20:2824–2833. doi: 10.1007/s00330-010-1880-x. [DOI] [PubMed] [Google Scholar]
- 87.Inoue K, Motoyama S, Sarai M, Sato T, Harigaya H, Hara T, et al. Serial coronary CT angiography-verified changes in plaque characteristics as an end point: evaluation of effect of statin intervention. JACC Cardiovasc Imaging. 2010;3:691–698. doi: 10.1016/j.jcmg.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 88.Zeb I, Li D, Nasir K, Malpeso J, Batool A, Flores F, et al. Effect of statin treatment on coronary plaque progression - a serial coronary CT angiography study. Atherosclerosis. 2013;231:198–204. doi: 10.1016/j.atherosclerosis.2013.08.019. [DOI] [PubMed] [Google Scholar]
- 89.Lo J, Lu MT, Ihenachor EJ, Wei J, Looby SE, Fitch KV, et al. Effects of statin therapy on coronary artery plaque volume and high-risk plaque morphology in HIV-infected patients with subclinical atherosclerosis: a randomised, double-blind, placebo-controlled trial. Lancet HIV. 2015;2:e52–e63. doi: 10.1016/S2352-3018(14)00032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Auscher S, Heinsen L, Nieman K, Vinther KH, Løgstrup B, Møller JE, et al. Effects of intensive lipid-lowering therapy on coronary plaques composition in patients with acute myocardial infarction: assessment with serial coronary CT angiography. Atherosclerosis. 2015;241:579–587. doi: 10.1016/j.atherosclerosis.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 91.Li Z, Hou Z, Yin W, Liu K, Gao Y, Xu H, et al. Effects of statin therapy on progression of mild noncalcified coronary plaque assessed by serial coronary computed tomography angiography: a multicenter prospective study. Am Heart J. 2016;180:29–38. doi: 10.1016/j.ahj.2016.06.023. [DOI] [PubMed] [Google Scholar]
- 92.Nou E, Lu MT, Looby SE, Fitch KV, Kim EA, Lee H, et al. Serum oxidized low-density lipoprotein decreases in response to statin therapy and relates independently to reductions in coronary plaque in patients with HIV. AIDS. 2016;30:583–590. doi: 10.1097/QAD.0000000000000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Smit JM, van Rosendael AR, El Mahdiui M, Neglia D, Knuuti J, Saraste A, et al. Impact of clinical characteristics and statins on coronary plaque progression by serial computed tomography angiography. Circ Cardiovasc Imaging. 2020;13:e009750. doi: 10.1161/CIRCIMAGING.119.009750. [DOI] [PubMed] [Google Scholar]
- 94.Foldyna B, Lo J, Mayrhofer T, Grinspoon SK, Hoffmann U, Lu MT. Individual coronary plaque changes on serial CT angiography: within-patient heterogeneity, natural history, and statin effects in HIV. J Cardiovasc Comput Tomogr. 2020;14:144–148. doi: 10.1016/j.jcct.2019.08.011. [DOI] [PubMed] [Google Scholar]
- 95.Motoyama S, Nagahara Y, Sarai M, Kawai H, Miyajima K, Sato Y, et al. Effect of omega-3 fatty acids on coronary plaque morphology—a serial computed tomography angiography study. Circ J. 2022;86:831–842. doi: 10.1253/circj.CJ-21-0615. [DOI] [PubMed] [Google Scholar]
- 96.Alfaddagh A, Elajami TK, Ashfaque H, Saleh M, Bistrian BR, Welty FK. Effect of eicosapentaenoic and docosahexaenoic acids added to statin therapy on coronary artery plaque in patients with coronary artery disease: a randomized clinical trial. J Am Heart Assoc. 2017;6:e006981. doi: 10.1161/JAHA.117.006981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Baumann S, Kettel L, Stach K, Özdemir GH, Renker M, Tesche C, et al. Serial changes in coronary plaque formation using CT angiography in patients undergoing PCSK9-inhibitor therapy with 1-year follow-up. J Thorac Imaging. 2022;37:285–291. doi: 10.1097/RTI.0000000000000666. [DOI] [PubMed] [Google Scholar]
- 98.Budoff MJ, Bhatt DL, Kinninger A, Lakshmanan S, Muhlestein JB, Le VT, et al. Effect of icosapent ethyl on progression of coronary atherosclerosis in patients with elevated triglycerides on statin therapy: final results of the EVAPORATE trial. Eur Heart J. 2020;41:3925–3932. doi: 10.1093/eurheartj/ehaa652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pérez de Isla L, Díaz-Díaz JL, Romero MJ, Muñiz-Grijalvo O, Mediavilla JD, Argüeso R, et al. Alirocumab and coronary atherosclerosis in asymptomatic patients with familial hypercholesterolemia: the ARCHITECT study. Circulation. 2023;147:1436–1443. doi: 10.1161/CIRCULATIONAHA.122.062557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Elnabawi YA, Dey AK, Goyal A, Groenendyk JW, Chung JH, Belur AD, et al. Coronary artery plaque characteristics and treatment with biologic therapy in severe psoriasis: results from a prospective observational study. Cardiovasc Res. 2019;115:721–728. doi: 10.1093/cvr/cvz009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Choi H, Uceda DE, Dey AK, Abdelrahman KM, Aksentijevich M, Rodante JA, et al. Treatment of psoriasis with biologic therapy is associated with improvement of coronary artery plaque lipid-rich necrotic core: results from a prospective, observational study. Circ Cardiovasc Imaging. 2020;13:e011199. doi: 10.1161/CIRCIMAGING.120.011199. [DOI] [PubMed] [Google Scholar]
- 102.Budoff MJ, Ellenberg SS, Lewis CE, Mohler ER, 3rd, Wenger NK, Bhasin S, et al. Testosterone treatment and coronary artery plaque volume in older men with low testosterone. JAMA. 2017;317:708–716. doi: 10.1001/jama.2016.21043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lee DH, Chun EJ, Hur JH, Min SH, Lee JE, Oh TJ, et al. Effect of sarpogrelate, a selective 5-HT2A receptor antagonist, on characteristics of coronary artery disease in patients with type 2 diabetes. Atherosclerosis. 2017;257:47–54. doi: 10.1016/j.atherosclerosis.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 104.Matsumoto S, Ibrahim R, Grégoire JC, L’Allier PL, Pressacco J, Tardif JC, et al. Effect of treatment with 5-lipoxygenase inhibitor VIA-2291 (atreleuton) on coronary plaque progression: a serial CT angiography study. Clin Cardiol. 2017;40:210–215. doi: 10.1002/clc.22646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Vaidya K, Arnott C, Martínez GJ, Ng B, McCormack S, Sullivan DR, et al. Colchicine therapy and plaque stabilization in patients with acute coronary syndrome: a CT coronary angiography study. JACC Cardiovasc Imaging. 2018;11(2 Pt 2):305–316. doi: 10.1016/j.jcmg.2017.08.013. [DOI] [PubMed] [Google Scholar]
- 106.Shaikh K, Kinninger A, Cherukuri L, Birudaraju D, Nakanishi R, Almeida S, et al. Aged garlic extract reduces low attenuation plaque in coronary arteries of patients with diabetes: a randomized, double-blind, placebo-controlled study. Exp Ther Med. 2020;19:1457–1461. doi: 10.3892/etm.2019.8371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aldana-Bitar J, Moore J, Manubolu VS, Dahal S, Verghese D, Lakshmanan S, et al. Plaque progression differences between apixaban and rivaroxaban in patients with atrial fibrillation measured with cardiac computed tomography and plaque quantification. Am J Ther. 2023;30:e313–e320. doi: 10.1097/MJT.0000000000001569. [DOI] [PubMed] [Google Scholar]
- 108.Heinsen LJ, Pararajasingam G, Andersen TR, Auscher S, Sheta HM, Precht H, et al. Liraglutide treatment is associated with progression of coronary artery fibrous plaque: a prospective 1-year follow-up study in asymptomatic patients with type 2 diabetes. BMC Cardiovasc Disord. 2023;23:214. doi: 10.1186/s12872-023-03228-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takx RA, Willemink MJ, Nathoe HM, Schilham AM, Budde RP, de Jong PA, et al. The effect of iterative reconstruction on quantitative computed tomography assessment of coronary plaque composition. Int J Cardiovasc Imaging. 2014;30:155–163. doi: 10.1007/s10554-013-0293-8. [DOI] [PubMed] [Google Scholar]
- 110.Williams MC, Earls JP, Hecht H. Quantitative assessment of atherosclerotic plaque, recent progress and current limitations. J Cardiovasc Comput Tomogr. 2022;16:124–137. doi: 10.1016/j.jcct.2021.07.001. [DOI] [PubMed] [Google Scholar]
- 111.Achenbach S, Boehmer K, Pflederer T, Ropers D, Seltmann M, Lell M, et al. Influence of slice thickness and reconstruction kernel on the computed tomographic attenuation of coronary atherosclerotic plaque. J Cardiovasc Comput Tomogr. 2010;4:110–115. doi: 10.1016/j.jcct.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 112.Min JK, Chang HJ, Andreini D, Pontone G, Guglielmo M, Bax JJ, et al. Coronary CTA plaque volume severity stages according to invasive coronary angiography and FFR. J Cardiovasc Comput Tomogr. 2022;16:415–422. doi: 10.1016/j.jcct.2022.03.001. [DOI] [PubMed] [Google Scholar]