Abstract
Copper ion homeostasis is complicated in that copper is an essential element needed for a variety of cellular processes but is toxic at excess levels. To identify Candida albicans genes that are involved in resistance to copper ion toxicity, a library containing inserts of C. albicans genomic DNA was used to complement the copper sensitivity phenotype of a Saccharomyces cerevisiae cup1Δ strain that is unable to produce Cup1p, a metallothionein (MT) responsible for high-level copper ion resistance. A P1-type ATPase (CPx type) that is closely related to the human Menkes and Wilson disease proteins was cloned. The gene encoding this pump was termed CRD1 (for copper resistance determinant). A gene encoding a 76-amino-acid MT similar to higher eukaryotic MTs in structure was also cloned, and the gene was termed CRD2. Transcription of the CRD1 gene was found to increase upon growth with increasing copper levels, while the CRD2 mRNA was expressed at a constant level. Strains with the CRD1 gene disrupted were extremely sensitive to exogenous copper and failed to grow in medium containing 100 μM CuSO4. These crd1 strains also exhibited increased sensitivity to silver and cadmium, indicating that Crd1p is somewhat promiscuous with respect to metal ion transport. Although strains with the CRD2 gene disrupted showed reduced growth rate with increasing copper concentration, the crd2 mutants eventually attained wild-type levels of growth, demonstrating that CRD2 is less important for resistance to copper ion toxicity. Crd1p is the first example of a eukaryotic copper pump that provides the primary source of cellular copper resistance, and its ability to confer silver resistance may enhance the prevalence of C. albicans as a nosocomial pathogen.
Copper is an essential cofactor for enzymes involved in diverse biological processes including respiration, destruction of free radicals, iron homeostasis, and neurological development. However, when in excess, copper is toxic as it generates reactive oxygen species via the Fenton reaction, disrupts metal ion binding and homeostasis, and binds macromolecules such as proteins inappropriately (25, 50).
Resistance to the toxicity of copper and other heavy metals is achieved through mechanisms such as reduced influx, facilitated efflux, sequestration, and modification. Eukaryotic organisms generally utilize small cysteine-rich proteins termed metallothioneins (MTs) to sequester metals (17). MTs bind heavy metals through thiolate bonds; the multiple cysteine residues are arranged in Cys-X-Cys or Cys-X-X-Cys repeats that coordinate metal binding. In contrast, prokaryotes generally achieve copper resistance by facilitated efflux utilizing transporters such as the P1-type ATPases (45).
The closely related yeasts Saccharomyces cerevisiae and Candida glabrata use MTs for copper homeostasis. The major copper resistance determinant in S. cerevisiae is CUP1, which encodes an MT (4, 10). CUP1 expression is induced to a high level when excess copper is present, and the gene can be amplified up to 20 times or more (5, 18, 52). These two factors combine to allow CUP1-amplified strains to grow in medium with high levels of copper (21). The S. cerevisiae CRS5 gene encodes a second MT, which is not amplified or expressed at a high level and which provides minimal resistance to copper ion toxicity (8). S. cerevisiae also utilizes the P1-type ATPases Ccc2p and Pca1p for copper homeostasis. Ccc2p is a homolog of the Menkes disease protein that is involved in exporting cytosolic copper to the secretory pathway and the extracytosolic domain of the copper-dependent oxidase Fet3p (54). In contrast, Pca1p is thought to play a role in resistance to copper ion toxicity as pca1Δ strains do not achieve wild-type growth levels in medium containing high concentrations of copper (39). C. glabrata contains three MT-encoding genes (28). The MTIIA/B genes are highly induced in the presence of copper and are most important in conferring resistance to copper (29, 30). While a few other fungal species have been shown to contain MTs, the fission yeast Schizosaccharomyces pombe utilizes an alternative mechanism to achieve resistance to copper ion toxicity. In S. pombe, (γ-Glu-Cys)n-Gly peptides termed phytochelatins are used to sequester copper (7).
This report describes mechanisms of resistance to copper ion toxicity employed by the diploid yeast C. albicans, an opportunistic pathogen of humans (43). C. albicans is a common commensal that is found associated with the gastrointestinal tracts of various mammalian species. In immunocompromised individuals this generally harmless yeast can cause both superficial and life-threatening disseminated diseases (37). This study of copper homeostasis in C. albicans demonstrates that the major resistance locus encodes a copper-inducible P1-type ATPase. In addition to conferring high-level copper resistance, this ATPase contributes to silver resistance. It is also shown that C. albicans contains an MT gene that is not amplified and that provides minimal copper resistance. Since silver is widely used as an antimicrobial agent, possession of a pump that confers resistance to silver ion toxicity may enhance the spread of C. albicans as a nosocomial pathogen.
MATERIALS AND METHODS
Strains and plasmids.
C. albicans strains are listed in Table 1. Parent strains were CAI4 (Δura3/Δura3) and SGY243 (Δura3/Δura3) (11, 23). To construct crd1 and crd2 null mutants, the parental strains were transformed with the disruption plasmids pPR300 and pPR400, respectively. The S. cerevisiae strain dRM110.18A (MATa leu2-3,112 ura3::HIS3 cup1Δ::ura3::THR3) was kindly provided by R. Boumil and D. Dawson. For plasmid cloning, E. coli XL1-Blue (Stratagene) was used.
TABLE 1.
C. albicans strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| CAI4 | Δura3::imm434/Δura3::imm434; congenic to clinical isolate SC5314 | 11 |
| SGY243 | ade2/ade2 Δura3::ADE2/Δura3::ADE2 | 23 |
| CAPR301 | CAI4, CRD1/CRD1/crd1::hisG-URA3-hisG | This study |
| CAPR302 | CAI4, CRD1/CRD1/crd1::hisG | This study |
| CAPR303 | CAI4, CRD1/crd1:hisG-URA3-hisG/crd1::hisG | This study |
| CAPR304 | CAI4, CRD1/crd1::hisG/crd1::hisG | This study |
| CAPR305 | CAI4, crd1::hisG-URA3-hisG/crd1::hisG/crd1::hisG | This study |
| CAPR307 | CAI4, crd1::hisG/crd1::hisG/crd1::hisG (pPR311) | This study |
| CAPR401 | CAI4, CRD2/crd2::hisG-URA3-hisG | This study |
| CAPR403 | CAI4, crd2::hisG-URA3/crd2::hisG | This study |
Growth media.
C. albicans and S. cerevisiae strains used in this study were routinely cultured on YPD (1% yeast extract, 2% Bacto peptone, 2% glucose), CM lacking uracil or uridine (Urd), or SD (0.67% yeast nitrogen base, 2% glucose) at 30°C (2). For solid media 2% agar (Difco) was added. Urd− auxotrophs were selected on medium containing 5-fluoroorotic acid (5-FOA) (2). Escherichia coli cells were cultured in L broth or on L plates (31). Ampicillin was added at a concentration of 100 μg/ml.
Metal sensitivity assays.
C. albicans strains were grown to saturation at 30°C in liquid SD medium and diluted 100-fold into SD medium with various concentrations of CuSO4, AgNO3, or CdSO4. Cell growth was monitored by determining the optical density at 600 nm (OD600) of the cultures.
DNA manipulations and analysis.
Plasmid isolation, PCR, restriction digestion, cloning, gel electrophoresis, and Southern hybridization analysis were performed by standard methods (2). C. albicans genomic DNA was isolated using a glass bead disruption procedure (2). Automated DNA sequencing was performed by Michael Berne and coworkers at the Tufts University Core Facility. DNA sequence analysis utilized the Lasergene sequence analysis software (DNAStar Inc., Madison, Wis.) or the Blast algorithm (National Center for Biotechnology Information).
RNA analysis.
Cells for RNA isolation were grown in SD liquid medium at 30°C to an OD600 of 1.0. Where appropriate CuSO4 or CdSO4 was added at 0.1 or 1.0 mM and the cultures were incubated for an additional 30 min. For RNA extraction, cells were lysed using glass beads and phenol (2). Ten micrograms of total RNA per sample was separated on a formaldehyde-agarose gel, transferred to a Nytran-plus membrane, and probed by standard Northern hybridization methods (2). Densitometric analysis was performed with a Molecular Dynamics computing densitometer and ImageQuant, version 3.3, software.
Transformation and screening of the C. albicans genomic library.
S. cerevisiae cells were transformed by the lithium acetate method of Gietz et al. (13). A C. albicans genomic library (42) was used to transform S. cerevisiae strain dRM110.18A, and transformants were replicated to SD medium containing 125 μM CuSO4. Approximately 50,000 transformants were screened, and 15 colonies that exhibited growth on 125 μM CuSO4 were chosen for further study. Plasmids were isolated from the resistant transformants and reintroduced into the cup1Δ strain dRM110.18A to confirm the heterologous complementation.
Plasmid construction.
Disruption constructs for CRD1 and CRD2 utilized the “URA blaster” plasmid pMB7 (11). In the primer sequences given below, underlined sequences are complementary to sequences from the CRD1 and CRD2 loci while sequences that are not underlined indicate restriction enzyme sites added to the primer. The CRD1 deletion construct primers NTRMDR (5′-GCCGAGCTCGAAGACGGTAATAGTTCAGTTGTTGG-3′) and NTRMRV (5′-GCCGAGCTCACACGATGTTGACATTGG-3′) yielded a 443-bp PCR fragment with SacI sites at the ends, and the primers CTRMDR (5′-GCCCTGCAGGTGAAAGCTCACTCTCG-3′) and CTRMPL (5′-GCCCTGCAGTCGGCGGATGTTGGAATTG-3′) yielded a 390-bp PCR fragment with PstI sites at the ends. The fragments were digested with SacI or PstI and ligated sequentially into the SacI and PstI sites flanking the URA blaster cassette in pMB7 to give the CRD1 disruption plasmid pPR300. The CRD2 deletion construct primers MTNDSR (5′-CGGGAGCTCGAAGACCTCCGCCTTTACTTTCAACG-3′) and MT5′RVP (5′-GCCGAGCTCGAGCACAGACACATTGAGC-3′) yielded a 318-bp fragment with SacI sites at the ends, and the primers MT3′UTRP (5′-GCCCTGCAGCTATACTAACCAACAACG-3′) and 3′DSRPT (GCCCTGCAGGAAGACTGAAAGATTATCTGAGTAC-3′) yielded a 549-bp fragment with PstI sites at the ends. The fragments were digested with SacI or PstI and ligated sequentially into the SacI and PstI sites flanking the URA blaster cassette in pMB7 to give the CRD2 disruption plasmid pPR400. To construct the CRD1-complementing plasmid pPR311, a 1.1-kb HindIII/SphI fragment containing the smaller C-terminal portion of the CRD1 open reading frame (ORF), some of the 3′-untranslated region, and 187 bp of the tet gene sequence (including the SphI site) from YEp13 was cloned into the HindIII/SphI-digested plasmid pCK66. Plasmid pCK66 is derived from pNEB193 (New England BioLabs) and contains the SacI/XbaI (blunted) fragment of the C. albicans URA3 gene cloned into the NdeI site (blunted). A 4.3-kbp HindIII fragment encompassing most of the CRD1 ORF and some of the 5′ upstream sequence was cloned in the proper orientation into the HindIII site of the above construct, reconstituting the CRD1 ORF. For directed integration into the CRD1 locus, pPR311 was digested with the restriction enzyme ClaI.
Gene disruption of the CRD genes.
The CRD genes were disrupted using the URA blaster method (1, 11). Prior to transformation, both the CRD1 and CRD2 disruption cassettes (from the disruption plasmids pPR300 and pPR400, respectively) were released from the pMB7 plasmid backbone by digestion with the restriction enzyme BbsI. The primer pairs were engineered so that digestion with the restriction enzyme BbsI would generate ends derived from the C. albicans sequence. To generate the CRD1 and CRD2 disruption strains, sequential transformations were done as described previously (1, 11). Strains that had resolved out the URA3 gene were selected on 5-FOA plates (2). Southern hybridization analysis was used to confirm all integration events. For analysis of CRD1 disruptions, chromosomal DNA was digested with EcoRI and XhoI and probed with an 1,126-bp XbaI/XhoI fragment from the CRD1 ORF. Sizes of the hybridizing bands were 2.4 kb for the CRD1 allele and 3.8 and 8.1 kb for Δcrd1::hisG-URA3-hisG and Δcrd1::hisG alleles, respectively. EcoRI- and XhoI-digested DNA from CAPR301 yielded fragments of the expected sizes of 2.4 and 3.8 kb for the wild-type and disrupted alleles, respectively. However, the fragment corresponding to the wild-type CRD1 allele was approximately twice as intense as that corresponding to the allele disrupted with the URA blaster cassette, suggesting that C. albicans contains three copies of the CRD1 gene. Several Urd− strains were isolated by plating these first-round disruptants on 5-FOA medium and selecting strains that had undergone recombination between the direct hisG repeats of the URA blaster cassette. EcoRI- and XhoI-digested DNA from CAPR302 yielded a fragment of approximately 8.1 kb due to the loss of the URA3 gene and its accompanying EcoRI site. Again, the hybridization signal of the wild-type allele was about twofold greater than that of the hisG-disrupted allele. Four independently constructed 5-FOAr heterozygotes were retransformed using the CRD1 disruption construct with URA3 as the selectable marker. EcoRI- and XhoI-digested DNA from several independent strains exhibited a banding pattern where the CRD1, crd1::hisG-URA3-hisG, and crd1::hisG alleles were all visible, indicating that C. albicans contains three copies of the CRD1 gene. These strains were plated on 5-FOA medium and screened for the desired intrachromosomal recombination event. Two of the 5-FOAr strains were chosen for a third round of transformation with the CRD1 disruption construct. The DNA from several third-round transformants gave the pattern of hybridizing fragments expected for homozygous null mutants. For analysis of CRD2 disruptions, chromosomal DNA was digested with NdeI and SacI and probed with a 420-bp PCR fragment corresponding to sequence that starts 759 bp downstream of the CRD2 ORF. DNA for this probe was amplified using the primer pair MTCTP1F (5′-GCTGTTGTTATTGTCAGG) and MTCTP2R (5′-ATGGATTGGGTTTGCTAG). Sizes of the hybridizing bands were approximately 8 kbp for the CRD2 allele and 7 and 6 kbp for the Δcrd2::hisG-URA3-hisG and Δcrd2::hisG alleles, respectively.
Nucleotide sequence accession number.
The sequences of the CRD1 and CRD2 genes have been deposited in GenBank under accession no. AF268098 and AF268099, respectively.
RESULTS
Isolation of C. albicans genomic sequences conferring copper resistance.
S. cerevisiae strains with the CUP1 locus deleted (cup1Δ) are extremely sensitive to exogenous copper ions (18). To isolate C. albicans genes that mediate resistance to copper ion toxicity, a 2 μm library containing C. albicans genomic fragments was screened for sequences that would heterologously complement the copper sensitivity phenotype of an S. cerevisiae cup1Δ strain (42). Fifteen plasmids that conferred the ability to grow on medium containing 125 μM copper sulfate were characterized. Restriction mapping and Southern hybridization analysis indicated that the plasmids were of two classes (six isolates for class I and nine isolates for class II), containing inserts of approximately 7.3 kb for class I and 4 kb for class II. The DNA sequences of subclones of the class I and class II plasmids were determined.
Class I clones encode a P1-type ATPase.
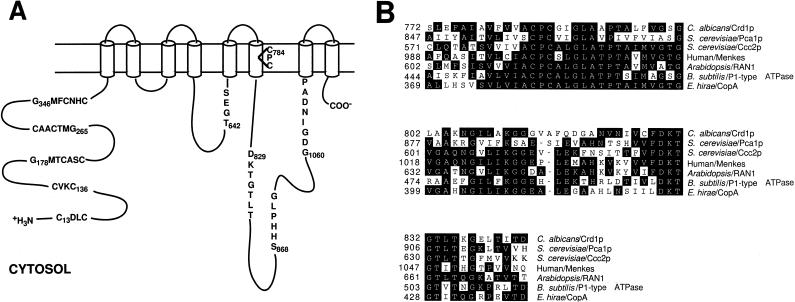
The nucleotide sequences of the class I clones were analyzed using the Blast algorithm, and a 3,591 bp ORF whose product exhibited significant sequence similarity to copper-transporting P1-type (CPx-type) ATPases was found (Fig. 1A) (26, 47). An essentially identical sequence was independently determined and deposited in GenBank by Weissman et al. (accession no. AAF04593) (51). As can be seen in Fig. 1B, an alignment of the most conserved region of P1-type ATPases reveals high similarity to ATP-driven copper pumps from humans, plants, and prokaryotes. Five copies of CXXC motifs, characteristic of proteins that bind copper, are found in the N-terminal region (6). The three most C-terminal CXXC sequences are part of the larger motif GMXCXXC, termed the HMA (heavy-metal-associated) sequence, which is seen in the N-terminal regions of most prokaryotic and eukaryotic P1-type ATPases. Additional sequences of approximately 60 amino acids surrounding the HMA motif exhibit extended sequence similarity to the copper binding modules of copper-transporting P1-type ATPases (3). The two most N-terminal CXXC sequences are not part of the classical HMA sequence and may not represent copper binding modules. Other features characteristic of P1-type ATPases included eight transmembrane domains (predicted from hydropathy analysis), the conserved CPC motif in the sixth transmembrane domain, thought to be involved in ion translocation, and a conserved histidine-proline sequence 41 residues C-terminal to the CPC motif (41).
FIG. 1.
(A) Two-dimensional model of the Crd1p P1-type ATPase depicting key features and putative membrane topology. Crd1p is predicted to have five N-terminal, cytosolic metal binding domains (CXXC) thought to be involved in Cu(I) binding. Hydropathy analysis is consistent with eight transmembrane (TM) domains. The following conserved motifs and their function are shown: TGES, phosphatase domain; CPC, ion translocation; DKTGT, aspartyl kinase domain; SHHPLG, unknown function (conserved HP dipeptide); GDGINDAP, ATP binding. The diagram is not to scale. (B) Protein sequence alignments of conserved domains involved in ion translocation from related proteins involved in copper transport. The most conserved region of all P1-type ATPases, encompassing a stretch of approximately 70 amino acids which contains the membrane helix with the CPx motif and the phosphorylation domain DKTGT, is shown. Regions of sequence identity to the consensus sequence are in black. Dashes, gaps in the amino acid sequence compared to other sequences. The sequences were aligned using the MegAlign program (Clustal method) of the DNAStar software package. GenBank accession numbers: C. albicans Crd1p, AF268098; S. cerevisiae Pca1p, Z29332; S. cerevisiae Ccc2p, AAC37425.1; human/Menkes AT7A, AAA35580.1; A. thaliana RAN1, AAD29109; Bacillus subtilis P1-type ATPase, CAB15335; E. hirae CopA, AAA61385.1.
Class II clones encode an MT.
Analysis of the DNA sequence of the class II clones indicated that they contained a small ORF exhibiting sequence similarity to higher eukaryotic MTs, and this gene has been termed CRD2 (17). The C. albicans MT is 76 amino acids in length and contains 12 cysteine residues with 10 of the Cys residues part of Cys-X-Cys motifs (Fig. 2). The CRD2 gene product contains 16% cysteine, 12% serine, 9% lysine, and only one aromatic amino acid residue, phenylalanine. These features are characteristic of MTs, which are typically 50 to 70 amino acid residues in length containing multiple Cys-X-Cys or Cys-Cys sequences and having 20 to 30% Cys, 20 to 30% Ser and Lys, and few aromatic amino acids. The C. albicans MT was quite similar to those of fish, such as carp and flounder, and to those of plants, such as Arabidopsis thaliana and tomato, but shared little sequence similarity with the S. cerevisiae Cup1p (data not shown).
FIG. 2.
Deduced amino acid sequence of the C. albicans MT encoded by CRD2. Cys-Xaa-Cys repeats thought to coordinate copper binding are underlined.
Expression of the CRD1 and CRD2 genes.
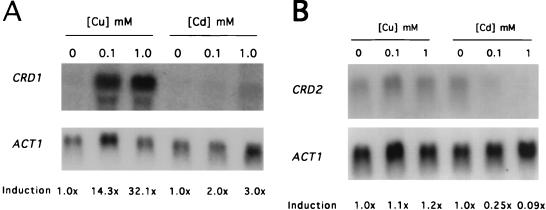
The MT genes of eukaryotes and the resistance pumps of prokaryotes that are involved in preventing copper ion toxicity exhibit copper-specific induction. To determine whether the CRD1 and CRD2 genes were regulated in response to changing copper levels, C. albicans strains SGY243 (Δura3/Δura3) and CAI4 (Δura3/Δura3) were grown in various concentrations of copper or cadmium and CRD1 and CRD2 mRNA levels were examined by Northern hybridization analysis. CRD1 mRNA was induced approximately 14- and 32-fold by exposure to 0.1 and 1 mM CuSO4, respectively (Fig. 3A). However, exposure of cells to 0.1 and 1 mM CdSO4 led only to two- to threefold induction. In contrast to the results seen with the CRD1 gene, CRD2 transcription was maintained at a basal level, as exposure of cells to 0.1 and 1 mM CuSO4 led to no significant induction of CRD2 mRNA over the levels seen with cells grown without added copper (Fig. 3B). Exposure to CdSO4 at 0.1 and 1 mM led to approximately 4- and 12-fold decreases, respectively, in steady-state mRNA levels of the CRD2 gene (Fig. 3B). Results similar to those shown in Fig. 3 were obtained from Northern hybridization analysis of CRD1 and CRD2 transcription in strain CAI4 (data not shown). These results indicate that the presence of copper leads to high-level induction of the CRD1 gene while transcription of the CRD2 locus is not induced, suggesting that the CRD1 gene may play the major role in resistance to copper ion toxicity.
FIG. 3.
Analysis of CRD1 and CRD2 transcription in response to metals. Strain SGY243 was grown to an OD600 of 1.0 in SD plus Urd and then treated for 30 min with the indicated concentration of CuSO4 or CdSO4 prior to RNA isolation for Northern hybridization analysis. Results were quantitated by densitometry. (A) Autoradiogram of a blot that was sequentially hybridized to CRD1- and ACT1-containing probes (the ACT1 mRNA was used as a loading control). (B) Autoradiogram of a blot that was sequentially hybridized to CRD2- and ACT1-containing probes.
Disruption of the CRD1 and CRD2 genes.
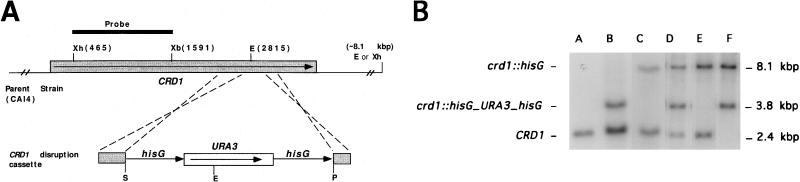
To determine the importance of the CRD1 and CRD2 genes in resistance to copper ion toxicity in C. albicans, the genes were sequentially disrupted using the URA blaster technique (1, 11). Recombination with the disruption fragment from pPR300 resulted in replacement of 740 bp of the CRD1 ORF with the URA blaster cassette, as illustrated in Fig. 4A. The deleted portion of the CRD1 ORF encoded the DKTGT (aspartyl kinase) and the GDGINDAP (ATP binding) motifs, which are expected to be essential for CRD1 function. C. albicans strain CAI4, a homozygous ura3 strain congenic to the clinical isolate SC5314, was transformed as described in Materials and Methods. Several independent transformants were selected and analyzed. Southern hybridization of chromosomal DNA from representative strains is shown in Fig. 4B.
FIG. 4.
Disruption of the C. albicans CRD1 gene. (A) A portion of the CRD1 ORF which encodes the conserved DKTGT (aspartyl kinase domain), HP, and GDGXNDXP (ATP binding domain) amino acid sequences was replaced by the hisG-URA3-hisG cassette. The 1,126-bp XhoI/XbaI restriction fragment (positions 464 and 1590 of the CRD1 sequence, respectively) was used as a probe. Shaded rectangles, CRD1 ORF; open rectangle, C. albicans URA3 gene that is part of the URA blaster cassette; unboxed arrows, Salmonella hisG repeat sequences that are part of the URA blaster cassette; heavy line, fragment used as probe. Only restriction sites relevant for analysis of the disruption strains are given. The sites are as follows: EcoRI (E), SacI (S), PstI (P), XbaI (Xb), and XhoI (Xh). Numbers in parentheses, positions in base pairs of relevant restriction sites with respect to the first nucleotide of the CRD1 ORF, unless indicated otherwise. (B) Southern hybridization analysis of the CRD1 disruption strains used in this study. Lane A, CAI4 (CRD1/CRD1/CRD1); lane B, CAPR301 (CRD1/CRD1/crd1::hisG-URA3-hisG); lane C, CAPR302 (CRD1/CRD1/crd1::hisG); lane D, CAP303 (CRD1/crd1::hisG-URA3-hisG/crd1::hisG); lane E, CAPR304 (CRD1/crd1::hisG/crd1::hisG); lane F, CAPR305 (crd1::hisG-URA3-hisG/crd1::hisG/crd1::hisG).
Because C. albicans is a diploid organism, typically two rounds of transformation are required to disrupt both copies of a gene of interest. However, for CRD1, three rounds of transformation were required to disrupt all copies of CRD1, indicating there were three copies of the CRD1 gene in the genome. The CRD1 gene was also disrupted in the C. albicans strain SGY243 (Δura3/Δura3) (data not shown). As for strain CAI4, three rounds of transformation were required to disrupt all copies the CRD1 gene, suggesting that this was not a strain-specific phenomenon.
Both copies of the CRD2 gene were disrupted using the URA blaster technique for the C. albicans strains CAI4 and SGY243 as described in Materials and Methods. The disrupted alleles retained only the first 11 amino acids of Crd2p. Both CAI4 and SGY243 contained only two copies of the CRD2 gene (data not shown).
Phenotypic analysis of the crd1 and crd2 disruption strains.
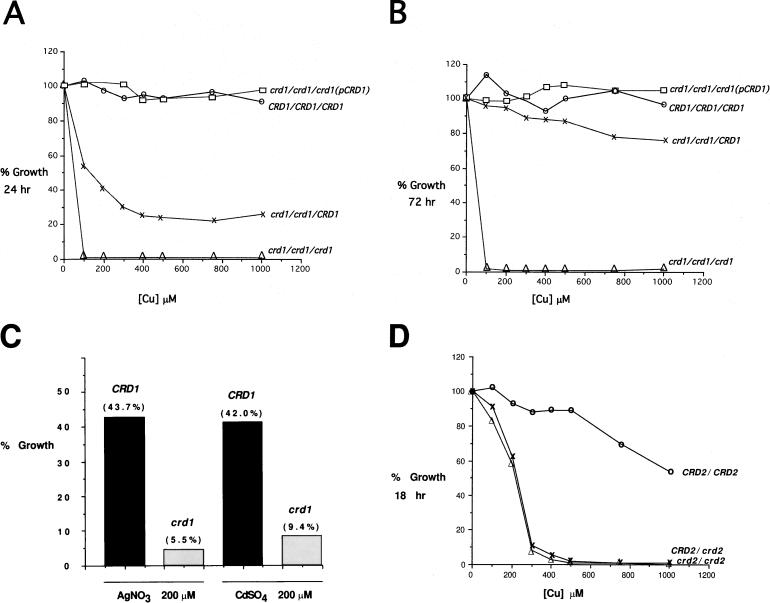
The crd1 homozygous null mutant CAPR305 was extremely sensitive to copper ions, exhibiting a complete lack of growth in SD medium containing 100 μM CuSO4 regardless of the time of incubation (Fig. 5B). In contrast, the strain that contains two disrupted alleles and one wild-type CRD1 allele exhibited growth in the presence of CuSO4, but its growth was clearly slower than that of wild-type strain CAI4 (Fig. 5A). After 24 h of growth in medium containing 1,000 μM CuSO4, the mutant strain CAPR303 (crd1/crd1/CRD1) grew to a density that was 26% of that observed in the absence of CuSO4. After 72 h, the level of growth seen with CAPR303 was close to that of the wild-type strain up to the highest levels of CuSO4 tested (Fig. 5B). Additionally, complementation of the homozygous null mutant with a single, integrated copy of the CRD1 gene (crd1/crd1/crd1/p3011[CRD1]) led to growth in copper-containing medium that was equal or greater than that seen with CAPR303 (Fig. 5A and B). These results indicate that C. albicans employs a copper-inducible P1-type ATPase transporter as the primary mechanism of resistance to high levels of copper.
FIG. 5.
Metal ion resistance of wild-type and crd disruption strains. Cells were grown overnight to saturation at 30°C in SD and diluted 1:100 into SD with various concentrations of CuSO4, AgNO3, or CdSO4. Cell growth was monitored by determining the OD600 of the cultures, with 100% growth equal to total cell growth in SD medium without added metal ions. (A and B) Strains CAI4 (○), CAPR303 (×), CAPR305 (▵), and CAPR307 (□). Growth at 24 (A) and 72 h (B) is shown. (C) Growth of strains CAI4 (CRD1) and CAPR305 (crd1) in 200 μM AgNO3 at 40 h and in 200 μM CdSO4 at 60 h. (D) Growth of wild-type and crd2 disruption strains at 18 h. Strains: CAI4 (○), CAPR401 (×), and CAPR403 (▵). For genotypes of strains, see Table 1.
The crd1 null mutant also exhibited increased sensitivity to silver and cadmium ions (Fig. 5C). This is consistent with the slight induction seen in medium containing CdSO4 and indicates some promiscuity with regards to metal ion transport by Crd1p. In contrast to the increased sensitivity of crd1 null mutants to silver and cadmium, crd1 mutants exhibited growth similar to that of the wild-type strain when exposed to high levels of zinc (data not shown).
Heterozygous and homozygous strains with the CRD2 gene disrupted also exhibited increased sensitivity to copper ions (Fig. 5D). This sensitivity was only observed at 18 h of incubation, and the crd2 disruption strains eventually reached wild-type growth levels after extended incubation (data not shown). Both the heterozygous and homozygous CRD2 disruption strains, CAPR401 and CAPR403, respectively, exhibited similar levels of growth inhibition at 18 h (Fig. 5D). These results indicate that Crd1p is the primary copper resistance determinant, while Crd2p may play a role in the initial buffering of excess copper ions until the CRD1 gene can be induced.
DISCUSSION
Cells need trace amounts of copper; but when copper is in excess, it is highly toxic (25, 50). To prevent copper toxicity, eukaryotes generally use MTs to chelate copper (17). In contrast, prokaryotes generally reduce influx and/or utilize efflux mechanisms to control intracellular copper levels (44). This work demonstrates that C. albicans primarily utilizes a P1-type ATPase (copper transporter) to resist copper ion toxicity because deletion of all copies of CRD1 resulted in a strain that exhibited greatly reduced resistance to copper ion toxicity, while deletion of all copies of CRD2 had a minor effect. This is the first example of a eukaryote utilizing a P1-type ATPase as its primary copper resistance mechanism.
Database analysis shows that the product of the CRD1 gene is highly similar to other P1-type ATPases involved in copper homeostasis in mammals, yeast, and prokaryotes. Examples of such proteins from mammals include the Menkes disease and Wilson disease proteins (3). Menkes disease is an X-linked disorder in which copper export is defective in many cell types, including the intestine, leading to systemic copper deficiency and ultimately neurological dysfunction and death. The autosomal recessive Wilson disease is due to mutations in a highly sequence-similar ATPase pump that is defective in hepatocytes, leading to liver and neurological damage due to copper ion toxicity. The Menkes and Wilson disease proteins each contain six CXXC motifs in their N-terminal regions, while Crd1p contains five CXXC repeats. In contrast, with the exception of E. coli CopA, which has two CXXC N-terminal repeats, most prokaryotic Cu-transporting ATPases have a single N-terminal HMA metal binding motif (40). The S. cerevisiae homolog of the Menkes disease and Wilson disease proteins is Ccc2p, which is required for export of cytosolic copper to the extracytosolic domain of the copper-dependent oxidase Fet3p (54). Prokaryotic genes exhibiting high levels of sequence similarity to the C. albicans CRD1 gene include known copper homeostatic genes copA of Enterobacter hirae (34), involved in copper import, and pacS of Synechococcus sp. strain PCC7942 (20), involved in copper export and resistance.
Previously characterized P1-type ATPases from yeast and prokaryotes involved in copper homeostasis exhibit tightly controlled transcriptional regulation that is consistent with their physiological roles. For example, the PacS protein of Synechococcus is induced upon exposure of the cells to excess copper (20). In contrast, the S. cerevisiae genes CTR1, CTR3, FRE1, and CCC2, which are involved in importing copper into the cell or delivering copper to the secretory pathway, are highly expressed in conditions of copper limitation; conditions of copper excess cause transcription of these genes to be strongly repressed (14, 24, 53). The CUP1 and MTIIA/B genes, encoding the major copper resistance MTs of S. cerevisiae and C. glabrata, respectively, are induced by growth in the presence of copper (5, 29, 30). C. albicans CRD1 is also induced by growth in the presence of copper, consistent with the observation that CRD1 confers copper resistance.
The cellular function(s) of the C. albicans MT gene CRD2 is undefined. However, physiological analyses of crd2 null mutants indicate a minor role for CRD2 in resistance to copper ion toxicity. The crd2/crd2 homozygous null strains exhibited a kinetic growth defect in that they grew more slowly in the presence of copper but ultimately reached wild-type levels of growth. Expression of the CRD2 gene is not induced in the presence of copper. Since intracellular copper can cause damage to DNA, proteins, and lipids, the basal expression of CRD2 may permit immediate binding of excess intracellular copper until the CRD1 efflux pump can be induced. The kinetic growth defect seen in the crd2 disruption strains may reflect the time required to synthesize and localize sufficient Crd1p and/or repair oxidative damage. Under normal growth conditions and low environmental copper concentrations, C. albicans synthesizes the CRD2 MT and does not express CRD1 at appreciable levels. This may permit C. albicans to maintain an intracellular reservoir of the essential trace element copper, and Crd2p may function in the proper distribution of copper to the various enzymes which require this trace element (36). The lack of induction in the presence of copper exhibited by the C. albicans CRD2 gene and its lack of amplification (data not shown) are consistent with a minor role in overall copper resistance. In addition to its role in copper homeostasis, C. albicans Crd2p may perform other functions in cellular metabolism. It has been demonstrated that S. cerevisiae Cu-Cup1p exhibits antioxidant activity, and Cu-Crd2p may also perform this role (36, 49).
Recently, a copper binding protein with significant sequence similarity to mammalian MTs was isolated from C. albicans cells grown in copper-containing medium (35). This protein is not identical to Crd2p reported in this study. While this work was in review, there appeared a paper by Weissman et al. describing the cloning of CaCRP1 (encoding a P1-type ATPase) and CaCUP1 (encoding a 33-amino-acid MT) involved in resistance to copper ion toxicity in C. albicans (51). The CaCUP1 gene was induced by copper and confers low-level resistance to copper ion toxicity. The deduced amino acid sequence of CaCup1p corresponded to that of the copper binding protein isolated by Oh et al. (35). CaCRP1 is essentially identical to CRD1 described in this study, and Weissman et al. demonstrated localization of CaCrp1p/Crd1p to the plasma membrane and the role of this P1-ATPase in maintaining low intracellular copper, consistent with our hypothesis that this protein functions in copper ion export.
Jensen et al. pointed out the striking similarities in terms of gene structure and regulation between the S. cerevisiae CUP1 and C. glabrata MTIIA/B genes and the S. cerevisiae CRS5 and C. glabrata MTI genes (19). The CUP1 gene of S. cerevisiae, the MTIIA/B gene of C. glabrata, and the CRD1 gene of C. albicans are all involved in high-level copper resistance and show high-level induction in conditions of excess copper (4, 21, 28, 30). In contrast, the S. cerevisiae CRS5 gene, C. glabrata MTI gene, and the C. albicans CRD2 gene contribute minimally to copper resistance, are single copy with no amplification, exhibit basal transcription in the absence of copper, and exhibit minimal transcriptional induction compared to that seen with the primary copper resistance determinants (8, 28, 30). Although the mechanism whereby high-level copper ion resistance is achieved in C. albicans is quite different from that of S. cerevisiae or C. glabrata, we suggest that the physiological role of the Crd1p ATPase of C. albicans is to detoxify copper ions, similar to the role of the S. cerevisiae Cup1p and C. glabrata MTIIA/B MTs, while the C. albicans Crd2p plays a role in copper homeostasis similar to that of the S. cerevisiae Crs5p and C. glabrata MTI MTs.
This hypothesis raises the question of why C. albicans utilizes an ATPase transporter as opposed to an MT as its primary means of copper resistance. Interestingly, the use of a P1-type ATPase may have important implications for nosocomial infections. The crd1 null mutant exhibits increased sensitivity to silver nitrate (Fig. 5C). Several Cu-transporting ATPases have been shown to be induced by and to transport silver ions in addition to copper (20, 33, 46). Generally this has been thought to be of no biological significance and a fortuitous occurrence due to the structural similarity between copper and silver ions (20, 46, 48). However, silver has been used as a microbiocidal agent for over a century and silver products are commonly used in the medical and dental fields (15). Silver-impregnated cloth and topicals containing silver sulfadiazine are used in hospital burn wards, and catheters containing silver as an antiseptic are widely used (9, 12, 22, 27, 32, 38). C. albicans is a frequent nosocomial infectious agent in burn patients and patients with indwelling catheters. Crd1p, which contributes to the ability of C. albicans to resist silver ion toxicity, may enhance the prevalence of C. albicans as a nosocomial pathogen.
Recently, a silver-resistant strain of Salmonella isolated from a hospital burn unit was shown to harbor a plasmid that contained multiple silver resistance genes linked in an approximately 14-kb region (16). One of the ORFs in this region encoded a P1-type ATPase (SilP) that contributes to silver resistance and that shares high sequence similarity with Cu-transporting ATPases (16). Thus, silver resistance mediated by ATPase pumps appears to make an important contribution to the virulence of nosocomial pathogens.
ACKNOWLEDGMENTS
We thank Dean Dawson and Rebecca Boumil for helpful discussions and the generous gift of S. cerevisiae strain dRM110.18A. We thank Yigal Koltin for the YEp13 C. albicans library. We are grateful to Ralph Isberg for helpful discussions and critical reading of the manuscript.
This work was supported by grant AI38591 from the National Institutes of Health (to C.A.K.). P.J.R. was supported during a portion of this work by HIV Pathogenesis Training Grant T32AI07389.
REFERENCES
- 1.Alani E, Cao L, Kleckner N. A method for gene disruption that allows repeated use of URA3 selection in the construction of multiply disrupted yeast strains. Genetics. 1987;116:541–545. doi: 10.1534/genetics.112.541.test. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F, Brent R, Kingston R, Moore D, Seidman J, Smith J, Struhl K, editors. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons; 1989. [Google Scholar]
- 3.Bull P C, Cox D W. Wilson disease and Menkes disease: new handles on heavy-metal transport. Trends Genet. 1994;10:246–252. doi: 10.1016/0168-9525(94)90172-4. [DOI] [PubMed] [Google Scholar]
- 4.Butt T R, Sternberg E, Herd J, Crooke S T. Cloning and expression of a yeast copper metallothionein gene. Gene. 1984;27:23–33. doi: 10.1016/0378-1119(84)90235-x. [DOI] [PubMed] [Google Scholar]
- 5.Butt T R, Sternberg E J, Gorman J A, Clark P, Hamer D, Rosenberg M, Crooke S T. Copper metallothionein of yeast, structure of the gene, and regulation of expression. Proc Natl Acad Sci USA. 1984;81:3332–3336. doi: 10.1073/pnas.81.11.3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Camakaris J, Voskoboinik I, Mercer J F. Molecular mechanisms of copper homeostasis. Biochem Biophys Res Commun. 1999;261:225–232. doi: 10.1006/bbrc.1999.1073. [DOI] [PubMed] [Google Scholar]
- 7.Clemens S, Kim E J, Neumann D, Schroeder J I. Tolerance to toxic metals by a gene family of phytochelatin synthases from plants and yeast. EMBO J. 1999;18:3325–3333. doi: 10.1093/emboj/18.12.3325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Culotta V C, Howard W R, Liu X F. CRS5 encodes a metallothionein-like protein in Saccharomyces cerevisiae. J Biol Chem. 1994;269:25295–25302. [PubMed] [Google Scholar]
- 9.Deitch E A, Marino A A, Gillespie T E, Albright J A. Silver-nylon: a new antimicrobial agent. Antimicrob Agents Chemother. 1983;23:356–359. doi: 10.1128/aac.23.3.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fogel S, Welch J W. Tandem gene amplification mediates copper resistance in yeast. Proc Natl Acad Sci USA. 1982;79:5342–5346. doi: 10.1073/pnas.79.17.5342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fonzi W A, Irwin M Y. Isogenic strain construction and gene mapping in Candida albicans. Genetics. 1993;134:717–728. doi: 10.1093/genetics/134.3.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gatter N, Kohnen W, Jansen B. In vitro efficacy of a hydrophilic central venous catheter loaded with silver to prevent microbial colonization. Zentralbl Bakteriol. 1998;287:157–169. doi: 10.1016/s0934-8840(98)80162-x. [DOI] [PubMed] [Google Scholar]
- 13.Gietz R D, Schiestl R H, Willems A R, Woods R A. Studies on the transformation of intact yeast cells by the LiAc/SS-DNA/PEG procedure. Yeast. 1995;11:355–360. doi: 10.1002/yea.320110408. [DOI] [PubMed] [Google Scholar]
- 14.Graden J A, Winge D R. Copper-mediated repression of the activation domain in the yeast Mac1p transcription factor. Proc Natl Acad Sci USA. 1997;94:5550–5555. doi: 10.1073/pnas.94.11.5550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guggenbichler J P, Boswald M, Lugauer S, Krall T. A new technology of microdispersed silver in polyurethane induces antimicrobial activity in central venous catheters. Infection. 1999;27(Suppl. 1):S16–S23. doi: 10.1007/BF02561612. [DOI] [PubMed] [Google Scholar]
- 16.Gupta A, Matsui K, Lo J F, Silver S. Molecular basis for resistance to silver cations in Salmonella. Nat Med. 1999;5:183–188. doi: 10.1038/5545. [DOI] [PubMed] [Google Scholar]
- 17.Hamer D H. Metallothionein. Annu Rev Biochem. 1986;55:913–951. doi: 10.1146/annurev.bi.55.070186.004405. [DOI] [PubMed] [Google Scholar]
- 18.Hamer D H, Thiele D J, Lemontt J E. Function and autoregulation of yeast copperthionein. Science. 1985;228:685–690. doi: 10.1126/science.3887570. [DOI] [PubMed] [Google Scholar]
- 19.Jensen L T, Howard W R, Strain J J, Winge D R, Culotta V C. Enhanced effectiveness of copper ion buffering by CUP1 metallothionein compared with CRS5 metallothionein in Saccharomyces cerevisiae. J Biol Chem. 1996;271:18514–18519. doi: 10.1074/jbc.271.31.18514. [DOI] [PubMed] [Google Scholar]
- 20.Kanamaru K, Kashiwagi S, Mizuno T. A copper-transporting P-type ATPase found in the thylakoid membrane of the cyanobacterium Synechococcus species PCC7942. Mol Microbiol. 1994;13:369–377. doi: 10.1111/j.1365-2958.1994.tb00430.x. [DOI] [PubMed] [Google Scholar]
- 21.Karin M, Najarian R, Haslinger A, Valenzuela P, Welch J, Fogel S. Primary structure and transcription of an amplified genetic locus: the CUP1 locus of yeast. Proc Natl Acad Sci USA. 1984;81:337–341. doi: 10.1073/pnas.81.2.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kearney J N, Arain T, Holland K T. Antimicrobial properties of antiseptic-impregnated biological dressings. J Hosp Infect. 1988;11:68–76. doi: 10.1016/0195-6701(88)90041-2. [DOI] [PubMed] [Google Scholar]
- 23.Kelly R, Miller S M, Kurtz M B, Kirsch D R. Directed mutagenesis in Candida albicans: one-step gene disruption to isolate ura3 mutants. Mol Cell Biol. 1987;7:199–208. doi: 10.1128/mcb.7.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Labbe S, Zhu Z, Thiele D J. Copper-specific transcriptional repression of yeast genes encoding critical components in the copper transport pathway. J Biol Chem. 1997;272:15951–15958. doi: 10.1074/jbc.272.25.15951. [DOI] [PubMed] [Google Scholar]
- 25.Linder M C, Hazegh-Azam M. Copper biochemistry and molecular biology. Am J Clin Nutr. 1996;63:797S–811S. doi: 10.1093/ajcn/63.5.797. [DOI] [PubMed] [Google Scholar]
- 26.Lutsenko S, Kaplan J H. Organization of P-type ATPases: significance of structural diversity. Biochemistry. 1995;34:15607–15613. doi: 10.1021/bi00048a001. [DOI] [PubMed] [Google Scholar]
- 27.MacMillan B G. The control of burn wound sepsis. Intensive Care Med. 1981;7:63–69. doi: 10.1007/BF01687262. [DOI] [PubMed] [Google Scholar]
- 28.Mehra R K, Garey J R, Butt T R, Gray W R, Winge D R. Candida glabrata metallothioneins. Cloning and sequence of the genes and characterization of proteins. J Biol Chem. 1989;264:19747–19753. [PubMed] [Google Scholar]
- 29.Mehra R K, Garey J R, Winge D R. Selective and tandem amplification of a member of the metallothionein gene family in Candida glabrata. J Biol Chem. 1990;265:6369–6375. [PubMed] [Google Scholar]
- 30.Mehra R K, Thorvaldsen J L, Macreadie I G, Winge D R. Disruption analysis of metallothionein-encoding genes in Candida glabrata. Gene. 1992;114:75–80. doi: 10.1016/0378-1119(92)90709-x. [DOI] [PubMed] [Google Scholar]
- 31.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 32.Modak S M, Sampath L, Fox C L., Jr Combined topical use of silver sulfadiazine and antibiotics as a possible solution to bacterial resistance in burn wounds. J Burn Care Rehabil. 1988;9:359–363. doi: 10.1097/00004630-198807000-00009. [DOI] [PubMed] [Google Scholar]
- 33.Odermatt A, Krapf R, Solioz M. Induction of the putative copper ATPases, CopA and CopB, of Enterococcus hirae by Ag+ and Cu2+, and Ag+ extrusion by CopB. Biochem Biophys Res Commun. 1994;202:44–48. doi: 10.1006/bbrc.1994.1891. [DOI] [PubMed] [Google Scholar]
- 34.Odermatt A, Suter H, Krapf R, Solioz M. Primary structure of two P-type ATPases involved in copper homeostasis in Enterococcus hirae. J Biol Chem. 1993;268:12775–12779. [PubMed] [Google Scholar]
- 35.Oh K B, Watanabe T, Matsuoka H. A novel copper-binding protein with characteristics of a metallothionein from a clinical isolate of Candida albicans. Microbiology. 1999;145:2423–2429. doi: 10.1099/00221287-145-9-2423. [DOI] [PubMed] [Google Scholar]
- 36.Palmiter R D. The elusive function of metallothioneins. Proc Natl Acad Sci USA. 1998;95:8428–8430. doi: 10.1073/pnas.95.15.8428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pfaller M A. Epidemiology and control of fungal infections. Clin Infect Dis. 1994;19(Suppl. 1):S8–S13. doi: 10.1093/clinids/19.supplement_1.s8. [DOI] [PubMed] [Google Scholar]
- 38.Raad I, Hachem R, Zermeno A, Dumo M, Bodey G P. In vitro antimicrobial efficacy of silver iontophoretic catheter. Biomaterials. 1996;17:1055–1059. doi: 10.1016/0142-9612(96)85905-9. [DOI] [PubMed] [Google Scholar]
- 39.Rad M R, Kirchrath L, Hollenberg C P. A putative P-type Cu(2+)-transporting ATPase gene on chromosome II of Saccharomyces cerevisiae. Yeast. 1994;10:1217–1225. doi: 10.1002/yea.320100910. [DOI] [PubMed] [Google Scholar]
- 40.Rensing C, Fan B, Sharma R, Mitra B, Rosen B P. CopA: an Escherichia coli Cu(I)-translocating P-type ATPase. Proc Natl Acad Sci USA. 2000;97:652–656. doi: 10.1073/pnas.97.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rensing C, Ghosh M, Rosen B P. Families of soft-metal-ion-transporting ATPases. J Bacteriol. 1999;181:5891–5897. doi: 10.1128/jb.181.19.5891-5897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rosenbluh A, Mevarech M, Koltin Y, Gorman J A. Isolation of genes from Candida albicans by complementation in Saccharomyces cerevisiae. Mol Gen Genet. 1985;200:500–502. doi: 10.1007/BF00425739. [DOI] [PubMed] [Google Scholar]
- 43.Scherer S, Magee P T. Genetics of Candida albicans. Microbiol Rev. 1990;54:226–241. doi: 10.1128/mr.54.3.226-241.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Silver S. Bacterial resistances to toxic metal ions—a review. Gene. 1996;179:9–19. doi: 10.1016/s0378-1119(96)00323-x. [DOI] [PubMed] [Google Scholar]
- 45.Silver S, Phung L T. Bacterial heavy metal resistance: new surprises. Annu Rev Microbiol. 1996;50:753–789. doi: 10.1146/annurev.micro.50.1.753. [DOI] [PubMed] [Google Scholar]
- 46.Solioz M, Odermatt A. Copper and silver transport by CopB-ATPase in membrane vesicles of Enterococcus hirae. J Biol Chem. 1995;270:9217–9221. doi: 10.1074/jbc.270.16.9217. [DOI] [PubMed] [Google Scholar]
- 47.Solioz M, Odermatt A, Krapf R. Copper pumping ATPases: common concepts in bacteria and man. FEBS Lett. 1994;346:44–47. doi: 10.1016/0014-5793(94)00316-5. [DOI] [PubMed] [Google Scholar]
- 48.Solioz M, Vulpe C. CPx-type ATPases: a class of P-type ATPases that pump heavy metals. Trends Biochem Sci. 1996;21:237–241. [PubMed] [Google Scholar]
- 49.Tamai K T, Gralla E B, Ellerby L M, Valentine J S, Thiele D J. Yeast and mammalian metallothioneins functionally substitute for yeast copper-zinc superoxide dismutase. Proc Natl Acad Sci USA. 1993;90:8013–8017. doi: 10.1073/pnas.90.17.8013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vulpe C D, Packman S. Cellular copper transport. Annu Rev Nutr. 1995;15:293–322. doi: 10.1146/annurev.nu.15.070195.001453. [DOI] [PubMed] [Google Scholar]
- 51.Weissman Z, Berdicevsky I, Cavari B Z, Kornitzer D. The high copper tolerance of Candida albicans is mediated by a P-type ATPase. Proc Natl Acad Sci USA. 2000;97:3520–3525. doi: 10.1073/pnas.97.7.3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welch J W, Fogel S, Cathala G, Karin M. Industrial yeasts display tandem gene iteration at the CUP1 region. Mol Cell Biol. 1983;3:1353–1361. doi: 10.1128/mcb.3.8.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yamaguchi-Iwai Y, Serpe M, Haile D, Yang W, Kosman D J, Klausner R D, Dancis A. Homeostatic regulation of copper uptake in yeast via direct binding of MAC1 protein to upstream regulatory sequences of FRE1 and CTR1. J Biol Chem. 1997;272:17711–17718. doi: 10.1074/jbc.272.28.17711. [DOI] [PubMed] [Google Scholar]
- 54.Yuan D S, Stearman R, Dancis A, Dunn T, Beeler T, Klausner R D. The Menkes/Wilson disease gene homologue in yeast provides copper to a ceruloplasmin-like oxidase required for iron uptake. Proc Natl Acad Sci USA. 1995;92:2632–2636. doi: 10.1073/pnas.92.7.2632. [DOI] [PMC free article] [PubMed] [Google Scholar]