Abstract
Phosphanorcaradienes are an appealing class of phosphorus compounds that can serve as synthons of transient phosphinidenes. However, the synthesis of such species is a formidable task owing to their intrinsic high reactivity. Herein we report straightforward synthesis, characterization and reactivity studies of a phosphanorcaradiene, in which one of the benzene rings in the flanking fluorenyl substituents is intramolecularly dearomatized through attachment to the phosphorus atom. It is facilely obtained by the reduction of phosphorus(III) dichloride precursor with potassium graphite. Despite being thermally robust, it acts as a synthetic equivalent of a transient phosphinidene. It reacts with trimethylphosphine and isonitrile to yield phosphanylidene-phosphorane and 1-phospha-3-azaallene, respectively. When it is treated with one and two molar equivalents of azide, iminophosphane and bis(imino)phosphane are isolated, respectively. Moreover, it is capable of activating ethylene and alkyne to afford [1 + 2] cycloaddition products, as well as oxidative cleavage of Si–H and N–H bonds to yield secondary phosphines. All the reactions proceed smoothly at room temperature without the presence of transition metals. The driving force for these reactions is most likely the high ring-constraint of the three-membered PC2 ring and recovery of the aromaticity of the benzene ring.
Subject terms: Chemical bonding, Ligands, Chemical bonding
Phosphanorcaradienes can serve as synthons for transient phosphinidene but the synthesis remains challenging. Here, the authors report a synthesis protocol for a phosphanorcaradiene, in which one of the benzene rings is intramolecularly dearomatized through attachment to the phosphorus atom.
Introduction
Carbene insertion into aromatic rings to produce cycloheptatrienes (CHTs) and norcaradienes (NCDs) developed by Büchner and Curtius in 1885 is a powerful method for the conversion of stable aromatic compounds to more reactive systems (Fig. 1a)1. The equilibria between CHTs and NCDs have been well-established2. Recent reports have shown that CHTs and NCDs can serve as carbene precursors via retro-Büchner reactions3,4. Phosphorus is viewed as carbon copy5. Phosphinidenes are neutral monocoordinate and monovalent phosphorus species possessing six valence electrons. Such species are isoelectronic to well-explored carbenes6–14 and tetrylenes15–17. In contrast, most phosphinidenes are transient species because of their exceptionally high reactivity18–21. The sole isolable example of phosphinidene was synthesized by Bertrand and coworkers22. The chemical properties of phosphinidenes have been mainly demonstrated by generating transient species with suitable precursors.
Fig. 1. Büchner ring expansion and selected P(III) compounds that act as synthons of transient phosphinidenes.
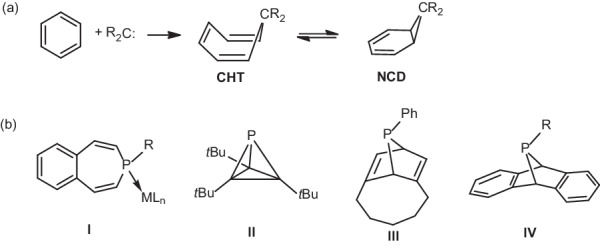
a Schematic depiction of Büchner ring expansion and the equilibria between cycloheptatriene (CHT) and norcaradiene (NCD); R is organic substituent. b Selected examples of P(III) precursors as synthons of transient phosphinidenes; R is organic substituent, and L is coordinating ligand.
Phosphepines, phosphorus analogs of CHTs, were first synthesized by Märkl et al. in the 1980s23,24, followed by the groups of Tsuchiya25,26 and Lammertsma27. The Lammertsma group showed that phosphepine transition metal (TM) complex I could act as a versatile metallo-phosphinidene precursor to activate a variety of substrates (Fig. 1b)28–31. In addition, several P(III) precursors, including phosphatetrahdrane II32, 7-phosphanorbornadiene III33, dibenzo derivatives IV34–36 and phosphirane-TM complexes37–41, have been utilized for phosphinidene reactivity studies. Notably, phosphinidene transfer with these materials must be promoted through heating or via TM catalysts.
In contrast to phosphepines, phosphanorcaradienes, which are phosphorus analogs of NCDs, have higher molecular-constraints and thereby exhibit inherent high reactivity, making their isolation in the condensed state a formidable task. On this basis, such compounds may be ideal synthons for accessing transient phosphinidenes via phosphorus-type retro-Büchner reactions. However, only one free phosphanorcaradiene has been reported by Stephan and coworkers. It was synthesized through demetalation of a phosphepine-gold complex, while its reactivity as a phosphinidene precursor for small molecule activation has not been fully disclosed42. Very recently, a ruthenophosphanorcaradiene acting as a synthon for an ambiphilic metallophosphinidene was reported by Scheer, Tilley and coworkers43.
Our group has continuous research interests in synthesizing low-coordinate main-group species, and has successfully isolated and structurally characterized several heavier analogs of free carbynes44–47, triplet stibinidene and bismuthinidenes (Fig. 2a)48–50 supported by sterically encumbered hydrindacene ligands51. Encouraged by these results, we continued our research to pursue isolable phosphinidenes. In this contribution, we report straightforward synthesis, characterization and reactivity studies of a phosphanorcaradiene 1 (Fig. 2b). Reactivity studies reveal that it can serve as an elegant synthon of phosphinidene due to the release of molecular-strain52,53.
Fig. 2. Triplet pnictinidenes and phosphanorcaradiene supported by hydrindacene ligands.
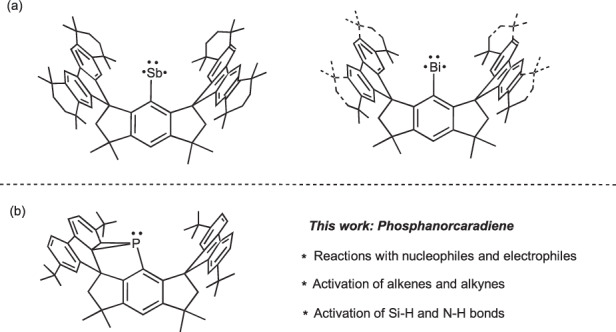
a Triplet stibinidene and bismuthinidenes; (b) phosphanorcaradiene in this work.
Results
Synthesis and characterization of 1
With the aim of synthesizing a stable phosphinidene, we carried out the reduction of the phosphorus(III) dichloride MsFluidtBu-PCl254 with two molar equivalents of potassium graphite in THF; however, phosphanorcaradiene 1 was obtained in 75% yield as a yellow solid instead of the expected product (Fig. 3a). One of the benzene rings in the flanking fluorenyl moieties is intramolecularly dearomatized through attachment to the phosphorus atom. Contrastingly, similar reduction reactions with less sterically hindered ligands afforded diphosphenes or other higher oligomers55–57. The formation of the PC2 three-membered ring leads to a decrease in the symmetry and complex 1H and 13C{1H} NMR spectra. The proton signal in the PC2 ring was shown at δ 2.54 ppm. A sharp singlet signal at δ–155.1 ppm was observed in the 31P{1H} NMR spectrum in C6D6 solution, similar to that of phosphiranes22,58. Interestingly, 1 can be heated to 100 oC in C6D6 solution for one hour without noticeable decomposition under an inert atmosphere, but it is highly air- and moisture-sensitive and yields intractable mixtures when exposed to air or dry oxygen. The synthesis of 1 is more straightforward in comparison to that of the phosphanorcaradiene reported by Stephan and coworkers.[18]
Fig. 3. Synthesis, molecular structure and selected frontier molecular orbitals of phosphanorcaradiene 1.
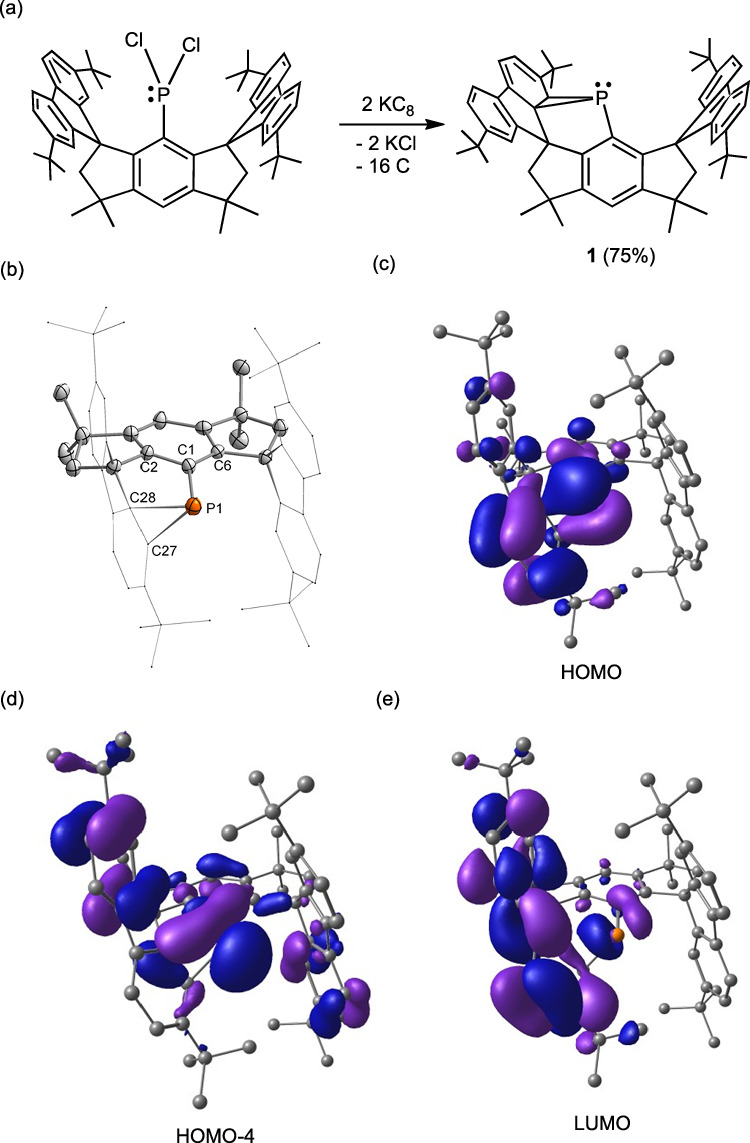
a Reduction of phosphorus(III) dichloride precursor with two molar equivalents of potassium graphite. b Thermal ellipsoid drawings of the molecular structure of 1 at 50% probability. All hydrogen atoms are omitted, and the fluorenyl moieties are shown in a wireframe style for clarity. Selected bond lengths (Å) and angles (o): P1–C1 1.847(4), P1–C27 1.973(5), P1–C28 1.985(5), C27–C28 1.476(6); C1–P1–C27 96.4(2), C1–P1–C28 88.06(19), C27–P1–C28 43.78(19), C27–C28–P1 67.7(3). c HOMO. d HOMO-4; (e) LUMO.
Single crystals of 1 suitable for X-ray diffraction analysis were obtained by layering n-hexane in a toluene solution at 4 oC59. It crystallizes in the triclinic space group P1. There are two independent enantiomers present in one crystal unit cell due to the chirality of the P atom. One of the molecules is shown in Fig. 3b, which unambiguously reveals the connection of the P atom to two C atoms of the flanking fluorenyl group. The distances of P1–C27 (1.973(5) Å) and P1–C28 (1.985(5) Å) are substantially longer than those of P1–C1 (1.847(4) Å) and the P–C distances of the PC2 ring in phosphiranes22,58. Consistently, the C27-P1-C28 angle (43.78(19)o) is more acute. Moreover, the P atom deviates from the position expected for atoms attached to a phenyl group as evidenced by the large difference between the bond angles of C2-C1-P1 (111.7(3)o) and C6-C1-P1 (131.7(4)o). These data suggest that the PC2 ring in 1 bears a greater ring-strain than phosphiranes.
Theoretical calculations of 1
The electronic structure of 1 was further elucidated by density functional theory (DFT) calculations at the BP86 + D3BJ/def2-SVP level. The HOMO represents the bonding orbitals of the C2P ring and the C–C π-orbitals of the linked C6 ring (Fig. 3c–e). The lone pair of P atom is shown in the HOMO-4, and the LUMO contains the π*-antibonding orbitals of the fluorenyl moiety linked to the P atom. Additionally, natural bond orbital (NBO) and intrinsic bond orbital (IBO)60 analyses were conducted to gain insight into the bonding character of 1. The Wiberg bond indices (WBIs) of the P1–C27 (0.74) and P1–C28 (0.72) bonds are substantially smaller than that of the P1–C1 bond (0.96), suggesting that they are relatively weaker than the P1–C1 bond. Moreover, they are smaller than those of the phosphanorcaradiene reported by Stephan and coworkers (0.79 and 0.76), implying a more fragile PC2 ring in 1. IBO analysis shows that P1–C27(C28) σ-bonds are formed by the overlap of the 3p orbitals at P1 and the 2p orbitals at C27(C28) (Supplementary Fig. 1). The 3p orbitals at the P atom and the 2p orbitals at the C atoms provided more than 90% of the bonding electrons in the P1–C27(C28) bonds. These orbitals are significantly different from the P1–C1 σ-bond, which is formed by the 3p orbital with the partial s character of P1 and the sp2 hybrid orbital of C1.
To further explore the electronic structure of the C2P ring of 1, the quantum theory of atoms in molecules (QTAIM) and electron localization function (ELF) analyses were carried out61,62. The QTAIM calculations indicate that there are three bond critical points (BCPs) among the P1, C27 and C28 atoms (Supplementary Fig. 2). In addition, the bond paths between P1 and C27(C28) slightly deviate away from the P1–C27(C28) axes. Furthermore, the ELF plots exhibit a twisted region with highly localized electron density between each C and P in the C2P ring (Supplementary Fig. 3).
The formation of 1 can be simplified to [2 + 1] cycloaddition of a transient phosphinidene with a C = C double of the flanking fluorenyl group. We previously reported the isolation of triplet stibinidene48 and bismuthinidenes49, which are heavy congeners of the proposed phosphinidene. A comparison of the calculated energies between triplet phosphinidene and 1 showed that 1 is 5.7 kcal/mol lower in energy than that of triplet phosphinidene (Supplementary Fig. 4). The conversion of triplet phosphinidene to 1 is facile with a predicted barrier no more than 5 kcal/mol.
To provide further understanding of 1, electronic and steric properties were theoretically studied. The proton affinity was analyzed to evaluate the electronic property, and meanwhile related calculations on PPh3 and Mes-P(CH2CH2) were also performed for comparison. As shown in Supplementary Fig. 5, the proton affinity of 1 is –156.8 kcal/mol, which is lower than those of PPh3 (–152.8 kcal/mol) and Mes-P(CH2CH2) (–141.8 kcal/mol). Compared with PPh3 and the analogous three-membered ring phosphirane Mes-P(CH2CH2), 1 has a better capability of electron donating. In addition, the steric property of 1 were studied with percent buried volume (%Vbur) analysis. The %Vbur of 1 is 76.6%, higher than those of PPh3 (65.0%) and Mes-P(CH2CH2) (54.3%). The results of percent buried volumes suggest that the steric hindrance around the phosphorus atom of 1 is greater than those of PPh3 and Mes-P(CH2CH2). Besides, steric maps shown in Supplementary Fig. 5 also display a larger steric hindrance of 1 than PPh3 and Mes-P(CH2CH2). Owing to the steric effect, this active phophanorcaradiene species 1 can be stabilized.
Reactions of 1 with nucleophiles and electrophiles
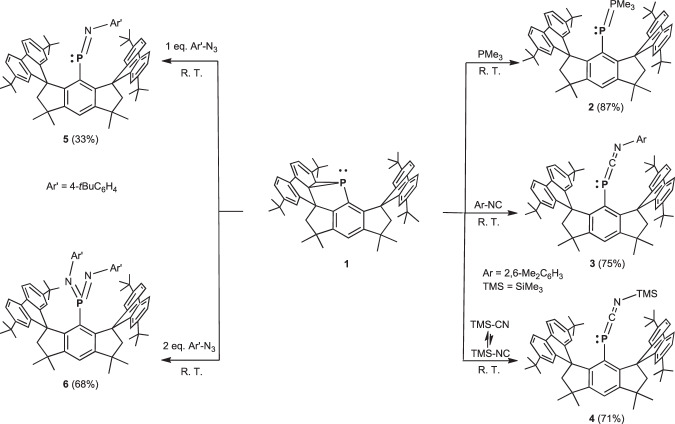
With understanding of the electronic structure, it is anticipated that 1 may act as a phosphinidene synthon through the dissociation of relatively weak P1–C27(C28) bonds and the release of molecular-strain. Accordingly, the reactivity of 1 toward nucleophiles and electrophile was tested (Fig. 4). It reacted smoothly at room temperature with trimethylphosphine to afford phosphanylidene-phosphorane 2, which was isolated in 87% yield. 2 exhibits 31P NMR resonance signals at δ –0.9 and –157.8 ppm with a coupling constant of 1JP-P = 603 Hz. The high-field signal is comparable to those of reported phosphanylidene-phosphoranes63,64, which have been utilized as phospha-Wittig reagents65–67. Moreover, treatment of 1 with 2,6-dimethylphenylisonitrile and trimethylsilylnitrile both afforded 1-phospha-3-azaallenes 3 and 4 in high yields, respectively. 31P resonances were observed at δ –152.3 and –190.5 ppm in the 31P{1H} NMR spectra. The formation of 4 can be interpreted by the equilibrium between trimethylsilylnitrile and trimethylsilylisonitrile68, and the latter species has a greater propensity to react with 1. A similar reaction pattern between disilyne and trimethylsilylnitrile has been reported by Sekiguchi and coworkers69. Additionally, 1 reacted with one and two molar equivalents of 4-tertbutylphenylazide yielded iminophosphorane 5 and bis(imino)phosphorane 6, respectively. Their 31P{1H} NMR spectra show singlet signals at δ 424.2 and 45.1 ppm, respectively.
Fig. 4. Reactions of 1 with nucleophiles and electrophiles.
Treatment of 1 with trimethylphosphine afforded phosphanylidene-phosphorane 2. Reactions of 1 with 2,6-dimethylphenylisonitrile and trimethylsilylnitrile yielded 1-phospha-3-azaallenes 3 and 4, respectively. 1 reacted with one and two molar equivalents of 4-tertbutylphenylazide afforded iminophosphorane 5 and bis(imino)phosphorane 6, respectively.
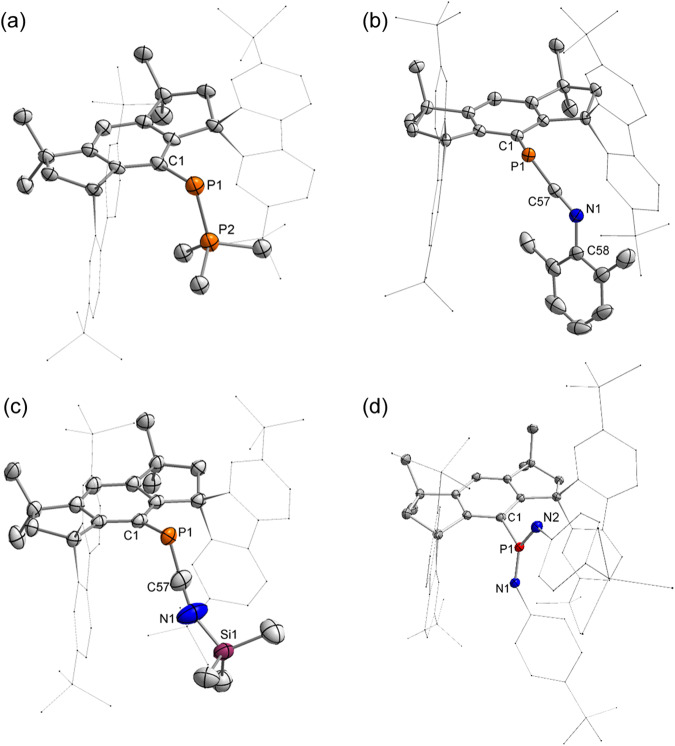
The dicoordinate phosphorus atoms in 2-4 were revealed by SC-XRD analysis (Fig. 5). The P–P distance of 2.1306(15) Å and the C1-P1-P2 bond angle of 102.03(11)o in 2 are comparable to those in DmpP=PMe3 (Dmp = 2,6-Mes2C6H3; 2.084(2) Å and 106.79(13)o, respectively)70. The P = C = N fragments in 3 and 4 are almost linear with P-C-N bond angles of 168.82(18)o and 168.0(3)o, respectively. Moreover, the P1–C57 (1.668(2) and 1.651(4) Å) and C57–N1 (1.200(3) and 1.212(5) Å) bonds feature double bond characteristics. These geometric parameters are reminiscent of (phosphino)phosphinidene-isonitriles reported by Bertrand and coworkers71, and are consistent with their cumulene-like properties. SC-XRD analysis shows that the phosphorus atom in 6 exhibits a trigonal planar geometry with a sum of angles around the P1 atom of 360o. Moreover, the distances of P1–N1 (1.5408(11) Å) and P1–N2 (1.5420(11) Å) suggest their double bond characters.
Fig. 5. Molecular structures of 2-4 and 6.
Thermal ellipsoid drawings of the molecular structures of 2 (a), 3 (b), 4 (c) and 6 (d) at 50% probability. All hydrogen atoms are omitted, and the fluorenyl moieties and the 4-tBuC6H4 groups are shown in a wireframe style for clarity.
Reactions of 1 with alkene and alkyne
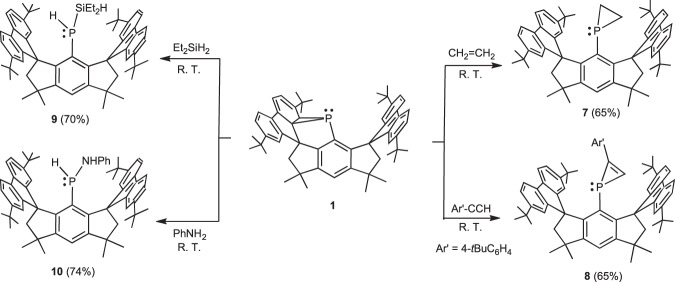
The ability of 1 to activate alkene and alkyne was further investigated (Fig. 6). Exposure of 1 to 1 atm. of ethylene atmosphere at room temperature led to the formation of phosphirane 7 in moderate yield. The 31P{1H} NMR resonance was shown at δ –229.2 ppm, similar to that of 1-mesitylphosphirane (δ –238.9 ppm)72. Complete consumption of 1 with an excess amount of 4-tertbutylphenylacetylene at room temperature was observed after 12 h, and phosphirene 8 was isolated in 65% yield. A single resonance signal was observed at δ –171.3 ppm in the 31P{1H} NMR spectrum of 8. The formation of 7 and 8 under mild conditions is most likely attributed to the decrease in the ring-strain and recovery of aromaticity at the six-membered ring in 1, which is striking since phosphinidene transfer to alkenes with II and IV developed by the Cummins group has to be promoted by heating or catalyzed by TM catalysts32,34,35. It is noteworthy that 7 and 8 do not react with 4-tertbutylphenylazide, most probably attributed to high steric hindrance around the phosphorus atoms.
Fig. 6. Activations of unactivated alkene, alkyne, silane and amine with 1.
The reaction of 1 with ethylene gave phosphirane 7; 1 reacted with 4-tertbutylphenylacetylene to afford phosphirene 8; 1 activated Si–H and N–H bonds to yield phosphines 9 and 10, respectively.
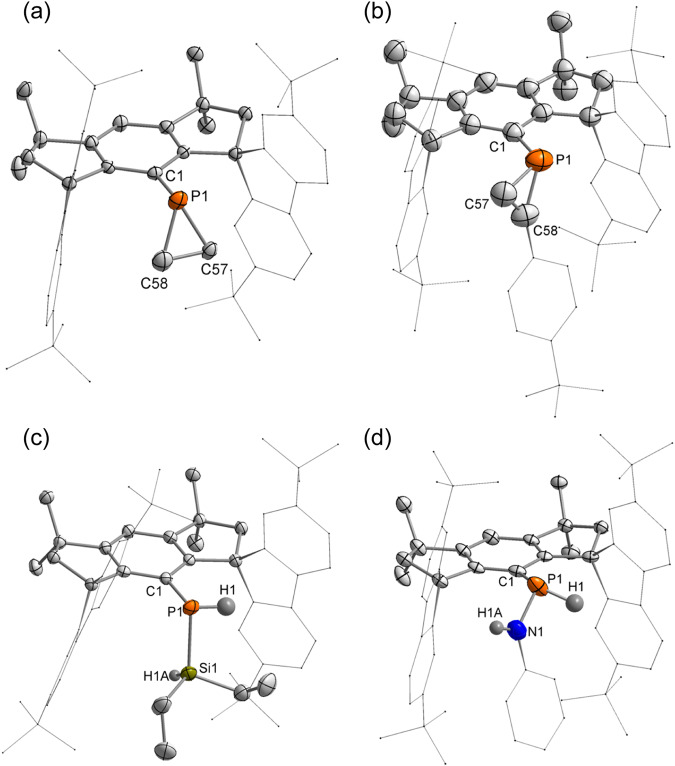
The molecular structures of 7 and 8 determined by SC-XRD analysis are shown in Fig. 7. New three-membered PC2 rings are shown in the structures. The C57–C58 distances are 1.513(4) and 1.308(5) Å, respectively, in accordance with their single and double bond nature. Moreover, the P–C bond lengths (1.847(3) and 1.848(3) Å for 7; 1.779(4) and 1.808(4) Å for 8) inside the PC2 ring are shortened in comparison to those in 1. In contrast to that of 1, the P atoms in 7 and 8 are in the expected positions for atoms attached to a phenyl ring.
Fig. 7. Molecular structures of 7-10.
Thermal ellipsoid drawings of the molecular structures of 7 (a), 8 (b), 9 (c) and 10 (d) at 50% probability. All hydrogen atoms except those at the P, Si and N atoms in 7 and 8 are omitted, and the fluorenyl moieties, the 4-tBuC6H4 and phenyl groups are shown in a wireframe style for clarity.
Activation of Si–H and N–H bonds with 1
Recently, geometrically constrained phosphines have shown to be capable of σ-bond activation73–76, prompting us to study the ability of 1 in σ-bond activation. Interestingly, we found that 1 could activate the Si–H and N–H bonds. The reactions of 1 with diethylsilane and phenylamine occurred smoothly at room temperature, and silylphosphine 9 and aminophosphine 10 were obtained in 70% and 74% yields, respectively (Fig. 6). Their structures were unambiguously determined by SC-XRD analysis (Fig. 7). The activation of Si–H and N–H bonds represents rare examples of intermolecular inert bond activation with metal-free transient phosphinidenes. Oxidation cleavage of Si–H bonds with electrophilic metallo-phosphinidene complexes was reported by Sterenberg et al.77,78. Stephan and coworkers showed hydrosilylation of low-valent P5Ph5 mediated by the Lewis acid B(C6F5)379. Hering-Junghans and coworkers recently revealed the first example of N–H activation at a metal-free P(I) center with phospha-Wittig reagents66. Additionally, the reactions of 1 with dioxygen and dihydrogen led to intractable mixtures.
Theoretical studies of the reaction mechanisms
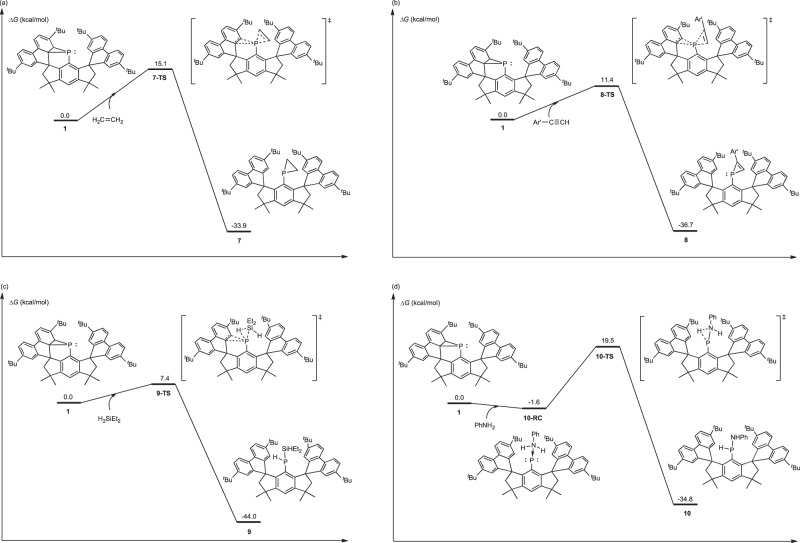
The mechanisms for the activation of alkene, alkyne, silane, and amine with 1 were studied by DFT calculations. For alkene, alkyne, and silane, as shown in Fig. 8a–c, respectively, the breakage of the PC2 three-membered ring structures and activations of the C = C bond, the C ≡ C bond, and the Si–H bond occur concertedly in respective transition states (7-TS, 8-TS, and 9-TS, respectively). Through respective transition states, the addition products 7, 8, and 9 are formed. While for the activation of amine, the phosphorus atom interacts with amine to form 10-RC firstly, in which the three-membered ring structure in 1 is broken (Fig. 8d). The N–H bond is activated through 10-TS to form product 10. The energy barriers for activations of alkene, alkyne, silane, and amine are 15.1, 11.4, 7.4, and 21.1 kcal/mol, respectively, which are in line with the reaction conditions of room temperature. As shown in Fig. 8, these transformations are exothermic processes through early transition states, and thus the activations of alkene, alkyne, silane, and amine are facile by the active phophanorcaradiene 1.
Fig. 8. Studies of the reaction mechanisms through DFT calculations.
Gibbs free energy profiles of activations of (a) alkene, (b) alkyne, (c) silane, and (d) amine with 1 (Ar’ = 4-tBuC6H4, Gibbs free energies are shown in kcal/mol). Calculations were performed at the BP86-D3/def2-TZVPP//BP86-D3/def2-SVP level of theory.
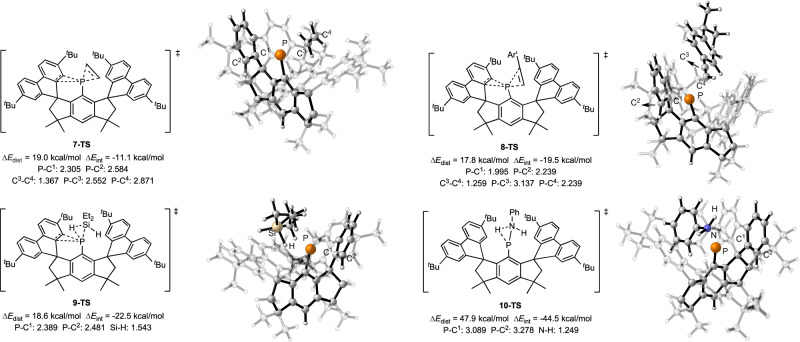
In addition, the structures of 7-TS, 8-TS, 9-TS, and 10-TS were analysed. As Fig. 9 shows, P–C1 and P–C2 distances in these transition states are all larger than those in 1 (1.980 and 2.002 Å for P-C1 and P-C2 respectively), indicating the breakage of the three-membered ring structure. The C3–C4 distance in 7-TS is 1.367 Å, and the C3–C4 distance in 8-TS is 1.259 Å. These C3–C4 distances respectively suggest that the C = C bond and the C ≡ C bond are activated. Besides, 7-TS and 8-TS are typical concerted asynchronous transition states. For 7-TS, the P–C3 and the P–C4 distances are 2.552 and 2.871 Å, respectively, and for 8-TS, the P–C3 and the P–C4 distances are 3.137 and 2.239 Å respectively. For 9-TS, the Si–H distance is 1.543 Å, and the N–H distance in 10-TS is 1.249 Å, suggesting the activations of the Si–H bond and the N–H bond. Furthermore, distortion analysis was performed on 7-TS, 8-TS, 9-TS, and 10-TS80,81. In the distortion analysis, these transition states were divided into the activated molecule moiety and the phophanorcaradiene moiety. The total deformation energies (ΔEdist) of 7-TS, 8-TS, 9-TS, and 10-TS are shown in Fig. 9. The deformations of 7-TS, 8-TS, and 9-TS compared to respective RCs are not as large as that of 10-TS, probably due to the aromatization driving force of the decyclization of P-ligand three-membered ring structure in the transition state. The ΔEdist of 7-TS, 8-TS, and 9-TS are 19.0, 17.8, and 18.6 kcal/mol respectively, which are all lower than that of 10-TS (47.9 kcal/mol). Therefore, the energy barrier of the activation of amine is relatively higher than those of activations of alkene, alkyne, and silane. In addition, the interaction energies (ΔEint) of transition states were also analyzed. ΔEint of 10-TS is –44.5 kcal/mol, which is much higher than those of 7-TS, 8-TS, and 9-TS (–11.1, –19.5, and –22.5 kcal/mol, respectively). Although the ΔEdist of 10-TS is high, the high ΔEint of 10-TS leads to a relatively low energy barrier for amine activation.
Fig. 9. Theoretical investigation of the structures of the transition states.
Structures of transition states in activations of alkene, alkyne, silane, and amine (distance are shown in Å). Calculations were performed at the BP86-D3/def2-TZVPP//BP86-D3/def2-SVP level of theory.
Discussion
In summary, we have described the synthesis and characterization of thermally robust phosphanorcaradiene 1 with high molecular-strain. It could react with nucleophiles and electrophiles. Strikingly, 1 is capable of activating C = C double and C ≡ C triple bonds, as well as Si–H and N–H bonds at room temperature. The driving force for these reactions is most likely the high ring-strain of the three-membered PC2 ring and recovery of aromaticity of the C6 ring. This work demonstrates the great potential of phosphanorcaradiene as a synthetic equivalent of phosphinidene in small molecule activation. The use of 1 to synthesize more phosphorus compounds including phosphinidene-TM catalysts is under investigation in our laboratory.
Methods
All experiments were carried out under a dry oxygen-free nitrogen atmosphere using standard Schlenk techniques or in a N2 filled-glove box. Solvents were dried by standard methods and stored in activated 4 Å molecule sieve in the glovebox. All reagents were purchased from commercial sources (Energy Chemical and TCI) and used without further purification unless otherwise noted. MsFluindtBu-PCl254, and KC882 were synthesized according to reported procedures. The 1H, 13C{1H} and 31P{1H} NMR spectra were recorded on Bruker spectrometers (AV400 and AV600). Chemical shift values for protons are referenced to the residual proton resonance of CDCl3 (δ: 7.26), C6D6 (δ: 7.16), THF-d8 (δ: 3.62); chemical shift values for carbons are referenced to the carbon resonance of CDCl3 (δ: 77.16), C6D6 (δ: 128.06), THF-d8 (δ: 67.21); chemical shift values for phosphorus are relative to 85% H3PO4. NMR multiplicities are abbreviated as follows: s = singlet, d = doublet, t = triplet, q = quartet, sept = septet, m = multiplet, br = broad signal. Chemical shifts are quoted in δ (ppm) and coupling constants in Hz. The samples were dissolved in deuterated solvents, and were sealed off in J-Young NMR tubes for measurements. For the single crystal X-ray structure analysis, the crystals were each mounted on a glass capillary in perfluorinated oil and measured in a cold N2 flow. The data for all compounds were collected on a Bruker D8 Venture or XtaLAB Synergy R, DW system, HyPix diffractometer at low temperatures.
Supplementary information
Source data
Acknowledgements
We thank the National Natural Science Foundation of China (Grants: 22322112 and 22071164, G.T.), Fundamental Research Funds for the Central Universities, Sun Yat-sen University (23qnpy35, G.T.), and the Suzhou Science & Technology NOVA Program (Grant: ZXL2022445, G.T.) for generous financial support. The computational work is supported by National Supercomputer Center (Tianhe-2 Supercomputer) in Guangzhou.
Author contributions
G.T. designed the project. Y.C. carried out the experimental and part theoretical work. Z.K. instructed the mechanistic studies, and P.S. performed the studies. D.W. performed single-crystal X-ray diffraction studies. Y.C. and P.S. contributed equally to the work.
Peer review
Peer review information
Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work. A peer review file is available.
Data availability
All data generated or analyzed during this study are included in the Supplementary Information. Details about materials and methods, experimental procedures, characterization data, and theoretical calculations are available in the Supplementary Information. The structures of 1–8 in the solid state were determined by single-crystal Xray diffraction studies and the crystallographic data have been deposited with the Cambridge Crystallographic Data Center under nos. CCDC 2323602 (1), 2323603 (2), 2323604 (3), 2323605 (4), 2349216 (6), 2323606 (7), 2323607 (8), 2323608 (9), and 2323609 (10). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data request/cif. All data are also available from corresponding authors upon request. Source data are present in this paper. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Yizhen Chen, Peifeng Su.
Change history
10/11/2024
A Correction to this paper has been published: 10.1038/s41467-024-53231-3
Contributor Information
Zhuofeng Ke, Email: kezhf3@mail.sysu.edu.cn.
Gengwen Tan, Email: tangw55@mail.sysu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-49042-1.
References
- 1.Buchner, E. & Curtius, T. Ueber die einwirkung von diazoessigäther auf aromatische kohlenwasserstoffe. Ber. Dtsch. Chem. Ges.18, 2377–2379 (1885). [Google Scholar]
- 2.Ciganek, E. The cycloheptatriene-norcaradiene system. I. 7,7-dicyanonorcaradienes. Preparation and structure proof. J. Am. Chem. Soc.89, 1454–1458 (1967). [Google Scholar]
- 3.Solorio-Alvarado, C. R. & Echavarren, A. M. Gold-catalyzed annulation/fragmentation: Formation of free gold carbenes by retro-cyclopropanation. J. Am. Chem. Soc.132, 11881–11883 (2010). [DOI] [PubMed] [Google Scholar]
- 4.Guo, Y., Nguyen, T. V. & Koenigs, R. M. Norcaradiene synthesis via visible-light-mediated cyclopropanation reactions of arenes. Org. Lett.21, 8814–8818 (2019). [DOI] [PubMed] [Google Scholar]
- 5.Dillon, K. B., Mathey, F. & Nixon, J. F., Phosphorus: The carbon copy. (John Wiley & Sons, New York, 1998).
- 6.Sau, S. C., Hota, P. K., Mandal, S. K., Soleilhavoup, M. & Bertrand, G. Stable abnormal N-heterocyclic carbenes and their applications. Chem. Soc. Rev.49, 1233–1252 (2020). [DOI] [PubMed] [Google Scholar]
- 7.Vivancos, Á., Segarra, C. & Albrecht, M. Mesoionic and related less heteroatom-stabilized N-heterocyclic carbene complexes: Synthesis, catalysis, and other applications. Chem. Rev.118, 9493–9586 (2018). [DOI] [PubMed] [Google Scholar]
- 8.Melaimi, M., Jazzar, R., Soleilhavoup, M. & Bertrand, G. Cyclic (alkyl)(amino)carbenes (CAACs): Recent developments. Angew. Chem. Int. Ed.56, 10046–10068 (2017). [DOI] [PubMed] [Google Scholar]
- 9.Nesterov, V. et al. NHCs in main group chemistry. Chem. Rev.118, 9678–9842 (2018). [DOI] [PubMed] [Google Scholar]
- 10.Loh, Y. K., Melaimi, M., Gembicky, M., Munz, D. & Bertrand, G. A crystalline doubly oxidized carbene. Nature623, 66–70 (2023). [DOI] [PubMed] [Google Scholar]
- 11.Hu, C. et al. Crystalline monometal-substituted free carbenes. Chem8, 2278–2289 (2022). [Google Scholar]
- 12.Wei, R., Wang, X.-F., Hu, C. & Liu, L. L. Synthesis and reactivity of copper carbyne anion complexes. Nat. Synth.2, 357–363 (2023). [Google Scholar]
- 13.Loh, Y. K., Melaimi, M., Munz, D. & Bertrand, G. An air-stable “masked” bis(imino)carbene: A carbon-based dual ambiphile. J. Am. Chem. Soc.145, 2064–2069 (2023). [DOI] [PubMed] [Google Scholar]
- 14.Hu, C., Wang, X.-F., Li, J., Chang, X.-Y. & Liu, L. L. A stable rhodium-coordinated carbene with a σ0π2 electronic configuration. Science383, 81–85 (2024). [DOI] [PubMed] [Google Scholar]
- 15.Yao, S., Saddington, A., Xiong, Y. & Driess, M. Chelating bis-silylenes as powerful ligands to enable unusual low-valent main-group element functions. Acc. Chem. Res.56, 475–488 (2023). [DOI] [PubMed] [Google Scholar]
- 16.Asay, M., Jones, C. & Driess, M. N-heterocyclic carbene analogues with low-valent group 13 and group 14 elements: Syntheses, structures, and reactivities of a new generation of multitalented ligands. Chem. Rev.111, 354–396 (2011). [DOI] [PubMed] [Google Scholar]
- 17.He, M., Hu, C., Wei, R., Wang, X.-F. & Liu, L. L., Recent advances in the chemistry of isolable carbene analogues with group 13–15 elements. Chem. Soc. Rev., 10.1039/D3CS00784G (2024). [DOI] [PubMed]
- 18.Grützmacher, H.-F., Silhan, W. & Schmidt, U. Über phosphinidene, 5. Notiz zum nachweis von phenyl- und methylphosphiniden beim thermischen zerfall von cyclophosphinen durch pyrolyse-massenspektrometrie. Chem. Ber.102, 3230–3232 (1969). [Google Scholar]
- 19.Schmidt, U. Formation, detection, and reactions of phosphinidenes (phosphanediyls). Angew. Chem. Int. Ed.14, 523–528 (1975). [Google Scholar]
- 20.Cowley, A. H., Gabbai, F., Schluter, R. & Atwood, D. New approaches to the generation of phosphinidenes. J. Am. Chem. Soc.114, 3142–3144 (1992). [Google Scholar]
- 21.Li, X., Lei, D., Chiang, M. Y. & Gaspar, P. P. General approaches to phosphinidenes via retroadditions. J. Am. Chem. Soc.114, 8526–8531 (1992). [Google Scholar]
- 22.Liu, L., Ruiz, D. A., Munz, D. & Bertrand, G. A singlet phosphinidene stable at room temperature. Chem1, 147–153 (2016). [Google Scholar]
- 23.Märkl, G. & Burger, W. 2,7-di-tert-butyl-1-phenyl-1h-phosphepine—the first stable, monocyclic phosphepine. Angew. Chem. Int. Ed.23, 894–895 (1984). [Google Scholar]
- 24.Märkl, G. & Burger, W. 3-phenyl-3-benzphospherin. Tetrahedron Lett.24, 2545–2548 (1983). [Google Scholar]
- 25.Kurita, J., Shiratori, S., Yasuike, S. & Tsuchiya, T., Synthesis of the first examples of 1-benzophosphepines. J. Chem. Soc., Chem. Commun. 1991, 1227–1228 (1991).
- 26.Yasuike, S., Ohta, H., Shiratori, S., Kurita, J. & Tsuchiya, T., Syntheses of the group 15 1-benzoheteroepines, dibenzo[b,d]heteroepines and dibenzo[b,f]heteroepines involving the first isolated examples of arsepines and bismepines. J. Chem. Soc., Chem. Commun. 1993, 1817–1819 (1993).
- 27.Lyaskovskyy, V. et al. Dibenzo[b,f]phosphepines: Novel phosphane–olefin ligands for transition metals. Organometallics32, 363–373 (2013). [Google Scholar]
- 28.Jansen, H. et al. Reactive intermediates: A transient electrophilic phosphinidene caught in the act. Chem. Eur. J.16, 1454–1458 (2010). [DOI] [PubMed] [Google Scholar]
- 29.Aktaş, H., Slootweg, J. C. & Lammertsma, K. Nucleophilic phosphinidene complexes: Access and applicability. Angew. Chem. Int. Ed.49, 2102–2113 (2010). [DOI] [PubMed] [Google Scholar]
- 30.Borst, M. L. G. et al. Phosphepines: Convenient access to phosphinidene complexes. J. Am. Chem. Soc.127, 5800–5801 (2005). [DOI] [PubMed] [Google Scholar]
- 31.Borst, M. L. G. et al. 3h-benzophosphepine complexes: Versatile phosphinidene precursors. J. Am. Chem. Soc.127, 16985–16999 (2005). [DOI] [PubMed] [Google Scholar]
- 32.Riu, M.-L. Y., Eckhardt, A. K. & Cummins, C. C. Reactions of tri-tert-butylphosphatetrahedrane as a spring-loaded phosphinidene synthon featuring nickel-catalyzed transfer to unactivated alkenes. J. Am. Chem. Soc.144, 7578–7582 (2022). [DOI] [PubMed] [Google Scholar]
- 33.van Eis, M. J. et al. The first free 7λ3-phosphanorbornadiene. J. Am. Chem. Soc.122, 3386–3390 (2000). [Google Scholar]
- 34.Xin, T. et al. Synthesis of phosphiranes via organoiron-catalyzed phosphinidene transfer to electron-deficient olefins. Chem. Sci.13, 12696–12702 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Velian, A. & Cummins, C. C. Facile synthesis of dibenzo-7λ3-phosphanorbornadiene derivatives using magnesium anthracene. J. Am. Chem. Soc.134, 13978–13981 (2012). [DOI] [PubMed] [Google Scholar]
- 36.Xin, T. & Cummins, C. C. Synthesis of phosphet-2-one derivatives via phosphinidene transfer to cyclopropenones. J. Am. Chem. Soc.145, 25989–25994 (2023). [DOI] [PubMed] [Google Scholar]
- 37.Wang, M. et al. The chemistry of phosphirane-substituted phosphinidene complexes. Chem. Commun.56, 9707–9710 (2020). [DOI] [PubMed] [Google Scholar]
- 38.Wit, J. B. M. et al. iPr2N–P═Fe(CO)4 in olefinic solvents: A reservoir of a transient phosphinidene complex capable of substrate hopping. Organometallics35, 1170–1176 (2016). [Google Scholar]
- 39.Wang, M. et al. Transition-metal-like reversible cycloadditions of [tBuSP-W(CO)5] with alkenes and alkynes. Chem. Eur. J.25, 15036–15039 (2019). [DOI] [PubMed] [Google Scholar]
- 40.Wong, J., Li, Y., Hao, Y., Tian, R. & Mathey, F. Isomerization of secondary phosphirane into terminal phosphinidene complexes: An analogy between monovalent phosphorus and transition metals. Angew. Chem. Int. Ed.54, 12891–12893 (2015). [DOI] [PubMed] [Google Scholar]
- 41.Hao, Y. et al. The chemistry of parent phosphiranide in the coordination sphere of tungsten. Dalton Trans.45, 8284–8290 (2016). [DOI] [PubMed] [Google Scholar]
- 42.Liu, L. L., Zhou, J., Andrews, R. & Stephan, D. W. A room-temperature-stable phosphanorcaradiene. J. Am. Chem. Soc.140, 7466–7470 (2018). [DOI] [PubMed] [Google Scholar]
- 43.Saint-Denis, T. G. et al. A ruthenophosphanorcaradiene as a synthon for an ambiphilic metallophosphinidene. J. Am. Chem. Soc.146, 4369–4374 (2024). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang, D. et al. An isolable germylyne radical with a one-coordinate germanium atom. Nat. Chem.15, 200–205 (2023). [DOI] [PubMed] [Google Scholar]
- 45.Wang, D. et al. Monosubstituted doublet Sn(I) radical featuring substantial unquenched orbital angular momentum. J. Am. Chem. Soc.145, 6914–6920 (2023). [DOI] [PubMed] [Google Scholar]
- 46.Wang, D. et al. An isolable phosphinogermylyne as a synthon of one-coordinate GeI radical. Chin. J. Chem.42, 736–742 (2024). [Google Scholar]
- 47.Chen, H. et al. An isolable one-coordinate lead(I) radical with strong g-factor anisotropy. Angew. Chem. Int. Ed. 63, e202402093 (2024). [DOI] [PubMed]
- 48.Wu, M. et al. A triplet stibinidene. Chem9, 2573–2584 (2023). [Google Scholar]
- 49.Wu, M. et al. Triplet bismuthinidenes featuring unprecedented giant and positive zero field splittings. Natl Sci. Rev.10, nwad169 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang, Y. et al. Synthesis and isolation of a triplet bismuthinidene with a quenched magnetic response. Science380, 1043–1048 (2023). [DOI] [PubMed] [Google Scholar]
- 51.He, Y., Dai, C., Wang, D., Zhu, J. & Tan, G. Phosphine-stabilized germylidenylpnictinidenes as synthetic equivalents of heavier nitrile and isocyanide in cycloaddition reactions with alkynes. J. Am. Chem. Soc.144, 5126–5135 (2022). [DOI] [PubMed] [Google Scholar]
- 52.Tang, M., Liang, Y., Liu, J., Bian, L. & Liu, Z. Mechanical trapping of the Phlorin intermediate. CCS Chem.4, 3230–3237 (2022). [Google Scholar]
- 53.Tang, M. et al. Molecular-strain engineering of double-walled tetrahedra. Chem.7, 2160–2174 (2021). [Google Scholar]
- 54.Chen, Y. et al. An isolable heteroleptic diarylphosphinyl radical. Organometallics42, 538–542 (2023). [Google Scholar]
- 55.Yoshifuji, M., Shima, I., Inamoto, N., Hirotsu, K. & Higuchi, T. Synthesis and structure of bis(2,4,6-tri-tert-butylphenyl)diphosphene: Isolation of a true phosphobenzene. J. Am. Chem. Soc.103, 4587–4589 (1981). [Google Scholar]
- 56.Weber, L. The chemistry of diphosphenes and their heavy congeners: Synthesis, structure, and reactivity. Chem. Rev.92, 1839–1906 (1992). [Google Scholar]
- 57.Twamley, B., Sofield, C. D., Olmstead, M. M. & Power, P. P. Homologous series of heavier element dipnictenes 2,6-Ar2H3C6E=EC6H3-2,6-Ar2 (E = P, As, Sb, Bi; Ar = Mes = C6H2-2,4,6-Me3; or Trip = C6H2-2,4,6-iPr3) stabilized by m-terphenyl ligands. J. Am. Chem. Soc.121, 3357–3367 (1999). [Google Scholar]
- 58.Liedtke, J., Loss, S., Alcaraz, G., Gramlich, V. & Grützmacher, H. Very stable phosphiranes. Angew. Chem. Int. Ed.38, 1623–1626 (1999). [DOI] [PubMed] [Google Scholar]
- 59.Sheldrick, G. M. Crystal structure refinement with shelxl. Acta Cryst.C71, 3–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Knizia, G. Intrinsic atomic orbitals: An unbiased bridge between quantum theory and chemical concepts. J. Chem. Theory Comput.9, 4834–4843 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Bader, R. F. W., Atoms in molecules: A quantum theory. (Oxford University Press, Oxford, 1990).
- 62.Lu, T. & Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem.33, 580–592 (2012). [DOI] [PubMed] [Google Scholar]
- 63.Urnėžius, E., Shah, S. & Protasiewicz, J. D. Diphosphene and phosphoranylidenephosphine formation from a terminal phosphinidene complex. Phosphorus, Sulfur Silicon Relat. Elem.144, 137–139 (1999). [Google Scholar]
- 64.Surgenor, B. A., Bühl, M., Slawin, A. M. Z., Woollins, J. D. & Kilian, P. Isolable phosphanylidene phosphorane with a sterically accessible two-coordinate phosphorus atom. Angew. Chem. Int. Ed.51, 10150–10153 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah, S. & D. Protasiewicz, J., ‘Phospha-wittig’ reactions using isolable phosphoranylidenephosphines ArP=PR3 (Ar = 2,6-Mes2C6H3 or 2,4,6-But3C6H2). Chem. Commun. 1998, 1585–1586 (1998).
- 66.Dankert, F., Siewert, J.-E., Gupta, P., Weigend, F. & Hering-Junghans, C. Metal-free N−H bond activation by phospha-wittig reagents. Angew. Chem. Int. Ed.61, e202207064 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shah, S. & Protasiewicz, J. D. ‘Phospha-variations’ on the themes of Staudinger and Wittig: Phosphorus analogs of Wittig reagents. Coord. Chem. Rev.210, 181–201 (2000). [Google Scholar]
- 68.Booth, M. R. & Frankiss, S. G. The constitution, vibrational spectra and proton resonance spectra of trimethylsilyl cyanide and isocyanide. Spectrochim. Acta, Part A26, 859–869 (1970). [Google Scholar]
- 69.Takeuchi, K., Ichinohe, M. & Sekiguchi, A. Reactivity of the disilyne RSi≡SiR (R = SiiPr[CH(SiMe3)2]2) toward silylcyanide: Two pathways to form the bis-adduct [RSiSir(CNSiMe3)2] with some silaketenimine character and a 1,4-diaza-2,3-disilabenzene analogue. J. Am. Chem. Soc.130, 16848–16849 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Shah, S., Yap, G. P. A. & Protasiewicz, J. D. Crystal structure of the phosphanylidene-σ4-phosphorane DmpP=PMe3 (Dmp=2,6-Mes2C6H3) and reactions with electrophiles. J. Organomet. Chem.608, 12–20 (2000). [Google Scholar]
- 71.Hansmann, M. M. & Bertrand, G. Transition-metal-like behavior of main group elements: Ligand exchange at a phosphinidene. J. Am. Chem. Soc.138, 15885–15888 (2016). [DOI] [PubMed] [Google Scholar]
- 72.Akimov, A. V., Ganushevich, Y. S., Korchagin, D. V., Miluykov, V. A. & Misochko, E. Y. The EPR spectrum of triplet mesitylphosphinidene: Reassignment and new assignment. Angew. Chem. Int. Ed.56, 7944–7947 (2017). [DOI] [PubMed] [Google Scholar]
- 73.Chulsky, K., Malahov, I., Bawari, D. & Dobrovetsky, R. Metallomimetic chemistry of a cationic, geometrically constrained phosphine in the catalytic hydrodefluorination and amination of Ar–F bonds. J. Am. Chem. Soc.145, 3786–3794 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Volodarsky, S., Bawari, D. & Dobrovetsky, R. Dual reactivity of a geometrically constrained phosphenium cation. Angew. Chem. Int. Ed.61, e202208401 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lin, Y.-C., Gilhula, J. C. & Radosevich, A. T. Nontrigonal constraint enhances 1,2-addition reactivity of phosphazenes. Chem. Sci.9, 4338–4347 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hannah, T. J. & Chitnis, S. S. Ligand-enforced geometric constraints and associated reactivity in p-block compounds. Chem. Soc. Rev.53, 764–792 (2024). [DOI] [PubMed] [Google Scholar]
- 77.Vaheesar, K., Bolton, T. M., East, A. L. L. & Sterenberg, B. T. Si−H bond activation by electrophilic phosphinidene complexes. Organometallics29, 484–490 (2010). [Google Scholar]
- 78.Rajagopalan, R. A. & Sterenberg, B. T. Formation and reactivity of a transient cationic alkyl phosphinidene complex. Organometallics30, 2933–2938 (2011). [Google Scholar]
- 79.Geier, S. J. & Stephan, D. W. Lewis acid mediated P–P bond hydrogenation and hydrosilylation. Chem. Commun.46, 1026–1028 (2010). [DOI] [PubMed] [Google Scholar]
- 80.Fernández, I. & Bickelhaupt, F. M. The activation strain model and molecular orbital theory: Understanding and designing chemical reactions. Chem. Soc. Rev.43, 4953–4967 (2014). [DOI] [PubMed] [Google Scholar]
- 81.Bickelhaupt, F. M. & Houk, K. N. Analyzing reaction rates with the distortion/interaction-activation strain model. Angew. Chem. Int. Ed.56, 10070–10086 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ottmers, D. M. & Rase, H. F. Potassium graphites prepared by mixed-reaction technique. Carbon4, 125–127 (1966). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in the Supplementary Information. Details about materials and methods, experimental procedures, characterization data, and theoretical calculations are available in the Supplementary Information. The structures of 1–8 in the solid state were determined by single-crystal Xray diffraction studies and the crystallographic data have been deposited with the Cambridge Crystallographic Data Center under nos. CCDC 2323602 (1), 2323603 (2), 2323604 (3), 2323605 (4), 2349216 (6), 2323606 (7), 2323607 (8), 2323608 (9), and 2323609 (10). These data can be obtained free of charge from The Cambridge Crystallographic Data Center via www.ccdc.cam.ac.uk/data request/cif. All data are also available from corresponding authors upon request. Source data are present in this paper. Source data are provided with this paper.